Abstract
Background and Aims
In this Botanical Briefing we describe how the interactions between plants and their biotic environment can change during range-expansion within a continent and how this may influence plant invasiveness.
Scope
We address how mechanisms explaining intercontinental plant invasions by exotics (such as release from enemies) may also apply to climate-warming-induced range-expanding exotics within the same continent. We focus on above-ground and below-ground interactions of plants, enemies and symbionts, on plant defences, and on nutrient cycling.
Conclusions
Range-expansion by plants may result in above-ground and below-ground enemy release. This enemy release can be due to the higher dispersal capacity of plants than of natural enemies. Moreover, lower-latitudinal plants can have higher defence levels than plants from temperate regions, making them better defended against herbivory. In a world that contains fewer enemies, exotic plants will experience less selection pressure to maintain high levels of defensive secondary metabolites. Range-expanders potentially affect ecosystem processes, such as nutrient cycling. These features are quite comparable with what is known of intercontinental invasive exotic plants. However, intracontinental range-expanding plants will have ongoing gene-flow between the newly established populations and the populations in the native range. This is a major difference from intercontinental invasive exotic plants, which become more severely disconnected from their source populations.
Keywords: Climate change, range expansion, exotic plant, plant invasion, plant defence, trophic interactions, enemy release, EICA, above-ground and below-ground interactions, nutrient cycling, litter decomposition
INTRODUCTION
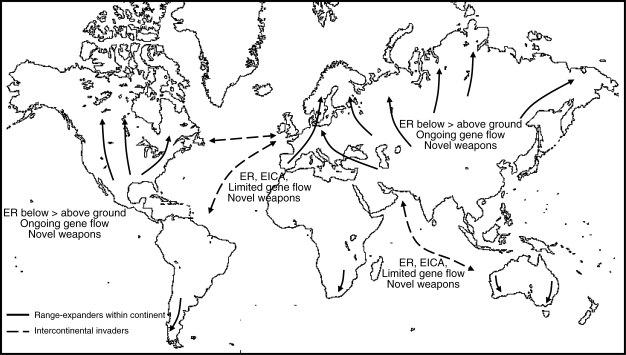
Owing to recent climate change, many plant species' ranges are expanding to higher latitudes (Walther et al., 2002). Such intracontinental range expansions can occur in any continent including those in the southern hemisphere (Fig. 1). However, they probably occur on a smaller scale in the southern hemisphere because less land mass is available for vascular plants. In their new environments, intracontinental range-expanding plants are exposed to above- and below-ground biotic interactions different from those in their original range. The same phenomenon of range expansion also occurs with plants that have been introduced first from other continents before moving pole-wards (Fig. 1). These species are called intercontinental range-expanding plants and a number of them are highly invasive in their new range. In this paper, we will discuss the possible consequences of plant range expansion for plant abundance, the evolution of plant defence and consequences for ecosystem processes. Our target is intracontinental range expansion, but since much of the ecology of intracontinental range-expanders in their new habitats is unknown, use will be made of the basic concepts developed for intercontinental plant invasions.
Fig. 1.
Differences between intercontinental invaders and intracontinental climate-warming-induced range-expanders. The effects of enemy release in range-expanders might be temporal: enemies may catch up, or existing generalist herbivores may switch hosts. As above-ground enemies are more mobile than below-ground enemies, range-expanding plants will be more likely to escape from their below-ground than from their above-ground enemies. Ongoing gene flow between populations in the new range and the native range, within a continent, affects evolutionary processes such as the evolution of increased competitive ability. Abbreviations: EICA = evolution of increased competitive ability; ER = enemy release.
The question is what ecological interactions intracontinental range-expanding plant species will experience in their new range. This is an aspect of climate change that has not yet received much attention from experimental ecologists. Thus far, in most studies on climate change effects, local communities have been exposed to novel conditions, such as warming, drought or elevated carbon dioxide (e.g. Körner, 2006). Those studies did not take into account that with the shift of species distribution through climate change, the biotic interactions between species will change as well.
