Abstract
Background and Aims
Salix nigra seeds are desiccation-tolerant, as are orthodox seeds, although in contrast to other orthodox seeds they lose viability in a few weeks at room temperature. They also differ in that the chloroplasts of the embryo tissues conserve their chlorophyll and endomembranes. The aim of this paper was to investigate the role of chlorophyll in seed deterioration.
Methods
Seeds were aged at different light intensities and atmospheric conditions. Mean germination time and normal and total germination were evaluated. The formation of free radicals was assessed using electronic spin resonance spectroscopy, and changes in the fatty acid composition from phospholipids, galactolipids and triglycerides using gas–liquid chromatography. Membrane integrity was studied with electronic spin resonance spin probe techniques, electrolyte leakage and transmission electron microscopy.
Key Results
Light and oxygen played an important role in free-radical generation, causing a decrease in normal germination and an increase in mean germination time. Both indices were associated with a decrease in polyunsaturated fatty acids derived from membrane lipids as phospholipids and galactolipids. The detection of damage in thylakoid membranes and an increase in plasmalemma permeability were consistent with the decrease in both types of lipids. Triglycerides remained unchanged. Light-induced damage began in outermost tissues and spread inwards, decreasing normal germination.
Conclusions
Salix nigra seeds were very susceptible to photooxidation. The thylakoid membranes appeared to be the first target of the photooxidative process since there were large decreases in galactolipids and both these lipids and the activated chlorophyll are contiguous in the structure of that membrane. Changes in normal germination and mean germination time could be explained by the deteriorative effects of oxidation.
Keywords: Embryo membrane integrity, free radicals, orthodox seed, photooxidation, Salicaceae seeds, Salix nigra, seed chlorophyll, seed lipid peroxidation, thylakoids, willow seeds
INTRODUCTION
Typical dry orthodox seeds exhibit longevities of several years (Roberts, 1973). During that time a very slow ageing process occurs, the main cause of which is auto-oxidation (Priestley, 1986; Wilson and McDonald, 1986; McDonald, 1999). This process generates free radicals (FRs) and reactive oxygen species (ROS) that produce damage to molecules, membranes and organelles (Priestley and Leopold, 1983; Priestley, 1986; Wilson and McDonald, 1986; Ponquett et al., 1992). Willow seeds possess the ability to tolerate desiccation to very low water contents, as do orthodox seeds, but differ from the latter in two aspects: (1) they lose viability in few weeks at room temperature (Maroder et al., 2000); and (2) chloroplasts in embryo tissues do not dedifferentiate during maturation drying, retaining chlorophyll and maintaining their endomembrane system intact (Maroder et al., 2003).
In fresh green tissues, chlorophyll excited by light can activate molecular oxygen either directly by excitation energy transference, forming the singlet oxygen, or indirectly by electron transference, forming the superoxide radical. Both oxygen types give way to FR and ROS generation (photo-oxidation) (Asada, 1994; Dalton, 1995; Foyer, 1996). Few studies on oxygen activation have been carried out on dry green tissues, such as those of Salicaceae seed embryos. Even so, it is possible to assume that electron transference does not occur, since photosystem I would not be operative in this state. However, it is important to emphasize that activation of molecular oxygen by energy transference, along with the emission of light with greater wavelength or heat liberation, constitute the possible paths of activation energy dissipation for light-activated chlorophyll (Golbeck, 1992; Chitnis, 1996).
In this paper, one willow species, i.e. Salix nigra has been selected, and complementary methods have been employed to study the possible role of chlorophyll in the short viability that characterizes willow seeds. It is proposed that FR and ROS generated by light-activated chlorophyll would first produce an intense lipid peroxidation at the thylakoid membrane level, since this is where the photosynthetic complexes containing chlorophyll are located, with further oxidative damage extending to other membranes and cellular structures. In the dehydrated state, molecular defences against these agents would be insufficient and/or scarcely efficient, and enzymatic defences inactive (Nandi et al., 1997; Bailly, 2004). As far as is known, no similar studies have been carried out on mature green seeds, or on seeds that reveal such rapid deterioration. To test this hypothesis, seed samples were aged at different light intensities and atmospheric compositions and measurements of normal and total germination (NG and TG, respectively), and mean germination time (MGT) were used to assess the extent of physiological ageing. Electronic spin resonance (ESR) spectroscopy evaluated FR production and changes in fatty acid composition of phospholipids were examined using gas–liquid chromatography (GLC). In order to assess any effects on membrane integrity, an extensive study was carried out using an ESR spin probe, electric conductivity and transmission electron microscopy (TEM).
MATERIALS AND METHODS
Seeds from Salix nigra Marsh. trees grown in the Delta area of the province of Buenos Aires, Argentina were collected and processed during mid-November 2004, as reported by Maroder et al. (2000). Seeds were processed under low light intensity to reduce the eventual FR and ROS generation until stored at −80 °C with 9·5–11 % RH.
Seed ageing
Seeds were aged at 45 % RH and 25 °C. A single layer of seeds was placed on Petri dishes and placed over a saturated solution of K2CO3 (45 % RH) contained in plastic boxes for 3 d under cool-white fluorescent light (1·75 and 16·1 µmol m−2 s−1) at 25 °C. During ageing the seed water content stabilized to 9 %. The treatments were carried out in a cooled incubator MIR-153 SANYO, in air or in a nitrogen atmosphere (nitrogen was previously bubbled into K2CO3-saturated solution). Control seeds correspond to those maintained at −80 °C.
