Abstract
Background and Aims
There is a need to evaluate the salt tolerance of plant species that can be cultivated as crops under saline conditions. Crambe maritima is a coastal plant, usually occurring on the driftline, with potential use as a vegetable crop. The aim of this experiment was to determine the growth response of Crambe maritima to various levels of airborne and soil-borne salinity and the ecophysiological mechanisms underlying these responses.
Methods
In the greenhouse, plants were exposed to salt spray (400 mm NaCl) as well as to various levels of root-zone salinity (RZS) of 0, 50, 100, 200 and 300 mm NaCl during 40 d. The salt tolerance of Crambe maritima was assessed by the relative growth rate (RGR) and its components. To study possible salinity effects on the tissue and cellular level, the leaf succulence, tissue Na+ concentrations, Na+ : K+ ratio, net K+/Na+ selectivity, N, P, K+, Ca2+, Mg2+, proline, soluble sugar concentrations, osmotic potential, total phenolics and antioxidant capacity were measured.
Key Results
Salt spray did not affect the RGR of Crambe maritima. However, leaf thickness and leaf succulence increased with salt spray. Root zone salinities up to 100 mm NaCl did not affect growth. However, at 200 mm NaCl RZS the RGR was reduced by 41 % compared with the control and by 56 % at 300 mm NaCl RZS. The reduced RGR with increasing RZS was largely due to the reduced specific leaf area, which was caused by increased leaf succulence as well as by increased leaf dry matter content. No changes in unit leaf rate were observed but increased RZS resulted in increased Na+ and proline concentrations, reduced K+, Ca2+ and Mg2+ concentrations, lower osmotic potential and increased antioxidant capacity. Proline concentrations of the leaves correlated strongly (r = 0·95) with RZS concentrations and not with plant growth.
Conclusions
Based on its growth response, Crambe maritima can be classified as a salt spray tolerant plant that is sensitive to root zone salinities exceeding 100 mm NaCl.
Keywords: Crambe maritima, halophyte, salt tolerance, ecophysiology, salt spray, root-zone salinity, relative growth rate, specific leaf area, leaf succulence, Na+, K+, osmotic potential, proline, phenolics, antioxidant capacity
INTRODUCTION
While fresh water is already a scarce resource in many countries, water shortage will increase in the near future, mainly due to the growing world population and rising prosperity (FAO, 2004; FAO and IFAD, 2006; Alcamo et al., 2007). Irrigation represents about 70 % of all water uses at present, but domestic use will claim an increasing part, resulting in lower fresh-water availability for agriculture (FAO and IFAD, 2006; Alcamo et al., 2007). Together with the ongoing global salinization, with 7 % of the world's total land area affected by salt and about 20 % of the irrigated land affected (Szabolcs, 1994; Yeo, 1999; Smedema and Shiati, 2002), this emphasizes the importance of exploring the use of brackish water and seawater for agricultural purposes (Rozema and Flowers, 2008). A promising approach is the domestication of halophytes to be used as salt-tolerant crops to adapt to the global salinity problem (Glenn et al., 1999; Lieth et al., 1999).
Crambe maritima or seakale, belonging to the Brassicaceae family, is such a potential salt-tolerant crop. It has been known as an edible plant for at least 300 years (Horwood, 1919). It is a long-lived perennial herb with an extensive, deep root system (up to 200 cm) and an underground stem resembling a tap root (Scott and Randall, 1976), referred to as tap root from here on in (for more details, see Péron, 1990). Leaves are covered with a thick waxy layer, which acts as an efficient water-repellent (Scott and Randall, 1976). At the end of the growing season the leaves and inflorescences die off, leaving only the underground root system and the leafless bud or tap root during the winter. When C. maritima is grown as a vegetable crop, etiolated sprouts are grown from this tap root, which are regarded as a highly prized and tasty vegetable (Péron, 1990; Briard et al., 2002).
Crambe maritima is mostly confined to coastal habitats with well-drained soils in north-west Europe (Clapham et al., 1962; Scott and Randall, 1976), a habitat occupied by only a few species. This is ascribed to considerable levels of airborne (Wells and Shunk, 1938; Rozema et al., 1982) and soil-borne salinity (Lee and Ignaciuk, 1985; Barbour et al., 1985), high substrate mobility, sand blasting and burial, low water-holding capacity and a low nutrient status of the substrate, except in the vicinity of detrital deposits (Boyce, 1954; Lee et al., 1983; Pakeman, 1990). This environmental variability of the habitat possibly explains the recorded variability in germination (Baron and Binet, 1964; Walmsley and Davy, 1997; Fusheng et al., 1998) and the great phenotypic and genetic variability within and between populations of C. maritima (Briard et al., 2002). Shingle beaches seem to be the preferred habitat of C. maritima (Scott and Randall, 1976). In The Netherlands establishment only occurred after construction of the basalt-stone sea dikes in the 1950s (Mennema et al., 1985). These dikes resemble an artificial pebble beach habitat. Since that time an explosive extension of C. maritima has been recorded, with the majority of the findings occurring on sea dikes (Mennema et al., 1985; Van der Meijden, 2005). Crambe maritima often occurs on the driftline (Clapham et al., 1962; Scott and Randall, 1976).
Plant species that occur on the driftline have rarely been evaluated for their salt tolerance and potential for saline agriculture. The driftline, or strandline, is the line immediately above high water and is characterized by deposition of drift material. This line is out of reach of the average high tide, but equinoctial tides or storms can increase soil salinity to a level that will affect the growth of most plant species (Ignaciuk and Lee, 1980; Barbour et al., 1985; Erickson and Young, 1995). The soil at this leading edge of the vegetation generally contains higher concentrations of salt than does soil further inland (De Jong, 1979; Greaver and Sternberg, 2007). So although C. maritima usually grows on well-drained soils where the salts can readily pass through the root zone, soil salinity levels can be elevated periodically and possibly it is able to cope with saline conditions in the root zone during such periods.
Salt spray is an important natural selective abiotic factor on coastal plant communities (Wells and Shunk, 1938; Boyce, 1954; Barbour, 1978; Rozema et al., 1982, 1983). Different levels of salt-spray tolerance can result in vegetation zonation; plants adapted to salt spray grow close to the ocean and are replaced by less salt-resistant plants further inland (Oosting and Billings, 1942; Oosting, 1945; Barbour, 1978; Yura, 1997). The salt-spray intensity at herbaceous plant height can range between 1 and 200 mg NaCl dm−2 leaf area d−1, with typical values between 10 and 50 mg NaCl dm−2 d−1 (Barbour, 1978; Griffiths, 2006). The salt may enter the aerial organs of the plants, especially where small surface injuries are present (Boyce, 1954), where it can disrupt the water balance of plants (Munns, 1993), causes necrosis or loss of leaves (Karschon, 1958) and leads to growth reduction (Tominaga et al., 1991). Because C. maritima grows close to the ocean, where it is exposed to considerable salt spray levels, it is expected to be adapted to salt spray.
The salt tolerance of a plant species is usually determined by assessing their growth performance under soil-borne salinity (Maas and Hoffman, 1977; Breckle, 2002). To understand the underlying principles of the salt tolerance of a given species, it is necessary to unravel the plant response on the cellular, tissue and whole-plant level (Flowers et al., 1977; Mansour, 2000; Flowers, 2004; Munns and Tester, 2008). The mechanisms of salt tolerance are many and plants growing naturally on saline soils have evolved various mechanisms to cope with salinity (Flowers et al., 1977; Zhu, 2001; Munns, 2001; Munns et al., 2002; Breckle, 2002; Ashraf and Harris, 2004; Parida and Das, 2005). In general, the tolerance of all halophytes to ionic as well as osmotic stress relies on controlled uptake, increased extrusion and compartmentalization of salts (Flowers et al., 1977; Zhu, 2001; Flowers and Colmer, 2008). The initial growth reduction is caused by the osmotic effect of salt outside the roots, and the subsequent growth reduction due to ionic stress is caused by the inability to prevent salt from reaching toxic levels in the transpiring leaves (Munns, 2005). As a consequence of these primary effects, secondary stresses such as oxidative damage and nutritional imbalance often occur (Zhu, 2001), also affecting plant growth. Halophytes, defined as plants that survive to reproduce in environments where the salt concentration is around 200 mm NaCl or more (Flowers and Colmer, 2008), are adapted to cope with these potential stresses on the whole plant, tissue and cellular level.
The objective of this study was to determine the effect of airborne and soil-borne salinity on the growth of C. maritima and to obtain an insight in the morphological and physiological adaptations underlying these growth responses. The species studied is a coastal plant and a potential crop for saline agriculture. In a first experiment, seedlings were exposed to salt spray to determine the effect of airborne salinity on plant growth and leaf thickness. In a second experiment, plants were exposed to airborne as well as soil-borne salinity and various responses at the whole plant and tissue levels were studied.
