Abstract
Background and Aims
Crop yield and nutritional quality are significantly reduced when potassium (K) in soil is deficient. As a beneficial element for plants, silicon (Si) is effective in alleviating the toxic effects of mineral nutrients. However, the roles played by Si in mediating deficiency in essential mineral nutrients in general and K in particular have not been investigated.
Methods
To evaluate the role of Si in K deficiency-induced inhibition of growth of soybean (Glycine max) seedlings, the effects of K deficiency on shoot and root growth, hydrogen peroxide accumulation, K contents, lipid peroxidation and activities of antioxidant enzymes in the absence and presence of 2 mm sodium silicate (Na2SiO3) were investigated.
Key Results
Both shoot and root biomass of soybean seedlings were markedly reduced when grown in K-deficient medium (1 mm K) compared with those grown in K-sufficient medium (5 mm). Addition of Na2SiO3 significantly ameliorated the K deficiency-induced reductions in shoot and root growth. Sodium silicate enhanced K concentrations in leaf, stem and root of K-deficient seedlings by 105·4, 83·4 and 58·8 %, respectively. Hydrogen peroxide (H2O2) and malondialdehyde (MDA) contents in soybean seedlings were increased by 25 and 97 %, respectively, when exposed to K-deficient medium. These increases in accumulation of H2O2 and MDA were removed by addition of Na2SiO3. Addition of Na2SiO3 reduced the K deficiency-induced increases in activities of superoxide dismutase, catalase and peroxidase.
Conclusions
Application of Si to soybean seedlings grown in K-deficient medium markedly enhanced K use efficiency. Therefore, Si not only increases tolerance to nutrient toxicity, but also ameliorates symptoms associated with deficiency in essential nutrients in plants.
Keywords: Antioxidant enzyme activities, oxidative stress, potassium deficiency, silicon, soybean, Glycine max
INTRODUCTION
Potassium (K) is the most abundant cation in plants, and plays important roles in many physiological processes such as photosynthesis, assimilate transport and enzyme activation. Potassium is essential for high-yield crop production, and can be a limiting factor for such crops under certain environmental conditions, for example drought (Liebersbach et al., 2004) and salinity (Qi and Spalding, 2004; Rus et al., 2004). Therefore, elucidation of the mechanisms underlying responses and adaptation of plants to K deficiency is of key importance. Although application of K fertilizers is an effective way to minimize K deficiency-induced loss of crop production, the economic burdens associated with the use of fertilizers, particularly in developing countries, are a limiting factor. Therefore, a better understanding of the mechanisms by which plants cope with K deficiency would provide a physiological basis for selecting crops with high K use efficiency (Rengel and Damon, 2008).
K deficiency reduces mechanical stability, nutritional quality and crop resistance to pathogens (Pettigrew, 2008). In this context, application of silicon (Si), which is one of the most abundant elements in the Earth's crust, has been shown to enhance lodging resistance through increased breaking strength and increased pushing tolerance (Uchimura et al., 2000). In addition, Si is capable of increasing plant tolerance to pests and diseases by acting as a physical barrier to protect plants from attack by insects and pathogens, and/or acting as a modulator of host resistance to pathogens (Ma and Yamaji, 2008). Although there has been no consensus regarding whether Si can be defined as an essential element for plant growth and development (Epstein and Bloom, 2003), ample evidence indicates that Si plays an important role in protecting plants from abiotic and biotic stresses (Liang et al., 2007; Ma and Yamaji, 2008). For instance, Si is effective in alleviating abiotic stresses, including salinity (Liang et al., 2003), drought (Gong et al., 2005), low temperature (Liang et al., 2008), and nutrient toxicity such as from magnesium (Iwasaki and Matsumura, 1999), cadmium (Liang et al., 2005; Vaculik et al., 2009), aluminium (Hodson and Evans, 1995) and boron (Gunes et al., 2007). The ameliorative effect of Si on plants suffering from abiotic stresses often occurs through counteracting oxidative stress by modulating antioxidant enzymes (Liang et al., 2007). Deficiency in macronutrients including K also leads to oxidative stress, as evidenced by accumulation of reactive oxygen species (ROS) and membrane lipid peroxidation (Cakmak, 1994; Shin and Schachtman, 2004; Tewari et al., 2004, 2007; Cakmak, 2005). ROS are strong oxidizing agents that cause oxidative damage to critical molecules such as lipids and proteins, leading to cell lesion. Malondialdehyde (MDA) is a decomposition product of polyunsaturated fatty acids, and has been widely used as a parameter for lipid peroxidation (Mittler, 2002). Given that Si can alleviate oxidative damage induced by various abiotic stresses (cf. Liang et al., 2007), it is conceivable that application of Si may mitigate K deficiency-induced inhibition of crop growth and development. However, there have been few studies to evaluate the role of Si in nutrient deficiency in general (Wallace et al., 1976) and of K deficiency in particular. The present study investigated the effect of Si on soybean seedlings suffering from K deficiency with emphasis on lipid peroxidation and activities of major antioxidant enzymes, including superoxide dismutase (SOD), catalase (CAT) and peroxidase (POD). To rule out the contributions of Na to the observed effect on soybean seedlings treated with Na2SiO3, the effect of NaCl on K-deficient soybean seedlings was also investigated.
