Abstract
The role of hydrogen sulfide (H2S) in inflammation is controversial, with both pro- and antiinflammatory effects documented. Many studies have used simple sulfide salts as the source of H2S, which give a rapid bolus of H2S in aqueous solutions and thus do not accurately reflect the enzymatic generation of H2S. We therefore compared the effects of sodium hydrosulfide and a novel slow-releasing H2S donor (GYY4137) on the release of pro- and antiinflammatory mediators in lipopolysaccharide (LPS)-treated murine RAW264.7 macrophages. For the first time, we show that GYY4137 significantly and concentration-dependently inhibits LPS-induced release of proinflammatory mediators such as IL-1β, IL-6, TNF-α, nitric oxide (•NO), and PGE2 but increased the synthesis of the antiinflammatory chemokine IL-10 through NF-κB/ATF-2/HSP-27–dependent pathways. In contrast, NaHS elicited a biphasic effect on proinflammatory mediators and, at high concentrations, increased the synthesis of IL-1β, IL-6, NO, PGE2 and TNF-α. This study clearly shows that the effects of H2S on the inflammatory process are complex and dependent not only on H2S concentration but also on the rate of H2S generation. This study may also explain some of the apparent discrepancies in the literature regarding the pro- versus antiinflammatory role of H2S. Antioxid. Redox Signal. 12, 1147–1154.
Introduction
Hydrogen sulfide (H2S) is a pungent gas that is formed endogenously in mammalian tissues from the amino acids cysteine and homocysteine by pyridoxal-5′-phosphate–dependent enzymes such as cystathionine-γ-lyase (CSE; E.C. 4.4.1.1) and cystathionine-β-synthetase (CBS; E.C. 4.2.1.22) (12, 28). To date, H2S biosynthesis has been identified in a variety of mammalian tissues, notably in the brain, heart, and the gastrointestinal tract, as well as in isolated vascular smooth muscle and endothelial cells and neurons (19, 29). A number of possible physiologic and pathophysiologic roles for this gas have been put forward, and a range of potential therapeutic uses of this gas has been proposed (10, 21, 28).
It is now becoming increasingly apparent that H2S exerts complex effects on inflammation. For example, we previously reported that administration of sodium hydrosulfide (NaHS), a “fast releasing” H2S donor, to mice (9) provokes an inflammatory reaction, as evidenced by increased liver and lung myeloperoxidase (MPO) activity (a marker for tissue leukocyte infiltration) and histologically by the presence of accumulated leukocytes extravascularly in the lung. These results suggest a proinflammatory effect of H2S, as does the finding that dl-propargylglycine (PAG), an irreversible inhibitor of CSE, exhibits antiinflammatory activity in a range of animal models of inflammation (2, 4, 14).
However, NaHS also has been reported to inhibit leukocyte adhesion to gastric mucosal blood vessels (30), which may be suggestive of an antiinflammatory effect. In addition, H2S “scavenges” proinflammatory oxidants such nitric oxide (•NO), peroxynitrite (ONOO−), hypochlorous acid (HOCl) (25, 26), superoxide, and hydrogen peroxide (3, 6, 15); such effects might be expected to alleviate inflammation. Finally, S-diclofenac (an H2S-releasing derivative of the nonsteroidal antiinflammatory drug, diclofenac) exhibits more-pronounced antiinflammatory activity in endotoxic shock (11) and against carrageenan-induced hindpaw edema (18) in the rat than does diclofenac. In each case, evidence has been presented that the augmented antiinflammatory action of this compound is secondary to the release of H2S from the parent molecule.
Recently, this group reported that GYY4137 [morpholin-4-ium 4 methoxyphenyl(morpholino) phosphinodithioate] releases H2S slowly over a period of hours both in vitro and after injection in the rat in vivo (13). In addition, GYY4137 exhibits antiinflammatory activity in vivo, as evidenced by a reduction in the lipopolysaccharide (LPS)-induced increase in plasma proinflammatory cytokines (TNF-α, IL-1β, IL-6), nitrite/nitrate, C-reactive protein, and l-selectin in the conscious rat (12).
H2S exerts complex and, at times, opposing effects on inflammation in whole animals. One possible explanation for these discrepant data may be the choice of H2S donor used in these various studies. The available H2S donors release H2S at different rates and therefore give rise to different concentrations of the gas over different time periods. In the present work, we therefore compared the effect on LPS-induced proinflammatory enzyme/metabolite generation in cultured RAW 264.7 macrophages of the fast-releasing H2S donor, NaHS, and the slow-releasing H2S donor, GYY4137.
Materials and Methods
Culture of RAW 264.7 cells
The murine RAW 264.7 macrophage cell line was purchased from the American Type Culture Collection (Rockville, MD). RAW 264.7 cells were chosen for the present experiments, as macrophages play an integral part in the etiology of inflammation, and their response to LPS has been intensively characterized. Cells were cultured in complete Dulbecco's Modified Eagle Medium (containing 10% vol/vol fetal bovine serum, 100 U/ml penicillin, and 100 mg/ml streptomycin, pH 7.4) at 37°C in 5% CO2 until ∼70–80% confluence. Cells (0.2 × 106 cells/ml) were then cultured overnight before the addition of either NaHS or GYY4137 (both 0–1,000 μM) along with an appropriate volume of vehicle, as well as LPS (1 μg/ml). After a further 24-h incubation period, medium or cells or both were harvested and assayed, as described later.