MATERIALS AND METHODS
Salt-spray experiment
Seeds of Crambe maritima were collected in Normandy, France in 2005 and stored dry at room temperature. Seeds were germinated in March 2006 on peat soil (seed plot soil; Jongkind, Aalsmeer, The Netherlands) and transplanted to individual pots also containing peat soil (potting soil No. 5; Jongkind). Seedlings were randomly assigned to either the salt-spray treatment or the control treatment. The experiment was initiated 4 weeks after germination started, with one group sprayed with seawater with a concentration of 400 mm NaCl [measured as electrical conductivity (dS m−1) and calculated as mm NaCl by means of a calibration curve] collected prior to the experiment at Zandvoort, The Netherlands (52°23′N 4°32′E) and one group sprayed with demineralized water as a control. Plants were sprayed with a fine mist of seawater four times per day, which equalled on average 160 mg NaCl dm−2 leaf area d−1, the controls were similarly sprayed four times per day with demineralized water. Plants were sprayed every 2·5 h between 0900 h and 1630 h. Plants were not sprayed at the weekend or during the holidays. Leaf length, number of leaves and leaf thickness were measured 8 weeks after germination, corresponding to 19 d of actual salt-spray treatment. These measurements were repeated 13 weeks after germination, corresponding to 45 d of actual salt spray. In addition, 11 weeks after germination with 34 d of salt spray the leaf thickness of the second youngest leaf was measured and harvested to obtain fresh weight and leaf area to calculate leaf succulence expressed as LFW/LA. The thickness of each leaf was measured manually between the veins at the tip of the leaf, with an analogue thickness gauge (No. 2046-08, accuracy 0·01 mm; Mitutoyo, Tokyo, Japan). Plants were watered with tap water during the experiment and grown under the same environmental conditions as the second experiment.
Salt-tolerance experiment
Seeds were collected in September 2007 from a large population of C. maritima located on the Afsluitdijk (52°58′N 5°06′E) in The Netherlands, and stored dry at room temperature. Prior to germination seeds were placed on moist vermiculite in a closed plastic bag for 58 d at 5 °C to break any potential dormancy and obtain uniform germination. After removal of the pericarp, seeds were placed on a Petri dish with filter paper (Whatman Grade 597) saturated with demineralized water for germination, which was defined by a visible, emerging radicle. Seeds that germinated within 9 d were transplanted into 4-L polyethylene pots filled with 2–5 mm gravel, after which seedlings were allowed to grow for another 17 d. After this period only plants with a visible, second true leaf (<2 cm in length) were used for the experiment so that the initial population was as uniform as possible. Plants were randomly divided into seven treatment groups consisting of five different root-zone salinity (RZS) treatments (0, 50, 100, 200 and 300 mm NaCl added to the nutrient solution), one salt-spray treatment (root zone with 0 mm NaCl) which was sprayed twice a day with seawater (similar to the salt-spray experiment) with an average of 80 mg NaCl dm−2 d−1, and one control treatment sprayed with demineralized water as a control. The effects of salt spray and RZS were analysed separately. The initial harvest consisted of five plants per treatment group just before salt addition started, 26 d after initial germination. This initial harvest was used to test for uniformity of the population at the start of the experiment and for calculations of relative growth rate (RGR). Plants were sprayed at 1000 h and 1600 h. Plants were not sprayed at the weekend or during holidays. Each pot contained one plant. Salt concentration was increased stepwise (50 mm NaCl per day) to avoid an osmotic shock. Plants were grown in a closed gravel/hydroponic system (Koyro, 2003), which was set up in a randomized block design (randomized weekly). The experiment was performed in April/May 2008 in a greenhouse with controlled temperature, humidity and light conditions: 22 ± 2/16 ± 2 °C day/night, relative humidity 60/80 % day/night, light intensity 300 PAR (μmol m−2 s−1) at plant level, 14 h light d−1. Nutrient solution, composed of 3 mm KNO3, 2 mm Ca(NO3)2, 1 mm NH4H2PO4, 0·5 mm MgSO4, 20 µm Fe(Na)-EDTA, 1 µm KCl, 25 µm H3BO3, 2 µm MnSO4, 2 µm ZnSO4, 0·1 µm CuSO4 and 0·1 µm (NH4)6Mo7O24 in demineralized water, buffered with 2 mm MES, pH 6·0, adjusted with KOH, was supplied by continuous drip-irrigation and surplus water drained back to each of the individual 50-L central reservoirs. Nutrient solutions were replaced every 2 weeks. The final harvest occurred 40 d after salt addition was started and five plants per treatment were harvested.
Sample preparation
Plants were carefully uprooted, washed with running demineralized water for 1 min and blotted dry and the fresh weight of the root, tap root, leaf stems and leaves were recorded. Following this, total leaf area was measured with a LI-COR 3100 (Li-Cor Inc., Lincoln, NE, USA) leaf area meter and expressed as cm2 plant−1. Leaves were separated into old and new leaves, with the first two true leaves as the old leaves and from the third true leaf onwards as new leaves. Directly after measuring leaf area, the leaves were frozen in liquid nitrogen and freeze-dried for 1 week. The rest of the plant material was oven-dried at 70 °C for 48 h. Dried plant material was ground to a fine powder using a ball-mill (MM200; Retsch GmbH, Haan, Germany). The freeze-dried leaves were used to analyse nutrient composition, total phenolics, antioxidant capacity, proline and soluble sugars. Roots were analysed for nutrient composition only.
Measurement of morphological parameters and RGR
The growth performance of C. maritima under airborne and soil-borne salinity was used to evaluate the salt tolerance. The specific leaf area (SLA), LWF, leaf succulence, leaf dry matter content (LDMC) and leaf water content (LWC) were determined to study the possible morphological adaptations. The RGR and the components unit leaf rate (ULR), LWF and SLA were estimated according to Hunt et al. (2002):
| 1 |
with
| 2 |
| 3 |
| 4 |
where Δ refers to the difference between values at the final and initial harvest, t is the salt treatment duration (days), W is the whole plant dry weight (g), LDW is the total leaf dry weight (g), and (loge)LA is the (natural logarithmic) value of leaf area (cm2). The components leaf density (LD) and leaf thickness (LTh) increase linearly with the inverse of SLA (Poorter and Garnier, 2007):
| 5 |
Leaf density was estimated by calculating LDMC because LD ≈ LDMC (Garnier and Laurent, 1994; Wilson et al., 1999), and LDMC = LDW/LFW (Garnier et al., 2001), with LDW as leaf dry weight (g) and LFW as leaf fresh weight (g).
Leaf thickness (LTh) can be estimated as:
| 6 |
according to Vile et al. (2005). This leaf thickness can also be expressed as leaf succulence because (SLA × LDMC)−1 = (LA/LDW × LDW/LFW)−1 = LFW/LA, and LFW/LA is often used as an estimate of leaf succulence (Jennings, 1976; Agarie et al., 2007). In this paper, leaf succulence is calculated as
| 7 |
with LFW as leaf fresh weight (g) and LA as leaf area (cm2).
Leaf water content was estimated according to the commonly used formula
| 8 |
with LFW as leaf fresh weight (g) and LDW as leaf dry weight (g).
Nutrient composition
Total C and N concentrations were determined by dry combustion with an elemental analyser (Perkin Elmer 2400 Series II; Wellesley, MA, USA). Samples for measuring total P, Ca2+, Mg2+, Na+ and K+ were prepared by digesting 100 mg plant material in 2 mL 37 % (v/v) HCl : 65 % (v/v) HNO3 (1/4, v/v) in a Teflon cylinder for 6 h at 140 °C, after which the volume was adjusted to 10 mL with demineralized water. Total P concentrations of the samples were determined using molybdenum blue as reagent, measured at 880 nm on a spectrophotometer (Shimadzu UV-1601PC, Japan) (Murphy and Riley, 1962). The Ca2+, Mg2+, Na+ and K+ concentrations were determined on a flame atomic absorption spectrophotometer (Perkin-Elmer 1100B; Perkin Elmer Inc., Waltham, MA, USA). Chloride was measured only in the supernatant of the extract of the osmotic potential, with a Sherwood Chloride Analyser 926 (Sherwood Scientific Ltd, Cambridge, UK). Samples were measured in duplicate. The net selectivity of ion accumulation for K+ over Na+ was estimated from ion contents using the commonly used formula
| 9 |
Osmolality and osmotic potential
Plant samples were frozen and stored at −18 °C directly after harvest. Leaf discs were collected from the centre parts of fresh leaves of the whole plant, the frozen plant tissues were put into a syringe to thaw after which leaf sap was extracted. Of the supernatant, 10 µL were utilized to determine osmolality. Osmolality was measured using a vapour pressure osmometer (5500; Wescor Inc., Logan, UT, USA). Samples were measured in duplicate. The osmotic potential was determined according to the Van't Hoff equation:
| 10 |
Ion contribution to plant osmotic potential was determined according to the Van't Hoff relationship:
| 11 |
with C = molarity of the solution (mol of solute kg−1 H2O), R = gas constant (0·00831 kg mol−1 K−1) and T = temperature (in K).
Soluble sugars
Soluble sugars were determined according to the anthrone method (Yemm and Willis, 1954). For this, 40 mg plant material was mixed with 5 mL 80 % ethanol and centrifuged at 4000 g for 10 min. This was repeated twice and the supernatants were combined and demineralized water was added to a total volume of 25 mL. Of this, 250 µL was added to 2·5 mL anthrone reagent (400 mg anthrone in 6 mL 96 % ethanol, 60 mL demineralized water and 200 mL concentrated H2SO4). Samples were placed in a water bath for 7 min at 100 °C. After the samples cooled down to room temperature the absorbance was measured at 625 nm using a spectrophotometer (UV-1601PC; Shimadzu, Kyoto, Japan). Sucrose was used to make the standard curve and results are expressed in μmol equivalent sucrose g−1 d. wt. Samples were measured in triplicate.
Proline
The proline content was measured according to the method of Bates et al. (1973). Approximately 20 mg plant material was mixed with 2 mL 3 % sulfosalicylic acid and centrifuged for 10 min at 4000 g. Of the supernatant, 0·5 mL was mixed with 0·5 mL ninhydrine reagent and 0·5 mL glacial acetic acid, and placed in a water bath for 1 h at 100 °C. After termination of the reaction in ice water, 1·0 mL toluene was added to extract the reaction mixture. The absorbance was measured at 520 nm using a spectrophotometer (LKB-Ultrospec3; Pharmacia, Cambridge, UK). Proline was used to make the standard curve and results are expressed in μmol proline g−1 d. wt. Samples were measured in triplicate.