MATERIALS AND METHODS
Plant materials
Soybean, Glycine max (L.) Merrill ‘Tiefen 35’ seedlings were grown in glasshouse conditions with maximum photosynthetic photon flux density (1200 h) of 1120–1330 mol m−2 s−1, and daily maximum and minimum temperatures of 32–38 and 20–30 °C, respectively. The average photoperiod was approx. 11 h. The seedlings were planted in pots (diameter 15 cm and height 30 cm) filled with vermiculite and watered with both K-sufficient and K-deficient solutions with and without 2 mm Na2SiO3 and 4 mm NaCl, respectively. The Si concentration (2 mm) used was comparable with those widely used in other studies investigating the ameliorative role of Si in abiotic stress (Hodson and Evans, 1995; Gong et al., 2005; Gunes et al., 2007; Kaya et al., 2006; Liang et al., 2003, 2005). The K-sufficient solution was half-strength Hoagland solution with 5 mm K and the K-deficient solution was half-strength Hoagland solution with K reduced to 1 mm. To maintain concentration of NO3− equal between the K-sufficient and K-deficient solutions, NO3− in K-deficient solution was reduced to 1 mm and supplemented with 4 mm NaNO3, and an additional 4 mm NaCl was added in the K-sufficient solution to cancel the potential effect of Na. Each pot with three seedlings was watered with 100 mL K-sufficient and K-deficient solutions with and without Na2SiO3 and NaCl every 3 d during the experimental period. The initial pH of the nutrient solution after addition of Na2SiO3 was approx. 7·8, and the pH was adjusted to 6·0 with HCl prior to irrigation. Each treatment had four pots. After the seedlings were grown in the two K regimes in the absence and presence of Na2SiO3 and NaCl for 60 d, physiological parameters such as root length, lateral root density, biomass, K contents and enzyme activities were determined. Lateral root density was determined by counting total visible lateral root number and was normalized on the basis of primary root length.
Determination of K contents
The 60-d-old soybean plants were harvested and separated into leaves (the third fully expanded), stems and roots for analysis of K contents in these organs. After thorough rinsing with distilled water, leaves, stems and roots were oven-dried for 4 d at 70 °C and digested with nitric acid. K contents were measured by atomic absorption spectrophotometry. Measurements were repeated four times.
Determination of hydrogen peroxide and lipid peroxidation
Hydrogen peroxide concentration was determined by the peroxidase-coupled assay protocols described by Veljovic-Jovanovic et al. (2002). Briefly, approx. 0·5 g of young soybean leaves (the third fully expanded) were ground in liquid N2 and the powder was extracted in 2 mL 1 m HClO4 in the presence of insoluble polyvinylpyrrolidone (PVP) (5 %). The homogenate was centrifuged at 12 000g for 10 min and the supernatant was neutralized with 5 m K2CO3 to pH 5·6 in the presence of 100 µL 0·3 m phosphate buffer (pH 5·6). The solution was centrifuged at 12 000g for 1 min and the sample was incubated for 10 min with 1 U ascorbate oxidase to oxidize ascorbate prior to assay. The reaction mixture comprised 100 mm phosphate buffer (pH 6·5), 3·3 mm DMAB (p-dimethylaminobenzaldehyde), 70 µM MBTH (3-methylbenzthiazolinone-2-hydrazone) and 0·3 units peroxidase. The reaction was initiated by addition of 200 µL sample. The absorbance change at 590 nm was monitored at 25 °C.
Lipid peroxidation was determined by measuring the MDA content via thiobarbituric acid reaction. The amount of MDA-equivalent TBA-reactive substance (TBARS) was derived from the difference in absorbance at 532 and 600 nm using an extinction coefficient of 155 mm cm−1 as described by Tewari et al. (2007).
