Abstract
Background and Aims
Although plant functional traits (PFTs) appear to be important indicators of species' responses to land use changes, there is no clear understanding of how the variations in traits and their plasticity determine variations in species performance. This study investigated the role of functional shoot traits and their plasticity for variation in above-ground net primary productivity (ANPP) due to changes in N supply and in cutting frequency for 13 native perennial C3 grass species.
Methods
Monocultures of the grass species were grown in a fully factorial block design combining plant species, cutting frequency and N supply as factors.
Key Results
Four major trait associations were obtained by reducing the dimensions of 14 PFTs with a principal component analysis (PCA).Variations in species' productivity in response to an increase in cutting frequency was mainly explained by traits linked to the first PCA axis, opposing high plant stature from lower shoot cellulose and lignin contents and high leaf N content. Variation in species productivity in response to change in N supply was mainly explained by a set of predictor variables combining traits (average flowering date) and a trait's plasticity (tiller density per unit land area and leaf dry matter content, i.e. mg dry matter g fresh mass−1). These traits involved are linked to the second PCA axis (‘nutrient acquisition–conservation’), which opposes distinct strategies based on response to nutrient supply.
Conclusions
Variations in ANPP of species in response to an increase in cutting frequency and a decrease in N supply are controlled by a group of traits, rather than by one individual trait. Incorporating plasticity of the individual traits into these trait combinations was the key to explaining species' productivity responses, accounting for up to 89 % of the total variability in response to the changes in N supply.
Keywords: C3 grasses, cutting, grassland, leaf traits, nitrogen, primary productivity, species strategy, trait plasticity
INTRODUCTION
Human activities influence land use and the more widespread management practices such as biomass exploitation by grazing or soil fertility management, and have a large impact on an ecosystem's primary productivity. In grasslands, plant species respond differently to these management practices, which may depend on their specific growth strategies, as Grime (1977) and Tilman (1991) have shown for grasses. However, the different specific processes by which species adapt to new environmental conditions (i.e. climate or management changes) are not well understood and need to be investigated, especially within productive grasslands, in order to predict the variations in productivity of dominant species.
Plant functional traits are useful tools to achieve this goal, because their values reflect the strategy whereby adaptation to variations in land use are achieved (Craine et al., 2001; Al Haj Khaled et al., 2005), resulting in changes in species performance, such as productivity (Lavorel et al., 1997; Reich et al., 2003; Pontes et al., 2007a). For example, several studies have shown that functional traits of dominant species vary along gradients of disturbance by grazing and cutting (Díaz et al., 2001; Cingolani et al., 2005; Louault et al., 2005; Díaz et al., 2006). Grazing tolerance would be favoured by a high specific leaf area which increases the ability of shoots to regrow (Westoby, 1999). At population level, traits which help avoid the effects of cutting, such as high leaf dry matter content, were associated with lower species productivity (Pontes et al., 2007a).
Species strategies towards nutrient availability have also been based on traits linked to leaf morphology and physiology (Wright et al., 2005). Species with quick returns on investments of nutrients, i.e. exploitative species, exhibit high leaf nutrient concentrations, high rates of photosynthesis and respiration, short leaf lifespan and low dry mass investment per leaf area, whereas conservative species, i.e. with a slower potential rate of return, displayed the opposite trait syndrome (i.e. associations between traits, sensu Lavorel et al., 1997). Exploitative species have been characterized by greater responses, in terms of productivity, to an increase in nutrient availability (Poorter and De Jong, 1999; Wright et al., 2005). On the other hand, conservative species may maintain their productivity when nutrients are limiting (Craine et al., 2002; Liancourt et al., 2005). However, at community and population scales, other trait syndromes interact with nutrient acquisition or conservation syndrome (e.g. size plants traits; Gross et al., 2007) and must be taken into account to predict variations in productivity within grassland communities (Westoby, 1998; Ackerly et al., 2000; Maire et al., 2009). Also, little is known about the minimal trait combinations and trait syndromes involved to enable the changes in plant performance under different management conditions to be successfully predicted.
Plasticity (i.e. variability of a particular trait linked both to phenotypic plasticity and to genotype selection; see Sultan, 2000) in functional traits can provide plants with a greater access to limiting resources (Funk, 2008). Thus, plasticity of plant traits may influence the success and fitness of species, and increase ecosystem functioning by reducing niche overlap along resource axes (Tilman et al., 1997; Sultan, 2000). While recent work supports this idea (Weijschedé et al., 2008; Schumacher and Roscher, 2009) and despite an increasing consensus that plant functional traits successfully explain the properties of populations and communities subjected to land-use changes, experiments evaluating plant trait plasticity and consequences for productivity under contrasting regimes are still very scarce. Moreover, previous studies that have identified simultaneous changes in functional traits and ecosystem properties with changes in land use (Garnier et al., 2004, Louault et al., 2005; Quétier et al., 2007) did not separate the effect of species replacement from that of trait plasticity within species. Therefore, it is not clear how the co-variations between traits and their plasticity determine variations in species productivity in the absence of interference between species.
Using perennial grass species which display different growth strategies (Maire et al. 2009), it was investigated whether individual plant traits or combinations of them (morphological, chemical composition or phenological), when averaged for a given species, and trait plasticity can predict variations in species' productivity due to different N supplies and cutting frequencies. Here, the term ‘trait plasticity’ describes the variations in the magnitude of the plant trait with changes in these management factors. It was hypothesized that: (a) plant traits and their plasticity will play a significant role in predicting species responses to management factors; (b) a set of many plant traits is needed to explain these species responses, because individual traits should not be considered in isolation, since different traits are related to different physiological processes involved in the plant response; and (c) different trait syndromes are involved in the response of species to nutrient availability and cutting regime. In this work, we adapted a methodological framework proposed by Díaz et al. (2007) that can test for the minimum combination of variables (traits averaged over species and traits' plasticity) needed to predict productivity differences.