Total phenolics
The amount of total phenolics in the leaf tissue was determined by means of the Folin–Ciocalteu method (Waterman and Mole, 1994). The phenolic compounds were extracted by mixing 30 mg plant material with 5 mL 50 % methanol, placed in a water bath for 1 h at 40 °C and centrifuged at 4000 g for 5 min. Of this, 100 µL were mixed with 3·90 mL demineralized water and 250 of μL reagent were added. After 8 min 750 µL of sodium bicarbonate solution were added to stop the reaction. After 1 h the absorbance was measured at 760 nm using a spectrophotometer (UV-1601PC; Shimadzu). Tannic acid was used as a standard and results are expressed in mg equivalent tanin g−1 d. wt. Samples were measured in duplicate.
Antioxidant capacity
Total antioxidant capacity was measured according to Re et al. (1999). For this method the preformed radical monocation of 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS+) is generated by oxidation of ABTS with potassium persulfate and is reduced in the presence of both lipophilic and hydrophilic antioxidants. This results in a decolorization which can be measured using a spectrophotometer. The extraction was made by mixing 30 mg plant material with 5 mL of 50 % methanol, placed in a water bath for 1 h at 40 °C and centrifuged at 4000 g for 5 min. Of the supernatant 30 µL was mixed with 2970 µL of diluted ABTS+ reagent. After 30 min the absorbance was measured at 734 nm using a spectrophotometer (UV-1601PC; Shimadzu). Trolox was used as a standard and results are expressed in mg trolox equivalent antioxidant capacity (TEAC) g−1 d. wt. Samples were measured in triplicate.
Statistical analysis
Data were analysed using the statistical program SPSS 15·0 for Windows (SPSS Inc., Chicago, IL, USA). Prior to statistical analysis, normality and homogeneity assumptions of the test were checked both by visual inspection of the residuals for undesired patterns and by Levene's test for homogeneity of variances. One-way ANOVAs were performed to test for differences between salt treatments for the different parameters. When necessary, values were log-transformed prior to analysis or evaluated by nonparametric tests. When significant differences between means were found, Tukey's multiple range test was used to perform post hoc pair-wise comparisons between individual treatments. Linear regression was used to test for relationships between variables. Possible significant differences between treatments in leaf thickness in time and within treatments between old and new leaf Na+ concentrations of the plant were evaluated by repeated measures ANOVA with Bonferroni adjusted pair-wise comparisons. In the salt-tolerance experiment the effects of salt spray and RZS were analysed separately as well as all treatments together which gave similar results. To obtain normality and homogeneity of the Na+ concentration of roots, old and new leaves, only differences between salt-exposed treatments were evaluated in the statistical analysis.
RESULTS
Salt-spray experiment
Leaf length and number of leaves per plant of 8-week-old and 13-week-old plants were not affected during 19 d or 45 d of salt spray, respectively (Table 1). The differences in number of leaves and leaf length between week 8 and week 13 were minimal, indicating that growth in the first 8 weeks was greater than that in weeks 8–13. Mean leaf thickness of the salt spray plants showed a significant increase in week 8, week 11 and week 13 as compared with the control treatment. A repeated measures ANOVA showed that there was no significant difference in leaf thickness for weeks 8, 11 and 13 (F = 0·004, P = 0·996) as a result of salt spray.
Table 1.
Mean leaf length, number of leaves and leaf thickness of Crambe maritima plants, 8 and 13 weeks after germination, measured non-destructively and leaf thickness plus leaf succulence of the second youngest leaf 11 weeks after germination
| Whole plant, 8 weeks |
Second youngest leaf, 11 weeks |
Whole plant, 13 weeks |
||||||
|---|---|---|---|---|---|---|---|---|
| Treatment | Leaf length (cm) | No. of leaves | Leaf thickness (mm) | Leaf thickness (mm) | Leaf succulence (mg f. wt mm−2) | Leaf length (cm) | No. of leaves | Leaf thickness (mm) |
| Control | 9·7 ± 0·3a | 4·0 ± 0·1a | 0·70 ± 0·02a | 0·67 ± 0·02a | 0·85 ± 0·02a | 11·7 ± 0·3a | 4·8 ± 0·1a | 0·69 ± 0·02a |
| Salt spray | 9·3 ± 0·3a | 3·9 ± 0·2a | 0·78 ± 0·02b | 0·79 ± 0·02b | 0·99 ± 0·02b | 12·4 ± 0·4a | 4·8 ± 0·1a | 0·77 ± 0·02b |
Plants were grown on potting soil. Measurements were performed on the same plants in weeks 8 and 13 with 19 and 45 d of salt spray, respectively. Leaf thickness was measured with a thickness gauge and expressed in millimetres.
Values are means ± s.e. (n = 30). Means in the same column that have the same letter are not significantly different at P < 0·05 (one-way ANOVA).
The second youngest leaf was a good indicator for whole plant leaf thickness because this leaf thickness in week 11 did not significantly differ from the whole plant leaf thickness in weeks 8 and 13. There was a strong positive relationship between leaf thickness and leaf succulence, with leaf thickness = 0·137 + 0·641 × leaf succulence ± 0·085 s.e., with r = 0·77, F = 85·4, P < 0·001, n = 61. Although mean leaf thickness showed a significant increase compared with the control in weeks 8, 11 and 13, this was only in the order of 15 %, 18 % and 12 %, respectively.
Salt-tolerance experiment
Growth
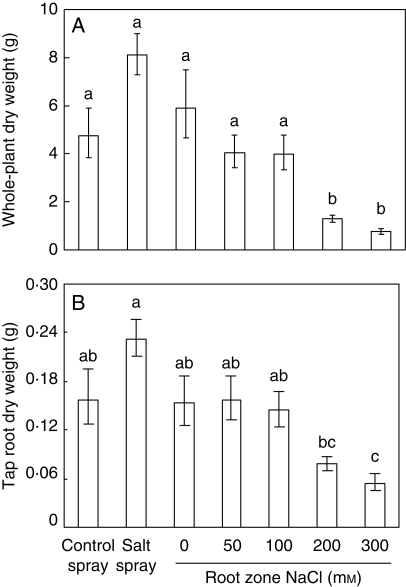
Cold stratification at 5 °C for 58 d resulted in the germination of 41 ± 2 % (mean ± s.e., n = 26 with 20 seeds per Petri dish as n) seeds within 9 d, of which 71 ± 3 % germinated in the last 3 d. The initial harvest prior to salt addition showed no significant differences between treatment groups (one-way ANOVA; F = 0·64, P = 0·70), demonstrating the uniformity of the population at the start of the experiment. The results of the final harvest of the 8-week-old plants, with either 20 d of actual salt-spray treatment or 40 d of RZS treatment, are listed in Fig. 1. The whole-plant dry-weight production (Fig. 1A) of the salt-spray treated plants showed no significant difference between the control and the 0, 50 and 100 mm NaCl RZS treatments. After 40 d of RZS exposure, whole-plant dry-weight production showed no significant difference between the 0, 50 and 100 mm treatments. Compared with the 0 mm RZS treatment the dry-weight production at 200 mm NaCl was reduced by 78 %. No significant difference occurred between the 200 and 300 mm NaCl treatments. Results of mean leaf dry-weight production and mean leaf area showed similar responses as whole plant dry-weight production (data not shown).
Fig. 1.
Dry-weight production of the whole-plant and the tap root of all treatments of the 8-week-old hydroponically grown Crambe maritima plants with 20 d of salt spray or 40 d of root-zone salinity ranging from 0 to 300 mm NaCl. Plants were grown on gravel culture with hydroponics. Values are the means of five replicates ± s.e. Different letters indicate a significant difference at P < 0·05 (Tukey's test).
Tap root dry-weight production (Fig. 1B) of the salt-spray treatment showed no difference between the control treatment and the 0, 50 and 100 mm NaCl RZS treatments. The 0, 50 and 100 mm treatments did not differ from the 200 mm NaCl RZS treatment. No difference occurred between the 200 and 300 mm treatments.
As expected, the RGR (Table 2) showed results similar to the whole-plant dry-weight production with no significant difference between the salt spray and the control spray treatments, as well as the 0, 50 and 100 mm RZS treatments. The RGR decreased 41 % between the 0 and 200 mm RZS NaCl treatment. Although the ULR showed a mean increase at moderate salinities and a decrease at the highest salinities, none of these differences was significant. The only difference occurred between the highest value of ULR (salt-spray treatment) and the lowest value (0 mm NaCl RZS treatment), although the difference in ULR between the 100 and 300 mm NaCl RZS was almost significant (P = 0·08). The LWF showed no differences between treatments. Of the components of RGR, it was SLA that showed the largest decline for both the salt spray and RZS treatments compared with the controls. The SLA of the 100, 200 and 300 mm NaCl RZS treatments significantly differed from the 0 mm treatment and showed a maximum decline of 44 % with increasing salinity. For leaf succulence the control spray treatment only differed from the 200 mm RZS treatment, although the P-values between the control spray and the salt-spray treatment (P = 0·051) and the 100 mm RZS (P = 0·054) almost showed significant differences. Within the LDMC only the 300 mm treatment showed an increase, similar to the LWC which was only affected at the 300 mm NaCl RZS level.
Table 2.