Electrolyte leakage
Electrolyte leakage was assessed as described by Lutts et al. (1996). Leaf samples were washed three times with deionized water to remove surface-adhered electrolytes. Leaf discs were placed in closed vials containing 10 mL of deionized water and incubated at 25 °C on a rotary shaker for 2 h; subsequently, electrical conductivity of the solution (Lt) was determined. Samples were then autoclaved at 120 °C for 20 min and the final electrical conductivity (L0) was obtained after equilibration at 25 °C. Electrolyte leakage was defined as:
Enzyme assays
SOD (EC 1·15·1·1) activity was measured as described by Giannopolitis and Ries (1977). Briefly, young leaves (approx. 0·60 g) were ground thoroughly with a cold mortar and pestle in 50 mm potassium phosphate buffer (pH 7·0) with 0·1 mm EDTA. The homogenate was centrifuged at 15 000g for 20 min at 4 °C. The supernatant was crude enzyme extraction. Activity of SOD was measured by the photochemical method with nitro-blue tetrazolium (NBT). One unit of SOD activity was defined as the amount of enzyme required to give 50 % inhibition of the rate of NBT reduction at 560 nm. Activities of SOD were represented on soluble protein bases.
POD (EC 1·11·1·7) activity was assayed following the protocols used by Liang et al. (2003). Briefly, fresh young soybean leaves (approx. 1 g) were homogenized in an ice bath in 5 mL 50 mm borate buffer (pH 8·7) containing 5 mm sodium hydrogen sulfite and 0·1 g PVP. Enzyme extract was obtained by centrifuging the homogenate at 10 000g at 4 °C for 25 min. A substrate mixture containing acetate buffer (0·1 mm, pH 5·4), ortho-dianisidine (0·25 % in ethyl alcohol) and 0·1 mm 0·75 % H2O2 was added to the enzyme extract (0·1 mL). POD activity was determined based on the change in absorbance of the brown guaiacol at 460 nm.
CAT (EC 1·11·1·6) activity was determined by monitoring the decrease in absorbance at 240 nm for 1 min following the decomposition of H2O2. The reaction mixture contained 50 mm phosphate buffer (pH 7·0), 15 mm H2O2 and 0·1 mL enzyme extract. CAT activity was calculated from the extinction coefficient (40 mm−1 cm−1) for H2O2.
RESULTS AND DISCUSSION
Root growth, dry mass of root and shoot
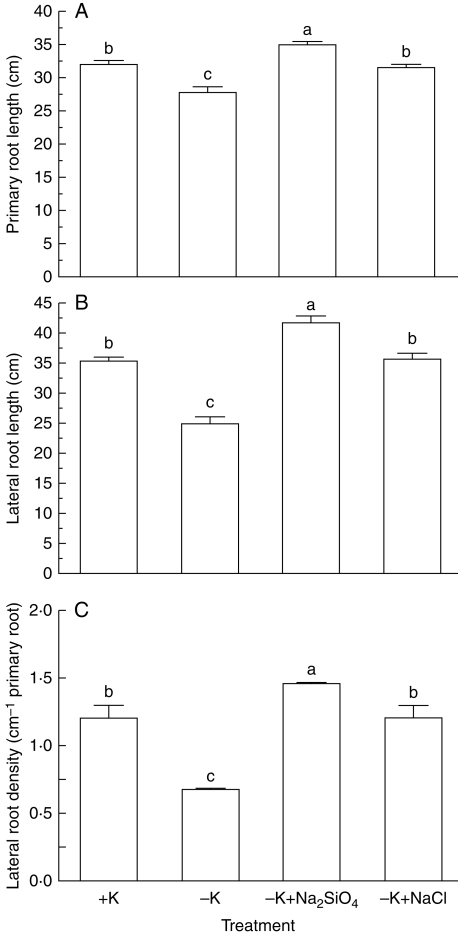
Previous studies have shown that deprivation of K leads to marked decreases in lateral root length, but not primary root length, and lateral root density in Arabidopsis thaliana (Shin and Schachtman, 2004). In contrast, in soybean both primary and lateral root length of seedlings was markedly inhibited when seedlings were grown in K-deficient medium (1 mm K) for 60 d in comparison with those grown in K-sufficient (5 mm K) medium (Fig. 1A, B). The K deficiency-induced reductions in length of primary and lateral roots were significantly ameliorated by the addition of 2 mm Na2SiO3 such that the length of primary and lateral roots was significantly longer than that in K-sufficient solutions (Fig. 1). In addition to root growth, lateral root density in seedlings grown in K-deficient medium was also significantly reduced under conditions of K deficiency and the decrease in lateral root density was enhanced substantially in the presence of Na2SiO3 (Fig. 1C). To test whether the ameliorative effect of Na2SiO3 on root growth of K-deficient seedlings had resulted from contributions of Na+ ions, the effect of equivalent Na+ ions on K-deficient root growth was also investigated by adding 4 mm NaCl to the K-deficient medium. As shown in Fig. 1, addition of NaCl to K-deficient solutions significantly enhanced growth of both primary and lateral roots and lateral root density of K-deficient seedlings. However, the ameliorative effect of NaCl on root growth and lateral root density was significantly less than that of Na2SiO3 (Fig. 1), suggesting that both Na and Si play a role in amelioration of root growth and development under K-deficient conditions with Si being more effective than Na. That Na+ alleviated K deficiency-induced inhibition of root growth implies that Na+ may partially substitute K+ for root growth under K-deficient conditions. In this context, it has been observed that K deficiency promotes accumulation of Na+ in maize (Jordan-Meille and Pellerin, 2008) and Trifolium repens (Henning, 2003). Because addition of NaCl alleviated K deficiency-induced root growth, treatment with NaCl under conditions of K deficiency were included throughout the study.