The main intracontinental gateways of plants into new ranges are via ruderal areas such as river banks, road verges and railway tracks. Therefore, the traits of the climate change-induced intracontinental range-expanders are those of quick colonizers, such as wind or water dispersers. Many of these plants first establish along the dispersal corridors, from which they may spread into adjacent habitats. Temperate regions are currently being colonized by both intercontinental and intracontinental range-expanding plant species. Intracontinental range-expanders may or may not have the same invasive traits as intercontinental invaders and they are probably facing biotic interactions that differ from those in their native range. On the other hand, the exposure of the range-expanders to biotic interactions in the new range can differ from that of related native species, for example because their associated above- and below-ground species do not migrate at the same rate (Berg et al., 2010).
The mechanisms that facilitate establishment of intercontinental invaders may vary from little competition on disturbed sites, to enhanced benefits from low-specific symbionts and reduced exposure to natural enemies (Colautti et al., 2004). On the other hand, the abundance of intercontinental exotics may be reduced when not recognized by symbionts or predators of their enemies (Verhoeven et al., 2009). Once the intercontinental invaders have become established, they may change above- and below-ground biotic interactions in their new range because the plants become so abundant that they dominate the vegetation and therefore strongly influence the quantity and quality of food supply for invertebrates, vertebrates and soil microbes (Parker and Hay, 2005).
Intercontinental exotic plants can introduce novel compounds, to which native plants and other biota have little or no tolerance or defences (e.g. Cappuccino and Arnason, 2006). Intercontinental invasive plants may also alter detritus chemistry, which influences nutrient cycling in the new range (Ehrenfeld, 2003). Therefore, besides changing biotic interactions in the new range, the intercontinental invaders may also be able to alter resource quality for soil phytophages and decomposers. This then may feed back to their capacity to become abundant. Finally, selection of plant traits may differ between the native species and the intercontinental exotics (Blossey and Nötzold, 1995; Müller-Schärer et al., 2004; Joshi and Vrieling, 2005). All these issues have been relatively well studied for invasive plant species of intercontinental origin, whereas little knowledge exists on intracontinental range-expanders.
Here, we review characteristics of intracontinental exotic plant species that expand their range into formerly temperate regions. Recently, it was shown that interactions of these intracontinental range-expanders with soil communities and above-ground polyphagous herbivores were not very different from the corresponding interactions of intercontinental invasive plant species (Engelkes et al., 2008). We will discuss whether or not these successful climate-warming-induced intracontinental range-expanding plant species may cause serious changes in the species composition, structure and trait composition of plant communities, the associated biota and their effects on ecosystem processes. First to be considered are how direct interactions between plants, above- and below-ground enemies and symbionts may be influenced by intracontinental range-expansions. This is followed by a discussion on how intracontinental range-expanding plants may introduce novel chemistry, and finally how this chemistry influences indirect interactions between plants and decomposer organisms is considered.
Above- and below-ground interactions
Above- and below-ground herbivores are both capable of influencing plant abundance by selectively consuming plant shoot and root material, thereby changing intra- and interspecific interactions among plants. Two concepts used for understanding the performance of intercontinental exotic plants in their new range can also be used for intracontinental range-expanding plants: enemy release and biotic resistance (Keane and Crawley, 2002). ‘Enemy release’ occurs when plants expand ranges faster than their enemies, and when the potential enemies already present in the new range do not recognize or feed on the exotic plants. This will provide the exotic species with an advantage in interspecific competition with natives that are under control of their natural enemies. ‘Biotic resistance’ to the invaders occurs when the enemies or plants in the new range control the invaders by herbivory, pathogenesis or competition.
Plant enemies can be specialists or generalists. Specialist enemies often feed on a limited number of related plant species. Specialists are generally adapted to the defences of their host plants and can exert strong control of plant population sizes. Although loss of specialists in particular could be beneficial to exotic plants in their new range (e.g. Wolfe, 2002), plants will still be exposed to generalist enemies in the new range (Müller-Schärer et al., 2004; Joshi and Vrieling, 2005).
Generalist herbivores are present in all habitats and colonize new hosts faster than specialists. Both matches and mismatches between plants and their novel herbivores have been reported (Parker and Hay, 2005), so that both enemy release and novel plant–enemy interactions can affect exotic plants in new ranges. Whether exotic plants experience reduced enemy impact depends on the net outcome of impacts from the enemies lost from their original range, and the enemies gained in the new range.