Germination tests
Seeds were tested for germination on three pieces of filter paper moistened with 3·5 cm3 distilled deionized water in 6-cm-diameter Petri dishes at 25 ± 1 °C and 16 h fluorescent light/8 h dark for 6 d. Four replicates of 30 seeds were used for each treatment. Germination was considered normal (NG) if the seedling developed cotyledons, a hypocotyl and a root, and was erect; seedlings not meeting these criteria were classified as abnormal. Normal plus abnormal seedlings correspond to total germination (TG). Most fresh seeds germinated in 12–24 h, although some slow germinators took up to 72 h. Definitive counting was carried out on the seventh day after sowing in order to be able to recognize abnormal seedlings beyond doubt. MGT was calculated by:
ni being the number of daily germinated seeds at time ti, from beginning of seed imbibition (Ellis and Roberts, 1980).
Chlorophyll determination
The chlorophyll analysis was carried out as described by Arnon (1949) and Lichtenthaler (1987). The pulverized seeds were soaked in a solution of chloroform and methanol (2 : 1 volume). The mix was sonicated for 10 min and then subjected to centrifugation at 8000 g. The extraction was repeated three times and the extract was dried in a nitrogen flow, re-dissolved in N, N-dimethylformamide and centrifuged for 10 min at 8000 g. Chlorophyll was quantified using the equations elaborated by Inskeep and Bloom (1985) and Lichtenthaler (1987).
TEM
For subcellular studies, seeds were fixed in 2·5 % glutaraldehyde in 0·1 m phosphate buffer, pH 6·8, at 4 °C, for 90 min. They were then post-fixed in 1 % OsO4 in the same buffer for 90 min, dehydrated in a graded ethanol-acetone series and embedded in Spurr's resin (Sigma–Aldrich, St Louis, MO, USA). Sections were mounted on grids coated with Formvar and then carbon, stained in uranyl acetate followed by lead citrate, and examined in a Zeiss M109 transmission electron microscope.
Conductivity tests
Electrolyte leakage from seeds was determined by placing each of three replicates of 300 mg seeds in 8 mL distilled water at 20 °C. Conductivity was measured after 3 h of soaking using an Altronix Model CTX-II conductivimeter (Brooklyn, NY, USA). Results were expressed as μS mg−1 of dry seeds (mean of three replicates ± s.d.).
Phospholipids lipid extraction and fatty acid analysis
For each treatment, three replicates of 75 mg of lyophilized seed tissues were transferred into 1·5-mL Eppendorf tubes and total lipids were extracted with chloroform–methanol mix using the procedure described by Folch et al. (1957). Phospholipids, triacylglycerol and galactolipids were separated from other lipids by TLC. The lipids were visualized under exposure to iodine vapour. Bands corresponding to phospholipids, triacylglycerol and galactolipids were isolated from chromatographic plates and washed three times with Folch reactive. Lipid extracts were dried, weighed, suspended in 2 mL of a fresh solution of 10 % KOH in ethanol and saponified for 60 min at 80 °C using stoppered glass tubes. Two millilitres of hexane were added and fatty acids were extracted by shaking. The upper organic phase (non-saponified) was discarded. The aqueous layer was acidified with 1·5 mL of concentrated HCl and fatty acids were extracted twice with 1·5 mL hexane. Extracts containing free fatty acids were dried under a nitrogen stream, dissolved in 1·5 mL BF3 (10 % in methanol) and 1·5 mL benzene, and esterified by heating to 100 °C and shaking for 1 h. Fatty acid methyl esters (FAME) were extracted twice with hexane and washed with distilled water. After washing, the organic phase was evaporated under a nitrogen stream, re-dissolved in hexane, and analysed by GLC. One microlitre of FAME solution was injected into an Omegawax X250 (Supelco Inc., Bellefonte, PA, USA) capillary column (30 m × 0·25 mm; 0·25-μm film) in a Hewlett Packard HP-6890 (Santa Clara, CA, USA) chromatograph equipped with a flame ionization detector. The column temperature was programmed for a linear increase of 3 °C min−1 from 175 to 230 °C. The chromatographic peaks of FAME were identified by comparing their retention times with standards under the same conditions.
ESR direct measurements
For each treatment, four replicates of 150 mg of seeds were transferred to an ESR quartz tube. ESR spectra were recorded at 20 °C in an X-band ESR spectrometer Bruker ECS 106 (Brucker Instruments, Inc., Berlin, Germany). The spectrometer settings were: microwave power 1 mW; centre field 3445; sweep width of 0·1 T; conversion time 5·12 ms; time constant 5·12 ms; modulation frequency 50 kHz; modulation amplitude 0·01 mT; gain 2·104; resolution 1024 points. The number of scans varied from 100 to 400 depending on signal intensity. The ESR signal is characteristic of quinone radicals (Wojtyla et al., 2006; Nandi et al., 1997). Signal intensity was measured as the total height of the free-radical peak in the first derivative spectrum signal intensity. Since all the ESR spectra had equivalent line-shapes and line-widths, the ESR signal intensities were considered proportional to the radical concentrations. Radical concentrations were estimated from the total heights of the respective peaks and expressed as relative measures, evaluating within each spectrum the relationship between the height of the FR peak and the height of the manganese low-field peak.