Relative growth rate and its components and leaf water content (LWC) of all treatments of the 8-week-old Crambe maritima plants with 20 d of salt spray or 40 d of root-zone salinity ranging from 0 to 300 mm NaCl
| Treatment | RGR (mg g−1 d−1) | ULR (mg cm−2 d−1) | LWF (g g−1) | SLA (cm2 g−1) | Succulence (g cm−2) | LDMC (mg g−1) | LWC (%) |
|---|---|---|---|---|---|---|---|
| Control spray | 101·2 ± 5·0a | 0·90 ± 0·11ab | 0·76 ± 0·02a | 153·0 ± 10·1ab | 0·087 ± 0·004a | 77·1 ± 5·3a | 92·3 ± 0·5a |
| Salt spray | 113·3 ± 3·5a | 1·18 ± 0·03b | 0·76 ± 0·00a | 127·5 ± 5·0bc | 0·108 ± 0·004ab | 73·3 ± 2·3a | 92·7 ± 0·2a |
| 0 | 110·2 ± 6·5a | 0·83 ± 0·08a | 0·78 ± 0·02a | 173·7 ± 11·3a | 0·092 ± 0·005ab | 63·6 ± 1·1a | 93·6 ± 0·1a |
| 50 | 97·9 ± 4·2a | 0·93 ± 0·09ab | 0·75 ± 0·01a | 145·4 ± 12·1ab | 0·100 ± 0·002ab | 70·9 ± 6·1a | 92·9 ± 0·6a |
| 100 | 94·7 ± 4·5a | 1·00 ± 0·07ab | 0·77 ± 0·01a | 125·2 ± 8·9bc | 0·108 ± 0·003ab | 75·5 ± 4·9a | 92·5 ± 0·2a |
| 200 | 65·2 ± 3·2b | 0·78 ± 0·04a | 0·72 ± 0·02a | 117·5 ± 5,8bc | 0·112 ± 0·006b | 76·8 ± 2·5a | 92·3 ± 0·2a |
| 300 | 48·9 ± 3·8b | 0·70 ± 0·04a | 0·72 ± 0·03a | 96·6 ± 2·2c | 0·103 ± 0·008ab | 102·7 ± 5·5b | 89·7 ± 0·6b |
Plants were grown on gravel culture with hydroponics.
Values are means ± s.e. (n = 5). Means in the same column that have the same letter are not significantly different at P < 0·05 (Tukey's test).
RGR = ULR × LWF × SLA, 1/SLA = leaf succulence (LFW/LA) × LDMC (LDW/LFW), LWC = (LFW − LDW)/LFW.
Na+ concentration
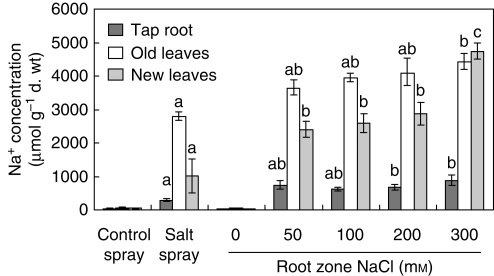
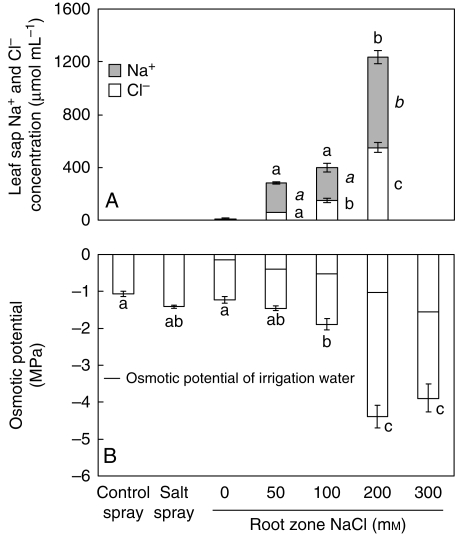
The tap root Na+ concentration of the salt-spray treatment showed no difference as compared with the 50 and 100 mm RZS treatments (Fig. 2). Also, the Na+ concentration of the old leaves of the salt-spray treatment did not differ from that of the 50, 100 and 200 mm RZS treatments. The largest differences in the Na+ concentration occurred in the new leaves. The Na+ concentration of the new leaves of the salt-spray treatment was lower than that of the RZS treatments. There were no differences in Na+ concentrations of the tap root and the old leaves between the RZS treatments. When comparing the Na+ concentrations of the new leaves there were no differences between the 50, 100 and 200 mm treatments, but all values differed from the 300 mm treatment. A repeated-measures ANOVA (results not shown in Fig. 2) was used to test for differences between the Na+ concentration of the old and new leaves. Only the salt spray 0, 50 and 100 mm NaCl RZS treatments showed significantly lower Na+ concentrations in the new leaves compared the Na+ concentration in the old leaves. The highest Na+ concentration occurred in the 300 mM NaCl treatment, reaching a concentration of 4·74 mmol g−1 d. wt in the new leaves, representing 11 % of the total dry weight. Chloride concentrations were only determined in the leaf sap (Fig. 3A).
Fig. 2.
Sodium concentrations of the tap root and old and new leaves of 8-week-old hydroponically grown Crambe maritima plants with 20 d of salt spray or 40 d of root-zone salinity ranging from 0 to 300 mm NaCl. Plants were grown on gravel culture with hydroponics. Values are means of four replicates ± s.e. Letters above the columns show possible significant differences in the Na+ concentrations in roots and old or new leaves between treatment groups. Different letters indicate a significant difference at P < 0·05 (Tukey's test).
Fig. 3.
Osmotic potential and Cl− and Na+ concentrations in the leaf sap of fresh leaves in 8-week-old Crambe maritima plants with 20 d of salt spray or 40 d of root-zone salinity ranging from 0 to 300 mm NaCl. Plants were grown on gravel culture with hydroponics. Differences between treatments in all NaCl concentrations were evaluated by repeated-measures ANOVA, the rest with one-way ANOVAs. Values are the means of three replicates ± s.e. Different letters indicate a significant difference at P < 0·05 (Tukey's test). Note that Na+ and Cl− concentrations combined do not give the total NaCl concentration per plant.
K+, Ca2+, Mg2+, N and P concentrations, Na+ : K+ ratio and net K+/Na+ selective uptake of the new leaves.
The Na+, K+, Ca2+, Mg2+, N and P concentrations were determined for the tap root and old and new leaves. No large differences occurred between these three compartments. Because the mineral or nutrient composition of the actively growing parts is most important, only results of the new leaves are shown (Table 3). Salt spray showed a negative effect on the K+ concentration of the new leaves, but only to a minor extent when compared with the RZS treatments. The effect of salt spray on the Na+, Ca2+, Mg2+, N and P concentrations, Na+ : K+ ratio and net K+/Na+ selective uptake of the new leaves also appears to be minimal when compared with the RZS treatments.
Table 3.
The Na+, K+, Ca2+ and Mg2+ concentrations, Na+ : K+ ratio and net K+/Na+ selectivity of the new leaves of the 8-week-old Crambe maritima plants with 20 d of salt spray or 40 d of root-zone salinity ranging from 0 to 300 mm NaCl
| Treatment | mmol Na+ g−1 | mmol K+ g−1 | mmol Ca2+ g−1 | mmol Mg2+ g−1 | Na+ : K+ ratio | K+ selectivity |
|---|---|---|---|---|---|---|
| Control spray | 0·05 ± 0·02a | 1·49 ± 0·06a | 1·06 ± 0·6a | 0·16 ± 0·00a | 0·03 ± 0·01a | 0·15 ± 0·04a |
| Salt spray | 1·01 ± 0·11b | 0·90 ± 0·19b | 0·99 ± 0·09a | 0·24 ± 0·00b | 1·44 ± 0·39b | 0·00 ± 0·00b |
| 0 | 0·03 ± 0·06a | 1·47 ± 0·09a | 1·16 ± 0·07a | 0·17 ± 0·01a | 0·02 ± 0·00a | 0·16 ± 0·03a |
| 50 | 2·40 ± 0·23c | 0·46 ± 0·04bc | 0·46 ± 0·03b | 0·08 ± 0·01c | 5·48 ± 0·91c | 1·55 ± 0·28c |
| 100 | 2·59 ± 0·13c | 0·49 ± 0·07bc | 0·43 ± 0·04b | 0·10 ± 0·01c | 5·68 ± 0·91c | 2·98 ± 0·51c |
| 200 | 2·87 ± 0·42c | 0·51 ± 0·10bc | 0·24 ± 0·02bc | 0·08 ± 0·00c | 7·18 ± 2·09c | 6·20 ± 1·70c |
| 300 | 4·74 ± 0·26d | 0·20 ± 0·06c | 0·12 ± 0·01c | 0·04 ± 0·00d | 29·41 ± 9·26d | 2·00 ± 0·75c |
Plants were grown on gravel culture with hydroponics. New leaves were defined as the third true leaf and onwards.
Values are means ± s.e. (n = 4) and are expressed per gram dry weight of the leaves. Means in the same column that have the same letter are not significantly different at P < 0·05 (Tukey's test). Concentrations of P and N were also determined with no significant differences in P concentrations and for the N concentration only a significant difference for the 300 mm NaCl treatment.
