Abstract
Background and Aims
Adventitious sprouting from the hypocotyle and roots in monocarpic herbs has been confirmed in previous experimental studies as a means to avoid bud limitation after severe injury in annual and biennial plants. Data regarding the role of adventitious sprouting in natural populations, however, were lacking. The aim of the present study was to assess whether adventitious sprouting occurs in natural populations and how it is affected by plant size, plant injury, plant cover and environmental characteristics.
Methods
Data were sampled from 14 037 individual plants from 389 populations belonging to 22 annual and biennial species. Growth parameters were measured in individual plants, species composition and plant cover in communities were evaluated, and environmental characteristics were estimated using Ellenberg indicator values.
Key Results
It was confirmed that adventitious sprouting occurs in natural populations of all but five species examined. Adventitious sprouting was positively affected by plant size and plant injury. Environmental factors including availability of soil nitrogen were not shown to affect adventitious sprouting. Annual and biennial plants did not differ in sprouting, but upright annuals had a lower number of and longer adventitious shoots than prostrate annuals.
Conclusions
Adventitious bud formation is used to overcome meristem limitation when stem parts are lost due to injury, and thus resprouting in short-lived monocarps should not be overlooked.
Keywords: Monocarpic herbs, annuals, biennials, weedy species, disturbance, Ellenberg indicator values, potential bud bank, resprouting, roots, hypocotyle
INTRODUCTION
Theory predicts that semelparous life history evolves when juvenile survivorship is relatively high compared with the probability of adult survivorship to the next reproductive event (Stearns, 1992). The majority of semelparous (monocarpic) plant species have an annual or biennial life cycle and dominate in ecosystems where severe but predictable disturbances detrimentally affect their populations yearly, typically in connection with a seasonal climate, for example summer drought, spring floods and ploughing of arable land. Monocarpic herbs adopt an avoidance strategy characterized by a short life cycle and numerous easily dispersible diaspores (Bellingham and Sparrow, 2000; Grime, 2001).
The ability of annual herbs to survive an injury is constrained by the scarcity of basal reserve meristems and poor carbon storage (Dina and Klikoff, 1974; Otzen, 1977; Krumbiegel, 1998). Monocarpic herbs with a biennial or short-lived perennial life cycle, by contrast, accumulate reserves and basal meristems (Krumbiegel, 1999; Vilela et al., 2008; Sosnová and Klimešová, 2009). However, their recovery from damage depends on life-history stage and diminishes with disturbance severity (Huhta et al., 2003; Boege and Marquis, 2005) as the costs of recovery may match the costs of intrinsically programmed life-history events (Klimešová et al., 2007).
Despite knowledge about the above-mentioned factors, monocarpic herbs can in reality be subjected to injury and regenerate vegetatively due to different disturbance events with varying intensity, timing and probability, such as herbivory, erosion or anthropogenic activity (Klimešová and Klimeš, 2003). Although overlooked in some theoretical studies (Bellingham and Sparrow, 2000; Grime, 2001), this is accepted, and monocarpic herbs represent a suitable model for studying the fitness consequences of damage (Lennartsson et al., 1997, 1998; Paige, 1999; Huhta et al., 2000a, b, c; Hellström et al., 2004; Piippo et al., 2005, 2009; Rautio et al., 2005).
Moreover, about 2 % of annuals and 13 % of biennial herbs of Central Europe possess the ability to form adventitious buds on the hypocotyle and/or roots (Klimešová and Klimeš, 2006). Such buds are formed de novo on organs originally lacking buds and thus provide a plant with additional meristems to those occurring in leaf axils on stem parts (Rauh, 1937). This trait contrasts with the expected avoidance strategy of monocarpic herbs, as it brings about a potential for overcoming meristem limitation (Klimešová and Klimeš, 2003; Klimešová and Martínková, 2004).
Experimental studies have tested whether adventitious bud formation, a morphological trait, might be considered as a pool of meristems for vegetative regeneration in the case of plant injury (potential bud bank sensu Klimešová and Klimeš, 2007). The results showed that the formation of adventitious buds in monocarpic short-lived herbs might be an important means to rescue an individual plant and ensure seed production after an injury that was far more severe than expected (Klimešová et al., 2008; Martínková et al., 2008; Latzel et al., 2009). Moreover, plant phenology, life-history stage, carbon storage and plant size are important characteristics constraining resprouting from adventitious buds; photoperiod, nutrient availability, disturbance severity and flooding stress are environmental variables that were found to affect the regeneration process or degree of compensation (Martínková et al., 2004a, b, 2006, 2008; Klimešová et al., 2007, 2008; Sosnová and Klimešová, 2009).
Although the capacity to deal with severe injury in monocarpic short-lived herbs was shown under experimental conditions, its role in nature remains unresolved. Apart from a few studies (Martínková et al., 2006; King et al., 2008), the occurrence of resprouting monocarps in the field remains only anecdotally documented in descriptive morphological studies (e.g. Wydler, 1850; Reichardt, 1857; Wittrock, 1884; Beijerinck, 1887; Holm, 1925; Rauh, 1937). Thus, we do not know whether the potential for resprouting is employed by plants in unmanipulated field conditions or if the occurrence of adventitious sprouting is restricted to certain rare situations and thus might be considered as a teratological feature (Penzig, 1921–1922).
