Abstract
This retrospective cohort study of HIV-infected women receiving highly active antiretroviral therapy (HAART) while pregnant assessed the effect of postpartum HAART discontinuation on maternal AIDS-defining events (ADEs), non-AIDS–defining events (non-ADEs), and death 1997–2008 in Nashville, Tennessee. Cox proportional hazards models compared rates of ADE or all-cause death and non-ADE or all-cause death, and competing risks analyses compared rates of ADE or ADE-related death and non-ADE or non-ADE–related death across the groups. There were two groups: women who stopped HAART postpartum (discontinuation, n = 54) and women who continued HAART postpartum (continuation, n = 69). Fifty percent were African American, 40% had prior non-HAART antiretroviral therapy (ART) use, and 38% had a history of illicit drug use. Median age was 27.5 years, baseline CD4(%) was 532 (34%) and CD4 nadir was 332 cells/mm3, baseline and peak HIV-1 RNA were 2.6 and 4.32 log10 copies per milliliter, respectively. Women in the continuation group were older, had lower baseline CD4, CD4%, and CD4 nadir, and had higher peak HIV-1 RNA. In multivariable proportional hazards models, the hazard ratios [95% confidence interval (CI)] of ADE or all-cause death and non-ADE or all-cause death were lower in the continuation group, but not statistically significantly: 0.50 (0.12, 2.12; p = 0.35) and 0.69 (0.24, 1.95; p = 0.48), respectively. The results were similar in competing risks analyses. Despite having characteristics associated with worse prognosis, women who continued HAART postpartum had lower hazard ratio point estimates for ADEs or death and non-ADEs or death than women who discontinued HAART. Larger studies with longer follow-up are indicated to assess this association.
Introduction
Highly active antiretroviral therapy (HAART) has significantly decreased HIV-1–associated morbidity and mortality1 and the risk of perinatal infection.2 Despite these findings, long-term therapy does not lead to eradication of HIV-1 infection,3 and continues to be associated with substantial toxicity,4 adherence difficulties,5 and drug resistance.6 Several studies have shown that therapy interruption among patients with controlled viremia and high CD4+ lymphocyte count may be safe.7,8 However, a large-scale randomized trial by the Strategies for Management of Antiretroviral Therapy (SMART) Study group determined that subjects undergoing therapy interruption when CD4+ lymphocyte count rose to >350 cells/mm3 had more than twice the risk of HIV disease progression compared with subjects continuing antiretroviral therapy.9 An unexpected finding of the trial was the excess risk of death associated with non-AIDS–related complications.
Pregnant women who start HAART for the prevention of mother-to-child transmission (PMTCT) of HIV and not for the mother's health constitute a special HIV treatment group. As a consequence of the primary reason for treatment initiation in this group, treatment is often discontinued in the postpartum period. The decision to discontinue treatment is made based on “adherence issues, current and nadir CD4+ lymphocyte count, HIV-1 RNA levels, and patient preferences.”10 The effect of treatment discontinuation in postpartum women is unknown, and a randomized control trial planned to investigate this effect is in its early stage of enrolling patients.11 In the meantime, only observational studies will be able to asses the effect of HAART discontinuation on maternal outcomes.
The goal of this study was to evaluate the effect of postpartum HAART discontinuation on maternal AIDS and non-AIDS outcomes. Our hypothesis was that the women continuing HAART postpartum would have lower rates of ADE, non-ADE, and death compared to women discontinuing HAART postpartum.
Methods
Study cohort
This was a retrospective cohort study. The study cohort was defined as HIV-1– infected women with at least one pregnancy while receiving care (>1 visit) at the Comprehensive Care Center in Nashville, Tennessee, between January 1, 1997 and December 31, 2008. All women included also received HAART during pregnancy and had follow-up for >90 days after pregnancy event. Pregnancy event included delivery, miscarriage, or abortion.
Clinical data were entered into an electronic medical record by medical providers at the time of the patient encounter, automated data upload (e.g., laboratory results), or clinic personnel (e.g., deaths). Laboratory and antiretroviral therapy data (including regimen and start and stop dates) were validated by systematic chart review. All study outcomes (defined below) were reviewed and confirmed by study investigators. The study was approved by the Vanderbilt Institutional Review Board.
Definition of study exposure
Study patients were classified according to timing of HAART discontinuation in relation to pregnancy event. Women who discontinued HAART any time during their first pregnancy while in care or <90 days after pregnancy event were included in the discontinuation group; women who continued HAART >90 days after the first pregnancy event while in care were included in the continuation group. The 90-day window after pregnancy event was chosen based on the data showing that the majority of women who discontinued HAART early postpartum did so within 90 days after pregnancy event.