Concentrations of K+, Ca2+ and Mg2+ were greatly reduced under all RZS treatments. The reductions of the K+ and Mg2+ concentrations were in the order of 50–70 % for the 50, 100 and 200 mm NaCl treatments and 75–85 % for the 300 mm treatment. The reduction in the Ca2+ concentration was in the order of 60 % for the 50 and the 100 mm NaCl treatments and 80 % for the 200 mm. The increase in the Na+ : K+ ratio was similar between the 50, 100 and 200 mm NaCl treatments and showed a 400 % increase at 300 mm RZS compared with the 100 mm treatment. The net K+/Na+ selectivity increased compared with the 0 mm NaCl treatment, but no differences occurred between the 50, 100, 200 and 300 mm NaCl RZS treatments. The difference in net K+/Na+ selectivity between the 50 mm and 200 mm NaCl RZS treatments was almost significant with P = 0·051.
No significant difference occurred in the P concentration between RZS treatments with values falling in the range of 0·20–0·30 mmol g−1 d. wt (data not shown). For the N concentration only the 300 mm NaCl treatment showed a significant reduction with a mean N concentration of 2·65 mmol g−1 d. wt, whereas the other treatments were in the range of 3·50–4·00 mmol N g−1 d. wt (data not shown).
Osmotic potential, leaf sap Na+ and Cl−, proline and soluble sugars
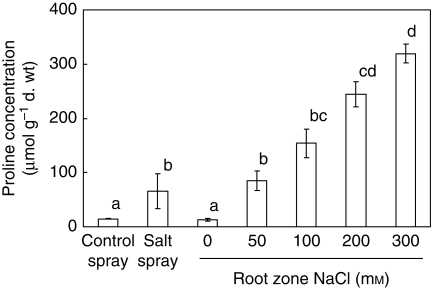
The osmotic potential of the leaf sap was lowered with increasing RZS. All values of the osmotic potential of the leaf sap were lower than that of the irrigation water (indicated by the horizontal line in the columns in Fig. 3B). Between the 100 and 200 mm NaCl RZS treatments the osmotic potential of the irrigation water increased by a factor of 2·0 and that of the leaf sap by a factor of 2·3. The total NaCl concentration of the leaf sap increased 3·1-fold between these two treatments. When comparing the total NaCl concentrations of the leaf sap (Fig. 3A) only the 200 mm differed from the 50 and 100 mm RZS treatments (indicated by the letters above each column). The Cl− concentration of the leaf sap was different for the RZS treatments, whereas the Na+ concentration between the 50 and 100 mm RZS did not differ (indicated by the letters beside the columns). Ion contribution to plant osmotic potential was 2, 10, 19 and 31 % for chloride and 1, 38, 32 and 39 % for sodium for the 0, 50, 100 and 200 mm NaCl RZS treatments, respectively (calculated according to eqn 11). Salt spray increased the proline concentration (Fig. 4) compared with the control spray and the 0 mm NaCl RZS treatment and was in the range of the proline concentrations of the 50 and 100 mm NaCl treatment. Proline concentrations of the RZS treatments showed a gradual and significant increase. The greatest increase in proline concentration compared with the control was found in the 300 mm NaCl treatment where proline concentrations increased 25-fold compared with the 0 mm NaCl treatment. A linear regression ANOVA showed that proline concentration (in μmol g−1 d. wt) was strongly correlated with RZS concentrations (NaCl in mm) (r = 0·95, F = 154·4, P < 0·001). The soluble sugar concentrations showed a gradual but not significant decrease with increasing salinities with values ranging from 116 to 175 µmol g−1 d. wt (data not shown).
Fig. 4.
Proline concentrations of the freeze-dried leaves of 8-week-old Crambe maritima plants with 20 d of salt spray or 40 d of root-zone salinity ranging from 0 to 300 mm NaCl. Plants were grown on gravel culture with hydroponics. Values are means ± s.e. with n = 4. Different letters indicate a significant difference at P < 0·05 (Tukey's test).
Antioxidant capacity and total phenolics
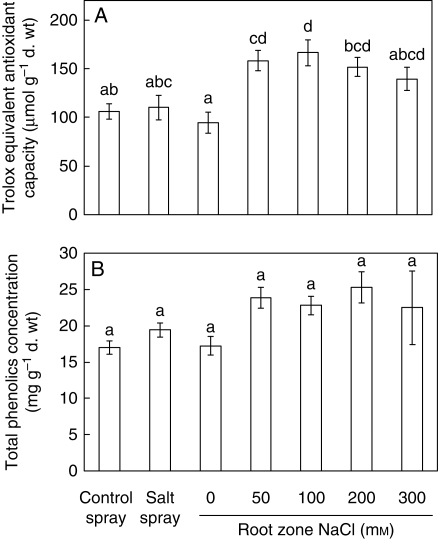
The mean antioxidant capacity of the leaves of C. maritima increased with increasing RZS compared with the 0 mm NaCl treatment with the exception of the 300 mm NaCl treatment, but no differences occurred amongst the 50, 100, 200 and 300 RZS treatments (Fig. 5A). The mean antioxidant capacity increased by 68, 77, 61 and 48 % in the 50, 100, 200 and 300 mm NaCl salt treatments compared with the 0 mM NaCl treatment, respectively. The antioxidant capacity of the salt-spray treatment showed no difference with the control spray treatment or the 0 mm NaCl RZS. No differences were found in the total phenolics concentration between the treatments (Fig. 5B).
Fig. 5.
Trolox equivalent antioxidant capacity and total phenolics concentration of the leaves of 8-week-old hydroponically grown Crambe maritima plants with 20 d of salt spray or 40 d of root-zone salinity ranging from 0 to 300 mm NaCl. Values are means ± s.e. with n = 4. Different letters indicate a significant difference at P < 0·05 (Tukey's test).
DISCUSSION
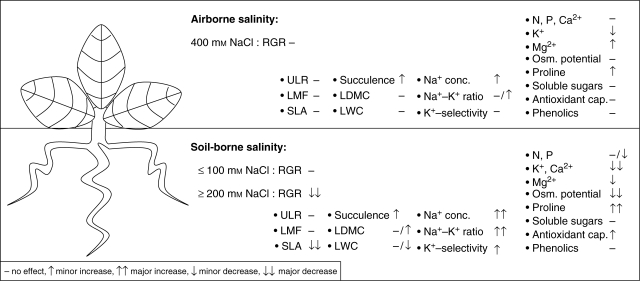
The aim of the experiments was to determine the growth response of C. maritima to various levels of airborne and soil-borne salinity and the ecophysiological mechanisms underlying these responses. For this, an overview of the overall effects on the measured parameters is given in Fig. 6. The salt spray-treated plants did not show growth reductions and showed only small physiological changes as compared with the RZS treatment. Because no growth reductions occurred up to 100 mM NaCl RZS only the effects of ≥200 mm RZS are listed in Fig. 6. The effects of airborne and soil-borne salt treatments are discussed separately.
Fig. 6.
The overall effects of airborne and soil-borne salinity on the measured parameters of Crambe maritima. For soil-borne salinity the effects of ≥200 mm NaCl are listed to evaluate the growth reduction observed in the 200 and 300 mm NaCl treatments.
Effect of salt spray on growth
The salt spray intensities in the experiments represented a moderate intensity (80 mg NaCl dm−2 d−1 during 20 d; salt-tolerance experiment) and a high intensity (160 mg NaCl dm−2 d−1 during 19 and 45 d; salt-spray experiment) compared with the levels found in the natural habitat of C. maritima (1–200 mg NaCl dm−2 d−1 (Barbour, 1978; Griffiths, 2006). The salt-spray experiment demonstrated that high salt-spray intensity applied during 19 or even 45 d did not affect leaf length and total leaf number per plant (Table 1). The salt-tolerance experiment showed that dry-weight production and RGR were not affected after 20 d exposure to moderate intensity salt spray (Table 2). The results even indicated a trend that salt spray might have affected the growth of C. maritima in a positive way, which has been reported for various strandline species by Rozema et al. (1982).
Ecophysiological adaptations to salt spray
Salt spray caused only minor physiological responses in the plants as compared with the RZS treatments and the salt-spray tolerance of C. maritima seemed to be based on preventing salt from entering the leaves. Leaves of C. maritima are covered with a thick waxy layer acting as an efficient water-repellent (Scott and Randall, 1976). Water droplets can roll off the leaves easily, but the salt spray in the present experiment was applied as a fine mist, which remained on the leaves. The salt-spray treatment resulted in lower Na+ concentrations in the plants as compared with the RZS treatments. Salt spray resulted in an increase in leaf thickness and leaf succulence (Table 1), which was comparable with the leaf thickness increase of another member of the Brassicaceae, the highly salt-tolerant Cakile maritima (Debez et al., 2008). The overall increase in leaf thickness is a typical response of salt-tolerant plants to salt spray (Martin and Clements, 1939; Boyce, 1951, 1954; Rozema et al., 1982) and increased leaf thickness or succulence has been interpreted as an adaptation of halophytes in terms of conservation of internal water, efficient water storage and dilution of accumulated salts (Storey and Wyn-Jones, 1979; Flowers et al., 1986; Breckle, 2002; Dimmit et al., 2005; Munns, 2005; Koyro and Lieth, 2008; Munns and Tester, 2008).
Effect of RZS on growth
Crambe maritima was relatively sensitive to soil-borne salinity above 100 mm NaCl and was only just capable of maintaining growth at salt concentrations of around 200 mm NaCl. Some halophytes show enhanced growth at moderate salinity levels around 50–100 mm NaCl RZS and are able to grow at salinity levels around half- or even full-strength seawater of around 500 mm NaCl (Flowers et al., 1986; Breckle, 2002; Bell and O'Leary, 2003; Koyro and Lieth, 2008), but C. maritima showed no enhanced growth at moderate salinities and growth was greatly reduced at the 200 mm NaCl level. Although the growth after 40 d of salt exposure did not differ at 300 mm NaCl compared with the 200 mm treatment, there are indications that C. maritima is not able to adapt to this level of salinity. Compared with the 200 mm treatment the mean RGR of the 300 mm treatment was 25 % lower, the leaf succulence started to decrease, the Na+ concentration and the Na : K ratio of the new leaves was considerably higher, whereas the net K+/Na+ selectivity and the nutrient concentrations of N, K+, Ca2+ and Mg2+ showed a sharp decrease.