MATERIALS AND METHODS
Plant material
The study was based on temperate C3 grasses that co-occur in upland semi-natural mesic grasslands, representative of a wide diversity of practices (cutting or grazing, with or without fertilizer, early or late use of the biomass). Thirteen species (Pontes et al., 2007b) were selected: Alopecurus pratensis (Ap), Anthoxanthum odoratum (Ao), Arrhenatherum elatius (Ae), Dactylis glomerata (Dg), Elytrigia repens (Er), Festuca arundinacea (Fa), Festuca rubra (Fr), Holcus lanatus (Hl), Lolium perenne (Lp), Phleum pratense (Php), Poa pratensis (Pp), Poa trivialis (Pt) and Trisetum flavescens (Tf). Henceforth, in the text, species are abbreviated (e.g. as F. rubra or Fr).
Experimental design
A factorial complete block design was used, with three factors (species, cutting frequency and N supply) and three replicates. Each block consisted of 56 individual plots of 4·2 m2 (2·8 × 1·5 m). The species were sown in pure stands in May 2001.
Two cutting frequencies (every 2 months and monthly, denoted C− and C+, respectively) and two rates of mineral N fertilizer (120 and 360 kg N ha−1 year−1, denoted N− and N+, respectively) were compared. The annual fertilizer N supply was chosen to provide limiting and non-limiting N nutrition (Pontes, 2006). The plots were cut at 6 cm height with a mower (Haldrup, Logstor, Denmark). The C+ plots were cut on 28 April, 26 May, 30 June, 11 August, 15 September and 4 November in 2003 and on 3 May, 3 June, 12 July, 12 August, 23 September and 21 October in 2004. The C− plots were cut only on the 2nd, 4th and 6th cuttings dates in each year. Cutting frequencies were selected to simulate defoliation frequencies found in hay meadows (C−) and in grazed pastures (C+). N fertilizer (ammonium nitrate) was applied in split applications after each cut. Phosphorus and potassium were applied in spring at non-limiting rates for growth. When soil water content was below 10 %, all plots were irrigated (for full details, see Pontes et al., 2007a).
Plant measurements
At each cutting date, the fresh harvested biomass of each individual plot was automatically collected by the mower and weighed. A subsample was immediately taken, weighed and dried at 60 °C for 48 h to determine the dry matter (DM) content of the harvested biomass and calculate the ANPP of each plot (g DM m−2). The annual ANPP (g DM m−2 year−1) was calculated as the sum of the six (C+) and three (C−) cuts taken each year.
The 14 traits measured and their codes are presented in Table 1. Seven of these traits (VE, SL, NM, LL, LDMC, SLA and leaf N content, i.e. LNC) were measured in June and in September 2003 and 2004, 3 weeks after a cut made on both cutting treatments, and from ten tillers collected at random in each plot, using standardized protocols (Garnier et al., 2001; Cornelissen et al., 2003). The leaf N content per unit fresh weight (LNCF, g N g−1 FM) was used rather than per unit dry weight (LNC), due to the better relationship of this trait with productivity (Pontes et al., 2007a). LNCF was calculated as the product of the LNC and the leaf dry matter content (LDMC).
Table 1.
Plant traits measured and their abbreviations
| Trait | Abbreviation | Units |
|---|---|---|
| Leaf traits | ||
| Leaf dry matter content | LDMC | mg d.wt g−1 f. wt |
| Specific leaf area | SLA | m2 kg−1 |
| Leaf length | LL | mm |
| Leaf lifespan | LLS | degree day, °Cd |
| Leaf N content per unit fresh matter | LNCF | mg g−1 |
| Plant traits | ||
| Number of mature leaves | NM | tiller−1 |
| Sheath length | SL | mm |
| Vegetative plant height elongated | VE | mm |
| Mature plant height elongated | ME | cm |
| Plant cellular content | CC | g kg−1 |
| Plant cellulose and lignin content | ADF | g kg−1 |
| Morphological traits | ||
| Earliness of growth | EG | – |
| Beginning of flowering period | BF | degree day, °Cd |
| Tiller density | TD | m−2 |
The phyllochron was determined in 2003 over two periods (3–13 June and 17 July to 8 August), on eight labelled tillers in each plot of the C− treatment. The phyllochron was calculated between two successive observations as the thermal time in degree-days (calculated as the daily temperature sum above 0 °C) between the appearance of two newly emerged leaves. The leaf lifespan (LLS) was then calculated according to Lemaire and Agnusdei (1999) as the product of the phyllochron and the average number of mature leaves per tiller.
The earliness of growth (EG) was determined each week during spring by visual evaluations of the fraction of green shoots in the standing herbage mass on a scale from 0 (<5 % green) to 6 (>95 % green).
The tiller density (TD) per unit ground area was determined every 2 months, at each C− cutting date, from the mean tiller mass and the harvested DM. In each plot, 16 tillers were sampled at random, cut at a height of 6 cm, dried at 60 °C for 48 h and weighed. The total tiller density (TD) was calculated as the ratio of the harvested DM to the mean individual tiller mass.
Chemical composition traits
Two quadrats (0·40 m2 each) were sampled at the same date as trait measurements in September 2004, for neutral detergent fibre (NDF; i.e. hemicellulose and cellulose plus lignin content) and acid detergent fibre (ADF; i.e. cellulose plus lignin content) determination. These sub-samples were ground using a sample mill (Cyclotec, Model 1093; FOSS TECATOR Inc., Höganäs, Sweden). Each forage subsample was analysed by near-infrared reflectance spectroscopy to determine the fibre fractions (NDF and ADF). Spectra were collected with a monochromator (FOSS-NIRSystems 6500, Silver Spring, MD, USA) which scans the spectral range of 400–2500 nm. Near-infrared reflectance-modified partial least square calibration equations were developed using 137 samples which were selected from the total spectra population collected, according to the procedure developed by Pontes et al. (2007b). Cell soluble content (CC; i.e. soluble carbohydrates plus proteins and organic acids content) was calculated as 1000 – NDF content. The statistical parameters of the calibration models obtained for NDF and ADF were, respectively: range (405–655 and 165–328 g kg−1), standard error of cross-validation (10·9 and 15·6 g kg−1) and r2 of cross-validation (0·92 and 0·98).