HAART was defined as regimens of more than 7 days duration that contained at least three antiretroviral medications. Any consecutive HAART regimens separated by <30 days or interruptions in HAART regimens of <30 days were counted as uninterrupted HAART. Thus, HAART interruption of >30 days was counted as HAART discontinuation. Non-HAART antiretroviral therapy (ART) included mono- or dual-nucleoside reverse transcriptase inhibitor (NRTI) therapy.
Definition of study outcomes
Outcomes of interest were time to ADE or all-cause death, time to non-ADE or all-cause death, time to ADE or ADE-related death, and time to non-ADE or non-ADE–related death at any time during follow-up. ADEs were based on the 1993 U.S. Centers for Disease Control and Prevention (CDC) classification criteria,12 excluding CD4+ lymphocytes <200 cells/mm3. Non-ADEs were based on the recommendations of an endpoints committee comprised of infectious diseases clinicians in the Vanderbilt-Meharry Center for AIDS Research Epidemiology and Outcomes group. The events included new established cardiovascular, renal, liver, and metabolic diseases, and non-AIDS–associated malignancies. Information on death was obtained from medical records. The records were routinely updated based on reports from families, local newspapers, hospitals, and the Social Security Death Index. A validated form ascertaining cause of death was used to identify causes of death.13 The immediate cause of death, if available, was classified as ADE-related or non-ADE–related, on the basis of the CDC list of AIDS-defining conditions.12
Maternal characteristics such as age, race, baseline CD4+ lymphocyte count and percentage (the most recent values <180 days prior to pregnancy event), CD4+ lymphocyte count nadir (the lowest CD4+ lymphocyte count prior to pregnancy event), baseline HIV-1 RNA, peak HIV-1 RNA prior to pregnancy event, HAART type [protease inhibitor (PI)-based, non-nucleoside reverse transcriptase inhibitor (NNRTI)-based, PI- and NNRTI-based, or neither], hepatitis C virus (HCV) serologic status, history of injection drug use (IDU) and other illicit drug use, history of ADE, prior use of non-HAART ART, history of cardiovascular, renal, liver, and metabolic diseases, history of non-AIDS–associated malignancies, history of preeclampsia, obesity (2009 ICD-9-CM code 278), and year of pregnancy event were also collected. Because data on adherence to HAART or medical care were not available for this study, we used frequency of provider visits during the study period as a surrogate measure of adherence.
Follow-up
Follow-up started 90 days after pregnancy event (T0). For the time to ADE or death (all-cause or ADE-related) analyses, follow-up ended with the first ADE or death (all-cause or ADE-related), last clinic encounter, or December 31, 2008. For the time to non-ADE or death (all-cause or non-ADE–related) analyses, follow-up ended with the first non-ADE or death (all-cause or non-ADE–related), last clinic encounter, or December 31, 2008. All events were reviewed and confirmed by study investigators (P.F.R., S.S.B., D.A.R., S.E.S.).
Statistical analyses
STATA IC (version 10.1; Stata Corporation, College Station, TX) and R statistical software (version 2.9.1) were used for all analyses. Continuous variables were compared with rank sum tests, and categorical variables were compared with Fisher's exact test.
ADE or death and non-ADE or death analyses
Cox proportional hazards models compared rates of ADE or all-cause death and rates of non-ADE/all-cause death according to timing of HAART discontinuation, both unadjusted and adjusted for the propensity of belonging to the discontinuation versus the continuation group. Competing risks analyses assessed the risk of ADE or ADE-related death conditional on not dying from non-ADE-related causes and the risk of non-ADE or non-ADE–related death conditional on not dying from ADE-related causes.14
The propensity scores of the estimated probability of belonging to the discontinuation group versus the continuation group were derived from logistic regression models that predicted belonging to the groups based on age, race, baseline CD4+ lymphocyte count and percentage, CD4+ lymphocyte count nadir, baseline HIV-1 RNA (log-transformed), peak HIV-1 RNA prior to pregnancy event (log-transformed), HAART type, HCV serologic status, history of IDU and other illicit drug use, history of ADE, prior use of non-HAART ART, history of cardiovascular and metabolic diseases, history of preeclampsia, obesity, and year of pregnancy event. There were no persons with history of renal or liver disease, or non-AIDS–associated malignancies, so these variables were not included in the model. The variables were chosen for inclusion in the model a priori. We also used different windows following pregnancy event (i.e., 60- and 120-day window) for the above analyses.