Fig. 1.
Effects of K deficiency on (A) primary root length, (B) lateral root length and (C) lateral root density of soybean seedlings in the absence and presence of Na2SiO3 and NaCl. Soybean seedlings were grown in 5 mm (+K) and 1 mm K (−K) supplemented with 2 mm Na2SiO3 or 4 mm NaCl for 60 d. Data are the mean ± s.e. of seven individual seedlings for each treatment. Different letters shown in the error bars indicate significant differences among control and treatments at P < 0·05.
In addition to measurements of root length, the effect of K deficiency on dry mass of both root and shoot was also investigated, as well as root/shoot ratio in the absence and presence of Na2SiO3 and NaCl. Similar to root growth, addition of Na2SiO3 and NaCl to the K-deficient medium markedly enhanced the dry weight of root, stem and leaf, with the effect of Na2SiO3 being much greater than that of NaCl (Table 1). It is of interest that the dry weight of root, stem and leaf for soybean seedlings grown in K-deficient medium were greater than those grown in K-sufficient medium when Na2SiO3 was added to the K-deficient solution (Table 1). K deficiency also reduced root/shoot ratio, and this decrease was markedly reversed by application of Na2SiO3 (Table 1). Although root/shoot ratio was also increased by addition of NaCl to the K-deficient medium, the magnitude of the increase was much less than that induced by application of Na2SiO3 (Table 1). In contrast to the K-deficient medium, addition of either NaCl or Na2SiO3 to the K-sufficient medium had no effect on shoot and root biomass (data not shown). These results suggest that Si can significantly ameliorate K deficiency-induced inhibition of both shoot and root growth.
Table 1.
Effect of Na2SiO3 and NaCl on dry weight of soybean seedlings under K deficiency
| Treatment | Root d. wt (mg plant−1) | Stem d. wt (mg plant−1) | Leaf d. wt (mg plant−1) | Root/shoot ratio |
|---|---|---|---|---|
| CK | 1·701 ± 0·071b | 0·729 ± 0·062b | 0·781 ± 0·011a | 0·284 ± 0·024b |
| −K | 1·120 ± 0·090c | 0·546 ± 0·023d | 0·489 ± 0·014c | 0·195 ± 0·020d |
| −K + Na2SiO3 | 2·460 ± 0·068a | 0·894 ± 0·011a | 0·717 ± 0·016a | 0·318 ± 0·010a |
| −K + NaCl | 1·723 ± 0·072b | 0·629 ± 0·013c | 0·633 ± 0·040b | 0·245 ± 0·019c |
Data are the mean ± s.e. of four replicates with each replicate containing at least three seedlings. Soybean seedlings were grown in medium watered with full nutrient solution (5 mm K) (CK) and K-deficient solution (1 mm) (−K) in the absence and presence of 2 mm Na2SiO3 (−K + Na2SiO3) and 4 mm NaCl (−K + Na2SiO3) for 60 d. Values with different letters within each column are significantly different at P < 0·05.
Potassium contents
There were significant decreases in K+ contents in roots, stems and leaves of soybean seedlings when grown in K-deficient medium compared with those grown in K-sufficient medium, with the decreases in K+ contents being greater in roots and stems than in leaves (Table 2). When Na2SiO3 was added to the K-deficient medium, K+ contents in roots, stems and leaves were increased by 105·4, 83·4 and 58·8 %, respectively, leading to greater K+ contents in the three organs of K-deficient seedlings than those in K-sufficient seedlings (Table 2). In contrast to Na2SiO3, addition of NaCl had no significant effect on K+ contents in roots, stems and leaves for soybean seedlings grown in K-deficient medium (Table 2). One interesting finding was that Si can facilitate K+ accumulation in K-deficient soybean seedlings such that K+ contents became greater than K-sufficient seedlings, with K contents in roots being mostly enhanced by Si (Table 2). Previous studies have shown that Si can reduce accumulation of cations such as Na+ (Liang, 1999; Liang et al., 2003), Cd2+ (Liang et al., 2005), Mn2+ (Iwasaki and Matsumura, 1999) and Al3+ (Hodson and Evans, 1995) due to deposition of Si in the roots, thus ameliorating salinity and heavy mental toxicity to plants. Liang (1999) reported that treatment of barley seedlings with NaCl markedly reduced K+ concentrations in shoots, and this inhibitory effect on K+ accumulation was ameliorated by addition of Si. The Si-dependent K+ accumulation under saline conditions has been ascribed to the enhancement of H+-ATPase activity in the presence of Si (Liang, 1999; Liang et al., 2003). A similar explanation may also account for the Si-induced increase in K accumulation under K-deficient conditions.