For some experiments, GYY4137 was prepared in aqueous solution and left unstoppered at room temperature for 5 days. Such “decomposed GYY4137” failed to release H2S on incubation and was therefore used as a control to assess the role of released H2S in the effect of GYY4137. To determine whether H2S donors were cytotoxic in these cells, cellular viability was assessed by using 3-(4,5-dimethyl-2-yl)-2,5-diphenyltetrazolium bromide (MTT), as described (25).
Assay of CSE/CBS enzyme activity and measurement of H2S
CSE and CBS recombinant protein (12.5 μg; Abnova Ltd, Taiwan) were added to Tris-HCl buffer (100 mM; pH 7.4; 25°C) containing l-cysteine (10 μM) and pyridoxal phosphate (10 μM). H2S generation was detected by using a World Precision Instruments H2S-selective membrane and electrode (ISO-H2S-2; 2 mm, Sarasota, FL), with four-channel TBR4100-416 radical detector equipped with a Lab-Trax-4 four-channel data-acquisition system, as described previously (13). In separate experiments, H2S generation from added NaHS (1 μM) and GYY4137 (100 μM) in phosphate-buffered saline (3 ml; pH 7.4; 25°C) also was determined for comparison.
Assay of nitrite PGE2, H2S, IL-1β, TNF-α, IL-6, and IL-10 in medium
Levels of nitrite (NO2−), PGE2, IL-1β, TNF-α, IL-6, and IL-10 were assayed in culture media. NO2− was determined spectrophotometrically in aliquots of culture medium by using the Griess reagent, as described elsewhere (17). H2S in culture medium was measured spectrophotometrically by using the methylene blue assay, as described previously (9). IL-1β, TNF-α, IL-6, and IL-10 were assayed with ELISA, according to the manufacturer's instructions (R&D Systems, Inc., Minneapolis, MN). PGE2 production was determined by using a PGE2 enzyme immunoassay kit according to the manufacturer's instructions (Cayman, Ann Arbor, MI).
Assay of NF-κB, HSP-27, and pATF-2 in cells
RAW 264.7 cells treated as described earlier were harvested, and the nuclear proteins extracted by using a nuclear extraction kit (Panomics, Fremont, CA), as described previously (11, 27). The nuclear extracts (10–20 μg) were assayed in duplicate for activity by using TransAM NF-κB p65 assay kit (Active Motif, Carlsbad, CA), according to the manufacturer's instructions. Data are shown as relative light units (RLUs). Phosphorylation of HSP-27 and ATF-2 was assayed quantitatively by using Fast Activated Cell-based ELISA (FACE) HSP27(S82) and ATF2 (T71) kits (Active Motif Europe, Rixensart, Belgium), again according to the manufacturer's instruction.
Chemicals and data analysis
GYY4137 was synthesised chemically by Dr. Choon-Hong Tan (Department of Chemistry, National University of Singapore), as described previously (13). Analytic kits were purchased from suppliers, as stated in the text. All drugs and chemicals were obtained from Sigma Chemical Company (Peele, U.K.). Data are show as mean ± SEM, with the number of observations indicated in parentheses. Statistical analysis was with one-way ANOVA followed by the post hoc Tukey test. A p value of < 0.05 was taken to indicate a statistically significant difference.
Results
Release of H2S from CSE/CBS, GYY4137, and NaHS in vitro
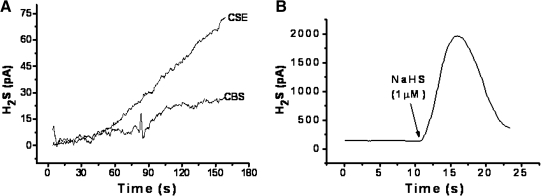
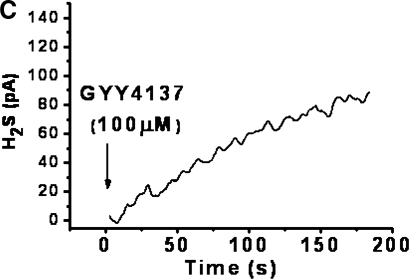
Incubation of either recombinant CSE or CBS enzyme with added l-cysteine and cofactor resulted in the time-dependent formation of H2S (Fig. 1A). CSE produced more H2S than CBS under these experimental conditions, with the amount generated still increasing at 180 s for both enzymes. Incubation of GYY4137 in aqueous solution also resulted in the release of similar amounts of H2S over a similar time frame (Fig. 1C). In contrast, release of H2S from incubated NaHS was much greater (∼200-fold) and occurred over a much shorter time period (Fig. 1B).
FIG. 1.
Time course of in vitro enzymatic formation of H2S from l-cysteine by recombinant CSE and CBS (A) and spontaneous H2S release from incubated NaHS (1 μM, B) and GYY4137 (100 μM, C). H2S was detected amperometrically. Results show representative traces from at least four separate experiments.