Due to the scarcity of data on resprouting of monocarpic herbs in field conditions, an analogical system was employed, namely woody resprouters in fire-prone areas, to make predictions. There is a tendency towards resprouting (i.e. survival and regeneration after fire from the bud bank) in nutrient-poor conditions and towards seeding (i.e. death after fire and regeneration from seeds) in nutrient-rich conditions (Iwasa and Kubo, 1997; Bellingham and Sparrow, 2000; Buhk et al., 2007; but see Clarke et al., 2005; Knox and Clarke, 2005). Resprouters are characterized by low stature and when disturbance is lacking they are overgrown by tall seeders (Midgley, 1996). This, however, may not be true in herbs where vertical growth starts each year from zero. When a large-scale severe disturbance affects a community of herbaceous monocarps during the growing season, those possessing adventitious buds will survive and resprout at the expense of storage carbon in roots and those lacking bud banks will die and regenerate from seed. However, as annual and biennial species prevail in habitats subjected to some predictable disturbance, for example ploughing, those plants regenerating from seed might fail to finish the life cycle by the end of the season and thus are not able to outcompete resprouters later on. Therefore, the success of herbaceous monocarpic resprouters will depend more on the ability to compensate for seed production, than just on the ability to survive because they are – contrary to woody resprouters – short living and their populations are dependent on regeneration from seeds.
Compensation ability (fitness and biomass production of injured versus untouched plants) is usually studied as a response of herbs to herbivory. Studies of the dependence on nutrient availability give contrasting, context-specific results (e.g. Ferraro and Osterheld, 2002; Wise and Abrahamson, 2007). On the other hand, vigour and compensation ability of regenerated root sprouting plants are in contrast to resprouting success supported by nutrient-rich conditions (Martínková et al., 2004a, b, 2008; Latzel and Klimešová, 2009). Thus, we may hypothesize that good growing conditions (high nitrogen, sufficient moisture and illumination) will support regeneration by adventitious sprouting in monocarpic short-lived herbs in contrast to resprouters in fire-prone areas (see also Eggers, 1946).
Additionally, as biennials usually have the ability to postpone reproduction to later seasons (Klimešová et al., 2007; Martínková et al., 2008), they possess larger carbon storage (Sosnová and Klimešová, 2009) and during a longer life cycle can experience more disturbance events than annuals. Thus, a second hypothesis is that adventitious sprouting will be more common in biennials than in annual herbs and in later phenological phases.
Due to a trade-off between apical dominance (competitive ability) and branching (Aarssen, 1995; Bonser and Aarssen, 1996; McPhee et al., 1997; Duffy et al., 1999), we expected that plants with reduced apical dominance, those which are not growing in a competitive environment or have a prostrate growth form will have a higher number of adventitious shoots.
The aim here is to test the following hypotheses: (1) good growing conditions (high nitrogen, sufficient moisture and illumination) will support regeneration by adventitious sprouting in short-lived monocarps; (2) adventitious sprouting will be more common in biennials compared with annual herbs and in later phenological phases; and (3) plants with reduced apical dominance, those which are not growing in a competitive environment or having a prostrate growth form will have a higher number of adventitious shoots. To test these hypotheses, the occurrence of adventitious buds and sprouts was assessed in numerous natural populations of 22 species of short-lived monocarpic herbs. As plant characteristics affecting adventitious sprouting, the effects of plant size, growth form, phenology, life history and plant injury were studied, and environmental characteristics studied were vegetation cover as a measure of the competitive milieu, soil compactness and, indirectly (using Ellenberg indicator values), nitrogen status, moisture, light availability and temperature.
METHODS
Field data
Monocarpic short-lived herbs that were reported as being capable of adventitious sprouting from the hypocotyle and/or roots (Klimešová and Klimeš, 2006; Klimešová and de Bello, 2009; Table 1) were studied in the field. Populations of annuals and biennials were sampled in different habitats and environmental conditions mainly in the Czech Republic (Central Europe) from 2005 to 2007.
Table 1.