While enemy release and biotic resistance have been tested widely for intercontinental invaders, there are only very few such reports on intracontinental range-expanding plants. In an experimental study, both inter- and intracontinental range-expanding plant species were compared with phylogenetically related native plant species in their response to polyphagous insects (Engelkes et al., 2008). Desert locusts (Schistocerca gregaria) were used as non-co-evolved polyphagous herbivores and it was predicted that these herbivores would feed equally well on native plant species and intra- and intercontinental range-expanders because all these plants were novel to the herbivores. Interestingly, both groups of exotic plants were less susceptible to the desert locusts than the natives, suggesting that the successful range-expanders were as well defended as the intercontinental invaders. Moreover, the results suggested that the exotic plants were better defended against non-co-evolved herbivores than related natives (Engelkes et al., 2008). This is particularly intriguing, as some of the native plant species were invasive elsewhere in the world. Exposure of the native and exotic plant species to aphids did not reveal any different responses of the aphids, so the effects of range-expanders may be herbivore-specific.
If intracontinental range expansion is promoted by climate warming, an indirect effect of warming will be that the incidence of plant invasions is enhanced. Thus far, climate-change effects on insects have been considered directly on the physiology of the insects (Bale et al., 2002) or indirectly from the perspective of plant quality (Bezemer and Jones, 1998). Here, we propose that climate change can also affect plant–insect relationships by introducing less-suitable host plant species originating from lower latitudes.
Aside from antagonist relationships, mutualisms like pollination and symbiosis may become altered by plant invasions. There are many different possible outcomes. For example, pollination of native plants can be reduced in the presence of invasive exotic plants that provide higher resources in their flowers (Traveset and Richardson, 2006). As a result, the exotic plants will receive higher incidences of pollination than the natives, ultimately causing loss of fitness of the natives. Plants that expand their original range within the continent may experience changes in the composition of the insect community that they encounter (Rohde, 1992). Moreover, the insects themselves may also shift range, which can result in all sorts of unpredictable effects (Hegland et al., 2009): in general, intracontinental range-expanding exotic plants may experience indirect advantages over natives if they are less dependent on pollinators than native plants.
Enemy release is best known for above-ground plant enemies. However, some recent studies have shown that plants may also become released from below-ground enemies. For example, in Canada, soil pathogen activity on Eurasian plant species developed as slowly as on dominant native plant species (Klironomos, 2002). In an intercontinental comparison, Prunus serotina (black cherry) suffered from soil pathogens in the root zone in the native range, whereas the plants from the invaded range were free from pathogenic effects (Reinhart et al., 2003). Other exotic plants were shown to accumulate local pathogens, as is demonstrated for above-ground viruses (Malmstrom et al., 2005) and soil pathogens (Mangla et al., 2008). Intracontinental range-expanding plant species are exposed to less soil-borne pathogenic activity than related natives (Van Grunsven et al., 2007; Engelkes et al., 2008). Moreover, in a first comparison between the native and new range of an intracontinental range-expander, the plants were shown to experience soil pathogenic activity in their native, but not in the new range (Van Grunsven et al., 2010).
Mutualistic relationships of plants and arbuscular mycorrhizal fungi (AMFs) can be quite non-specific, so that alterations in these mutualisms are supposed to be less involved in plant invasions (Richardson et al., 2000). Whether or not this is a general pattern during range shift remains to be investigated. In some cases, local mutualistic symbionts can even be strongly suppressed by invasive non-mycorrhizal exotic plants. These exotics may use an active process by which phytochemicals are excreted that suppress AMFs in the soil (Stinson et al., 2006) or a passive process by which the abundant non-mycorrhizal exotics do not support the AMFs in the soil leading to their decline (Vogelsang and Bever, 2009). In both cases the capacity of native mycorrhizal-dependent plant species to persist and survive in the invaded community was reduced. This has been studied for intercontinental exotic plants only.
Plant defence chemistry
Interactions between plants and their environments are mediated through plant secondary product chemistry. For example, plant secondary compounds can act as toxins or deterrents towards herbivores and pathogens, can attract predators of the herbivores and pollinators and can also be phytotoxic. Plant secondary product chemistry plays a role in at least two different hypotheses explaining biological invasions. The first is the novel weapons hypothesis; the second is the evolution of increased competitive ability (EICA) hypothesis. Here, the role and consequences of plant secondary compounds in the invasion of intracontinental range-expanders will be explored.