ESR spin probe studies
The detection of electron spin resonance (ESR) spectra using the spin probe technique and a broadening agent, to study changes in the barrier properties of membranes, has been described in previous reports and will only briefly be discussed here (Golovina and Tikhonov, 1994; Golovina et al., 1997).
Seeds were treated with a solution of TEMPONE (4-oxo-2,2,6,6-tetramethyl-1-piperidyneloxy) (Sigma), an amphiphilic and stable nitroxide radical that permeates the plasma membrane and partitions between the hydrophilic and hydrophobic phase of cytoplasm, giving each phase a specific ESR signal. Ferricyanide is water-soluble and does not permeate the plasma membrane except in the presence of damaged areas, in which case the amplitude signal (broadening) experiences a decrease corresponding to the hydrophilic phase, proportional in amount to membrane damage. The ratio between the two signal amplitudes represents the amount of plasma membrane damage.
Three replicates of 150 mg of seeds from each treatment were left to soak for 6 h at 4 °C in 1 mL of water. Then the water was removed, 0·5 mL of a 2 mm TEMPONE solution was added, samples were mixed for 2 min, 0·5 mL of potassium ferricyanide was added to a final concentration of 120 mm, and seeds were incubated for 15 min. After that, the solutions were removed and seeds were dried at 20 °C for 2 h at ambient relative humidity. Dried seeds were sealed in a 2-mm-diameter capillary for ESR measurements. ESR was recorded at room temperature with the above-mentioned X-band ESR spectrometer. The standard spectrometer settings for the nitroxide radicals were: centre field 348·3 mT, sweep width 80 G, microwave power 6·35×10−1 mW, microwave frequency 9·62 GHz, conversion time 2·56 ms, time constant 2·56 ms, modulation frequency 50 kHz, modulation amplitude 9·52×10−6 T, and gain 1·12 T. Twenty spectra were accumulated to improve the signal : noise ratio.
Statistical analysis
Values corresponded to means ± standard deviation. The differences in treatment means were determined with Tukey's test at α ≤ 0·05.
RESULTS
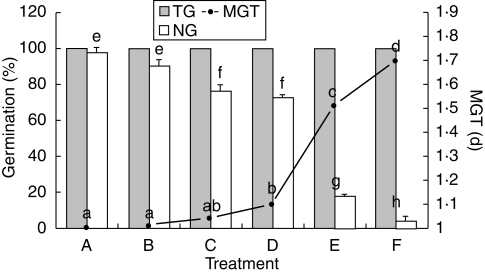
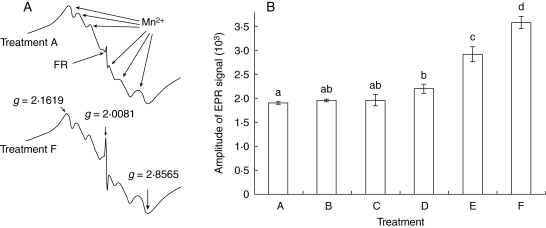
There was a decrease in the NG and an increase in MGT of Salix nigra seeds when they were aged in the presence of light and oxygen (Fig. 1). FRs were detected in both control seeds and in those submitted to the different ageing treatments. Control seeds, with nearly 100 % NG (Fig. 1), revealed a significant amount of FRs, approx. 50 % of that found in seeds in which NG decreased to nearly 0 % (Fig. 2, treatments A and F). The effect of ageing in the dark, plus nitrogen (treatment B) was not significantly different from the control with respect to both NG and FRs. When ageing took place in the dark with air (treatment C) there was only a small decrease in NG while the FR level remained unchanged (Figs 1 and 2). However, in treatment D (light and nitrogen) there was a decrease in NG, which was associated with an increase in FR level (Figs 1 and 2). An even greater increase in FRs was clearly evident following ageing with light and air (treatments E and F, Fig 2) and this was associated with a reduction in NG; in treatment E, NG fell to 18 % (Fig. 1). A light intensity approx. ten times greater (1·75 µmol m−2 s−1; treatment F) resulted in a large decrease in NG, but failed to reduce NG to zero and TG was not affected (Fig. 1).
Fig. 1.
Normal germination (NG), mean germination time (MGT) and total germination (TG) of Salix nigra seeds after 3 d at 45 % RH, 25 °C and different storage conditions: A, control; B, dark and N2 atmosphere; C, dark and air; D, light (1·75 µmol m−2 s−1) and N2 atmosphere; E, light (1·75 µmol m−2 s−1) and air; F, light (16·1 µmol m−2 s−1) and air. Treatments with the same letters do not significantly vary at P = 0·05 according to Tukey's test.
Fig. 2.
(A) ESR spectra of treatments A and F from Fig. 1. The sextet signal corresponds to the naturally occurring manganese ions (Mn2+) with G-values lying between 1·8565 and 2·1619. The radical spectrum is a signal whose g-value of 2·0081 suggests that is a radical apparently derived from quinone (Nandi et al., 1997). (B) Mean amplitude of the ESR signals from Salix nigra seeds for treatments A–F (Fig. 1). Treatments with the same letters above columns are not significantly different at P = 0·05 according to Tukey's test.