List of the 22 studied species with their status in the flora of the Czech Republic, number of sampled populations and total number of sampled and injured individuals during field seasons 2005 and 2006
| Species | Family | Growth form in annuals | Number of populations sampled | Total number of individuals sampled | Numbers of injured individuals |
|---|---|---|---|---|---|
| Annuals | |||||
| Anagallis arvensis L. | Primulaceae | prostrate | 30 | 1114 | 78 |
| Arabidopsis thaliana (L.) Heynh. | Brassicaceae | upright | 30 | 1121 | 66 |
| Euphorbia exigua L. | Euphorbiaceae | upright | 6 | 208 | 33 |
| Euphorbia helioscopia L. | Euphorbiaceae | upright | 30 | 1085 | 86 |
| Euphorbia peplus L. | Euphorbiaceae | upright | 31 | 1407 | 191 |
| Kickxia spuria (L.) Dumort. | Scrophulariaceae | prostrate | 3 | 61 | 0 |
| Kickxia elatine (L.) Dumort. | Scrophulariaceae | prostrate | 2 | 71 | 0 |
| Microrrhinum minus (L.) Fourr. | Scrophulariaceae | upright | 31 | 1257 | 53 |
| Biennials | |||||
| Arabis glabra (L.) Bernh. | Brassicaceae | 6 | 176 | 13 | |
| Arabis hirsuta (L.) Scop. sensu stricto | Brassicaceae | 2 | 68 | 31 | |
| Alliaria petiolata (M. Bieb.) Cavara et Grande | Brassicaceae | 27 | 993 | 73 | |
| Barbarea vulgaris W.T. Aiton | Brassicaceae | 31 | 1112 | 12 | |
| Barbarea stricta Andrz. | Brassicaceae | 10 | 339 | 4 | |
| Daucus carota L. | Apiaceae | 35 | 1074 | 162 | |
| Diplotaxis muralis (L.) DC. | Brassicaceae | 1 | 30 | 0 | |
| Isatis tinctoria L. | Brassicaceae | 2 | 40 | 13 | |
| Jasione montana L. | Campanulaceae | 5 | 176 | 1 | |
| Medicago lupulina L. | Fabaceae | 29 | 1090 | 87 | |
| Potentilla supina L. | Rosaceae | 30 | 1029 | 26 | |
| Reseda lutea L. | Resedaceae | 15 | 448 | 28 | |
| Reseda luteola L. | Resedaceae | 1 | 20 | 0 | |
| Rorippa palustris (L.) Besser | Brassicaceae | 32 | 1118 | 45 | |
The list of studied species, number of sampled populations and individuals is given in Table 1. The aim to assess the morphology in at least 30 natural populations per species and at least 20 individuals per population was not fulfilled in some rare species. Measured plant traits were as follows: plant height, base width, number of axilary branches, cumulative length of adventitious shoots, length of the longest adventitious shoot, number of adventitious buds and number of adventitious shoots. A disturbance was assessed as an injured or removed main shoot, while phenology was assessed as the main phenological stages (i.e. vegetative, flowering, fruiting).
Communities where sufficient numbers of individuals of a target species occurred were described using phytosociological relevés (van der Maarel, 2007): all species in a relevé were determined and their cover estimated (Braun–Blanquet scale r = 0·05–0·5 %, += 0·5–2·5 %, 1 = 2·5–7·5 %, 2a = 7·5–15 %, 2m = 15–22·5 %, 2b = 22·5–37·5 %, 3 = 37·5–62·5 %, 4 = 62·5–87·5 %, 5 = 87·5–100 %). Thus, the following community characteristics were also assessed: total vegetation cover, cover of individual species and species richness.
Soil cementation was determined using a semiquantitative scale, low, medium and high, according to permeability assessed using a pencil. Other environmental characteristics for the studied populations were assessed using Ellenberg indicator values for light, temperature, humidity and nitrogen (Ellenberg, 1986). Values of the environmental characteristics for individual populations were calculated as a weighted average of indicator values for individual species, weighted by the estimated species abundance.
Data analysis
The collected dataset (all species were included) was strictly hierarchical in nature, with individual species represented by multiple populations, each with many individuals. Consequently, our hypotheses were tested using linear mixed-effect models or generalized linear mixed-effect models, depending on the nature of a particular response variable (assuming Gaussian, quasi-Poisson or quasi-binomial distributions), with species identity as a random effect and population as a nested effect. The tests were based on the likelihood-ratio approach, approximating the difference in model deviances with a χ2 distribution. The two models were fitted using the lme4 package in R, version 2·8 (R Development Core Team, 2008).
Due to the possibility that phylogenetic inertia could affect both the parameters of adventitious resprouting behaviour and the explanatory variables implied in the hypotheses tested, the tests were also done with phylogenetic correction, using the method of Desdevises et al. (2003).
As the attributes of adventitious sprouting, representing individual response variables in the models, are at least partly related, the results for a particular predictor represent a family of statistical tests, for which Type I errors should be corrected to control for family-level errors. Holm's procedure (Holm, 1979) was employed, which is a more powerful alternative to the traditionally used Bonferroni correction.
RESULTS
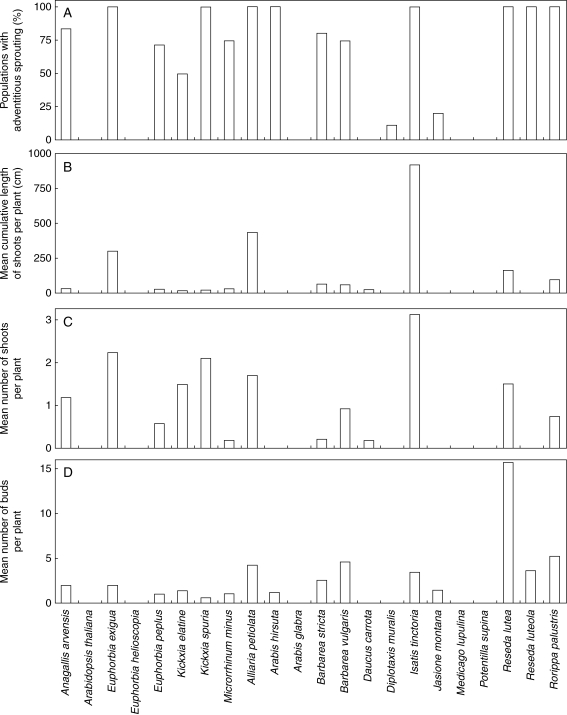
Adventitious sprouting was not observed (neither adventitious buds nor shoots were recorded) in five of the 22 studied species: Euphorbia helioscopia, Arabidopsis thaliana, Arabis glabra, Medicago lupulina and Potentilla supina. The species with the highest number of buds was Reseda lutea whereas the species with highest number of shoots was Isatis tinctoria (Fig. 1).