Results
Patient characteristics
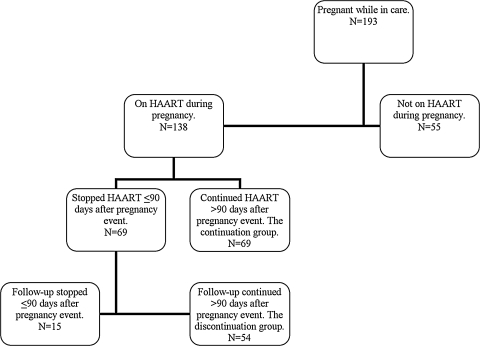
Of 193 women with more than one pregnancy while in care at the Comprehensive Care Center between January 1, 1997 and December 31, 2008, 138 women received HAART during their first pregnancy. Of these 138 women, 69 stopped HAART >90 days after pregnancy event (the continuation group), and 54 stopped HAART during pregnancy or <90 days after pregnancy event and were in care >90 days after pregnancy event (the discontinuation group). The remaining 15 women were in care <90 days after pregnancy event, and, thus, were excluded from the survival analyses (Fig. 1).
FIG. 1.
Flow chart for patient selection. HAART, highly active antiretroviral therapy.
Compared to the 54 women in the discontinuation group, the 15 women who stopped HAART <90 days after pregnancy event but had <90 days of follow-up had lower baseline CD4+ lymphocyte count and percent, lower CD4+ lymphocyte nadir, higher peak HIV-1 RNA level, shorter pregnancy duration, and a higher proportion with history of IDU (4/15; 26.7% versus 1/54; 1.9%). There were no differences in the proportion of women with ADE or all-cause death, or non-ADE or all-cause death (data not shown).
Table 1 shows demographic and clinical characteristics of the 123 study subjects. Women in the continuation group were older, had lower baseline CD4+ lymphocyte count and percentage, lower CD4+ lymphocyte nadir, higher peak HIV-1 RNA, were more likely to be on ART in the past, and were less likely to have initiated their first HAART regimen during pregnancy. Hypertension and diabetes were the only prior cardiovascular and metabolic disease diagnoses identified. There were no persons with a history of renal or liver disease, or non-AIDS–associated malignancies.
Table 1.
Demographic and Clinical Characteristics of the Study Population (n = 123)
| Characteristic |
Study population n = 123 |
Discontinuation group n = 54 |
Continuation group n = 69 |
pa |
|---|---|---|---|---|
| Age at pregnancy event, median (IQR), years | 27.5 (24.6–32.7) | 26.1 (24.0–28.9) | 29.7 (25.9–35.1) | 0.001 |
| Race/Ethnicity, no. (%) | 0.95 | |||
| Caucasian | 38 (30.9) | 15 (27.8) | 23 (33.3) | |
| African American | 62 (50.4) | 28 (51.9) | 34 (49.3) | |
| Hispanic | 13 (10.6) | 7 (13) | 6 (8.7) | |
| African | 7 (5.7) | 3 (5.6) | 4 (5.8) | |
| Unidentified | 2 (1.6) | 1 (1.9) | 1 (1.5) | |
| Baseline CD4+ lymphocyte count,b median (IQR), cells/mm3 | 532 (352–739) | 577 (450–750) | 441 (306–641) | 0.008 |
| Baseline CD4+ lymphocyte percentage,b median (IQR), percent | 34 (27–42) | 36 (32–44) | 32 (23–38) | 0.007 |
| CD4+ lymphocyte count nadir,c median (IQR), cells/mm3 | 332 (222–448) | 398 (306–476) | 270 (198–392) | <0.001 |
| Baseline HIV-1 RNA,b median (IQR), log10 copies per milliliter | 2.60 (2.60–2.60) | 2.60 (2.60–2.60) | 2.60 (2.60–2.60) | 0.99 |
| Peak HIV-1 RNA,d median (IQR), log10 copies per milliliter | 4.37 (3.66–4.97) | 4.04 (3.37–4.53) | 4.71 (4.10–5.09) | <0.001 |
| HAART type | 0.20 | |||
| PI-based | 68 (55.3) | 31 (57.4) | 37 (53.6) | |
| NNRTI-based | 28 (22.8) | 12 (22.2) | 16 (23.2) | |
| PI- and NNRTI-based | 14 (11.4) | 3 (5.6) | 11 (16) | |
| Other | 13 (10.6) | 8 (14.8) | 5 (7.3) | |
| History of ADE, no. (%) | 7 (5.7) | 2 (3.7) | 5 (7.3) | 0.47 |
| Prior non-HAART ART use, no. (%) | 49 (39.8) | 14 (25.9) | 35 (50.7) | 0.006 |
| Started first HAART during pregnancy, no. (%) | 87 (70.7) | 47 (87) | 40 (58) | 0.001 |
| Injection drug use,e no. (%) | 7 (5.7) | 1 (2) | 6 (8.7) | 0.13 |
| Illicit drug use,e no. (%) | 47 (38.2) | 16 (29.6) | 31 (45) | 0.10 |
| History of HCV infection, no. (%) | 3 (2.4) | 2 (3.7) | 1 (1.5) | 0.58 |
| History of preeclampsia, no. (%) | 7 (5.7) | 5 (9.3) | 2 (2.9) | 0.24 |
| History of cardiovascular diseases,f no. (%) | 7 (5.7) | 2 (3.7) | 5 (7.3) | 0.47 |
| History of metabolic diseases,g no. (%) | 2 (1.6) | 0 | 2 (2.9) | 0.50 |
| Obesity,h no. (%) | 36 (29.3) | 15 (27.8) | 21 (30.4) | 0.84 |
| Year of pregnancy event, median (IQR), year | 2003 (2001–2006) | 2004 (2002–2006) | 2002 (2000–2006) | 0.07 |
| Pregnancy duration, median (IQR), days | 266 (252–269) | 266 (252–270) | 263 (252–267) | 0.74 |
Continuous data were compared by Kruskal-Wallis test. Categorical data were compared by two-sided Fisher's exact test.