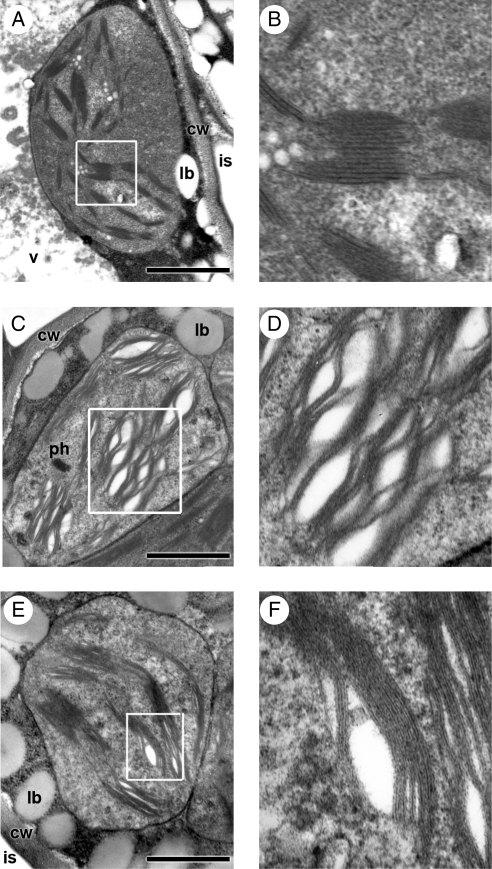
TEM studies showed that in the seeds exposed to 16·1 µmol m−2 s−1 for 3 d, the detrimental effects of light were especially evident in the superficial seed tissues, i.e. the epidermis and the subjacent chlorophyll parenchyma layers. The damage was clearly visible in the chloroplasts of the cells of these tissues, especially in thylakoid membranes (Fig. 3). Essentially, granal thylakoids appeared dilated forming intergranal vesicles, and thylakoid membranes lost their sharpness (Fig. 3A–F). In contrast, only slight alterations were detected in chloroplasts from the central and adaxial mesophyll of cotyledons (Fig. 3E, F) and no damage was observed in the central axis tissues and shoot apical meristems. The level of total chlorophyll decreased significantly from 1373 mg 100 g−1 in the control to 1113 and 890 mg 100 g−1 for the 1·75 and 890 µmol m−2 s−1 treatments, respectively.
Fig. 3.
TEM images of detrimental effects of light on Salix nigra chloroplasts. (A) In a control seed, chloroplast from a subdermal (abaxial) cell of cotyledon; (B) detail of (A). (C, E) Seed aged for 3 d at 25 °C and 16·1 µmol m−2 s−1: (C) chloroplast from a subdermal (abaxial) cell; (D) detail of (C); (E) chloroplast of a cell from the centre of the cotyledon mesophyll; (F) detail of (E). Note differences in thylakoid membranes between subdermal and central cells. Abbreviations: cw, cell wall; is, intercellular space; lb, lipid body; ph, phytopherritin deposit; v, vacuole. Scale bars = 1 µm.
During germination the damage produced in the seed aged at 16·1 µmol m−2 s−1 was reflected as abnormal development: (a) smaller seedling size; (b) underdeveloped radicles; and (c) pale green cotyledons with brown-red areas in both their abaxial surfaces and borders. All of these aspects were taken into account in the evaluation of NG (Fig. 4B). The seeds aged in the dark produced seedlings smaller in size than those of the control and with cotyledons pale green in colour (Fig. 4C). The effects of light were weaker in seeds exposed to 1·75 µmol m−2 s−1 (data not shown).
Fig. 4.
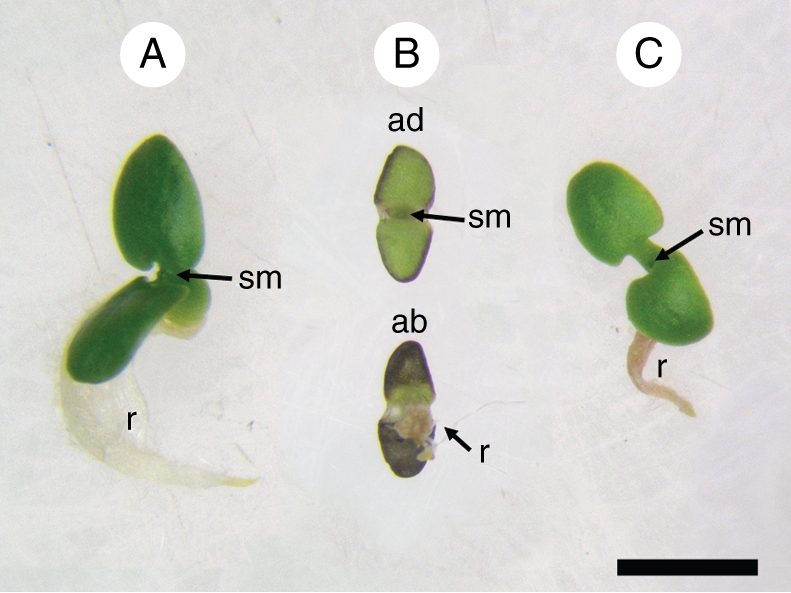
Seven-day-old Salix nigra seedlings. (A) Control seedling from seeds which, following removal from the freezer, had been immediately left to germinate; (B) seedling from a seed aged for 3 d, under 16·1 µmol m−2 s−1, at 25 °C; (C) seedling from seed aged in the dark, for 3 d, at 25 °C. Abbreviations: ab, seedling as seen from below (abaxial face of cotyledons); ad, seedling as seen from above (adaxial surface of cotyledons); r, radicle; sm, shoot apical meristem. Scale bar = 2 mm.