Fig. 1.
Characteristics of adventitious sprouting in the studied species. (A) Populations with adventitious sprouting (%); (B) mean cumulative length of shoots per plant (cm); (C) mean number of shoots per plant; (D) mean number of buds per plant.
Effect of plant characteristics on adventitious sprouting
Plant size, measured as shoot base diameter and branch number, was positively correlated with the resprouting intensity of plants, whereas plant height affected resprouting only marginally. Phenological stage and plant injury (defined as the loss of the primary shoot) affected all studied resprouting characteristics, with resprouting being more intensive in late phenological stages and injured plants (Table 2). Differences between biennials and annuals were found only in the length of adventitious shoots, being larger in biennials, whereas the numbers of buds and shoots were not different between the two life-history modes. The species investigated differed in all studied characteristics (Table 3).
Table 2.
Effect of plant size characteristics, developmental stage and damage on the attributes of adventitious sprouting
| Response variable | Plant height | Base width | No. of axillary branches | Phenology | Disturbance |
|---|---|---|---|---|---|
| No. of adventitious buds and shoots | 2·1 (n.s.) | 132·5 (<0·001) ↑ | 43·3 (<0·001) ↑ | 28·8 (<0·001) ↑ | 54·5 (<0·001) ↑ |
| No. of adventitious shoots/no. of adventitious buds and shoots | 4·7 (n.s.) | 18·1 (<0·001) ↓ | 2·4 (n.s.) | 5·6 (0·018) ↑ | 70·9 (<0·001) ↑ |
| Presence of adventitious buds or shoots | 2·1 (n.s.) | 27·1 (<0·001) ↑ | 15·5 (<0·001) ↑ | 11·5 (0·003) ↑ | 63·8 (<0·001) ↑ |
| Cumulative length of adventitious shoots | 0·4 (n.s.) | 0·9 (n.s.) | 10·8 (0·003) ↑ | 8·3 (0·0012) ↑ | 8·6 (0·003) ↑ |
| Length of the longest adventitious shoot | 0·01 (n.s.) | 2·0 (n.s.) | 3·8 (n.s.) | 8·0 (0·0012) ↑ | 10·8 (0·002) ↑ |
Effect of individual predictors (columns) was examined in two separate models for each response variable (rows): one for plant stature parameters (plant height, base width, number of axillary branches), and the other for plant phenological and damage status (phenology, disturbance). The χ2 statistic value is given first, with the corresponding Type I error estimate for a likelihood-ratio test of the particular model term in parentheses; the symbol (present only for significant predictors) summarizes the direction of the effect: ↑, a positive correlation between the predictor and response values; ↓, a negative correlation.
Table 3.
Test of differences among species, between annuals and biennials (life form), and between prostrate and upright annual species (growth form) in the attributes of adventitious sprouting
| Response variable | Species | Life form | Growth form |
|---|---|---|---|
| No. of adventitious buds and shoots | 390·6 (<0·001) | 0·5 (n.s.) | 12 202 (<0·001) |
| 12 187 (<0·001) | |||
| smaller for upright | |||
| No. of adventitious shoots/no. of adventitious buds and shoots | 36·5 (<0·001) | 1·9 (n.s.) | 1905 (<0·001) |
| 1900 (<0·001) | |||
| smaller for upright | |||
| Presence of adventitious buds or shoots | 403·2 (<0·001) | 0·00 (n.s.) | 3290 (<0·001) |
| 3290 (<0·001) | |||
| probability smaller for upright | |||
| Cumulative length of adventitious shoots | 34·6 (<0·001) | 7·1 (0·038) | 827 (<0·001) |
| 1·8 (n.s.) | 825 (<0·001) | ||
| larger for biennials | longer for upright | ||
| Length of the longest adventitious shoot | 45·6 (<0·001) | 9·9 (0·010) | 762 (<0·001) |
| 1·2 (n.s.) | 760 (<0·001) | ||
| larger for biennials | longer for upright |
Effect of individual predictors (columns) was examined in a separate model for each response variable (rows). The first row for each variable provides the χ2 statistic and corresponding Type I error estimate for a likelihood-ratio test of the particular model term; the next row provides results from the corresponding model with phylogenetic correction (fitted only for previously significant effects, except for ‘Species’, where the correction was not appropriate). If any of the two models found a significant effect, then below is described the direction of the effect (for ‘Life form’ and ‘Growth form’ predictors).
The annual plant species studied differed in their growth form; some are prostrate whereas others have upright stems. This growth form characteristic influenced significantly all measured characteristics of resprouting: upright annuals had a lower number of adventitious buds and shoots, but shoot length was higher than in the prostrate plants (Table 3).