Phenology trait
The developmental stage of each species in each plot was assessed visually in 2005, once a week and only in the C− plots, from April to June, to determine the beginning of flowering (BF) stage (visible anthers) for all species. To allow for a full reproductive development of the grasses, the spring cuts were omitted. The mature plant height elongated (ME) was measured on ten mature individuals for each grass population in both C−N− and C−N+ treatments. Because of its low perennity, P. trivialis could not be evaluated. For this species the values from Grime et al. (1988) for ME were used.
Data analysis
Two-year means were calculated from annual means. Annual means of traits are means for two measurement dates (June and September). An analysis of variance (ANOVA) was performed for traits and ANPP (means of two growing periods and two years, n = 156), with the species (12 d.f.), blocks (2 d.f.), cutting regime (1 d.f.) and nitrogen supply (1 d.f.) factors. All interactions were initially included in the statistical model, except interactions with the ‘block’ factor since these cannot be studied in a block design. All non-significant interactions were removed from the models (Dagnélie, 1986). Prior to ANOVA, CC and ADF data were normalized using the arcsin transformation.
A principal component analysis (PCA, Statistica 6 package, StatSoft Inc., Tulsa, OK, USA) was conducted on the traits of species in C−N+ treatment (n = 39, i.e. 13 species × 3 blocks) to evaluate trait syndromes characterizing species strategies (Suding et al., 2003).
Simple regression analyses, with Statgraphics Plus (Manugistics, Rockville, MD, USA), were carried out to assess the predictive ability of traits, with their average values in C−N+ treatment (i.e. lower cutting frequency and higher nutrient application) or with their plasticity (Δtraits) to the changes in N supply and cutting frequency, for ANPP response to these same two factors (ΔANPP). Four multiple regressions with stepwise procedure were done using JMP 8·0 (SAS Institute Inc., Cary, NC, USA). First, a model with 14 traits (average values in C−N+ treatments, see Table 1 for traits list) and ΔANPP were tested. Secondly, the model configured the relationship between Δtraits and ΔANPP. Then, a model where both average trait values and Δtraits were incorporated was tested. Finally, a multiple regression model was compiled with only the significant variables from the previous simple regression analyses. In a step-wise procedure, the most parsimonious model was selected by using Akaike information criterion (AIC; Akaike, 1973), according to Díaz et al. (2007). The aim was to find a model that best explains ANPP response with a minimum number of predictor variables.
The effect of N supply was studied by comparing C−N+ and C−N− treatments, i.e. within a low-cutting frequency treatment. Similarly, for cutting effect, C−N+ and C+N+ treatments were compared, because of their higher N availability. By comparing C−N+ and C+N− treatments, it was possible to study both cutting and N effects. Therefore, variations in ANPP and in traits (i.e. traits plasticity) in response to the cutting frequency (C) and N supply (N) factors were calculated as follows:
RESULTS
N supply and cutting frequency effects
The cutting frequency was significant for all variables (Table 2) but plant size traits (SL and VE) and tiller density (TD). NM, ME, BF and TD were not affected by N supply. The species × N supply interaction was significant for ten traits and ANPP, species × cut interaction was significant for four traits and ANPP, and the cut × N supply interaction was significant for LL and LNCF (Table 2). However, a maximum of 7·7 % (for NM) of the total variance was explained by these interactions.
Table 2.
F-ratios and statistical significance of ANOVAs for traits and above-ground dry matter productivity (ANPP)
| Trait | Species (d.f. 12) | C (d.f. 1) | N (d.f. 1) | C × species (d.f. 12) | N × species (d.f. 12) | C × N (d.f. 1) |
|---|---|---|---|---|---|---|
| LDMC | 54*** | 20*** | 47*** | n.s. | n.s. | n.s. |
| SLA | 167*** | n.s. | 34*** | n.s. | n.s. | n.s. |
| LL | 246*** | 4·4* | 111*** | 4·7*** | 3·2*** | 5·3* |
| LLS | 19*** | – | 20*** | – | 2·4** | n.s. |
| LNCF | 74*** | 42*** | 392*** | n.s. | 2·4** | 10** |
| NM | 26*** | 7·3** | n.s. | 3·3*** | 2·8** | n.s. |
| SL | 56*** | n.s. | 163*** | 2·9** | 2·8** | n.s. |
| VE | 178*** | n.s. | 211*** | 5·9*** | 3·6*** | n.s. |
| ME | 153*** | – | n.s. | – | n.s. | – |
| CC | 63*** | 24*** | 7·0** | n.s. | 3·4*** | n.s. |
| ADF | 51*** | 30*** | 11** | n.s. | 3·2*** | n.s. |
| EG | 18*** | 54*** | 29*** | n.s. | 2·2* | n.s. |
| BF | 143*** | – | n.s. | – | n.s. | – |
| TD | 49*** | n.s. | n.s. | n.s. | 2·7** | n.s. |
| ANPP | 84*** | 64*** | 112*** | 4·4*** | 2·7** | 6·8* |
*, P < 0·05; **, P < 0·01; ***, P < 0·001; n.s., not significant; –, not appropriate. The second-order interaction between species, cutting frequency and N supply was never significant.