Baseline values were defined as the latest values <180 days prior to pregnancy event.
CD4+ lymphocyte count nadir was defined as the lowest CD4+ lymphocyte count prior to pregnancy event.
Peak HIV-1 RNA prior to pregnancy event.
History of injection drug use and other illicit drug use.
Hypertension was the only prior cardiovascular disease diagnosis identified.
Diabetes was the only prior metabolic disease diagnosis identified.
Obesity diagnosis (2009 ICD-9-CM code 278).
IQR, interquartile range; HAART, highly active antiretroviral therapy; PI, protease inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; HCV, hepatitis C virus infection; ADE, AIDS-defining event; ART, antiretroviral therapy. HAART discontinuation group; women who discontinued HAART any time during pregnancy or ≤90 days after pregnancy event (defined as delivery, miscarriage, or abortion); HAART continuation group, women who continued HAART >90 days after pregnancy event.
Median follow-up [interquartile range (IQR)] was 36.7 (18.3–59.5) months in the discontinuation group and 54.5 (19.4–87) months in the continuation group (p = 0.09). Frequency (IQR) of visits during the study period did not differ between the study groups: 0.66 (0.49–1.01) visits/month in the discontinuation group and 0.79 (0.54–1.27) visits/month in the continuation group (p = 0.86).
Number of subsequent pregnancies during the study period also did not differ between the groups: 10 (18.5%) in the discontinuation group and 15 (21.7%) in the continuation group (p = 0.16). There were no cases of mother-to-child transmission of HIV-1.
Over the course of the study period, use of PI-based HAART regimens increased from 23% during 1997–2000 to 48% during 2001–2005 and 91% during 2006–2008; NNRTI-based HAART use was the highest (50%) during 1997–2000 compared to 2001–2005 (24%) and 2006–2008 (0%); NRTI-based HAART use was the highest during 2001–2005 (19%) compared to 1997–2000 (7%) and 2006–2008 (0%).
Twenty-eight (52%) women in the discontinuation group went back on HAART during the study period, with the median time (IQR) between the HAART regimens 1.89 (1.21–3.23) years. Those who went back on HAART did not differ from those who remained off HAART in age, ethnicity, baseline CD4 percentage and HIV-1 RNA level, peak HIV-1 RNA, proportion with prior-ADE, hepatitis C, injection drug use, illicit drug use, history of preeclampsia, hypertension, HAART type, obesity, and pregnancy duration. However, those who went back on HAART compared to those who remained off of HAART had lower baseline CD4+ lymphocyte count (478.5 versus 709 cells/mm3, p = 0.02) and CD4+ lymphocyte nadir (374.5 versus 428.5 cells/mm3, p = 0.04), had higher proportion of persons with subsequent pregnancies while in care (35.7 versus 0%, p = 0.001) and prior non-HAART ART use (39.3 versus11.5%, p = 0.03), and were pregnant during an earlier era (2003 versus 2005.5, p = 0.001). The proportion of women who developed ADE/all-cause death and non-ADE did not differ between the groups. Women who restarted HAART did so at median (IQR) CD4+ lymphocyte count 288 (227–454) cells/mm3, CD4+ lymphocyte percentage 24 (19–31), and HIV-1 RNA 4.01 (3.44–4.83) log10 copies/mL.