The light promoted significant changes in fatty acid composition. The most important changes detected in S. nigra seeds exposed to both 1·75 and 16·1 µmol m−2 s−1 corresponded to polyunsaturated fatty acids (PUFAs) derived from phospholipid and glycolipid, mostly the latter (Table 1). The intensity of 1·75 µmol m−2 s−1 was sufficient to produce a great decrease in the PUFAs (Table 1), with a smaller decrease at higher light intensity. The fatty acid composition from triglyceride was not significantly affected by light (Table 1).
Table 1.
Effect of light intensity on fatty acid compositions of triglycerides (TG), phospholipids (PL), and glycolipids (GL) from Salix nigra seeds
| Fatty acid* composition (%) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Treatment | Lipid class | C14:00 | C16:00 | C18:00 | C18:1w9 | C18:2w6 | C18:3w3 | Others |
| Control | TG | 1·2 ± 0·9 | 31·5 ± 2·4 | 4·5 ± 0·4 | 12·8 ± 1·1 | 44·6 ± 1·5 | 4·6 ± 0·2 | 0·8 |
| PL | 0·1 ± 0·2 | 30·8 ± 0·3 | 0·7 ± 1·2 | 3·6 ± 0·1 | 55·4 ± 1·6 | 9·3 ± 0·3 | 0·1 | |
| GL | 0·5 ± 0·5 | 18·3 ± 1·1 | 2·2 ± 1·6 | 4·3 ± 1·2 | 56·5 ± 2·2 | 18·2 ± 2·8 | ND† | |
| 1·75 µmol m−2 s−1 | TG | 1·0 ± 0·4 | 32·4 ± 2·3 | 4·8 ± 0·1 | 13·0 ± 0·3 | 43·6 ± 0·5 | 3·8 ± 0·5 | 1·4 |
| PL | 2·3 ± 1·4 | 32·3 ± 1·2 | 5·3 ± 1·4 | 4·3 ± 0·1 | 46·3 ± 2·8 | 6·1 ± 0·9 | 3·4 | |
| GL | 3·0 ± 0·2 | 32·3 ± 5·6 | 4·8 ± 0·7 | 4·1 ± 1·1 | 38·6 ± 3·4 | 12·6 ± 0·4 | 4·6 | |
| 16·1 µmol m−2 s−1 | TG | 2·0 ± 2·8 | 33·4 ± 5·7 | 4·8 ± 1·4 | 14·0 ± 2·2 | 40·6 ± 1·2 | 1·5 ± 2·2 | 3·7 |
| PL | 3·6 ± 1·9 | 34·6 ± 0·6 | 7·2 ± 1·8 | 7·0 ± 1·0 | 40·0 ± 0·2 | 4·0 ± 1·2 | 3·6 | |
| GL | 7·8 ± 1·4 | 38·2 ± 2·9 | 5·5 ± 1·1 | 7·9 ± 2·7 | 27·4 ± 3·6 | 7·5 ± 0·8 | 5·7 | |
Seeds were aged at 25 °C for 3 d at the two different light intensities.
Values correspond to mean ± s.d. (n = 3).
* Fatty acid notation: C14:00 myristic; C16:00 palmitic; C18:00 stearic; C18:1w9 oleic; C18:2w6 linoleic; C18:3w3 linolenic.
† ND, Not determined.
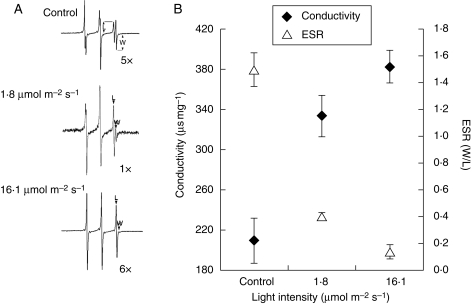
Plasma membrane damage evaluated by both ESR and electrolyte loss was apparent in illuminated seeds. Figure 5A represents the paramagnetic resonant spectra of TEMPONE. The signals from the right side of the spectrum represent the L and W components that correspond to the probe signals found in the hydrophilic and hydrophobic phases of the cytoplasm, respectively. These spectra clearly show that at high light intensity the W signal was nearly zero because the broadening agent was able to penetrate the damaged plasma membrane, broadening the hydrophilic signal (Fig. 5A). The W : L ratio, an index of plasma membrane damage, decreased from 1·47 to 0·39 with 1·75 µmol m−2 s−1 and only an additional 21 % with 16·1 µmol m−2 s−1 (Fig. 6B). The decrease in the W : L ratio was associated with an increase in electrolyte leakage (Fig. 6B).
Fig. 5.
In Salix nigra seeds, the effect of light intensity on membrane permeability, was measured by both ESR spin probe and conductivity. Seeds were aged at 25 °C for 3 d at two different light intensities (1·75 and 16·1 µmol m−2 s−1). (A) ESR spectra of the spin-probe TEMPONE; (B) comparison chart between ESR and conductivity. Values correspond to means ± s.d.