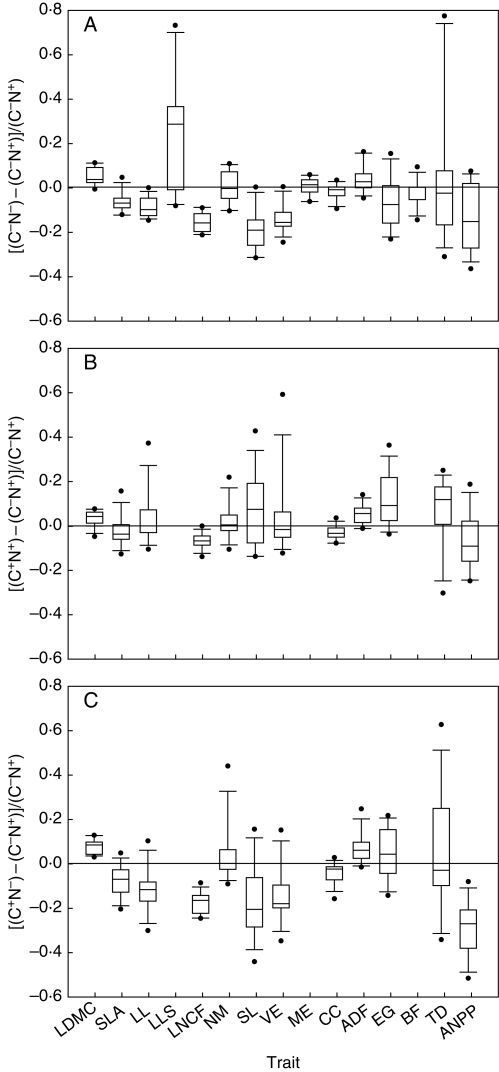
Trait plasticity to N and cutting frequency were plotted as box plots indicating the variability among species (Fig. 1). On average, trait plasticity ranged from –0·19 (ΔSL, see centre lines within each box in Fig. 1) to 0·25 (ΔLLS) in response to a decrease in N supply, and from –0·07 (ΔLNCF) to 0·12 (ΔEG) in response to an increase in cutting frequency. Positive values indicated increases in trait values with a decrease in N availability (Fig. 1A). Hence, a decrease in N supply resulted in: (a) a decrease in individual leaf length (LL), LNCF and plant size (VE) for all species; (b) a decrease in SL (except P. trivialis), SLA (except D. glomerata), CC (except A. pratensis, Ph. pratense and P. trivialis), EG (except F. rubra, H. lanatus and T. flavescens), TD (except D. glomerata, F. arundinacea, F. rubra and H. lanatus) and ANPP (except A. odoratum, F. rubra, H. lanatus, L. perenne) for most, but not all species; and (c) an increase in shoot cellulose and lignin content (ADF), LDMC, NM, ME and leaf lifespan (LLS), on average.
Fig. 1.
Box-and-whisker plots of the responses to (A) nitrogen supply (N), (B) cutting frequency (C−, C+), and (C) N × C factors of traits and above-ground dry matter productivity (ANPP). Values are means of 2 years per species (n = 13). The centre lines within each box show the location of the sample medians. The lower whisker is drawn from the lower quartile to the smallest point within 1·5 interquartile ranges from the lower quartile. The other whisker is drawn from the upper quartile. See Table 1 for trait code.
In response to an increase in cutting frequency (Fig. 1B), positive values show an increase in trait values. Therefore, on average, an increase in cutting frequency increased the LDMC, NM, SL, ADF, EG and TD. In contrast, the relative responses to cutting frequency of LL, SLA, VE, CC and ANPP were, on average, negative. Only three species (A. odoratum, L. perenne and P. trivialis) gave higher production at the higher cutting frequency (in N+ treatments).
Only one trait (LDMC) apart from ANPP had its values modified in the same way for all species with the changes in both factors, i.e. increase in cutting frequency and decrease in N supply (Fig. 1C).
Trait syndromes
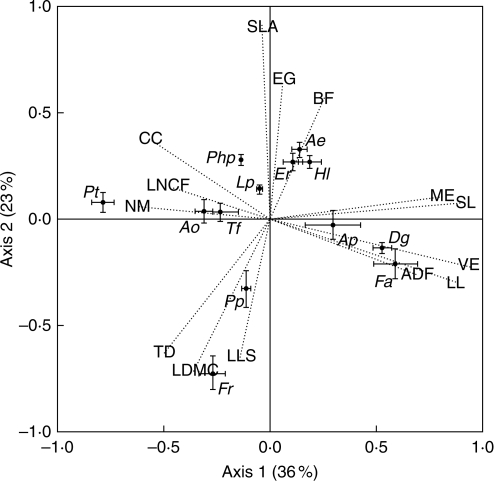
The first axis of the PCA (Fig. 2) explained 36 % of the variance and was positively correlated with all plant size traits (LL, SL, VE and ME) and cell wall content (ADF), as underlined by the position of D. glomerata and F. arundinacea, and negatively correlated with NM, CC and LNCF, close to the position of P. trivialis. The second axis explained an additional 23 % of the variance. It was positively correlated with SLA, EG and BF, negatively correlated with LLS, TD and LDMC and associated with species such as F. rubra and E. repens. Data used in PCA analysis are provided in the Supplementary Data (available online).
Fig. 2.
Principal components analysis between traits measured under high N supply and low cutting frequency. The two orthogonal axes explain 36 % and 23 % of the variance, respectively. See details of abbreviations in Table 1. Error bars in species indicate the s.e. for each species along axes 1 and 2 (n = 39). Ap, Alopecurus pratensis; Ao, Anthoxanthum odoratum; Ae, Arrhenatherum elatius; Dg, Dactylis glomerata; Er, Elytrigia repens; Fa, Festuca arundinacea; Fr, Festuca rubra; Hl, Holcus lanatus; Lp, Lolium perenne; Php, Phleum pratense; Pp, Poa pratensis; Pt, Poa trivialis; Tf, Trisetum flavescens.
Relationship between trait values and the variations in ANPP
A simple regression analyses was done between trait values in C−N+ and the variations in ANPP in response to the changes in cutting frequency and N supply (Table 3). In response to a decrease in N supply, variations in ANPP were negatively correlated only with flowering date (BF; r = –0·41, P < 0·05). The ANPP response to an increase in cutting frequency was negatively correlated to plant size traits (LL, SL, VE and ME; Table 3) and ADF (r = –0·51, P < 0·001), and was positively correlated to NM (r = 0·31, P < 0·10) and CC (r = 0·54, P < 0·001). Variations in ANPP in response to both N supply decrease and cutting frequency increase were positively correlated with NM (r = 0·32, P < 0·05) and negatively correlated with plant size traits (LL, VE and ME; Table 3).
Table 3.