The novel weapons hypothesis (Callaway and Aschehoug, 2000) states that invasive exotic plants may release from the roots compounds to which the native plants in the new range are not adapted. This gives the invasive exotic plant a competitive advantage over the native competitors. Initially, the novel weapons hypothesis was posed for allelopathic effects in the soil but this can be extended to above-ground interactions as well. Native above-ground herbivores may also not be adapted to the novel chemistry of invasive exotic plants. Cappuccino and Arnason (2006) showed that invasive exotic plants are more likely to have unique compounds compared with non-invasive exotic plants and natives, suggesting that novel chemistry indeed leads to invasion success.
Similar to intercontinental invasions, the success and invasion of intracontinental range-expanding exotics could, at least in part, be due to whether or not these plants contain novel chemistry. In Europe, climate warming is one of the factors enabling plants to shift their distribution northwards. It is often assumed that plants from lower latitudes are better defended against herbivores owing to higher consumer pressures (Coley and Aide, 1991). Lower-latitude salt-marsh plants indeed are less palatable than high-latitude genotypes (Salgado and Pennings, 2005). If these better-defended low-latitude plants moved northwards (in the northern hemisphere), they might thus experience less negative impact of herbivores and pathogens compared with the native plants of the north. This better defence of lower-latitudinal plants can be due to both novel chemistry and higher concentrations of chemical compounds. Successful intracontinental range-expanders showed a higher induction of phenolic compounds than related native plants after herbivory (Engelkes et al., 2008). The release from herbivores and consequently the loss of top-down control of plant population sizes in the new range can promote plant invasiveness (Keane and Crawley, 2002).
The release from herbivores also has another consequence. Plants can afford to invest less in plant defences and more into plant growth, which can further lead to invasion success as is stated in the EICA hypothesis (Blossey and Nötzold, 1995). The EICA hypothesis was extended by incorporation of the different selection of generalist versus specialist enemies (Müller-Schärer et al., 2004). While specialists can be attracted to plant secondary metabolites, generalists are thought to be deterred by these compounds. With intercontinental invasive plants, usually mainly the specialist herbivores are lacking in the introduced range. Hence, an increase of plant secondary metabolites can be expected in the new range if the costs of producing these compounds are low (Joshi and Vrieling, 2005).
In intracontinental range-expanding plants, differences in selection between the old and new range could be less dramatic than for intercontinental invaders. Herbivores may be present in the new range that feed on closely related plant species and can perhaps easily switch to a novel host plant from lower latitudes. Furthermore, herbivores, at least those acting above ground, could move at the same rate as their host plants (Berg et al., 2010). Nevertheless, the chance is high that the multitrophic interactions between the plant and its biotic environment will change during range expansion and therefore selection on plant chemistry will also change in the new range. If selection in the new range is mainly exerted by generalist herbivores, selection towards higher concentrations of plant secondary metabolites can be expected, as has been observed for intercontinental invasive plants.
In contrast to the situation with invaders from other continents, there is continuing gene flow between the source populations in the native range and the populations in the new range of intracontinental range-expanding plants. On the one hand, this can hamper evolution of plant chemistry through random processes such as genetic drift or founder effects and also natural selection by ‘diluting’ any local adaptation in the new range (Kirkpatrick and Barton, 1997). On the other hand, gene flow will increase genetic variation in the newly founded populations, providing more opportunities for natural selection to act. Consequently, evolutionary processes will be different between classic invaders from other continents (Sakai et al., 2001) and climate-warming-induced range-expanders.
Soil nutrient cycling
Root exudates and litter inputs mediate the interactions between plant composition and soil nutrient cycling (Aerts and Chapin, 2000). Intercontinental exotic plants can change these interactions when their litter or their root exudates differ qualitatively or quantitatively from those of native plants (Ehrenfeld, 2003). For example, litter with a high nutrient concentration is decomposed at a faster rate than litter with high lignin content (Cornwell et al., 2008). This can result in faster nutrient cycling of higher-quality litter and slower nutrient cycling of lower-quality litter. When exotic plants alter soil nutrient cycling after establishment this can have consequences for the whole ecosystem, so that native plants will have to deal with the changed nutrient-cycling conditions (Ehrenfeld, 2003). To date, no study has examined the effects of intracontinental climate-warming-induced range-expanding plants on soil nutrient cycling.