Effect of GYY4137 and NaHS on LPS-evoked PGE2 and NO2− formation
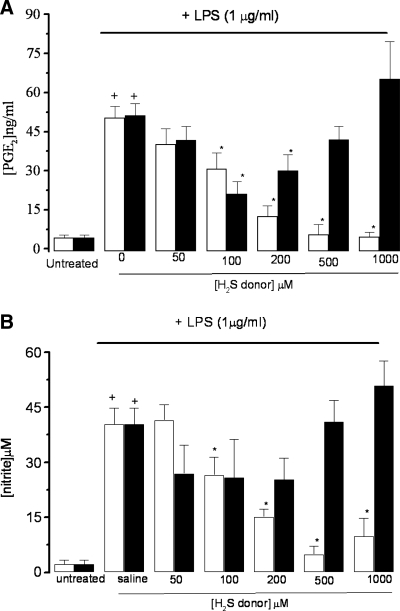
As expected (17), treatment of RAW 264.7 cells with LPS resulted in a significant increase in both PGE2 and NO2− concentrations in the medium. Treatment of LPS-exposed RAW 264.7 cells with GYY4137 (0–1,000 μM) resulted in a concentration-dependent inhibition of the biosynthesis of both PGE2 (Fig. 2A) and NO2− (Fig. 2B), with half-maximal inhibitory concentration (IC50) values of 210.9 ± 4.5 and 127.2 ± 32.4 μM (n = 5). Furthermore, similar treatment of RAW 264.7 cells with GYY4137 (100 μM) resulted in a significant increase in the concentration of H2S detected in the culture medium after 24 h (29.2 ± 1.8 μM, c.f. 1.6 ± 0.7 μM; n = 5; p < 0.05). Inhibition of LPS-evoked formation of both PGE2 and NO2− was >90% inhibition at the higher concentration (>500 μM) of GYY4137 used. “Decomposed GYY4137” did not significantly affect LPS-evoked formation of either PGE2 or NO2− (p > 0.05). In contrast, the effect of NaHS (0–1,000 μM) on LPS-evoked PGE2 formation in cultured RAW 264.7 cells was seemingly biphasic, with modest inhibition (∼40% of control values) apparent at lower concentrations (i.e., 200 μM). This effect was gradually reversed as the concentration of NaHS was increased, with no significant effect noted at concentrations in excess of 500 μM (Fig. 2A and B). In contrast, NaHS (0–1,000 μM) did not significantly affect LPS-evoked NO2− formation, although a trend toward activation of LPS-evoked NO2− generation was evident at higher concentrations. Interestingly, treatment of RAW 264.7 cells with NaHS (100 μM) did not increase the concentration of H2S detected in the culture medium after 24 h (1.8 ± 0.6 μM, c.f. 1.6 ± 0.8 μM; n = 5; p > 0.05). Control experiments showed that neither GYY4137 (1 mM) nor NaHS (1 mM) induced a significant loss of cell viability (percentage) assessed by using the MTT assay; GYY4137, 98.3 ± 2.5%; NaHS, 95.4 ± 8.3%; vehicle-treated control cells, 102.9 ± 3.2% (all n = 5; p > 0.05).
FIG. 2.
Effect of NaHS (black columns) and GYY4137 (open columns) on LPS (1 μg/ml)-evoked release of PGE2 (A) and nitrite (B) in incubated (24 h) RAW 264.7 cells. Results show concentration of PGE2 (ng/ml) or nitrite (micromolar) and are expressed as mean ± SEM; n = 5; *p < 0.05 (c.f. LPS group); +p < 0.05 (c.f. saline group); ANOVA plus post hoc Tukey test.
Effect of GYY4137 and NaHS on LPS-evoked cytokine formation
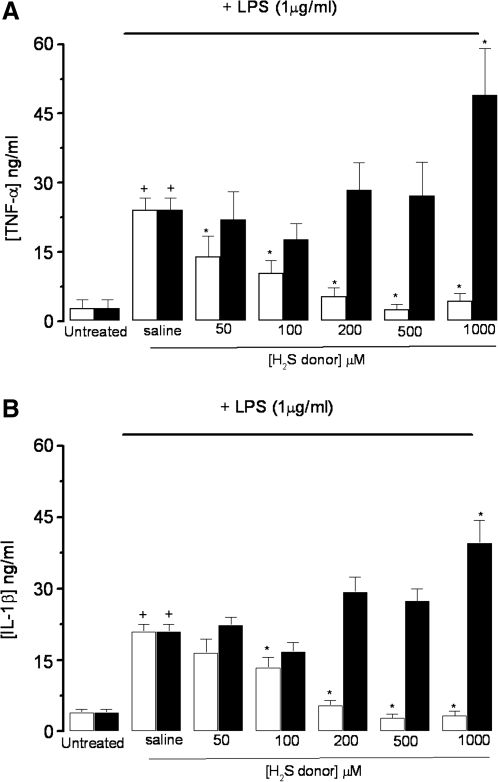
Treatment of RAW 264.7 cells with LPS also resulted in a significant increase in both TNF-α and IL-1β concentrations in the medium. Co-treatment of LPS-exposed RAW 264.7 cells with GYY4137 (0–1,000 μM) resulted in a concentration-related inhibition of the formation of both TNF-α (Fig. 3A) and IL-1β (Fig. 3B) with IC50 values of 70.4 ± 4.4 and 134.1 ±10.1 μM (n = 5), respectively. In both cases, substantial inhibition was achieved at higher concentrations of GYY4137 (>500 μM). In contrast, NaHS (0–1,000 μM) did not inhibit the biosynthesis of either cytokine (Fig. 3A and B). Indeed, at the highest concentration of NaHS used, a significant enhancement of the LPS-evoked generation of both TNF-α and IL-1β.