Ecophysiological adaptations to RZS
Many of the physiological traits measured, proline concentration, Na+ concentration and Na+ : K+ ratio all responded to the RZS treatments but none of these appeared to result in a change in ULR. The reduction in RGR with increasing salinity was mainly caused by a decreasing SLA. All other determined parameters responded to increasing salinity, but it appeared that no single parameter could explain the observed growth reduction between 100 and 200 mm NaCl RZS.
The reduction in RGR with increasing salinity was mainly caused by a decreasing SLA, because decreases in ULR and LMF were only minor and not significant (Table 2). In general, variation in RGR is strongly correlated with SLA and can be considered as the prime factor determining interspecific variation in RGR (Lambers and Poorter, 2004) and shoot and leaf morphology are more plastic and more important determinants of leaf assimilation capacities than leaf chemistry and assimilation rates (Niinemets, 1999). Also, a gradual decrease in SLA with increasing salinity has been reported for Lycopersicon esculentum (Knight et al., 1992) and for Aster tripolium (Shennan et al., 1987). The inverse of SLA, 1/SLA, is the product of leaf succulence and LDMC (Poorter and Garnier, 2007) and leaf succulence in our RZS experiment showed a maximum increase of 22 % with increasing salinity. Since the photosynthetic carbon acquisition by a leaf depends not only on leaf area, but also on leaf thickness (Vile et al., 2005), an increase in leaf succulence can compensate for the negative effects of salinity on leaf cell metabolism to some extent. This also seems to be true for the 50 and 100 mm NaCl RZS where there was a trend for the reduction in SLA to be compensated by an increase in ULR. However, the increased internal surface for CO2 absorption does not necessarily lead to higher CO2 uptake rates because the CO2 resistance, as well as stomatal resistance, can increase even more with salinity, resulting in a decrease in photosynthesis (Longstretch and Nobel, 1979; Flowers, 1985; Geissler et al., 2009). Although the SLA showed the greatest reductions with increasing salinity, the SLA of the 200 mm NaCl RZS treatment only showed a minor decrease compared with the 100 mm NaCl RZS treatment, whereas the reduction in RGR showed a major decrease. The ULR changed from 120 % to 94 % (compared with the 0 mm NaCl RZS treatment) for the 100 mm and 200 mm treatments, respectively, so it appears that the observed growth reduction between the 100 mm and the 200 mm NaCl RZS was caused by the combined reduction in SLA and ULR. The SLA and LDMC are considered to reflect a fundamental trade-off in plant functioning between a rapid production of dry weight (high SLA, low LDMC species) and an efficient conservation of nutrients (low SLA, high LDMC species) (Garnier et al., 2001). In this regard, C. maritima adapted to increased salinity by changing from fast-growth to slow-growth with conservation of water and nutrients.
The ability of controlled uptake, increased extrusion and compartmentalization of Na+ is likely to be the primary factor determining salt tolerance of halophytes (Flowers et al., 1977; Zhu, 2001; Munns, 2005; Flowers and Colmer, 2008; Munns and Tester, 2008). No visible salt glands or bladders were detected on the leaves of C. maritima and no mention of such structures was found in the literature (Scott and Randall, 1976). Increased root zone salinities resulted in increased Na+ concentrations, decreased K+ concentrations and increased Na+ : K+ ratios in C. maritima. The results showed that Na+ readily entered the plants at 50 mm NaCl RZS but concentrations up to 200 mm NaCl were comparable with concentrations found in the highly salt-tolerant Brassicaceae species Cakile maritima (Debez et al., 2004; Megdiche et al., 2007) and Thellungiella halophila (Ghars et al., 2008). So the measured concentration of Na+ on the leaf tissue level does not inevitably result in major growth reduction as observed in C. maritima. Possibly, the amount of Na+ transported into the vacuoles differed amongst the species. Halophytes must be able to select K+ from a medium in the root zone dominated by Na+ to maintain adequate K+ nutrition or, in other words, to maintain a low Na+ : K+ ratio in the cytosol (Breckle, 2002; Xiong and Zhu, 2002; Wyn Jones and Gorham, 2002; Flowers and Colmer, 2008). Dependent on the plant species this ratio can range from 1 to 46 when concentrations of the whole leaf are considered (Flowers et al., 1986; Flowers and Colmer, 2008), although in the Brassicaceae species Cakile maritima (Debez et al., 2004; Megdiche et al., 2007) and Thellungiella halophila (Ghars et al., 2008) this ratio is around 4 at 200 mm NaCl and around 8 at 500 mm NaCl. The Na+ : K+ ratio of C. maritima was about 7 at 200 mm NaCl and a similar ratio resulted in considerable growth reductions in Cakile maritima and Thellungiella halophila. Although this ratio was reached at different salt concentrations for the different species, it does emphasize the importance of a low Na+ : K+ ratio to maintain high growth rates. Based on these results the growth reduction of C. maritima at 200 mm NaCl seems to be, at least partially, due to an increased Na+ : K+ ratio. However, it was expected that growth reduction by an increased Na+ : K+ ratio would result in ionic stress of which the physiological effects would reduce the ULR. The results (Table 2) showed no effect on the ULR, making it unlikely that ionic effects caused the observed growth reductions.
It is likely that osmotic effects are not responsible for the observed growth reductions with increasing root zone salinities. Crambe maritima was able to sufficiently lower its osmotic potential with NaCl present in the leaf tissue. All values of the osmotic potential of the leaf sap were lower (more negative) than that of the irrigation water and at the 200 mm NaCl treatment level the leaf sap NaCl concentration accounted for 70 % of the leaf sap osmotic potential. Compatible solutes like proline must accumulate in the cytosol to balance the osmotic pressure and allow turgor maintenance of cells (Yeo, 1983; Zhu, 2001; Breckle, 2002; Wyn Jones and Gorham, 2002; Munns and Tester, 2008). It is likely that proline is an important solute for C. maritima, but the proline concentration in the present experiment was strongly correlated with the soil-borne NaCl concentration and not to the RGR of C. maritima.
Salinity could also have directly affected nutrient uptake (Grattan and Grieve, 1999; Ullrich, 2002; Xiong and Zhu, 2002; Abdelgadir et al., 2005). The differences in K+ and Mg2+ concentrations between the 100 and 200 mm NaCl RZS were relatively small, so it is unlikely that nutrient deficiencies were responsible for the observed growth reductions. The decrease in Ca2+ concentration between the 100 and 200 mm NaCl RZS indicated that the role of Ca2+ may be important in the salt tolerance in C. maritima.
Salinity can induce oxidative stress by the production of toxic reactive oxygen species (ROS) in plants. Compounds that detoxify ROS, like enzymes, small molecule antioxidants and polyphenols, may have an essential role in adapting plants to salinity stress (Zhu, 2001; Xiong and Zhu, 2002; Ashraf and Harris, 2004; Parida and Das, 2005; Halliwell, 2006; Munns and Tester, 2008). The present results showed that with increasing salinity there was an increase in TEAC but not in total phenolics (also used as a measurement of oxidative activity). The level of increase of TEAC was similar amongst salt treatments, showing that this reaction was not specific to the actual salt concentration. It is not known if these levels are the maximum that C. maritima can produce or that these levels were sufficient to cope with the potential oxidative stress. Salt-sensitive plant species, like Lactuca sativa, show a decrease in total phenolics and antioxidant capacity with increasing salinity (Chisari et al., 2010). This will reduce the ability of a plant species to detoxify ROS whereas this detoxification can improve plant salt tolerance (Zhu, 2001). However, genetic differences in salinity tolerance are not necessarily due to differences in the ability to detoxify ROS (Munns and Tester, 2008) and further work is required to establish the general validity of these protective mechanisms in salinity tolerance (Ashraf and Harris, 2004).
Suitability as a crop for saline agriculture
When C. maritima is grown as an agricultural crop, root cuttings are used as the propagation method and the etiolated sprouts that grow from the root stem form the actual crop (Péron, 1990; Briard et al., 2002). It is not known if these root cuttings and etiolated sprouts respond in the same way to increased salinity as the seedlings in the present experiment. However, no growth or tap root dry weight reduction occurred up to the 100 mm NaCl RZS level in the present experiment, indicating that C. maritima can be cultivated under this salt concentration without loss in yield. Also, no growth reductions are expected when C. maritima is grown in a salt spray-rich environment.
Conclusions
Based on the growth performance it can be concluded that C. maritima is salt spray tolerant and can endure the salt spray levels which it encounters in its natural habitat. Salt spray caused only minor physiological responses in the plants as compared with the RZS treatments, although leaf thickness and leaf succulence significantly increased with salt spray.
Root zone salinities up to 100 mm NaCl did not result in a reduction in growth, but a sharp reduction in growth was observed at 200 mm NaCl. Crambe maritima responded to increasing soil salinity by lowering the RGR. The observed growth reduction at 200 mm NaCl RZS was mainly caused by the reduction in SLA. The lower SLA was caused by increased leaf succulence as well as increased LDMC. All other determined parameters responded to increasing salinity, but it appeared that no single parameter was clearly linked with the observed growth reduction between 100 and 200 mm NaCl RZS.