Table 2.
Changes in K contents in roots, stems and the third fully expanded leaves of soybean under K deficiency in response to addition of 2 mm Na2SiO3 and 4 mm NaCl to K-deficient medium
| Treatment | Root K (mg g−1) | Stem K (mg g−1) | Leaf K (mg g−1) |
|---|---|---|---|
| Control (CK) | 18·92 ± 0·39b | 16·77 ± 1·13b | 20·49 ± 1·18b |
| K deficiency (−K) | 10·32 ± 0·82c | 10·49 ± 0·89c | 17·14 ± 0·72c |
| −K + Na2SiO3 | 23·50 ± 1·52a | 19·24 ± 0·95a | 27·21 ± 2·27a |
| −K + NaCl | 12·09 ± 1·01c | 11·60 ± 0·34c | 17·37 ± 1·72c |
Data are from seedlings grown under different treatments for 60 d and expressed as mean ± s.d. of four replicates. Values with different letters within each column are significantly different at P < 0·05.
MDA and hydrogen peroxide contents
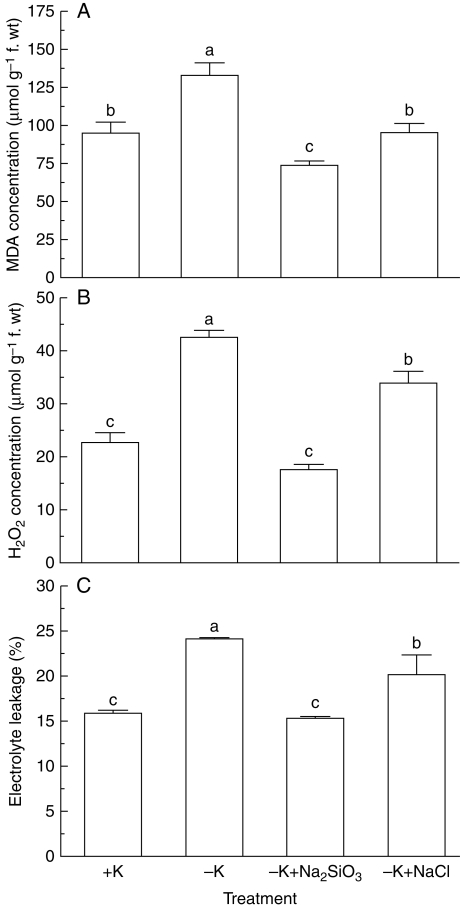
There was a significant increase in MDA concentrations in soybean leaves when grown in K-deficient medium, and the K deficiency-induced increase in concentrations of MDA was abolished in the presence of Na2SiO3 such that MDA concentrations in K-deficient leaves were lower than those in K-sufficient leaves (Fig. 2A). In contrast to treatment with Na2SiO3, addition of NaCl had no effect on MDA concentrations in K-deficient soybean leaves (Fig. 2A), suggesting that Si is responsible for alleviating the effect of Na2SiO3 on K deficiency-induced MDA accumulation. In addition to MDA, K deficiency also evoked accumulation of H2O2 in soybean leaves, and the increase in H2O2 concentrations was markedly reduced by Na2SiO3 (Fig. 2B). Although the K deficiency-induced elevation of H2O2 was reduced by addition of NaCl to the K-deficient medium (Fig. 2B), the reduction in H2O2 concentrations in K-deficient leaves by NaCl was significantly less than that caused by addition of Na2SiO3 (Fig. 2B). There was a significant increase in electrolyte leakage in soybean leaves when exposed to the K-deficient medium, and this increase was reversed by application of Na2SiO3 (Fig. 2C). Moreover, the K deficiency-dependent electrolyte leakage was attenuated by NaCl (Fig. 2C), but the effect of NaCl on electrolyte leakage was significantly less than that of Na2SiO3 (Fig. 2C). Similar ameliorative effects of Si on electrolytic leakage induced by salinity (Liang et al., 2003), boron toxicity (Gunes et al., 2007) and osmotic stress (Kaya et al., 2006) have been reported. These results indicate that Si can stabilize the structure and integrity of plasma membranes by affecting the stress-dependent peroxidation of membrane lipids (Liang et al., 2003). Similar to the present findings, Shin and Schachtman (2004) reported K deficiency-induced accumulation of H2O2 in Arabidopsis leaves. Potassium deficiency has been shown to elicit lipid peroxidation in mulberry (Morus alba) leaves, as evidenced by accumulation of MDA (Tewari et al., 2007). Therefore, the present results indicate that Si can effectively ameliorate membrane lipid peroxidation, thus protecting plants from oxidative stress.