For intercontinental invasions, 70 % of all studies determine impacts of invasive plants on soil nutrient cycling based on field observations (Ehrenfeld, 2003). These may include hidden factors, for example that sites invaded by the exotic plants already had higher nutrient cycling prior to invasion. In general, invasive exotic plants can have positive, negative or neutral effects on nutrient cycling (Ehrenfeld, 2003). The effects are site-dependent (Dassonville et al., 2008), and the traits of the exotic plants, especially nitrogen fixation, play a major role in the responses of soil nutrient cycling to invasion (Liao et al., 2008).
In some experimental studies, effects of invasive plants on soil nutrient cycling operated through altering soil microbial community structure (Kourtev et al., 2003). In other studies, invasive exotic plants increased gross nitrification rates by increasing the quantity and diversity of bacteria involved through altered inputs from roots and litter (Hawkes et al., 2005). However, most intercontinental plant invaders tend to have higher decomposition rates of dead leaves than natives that are replaced by the invaders (Ehrenfeld, 2003). This is probably due to higher litter quality, as invasive exotic plants have been observed with higher shoot nutrient concentrations (Dassonville et al., 2008). In some cases, litter quality of the exotic plants was not higher than of the natives, whereas litter from the exotics still decomposed faster than that of the natives (Allison and Vitousek, 2004).
Although it is expected that invasive plants increase nutrient cycling and availability through litter inputs, this mechanism has rarely been studied experimentally in comparison with related natives. On average, faster decomposition of invasive plant litter can result in increased nitrogen loss from this litter (Allison and Vitousek, 2004; Ashton et al., 2005). However, pair-wise comparisons between litter from related native plants and exotic invaders do not necessarily support this view (Ashton et al., 2005). Moreover, nitrogen concentrations in soils are not always increased when litter decomposition rates are increased (e.g. Ashton et al., 2005), suggesting that instead of making nitrogen directly available to plants, soil microbes first immobilize and store the nitrogen in their own biomass. This immobilized nitrogen can later be released. Because altered nutrient cycling can affect whole ecosystems, experimental studies are needed to answer the question of how intracontinental range-expanding plants may alter nutrient cycling via root and litter inputs and how this compares with intercontinental exotic invaders.
DISCUSSION
Thus far, consequences of warming have been studied experimentally by warming, drying, or exposure of field plots to increased concentrations of carbon dioxide (e.g. Körner, 2006). Modelling studies have assessed changing vegetation zones (Schröter et al., 2005), or responses of species to changing climate envelopes (Guisan and Thuiller, 2005). However, in spite of a number of studies pointing to climate-warming-induced intracontinental range-expansions (e.g. Walther et al., 2002), very few studies have actually assessed the impacts of such range-expansion on the biology and ecology of plant species, and on ecosystem properties and functions.
Here, we point out a number of characteristics that intracontinental range-expanding plants have in common with intercontinental invaders. Range-expansions within a continent may enable plants from warmer climate regions to become released from their above- and below-ground natural enemies. Possibly, above-ground enemy species more easily co-migrate to higher latitudes (Berg et al., 2010), although it is not necessarily so that when all species migrate to higher latitudes the original species interactions become re-established in the new range (Menendez et al., 2008). For example, the herbivores may not recognize their original hosts in the new environment, or they may prefer native plant species over their original hosts. Soil organisms have poorer active dispersal and host location capacity, which makes their re-interacting with original hosts a matter of chance. Therefore, intracontinental climate-warming-induced range-expansion may result in both above- and below-ground enemy release, but the chance of below-ground enemy release is probably higher owing to the limited dispersal and search capacities of most root herbivores and soil pathogens (Fig. 1).
Since most intracontinental range-expanding plant species originate from warmer climate regions with higher insect abundance, they will be better defended against high insect feeding pressure than the native species from the more temperate areas. Thus, intracontinental range-expansions may introduce novel chemicals into the former temperate regions, which can have strong consequences for ecological interactions. Specialist enemies may not be able to recognize the exotic plants, whereas the high concentrations of defensive compounds in plant tissues may prevent abundance control of the range-expanding plants by generalists. To what extent these effects of novel chemistry also apply to below-ground interactions is not known; above- and below-ground defensive chemistry is not necessarily coupled (Van Dam et al., 2003). It is difficult to predict the consequences of intracontinental plant range-expansions for nutrient cycling processes in the new range because of the paucity of studies on the decomposability of leaf litter and root exudates of lower- versus higher-latitude species.