ACKNOWLEDGEMENTS
The authors are grateful to Prof. Dr Rien Aerts and Dr Ir Bert de Boer for useful remarks on a draft of the manuscript, to Dr Peter van Bodegom for advice on the experimental set-up and statistical analyses and to Prof. Dr Tim Colmer for constructive and detailed comments. The fruitful co-operation with the company Texelse Milieuvriendelijke NatuurProducten, led by Marc van Rijsselberge, is gratefully acknowledged. The study was supported by grant TA&G9356-R020 Zilte Landbouw Texel-BSIK-Transforum Agro&Groen-Leven met Water.
LITERATURE CITED
- Abdelgadir EM, Oka M, Fujiyama H. Characteristics of nitrate uptake by plants under salinity. Journal of Plant Nutrition. 2005;28:33–46. [Google Scholar]
- Agarie S, Shimoda T, Shimizu Y, et al. Salt tolerance, salt accumulation, and ionic homeostasis in an epidermal bladder-cell-less mutant of the common ice plant Mesembryanthemum crystallinum. Journal of Experimental Botany. 2007;58:1957–1967. doi: 10.1093/jxb/erm057. [DOI] [PubMed] [Google Scholar]
- Alcamo J, Florke M, Marker M. Future long-term changes in global water resources driven by socio-economic and climatic changes. Hydrological Sciences. 2007;52:247–275. [Google Scholar]
- Ashraf M, Harris PJC. Potential biochemical indicators of salinity tolerance in plants. Plant Science. 2004;166:3–16. [Google Scholar]
- Barbour MG. Salt spray as a microenvironmental factor in the distribution of beach plants at Point Reyes, California. Oecologia. 1978;32:213–224. doi: 10.1007/BF00366073. [DOI] [PubMed] [Google Scholar]
- Barbour MG, De Jong TM, Pavlik BM. Marine beach and dune communities. In: Chabot BF, Mooney HA, editors. Physiological ecology of North American plant communities. New York, NY: Chapman and Hall; 1985. pp. 296–322. [Google Scholar]
- Baron M, Binet P. Quelques aspects physiologiques de la germination des semences de Crambe maritima L. Bulletin de la Societe Francaise de Physiologie Vegetale. 1964;10:263–267. [Google Scholar]
- Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant and Soil. 1973;39:205–207. [Google Scholar]
- Bell HL, O'Leary JW. Effects of salinity on growth and cation accumulation of Sporobolus virginicus (Poaceae) American Journal of Botany. 2003;90:1416–1424. doi: 10.3732/ajb.90.10.1416. [DOI] [PubMed] [Google Scholar]
- Breckle S-W. Salinity, halophytes and salt affected natural ecosystems. In: Läuchli A, Lüttge U, editors. Salinity: environment–plants–molecules. Dordrecht: Kluwer; 2002. pp. 53–77. [Google Scholar]
- Briard M, Horvais A, Péron J.-Y. Wild seakale (Crambe maritima L.) diversity as investigated by morphological and RAPD markers. Scientia Horticulturae. 2002;95:1–12. [Google Scholar]
- Boyce SG. Salt hypertrophy in succulent dune plants. Science. 1951;114:544–545. doi: 10.1126/science.114.2969.544. [DOI] [PubMed] [Google Scholar]
- Boyce SG. The salt spray community. Ecological Monographs. 1954;24:29–67. [Google Scholar]
- Chisari M, Todaro A, Barbagallo RN, Spagna G. Salinity effects on enzymatic browning and antioxidant capacity of fresh-cut baby Romaine lettuce (Lactuca sativa L. cv. Duende) Food Chemistry. 2010;119:1502–1506. [Google Scholar]
- Clapham AR, Tutin TG, Warburg EF. Flora of the British Isles. Cambridge: Cambridge University Press; 1962. p. 135. [Google Scholar]
- Debez A, Ben Hamed K, Grignon C, Abdelly C. Salinity effects on germination, growth, and seed production of the halophyte Cakile maritima. Plant and Soil. 2004;262:179–189. [Google Scholar]
- Debez A, Koyro H-W, Grignon C, Abdelly C, Huchzermeyer B. Relationship between the photosynthetic activity and the performance of Cakile maritima after log-term salt treatment. Physiologia Plantarum. 2008;133:373–385. doi: 10.1111/j.1399-3054.2008.01086.x. [DOI] [PubMed] [Google Scholar]
- De Jong TM. Water and salinity relations of Californian beach species. Journal of Ecology. 1979;67:647–663. [Google Scholar]
- Dimmit MA, Wiens JF, Van Devender TR. Extreme succulent plant diversity on cerro Colorado near San Ignacio, Baja California Sur. In: Cartron J-LE, Ceballos G, Felger RS, editors. Biodiversity, ecosystems, and conservation in Northern Mexico. New York, NY: Oxford University Press; 2005. pp. 249–263. [Google Scholar]
- Erickson DL, Young DR. Salinity response, distribution, and possible dispersal of a barrier island strand glycophyte, Strophostyles umbellate (Fabaceae) Bulletin of the Torrey Botanical Club. 1995;122:95–100. [Google Scholar]
- FAO. Economic valuation of water resources in agriculture. Rome: FAO; 2004. FAO Water Report No. 27. [Google Scholar]
- FAO and IFAD. Water a shared responsibility. Paris: UNESCO; 2006. Water for food, agriculture and rural livelihoods; pp. 243–274. World Water Development Report No. 2. [Google Scholar]
- Flowers TJ. Physiology of halophytes. Plant and Soil. 1985;89:41–56. [Google Scholar]
- Flowers TJ. Improving crop salt tolerance. Journal of Experimental Botany. 2004;55:307–319. doi: 10.1093/jxb/erh003. [DOI] [PubMed] [Google Scholar]
- Flowers TJ, Colmer TD. Salinity tolerance in halophytes. New Phytologist. 2008;179:945–963. doi: 10.1111/j.1469-8137.2008.02531.x. [DOI] [PubMed] [Google Scholar]
- Flowers TJ, Troke PF, Yeo AR. The mechanism of salt tolerance in halophytes. Annual Review of Plant Physiology. 1977;28:89–121. [Google Scholar]
- Flowers TJ, Hajibagheri MA, Clipsom NJW. Halophytes. The Quarterly Review of Biology. 1986;61:313–337. [Google Scholar]
- Fusheng L, Peron J, Blanchard N. Effect of different pre-treatments to overcome the dormancy of seakale (Crambe maritima L.) seeds. Acta Horticulturae. 1998;467:233–244. [Google Scholar]
- Garnier E, Laurent G. Leaf anatomy, specific mass and water content in congeneric annual and perennial grass species. New Phytologist. 1994;128:725–736. [Google Scholar]
- Garnier E, Shipley B, Roumet C, Laurent G. A standardized protocol for the determination of specific leaf area and leaf dry matter content. Functional Ecology. 2001;15:688–695. [Google Scholar]
- Ghars MA, Parre E, Debez A, et al. Comparative salt tolerance analysis between Arabidopsis thaliana and Thellungiella halophila, with special emphasis on K+/Na+ selectivity and proline accumulation. Journal of Plant Physiology. 2008;165:588–599. doi: 10.1016/j.jplph.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Geissler N, Hussin S, Koyro H-W. Elevated atmospheric CO2 concentration ameliorates effects of NaCl salinity on photosynthesis and leaf structure of Aster tripolium L. Journal of Experimental Botany. 2009;60:137–151. doi: 10.1093/jxb/ern271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn EP, Brown JJ, Blumwald EJ. Salt tolerance and crop potential of halophytes. Critical Reviews in Plant Sciences. 1999;18:227–255. [Google Scholar]
- Grattan SR, Grieve CM. Salinity-mineral nutrient relations in horticultural crops. Scientia Horticulturae. 1999;78:127–157. [Google Scholar]
- Greaver TL, Sternberg LSL. Fluctuating deposition of ocean water drives plant function on coastal sand dunes. Global Change Biology. 2007;13:216–223. [Google Scholar]
- Griffiths ME. Salt spray and edaphic factors maintain dwarf stature and community composition in coastal sandplain heathlands. Plant Ecology. 2006;186:69–86. [Google Scholar]
- Halliwell B. Polyphenols: antioxidant treats for healthy living or covert toxins? Journal of the Science of Food and Agriculture. 2006;86:1992–1995. [Google Scholar]
- Horwood AR. British wild flowers in their natural haunts. Vols 2–4. London: Gresham Publishing; 1919. [Google Scholar]
- Hunt R, Causton DR, Shipley B, Askew AP. A modern tool for classical plant growth analysis. Annals of Botany. 2002;90:485–488. doi: 10.1093/aob/mcf214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignaciuk R, Lee JA. The germination of four annual strand-line species. New Phytologist. 1980;84:581–591. [Google Scholar]
- Jennings DH. The effects of sodium chloride on higher plants. Biological Reviews. 1976;51:433–486. [Google Scholar]
- Karschon R. Leaf absorption of wind-borne salt and leaf scorch in Eucalyptus camaldulensis Dehn. Ilanoth. 1958;4:5–25. [Google Scholar]
- Knight SL, Rogers RB, Smith MAL, Spomer LA. Effects of NaCl salinity on miniature dwarf tomato Micro-Tom'. I. Growth analysis and nutrient composition. Journal of Plant Nutrition. 1992;15:2315–2327. [Google Scholar]
- Koyro H-W. Study of potential cash crop halophytes by a quick check system: determination of the threshold of salinity tolerance and the ecophysiological demands. In: Lieth H, Mochtchenko M, editors. Cash crop halophytes: recent studies. Dordrecht: Kluwer; 2003. pp. 5–17. Tasks for Vegetation Science No. 38. [Google Scholar]
