Abstract
Background and Aims
The smoke-derived compound karrikinolide (KAR1) shows significant potential as a trigger for the synchronous germination of seeds in a variety of plant-management contexts, from weed seeds in paddocks, to native seeds when restoring degraded lands. Understanding how KAR1 interacts with seed physiology is a necessary precursor to the development of the compound as an efficient and effective management tool. This study tested the ability of KAR1 to stimulate germination of seeds of the global agronomic weed Brassica tournefortii, at different hydration states, to gain insight into how the timing of KAR1 applications in the field should be managed relative to rain events.
Methods
Seeds of B. tournefortii were brought to five different hydration states [equilibrated at 15 % relative humidity (RH), 47 % RH, 96 % RH, fully imbibed, or re-dried to 15 % RH following maximum imbibition] then exposed to 1 nm or 1 µm KAR1 for one of five durations (3 min, 1 h, 24 h, 14 d or no exposure).
Key Results
Dry seeds with no history of imbibition were the most sensitive to KAR1; sensitivity was lower in seeds that were fully imbibed or fully imbibed then re-dried. In addition, reduced sensitivity to KAR1 was associated with an increased sensitivity to exogenously applied abscisic acid (ABA).
Conclusions
Seed water content and history of imbibition were found to significantly influence whether seeds germinate in response to KAR1. To optimize the germination response of seeds, KAR1 should be applied to dry seeds, when sensitivity to ABA is minimized.
Keywords: Karrikinolide, karrikins, butenolide, smoke, germination stimulant, seed water content, abscisic acid, ABA, gibberellin, weed, Brassica tournefortii
INTRODUCTION
When seeds are dispersed from the parent plant, they can be dormant and unable to germinate despite exposure to environmental conditions that might otherwise be suitable for germination. Seeds that are physiologically dormant will not germinate until dormancy is alleviated by processes such as dry after-ripening, wet–dry cycles, or cold- or warm-stratification (Merritt et al., 2007; Hoyle et al., 2008). Such dormancy alleviation processes are mediated by seed water content and the temperature of the environment (Merritt et al., 2007). Seeds with a permeable seed coat will equilibrate with ambient moisture conditions (Wuest, 2007), whether seeds are on the surface of the soil, buried or retained serotinously on the parent plant.
As an alternative to natural alleviation of dormancy, seeds can be stimulated to germinate by chemical cues, such as nitrates (Finch-Savage et al., 2007), strigolactones (Goldwasser et al., 2008) and a suite of chemicals present in smoke (Chiwocha et al., 2009). One such seed germination stimulant identified from smoke is karrikinolide (KAR1 – a butenolide) (Flematti et al., 2004). Karrikinolide triggers the germination of numerous species from fire-prone (Dixon et al., 2009), non-fire-prone (Merritt et al., 2007) and agricultural environments (Daws et al., 2007; Stevens et al., 2007), highlighting its potential for use in a range of plant-management contexts. Applying KAR1 to mined and degraded land could assist with regenerating flora from broadcast seed or seeds within replaced topsoil (Commander et al., 2008, 2009). In a commercial-seed context, KAR1 may be used as a priming agent to enhance the subsequent germination characteristics of crop seeds (Jain and Van Staden, 2007). Furthermore, in agricultural systems, weeds could be triggered to germinate on demand, maximizing the efficiency of herbicide applications, and reducing the burden of pesticides in the environment (Davis et al., 2005; Stevens et al., 2007). To realize such valuable applications for KAR1 and ensure its efficacy, it is necessary to explore its effect under a variety of environmental conditions. In particular, studies that optimize the timing of application of KAR1 relative to when seeds are most receptive would contribute to the efficient use of KAR1 in agricultural and restoration environments.
Environmental conditions may alter the receptivity of seeds to smoke signals (Roche et al., 1998; Baker et al., 2005). For example, smoke has been shown to be more effective at eliciting germination when applied during the dry autumn of a Mediterranean climate than when applied during the wetter months of winter and spring (Roche et al., 1998). These observations imply that there may be an interaction between the hydration state of seeds and the efficacy of smoke-derived chemicals such as KAR1. To date, studies of KAR1 and related smoke-extracts have not considered how the hydration state of seeds interacts with stimulatory chemicals to influence germination outcomes. However, an influence of hydration state on germination is likely, because seed water content affects both the physical and the physiological characteristics of seeds.
Physical studies of imbibing seeds indicate that the rate and volume of solution uptake is greatly influenced by seed water potential (Bradford, 1995). Drier seeds have more negative matric water potentials and absorb water more quickly than moister seeds with less negative water potentials (Wolk et al., 1989). In contrast, uptake of solutions by fully imbibed seeds is driven by differences in osmotic potential between the seed and the surrounding solution (Roberts and Ellis, 1989). Thus, the water content of seeds may be an important factor affecting the uptake of aqueous stimulants and subsequent germination response.