FIG. 3.
Effect of NaHS (black columns) and GYY4137 (open columns) on LPS (1 μg/ml)-evoked release of TNF-α (A) and IL-1β (B) in incubated (24 h) RAW 264.7 cells. Results show concentration of each cytokine (ng/ml) and are expressed as mean ± SE; n = 5; *p < 0.05 (c.f, LPS group); +p < 0.05 (c.f. saline group); ANOVA plus post hoc Tukey test.
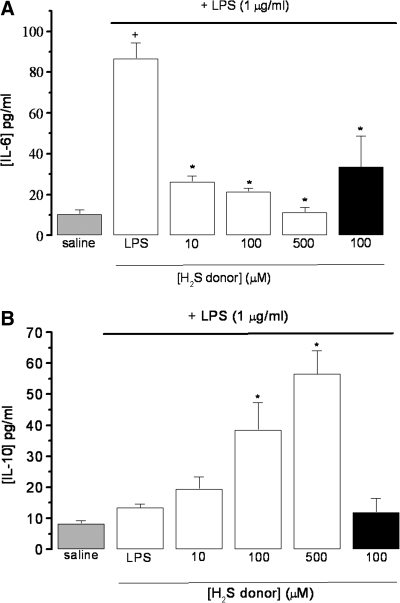
Because GYY4137 elicited concentration-dependent inhibition of LPS-induced TNF-α and IL-1β formation, we also investigated the effect of this H2S donor on the generation of both proinflammatory IL-6 and antiinflammatory IL-10 in cultured RAW 264.7 cells under identical experimental conditions. GYY4137 (10–500 μM) inhibited the LPS-evoked increase in IL-6 concentration (Fig. 4A), while potentiating the LPS-evoked increase in biosynthesis of IL-10 (Fig. 4B). Even the lowest concentration of GYY4137 used (i.e., 10 μM) reduced IL-6 formation by >50%. In comparison, NaHS (100 μM) also inhibited IL-6 production but failed to affect the generation of IL-10 (Fig. 4A and B).
FIG. 4.
Effect of NaHS (black column) and GYY4137 (open columns) on LPS (1 μg/ml)-evoked release of IL-6 (A) and IL-10 (B) in incubated (24 h) RAW 264.7 cells. Saline-treated control cells are shown by the grey column. Results show concentration of cytokines (pg/ml) and are expressed as mean ± SEM; n = 5–9; *p < 0.05 (c.f. LPS group); +p < 0.05 (c.f. saline group); ANOVA plus post hoc Tukey test.
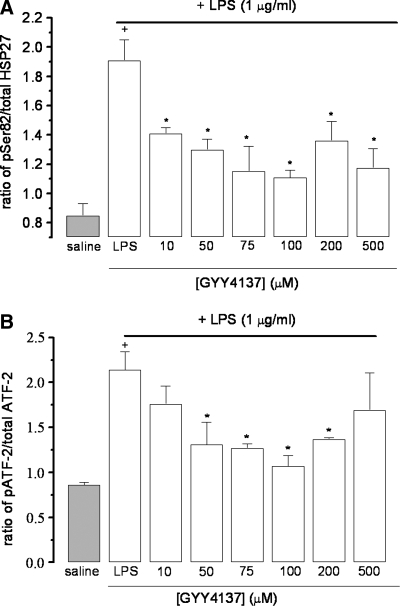
Effect of GYY4137 on phosphorylation of HSP 27 and ATF-2
Incubation of RAW 264.7 cells with LPS resulted in marked phosphorylation of both HSP27 and ATF-2 (Fig. 5A and B). In both cases, inclusion of GYY4137 (10–500 μM) significantly inhibited LPS-evoked phosphorylation. GYY4137 was particularly effective as an inhibitor of HSP-27 phosphorylation with an IC50 of 14.0 ± 1.1 μM (n = 5). Similarly, relatively low concentrations of GYY4137 (10–100 μM) also inhibited ATF-2 phosphorylation, with an IC50 of 35.1 ± 6.7 μM (n = 5). However, in this case, inhibition declined and was partially reversed at the highest concentration (500 μM).
FIG. 5.
Effect of GYY4137 on phosphorylation of ATF-2 (A) and HSP-27 (B) in LPS (1 μg/ml)-treated (24 h) RAW 264.7 cells. Saline-treated control cells are shown by the grey column. Results show ratio of phosphorylated to nonphosphorylated product and are expressed as mean ± SEM; n = 5; *p < 0.05 (c.f. LPS group); +p < 0.05 (c.f. saline group); ANOVA plus post hoc Tukey test.
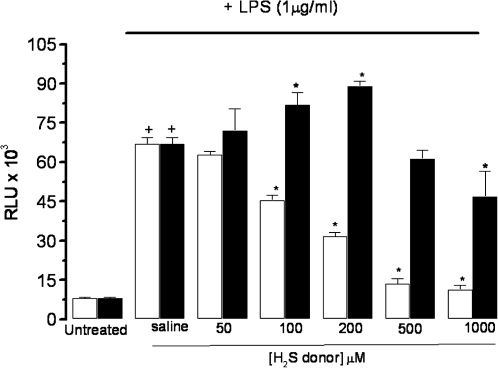
Effect of GYY4137 and NaHS on LPS-evoked activation of NF-κB
Treatment of RAW 264.7 cells with LPS resulted in a significant increase in NF-κB activation. Treatment of LPS-exposed RAW 264.7 cells with GYY4137 (0–1,000 μM) caused a concentration-related inhibition of the activation of NF-κB (Fig. 5), with an IC50 value of 214.8 ± 10.0 μM (n = 5). Interestingly, the effect of NaHS (0–1,000 μM) was biphasic, with lower concentrations (100–200 μM) promoting NF-κB activation, whereas a high concentration (1,000 μM) caused inhibition (Fig. 6).