Effect of environmental characteristics
Environmental characteristics were tested with shoot diameter, number of branches, phenology and injury as covariates, i.e. the effect of plant developmental state was removed from the analysis. None of the tested characteristics (light, soil nitrogen, moisture, soil cementation, total cover of the herb layer, temperature and species richness of the community) had any effect on adventitious sprouting. Similar results were obtained when only phenological stage was used as a covariate.
Non-disturbed plants were analysed separately to assess the role of environmental variables on the presence of adventitious bud formation. Again, environmental variables had no effect on adventitious sprouting.
Phylogenetic correction
The difference between annuals and biennials disappeared when taking into account the phylogenetic relatedness of the species studied. Moreover, the results obtained for environmental variables remained unaffected and non-significant.
DISCUSSION
Adventitious sprouting in short-lived monocarpic herbs was found in natural communities, but its extent differed among species and was generally enhanced by injury. The effect of environmental variables on adventitious sprouting was not significant. Sprouting was more vigorous in large, branched plants and their later phenological phases (i.e. accumulation of disturbance with life span). Biennials tended to produce longer adventitious shoots, but this effect was affected by phylogenetic relatedness within life-history modes and disappeared after phylogenetic correction. Prostrate annuals formed more buds whereas upright annuals had fewer but longer shoots; this indicates that apical dominance was more pronounced in upright forms.
The fact that adventitious sprouting was not observed in all studied plant species should not be considered as proof that they do not have any resprouting ability. However, at least six populations were studied in those species, suggesting that any adventitious sprouting would probably be very rare in the species lacking adventitious sprouting in the present study.
Effect of plant characteristics
The results on the effect of plant characteristics on adventitious sprouting are in accordance with the expectations based on experimental studies listed in the Introduction, with the exception of a lack of difference between biennials and annuals. This surprising result may be caused by the presence of carbon storage connected with potential bud bank formation in both life-history modes. Restriction of monocarpic root-sprouters to places affected by human activity and under-representation in more pristine communities in comparison with non-sprouters (J. Martínková et al., Institute of Botany ASCR, Czech Republic, unpubl. res.) implies that there are costs of unrealized resprouting when there is a lack of disturbance. These costs may be interpreted as carbohydrate storage in below-ground parts at the expense of growth in above-ground parts. Although differences in the storage economy of root-sprouters versus non-sprouters in monocarpic herbs were not directly tested, the root-sprouting monocarp Rorippa palustris builds larger carbohydrate reserves in comparison with some other annuals (Dina and Klikoff, 1974; Clark and Burk, 1980; Chiariello and Roughgarden, 1984; Sosnová and Klimešová, 2009).
Another factor responsible for the lack of difference between adventitious sprouting in annuals and biennials might be the fact that those two life-history modes are rather plastic and many species are characterized by life-history variation (Rauh, 1937; Klimešová, 2003; Klimešová et al., 2007). Adventitious buds provide a bud bank for production of additional shoots after flowering or over-wintering; many species of short-lived root sprouters can behave as short-lived perennials (MacDonald and Cavers, 1974; Klimešová et al., 2007).
The present study compared only surviving plants, and therefore it was not possible to disentangle whether adventitious sprouters were larger because injury and consequent resprouting led to over-compensation and huge growth, or simply because smaller plants were more prone to mortality after injury than larger plants (King et al., 2008). Because successful resprouting of the largest plants is in accordance with experimental studies (Martínková et al., 2004a; M. Sosnová, Institute of Botany ASCR, Czech Republic, unpubl. res.), injury can be considered as the principal factor affecting adventitious sprouting, which is successful in plants exceeding some site- and species-specific size threshold.
The significant effect of later phenological phases on the degree of adventitious sprouting might be explained by the longer time available for accumulation of disturbance events as proposed in the Introduction. This accumulation process, however, did not result in a difference between annuals and biennials. This may be due to the fact that biennial plants occur in less disturbed habitats (Grime, 2001) and are less prone to disturbance during the first year of life due to a prostrate growth form (usually a rosette of leaves; Krumbiegel, 1999). The accumulation process outweighed the decreasing ability to form adventitious buds on the hypocotyle with plant age in annuals and decreasing resprouting success with advanced phenological phases reported in some biennials (Link and Eggers, 1946; Martínková et al., 2004a).
Biennials and upright annuals characterized by strong apical dominance tended to have less numerous but longer adventitious shoots. It is possible that rapid re-establishment of a secondary dominant shoot resumes the role of lateral meristem inhibition (as suggested by Aarssen, 1995). On the other hand, prostrate annuals had more buds and shoots, which indicates lower apical dominance and supports our hypothesis.
Effect of environmental characteristics
Contrary to expectation, environmental characteristics such as light, soil nitrogen, moisture, soil cementation, total cover of the herb layer, temperature and species richness of the community were not found to affect adventitious sprouting of plants in the present dataset; only plant size was responsible for the observed variability. Two important points need to be stressed from this result: (1) compensation for lost biomass and fitness seems to be important for resprouting success of adventitious short-lived sprouters rather than survival per se as hypothesized in the Introduction, because populations of short-lived monocarps are dependent on seed regeneration contrary to perennial polycarpic species; and (2) benign conditions, especially higher nitrogen availability, were probably counter-balanced by higher competition and thus did not lead to a larger size of target plants and consequently to their enhanced survival and resprouting. Whether the effect of plant size is removed from the analysis or not, the effect of environmental conditions is non-significant, which suggests that the experimentally shown effect of nutrients on resprouting in short-lived adventitious sprouters (Martínková et al., 2004a, b) or axillary sprouters (Benner, 1988; Huhta et al., 2000a, b) was due to larger plant size. This view is supported also by the fact that, in studies where there are contrasting results for the relationship between nutrient status and resprouting from roots (Klimeš and Klimešová, 1999; Klimešová et al., 2009; Latzel and Klimešová, 2009), plant size was controlled for.