Whilst the hydration state of seeds may impart physical effects on the response of seeds to germination stimulants, it may also influence the response physiologically. The water content of seeds directly affects molecular mobility within cells, and consequently the types and rates of reactions that occur (Walters et al., 2005). Moderate seed hydration [e.g. −100 to −10 MPa, 40–92 % relative humidity (RH)] favours degradative reactions, whilst lower seed water contents (10–20 % RH) can induce a state of relative molecular paralysis that optimizes seed longevity (Walters et al., 2005). Imbibition to seed hydration states above approx. −10 MPa provides sufficient water for reparative reactions to occur (Bewley, 1997; Walters et al., 2005). Indeed, in priming, seeds can be fully imbibed then re-dried to improve subsequent germination percentages and rates (Gurusinghe et al., 2002; Demir et al., 2009). The increased physiological activity associated with imbibition can alter the balance of compounds that regulate germination, or the sensitivity of seeds to these compounds (Bu et al., 2009). Pertinently for seed dormancy, imbibition permits synthesis or breakdown of plant growth regulators such as abscisic acid (ABA; Grappin et al., 2000; Ali-Rachedi et al., 2004), which maintains dormancy and inhibits germination, and gibberellins (GA), which promote germination (Nelson et al., 2009). These plant growth regulators may in turn interact antagonistically or synergistically with germination stimulants such as KAR1, although the nature and mechanisms of these interactions remain unclear (Nelson et al., 2009).
Considering the many ways that the hydration state of seeds affects their physical and physiological characteristics, we reasoned that the hydration state of seeds will influence their germination response to KAR1. More specifically, KAR1 will stimulate more germination when applied to dry seeds than to seeds with higher water contents. In addition, based on the key roles of ABA and GA in maintaining dormancy and inducing seed germination, the interaction of these plant growth regulators with seed hydration state and sensitivity to KAR1 was examined.
MATERIALS AND METHODS
Seed material
The annual weed Brassica tournefortii was chosen for this study as it has been shown to be highly responsive to KAR1 (Stevens et al., 2007). Seeds of B. tournefortii were collected from wild plants growing in semi-natural dune vegetation at City Beach, Perth, Western Australia (31 °53′26″S, 115 °45′21″E) in October, 2006. Following drying at 15 % RH and 15 °C for 4 weeks, seeds were sealed in a foil bag and stored at 5 °C until used in experiments.
Initial seed hydration states
Brassica tournefortii seeds were prepared according to one of five initial seed hydration treatments: 15 % RH, 47 % RH, 96 % RH, fully imbibed, or fully imbibed then dried to 15 % RH (‘re-dried’) prior to exposure to KAR1. For all hydration treatments, multiple sets of 50 seeds were sealed in nylon mesh bags. Samples for the 15 % RH treatment were stored in a controlled-environment room at 15 % RH and 15 °C. Samples for the 47 and 96 % RH treatments were placed in sealed boxes (polycarbonate electrical enclosure boxes; 270 × 190 × 100 mm; NHP Fibox, Perth, Australia) and suspended above solutions of LiCl (330 g L−1) and saturated KNO3, respectively, to equilibrate for 4 weeks at 15 °C. For the fully imbibed treatment, samples were placed in tissue culture-grade deionized water (TC-H2O) at room temperature (21 °C) for 18 h, using an automatic shaker (40 r.p.m.; Orbital Mixer, Ratek Instruments, Sydney, Australia) to gently agitate the water. Similarly, seeds in the re-dried treatment were allowed to imbibe for 18 h then returned to the 15 % RH environment (15 °C) to dry for 26 h.
KAR1 sensitivity
For each hydration treatment (see above) three replicate samples were exposed to 200 mL of 1 nm or 1 µm KAR1 (synthesized from pyromeconic acid according to Flematti et al., 2005) for 0 min, 3 min, 1 h, 24 h or 14 d. Three samples for each combination of hydration treatment and KAR1 exposure duration were placed in separate replicate containers of 1 nm or 1 µm KAR1 and agitated on an automated shaker. Samples exposed to KAR1 for ≤24 h were subsequently rinsed in 400 mL TC-H2O and transferred to individual 90-mm Petri dishes containing 20 mL TC-H2O for the remainder of the 24-h period, so that all samples were hydrated for the same period. Samples were then patted dry and transferred to 90-mm Petri dishes containing 1 % (w/v) agar for germination. For samples exposed to KAR1 for 14 d, seeds were removed from the KAR1 soaking solution after 24 h, patted dry and transferred directly to germination media containing either 1 nm or 1 µm KAR1.
Germination testing
Petri dishes were wrapped in two layers of aluminium foil to exclude light and incubated at 20 °C (Thermoline, Brisbane, Queensland, Australia). Germination was scored once after 14 d, and seeds were scored as germinated if the radicle had visibly protruded (>1 mm). Seedlings that germinated but which failed to develop normally (e.g. radicle appeared glassy) were not included in germination totals. Any seeds that did not germinate were inspected for obvious necrosis and squeezed with forceps to assess viability; seeds with firm white embryos were scored as viable.
Imbibition curves
Seed water uptake was monitored over a 24-h period for each of the initial hydration states (see above) to create imbibition curves. Due to the mucilaginous layer surrounding B. tournefortii seeds, which was degraded when patted dry prior to weighing samples, a destructive method of sampling was adopted. Thirty nylon mesh bags containing 50 seeds were prepared for each initial hydration state. The weight of seeds (at 15 % RH) in each bag, and the seed + bag weights were recorded prior to applying the hydration treatments. Ten bags of each hydration treatment were placed in each of three replicate solutions of TC-H2O to imbibe. Periodically, one bag for each hydration treatment and replicate solution was removed from the solution, patted dry to remove excess water then weighed. For each sample, seed weight gain was calculated as (current weight − original weight at 15 % RH)/(original weight at 15 % RH) × 100 %.