FIG. 6.
Effect of NaHS (black columns) and GYY4137 (open columns) on activation of NF-κB in LPS (1 μg/ml)-treated (24 h) RAW 264.7 cells. Results show relative light units (RLU) and are expressed as mean ± SEM; n = 5; *p < 0.05 (c.f. LPS group); +p < 0.05 (c.f. saline group); ANOVA plus post hoc Tukey test.
Discussion
The role of H2S as an inflammatory mediator is clearly complex. The vast majority of studies carried out used simple sulfide salts such as sodium hydrosulfide (NaHS) and sodium sulfide (Na2S), which generate H2S instantaneously in aqueous solutions. Indeed, we show here, by using an H2S-selective probe, that NaHS releases large amounts of H2S over a period of a few seconds. Although undoubtedly useful in that these salts are convenient and circumvent the necessity for the somewhat more-complex preparation of authentic H2S gas solutions, the manner in which cells and tissues are exposed to the gas via NaHS, Na2S, and H2S gas solutions is unlikely to reflect accurately either the physiologic or the pathophysiologic situation. Thus, these approaches generate an instant “bolus” of H2S rather than release H2S in a slow and sustained manner, as occurs enzymatically from CSE and CBS and as would be expected to occur in intact cells/tissues.
Therefore, we investigated whether the effects of bolus H2S (generated by NaHS) or slow and sustained H2S release (via GYY4137) elicited differential responsiveness to LPS in murine RAW264.7 macrophages. In contrast to NaHS, the present data reveal a very much slower and sustained release of H2S, again measured by using an H2S-selective probe, from incubated GYY4137. The present study serves, (a) to highlight important differences in the effect of these two H2S donors on the inflammatory response of cultured macrophages to LPS, and (b) to shed new light on the possible mechanism(s) underlying the recently reported antiinflammatory effect of the slow-releasing H2S donor GYY4137 in LPS-evoked endotoxic shock in vivo (12).
GYY4137 consistently inhibited LPS-evoked formation of PGE2, •NO (as measured by NO2− accumulation), TNF-α, IL-1β, IL-6, and consistently augmented LPS-induced formation of IL-10 in cultured RAW 264.7 cells. In contrast, the effect of NaHS was very much less consistent, with a biphasic (inhibition at a concentration of 200 μM; no action at higher concentrations) effect on LPS-induced PGE2 and NO2− formation and no statistically significant inhibitory effect on the evoked biosynthesis of either TNF-α or IL-1β. Indeed, at the highest concentration studied, NaHS actually promoted LPS-evoked cytokine generation in these cells. Whether such an effect might contribute to the reported proinflammatory effect of this H2S donor (e.g., 9) is not yet clear.
Decomposed GYY4137 (left at room temperature for 5 days) did not affect LPS-induced biosynthesis of either PGE2 or NO2−, demonstrating that the effects of GYY4137 observed in this study were largely due to released H2S. Furthermore, GYY4137 caused a concentration-dependent inhibition of the LPS-induced NF-κB activation in RAW 264.7 cells, together with a concentration-dependent reduction of the phosphorylation of both ATF-2 and HSP-27. In contrast, the effects of NaHS on NF-κB activation was biphasic, in that lower concentrations increased activation, but higher concentrations were inhibitory (500 μM or greater). Neither GYY4137 nor NaHS at the highest concentration used in this study (1,000 μM) affected cell viability and thus the observed effect of these H2S donors on macrophage inflammatory mediator release are unlikely to be secondary to any toxic effect of H2S, at least under the experimental conditions used in the present work.
We previously reported that GYY4137 reduced LPS-evoked hypotension and organ damage while reducing plasma cytokine levels in the rat in vivo (12). The present data confirm that GYY4137 inhibits LPS-induced release of inflammatory mediators (i.e., PGE2, •NO, TNF-α, and IL1β) from macrophages in vitro and show for the first time that this H2S donor increases the release of antiinflammatory IL-10 under the same experimental conditions. The finding that GYY4137 also inhibited LPS-induced NF-κB activation is consistent with previous reports in the literature suggesting an inhibitory effect of H2S on transcription via NF-κB. For example, H2S (derived from NaHS) inhibited NF-κB activation in LPS-challenged RAW 264.7 macrophages maintained in culture (16), whereas exposure of rats to gaseous H2S reduced brain (cortical) NF-κB mRNA (5). The H2S donor drug, S-diclofenac, also reduced liver NF-κB activation in LPS-injected rats (11). In addition, H2S reduced kidney NF-κB activation in a rat model of renal ischemia/reperfusion injury (22). Other potential H2S donors, such as the garlic constituent, diallysulfide, also inhibit NF-κB activation in primary cultures of human articular chondrocytes (8) and in lung fibrosis induced by bleomycin in rats (7).