ADE or death analyses
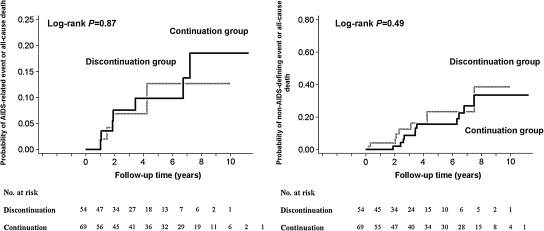
Outcomes by study group are listed in Table 2. The rates of ADE or all-cause death did not differ significantly between women who continued and discontinued HAART postpartum: the hazard ratio (HR) for the continuation group 1.11, 95% CI 0.32, 3.86, p = 0.87 (Table 3 and Fig. 2). In univariate analyses, higher baseline CD4+ lymphocyte count and percentage, higher CD4+ lymphocyte count nadir, and pregnancy event during a later era were associated with slower progression to ADE or death. Higher baseline HIV-1 RNA and peak HIV-1 RNA while in care, history of ADE, IDU, illicit drug use, and HCV infection were associated with higher rates of progression to ADE or death (Table 3).
Table 2.
Outcomes in Study Patients, by Study Group
| Outcome |
Discontinuation group n = 54 |
Continuation group n = 69 |
pa |
|---|---|---|---|
| ADE or all-cause death, no. (%) | 4 (7.4) | 7 (10.1) | 0.75 |
| Non-ADE or all-cause death, no. (%) | 9 (16.7) | 12 (17.4) | 1.00 |
| ADE, no. (%) | 3 (5.6) | 6 (8.7) | 0.73 |
| 1 (1.9) Mycobacterium avium complex infection | 4 (5.8) Wasting | ||
| 1 (1.9) Pneumocystis jirovecii pneumonia | 1 (1.5) Pneumocystis jirovecii pneumonia | ||
| 1 (1.9) Pneumonia | 1 (1.5) AIDS dementia | ||
| Non-ADE, no. (%) | 7 (13) | 10 (14.5) | 1.00 |
| 2 (3.7) Diabetes | 8 (11.6) Hypertension | ||
| 5 (9.3) Hypertension | 1 (1.5) Renal disease | ||
| 1 (1.5) Non-AIDS–associated malignancy | |||
| All-cause death, no. (%) | 3 (5.6) | 5 (7.3) | 1.00 |
| ADE-related deaths | 3 (5.6) | 3 (4.4) | 1.00 |
| Non-ADE–related deaths | 0 | 2 (2.9) | 0.50 |
Categorical data were compared by two-sided Fisher's exact test.
ADE, AIDS-defining event.
Table 3.
Univariate Cox Proportional Hazards Models: Progression to AIDS-Defining Event or All-Cause Death and to Non-AIDS-Defining Event or All-Cause Death
| Variables | Hazard ratio (95% CI), ADE or all-cause death | p | Hazard ratio (95% CI), Non-ADE or all-cause death | p |
|---|---|---|---|---|
| HAART continuation group | 1.11(0.32, 3.86) | 0.87 | 0.72 (0.29, 1.82) | 0.49 |
| Age at pregnancy event, per year | 1.05 (0.97, 1.13) | 0.24 | 1.04 (0.98, 1.10) | 0.23 |
| Race/Ethnicity | 0.82 (0.52, 1.30) | 0.41 | 0.83 (0.56, 1.22) | 0.34 |
| Baseline CD4+ lymphocyte count, per 1 cell/mm3 increase | 0.99 (0.99, 1.00) | <0.001 | 1.00 (1.00, 1.00) | 0.59 |
| Baseline CD4+ lymphocyte percentage, per 1% increase | 0.92 (0.88, 0.96) | <0.001 | 0.98 (0.95, 1.02) | 0.27 |
| CD4+ lymphocyte count nadir, per 1 cell/mm3 increase | 1.00 (0.99, 1.00) | 0.001 | 1.00 (1.00, 1.00) | 0.23 |
| Baseline HIV-1 RNA, per 1 log10 copies per milliliter increase | 2.61 (1.60, 4.23) | <0.001 | 1.96 (1.03, 2.47) | 0.04 |
| Peak HIV-1 RNA, per1 log10 copies per milliliter increase | 2.57 (1.41, 4.69) | 0.002 | 1.55 (0.98, 2.45) | 0.06 |
| HAART type | 1.83 (0.90, 3.74) | 0.10 | 1.03 (0.61, 1.74) | 0.90 |
| Prior ADE | 8.90 (2.95, 26.88) | <0.001 | 6.35 (2.16, 18.61) | 0.001 |
| Prior non-HAART ART use | 2.33 (0.96, 5.62) | 0.06 | 1.68 (0.84, 3.38) | 0.14 |
| Injection drug use | 5.64 (2.34, 13.62) | <0.001 | 3.10 (1.39, 6.92) | 0.01 |
| Illicit drug use | 2.73 (1.10, 6.27) | 0.03 | 1.64 (0.82, 3.28) | 0.17 |
| History of HCV infection | 6.19 (1.43, 26.85) | 0.02 | 6.51 (1.52, 27.84) | 0.01 |
| History of preeclampsia | —a | 2.35 (0.31, 17.56) | 0.41 | |
| History of cardiovascular disease | —a | —a | ||
| History of metabolic disease | —a | —a | ||
| Obesity | 0.76 (0.28, 2.07) | 0.59 | 1.55 (0.76, 3.19) | 0.23 |
| Year of pregnancy event | 0.79 (0.63, 0.99) | 0.04 | 0.94 (0.79, 1.13) | 0.53 |
ADE, AIDS-defining event; Non-ADE, non-AIDS-defining event; HAART, highly active antiretroviral therapy; ART, antiretroviral therapy; IQR, interquartile range; HCV, hepatitis C virus infection.