DISCUSSION
This study supports the hypothesis that light and oxygen promote strong photooxidative processes mediated by FRs and ROS in Salix nigra seeds. The production of FRs and ROS is well documented for fresh tissues (Asada, 1994; Dalton, 1995; Foyer, 1996), but insufficient information is available on the occurrence of these processes in dry green tissues, such as those of willow seed embryos. Control seeds, with nearly 100 % NG (Fig. 1), contained 50 % of the FRs found in seeds in which NG decreased to nearly 0 % (Fig. 2, treatments A and F). This suggests that (a) this amount of FRs does not affect NG, and (b) during imbibition and early germination of control seeds, FRs could be eliminated and damage repaired as a consequence of molecular and enzymatic defences against FRs and supports the proposal by Bailly (2004) that these defences would be actively operating in hydrated seeds.
This leads to the question of the origin of the FRs in control seeds. One hypothesis is that most of the FRs could have been generated by the superoxide radical formed as a consequence of the deregulation of electron transport of the respiratory chain produced during dehydration, which naturally occurs in the last stages of orthodox seed development (Vertucci and Farrant, 1995; Bailly, 2004). Since these seeds show high sensitivity to light, the possibility that FRs could have formed during the lapse of time between the expulsion of the seeds from the fruit and their storage cannot be discarded, even though the light intensity was maintained at a low level in the room where the fruit and seeds were manipulated.
The decrease in NG in treatment C (dark and air) could reflect the damage produced by the reaction of the oxygen with the pre-existing radicals, as suggested by Ohlrogge and Kernan (1982) and Justin and Bass (1978), especially considering that the seeds were treated for 3 d at 25 °C, a temperature conducive to ageing (Maroder et al., 2000). In accordance with Benson (1990), the decrease in NG produced in treatment D (light and nitrogen) could be attributed to the light-activated chlorophyll reacting directly with the substrate to form FRs, excluding oxygen from the reaction. However, the formation of some oxi-radicals is not completely impossible since the replacement of oxygen by the nitrogen could have been partial. An increase in the deteriorative action of the FRs was clearly demonstrated in the presence of light and air (treatments E and F; Fig. 1). This suggests that a strong photooxidative process occurred in spite of the low light intensity (treatment E) that reduced NG to very low values. Since these seeds have a thin and transparent seed coat (Maroder et al., 2003), the attack of the FRs induced by the light in the presence of oxygen began in superficial tissues of the embryo. In fact, the outermost embryo tissues, i.e. those of the abaxial side of the cotyledons and the most-external tissues of the axis and the root tip, were those most damaged in these conditions (Figs 3 and 4). During germination, the damage produced in the seeds was manifested as abnormal development in the roots and cotyledons, resulting in irregularities in size and colour for the latter (Fig. 4), aspects considered in the evaluation of NG. The oxidative damage spreads from the outermost to the innermost tissues in such a way that the shoot apical meristem remained sheltered, delaying the fall in TG (beginning of seed mortality). By the time TG was affected by the damage, NG had already been reduced to zero, i.e. decreases in the two parameters occurred independently. In typical orthodox seeds, which are not susceptible to light, the ageing process occurs simultaneously in all of the tissues with only slight variation in intensity; as a result the decrease in the two parameters (TG and NG) is almost simultaneous and, consequently, the decrease curves overlap (Ellis and Roberts, 1981).
Such an interpretation of the events based on an oxidative process that begins in the outermost tissues and spreads inwards could also explain why, as seen in Fig. 1, a light intensity of 1·75 µmol m−2 s−1 reduced NG to 20 % while an intensity approx. ten times greater not only did not reduce NG to zero, but also failed to affect TG (Fig. 1). Similarly, 16·1 µmol m−2 s−1 damaged the external embryo tissues to such an extent that it would destroy chlorophyll and reduce light penetration and photoactivable capacity of the tissues. Therefore, there would be no relationship between the intensity of the light and its effect on NG and the presence of FRs. This interpretation of the photooxidative process that occurred in these seeds is supported by a decrease in chlorophyll content, the TEM study and observations of seedlings (Figs 3 and 4, respectively). These studies showed that the outermost parts of the seed, i.e. abaxial cotyledon surfaces and radicle (including the caliptra), were the most exposed to detrimental action by incident light and hence those most affected. Therefore, the innermost tissues, i.e. those of the adaxial surface of cotyledons (adaxial epidermis and underlying mesophyll), the centre of the axis, and the shoot apical meristem, were barely affected, if at all. This explains why TG had still not been affected at 3 d (Fig. 1).
A metabolic indicator closely associated with the deteriorative action of FRs on lipids is peroxidation (Wilson and McDonald, 1986). Since PUFAs (important lipid membrane components) are the most easily oxidized, a greater reduction of these in the lipid fraction provides evidence of that process (Wilson and McDonald, 1986). In this study, peroxidation was not assessed using malondialdehyde (MDA) because the amount of linolenic acid (from which MDA is derived) was low, making the determination of changes in PUFAs by GLC a better indicator of peroxidation (Table 1). In this respect, Chiu et al. (1995) do not consider MDA an appropriate method when the linolenic acid content is low.
Significant damage to the thylakoid membranes caused by light was observed by TEM, therefore these studies concentrated on the changes observed in the fatty acids of galactolipids, the main component of the thylakoids exclusively found in chloroplasts (Wintermans, 1960; Shibuya et al., 1965). The strong decrease in the PUFAs of the glycolipid fraction (Table 1) point to the thylakoid membranes as a potential target for peroxidation due to the intimate relationship between polyunsaturated acids and the chlorophyll contained in the light receptors (Halliwell and Gutteridge, 1999). In this regard, the thylakoid membranes would be the first target for attack by the activated oxygen, leading to peroxidation, which would destroy fatty acids and the membranes. The FRs and ROS would then spread oxidative damage to other membranes. This is consistent with the decrease in PUFAs corresponding to the phospholipid fraction (Table 1), a distinguishing component of the membrane (Pukacka and Kuiper, 1988; Corbineau et al., 2002).