Scanning electron microscopy
Scanning electron microscopy was used to compare the surface features of seeds prepared according to the re-dried treatment and seeds at 15 % RH with no history of imbibition; fully imbibed samples could not be imaged as only dry samples could be tested. Nine seeds of each treatment were gold-plated (Emitech K550X sputter-coater, Quorum Technologies Ltd, East Grinstead, UK) and viewed with a Jeol NeoScope JCM 5000 scanning electron microscope (10 kV acceleration voltage, 10−2 Pa; Tokyo, Japan).
ABA and GA concentrations
Three samples of seeds (approx. 500 mg at 15 % RH, actual weight recorded) were pre-equilibrated to each of the five target hydration states in nylon mesh bags, as described above. Samples were ground to a fine powder in a mortar and pestle with liquid nitrogen and analysed for their ABA, GA1, GA3 and GA4 content according to the methods of Nelson et al. (2009). Only ABA, GA3 and GA4 were detected in the samples.
ABA, GA3 and GA4 sensitivity
To investigate further the effect of seed hydration on seed responses to KAR1, the sensitivity of seeds to exogenously applied ABA and GA3 and GA4 were assessed for the 15 % RH, fully imbibed and re-dried treatments. Germination of B. tournefortii was assessed in response to six concentrations of biologically active (+)-ABA (0, 0·5, 1, 5, 10 or 50 µm, Sigma-Aldrich Company, St Louis, MO, USA) in combination with 1 µm KAR1. Six concentrations of GA3 and GA4 (Sigma-Aldrich) were also tested to assess the sensitivity of seeds to GA. The GA3 concentrations used were 0, 0·5, 1, 5, 10 and 100 µm, and the GA4 concentrations were 0, 0·1, 0·5, 1, 5 and 10 µm. All ABA and GA media were prepared by adding stock solutions of the plant-growth regulators to 1 % (w/v) agar, after the agar was autoclaved (121 °C for 20 min) and allowed to cool to approx. 60 °C. Three replicates of 50 seeds for each hydration state were assigned to each treatment in the ABA and GA sensitivity experiments. Conditions for germination were consistent with the KAR1 sensitivity experiment.
Seed water content assessment
For each experiment, all initial hydration treatments were tested for their water content and RH to verify that the treatments were consistent. Water content was assessed gravimetrically by weighing three samples of each treatment before and after drying them in an oven at 105 °C for 48 h. Seed water content was calculated on a dry-weight percentage basis: [(fresh weight − dry weight)/dry weight] × 100 (Table 1).
Table 1.
Initial dry-weight water content (%, mean ± s.e.) of Brassica tournefortii seeds prior to treatment with karrikinolide
| Treatment | Dry-weight seed water content |
|---|---|
| 15 % RH | 6·6 ± 0·5 |
| 47 % RH | 10·1 ± 0·6 |
| 96 % RH | 30·9 ± 0·2 |
| Fully imbibed | 86·3 ± 5·7 |
| Re-dried (15 % RH) | 7·0 ± 0·2 |
RH, relative humidity.
* Water content with mucilage was 319·7 ± 33·1 % d. wt.
Statistical analyses
A generalized linear model with a logit link-function was fitted to germination data using step-wise regression (GenStat 10th Edition; McCullagh and Nelder, 1989). For the results of the KAR1 exposure experiment, the terms included in the model were KAR1 concentration, exposure time, seed hydration state, KAR1 concentration × exposure time, and seed hydration state × exposure time (all terms, P < 0·001). For the analysis of the ABA sensitivity experiment, the terms included in the model were seed hydration state, concentration and seed hydration state × concentration (all terms, P < 0·001); the only term that had a significant effect on modelling the GA sensitivity data was GA concentration. To test for differences in the endogenous concentration of ABA, GA3 and GA4, a generalized linear model with a logarithm link-function was fitted to the data. In all experiments, there was no bias based on replicate results. Comparisons of individual hydration states were conducted using parameter estimates from models including only the seed hydration state term. Data for the effect of hydration state on the germination response to KAR1 (Fig. 1), ABA sensitivity (Fig. 5A) and GA sensitivity (Fig. 5B, C) were transformed via log(x + 1) to facilitate graphing, but non-transformed data were used in all statistical analyses.
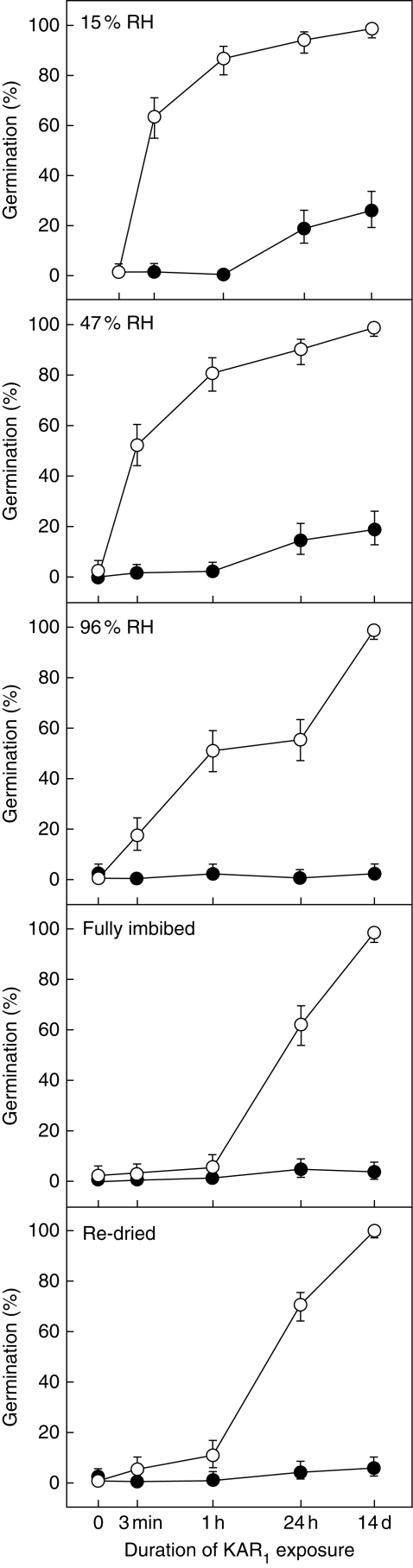
Fig. 1.