Correlation statistics (r values) for the relationships (n = 39): (A) between the traits' average values in the C−N+ treatment and the variations in above-ground dry matter productivity (ANPP) in response to changes in N supply (N) and cutting frequency (C), and (B) between trait plasticity (ΔTraits) and the variations in ANPP in response to the changes in N and C factors
| (A) Traits |
(B) ΔTraits |
|||||
|---|---|---|---|---|---|---|
| Trait | N | C | N × C | N | C | N × C |
| LDMC | n.s. | n.s. | n.s. | −0·37* | −0·35* | n.s. |
| SLA | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| LL | n.s. | −0·41** | −0·33* | n.s. | 0·40* | 0·43** |
| LLS | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| LNCF | n.s. | n.s. | n.s. | n.s. | −0·36* | n.s. |
| NM | n.s. | 0·31# | 0·32* | n.s. | −0·38* | −0·44** |
| SL | n.s. | −0·34* | n.s. | n.s. | n.s. | 0·51*** |
| VE | n.s. | −0·40* | −0·29# | n.s. | 0·32* | 0·43** |
| ME | n.s. | −0·54*** | −0·34* | n.s. | – | – |
| CC | n.s. | 0·54*** | n.s. | n.s. | −0·31# | n.s. |
| ADF | n.s. | −0·51*** | n.s. | n.s. | 0·33# | n.s. |
| EG | n.s. | n.s. | n.s. | 0·39* | n.s. | n.s. |
| BF | −0·41* | n.s. | n.s. | n.s. | – | – |
| TD | n.s. | n.s. | n.s. | 0·64*** | n.s. | 0·45** |
See Materials and methods for calculation of variation in ANPP.
For abbreviations, see Table 1.
#, P < 0·10; *, P < 0·05; **, P < 0·01; ***, P < 0·001; n.s., not significant; –, not suitable.
Relationship between trait plasticity (Δtraits) and the variations in ANPP
In response to a decrease in N supply, variations in ANPP were positively correlated with EG and TD plasticity and negatively correlated with LDMC plasticity (Table 3). The variation in ANPP in response to an increase in cutting frequency was also negatively correlated with trait plasticity for LDMC, LNCF, NM and CC (P < 0·10) and was positively correlated with the plasticity of plant size traits (LL and VE) and ADF (P < 0·10). There was a significant negative relationship between NM plasticity and variations in ANPP in response to both N supply decrease and cutting frequency increase. Similarly, a significant positive relationship was found between plant size trait (LL, SL and VE) plasticity and tiller density plasticity and variations in ANPP associated with a decrease in N supply and an increase in cutting frequency.
Relationship between combinations of traits and variations in ANPP
Combinations of traits were found to predict the variations in ANPP better than a single plant trait (Table 4). Six predictor variables (averaging traits in C−N+ and their plasticity to the changes in N supply) were required to explain 89 % of total variance in ANPP (AIC = –64·9, Table 4C). However, with only three of them (BF, ΔLDMC and ΔTD) 58 % of the total variance in ANPP in response to the changes in N supply was accounted for (AIC = –43·5). When all variable predictors (averaging traits in C−N+ treatment and trait plasticity; Table 4C) or only significant variable predictors from the two previous simple regression analyses (Table 4D) were introduced into the multiple regression, the combination of NM, CC, ADF, ME and ΔADF (i.e. plasticity of ADF trait to cutting frequency) accounted for 66 % of the total variance of ANPP response to an increase in cutting frequency (AIC = –53·6). Finally, the combination of VE, ME, ΔNM, ΔSL, ΔTD accounted for 60 % of the total variance of ANPP response to the changes in both management factors, i.e. cutting frequency and N supply (AIC = –46·3; Table 4D).
Table 4.
Coefficient of determination and Akaike (AIC) value between the variations in above-ground dry matter productivity in response to changes in N supply and cutting frequency and trait values or trait plasticity (Δtraits)
| Trait combination | r2 | AIC | |
|---|---|---|---|
| (A) With trait values on average of C−N+ treatment | |||
| N supply | SL, LL, LDMC, SLA, TD, BF | 0·43 | −20·52 |
| Cutting frequency | VE, LL, SLA, CC, ME | 0·64 | −51·43 |
| N supply and cutting frequency | NM, TD, ME | 0·27 | −27·87 |
| (B) With traits plasticity (Δtraits) | |||
| N supply | ΔSL, ΔLDMC, ΔTD | 0·60 | −37·51 |
| Cutting frequency | ΔNM, ΔLL, ΔLDMC, ΔEG | 0·44 | −37·72 |
| N supply and cutting frequency | ΔNM, ΔSL, ΔTD, ΔLNCF | 0·55 | −43·17 |
| (C) With all variables (traits values on average of C−N+ treatment and traits plasticity) | |||
| N supply | SL, LL, SLA, CC, ΔNM, ΔSL, ΔCC, ΔTD | 0·89 | −64·85 |
| Cutting frequency | NM, CC, ADF, ME, ΔADF | 0·66 | −53·63 |
| N supply and cutting frequency | SL, SLA, CC, ME, ΔNM, ΔSL, ΔTD | 0·68 | −46·17 |
| (D) With only the significant variables in the two previous simple regression analyses (see Table 3) | |||
| N supply | ΔLDMC, ΔTD, BF | 0·58 | − 43·53 |
| Cutting frequency | NM, CC, ADF, ME, ΔADF | 0·66 | − 53·63 |
| N supply and cutting frequency | VE, ME, ΔNM, ΔSL, ΔTD | 0·60 | − 46·33 |
The models selected are shown in bold (with the lowest AIC value and with the lowest number of traits). See Materials and methods for calculation of variation in ANPP and trait plasticity.
For trait abbreviations, see Table 1.
DISCUSSION
Trait plasticity in response to N supply and cutting frequency
Taller plants with less dense leaves and with a short lifespan (e.g. A. elatius, H. lanatus) were observed in more heavily fertilized treatments (Fig. 1A). Further, an earlier start of growth in spring was also observed in these N+ treatments. These responses suggest species strategies which could favour resource capture (Eckstein et al., 1999; Wilson et al., 1999; Kazakou et al., 2007). They are consistent with other studies in monocultures (e.g. Al Haj Khaled et al., 2005) and in plant communities (e.g. Quétier et al., 2007).