The significant FR-mediated damage induced by low light intensity suggests that a considerable amount of activated chlorophyll molecules is involved in that process. In the cotyledons of dry, green, pea seeds, Vertucci et al. (1985) found that the amount of energy transferred to chlorophyll by other light-harvesting pigments was limited. However, in Salix seeds the strong photooxidation process that occurs could be accounted for, at least partially, by their high chlorophyll content. Indeed, the amount of chlorophyll in Salix seeds is approx. ten times greater than that in pea cotyledons (Cheng et al., 2004).
Nevertheless, the damage determined at the plasmalemma evaluated by changes in membrane permeability (Fig. 5) corresponded with a decrease in those PUFAs. In seeds submitted to drying, Leprince et al. (1999) found that ESR is a more sensitive and accurate indicator than electrical conductivity when it comes to assessing membrane integrity. However, in the present case both methods were found to be closely correlated (Fig. 5).
According to Smith and Berjak (1995), TEM can detect changes produced in the membranes of radicle meristems (like the separation of the plasma membrane from the cell wall) when the seeds are soaked. The present study also revealed an incipient separation of the plasma membrane from the cell walls, which in a dry state and due to the intense photooxidation process could cause severe damage (Fig. 3). It is worth mentioning that separation of the plasma membrane was detected in dry seeds.
In conclusion, results of different light treatments revealed that the increase in light intensity was accompanied by FR formation, leading to a decrease in polyunsaturated acids. The latter was associated with significant thylakoid membrane damage and with alteration of plasma membrane permeability, all of which constitute a sequence of strongly linked processes reflected in the decrease of NG. At high light intensity TG was also affected. These results contrast with those observed in Tortula ruraliformis (Seel et al., 1991), a moss species containing chlorophyll which is tolerant of desiccation, and, therefore, similar to willow seeds. However, in mosses the action of light produces FR without causing damage. This difference could be attributed to the high level of molecular antioxidant defences, which in a dry state would protect the tissues.
Previous research has revealed similar behaviour among Salix nigra, Salix alba (Maroder et al., 2000) and Populus nigra (G. Roqueiro et al., unpubl. res.) seeds, suggesting that these conclusions could be extended to the remaining species of Salicaceae.
ACKNOWLEDGEMENTS
This work was supported by a grant from the Agencia Nacional de Promoción Científica y Tecnológica, Ministerio de Ciencia y Tecnología (PICT 01-13368 to S.M. and H.M.) and Universidad de Buenos Aires (UBACYT B130) to E.R.C. and G.B.F.
LITERATURE CITED
- Arnon DI. Copper enzymes in isolated cloroplasts: polyphenol oxidase in Beta vulgaris. Plant Physiology. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asada K. Production and action of active oxygen species in photosynthetic tissues. In: Foyer CH, Mullineaux PM, editors. Cause of photooxidative stress and amelioration of defense system in plants. Boca Raton, FL: CRC Press; 1994. pp. 77–104. [Google Scholar]
- Bailly C. Active oxygen species and antioxidants in seed biology. Seed ScienceResearch. 2004;14:93–107. [Google Scholar]
- Benson EE. Free radicals damage in stored plant germoplasm. Rome: International Board or Plant Genetic Resources; 1990. [Google Scholar]
- Cheng M, Mcphee KE, Baik B. Bleaching of green peas and changes in enzyme activities of seeds under simulated climatic conditions. Journal of Food Science. 2004;69:511–518. [Google Scholar]
- Chitnis PR. Photosystem I. Plant Physiology. 1996;111:661–669. doi: 10.1104/pp.111.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu KY, Wang CS, Sung JM. Lipid peroxidation and peroxide-scavenging enzymes associated with accelerated aging and hydration of watermelon seeds differing in ploid. Physiologia Plantarum. 1995;94:441–446. [Google Scholar]
- Corbineau F, Gay-Mathieu C, Vinel D, Côme D. Decrease in sunflower (Helianthus annuus) seed viability caused by high temperature as related to energy metabolism, membrane damage and lipid composition. Physiologia Plantarum. 2002;116:489–496. [Google Scholar]
- Dalton DA. Antioxidant defenses of plants and fungi. In: Ahmad S, editor. Oxidative stress and antioxidant defenses in biology. New York, NY: Chapman and Hall; 1995. pp. 298–355. [Google Scholar]
- Ellis RH, Roberts EH. The influence of temperature and moisture on seed viability period in barley (Hordeum distichum L.) Annals of Botany. 1980;45:31–37. [Google Scholar]
- Ellis RH, Roberts EH. The quantification of ageing and survival in orthodox seeds. Seed Science and Technology. 1981;9:373–409. [Google Scholar]
- Folch J, Lees M, Stanley GHS. A simple method for the isolation and purification of total lipids from animal tissues. Journal of Biological Chemistry. 1957;226:497–509. [PubMed] [Google Scholar]