Germination of Brassica tournefortii seeds following exposure to 1 nm (closed circles) and 1 µm (open circles) KAR1. Seeds were equilibrated to 15 % RH, 47 % RH, 96 % RH, were fully imbibed, or were fully imbibed then dried to 15 % (‘re-dried’) prior to exposure to KAR1. Exposure durations were 0, 3 min, 1 h, 24 h or 14 d. Data are presented on a log scale as binomial estimates with asymmetrical error bars representing a 95 % confidence interval (n = 150 seeds per data point).
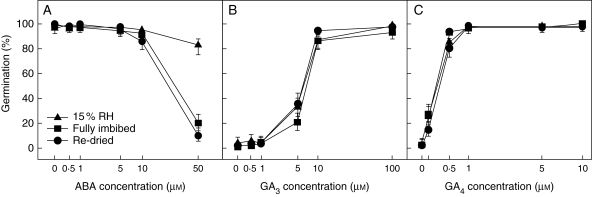
Fig. 5.
Effect of exogenously applied ABA (A; ABA with 1 µm KAR1), GA3 (B) and GA4 (C) on the germination of Brassica tournefortii seeds. Prior to plating on the relevant agar media, seeds were either dry with no history of imbibition (‘15 % RH’), soaked in water for 18 h prior to treatment (‘fully imbibed’) or soaked for 18 h then re-dried to 15 % RH (‘re-dried’). Data are presented as binomial estimates with asymmetrical error bars representing the 95 % confidence interval (n = 150 seeds per data point).
RESULTS
KAR1 sensitivity
For the seeds with no history of imbibition, seeds with lower seed water content were more sensitive to KAR1, as more seeds germinated following shorter exposure times (Fig. 1). Seeds that were pre-equilibrated to 15 and 47 % RH germinated to similar levels (t = 1·73, P = 0·083, d.f. = 4) and were the most sensitive to both 1 nm and 1 µm KAR1; more than 50 % of seeds germinated when exposed to 1 µm KAR1 for as little as 3 min. In contrast, seeds from the 96 % RH and fully imbibed treatments only germinated to levels above 50 % when exposed to 1 µm KAR1 for 1 h or longer. Across all treatments, the germination response to 1 nm KAR1 was limited, yet for this concentration the effect of seed water content on KAR1 sensitivity was still clear; only the 15 and 47 % RH treatments germinated in response to 1 nm KAR1, and only when exposed for longer than 1 h. All hydration treatments germinated to 100 % following exposure to 1 µm KAR1 for 14 d, confirming that the hydration treatments per se did not affect seed viability (P ≥0·837, d.f. = 4).
A history of imbibition was found to influence the seed responses to KAR1, as seeds that had been imbibed were less sensitive to KAR1. Although the 15 % RH seeds, which had never been imbibed, and the re-dried seeds had the same seed water content before exposure to the KAR1 solutions (approx. 6·8 % d. wt; Table 1), the re-dried seeds were less responsive to KAR1 (P < 0·001, d.f. = 4). Indeed, the response of the re-dried seeds was indistinguishable from that of the fully imbibed seeds (P = 0·189, d.f. = 4), and only marginally different from the 96 % RH seeds (P = 0·049, d.f. = 4), despite having a lower seed water content.
Seed imbibition
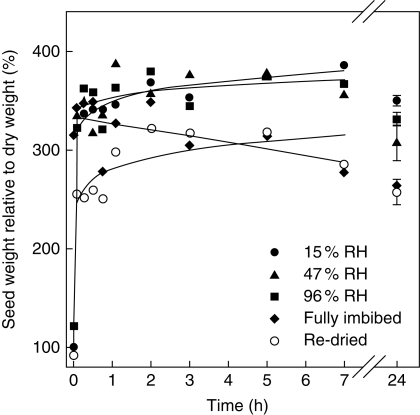
Seed water uptake was influenced by the initial hydration state of seeds. Seeds equilibrated at 15, 47 and 96 % RH gained weight similarly, and were indistinguishable from the fully imbibed treatment within 3 min of imbibition (P ≥0·417, d.f. = 4; Fig. 2). The re-dried seeds absorbed less water than the 15, 47 and 96 % RH treatments in the first 45 min of imbibition (P = 0·022, d.f. = 1). All treatments reached their maximum seed weight, which was at least three times their dry-seed weight, within 3 h of imbibition. At 24 h, the re-dried seeds and fully imbibed seeds equilibrated at a lower weight than the other three treatments (P < 0·001, d.f. = 1), which had not previously imbibed, but all treatments lost weight between 3 and 24 h (P = 0·01, d.f. = 1).