Some avoidance mechanisms in response to an increase in cutting frequency were noted. They consisted of changes in architectural attributes, such as a reduction in plant size (Fig. 1B) which limits tissue accessibility to herbivores, and changes in tissue composition, such as reductions in leaf lamina N content, increases in leaf dry matter content and cellulose and lignin content, which reduce tissue palatability (Westoby, 1999; Díaz et al., 2001; Cingolani et al., 2005).
Trait syndromes
The first PCA axis separates tall species (e.g. F. arundinacea; Fig. 2) with high cell-wall content from short species, richer in N and in cell soluble content (e.g. P. trivialis). This ‘size traits axis’, because it was correlated with all plant size traits (LL, SL, VE and ME), can be related to species strategies in response to disturbance, since small species are associated with avoidance mechanisms to cutting or grazing (Díaz et al., 2006), and tall species can be associated with resistance mechanisms to grazing (Briske, 1996) attributable to lower palatability due to high ADF and low LNCF.
The second PCA axis (Fig. 2) separates species in relation to nutrient acquisition or conservation strategies, since it opposes plants with long leaf lifespan and high vegetative tillering (e.g. F. rubra) and those with high SLA (e.g. A. elatius) and a long vegetative stage, i.e. early growth and late flowering, such as E. repens (Reich et al., 1992; Ryser, 1996; Wilson et al., 1999; Wright et al., 2004). The combination of these last sets of traits is likely to increase both the duration and the intensity of photosynthetic activity (Lavorel and Garnier, 2002).
Combinations of trait values and trait plasticity to predict variations in ANPP in response to N supply and cutting frequency
The hypothesis that different trait and trait plasticity combinations are involved in the response of species to nutrient availability and cutting regime, and hence can be used to predict variations in species' productivity, was confirmed by the present experiment. Variations in ANPP due to the changes in cutting frequency were predicted from a combination of traits correlated with axis 1 of the PCA (Fig. 2), i.e. NM, ME, CC and ADF, on average, and ΔADF (trait plasticity). As cited above, chemical composition traits (cell soluble content, CC and shoot cellulose and lignin, ADF) are important indicators of avoidance mechanisms because they affect the species' palatability. Height at maturity (ME) is also an important trait used as a predictor of the response to cutting [LHS, leaf height–seed; Westoby's scheme (Westoby 1998)], because it expresses the amount of growth made between disturbances. A larger number of leaves (NM) is linked to an increased opportunity for photosynthesis (Gutschick, 1999; Franklin and Ågren, 2002). Therefore, the ANPP response to cutting frequency can be better explained by traits linked to both tolerance (by the changes in NM) and avoidance mechanisms (by the changes in plant stature and palatability). Del-Val and Crawley (2005) has argued that these two mechanisms are not mutually exclusive.
To explain variations in ANPP in response to the changes in N supply, three variables were highlighted: BF (trait values averaged for each species), ΔLDMC and ΔTD (trait plasticity). All these traits were characterized by the ‘acquisition–conservation’ PCA axis (axis 2, Fig. 2), which opposes these two distinct strategies based on response to nutrient supply (Díaz et al., 2004; Wright et al., 2004). Later species (in terms of BF) showed a higher response to nutrient addition (r = –0·41; Table 3). Decrease in LDMC and increase in TD, ssociated with higher N availability, contributed to increased ANPP (Table 3). These relationships seem to reflect the stimulation of total shoot meristem activity by an increase in N availability, mainly for species with a longer vegetative stage. Shoot meristem activity is directly reflected in the dynamics of size (by an increase in leaf meristem length, which is negatively correlated with LDMC; Arredondo and Schnyder, 2003) and number of tillers (by an increase in meristem density; Sugiyama, 2005).
The greatest trait plasticity observed was in response to N supply rather than to cutting frequency (Fig. 1). This could be one possible explanation why more of the total variability was explained by including trait plasticity (e.g. ΔLDMC and ΔTD) in the predictor variable group for species productivity variations to changes in N supply (Table 4). Thus, thanks to trait plasticity, native grass species benefited from N enrichment, increasing their above-ground productivity. Furthermore, small differences in trait values affected productivity. For example, despite small differences in LDMC (<5 %, on average for all species) due to an increase in N supply, the plasticity of this trait was correlated to variations in ANPP. This highlights the importance of exploring the functional significance of traits (rather than simply quantifying the amount of plasticity) in species-level studies (Funk, 2008). On the other hand, species seem to make more use of their tolerance or avoidance trait values than their trait plasticity to adapt to an increase in cutting frequency. For example, in the present trial, smaller variations in ANPP as a result of an increase in cutting frequency (see Pontes et al., 2007b) were found for species (e.g. A. odoratum, L. perenne, P. trivialis and T. flavescens; Fig. 1) which display characteristics of both tolerance (high NM) and avoidance mechanisms (smaller stature) to cutting. However, since only leaf-level or plant-level traits were examined, this could not represent all the differences in plasticity among contrasting regimes.
Finally, it was found that the combination of VE and ME (vegetative and reproductive plant height, respectively), on average, and ΔNM, ΔSL andΔTD, i.e. trait plasticity, which represent both ‘size traits axis’ and ‘acquisition–conservation axis’, appear to be the best predictors of the variations in ANPP in response to both N supply decrease and cutting frequency increase. Hence, both PCA trait axes, i.e. different trait syndromes, are required to capture variations in species productivity, such as with plant strategy schemes (Grime, 1977; Westoby, 1998).