- Foyer CH. Free radical processes in plants. Biochemical Society Transactions. 1996;24:427–433. doi: 10.1042/bst0240427. [DOI] [PubMed] [Google Scholar]
- Golbeck JH. Structure and function of photosystem I. Annual Review of Plant Physiology and Plant Molecular Biology. 1992;43:293–324. [Google Scholar]
- Golovina EA, Tikhonov AN. The structural differences between the embryos of viable and nonviable wheat seeds as studied with the ESR spectroscopy of lipid soluble spin labels. Biochimica et Biophysica Acta. 1994;1190:385–392. doi: 10.1016/0005-2736(94)90098-1. [DOI] [PubMed] [Google Scholar]
- Golovina EA, Tikhonov AN, Hoekstra FA. An electron paramagnetic resonance spin-probe study of membrane permeability changes with seed aging. Plant Physiology. 1997;114:383–389. doi: 10.1104/pp.114.1.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC. Free radical in biology and medicine. 3rd edn. New York, NY: Oxford University Press; 1999. [Google Scholar]
- Inskeep WP, Bloom PR. Extinction coefficients of chlorophyll a and b in N,N-dimethylformamide and 80 % acetone. Plant Physiology. 1985;77:483–485. doi: 10.1104/pp.77.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justin OL, Bass LN. Principles and practices of seed storage. US Dept of Agriculture Handbook. 1978:57–77. No. 506. [Google Scholar]
- Leprince O, Buitinik J, Hoekstra FA. Axes and cotyledons of recalcitrant seeds of Castanea sativa Mill. exhibit contrasting responses of respiration to drying in relation to desiccation sensitivity. Journal of Experimental Botany. 1999;50:1515–1524. [Google Scholar]
- Lichtenthaler HK. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods in Enzymology. 1987;148:350–382. [Google Scholar]
- McDonald MB. Seed deterioration: physiology, repair and assessment. Seed Science and Technology. 1999;27:177–237. [Google Scholar]
- Maroder HL, Prego IA, Facciuto GR, Maldonado SB. Storage behaviour of Salix alba and Salix matsudana seeds. Annals of Botany. 2000;86:1017–1021. [Google Scholar]
- Maroder HL, Prego IA, Maldonado SB. Histochemical and ultrastructural studies on Salíx alba and Salíx matsudana seeds. Trees. 2003;17:193–199. [Google Scholar]
- Nandi S, Sen-Mandi S, Sinha TP. Active oxygen and their scavengers in rice seeds (Oryza sativa cv. IET 4094) aged under tropical environmental conditions. Seed Science Research. 1997;7:253–259. [Google Scholar]
- Ohlrogge JB, Kernan TP. Oxygen-dependent aging of seeds. Plant Physiology. 1982;70:791–794. doi: 10.1104/pp.70.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponquett RT, Smith MT, Ross G. Lipid autoxidation and seed ageing: putative relationships between seed longevity and lipids stability. Seed Science Research. 1992;2:51–54. [Google Scholar]
- Priestley DA. Seed aging: implications for seed storage and persistence in the soil. Ithaca, NY: Cornell University Press; 1986. [Google Scholar]
- Priestley DA, Leopold AC. Lipid changes during natural aging of soybean seeds. Physiologia Plantarum. 1983;59:467–470. [Google Scholar]
- Pukacka S, Kuiper PJC. Phospholipid composition and fatty acid peroxidation during ageing of Acer platanoides seeds. Physiologia Plantarum. 1988;72:89–93. [Google Scholar]
- Roberts EH. Loss of viability: ultrastructural and physiological aspects. Seed Science and Technology. 1973;1:29–34. [Google Scholar]
- Seel WE, Hendry GAF, Atherton NR, Lee JA. Radical formation and accumulation in vivo, in desiccation-tolerant and intolerant mosses. Free Radical Research Communications. 1991;15:133–141. doi: 10.3109/10715769109049133. [DOI] [PubMed] [Google Scholar]
- Shibuya I, Maruo B, Benson AA. Sulfolipid localization in lamellar lipoprotein. Plant Physiology. 1965;40:1251–1256. doi: 10.1104/pp.40.6.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MT, Berjak P. Deteriorative changes associated with the loss viability of stored desiccation tolerant and desiccation-sensitive seeds. In: Kigel J, Galili G, editors. Seed development and germination. New York, NY: Marcel Dekker; 1995. pp. 701–46. [Google Scholar]
- Vertucci CW, Farrant JM. Acquisition and loss of desiccation tolerance. In: Kigel J, Galili G, editors. Seed development and germination. New York, NY: Marcel Dekker; 1995. pp. 237–271. [Google Scholar]
- Vertucci CW, Ellenson JL, Leopold AC. Chlorophyll fluorescence characteristics associated with hydration level in pea cotyledons. Plant Physiology. 1985;79:248–252. doi: 10.1104/pp.79.1.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DO, McDonald MB. The lipid peroxidation model of seed ageing. Seed Science and Technology. 1986;14:269–300. [Google Scholar]
- Wintermans JFGM. Concentrations of phosphatides and glycolipids in leaves and chloroplasts. Biochimica et Biophysica Acta. 1960;44:49–54. doi: 10.1016/0006-3002(60)91521-3. [DOI] [PubMed] [Google Scholar]
- Wojtyla L, Garnczarska M, Zalewski T, Bednarski W, Ratajczak L, Jurga S. A comparative study of water distribution, free radical production and activation of antioxidative metabolism in germinating pea seeds. Journal of Plant Physiology. 2006;16:1207–1220. doi: 10.1016/j.jplph.2006.06.014. [DOI] [PubMed] [Google Scholar]