Thus far, climate change research has resulted in predictions based on data from both latitudes and altitudes. Using altitudinal data in order to predict latitudinal responses may underestimate the differences in dispersal capacities of below-ground and above-ground biota (Berg et al., 2010). Therefore, in order to enhance the predictions for intracontinental latitudinal range-expansions, the consequences of above- and below-ground biotic interactions for plant abundance and invasiveness need to be considered more explicitly. There is some evidence now showing that intracontinental plant range-expansions along latitudinal gradients indeed involve enemy release (Van Grunsven et al., 2010) similar to that already found for intercontinental invasions. It will be important to study further above- and below-ground biotic interactions in relation to plant range expansion and to relate these findings to altitudinal range-expansions and to intercontinental plant invasions.
In conclusion, we think that the recent intracontinental climate-warming-induced range-expansions of many plant species from lower to higher latitudes may introduce novel plant traits that change ecological interactions with the potential of changing ecosystem processes. These range-expansions within continents provide many new questions for botanists, plant biologists and ecologists, such as how these exotic plants are performing in their new range, how they change ecological relationships in the new range, what their direct and indirect influences are on the native plant species, and how ecosystem processes will change. To some degree, the concepts developed in studies on classic intercontinental exotic invaders can be used for the studies on intracontinental range-expanding plants. However, as we have outlined, there are also substantial differences between intra- and intercontinental invasions. Within continents, there are more opportunities for ongoing gene flow between plants from the original and new ranges (Fig. 1), which will influence the rate of adaptation to novel conditions. Moreover, natural enemies, symbionts and carnivores from the native range may, in principle, migrate as well, although not all species will be able to migrate to higher latitudes. Sooner or later, however, some species assemblages may become re-established, but the question is whether they will interact similarly to how they did in the original range: are the species still the same and how does their interaction depend on other environmental conditions? As some of the conditions for intracontinental range-expanders are in strong contrast to those of intercontinental invasions, understanding and predicting the consequences of rapid range shifts requires new studies that will further develop current ecological hypotheses and concepts. Clearly, the recent experimental findings on ecological consequences of climate-warming-induced plant range-expansion result in a wealth of new research questions to ecology.
ACKNOWLEDGEMENTS
This study was funded by an ALW-VICI grant to W.H.v.d.P.
LITERATURE CITED
- Aerts R, Chapin FS. The mineral nutrition of wild plants revisited: a re-evaluation of processes and patterns. Advances in Ecological Research. 2000;30:1–67. [Google Scholar]
- Allison SD, Vitousek PM. Rapid nutrient cycling in leaf litter from invasive plants in Hawai'i. Oecologia. 2004;141:612–619. doi: 10.1007/s00442-004-1679-z. [DOI] [PubMed] [Google Scholar]
- Ashton IW, Hyatt LA, Howe KM, Gurevitch J, Lerdau MT. Invasive species accelerate decomposition and litter nitrogen loss in a mixed deciduous forest. Ecological Applications. 2005;15:1263–1272. [Google Scholar]
- Bale JS, Masters GJ, Hodkinson ID, et al. Herbivory in global climate change research: direct effects of rising temperature on insect herbivores. Global Change Biology. 2002;8:1–16. [Google Scholar]
- Berg MP, Kiers ET, Driessen G, et al. Adapt or disperse: understanding species persistence in a changing world. Global Change Biology. 2010;16:587–598. [Google Scholar]