Fig. 2.
Imbibition curves for Brassica tournefortii seeds pre-equilibrated to five different hydration states: 15 % RH, 47 % RH, 96 % RH, fully imbibed, or fully imbibed then re-dried to 15 % RH (‘Re-dried’). For clarity, mean data (n = 3 replicates of 50 seeds) are presented with fitted exponential curves (except for fully imbibed, for which a linear curve was fitted) and ±1 s.e. bars are only shown for the final time point.
Scanning electron microscopy
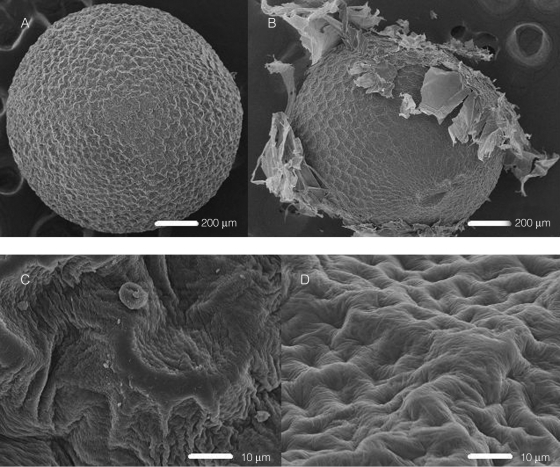
Scanning electron microscopy images showed that the mucilage layer of re-dried seeds was damaged relative to that of seeds at 15 % RH with no history of imbibition (Fig. 3). The mucilage layer visibly flaked away from the surface of re-dried seeds, and the morphology of the underlying testa of re-dried seeds appeared to be smoother.
Fig. 3.
Scanning electron microscope images of Brassica tournefortii seeds at 15 % RH: seed with no history of imbibition (A, C) and seed that was re-dried following 18 h of imbibition (B, D).
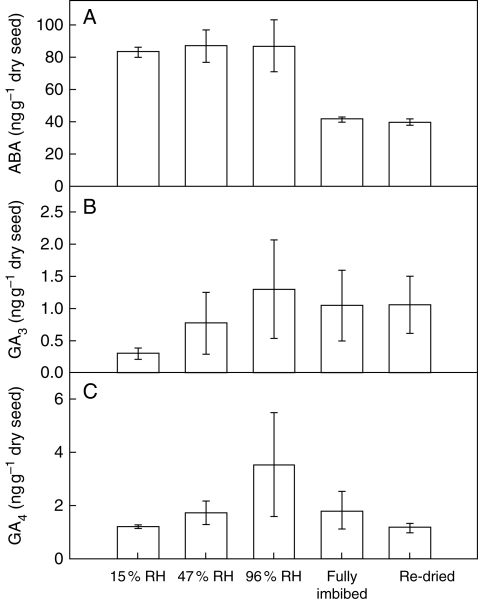
Endogenous ABA, GA3 and GA4 concentrations
Seeds that had been imbibed contained approximately half the ABA concentration found in dry seeds with no history of imbibition (Fig. 4A), which was surprising given the imbibed seeds were more dormant. For the different hydration treatments without prior imbibition, ABA concentrations were found to be similar (P ≥0·765, d.f. = 4); the fully imbibed and re-dried samples were found to have indistinguishable ABA concentrations (P = 0·888, d.f. = 4). Both GA3 and GA4 were present at low concentrations in all the seed batches, and were found not to differ significantly between the different hydration treatments (Fig. 4B, C; P ≥0·335 and P ≥0·366).
Fig. 4.
The endogenous concentration (mean ± 1 s.e., n = 3) of (A) ABA, (B) GA3 and (C) GA4 in Brassica tournefortii seeds pre-equilibrated to the five different hydration states: 15 % RH, 47 % RH, 96 % RH, fully imbibed, or fully imbibed then re-dried to 15 % RH (‘Re-dried’).
ABA, GA3 and GA4 sensitivity
Seeds with a history of being imbibed were more sensitive to exogenously applied ABA than seeds that had not been imbibed prior to exposure, but there were no differences in sensitivity to either GA3 or GA4 (Fig. 5). Compared with the 15 % RH treatment, germination of the re-dried treatment was inhibited by 10 and 50 µm ABA (P = 0·017 and <0·001; d.f. = 2). Seeds in the fully imbibed treatment responded in the same way as the re-dried treatment (P = 0·254), but their germination was only distinguishable from the 15 % RH treatment at 50 µm ABA (P < 0·001). All hydration treatments responded equally to GA3 and GA4 (P ≥0·063 and P ≥0·212; d.f. = 2), with GA4 stimulating germination at lower concentrations than GA3.
DISCUSSION
Our hypothesis that KAR1 would stimulate more germination when applied to dry seeds than to seeds with higher water contents was accepted, but only for cases when the seeds had not previously imbibed water. Seeds with a history of imbibition were less sensitive to KAR1, even when re-dried. Furthermore, re-dried seeds behaved similarly to fully imbibed seeds in germination assays at both concentrations of KAR1, even though the fully imbibed seeds had higher water contents at the point of exposure to KAR1. Accordingly, water content alone does not explain why the treatments responded differently to KAR1. Thus, we conclude that both water content and the history of imbibition are important factors that influence the ability of KAR1 to stimulate seed germination.