CONCLUSIONS
Field assessment suggests that the potential bud bank on the hypocotyle and roots of annual and biennial herbs supports vegetative regeneration of injured plants in natural populations. This result indicates that adventitious bud formation is a functional trait in the studied plant species and should not be considered only as a teratological feature. This finding raises many questions about the ecology and evolution of this trait. For example, what are the consequences of potential bud bank formation for plant distribution and occurrence in different communities or crop cultures with specific types of disturbance? Is there an evolutionary trade-off between the potential bud bank providing persistence after disturbance and seed traits, such as seed dispersion method and longevity of the seed bank? How does adventitious sprouting affect the allometry of annual and biennial species? How does adventitious sprouting contribute to compensation of plant body damage? What are the costs of adventitious bud formation?
That some annuals and biennials possess a potential bud bank implies that this feature should be considered not only in ecological studies, but also in the management of weedy and invasive plants, because mechanical disturbance instead of eradication can lead to vegetative regeneration.
ACKNOWLEDGEMENTS
We are indebted to Keith Edwards for linguistic correction. Vít Latzel and two anonymous referees provided valuable comments on previous versions of the manuscript. This work was supported by the Grant Agency of the Czech Republic (GD206/08/H044), Academy of Sciences of the Czech Republic (AV 0Z60050516) to L.M. and J.K., and by the Czech Ministry of Education (MSM-6007665801) to P.S.
LITERATURE CITED
- Aarssen LW. Hypotheses for the evolution of apical dominance in plants: implications for the interpretation of overcompensation. Oikos. 1995;74:149–156. [Google Scholar]
- Beijerinck MW. Wurzelknospen und Nebenwurzeln. Verhanelingen der Koninklijke Akademie van Wetenschappen. 1887;25/3:1–150. [Google Scholar]
- Bellingham PJ, Sparrow AD. Resprouting as a life history strategy in plant communities. Oikos. 2000;98:409–416. [Google Scholar]
- Benner BL. Effect of apex removal and nutrient supplementation on branching and seed production in Thlaspi arvense (Brassicaceae) American Journal of Botany. 1988;75:645–651. doi: 10.1002/j.1537-2197.1988.tb13487.x. [DOI] [PubMed] [Google Scholar]
- Boege K, Marquis RJ. Facing herbivory as you grow up: the ontogeny of resistance in plants. Trends in Ecology and Evolution. 2005;20:441–448. doi: 10.1016/j.tree.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Bonser SP, Aarssen LW. Meristem allocation: a new classification theory for adaptive strategies in herbaceous plants. Oikos. 1996;77:347–352. [Google Scholar]
- Buhk C, Meyn A, Jentsch A. The challenge of plant regeneration after fire in the Mediterranean Basin: scientific gaps in our knowledge on plant strategies and evolution of traits. Plant Ecology. 2007;192:1–19. [Google Scholar]
- Chiariello N, Roughgarden J. Storage allocation in seasonal races of an annual plant: optimal versus actual allocation. Ecology. 1984;65:1290–1301. [Google Scholar]
- Clark DD, Burk JH. Resource allocation patterns of two California-Sonoran desert ephemerals. Oecologia. 1980;46:86–91. doi: 10.1007/BF00346971. [DOI] [PubMed] [Google Scholar]
- Clarke PJ, Knox KJE, Wills KE, Campbell M. Landscape patterns of woody plant response to crown fire: disturbance and productivity influence sprouting ability. Journal of Ecology. 2005;93:544–555. [Google Scholar]
- Dina SJ, Klikoff LG. Carbohydrate cycle of Plantago insularis var. fastigiata, a winter annual from the Sonoran desert. Botanical Gazette. 1974;135:13–18. [Google Scholar]
- Duffy NM, Bonser SP, Aarssen LW. Patterns of variation in meristem allocation across genotypes and species in monocarpic Brassicaceae. Oikos. 1999;84:284–292. [Google Scholar]
- Desdevises Y, Legendre P, Azouzi L, Morand S. Quantifying phylogenetically-structured environmental variation. Evolution. 2003;57:2647–2652. doi: 10.1111/j.0014-3820.2003.tb01508.x. [DOI] [PubMed] [Google Scholar]
- Eggers V. Influence of carbohydrate and nitrate-nitrogen nutrition on development of hypocotydonary buds in flax. Botanical Gazette. 1946;107:385–390. [Google Scholar]
- Ellenberg H. Vegetation ecology of central Europe. 4th edn. Cambridge: Cambridge University Press; 1986. [Google Scholar]
- Ferraro DO, Oesterheld M. Effect of defoliation on grass growth. A quantitative review. Oikos. 2002;98:125–133. [Google Scholar]
- Grime JP. Plant strategies, vegetation processes and ecosystem properties. 2001 Chichester: John Wiley & Sons. [Google Scholar]