- Koyro H-W, Lieth H. Global water crisis: the potential of cash crop halophytes to reduce the dilemma. In: Lieth H, Garcia Sucre M, Herzog B, editors. Mangroves and halophyes: restoration and utilisation. The Netherlands: Springer; 2008. pp. 7–19. Tasks for Vegetation Science No. 43. [Google Scholar]
- Lambers H, Poorter H. Inherent variation in growth rate between higher plants: a search for physiological causes and ecological consequences. In: Caswell H, editor. Advances in ecological research. Vol. 34. Amsterdam: Elsevier Academic Press; 2004. pp. 283–362. [Google Scholar]
- Lee JA, Ignaciuk R. The physiological ecology of strandline plants. Vegetatio. 1985;62:319–326. [Google Scholar]
- Lee JA, Harmer R, Ignaciuk R. Nitrogen as a limiting factor in plant communities. In: Lee JA, McNeill S, Rorison IH, editors. Nitrogen as an ecological factor. Oxford: Blackwell; 1983. pp. 95–112. [Google Scholar]
- Lieth H, Moschenko M, Lohmann M, Koyro H-W, Hamdy A. Leiden: Backhuys; 1999. Halophyte uses in different climates. I. Ecological and ecophysiological studies. [Google Scholar]
- Longstretch DJ, Nobel PS. Salinity effects on leaf anatomy. Plant Physiology. 1979;63:700–703. doi: 10.1104/pp.63.4.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas EV, Hoffman GJ. Crop salt tolerance-current assessment. Journal of the Irrigation and Drainage Division, American Society of Civil Engineers. 1977;103:115–134. [Google Scholar]
- Mansour MMF. Nitrogen containing compounds and adaptation of plants to salinity stress. Biologia Plantarum. 2000;43:491–500. [Google Scholar]
- Martin WE, Clements FE. 1939 Adaptations and origin in the plant world. 1. Factors and functions in coastal dunes. Carnegie Institution of Washington Publication No. 521. [Google Scholar]
- Megdiche W, Ben Amor N, Debez A, et al. Salt tolerance of the annual halophyte Cakile maritima as affected by the provenance and the developmental stage. Acta Physiologiae Plantarum. 2007;29:375–384. [Google Scholar]
- Mennema J, Quené-Boterenbrood AJ, Plate CL. Utrecht: Bohn, Scheltema & Holkema; 1985. Atlas van de Nederlandse Flora 2. [Google Scholar]
- Munns R. Physiological processes limiting plant growth in saline soils: some dogmas and hypotheses. Plant, Cell & Environment. 1993;16:15–24. [Google Scholar]
- Munns R. Avenues for increasing salt tolerance of crops. In: Horst WJ, Schenk MK, Burkert A, et al., editors. Plant nutrition-food security and sustainability of agro-ecosystems. Dordrecht: Kluwer; 2001. pp. 370–371. [Google Scholar]
- Munns R. Genes and salt tolerance: bringing them together. New Phytologist. 2005;167:645–663. doi: 10.1111/j.1469-8137.2005.01487.x. [DOI] [PubMed] [Google Scholar]
- Munns R, Tester M. Mechanisms of salinity tolerance. Annual Review of Plant Biology. 2008;59:651–681. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- Munns R, Husain S, Rivelli AR, et al. Avenues for increasing salt tolerance of crops, and the role of physiologically based selection traits. Plant and Soil. 2002;247:93–105. [Google Scholar]
- Murphy J, Riley JP. A modified single solution method for determination of phosphate in natural waters. Analytica Chimica Acta. 1962;27:31–36. [Google Scholar]
- Niinemets Ü. Components of leaf dry mass per area, thickness and density, alter leaf photosynthetic capacity in reverse directions in woody plants. New Phytologist. 1999;144:35–47. [Google Scholar]
- Oosting HJ. Tolerance of salt spray of plants of coastal dunes. Ecology. 1945;26:85–89. [Google Scholar]
- Oosting HJ, Billings WD. Factors affecting vegetational zonation on coastal dunes. Ecology. 1942;23:131–142. [Google Scholar]
- Pakeman RJ. Mineral nutrition of strandline annuals. 1990 PhD Thesis, University of Manchester. [Google Scholar]
- Parida AK, Das AB. Salt tolerance and salinity effects on plants: a review. Ecotoxicology and Environmental Safety. 2005;60:324–349. doi: 10.1016/j.ecoenv.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Péron J-Y. Seakale: a new vegetable produced as etiolated sprouts. In: Janick J, Simon JE, editors. Advances in new crops. Portland, OH: Timber Press; 1990. pp. 419–422. [Google Scholar]
- Poorter H, Garnier E. Ecological significance of inherent variation in relative growth rate and its components. In: Pugnaire FI, Valladares F, editors. Functional plant ecology. New York, NY: CRC Press; 2007. pp. 67–100. [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology & Medicine. 1999;26:1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Rozema J, Flowers T. Crops for a salinized world. Science. 2008;322:1478–1480. doi: 10.1126/science.1168572. [DOI] [PubMed] [Google Scholar]
- Rozema J, Bijl F, Dueck T, Wesselman H. Salt-spray stimulated growth in strandline species. Physiologia Plantarum. 1982;56:204–210. [Google Scholar]
- Rozema J, Dueck T, Wesselman H, Bijl F. Nitrogen dependent growth stimulation by salt in strand-line species. Acta Oecologica/Oecologia Plantarum. 1983;4:41–52. [Google Scholar]
- Scott GAM, Randall RE. Biological flora of the British isles: Crambe maritima L. Journal of Ecology. 1976;64:1077–1091. [Google Scholar]
- Shennan C, Hunt R, Macrobbie EAC. Salt tolerance in Aster tripolium L. I. The effect of salinity on growth. Plant, Cell & Environment. 1987;10:59–65. doi: 10.1111/j.1365-3040.1987.tb02080.x. [DOI] [PubMed] [Google Scholar]
- Smedema LK, Shiati K. Irrigation and salinity: a perspective review of the salinity hazards of irrigation development in the arid zone. Irrigation and Drainage Systems. 2002;16:161–174. [Google Scholar]
- Storey R, Wyn-Jones RG. Responses of Atriplex spongiosa and Suaeda monoica to salinity. Plant Physiology. 1979;63:156–162. doi: 10.1104/pp.63.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabolcs I. Soils and salinisation. In: Pessarakali M, editor. Handbook of plant and crop stress. New York, NY: Marcel Dekker; 1994. pp. 3–11. [Google Scholar]
- Tominaga T, Kobayashi H, Ueki K. Clonal variation in salt tolerance of Imperata cylindrical L. Beauv. var. koenigii (Retz.) et Schinz. Journal of Japanese Grassland Science. 1991;37:69–75. [Google Scholar]
- Ullrich WR. Salinity and nitrogen nutrition. In: Läuchli A, Lüttge U, editors. Salinity: environment–plants–molecules. Dordrecht: Kluwer; 2002. pp. 229–248. [Google Scholar]
- Van der Meijden R. Heukels' Flora van Nederland. 23st edn. Groningen/Houten: Wolters-Noordhoff; 2005. [Google Scholar]
- Vile D, Garnier E, Shipley B, et al. Specific leaf area and dry matter content estimate thickness in laminar leaves. Annals of Botany. 2005;96:1129–1136. doi: 10.1093/aob/mci264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walmsley CA, Davy AJ. Germination characteristics of shingle beach species, effect of seed ageing and their implications for vegetation restoration. Journal of Applied Ecology. 1997;34:131–142. [Google Scholar]
- Waterman PG, Mole S. Analysis of phenolic plant metabolites. In: Lawton JH, Likens GE, editors. Methods in ecology. Oxford: Blackwell Scientific; 1994. pp. 80–90. [Google Scholar]
- Wells BW, Shunk IV. Salt spray: an important factor in coastal ecology. Bulletin of the Torrey Botanical Club. 1938;65:485–492. [Google Scholar]
- Wilson PJ, Thompson K, Hodgson JG. Specific leaf area and leaf dry matter content as alternative predictors of plant strategies. New Phytologist. 1999;143:155–162. [Google Scholar]
- Wyn Jones G, Gorham J. Intra- and inter-cellular compartments of ions. In: Läuchli A, Lüttge U, editors. Salinity: environment–plants–molecules. Dordrecht: Kluwer; 2002. pp. 159–180. [Google Scholar]
- Xiong L, Zhu J-K. Salt tolerance. In: Meyerowitz EM, Somerville CR, editors. The arabidopsis book. issue 1. Vol. 24. Rockville, MD: American Society of Plant Biologists; 2002. pp. 1–22. [Google Scholar]
- Yemm EW, Willis AJ. The estimation of carbohydrates in plant extracts by anthrone. Biochemical Journal. 1954;57:508–514. doi: 10.1042/bj0570508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo AR. Salinity resistance: physiologies and prices. Physiologia Plantarum. 1983;58:214–222. [Google Scholar]
- Yeo AR. Predicting the interaction between the effects of salinity and climate change on crop plants. Scientia Horticulturae. 1999;78:159–174. [Google Scholar]
- Yura H. Comparative ecophysiology of Chrysanthemum pacificum Nakai and Solidago altissima L. 1. Why S. altisimma cannot be established on the seashore. Ecological Research. 1997;12:313–323. [Google Scholar]
- Zhu J-K. Plant salt tolerance. Trends in Plant Science. 2001;6:66–71. doi: 10.1016/s1360-1385(00)01838-0. [DOI] [PubMed] [Google Scholar]