Our observations that the water content and history of imbibition of seeds influenced the sensitivity of B. tornefortii to KAR1 may be explained by physical and physiological changes that occur when seeds imbibe. In a physical sense, the hydration state of seeds could influence how seeds respond to KAR1 by dictating the nature of the forces driving the uptake of the stimulant in solution, and the quantity of solution absorbed. Additionally, the amount of water in seeds can determine their physiological activity by influencing the types and rates of reactions that occur (Walters et al., 2005). In particular, when seeds imbibe they can synthesize, break down or change sensitivity to ABA and GA, which mediate germination (Grappin et al., 2000; Ali-Rachedi et al., 2004). Thus, we proposed three explanations for why hydration state influenced germination in response to KAR1, namely that it affected (1) the physical uptake of the KAR1 solution, (2) the endogenous concentration of ABA or sensitivity to ABA, and (3) the endogenous concentration of GA3 and GA4 or sensitivity to GA3 and GA4.
Imbibition curves for seeds of each hydration state afforded insight into the physical uptake of the KAR1 solutions. The re-dried, 15, 47 and 96 % RH seeds at least doubled their weight in the first hour – probably due to having more negative matric potentials – whereas any uptake of the solution by the fully imbibed seeds was driven by osmosis. Yet the quantity and rate of solution absorption was not associated with germination, as the re-dried seeds germinated like the fully imbibed seeds, despite having different water contents when initially exposed to KAR1. The fully imbibed and re-dried seeds equilibrated at lower water contents relative to the other hydration treatments at 24 h, probably due to mucilage loss. This loss of mucilage also did not correlate with differences in germination as the fully imbibed and re-dried treatments had the same germination response as the 96 % RH treatment following 24 h of exposure to the KAR1 solutions. Thus, differences in the volume of uptake and physical forces driving the uptake of solutions did not fully explain why the history of hydration had a significant effect on the germination response of seeds to KAR1.
One possible physiological explanation for the results is that KAR1 interacts with ABA concentration or ABA sensitivity, which change according to the hydration state of seeds (Finch-Savage and Leubner-Metzger, 2006). No link was found in the present study between endogenous concentrations of ABA and germination response, as ABA levels decreased with imbibition. Indeed, the lower concentration of ABA in the fully imbibed and re-dried treatments implies that dormancy is partially alleviated in these seeds (Ali-Rachedi et al., 2004; Feurtado et al., 2007), yet fewer of these seeds germinated with KAR1. On the one hand, these results were surprising as we had reasoned that the poorer germination of previously imbibed seeds may be due to increased endogenous concentrations of ABA, which could override the stimulatory effect of KAR1. On the other hand, the results are supported by other reports that ABA concentrations decline as seeds imbibe (Seo et al., 2006; Toh et al., 2008; Nelson et al., 2009), and are not always reflected in increased germination outcomes (Millar et al., 2006).
Despite the apparent lack of association between endogenous concentrations of ABA and the germination response of seeds, a different pattern with ABA sensitivity was observed. That is, seeds that had previously been imbibed were more sensitive to exogenous applications of ABA than were dry seeds. ABA sensitivity is moderated by a range of proteins that facilitate ABA signalling (Finkelstein et al., 2002), and it is plausible that transcription of genes for intermediates involved in signalling is favoured when seeds imbibe. Therefore, we reason that seeds become more sensitive to ABA during imbibition, and so become more receptive to what little ABA might be present in the seeds. In turn, the heightened sensitivity to ABA may obstruct the stimulatory effect of KAR1, affording seeds a poorer germination response.
A second possible physiological explanation for the observed link between seed hydration state and germination in response to KAR1 is that endogenous concentrations of gibberellins or the sensitivity of seeds to gibberellins are lower in imbibed seeds. Other studies have proposed interactions between gibberellins and smoke or KAR1 signals (Schwachtje and Baldwin, 2004; Nelson et al., 2009). Schwachtje and Baldwin (2004) showed that exposure to smoke water enhanced the sensitivity of Nicotiana attenuata seeds to exogenous applications of GA3, while Nelson et al. (2009) found no influence of KAR1 on the sensitivity of Arabidopsis thaliana seeds to gibberellins. In the present study, there was no apparent connection between gibberellins and the germination response of seeds to KAR1; neither the endogenous concentrations of GA3 and GA4 nor the sensitivity of seeds to exogenous applications of GA3 and GA4 declined during imbibition or re-drying. Moreover, in contrast to the theory of Finch-Savage and Leubner-Metzger (2006) that high ABA/GA ratios pertain to greater dormancy and reduced germinability, the ratio of ABA to GA did not reflect the dormancy state and germinability of B. tournefortii seeds in this study. We conclude that ABA sensitivity is more important in determining seed germination characteristics than are the relative endogenous concentrations of ABA and gibberellins or the sensitivity to gibberellins. This finding is supported by Goggin et al. (2009) for Lolium rigidum seeds, who also identified ABA sensitivity as a key moderator of germinability.