In conclusion, while ANPP can be well predicted by a single trait (LNCF; see Pontes et al., 2007a), a group of traits may best predict variations in ANPP in response to management factors. Here, trait values averaged for a species and trait plasticity were combined to predict variations in ANPP (Table 4) to the changes in cutting frequency and N supply. However, trait combinations that explain ANPP variations due the changes in cutting frequency are not the same as those that explain ANPP variations to the changes in N supply. Interestingly enough, each of these trait combinations represents a different species strategy according to the PCA axes, i.e. avoidance or resistance mechanisms to cut (axis 1) and nutrients acquisition or conservation (axis 2). In addition, including trait plasticity to management factors in the regressions led to a considerable increase in the explained variance of ANPP, mainly in response to the changes in N supply. Therefore, both trait values and trait plasticity were chosen to explain ANPP variations, accounting for >60 % of the total variance. As far as is know, these relationships have never been reported before. However, further studies are needed to understand if the combinations of traits identified here can assess species abundance at the community scale (the ‘selection effect’, sensu Loreau and Hector, 2001) and the impact of interspecific competition on productivity.
SUPPLEMENTARY DATA
Supplementary Material
ACKNOWLEDGEMENTS
We thank B. Pons, S. Toillon and M. Lafarge for their skilful assistance with the experiment and N. Gross, who provided helpful comments on this manuscript. L. da S. Pontes acknowledges the support of INRA for a doctoral grant.
LITERATURE CITED
- Ackerly DD, Dudley SA, Sultan SE, et al. The evolution of plant ecophysiological traits: recent advances and future directions. BioScience. 2000;50:979–995. [Google Scholar]
- Akaike H. ‘Information Theory and an extension of the Maximum Likelihood Principle. In: Petrov BN, Csaki F, editors. The 2nd International Symposium on Information Theory. Budapest: Akademia Kiado; 1973. pp. 267–281. [Google Scholar]
- Al Haj Khaled R, Duru M, Theau JP, Plantureux S, Cruz P. Variations in leaf traits through seasons and N-availability levels and its consequences for ranking grassland species. Journal of Vegetation Science. 2005;16:391–398. [Google Scholar]
- Arredondo JT, Schyder H. Components of leaf elongation rate and their relationship to specific leaf area in contrasting grasses. New Phytologist. 2003;158:305–314. [Google Scholar]
- Briske D. Strategies of plant survival in grazed systems: a functional interpretation. In: Hodgson J, Illius AW, editors. The ecology and management of grazing systems. Wallingford: CAB International; 1996. pp. 37–67. [Google Scholar]
- Cingolani AM, Posse G, Collantes MB. Plant functional traits, herbivore selectivity and response to sheep grazing in Patagonian steppe grasslands. Journal of Applied Ecology. 2005;42:50–59. [Google Scholar]
- Cornelissen JHC, Lavorel S, Garnier E, et al. A handbook of protocols for standardised and easy measurements of plant functional traits worldwide. Australian Journal of Botany. 2003;51:335–380. [Google Scholar]
- Craine JM, Froehle J, Tilman GD, Wedin DA, Chapin FS. The relationships among root and leaf traits of 76 grassland species and relative abundance along fertility and disturbance gradients. Oikos. 2001;93:274–285. [Google Scholar]
- Craine JM, Tilman D, Wedin D, Reich P, Tjoelker M, Knops J. Functional traits, productivity and effects on nitrogen cycling of 33 grassland species. Functional Ecology. 2002;16:563–574. [Google Scholar]
- Dagnélie P. Théorie et méthodes statistiques. 2nd edn. Vol. II. Gembloux, Belgique: Les Presses Agronomiques de Gembloux; 1986. Les méthodes de l'inférence statistique. [Google Scholar]
- Del-Val E, Crawley MJ. Are grazing increaser species better tolerators than decreasers? An experimental assessment of defoliation tolerance in eight British grassland species. Journal of Ecology. 2005;93:1005–1016. [Google Scholar]
- Díaz S, Noy-Meir I, Cabido M. Can grazing response of herbaceous plants be predicted from simple vegetative traits? Journal of Applied Ecology. 2001;38:497–508. [Google Scholar]
- Díaz S, Lavorel S, de Bello F., Quetier F, Grigulis K, Robson M. Incorporating plant functional diversity effects in ecosystem service assessments. Proceedings of the National Academy of Sciences of the USA. 2007;104:20684–20689. doi: 10.1073/pnas.0704716104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz S, Lavorel S, McIntyre S, et al. Plant trait responses to grazing – a global synthesis. Global Change Biology. 2006;12:1–29. [Google Scholar]
- Eckstein RL, Karlsson PS, Weih M. Leaf life span and nutrient resorption as determinants of plant nutrient conservation in temperate-arctic regions. New Phytologist. 1999;143:177–189. [Google Scholar]
- Franklin O, Ågren GI. Leaf senescence and resorption as mechanisms of maximizing photosynthetic production during canopy development at N limitation. Functional Ecology. 2002;16:727–733. [Google Scholar]
- Funk JL. Differences in plasticity between invasive and native plants from a low resource environment. Journal of Ecology. 2008;96:1162–1173. [Google Scholar]
- Garnier E, Shipley B, Roumet C, Laurent G. A standardized protocol for the determination of specific leaf area and leaf dry matter content. Functional Ecology. 2001;15:688–695. [Google Scholar]
- Garnier E, Cortez J, Billes G, et al. Plant functional markers capture ecosystem properties during secondary succession. Ecology. 2004;85:2630–2637. [Google Scholar]
- Grime JP. Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. American Naturalist. 1977;111:1169–1194. [Google Scholar]
- Grime JP, Hodgson JG, Hunt R. Comparative plant ecology: a functional approach to common British species. London: Unwin Hyman; 1988. [Google Scholar]
- Gross N, Suding KN, Lavorel S. Leaf dry matter content and lateral spread predict response to land use change for six subalpine grassland species. Journal of Vegetation Science. 2007;18:289–300. [Google Scholar]