HR <4.55e-15, P = 1.00.
FIG. 2.
Kaplan-Meier failure curve of progression to new AIDS-defining event or all-cause death (left panel) or non-AIDS-defining event or all-cause death (right panel) according to the timing of HAART discontinuation. The numbers of women at risk each year are also given.
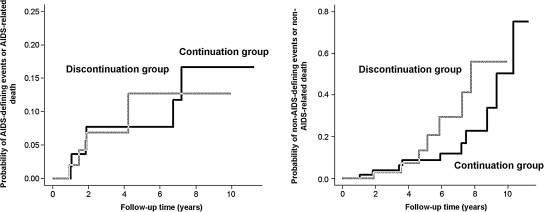
In multivariable Cox proportional hazards models that included the propensity score, the HR of progressing to ADE or all-cause death for the continuation group was 0.50, 95% CI 0.12, 2.12; p = 0.35. In multivariable competing risks analysis that included the propensity score, the HR of progressing to ADE or ADE-related death for the continuation group was 0.46, 95% CI 0.15, 1.45; p = 0.15 (Fig. 3). Use of different windows following pregnancy event (i.e., 60- and 120-day window) did not change the results (data not shown).
FIG. 3.
Competing risk analyses: cumulative incidences of AIDS-defining event or AIDS-related death (left panel) and of non-AIDS-defining events or non-AIDS-related death (right-panel).
Non-ADE or death analyses
Non-ADEs by study group are listed in Table 2. The rates of non-ADE or all-cause death according to group did not differ significantly between women who continued and discontinued HAART: the HR for the continuation group was 0.72, 95% CI 0.29, 1.82; p = 0.49 (Table 3 and Fig. 2). In univariate Cox proportional hazards models, higher baseline HIV-1 RNA, prior ADE, history of IDU and HCV infection were associated with higher rates of progression to non-ADE or death (Table 3).
In multivariable Cox proportional hazards models that included the propensity score, the HR of progressing to non-ADE or all-cause death for the continuation group was 0.69, 95% CI 0.24, 1.95; p = 0.48. In a multivariable competing risks analysis that included the propensity score, the HR of progressing to non-ADE or non-ADE-related death for the continuation group was 0.70, 95% CI 0.27, 1.82; p = 0.46 (Fig. 3). Use of different windows following pregnancy event (i.e., 60- and 120-day window) did not change the results (data not shown).
Discussion
In this study, we compared long-term rates of ADE or death and non-ADE or death in women who discontinued HAART <90 days after pregnancy event with rates among those who continued HAART >90 days after pregnancy event. In our cohort, women who continued HAART beyond 90 days had characteristics generally associated with increased risk of progression to ADE or death and non-ADE or death (Table 1): lower CD4+ lymphocyte counts, higher HIV-1 RNA, and older age. However, after adjusting for these characteristics, our HR estimates suggested that it may be beneficial to continue HAART postpartum. Our findings were not statistically significant, with wide confidence intervals including HR of one; therefore, these results do not provide strong evidence that continuation of HAART postpartum decreases the risk of ADE, non-ADE, and death. However, taken with data from other studies, particularly the SMART study, our findings warrant further investigation of the effects of HAART continuation postpartum in larger population.
The lack of statistical evidence is likely due to the small number of women in our study who progressed to ADE or death and non-ADE or death. If indeed continuing therapy causes a 50% reduction in the hazard of ADE or all-cause death, it would require approximately 740 women with similar HAART continuation/discontinuation rates to those seen in our study to have 80% power to detect a reduction at the 0.05 significance level. To detect a 30% reduction in the hazard of non-ADE or all-cause death, it would require nearly 1800 subjects.