In contrast, we show here that only a high concentration of NaHS inhibits NF-κB activation. Indeed, at lower concentrations of NaHS (e.g., 100 and 200 μM), a small but significant activation of NF-κB is apparent. Interestingly, a similar activating effect of this H2S donor was reported in an interferon-γ (IFN-γ) − primed human monocytic cell line (U937), most likely by rapid degradation of IκBα (31). It is paradoxic that NaHS (1 mM) inhibits NF-κB activation in RAW 264.7 cells but promotes the LPS-evoked formation of TNF-α and IL-1β without significantly altering PGE2 or NO2− generation. These data suggest that NaHS at such high concentrations may also be able to affect the function of transcription factors other than NF-κB. In this respect, we previously reported that administration of the H2S donor, S-diclofenac, reduced liver AP-1 activation in LPS-injected rats. An identical effect also was observed with the parent compound, diclofenac (11), which would argue against a direct effect of H2S on AP-1. However, other H2S donors, such as diallysulfide and diallytrisulfide, have both been reported to increase the DNA-binding activity of AP-1 in rat epithelial clone 9 cells (23). Certainly, the present data suggest that the effect of NaHS on NF-κB activation under these experimental conditions is biphasic, and further experiments are required to determine whether high concentrations of NaHS are able to affect other transcription factors in these cells.
To the best of our knowledge, no other reports exist of the effect of H2S donors on either ATF-2 or HSP-27. As such, both should now be considered as potential targets for GYY4137/H2S. In this respect, HSP-27 was recently implicated as a regulator of the increased expression of both cyclooxygenase-2 (COX-2) and IL-6 in inflammatory cells exposed to LPS (1), most probably by modulating NF-κB signaling (20), whereas ATF-2 is a member of the ATF/cAMP-response element–binding protein family, which play an important role in the cellular stress response. Interestingly, TNF-α is one of the major target genes for ATF-2 (24). Growing evidence suggests that ATF-2 plays an important role in the stress response, cell growth and differentiation, as well as the immune response, and the finding that it is targeted by GYY4137 is potentially of wider interest.
An important feature of the present study is the finding that, although GYY4137 consistently reduced LPS-evoked inflammatory mechanisms in RAW 264.7 cells, the response to NaHS was less consistent. It should perhaps be noted that other authors have detected an effect of NaHS on LPS-evoked inflammatory changes (e.g., NO2− formation, iNOS expression, and NF-κB activation) in RAW 264.7 cells in culture (16). The reason for the discrepancy between the two studies in not clear. However, important differences exist in the experimental conditions used. For example, we incubated cells with either GYY4137 or NaHS concurrent with LPS for 24 h, whereas in the previous study, macrophages were preincubated with NaHS for 12 h before addition of LPS, and further incubation for an additional 18 h. Bearing in mind the transient stability of NaHS in culture medium, it is likely that the time course of exposure of cells to NaHS will be a very important factor in determining the effect of H2S on LPS-induced inflammatory mediator release under these experimental conditions. With this in mind, it is interesting that, in the present experiments, H2S was detectable in the culture medium at the end of the incubation period when macrophages were incubated with GYY4137 but not with an equivalent concentration of NaHS.
We previously reported that GYY4137 releases H2S slowly (i.e., over a period of several hours) in aqueous buffer and produces a sustained increase in plasma H2S concentration in the anesthetized rat after parenteral injection (13). When dissolved in water, H2S rapidly forms the hydrosulfide anion (HS−), which enters into an equilibrium with H+ to yield H2S. Consequently, GYY4137 is best considered a “slow releasing” H2S donor. In contrast, release of H2S from NaHS is rapid. Indeed, NaHS injection did not result in measurable increase in plasma H2S in the anesthetized rat. Thus, NaHS is considered a “fast releasing” donor of this gas (13). With this in mind, it is conceivable that RAW 264.7 cells were exposed to very much higher concentrations of H2S but for a very much shorter time in the presence of NaHS (c.f. GYY4137).
In conclusion, the effect of H2S on inflammatory mechanisms in isolated macrophages seems to be dependent to a large extent on the choice of H2S donor. It is known that different donors release H2S at different rates. It is likely that both the absolute concentration of this gas and the time course of its presence after provocation of an inflammatory response by LPS, in this instance, are critical. Drugs that release small amounts of H2S over an extended time appear to be more effective than drugs that release larger amounts of the gas over a shorter time. This should perhaps be borne in mind in the search for novel H2S donors with potential antiinflammatory activity in the clinic. Furthermore, the antiinflammatory effect of GYY4137, which we previously identified in intact rats, is likely to be dependent on inhibition of transcription through the NF-κB pathway. The possibility that GYY4137 may also interfere with both ATF-2 and HSP-27 is an intriguing one and warrants further study.
Abbreviations Used
- ATF-2
activating transcription factor-2
- CBS
cystathionine β synthetase
- CSE
cystathionine γ lyase
- GYY4137
morpholin-4-ium 4-methoxyphenyl(morpholino) phosphinodithioate
- H2S
hydrogen sulfide
- HSP-27
heat-shock protein-27
- IL-1β
interleukin-1 beta
- LPS
lipopolysaccharide
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NaHS
sodium hydrosulfide
- NF-κB
nuclear factor κB
- •NO
nitric oxide
- NO2−
nitrite
- PGE2
prostaglandin E2
- TNFα
tumor necrosis factor alpha
Acknowledgments
We are grateful to the Arthritis Research Campaign (MP/8471, U.K.) and the Northcott Devon Medical Foundation for their continued and generous research support.