- Bezemer TM, Jones TH. Plant-insect herbivore interactions in elevated atmospheric CO2: quantitative analyses and guild effects. Oikos. 1998;82:212–222. [Google Scholar]
- Blossey B, Nötzold R. Evolution of increased competitive ability in invasive nonindigenous plant – a hypothesis. Journal of Ecology. 1995;83:887–889. [Google Scholar]
- Callaway RM, Aschehoug ET. Invasive plants versus their new and old neighbors: a mechanism for exotic invasion. Science. 2000;290:521–523. doi: 10.1126/science.290.5491.521. [DOI] [PubMed] [Google Scholar]
- Cappuccino N, Arnason JT. Novel chemistry of invasive exotic plants. Biology Letters. 2006;2:189–193. doi: 10.1098/rsbl.2005.0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colautti RI, Ricciardi A, Grigorovich IA, MacIsaac HJ. Is invasion success explained by the enemy release hypothesis? Ecology Letters. 2004;7:721–733. [Google Scholar]
- Coley PD, Aide TM. Comparison of herbivory and plant defenses in temperate and tropical broad-leaved forests. In: Price PW, Lewinsohn TM, Fernandes GW, Benson WW, editors. Plant–animal interactions: evolutionary ecology in tropical and temperate regions. New York, NY: John Wiley and Sons; 1991. [Google Scholar]
- Cornwell WK, Cornelissen JHC, Amatangelo K, et al. Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecology Letters. 2008;11:1065–1071. doi: 10.1111/j.1461-0248.2008.01219.x. [DOI] [PubMed] [Google Scholar]
- Dassonville N, Vanderhoeven S, Vanparys V, Hayez M, Gruber W, Meerts P. Impacts of alien invasive plants on soil nutrients are correlated with initial site conditions in NW Europe. Oecologia. 2008;157:131–140. doi: 10.1007/s00442-008-1054-6. [DOI] [PubMed] [Google Scholar]
- Ehrenfeld JG. Effects of exotic plant invasions on soil nutrient cycling processes. Ecosystems. 2003;6:503–523. [Google Scholar]
- Engelkes T, Morriën E, Verhoeven KJF, et al. Successful range-expanding plants experience less above-ground and below-ground enemy impact. Nature. 2008;456:946–948. doi: 10.1038/nature07474. [DOI] [PubMed] [Google Scholar]
- Guisan A, Thuiller W. Predicting species distribution: offering more than simple habitat models. Ecology Letters. 2005;8:993–1009. doi: 10.1111/j.1461-0248.2005.00792.x. [DOI] [PubMed] [Google Scholar]
- Hawkes CV, Wren IF, Herman DJ, Firestone MK. Plant invasion alters nitrogen cycling by modifying the soil nitrifying community. Ecology Letters. 2005;8:976–985. doi: 10.1111/j.1461-0248.2005.00802.x. [DOI] [PubMed] [Google Scholar]
- Hegland SJ, Nielsen A, Lázaro A, Bjerknes AL, Totland O. How does climate warming affect plant–pollinator interactions? Ecology Letters. 2009;12:184–195. doi: 10.1111/j.1461-0248.2008.01269.x. [DOI] [PubMed] [Google Scholar]
- Joshi J, Vrieling K. The enemy release and EICA hypothesis revisited: incorporating the fundamental difference between specialist and generalist herbivores. Ecology Letters. 2005;8:704–714. [Google Scholar]
- Keane RM, Crawley MJ. Exotic plant invasions and the enemy release hypothesis. Trends in Ecology & Evolution. 2002;17:164–170. [Google Scholar]
- Kirkpatrick M, Barton NH. Evolution of a species' range. American Naturalist. 1997;150:1–23. doi: 10.1086/286054. [DOI] [PubMed] [Google Scholar]
- Klironomos JN. Feedback with soil biota contributes to plant rarity and invasiveness in communities. Nature. 2002;417:67–70. doi: 10.1038/417067a. [DOI] [PubMed] [Google Scholar]
- Körner C. Plant CO2 responses: an issue of definition, time and resource supply. New Phytologist. 2006;172:393–411. doi: 10.1111/j.1469-8137.2006.01886.x. [DOI] [PubMed] [Google Scholar]
- Kourtev PS, Ehrenfeld JG, Häggblom M. Experimental analysis of the effect of exotic and native plant species on the structure and function of soil microbial communities. Soil Biology & Biochemistry. 2003;35:895–905. [Google Scholar]
- Liao C, Peng R, Luo Y, et al. Altered ecosystem carbon and nitrogen cycles by plant invasion: a meta-analysis. New Phytologist. 2008;177:706–714. doi: 10.1111/j.1469-8137.2007.02290.x. [DOI] [PubMed] [Google Scholar]