To our knowledge, this is the first study that has assessed rates of ADE, non-ADE, and death according to timing of HAART discontinuation postpartum. In larger randomized control studies of HIV-1-infected patients that excluded pregnant women (the SMART and the Trivacan studies), HAART interruption was found to lead to higher rates of morbidity and mortality.9,15 A randomized study, the HAART Standard Version of the Promoting Maternal and Infant Survival Everywhere (PROMISE) Study, is planned and will assess maternal health outcomes among women who initiate HAART during pregnancy for PMTCT and not for their own health.11 Results of this interventional trial are not expected for several years. Until then, only observational studies can provide information on the effect of postpartum HAART discontinuation on maternal outcomes. However, most large observational cohort studies do not collect data on pregnancy (e.g., NA-ACCORD and ART-CC).
A few retrospective studies looked at maternal HIV outcomes after pregnancy according to receipt of ART postpartum. These studies included women who received mono- or dual-NRTI therapy during pregnancy,16 did not assess for non-HIV outcomes,16,17 and had shorter study periods.18 These studies showed that women discontinuing ART postpartum either had an increased risk of progression to ADE or death,16 no difference in progression to ADE or death,17 or increased risk of class B events.18
HAART use in our cohort was similar to the use in another study in a resource-rich setting over comparable period of time19: PI-based HAART regimen use increased and NNRTI- and NRTI-based HAART regimen use decreased during the study period.
Our study was an observational study and had several limitations. We had no data on duration of HIV-1 infection. There is evidence that in early HIV infection and in persons with CD4+ lymphocyte nadir >350 cells/mm3, HAART interruption is less detrimental to short-term HIV disease progression.8,20,21 We attempted to account for these factors in part by adjusting for CD4+ lymphocyte nadir and peak HIV-1 RNA levels.
In addition, we did not have data on other medications the patients received during pregnancy that could have influenced non-ADE outcomes, such as lipid-lowering medications and antihypertensives. However, it is unlikely that many of the patients were on these medications without indication by a relevant diagnosis at baseline.
Although a surrogate measure of adherence (i.e., frequency of provider visit during the follow-up) did not differ between the study groups, a validated measure of adherence was not available for this study.22 Potentially poorer adherence to HAART among women in the discontinuation group may have led to the poorer HIV-related outcomes in that group.
Finally, residual confounding by indication might remain despite statistical methods to account for the differences between the groups. For example, women stopping HAART postpartum might have discontinued HAART due to poor adherence or a desire to be off of HAART. These women may have been less likely to resume HAART during follow-up due to these factors. This might have led to poorer outcomes in those who discontinued HAART postpartum.
With the above limitations noted, this study identifies the need both for larger observational studies and randomized controlled trials to assess the long-term safety of the current guidelines to consider postpartum HAART discontinuation among women who do not require HAART for their own health. Since a growing proportion of HIV-1–infected women are of child-bearing age,23 it will become increasingly important to understand the effects of postpartum HAART discontinuation on maternal health.
Acknowledgments
This research was supported by the National Institutes of Health: the Vanderbilt-Meharry Center for AIDS Research (P30 AI54999 to B.E.S., S.E.S., P.F.R., S.S.B., D.A.R., T.R.S.; K24 AI065298 to S.E.S., T.R.S; the Vanderbilt Physician Scientist Development Award to V.V.M. We would like to thank providers and all patients at the Comprehensive Care Center whose participation made these analyses possible.
T.R.S. has received a research grant from Pfizer.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Palella FJ., Jr. Delaney KM. Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 2.Achievements in public health. Reduction in perinatal transmission of HIV infection—United States, 1985-2005. MMWR Morb Mortal Wkly Rep. 2006;55:592–597. [PubMed] [Google Scholar]
- 3.Siliciano JD. Kajdas J. Finzi D, et al. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat Med. 2003;9:727–728. doi: 10.1038/nm880. [DOI] [PubMed] [Google Scholar]