Author Disclosure Statement
No competing financing interests exist.
References
- 1.Alford KA. Glennie S. Turrell BR. Rawlinson L. Saklatvala J. Dean JL. Heat shock protein 27 functions in inflammatory gene expression and transforming growth factor-beta-activated kinase-1 (TAK1)-mediated signaling. J Biol Chem. 2007;282:6232–6241. doi: 10.1074/jbc.M610987200. [DOI] [PubMed] [Google Scholar]
- 2.Bhatia M. Wong FL. Fu D. Lau HY. Moochhala SM. Moore PK. Role of hydrogen sulphide in acute pancreatitis in the mouse and rat. FASEB J. 2004;19:623–625. doi: 10.1096/fj.04-3023fje. [DOI] [PubMed] [Google Scholar]
- 3.Chang L. Geng B. Yu F. Zhao J. Jiang H. Du J. Tang C. Hydrogen sulfide inhibits myocardial injury induced by homocysteine in rats. Amino Acids. 2008;34:573–585. doi: 10.1007/s00726-007-0011-8. [DOI] [PubMed] [Google Scholar]
- 4.Collin M. Anuar F. Murch O. Bhatia M. Moore PK. Thiemermann C. Inhibition of endogenous hydrogen sulfide formation reduces the organ injury caused by endotoxemia. Br J Pharmacol. 2005;146:498–505. doi: 10.1038/sj.bjp.0706367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Florian B. Vintilescu R. Balseanu AT. Buga AM. Grisk O. Walker LC. Kessler C. Popa-Wagner A. Long-term hypothermia reduces infarct volume in aged rats after focal ischemia. Neurosci Lett. 2008;438:180–185. doi: 10.1016/j.neulet.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 6.Geng B. Chang L. Pan C. Qi Y. Zhao J. Pang Y. Du J. Tang C. Endogenous hydrogen sulfide regulation of myocardial injury induced by isoproterenol. Biochem Biophys Res Commun. 2004;318:756–763. doi: 10.1016/j.bbrc.2004.04.094. [DOI] [PubMed] [Google Scholar]
- 7.Kalayarasan S. Sriram N. Sudhandiran G. Diallyl sulfide attenuates bleomycin-induced pulmonary fibrosis: critical role of iNOS, NF-kappaB, TNF-alpha and IL-1 beta. Life Sci. 2008;82:1142–1153. doi: 10.1016/j.lfs.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 8.Lee HS. Lee CH. Tsai HC. Salter DM. Inhibition of cyclooxygenase 2 expression by diallyl sulfide on joint inflammation induced by urate crystal and IL-1beta. Osteoarthritis Cartilage. 2009;17:91–99. doi: 10.1016/j.joca.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 9.Li L. Bhatia M. Zhu YZ. Zhu Y. Ramnath RD. Wang ZJ. Anaur F. Whiteman M. Salto-Tellez M. Moore PK. Hydrogen sulfide is a novel mediator of endotoxic shock. FASEB J. 2005;119:1196–1198. doi: 10.1096/fj.04-3583fje. [DOI] [PubMed] [Google Scholar]
- 10.Li L. Hsu A. Moore PK. Actions and interactions of nitric oxide, carbon monoxide and hydrogen sulphide in the cardiovascular system and in inflammation: a tale of three gases! Pharmacol Ther. 2009;123:386–400. doi: 10.1016/j.pharmthera.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Li L. Rossoni G. Sparatore A. Lee LC. Del Soldato P. Moore PK. Anti-inflammatory and gastrointestinal sparing activity of a novel H2S-releasing diclofenac agent: new insights into the biological roles of H2S. Free Radic Biol Med. 2006;42:706–719. doi: 10.1016/j.freeradbiomed.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 12.Li L. Salto-Tellez M. Tan CH. Whiteman M. Moore PK. GYY4137, a novel hydrogen sulfide-releasing molecule, protects against endotoxic shock in the rat. Free Radic Biol Med. 2009;47:103–113. doi: 10.1016/j.freeradbiomed.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 13.Li L. Whiteman M. Guan Y. Neo KL. Cheng Y. Lee SW. Zhao Y. Baskar R. Tan CH. Moore PK. Characterisation of a novel, water soluble hydrogen sulfide releasing molecule (GYY4137): new insights into the biology of hydrogen sulphide. Circulation. 2008;117:2351–2360. doi: 10.1161/CIRCULATIONAHA.107.753467. [DOI] [PubMed] [Google Scholar]
- 14.Mok YYP. Atan MS. Cheung YP. Wang ZJ. Bhatia M. Moochhala S. Moore PK. Role of hydrogen sulphide in haemorrhagic shock in the rat: protective effect of inhibitors of hydrogen sulphide biosynthesis. Br J Pharmacol. 2004;143:881–889. doi: 10.1038/sj.bjp.0706014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muzzaffar S. Shukla N. Bond M. Newby AC. Angelini GD. Sparatore A. Del Soldato P. Jeremy J. Exogenous hydrogen sulfide inhibits superoxide formation, NOX-1 expression and Rac1 activity in human vascular smooth muscle cells. J Vasc Res. 2008;45:521–528. doi: 10.1159/000129686. [DOI] [PubMed] [Google Scholar]