The finding that higher seed water content and a history of imbibition can reduce the sensitivity of seeds to KAR1 has clear implications for practice and research. First, we recommend that KAR1 should be applied when seeds are dry to optimize the germination response. In a Mediterranean climate, where seeds experience an extended dry period following dispersal in late spring or early summer, KAR1 may be most effective if applied prior to the onset of the late autumn rains, as was also observed for smoke applications in Roche et al. (1998). We recommend that future studies investigate how multiple hydration cycles, and hydration conditions that are common to other climates, such as cold- and warm-stratification, influence the receptivity of seeds to KAR1. Finally, researchers should consider the hydration state and history of imbibition of seeds when conducting experiments using germination stimulants. As seen here, using only seeds in higher hydration states or those that had previously been imbibed could have led to the erroneous conclusion that the seed lot was not responsive to 1 nm KAR1. It is plausible that germination with other commonly used stimulants such as smoke-water and nitrates may also depend on hydration state and history, especially given the observed effects of hydration state on ABA concentrations and sensitivity.
ACKNOWLEDGMENTS
We thank David Nelson, Steven Smith, Danica Goggin and Janine Croser for helpful discussions. David Symons and Matthew Barrett assisted with scanning electron microscopy. This research was supported under the Australian Research Council's Linkage Projects funding scheme (LP0776951), Kings Park and Botanic Garden – Alcoa of Australia Limited Seed Conservation Partnership, the California State University San Bernadino, and the US Department of Agriculture (2008-38422-19133).
LITERATURE CITED
- Ali-Rachedi S, Bouinot D, Wagner MH, et al. Changes in endogenous abscisic acid levels during dormancy release and maintenance of mature seeds: studies with the Cape Verde Islands ecotype, the dormant model of Arabidopsis thaliana. Planta. 2004;219:479–488. doi: 10.1007/s00425-004-1251-4. [DOI] [PubMed] [Google Scholar]
- Baker KS, Steadman KJ, Plummer JA, Merritt DJ, Dixon KW. The changing window of conditions that promotes germination of two fire ephemerals, Actinotus leucocephalus (Apiaceae) and Tersonia cyathiflora (Gyrostemonaceae) Annals of Botany. 2005;96:1225–1236. doi: 10.1093/aob/mci274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewley JD. Seed germination and dormancy. The Plant Cell. 1997;9:1055–1066. doi: 10.1105/tpc.9.7.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford KJ. Water relations in seed germination. In: Kigel J, Galili G, editors. Seed development and germination. New York: Marcel Dekker; 1995. pp. 351–396. [Google Scholar]
- Bu QY, Li HM, Zhao QZ, et al. The Arabidopsis RING Finger E3 Ligase RHA2a is a novel positive regulator of abscisic acid signaling during seed germination and early seedling development. Plant Physiology. 2009;150:463–481. doi: 10.1104/pp.109.135269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiwocha SDS, Dixon KW, Flematti GR, et al. Karrikins: a new family of plant growth regulators in smoke. Plant Science. 2009;177:252–256. [Google Scholar]
- Commander LE, Merritt DJ, Rokich DP, Flematti GR, Dixon KW. Seed germination of Solanum spp. (Solanaceae) for use in rehabilitation and commercial industries. Australian Journal of Botany. 2008;56:333–341. [Google Scholar]
- Commander LE, Merritt DJ, Rokich DP, Dixon KW. Seed biology of Australian arid zone species: Germination of 18 species used for rehabilitation. Journal of Arid Environments. 2009;73:617–625. [Google Scholar]
- Davis AS, Cardina J, Forcella F, et al. Environmental factors affecting seed persistence of annual weeds across the US corn belt. Weed Science. 2005;53:860–868. [Google Scholar]
- Daws MI, Davies J, Pritchard HW, Brown NAC, Van Staden J. Butenolide from plant-derived smoke enhances germination and seedling growth of arable weed species. Plant Growth Regulation. 2007;51:73–82. [Google Scholar]
- Demir I, Light ME, Van Staden J, Kenanoglu BB, Celikkol T. Improving seedling growth of unaged and aged aubergine seeds with smoke-derived butenolide. Seed Science and Technology. 2009;37:255–260. [Google Scholar]
- Dixon KW, Merritt DJ, Flematti GR, Ghisalberti EL. Karrikinolide – a phytoreactive compound derived from smoke with applications in horticulture, ecological restoration and agriculture. Acta Horticulturae. 2009;813:155–170. [Google Scholar]
- Feurtado JA, Yang J, Ambrose SJ, Cutler AJ, Abrams SR, Kermode AR. Disrupting abscisic acid homeostasis in western white pine (Pinus monticola Dougl. Ex D. Don) seeds induces dormancy termination and changes in abscisic acid catabolites. Journal of Plant Growth Regulation. 2007;26:46–54. [Google Scholar]
- Finch-Savage WE, Leubner-Metzger G. Seed dormancy and the control of germination. New Phytologist. 2006;171:501–523. doi: 10.1111/j.1469-8137.2006.01787.x. [DOI] [PubMed] [Google Scholar]