- Malmstrom CM, McCullough AJ, Johnson HA, Newton LA, Borer ET. Invasive annual grasses indirectly increase virus incidence in California native perennial bunchgrasses. Oecologia. 2005;145:153–164. doi: 10.1007/s00442-005-0099-z. [DOI] [PubMed] [Google Scholar]
- Mangla S, Inderjit, Callaway RM. Exotic invasive plant accumulates native soil pathogens which inhibit native plants. Journal of Ecology. 2008;96:58–67. [Google Scholar]
- Menendez R, Gonzalez-Megias A, Lewis OT, Shaw MR, Thomas CD. Escape from natural enemies during climate-driven range expansion: a case study. Ecological Entomology. 2008;33:413–421. [Google Scholar]
- Müller-Schärer H, Schaffner U, Steinger T. Evolution in invasive plants: implications for biological control. Trends in Ecology & Evolution. 2004;19:417–422. doi: 10.1016/j.tree.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Parker JD, Hay ME. Biotic resistance to plant invasions? Native herbivores prefer non-native plants. Ecology Letters. 2005;8:959–967. doi: 10.1111/j.1461-0248.2005.00799.x. [DOI] [PubMed] [Google Scholar]
- Reinhart KO, Packer A, Van der Putten WH, Clay K. Plant–soil biota interactions and spatial distribution of black cherry in its native and invasive ranges. Ecology Letters. 2003;6:1046–1050. [Google Scholar]
- Richardson DM, Allsopp N, D'Antonio CM, Milton SJ, Rejmanek M. Plant invasions – the role of mutualisms. Biological Reviews. 2000;75:65–93. doi: 10.1017/s0006323199005435. [DOI] [PubMed] [Google Scholar]
- Rohde K. Latitudinal gradients in species-diversity: the search for the primary cause. Oikos. 1992;65:514–527. [Google Scholar]
- Sakai AK, Allendorf FW, Holt JS, et al. The population biology of invasive species. Annual Review of Ecology and Systematics. 2001;32:305–332. [Google Scholar]
- Salgado CS, Pennings SC. Latitudinal variation in palatability of salt-marsh plants: are differences constitutive? Ecology. 2005;86:1571–1579. [Google Scholar]
- Schröter D, Cramer W, Leemans R, et al. Ecosystem service supply and vulnerability to global change in Europe. Science. 2005;310:1333–1337. doi: 10.1126/science.1115233. [DOI] [PubMed] [Google Scholar]
- Stinson KA, Campbell SA, Powell JR, et al. Invasive plant suppresses the growth of native tree seedlings by disrupting belowground mutualisms. PLoS Biology. 2006;4:727–731. doi: 10.1371/journal.pbio.0040140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traveset A, Richardson DM. Biological invasions as disruptors of plant reproductive mutualisms. Trends in Ecology & Evolution. 2006;21:208–216. doi: 10.1016/j.tree.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Van Dam NM, Harvey JA, Wäckers FL, Bezemer TM, Van der Putten WH, Vet LEM. Interactions between aboveground and belowground induced responses against phytophages. Basic and Applied Ecology. 2003;4:63–77. [Google Scholar]
- Van Grunsven RHA, Van Der Putten WH, Bezemer TM, Tamis WLM, Berendse F, Veenendaal EM. Reduced plant–soil feedback of plant species expanding their range as compared to natives. Journal of Ecology. 2007;95:1050–1057. [Google Scholar]
- Van Grunsven RHA, Van der Putten WH, Bezemer TM, Berendse F, Veenendaal EM. Plant–soil interactions in the expansion and native range of a poleward shifting plant species. Global Change Biology. 2010;16:380–385. [Google Scholar]
- Verhoeven KJF, Biere A, Harvey JA, van der Putten WH. Plant invaders and their novel natural enemies: who is naive? Ecology Letters. 2009;12:107–117. doi: 10.1111/j.1461-0248.2008.01248.x. [DOI] [PubMed] [Google Scholar]
- Vogelsang KM, Bever JD. Mycorrhizal densities decline in association with nonnative plants and contribute to plant invasion. Ecology. 2009;90:399–407. doi: 10.1890/07-2144.1. [DOI] [PubMed] [Google Scholar]
- Walther GR, Post E, Convey P, et al. Ecological responses to recent climate change. Nature. 2002;416:389–395. doi: 10.1038/416389a. [DOI] [PubMed] [Google Scholar]
- Wolfe LM. Why alien invaders succeed: support for the escape-from-enemy hypothesis. American Naturalist. 2002;160:705–711. doi: 10.1086/343872. [DOI] [PubMed] [Google Scholar]