Fig. 2.
Effects of K deficiency on accumulation of (A) malondialdehyde (MDA), (B) H2O2 and (C) electrolyte leakage of soybean seedlings in the absence and presence of Na2SiO3 and NaCl. Soybean seedlings were grown in 5 mm (+K) and 1 mm K (−K) supplemented with 2 mm Na2SiO3 or 4 mm NaCl for 60 d. Data are the mean ± s.e. of four replicates. Different letters shown in the error bars indicate significant differences among control and treatments at P < 0·05.
Antioxidant enzyme activities
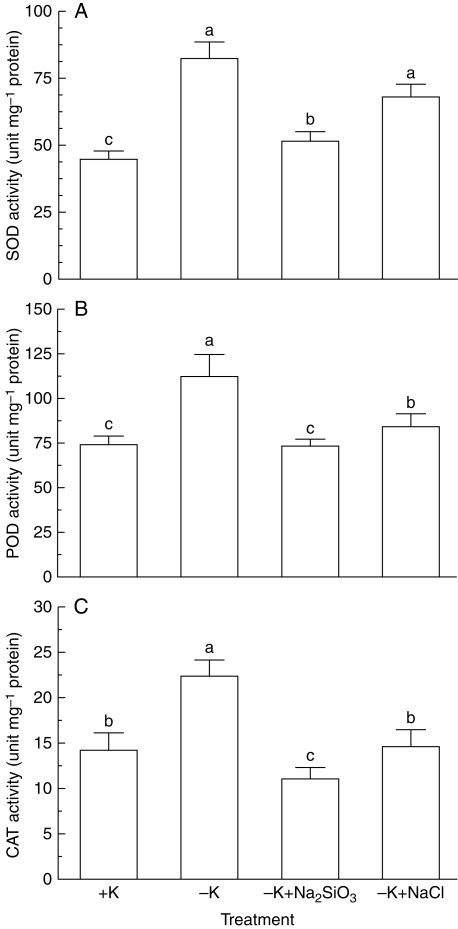
Previous studies have demonstrated that Si can reduce salinity-induced lipid peroxidation by regulating antioxidant enzymes in plants (Liang et al., 2003). As Si reduced lipid peroxidation and H2O2 contents in K-deficient seedlings, the effect of K deficiency on activities of antioxidant enzymes in the absence and presence of Si was investigated. Activities of SOD, POD and CAT were increased substantially when soybean seedlings were exposed to K-deficient medium (Fig. 3). The K deficiency-induced increases in activities of SOD, POD and CAT were inhibited markedly by addition of Na2SiO3 to K-deficient medium (Fig. 3A). In contrast to Na2SiO3, the K deficiency-induced increase in SOD activity was not affected by addition of NaCl to K-deficient medium (Fig. 3A). Activities of POD and CAT were reduced by addition of NaCl to the K-deficient medium, but the inhibitory effect of NaCl on K deficiency-induced increases in POD and CAT activities was significantly less than that of Na2SiO3 (Fig. 3B, C). Changes in antioxidant enzyme activities such as SOD, POD and CAT have been widely reported in plants in response to deficiency in macronutrients, including K, to modulate toxic levels of ROS (Cakmak, 1994; Tewari et al., 2004, 2007). For instance, a K deficiency-induced increase in SOD activity has been observed in mulberry (Tewari et al., 2007) and maize leaves (Tewari et al., 2004), while CAT was stimulated by K deficiency in maize leaves (Tewari et al., 2004), but not in mulberry leaves (Tewari et al., 2007). In contrast to the present findings, Tewari et al. (2004, 2007) noted that POD activity was not affected by K deficiency in both mulberry and maize. This discrepancy may be accounted for by the differences in plant species, K-deficient regimes and treatment periods. For instance, in the present study, soybean plants grown in 5 and 1 mm K medium were referred to as K-sufficient and K-deficient seedlings, respectively. In contrast, K deficiency was taken as removal of all K in the growth medium in both Arabidopsis (Shin and Schachtman, 2004) and mulberry (Tewari et al., 2007). In addition, the relatively long-term effect of K deficiency on soybean seedlings (60 d) was investigated herein, whereas responses of Arabidopsis and mulberry to K starvation for 10 and 30 d were investigated, respectively (Shin and Schachtman, 2004; Tewari et al., 2007). The longer treatment period with relatively high K concentrations employed here is of more physiological relevance, and thus the present findings may have important significance in farming practice. Moreover, the effects of Si on the K deficiency-induced changes in antioxidant enzymes activities, membrane lipid peroxidation and H2O2 concentrations are comparable with the effects of Si on those parameters evoked by boron toxicity (Gunes et al., 2007). For instance, excess B-induced accumulation of H2O2 and MDA, and increased activities of SOD and CAT were markedly reduced by Si (Gunes et al., 2007). The findings that Si alleviated K deficiency-induced oxidative damage to soybean plants by modulating antioxidant enzyme activities may underlie the observed ameliorative effect of Si on shoot and root growth (Table 1).