- Hellström K, Rautio P, Huhta AP, Tuomi J. Tolerance of an annual hemiparasite, Euphrasia stricta agg., to simulated grazing in relation to the host environment. Flora. 2004;199:247–255. [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics. 1979;6:65–70. [Google Scholar]
- Holm T. On the development of buds upon roots and leaves. Annals of Botany. 1925;39:867–881. [Google Scholar]
- Huhta AP, Hellström K, Rautio P, Tuomi J. A test of the compensatory continuum: fertilization increases and below-ground competition decreases the grazing tolerance of tall wormseed mustard (Erysimum strictum) Evolutionary Ecology. 2000a;14:353–372. [Google Scholar]
- Huhta AP, Lennartsson T, Tuomi J, Rautio P, Laine K. Tolerance of Gentianella campestris in relation to damage intensity: an interplay between apical dominance and herbivory. Evolutionary Ecology. 2000b;14:373–392. [Google Scholar]
- Huhta AP, Tuomi J, Rautio P. Cost of apical dominance in two monocarpic herbs, Erysimum strictum and Rhinanthus minor. Canadian Journal of Botany. 2000c;78:591–599. [Google Scholar]
- Huhta AP, Hellström K, Rautio P, Tuomi J. Grazing tolerance of Gentianella amarella and other monocarpic herbs: why is tolerance highest at low damage levels? Plant Ecology. 2003;166:49–61. [Google Scholar]
- Iwasa Y, Kubo T. Optimal size of storage for recovery after unpredictable disturbances. Evolutionary Ecology. 1997;11:41–65. [Google Scholar]
- King GE, Eckhart MV, Mohl CE. Magnitudes and mechanism of shoot-damage compensation in annual species of Linum (Linaceae) in Iowa. American Midland Naturalist. 2008;159:200–213. [Google Scholar]
- Klimeš L, Klimešová J. Root sprouting in Rumex acetosella under different nutrient levels. Plant Ecology. 1999;141:33–39. [Google Scholar]
- Klimešová J. Monokarpické rostliny schopné přežít silnou disturbanci (Monocarpic plants surviving severe disturbance) Zprávy České Botanické Společnosti, Praha, 38, Materiály. 2003;19:37–48. [Google Scholar]
- Klimešová J, de Bello F. CLO-PLA: the database of clonal and bud bank traits of Central European flora. Journal of Vegetation Science. 2009;20:511–516. [Google Scholar]
- Klimešová J, Klimeš L. Resprouting of herbs in disturbed habitats: it is adequately described by Bellingham-Sparow's model? Oikos. 2003;103:225–229. [Google Scholar]
- Klimešová J, Klimeš L. CLO-PLA3–the database of clonal and bud bank traits of Central European flora. 2006 doi: 10.1002/ecy.1745. http://clopla.butbn.cas.cz . [DOI] [PubMed] [Google Scholar]
- Klimešová J, Klimeš L. Bud banks and their role in vegetative regeneration–a literature review and proposal for simple classification and assessment. Perspectives in Plant Ecology Evolution and Systematics. 2007;8:115–129. [Google Scholar]
- Klimešová J, Martínková J. Intermediate growth forms as a model for the study of plants clonality functioning: an example with root sprouters. Evolutionary Ecology. 2004;18:669–681. [Google Scholar]
- Klimešová J, Sosnová M, Martínková J. Life-history variation in the short-lived herb Rorippa palustris: effects of germination date and injury timing. Plant Ecology. 2007;189:237–246. [Google Scholar]
- Klimešová J, Kociánová A, Martínková J. Weeds that can do both tricks: vegetative versus generative regeneration of short-lived root-sprouting herbs Rorippa palustris and Barbarea vulgaris. Weed Research. 2008;48:131–135. [Google Scholar]
- Klimešová J, Pokorná A, Klimeš L. Establishment growth and bud bank formation in Epilobium angustifolium: the effects of nutrient availability, plant injury and environmental heterogeneity. Botany. 2009;87:195–201. [Google Scholar]
- Knox KJE, Clarke PJ. Nutrient availability induces contrasting allocation and starch formation in resprouting and obligate seeding shrubs. Functional Ecology. 2005;19:690–698. [Google Scholar]
- Krumbiegel A. Growth forms of annual vascular plants in central Europe. Nordic Journal of Botany. 1998;18:563–576. [Google Scholar]
- Krumbiegel A. Growth forms of biennial and pluriennial vascular plants in central Europe. Nordic Journal of Botany. 1999;19:217–226. [Google Scholar]
- Latzel V, Klimešová J. Fitness of resprouters versus seeders in relation to nutrient availability in two Plantago species. Acta Oecologica. 2009;35:541–547. [Google Scholar]
- Latzel V, Dospělová L, Klimešová J. Annuals sprouting adventitiously from the hypocotyl: their compensatory growth and implications for weed management. Biológia. 2009;64:923–929. [Google Scholar]
- Lennartsson T, Tuomi J, Nilsson P. Evidence for an evolutionary history of overcompensation in the grassland biennial Gentianella campestris (Gentianaceae) American Naturalist. 1997;149:1147–1155. doi: 10.1086/286043. [DOI] [PubMed] [Google Scholar]