- Gutschick VP. Biotic and abiotic consequences of differences in leaf structure. New Phytologist. 1999;143:3–18. [Google Scholar]
- Kazakou E, Garnier E, Navas ML, Roumet C, Collin C, Laurent G. Components of nutrient residence time and the leaf economics spectrum in species from Mediterranean old-fields differing in successional status. Functional Ecology. 2007;21:235–245. [Google Scholar]
- Lavorel S, Garnier E. Predicting changes in community composition and ecosystem functioning from plant traits: revisiting the Holy Grail. Functional Ecology. 2002;16:545–556. [Google Scholar]
- Lavorel S, McIntyre S, Landsberg J, Forbes TDA. Plant functional classifications: from general groups to specific groups based on response to disturbance. Trends in Ecology & Evolution. 1997;12:474–478. doi: 10.1016/s0169-5347(97)01219-6. [DOI] [PubMed] [Google Scholar]
- Lemaire G, Agnusdei M. Leaf tissue turn-over and efficiency of herbage utilisation. In: Moraes A, Nabinger C, Carvalho PCF, Alves SJ, Lustosa SBC, editors. Grassland ecophysiology and grazing ecology. Curitiba, Brasil: Imprensa da Universidade Federal do Parana; 1999. pp. 165–186. [Google Scholar]
- Liancourt P, Corcket E, Michalet R. Stress tolerance abilities and competitive responses in a watering and fertilization field experiment. Journal of Vegetation Science. 2005;16:713–722. [Google Scholar]
- Loreau M, Hector A. Partitioning selection and complementarity in biodiversity experiments. Nature. 2001;412:72–76. doi: 10.1038/35083573. [DOI] [PubMed] [Google Scholar]
- Louault F, Pillar VD, Aufrère J, Garnier E, Soussana JF. Plant traits functional types in response to reduced disturbance in a semi-natural grassland. Journal of Vegetation Science. 2005;16:151–160. [Google Scholar]
- Maire V, Gross N, Pontes L da S, Picon-Cochard C, Soussana JF. Trade-off between root nitrogen acquisition and shoot nitrogen utilization across 13 co-occurring pasture grass species. Functional Ecology. 2009;23:668–679. [Google Scholar]
- Pontes L da S. Diversité fonctionnelle des graminées prairiales: conséquences pour la productivité et pour la valeur nutritive. 2006 Thesis, Université Blaise Pascal, Clermont-Ferrand. [Google Scholar]
- Pontes L da S, Soussana JF, Louault F, Andueza D, Carrère P. Leaf traits affect the above-ground productivity and quality of pasture grasses. Functional Ecology. 2007a;21:844–853. [Google Scholar]
- Pontes L da S, Carrère P, Andueza D, Louault F, Soussana JF. Seasonal productivity and nutritive value of native temperate grasses. Responses to cuting frequency and N supply. Grass and Forage Science. 2007b;62:485–496. [Google Scholar]
- Poorter H, De Jong R. A comparison of specific leaf area, chemical composition and leaf construction costs of field plants from 15 habitats differing in productivity. New Phytologist. 1999;143:163–176. [Google Scholar]
- Quétier F, Thébault A, Lavorel S. Plant traits in a state and transition framework as markers of ecosystem response to land-use change. Ecological Monographs. 2007;77:33–52. [Google Scholar]
- Reich PB, Walters MB, Ellsworth DS. Leaf life-span in relation to leaf, plant, and stand characteristics among diverse ecosystems. Ecological Monographs. 1992;62:365–392. [Google Scholar]
- Reich PB, Buschena C, Tjoelker MG, et al. Variation in growth rate and ecophysiology among 34 grassland and savannas' species under contrasting N supply: a test of functional group differences. New Phytologist. 2003;157:617–631. doi: 10.1046/j.1469-8137.2003.00703.x. [DOI] [PubMed] [Google Scholar]
- Ryser P. The importance of tissue density for growth and life span of leaves and roots: a comparison of five ecologically contrasting grasses. Functional Ecology. 1996;10:717–723. [Google Scholar]
- Schumacher J, Roscher C. Differential effects of functional traits on aboveground biomass in semi-natural grasslands. Oikos. 2009;118:1659–1668. [Google Scholar]
- Suding KN, Goldeberg DE, Hartman KM. Relationships among species traits: separating levels of response and identifying linkages to abundance. Ecology. 2003;84:1–16. [Google Scholar]
- Sugiyama S. Relative contribution of meristem activities and specific leaf area to shoot relative growth rate in C3 grass species. Functional Ecology. 2005;19:925–931. [Google Scholar]
- Sultan SE. Phenotypic plasticity for plant development, function and life history. Trends in Plant Science. 2000;5:537–542. doi: 10.1016/s1360-1385(00)01797-0. [DOI] [PubMed] [Google Scholar]
- Tilman D. Relative growth rates and plant allocation patterns. American Naturalist. 1991;138:1269–1275. [Google Scholar]
- Tilman D, Knops J, Wedin D, Reich P, Ritchie M, Sieman E. The influence of functional diversity and composition on ecosystem processes. Science. 1997;277:1300–1302. [Google Scholar]
- Weijschedé J, Berentsen R, de Kroon H, Huber H. Variation in petiole and internode length affects plant performance in Trifolium repens under opposing selection regimes. Evolutionary Ecolgy. 2008;22:383–397. [Google Scholar]
- Westoby M. A leaf-height-seed (LHS) plant ecology strategy scheme. Plant and soil. 1998;199:213–227. [Google Scholar]
- Westoby M. A LHS strategy scheme in relation to grazing and fire. In: Eldridge D, Freudenberger D, editors. Proceedings of the VIth International Rangeland Congress. Queensland, Australia: Australian Rangeland Society; 1999. pp. 893–896. [Google Scholar]
- Wilson PJ, Thompson K, Hodgson JG. Specific leaf area and leaf dry matter content as alternative predictors of plant strategies. New Phytologist. 1999;143:155–162. [Google Scholar]
- Wright IJ, Reich PB, Westoby M, et al. The worldwide leaf economics spectrum. Nature. 2004;428:821–827. doi: 10.1038/nature02403. [DOI] [PubMed] [Google Scholar]
- Wright IJ, Reich PB, Cornelissen JHC, et al. Assessing the generality of global leaf traits relationships. New Phytologist. 2005;166:485–496. doi: 10.1111/j.1469-8137.2005.01349.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.