Fig. 3.
Effects of K deficiency on activities of (A) superoxide dismutase (SOD), (B) peroxidase (POD) and (C) catalse (CAT) of soybean seedlings in the absence and presence of Na2SiO3 and NaCl. Soybean seedlings were grown in 5 mm (+K) and 1 mm K (−K) supplemented with 2 mm Na2SiO3 or 4 mm NaCl for 60 d. Data are the mean ± s.e. of four replicates. Different letters shown in the error bars indicate significant differences among control and treatments at P < 0·05.
Conclusions
The present study demonstrates that application of Si to K-deficient medium markedly alleviated K deficiency-induced inhibition of both root and shoot growth in soybean seedlings. In addition, Si significantly enhanced K accumulation in soybean plants exposed to K-deficient medium, thus alleviating K deficiency-induced membrane lipid peroxidation and oxidative stress by modulating antioxidant enzymes. These results highlight that Si is not only involved in amelioration of nutrient toxicity, but can also improve nutrient use efficiency under nutrient-deficient conditions. These findings may also have important implications in agronomic practice to improve nutrient efficiency by application of Si fertilizer under nutrient-deficient conditions.
ACKNOWLEDGEMENTS
This work was supported by the State Key Basic Research Development Programme of China (2007CB106800) and National Natural Science Foundation of China (No. 30821062). We thank the two anonymous reviewers for their constructive suggestions made on an earlier version of the manuscript.
LITERATURE CITED
- Cakmak I. Activity of ascorbate-dependent H2O2-scavenging enzymes and leaf chlorosis are enhanced in magnesium- and potassium-deficient leaves, but not in phosphorus-deficient leaves. Journal of Experimental Botany. 1994;45:1259–1266. [Google Scholar]
- Cakmak I. The role of potassium in alleviating detrimental effects of abiotic stresses in plants. Journal of Plant Nutrition and Soil Science. 2005;168:521–530. [Google Scholar]
- Epstein E, Bloom AJ. Mineral Nutrition of Plants: Principles and Perspectives. 2nd edn. New York: John Wiley & Sons; 2003. [Google Scholar]
- Giannopolitis CN, Ries SK. Superoxide dismutase in higher plants. Plant Physiology. 1977;59:309–314. doi: 10.1104/pp.59.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong H, Zhu X, Chen K, Wang S, Zhang C. Silicon alleviates oxidative damage of wheat plants in pots under drought. Plant Science. 2005;169:313–321. [Google Scholar]
- Gunes A, Inal A, Baggi EG, Coban S, Pilbean DJ. Silicon mediates changes to some physiological and enzymatic parameters symptomatic for oxidative stress in spinach (Spinacia oleracea L.) grown under B toxicity. Scientia Horticulture. 2007;113:113–119. [Google Scholar]
- Henning GH. The effect of potassium deficiency on growth and N2-fixing in Trifolium repens. Physiologia Plantrum. 2003;129:440–449. [Google Scholar]
- Hodson MJ, Evans DE. Aluminum/silicon interactions in higher plants. Journal of Experimental Botany. 1995;46:161–171. doi: 10.1093/jxb/eraa024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki K, Matsumura A. Effect of silicon on alleviation of manganese toxicity in pumpkin (Cucurbita moschata Duch cv. Shintosa) Soil Science & Plant Nutrition. 1999;45:909–920. [Google Scholar]
- Jordan-Meille L, Pellerin S. Shoot and root growth of hydroponic maize (Zea mays L.) as influenced by K deficiency. Plant Soil. 2008;304:157–168. [Google Scholar]
- Kaya C, Tuna L, Higgs D. Effect of silicon on plant growth and mineral nutrition of maize grown under water-stress conditions. Journal of Plant Nutrition. 2006;29:1469–1480. [Google Scholar]
- Liang YC. Effects of silicon on enzyme activity and sodium, potassium and calcium concentrations in barley under salt stress. Plant Soil. 1999;209:217–224. [Google Scholar]
- Liang YC, Chen Q, Liu Q, Zhang WH, Ding RX. Exogenous silicon (Si) increases antioxidant enzyme activity and reduces lipid peroxidation in roots of salt-stressed barley (Hordeum vulgare L.) Journal of Plant Physiology. 2003;160:1157–1164. doi: 10.1078/0176-1617-01065. [DOI] [PubMed] [Google Scholar]