- Finch-Savage WE, Cadman CSC, Toorop PE, Lynn JR, Hilhorst HWM. Seed dormancy release in Arabidopsis Cvi by dry after-ripening, low temperature, nitrate and light shows common quantitative patterns of gene expression directed by environmentally specific sensing. Plant Journal. 2007;51:60–78. doi: 10.1111/j.1365-313X.2007.03118.x. [DOI] [PubMed] [Google Scholar]
- Finkelstein RR, Gampala SSL, Rock CD. Abscisic acid signaling in seeds and seedlings. Plant Cell. 2002;(Supplement 2002):S15–S45. doi: 10.1105/tpc.010441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flematti GR, Ghisalberti EL, Dixon KA, Trengove RD. A compound from smoke that promotes seed germination. Science. 2004;305:977. doi: 10.1126/science.1099944. [DOI] [PubMed] [Google Scholar]
- Flematti GR, Ghisalberti EL, Dixon KW, Trengove RD. Synthesis of the seed germination stimulant 3-methyl-2H-furo[2,3-c]pyran-2-one. Tetrahedron Letters. 2005;46:5719–5721. [Google Scholar]
- Goggin DE, Steadman KJ, Emery RJN, Farrow SC, Benech-Arnold RL, Powles SB. ABA inhibits germination but not dormancy release in mature imbibed seeds of Lolium rigidum Gaud. Journal of Experimental Botany. 2009;60:3387–3396. doi: 10.1093/jxb/erp175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldwasser Y, Yoneyama K, Xie X, Yoneyama K. Production of strigolactones by Arabidopsis thaliana responsible for Orobanche aegyptiaca seed germination. Plant Growth Regulation. 2008;55:21–28. [Google Scholar]
- Grappin P, Bouinot D, Sotta B, Miginiac E, Jullien M. Control of seed dormancy in Nicotiana plumbaginifolia: post-imbibition abscisic acid synthesis imposes dormancy maintenance. Planta. 2000;210:279–285. doi: 10.1007/PL00008135. [DOI] [PubMed] [Google Scholar]
- Gurusinghe S, Powell ALT, Bradford KJ. Enhanced expression of BiP is associated with treatments that extend storage longevity of primed tomato seeds. Journal of the American Society of Horticultural Science. 2002;127:528–534. [Google Scholar]
- Hoyle GL, Daws MI, Steadman KJ, Adkins SW. Mimicking a semi-arid tropical environment achieves dormancy alleviation for seeds of Australian native Goodeniaceae and Asteraceae. Annals of Botany. 2008;101:701–708. doi: 10.1093/aob/mcn009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain N, Van Staden J. The potential of the smoke-derived compound 3-methyl-2H-furo[2,3-c]pyran-2-one as a priming agent for tomato seeds. Seed Science Research. 2007;17:175–181. [Google Scholar]
- McCullagh P, Nelder JA. Generalized linear models. 2nd edn. London: Chapman and Hall; 1989. [Google Scholar]
- Merritt DJ, Turner SR, Clarke S, Dixon KW. Seed dormancy and germination stimulation syndromes for Australian temperate species. Australian Journal of Botany. 2007;55:336–344. [Google Scholar]
- Millar AA, Jacobson JV, Helliwell CA, et al. Seed dormancy and ABA metabolism in Arabidopsis and barley: the role of ABA 8′;-hydroxylase. The Plant Journal. 2006;45:942–954. doi: 10.1111/j.1365-313X.2006.02659.x. [DOI] [PubMed] [Google Scholar]
- Nelson DC, Riseborough J-A, Flematti GR, et al. Karrikins discovered in smoke trigger Arabidopsis seed germination by a mechanism requiring gibberellic acid synthesis and light. Plant Physiology. 2009;149:863–873. doi: 10.1104/pp.108.131516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts E, Ellis R. Water and seed survival. Annals of Botany. 1989;63:39–52. [Google Scholar]
- Roche S, Dixon KW, Pate JS. For everything a season: smoke-induced seed germination and seedling recruitment in a Western Australian Banksia woodland. Australian Journal of Ecology. 1998;23:111–120. [Google Scholar]
- Schwachtje J, Baldwin IT. Smoke exposure alters endogenous gibberellin and abscisic acid pools and gibberellin sensitivity while eliciting germination in the post-fire annual, Nicotiana attenuata. Seed Science Research. 2004;14:51–60. [Google Scholar]
- Seo M, Hanada A, Kuwahara A, et al. Regulation of hormone metabolism in Arabidopsis seeds: phytochrome regulation of abscisic acid metabolism and abscisic acid regulation of gibberellin metabolism. Plant Journal. 2006;48:354–366. doi: 10.1111/j.1365-313X.2006.02881.x. [DOI] [PubMed] [Google Scholar]
- Stevens JC, Merritt DJ, Flematti GR, Ghisalberti EL, Dixon KW. Seed germination of agricultural weeds is promoted by the butenolide 3-mthyl-2H-furo[2,3-c]pyran-2-one under laboratory and field conditions. Plant Soil. 2007;298:113–124. [Google Scholar]
- Toh S, Imamura A, Watanabe A, et al. High temperature-induced abscisic acid biosynthesis and its role in the inhibition of gibberellin action in Arabidopsis seeds. Plant Physiology. 2008;146:1368–1385. doi: 10.1104/pp.107.113738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters C, Hill LM, Wheeler LJ. Dying while dry: kinetics and mechanisms of deterioration in desiccated organisms. Integrative and Comparative Biology. 2005;45:751–758. doi: 10.1093/icb/45.5.751. [DOI] [PubMed] [Google Scholar]
- Wolk WD, Dillon PF, Copeland LF, Dilley DR. Dynamics of imbibition in Phaseolus vulgaris L. in relation to initial seed moisture content. Plant Physiology. 1989;89:805–810. doi: 10.1104/pp.89.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuest SB. Vapour is the principal source of water imbibed by seeds in unsaturated soils. Seed Science Research. 2007;17:3–9. [Google Scholar]