- Lennartsson T, Nilsson P, Tuomi J. Induction of overcompensation in the field gentian, Gentianella campestris. Ecology. 1998;79:1061–1072. [Google Scholar]
- Link GKK, Eggers V. Mode, site and time of initiation of hypocotyledonary bud primordia in Linum ussitatissimum. Botanical Gazette. 1946;107:441–454. [Google Scholar]
- van der Maarel E. Transformation of cover-abundance values for appropriate numerical treatment–Alternatives to the proposals by Podani. Journal of Vegetation Science. 2007;18:767–770. [Google Scholar]
- MacDonald MA, Cavers PB. Cauline rosettes–an asexual means of reproduction and dispersal occurring after seed formation in Barbarea vulgaris (yellow rocket) Canadian Journal of Botany. 1974;52:913–918. [Google Scholar]
- Martínková J, Klimešová J, Mihulka S. Resprouting after disturbance: an experimental study with short-lived monocarpic herbs. Folia Geobotanica. 2004a;39:1–12. [Google Scholar]
- Martínková J, Kočvarová M, Klimešová J. Resprouting after disturbance in the short-lived herb Rorippa palustris (Brassicaceae): an experiment with juveniles. Acta Oecologica. 2004b;25:143–150. [Google Scholar]
- Martínková J, Klimešová J, Mihulka S. Vegetative regeneration of biennial Oenothera species after a disturbance: field observations and an experimental study. Flora. 2006;201:287–297. [Google Scholar]
- Martínková J, Klimešová J, Mihulka S. Compensation of seed production after severe injury in the short-lived herb Barbarea vulgaris. Basic Applied Ecology. 2008;9:44–54. [Google Scholar]
- McPhee CS, Bonser SP, Aarssen LW. The role of apical dominance in the interpretation of adaptive architecture in prostrate plant species. Ecoscience. 1997;4:490–500. [Google Scholar]
- Midgley JJ. Why the world's vegetation is not totally dominated by resprouting plants; because resprouters are shorter than reseeders. Ecography. 1996;19:92–95. [Google Scholar]
- Otzen D. Life forms of three Senecio species in relation to accumulation and utilization of non-structural carbohydrates. Acta Botanica Neerlandica. 1977;26:401–409. [Google Scholar]
- Paige KN. Regrowth following ungulate herbivory in Ipomopsis aggregata: geographic evidence for overcompensation. Oecologia. 1999;118:316–323. doi: 10.1007/s004420050732. [DOI] [PubMed] [Google Scholar]
- Penzig O. Pflanzen Teratologie. 2nd edn. Berlin: Verlag von Gebrüder Borntraeger; 1921–1922. [Google Scholar]
- Piippo S, Huhta AP, Rautio P, Tuomi J. Resource availability at the rosette stage and apical dominance in the strictly biennial Erysimum strictum (Brassicaceae) Canadian Journal of Botany. 2005;83:405–412. [Google Scholar]
- Piippo S, Hellstrom K, Huhta AP, Rautio P, Tuomi J. Delayed flowering as a potential benefit-decreasing cost of compensatory regrowth. Botany. 2009;87:837–844. [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2008. http://www.R-project.org . [Google Scholar]
- Rauh W. Die Bildung von Hypocotyl- und Wurzelsprossen und ihre Bedeutung fur die Wuchsformen der Phlanzen. Nova Acta Leopoldiana. 1937;4/24:395–553. [Google Scholar]
- Rautio P, Huhta AP, Piippo S, et al. Overcompensation and adaptive plasticity of apical dominance in Erysimum strictum (Brassicaceae) in response to simulated browsing and resource availability. Oikos. 2005;111:179–191. [Google Scholar]
- Reichardt HW. Beiträge zur Kentniss hypokotylischer Adventivknospen und Wurzelsprosse bei krautigen Dikotylen. Verhandlungen der Zoologisch-botanischen Vereins in Wien. 1857;7:235–244. [Google Scholar]
- Sosnová M, Klimešová J. Storage of carbon in relation to life history mode and resprouting ability in short-lived root-sprouter Rorippa palustris. Acta Oecologica. 2009;5:691–697. [Google Scholar]
- Stearns SC. The evolution of life histories. Oxford: Oxford University Press; 1992. [Google Scholar]
- Vilela A, Cariaga R, Gonzalez-Paleo L, Ravetta D. Trade-offs between reproductive allocation and storage in species of Oenothera L. (Onagraceae) native to Argentina. Acta Oecologica. 2008;33:85–92. [Google Scholar]
- Wise MJ, Abrahamson WG. Effects of resource availability on tolerance of herbivory: a review and assessment of three opposing models. American Naturalist. 2007;169:443–454. doi: 10.1086/512044. [DOI] [PubMed] [Google Scholar]
- Wittrock VB. Ueber Wurzelsprossen bei krautigarten Gewächsen, mit besonderer Rücksicht auf ihre verschiedene biologische Bedeutung. Botanisches Centralblatt. 1884;17/8:227–232. 17/9: 257–264. [Google Scholar]
- Wydler H. Über subcotyledonare Sprossbildung. Flora. 1850;8/22:337–338. [Google Scholar]