- 4.Morse CG. Kovacs JA. Metabolic and skeletal complications of HIV infection: The price of success. JAMA. 2006;296:844–854. doi: 10.1001/jama.296.7.844. [DOI] [PubMed] [Google Scholar]
- 5.Harrington M. Carpenter CC. Hit HIV-1 hard, but only when necessary. Lancet. 2000;355:2147–2152. doi: 10.1016/S0140-6736(00)02388-6. [DOI] [PubMed] [Google Scholar]
- 6.Johnson VA. Brun-Vezinet F. Clotet B, et al. Update of the Drug Resistance Mutations in HIV-1. Top HIV Med. 2008;16:138–145. [PubMed] [Google Scholar]
- 7.Cardiello PG. Hassink E. Ananworanich J, et al. A prospective, randomized trial of structured treatment interruption for patients with chronic HIV type 1 infection. Clin Infect Dis. 2005;40:594–600. doi: 10.1086/427695. [DOI] [PubMed] [Google Scholar]
- 8.Krolewiecki AJ. Zala C. Vanzulli C, et al. Safe treatment interruptions in patients with nadir CD4 counts of more than 350 cells/μL: A randomized trial. J Acquir Immune Defic Syndr. 2006;41:425–429. doi: 10.1097/01.qai.0000219984.27824.6d. [DOI] [PubMed] [Google Scholar]
- 9.El-Sadr WM. Lundgren JD. Neaton JD, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 10.Perinatal HIV Guidelines Working Group. Public Health Service Task Force Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV Transmission in the United States. Jul 8, 2008. http://aidsinfo.nih.gov/ContentFiles/PerinatalGL.pdf. [Sep 30;2009 ]. pp. 1–98.http://aidsinfo.nih.gov/ContentFiles/PerinatalGL.pdf
- 11.National Institute of Allergy and Infectious Diseases. HAART Standard Version of the Promoting Maternal and Infant Survival Everywhere (PROMISE) Study. National Library of Medicine (US) Nov 25, 2009. http://clinicaltrials.gov/ct2/show/NCT00955968?term=promise&rank=12. [Sep 30;2009 ]. http://clinicaltrials.gov/ct2/show/NCT00955968?term=promise&rank=12
- 12.Centers for Disease Control and Prevention. 1993 revised classification system for HIV infection and expanded surveillance care definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41:1–19. [PubMed] [Google Scholar]
- 13.Fusco GP. Jusitce AC. Becker SL. Raffanti SP. Coll-Erickson P. Fusco JS. Strategies for obtaining consistent diagnoses for cause of death in an HIV observational cohort study. Poster Exhibit: The XIV International AIDS Conference; 2002. Abstract no. TuPeG5686. [Google Scholar]
- 14.Fine JP. Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 15.Danel C. Moh R. Minga A, et al. CD4-guided structured antiretroviral treatment interruption strategy in HIV-infected adults in west Africa (Trivacan ANRS 1269 trial): a randomized trial. Lancet. 2006;367:1981–1989. doi: 10.1016/S0140-6736(06)68887-9. [DOI] [PubMed] [Google Scholar]
- 16.Onen NF. Nurutdinova D. Sungkanuparph S. Gase D. Mondy K. Overton ET. Effect of postpartum HIV treatment discontinuation on long-term maternal outcome. J Int Assoc Physicians AIDS Care (Chic Ill) 2008;7:245–251. doi: 10.1177/1545109708325466. [DOI] [PubMed] [Google Scholar]
- 17.Martin F. Navaratne L. Khan W, et al. Pregnant women with HIV infection can expect healthy survival: Three-year follow-up. J Acquir Immune Defic Syndr. 2006;43:186–192. doi: 10.1097/01.qai.0000233311.28602.4d. [DOI] [PubMed] [Google Scholar]
- 18.Watts DH. Lu M. Thompson B, et al. Treatment interruption after pregnancy: Effects on disease progression and laboratory findings. Infect Dis Obstet Gynecol. 2009;2009:456717. doi: 10.1155/2009/456717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baroncelli S. Tamburrini E. Ravizza M, et al. Antiretroviral treatment in pregnancy: A six-year perspective on recent trends in prescription patterns, viral load suppression, and pregnancy outcomes. AIDS Patient Care STDs. 2009;23:513–520. doi: 10.1089/apc.2008.0263. [DOI] [PubMed] [Google Scholar]
- 20.Pogany K. van Valkengoed IG. Prins JM, et al. Effects of active treatment discontinuation in patients with a CD4+ T-cell nadir greater than 350 cells/mm3: 48-week Treatment Interruption in Early Starters Netherlands Study (TRIESTAN) J Acquir Immune Defic Syndr. 2007;44:395–400. doi: 10.1097/QAI.0b013e31802f83bc. [DOI] [PubMed] [Google Scholar]
- 21.Steingrover R. Pogany K. Fernandez Garcia E, et al. HIV-1 viral rebound dynamics after a single treatment interruption depends on time of initiation of highly active antiretroviral therapy. AIDS. 2008;22:1583–1588. doi: 10.1097/QAD.0b013e328305bd77. [DOI] [PubMed] [Google Scholar]
- 22.Miller LG. Hays RD. Measuring adherence to antiretroviral medications in clinical trials. HIV Clin Trials. 2000;1:36–46. doi: 10.1310/enxw-95pb-5ngw-1f40. [DOI] [PubMed] [Google Scholar]
- 23.United Nations Joint Programme on HIV/AIDS. 2007 AIDS epidemic update. 2008. www.unaids.org/en/ [Sep 30;2009 ]. www.unaids.org/en/