- Liang YC, Wong JWC, Wei L. Silicon-mediated enhancement of cadmium tolerance in maize (Zea mays L.) grown in cadmium contaminated soil. Chemosphere. 2005;58:475–483. doi: 10.1016/j.chemosphere.2004.09.034. [DOI] [PubMed] [Google Scholar]
- Liang YC, Sun WC, Zhu YG, Christie P. Mechanisms of silicon-mediated alleviation of abiotic stresses in higher plants: a review. Environmental Pollution. 2007;147:422–428. doi: 10.1016/j.envpol.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Liang YC, Zhu J, Li ZJ, et al. Role of silicon in enhancing resistance to freezing stress in two contrasting winter wheat cultivars. Environmental and Experimental Botany. 2008;64:286–294. [Google Scholar]
- Liebersbach H, Steingrobe B, Claassen N. Roots regulate ion transport in the rhizosphere to counteract reduced mobility in dry soil. Plant Soil. 2004;260:79–88. [Google Scholar]
- Lutts S, Kinet JM, Bouharmont J. NaCl-induced senescence in leaves of rice (Oryza sativa L.) cultivars differing in salinity resistance. Annals of Botany. 1996;78:389–398. [Google Scholar]
- Ma JF, Yamaji N. Functions and transport of silicon in plants. Cellular and Molecular Life Science. 2008;65:3049–3057. doi: 10.1007/s00018-008-7580-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends in Plant Science. 2002;7:405–410. doi: 10.1016/s1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- Pettigrew WT. Potassium influences on yield and quality production for maize, wheat, soybean and cotton. Physiologia Plantarum. 2008;133:670–681. doi: 10.1111/j.1399-3054.2008.01073.x. [DOI] [PubMed] [Google Scholar]
- Qi Z, Spalding EP. Protection of plasma membrane K+ transport by the salt overly sensitive1 Na+-H+ antiporter during salinity stress. Plant Physiology. 2004;136:2547–2555. doi: 10.1104/pp.104.049213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rengel Z, Damon PM. Crops and genotypes differ in efficiency of potassium uptake and use. Physiologia Plantarum. 2008;133:624–636. doi: 10.1111/j.1399-3054.2008.01079.x. [DOI] [PubMed] [Google Scholar]
- Rus A, Lee BH, Munoz-Mayor A, et al. AtHKT1 facilities Na+ homeostasis and K+ nutrition in planta. Plant Physiology. 2004;136:2500–2511. doi: 10.1104/pp.104.042234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin R, Schachtman DP. Hydrogen peroxide mediates plant root cell response to nutrient deprivation. Proceedings of the National Academy of Sciences USA. 2004;101:8827–8832. doi: 10.1073/pnas.0401707101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari RK, Kumar P, Tewari N, Srivastava S, Sharma PN. Macronutrient deficiencies and differential antioxidant responses – influence on the activity and expression of superoxide dismutase in maize. Plant Science. 2004;66:687–694. [Google Scholar]
- Tewari RK, Kumar P, Sharma PN. Oxidative stress and antioxidant responses in young leaves of mulberry plants grown under nitrogen, phosphorus or potassium deficiency. Journal of Integrative Plant Biology. 2007;49:313–322. [Google Scholar]
- Uchimura Y, Ogata T, Sato H, Matsue Y. Effects of silicate application on lodging, yield and palatability of rice grown by direct sowing culture. Japan Journal of Crop Science. 2000;69:487–492. [Google Scholar]
- Vaculik M, Luxa A, Luxovac M, Tanimoto E, Lichtscheidle I. Silicon mitigates cadmium inhibitory effects in young maize plants. Environmental and Experimental Botany. 2009;67:52–58. [Google Scholar]
- Veljovic-Jovanovic S, Noctor G, Foyer CH. Are leaf hydrogen peroxide concentrations commonly overestimated? The potential influence of artefactual interference by tissue phenolics and ascorbate. Plant Physiology and Biochemistry. 2002;40:501–507. [Google Scholar]
- Wallace A, Romney EM, Muller RT. Nitrogen–silicon interaction in plants grown in desert soil with nitrogen deficiency. Agronomy Journal. 1976;68:529–530. [Google Scholar]