- 16.Oh GS. Pae HO. Lee BS. Kim BN. Kim JM. Kim HR. Jeon SB. Jeon WK. Chae HJ. Chung HT. Hydrogen sulfide inhibits nitric oxide production and nuclear factor-kappaB via heme oxygenase-1 expression in RAW264.7 macrophages stimulated with lipopolysaccharide. Free Radic Biol Med. 2006;41:106–119. doi: 10.1016/j.freeradbiomed.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 17.Rose P. Won YK. Ong CN. Whiteman M. Beta-phenylethyl and 8-methylsulphinyloctyl isothiocyanates, constituents of watercress, suppress LPS induced production of nitric oxide and prostaglandin E2 in RAW 264.7 macrophages. Nitric Oxide. 2005;12:237–243. doi: 10.1016/j.niox.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Sidhapuriwala J. Li L. Sparatore A. Moore PK. Bhatia M. Effect of S-diclofenac, a novel hydrogen sulfide releasing derivative, on carrageenan-induced hindpaw oedema formation in the rat. Eur J Pharmacol. 2007;569:149–154. doi: 10.1016/j.ejphar.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Stipanuk MH. Sulfur amino acid metabolism: pathways for production and removal of homocysteine and cysteine. Annu Rev Nutr. 2004;24:539–577. doi: 10.1146/annurev.nutr.24.012003.132418. [DOI] [PubMed] [Google Scholar]
- 20.Sur R. Lyte PA. Southall MD. Hsp27 regulates pro-inflammatory mediator release in keratinocytes by modulating NF-kappaB signaling. J Invest Dermatol. 2008;128:1116–1122. doi: 10.1038/sj.jid.5701157. [DOI] [PubMed] [Google Scholar]
- 21.Szabo C. Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Disc. 2007;6:917–935. doi: 10.1038/nrd2425. [DOI] [PubMed] [Google Scholar]
- 22.Tripatara P. Patel NS. Collino M. Gallicchio M. Kieswich J. Castiglia S. Benetti E. Stewart KN. Brown PA. Yaqoob MM. Fantozzi R. Thiemermann C. Generation of endogenous hydrogen sulfide by cystathionine gamma-lyase limits renal ischemia/reperfusion injury and dysfunction. Lab Invest. 2008;88:1038–1048. doi: 10.1038/labinvest.2008.73. [DOI] [PubMed] [Google Scholar]
- 23.Tsai CW. Chen HW. Yang JJ. Sheen LY. Lii CK. Diallyl disulfide and diallyl trisulfide up-regulate the expression of the pi class of glutathione S-transferase via an AP-1-dependent pathway. J Agric Food Chem. 2007;55:1019–1026. doi: 10.1021/jf061874t. [DOI] [PubMed] [Google Scholar]
- 24.Tsai EY. Jain J. Pesevanto PA. Rao A. Goldfield AE. Tumor necrosis factor alpha gene regulation in activated T cells involves ATF-2/Jun and NFATp. Mol Cell Biol. 1996;16:459–467. doi: 10.1128/mcb.16.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whiteman M. Armstrong JS. Chu SH. Jia-Ling S. Wong BS. Cheung NS. Halliwell B. Moore PK. The novel neuromodulator hydrogen sulfide: an endogenous peroxynitrite “scavenger”? J Neurochem. 2004;90:765–768. doi: 10.1111/j.1471-4159.2004.02617.x. [DOI] [PubMed] [Google Scholar]
- 26.Whiteman M. Cheung NS. Zhu YZ. Chu SH. Siau JL. Wong BS. Armstrong JS. Moore PK. Hydrogen sulfide: a novel mediator of hypochlorous acid mediated oxidative damage in the brain. Biochem Biophys Res Commun. 2004;326:794–798. doi: 10.1016/j.bbrc.2004.11.110. [DOI] [PubMed] [Google Scholar]
- 27.Whiteman M. Spencer JP. Zhu YZ. Armstrong JS. Schantz JT. Peroxynitrite-modified collagen-II induces p38/ERK and NF-kappaB-dependent synthesis of prostaglandin E2 and nitric oxide in chondrogenically differentiated mesenchymal progenitor cells. Osteoarthritis Cartilage. 2006;4:460–470. doi: 10.1016/j.joca.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 28.Whiteman M. Moore PK. Hydrogen sulfide and the vasculature: a novel vasculoprotective entity and regulator of nitric oxide bioavailability? J Cell Mol Med. 2009;13:488–507. doi: 10.1111/j.1582-4934.2009.00645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang G. Wu L. Jiang B. Yang W. Qi J. Cao K. Meng Q. Mustafa AK. Mu W. Zhang S. Snyder SH. Wang R. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008;322:587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zanardo RC. Brancaleone V. Distrutti E. Fiorucci S. Cirino G. Wallace JL. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. FASEB J. 2006;20:2118–2120. doi: 10.1096/fj.06-6270fje. [DOI] [PubMed] [Google Scholar]
- 31.Zhi L. Ang AD. Zhang H. Moore PK. Bhatia M. Hydrogen sulfide induces the synthesis of proinflammatory cytokines in human monocyte cell line U937 via the ERK-NF-kappaB pathway. J Leukoc Biol. 2007;81:1322–1332. doi: 10.1189/jlb.1006599. [DOI] [PubMed] [Google Scholar]