Abstract
Background and Aims
Physical dormancy in seeds of species of Geraniaceae is caused by a water-impermeable palisade layer in the outer integument of the seed coat and a closed chalaza. The chalazal cleft has been reported to be the water gap (i.e. location of initial water entry) in innately permeable seeds of Geraniaceae. The primary aim of this study was to re-evaluate the location of the water gap and to characterize its morphology and anatomy in physically dormant seeds of Geraniaceae, with particular reference to G. carolinianum.
Methods
Length, width, mass, anatomy and germination of two seed types (light brown and dark brown) of G. carolinianum were compared. Location, anatomy and morphology of the water gap were characterized using free-hand and microtome tissue sectioning, light microscopy, scanning electron microscopy, dye tracking, blocking and seed-burial experiments.
Key Results
Treatment with dry heat caused a colour change in the palisade cells adjacent to the micropyle. When placed in water, the ‘hinged valve’ (blister) erupted at the site of the colour change, exposing the water gap. The morphology and anatomy in the water-gap region differs from those of the rest of the seed coat. The morphology of the seed coat of the water-gap region is similar in G. carolinianum, G. columbinum, G. molle and G. pusillum and differs from that of the closely related species Erodium cicutarium.
Conclusions
Dislodgment of swollen ‘hinged valve’ palisade cells adjacent to the micropyle caused the water gap to open in physically dormant seeds of G. carolinianum, and it was clear that initial water uptake takes place through this gap and not via the chalazal opening as previously reported. This water gap (‘hinged valve gap’) differs from water gaps previously described for other families in morphology, anatomy and location in the seed coat.
Keywords: Erodium, Geraniaceae, Geranium, physical dormancy, seed-coat anatomy, seed burial, seed germination, water gap
INTRODUCTION
Physical dormancy (PY) is caused by one or more water-impermeable layers of palisade cells in the seed (or fruit) coat (Baskin and Baskin, 1998; Baskin et al., 2000) along with a closed chalaza and micropyle. PY is known to occur only in angiosperms and thus is unknown in gymnosperms (Baskin and Baskin, 1998). One monocot and 16 eudicot families have been demonstrated or inferred to contain species that have PY (Baskin and Baskin, 1998; Nandi, 1998; Baskin, 2003; Horn, 2004; Baskin et al., 2000, 2006). Of the 16 eudicots, occurrence of PY in Dipterocarpaceae, Sarcolaenaceae and Sphaerosepalaceae has been based only on seed-coat anatomy (Nandi, 1998; Horn, 2004). Some families that contain species with PY also have species with physiological dormancy (PD), combinational dormancy (PY + PD) or non-dormancy (Baskin et al., 2000).
Seeds with PY cannot imbibe water even under favourable environmental conditions due to a water-impermeable layer(s) of cells. Specialized structures are involved in occlusion of the water gaps (see table 3·5, Baskin and Baskin, 1998; table 1, Baskin et al., 2000). The breaking of PY involves disruption or dislodgement of ‘water-gap’ structures, which act as environmental ‘signal detectors’ for germination (Baskin et al., 2000). Once the closed water gap opens, a seed can imbibe water rapidly and germinate under a wide range of conditions (Baskin and Baskin, 1998; Baskin et al., 2000).
Table 1.
Mass, length and width of the two seed types of G. carolinianum
| Seed type | Seed mass (mg) | Seed length (mm) | Seed width (mm) |
|---|---|---|---|
| Light brown | 2·44 ± 0·03 | 1·92 ± 0·02 | 1·26 ± 0·01* |
| Dark brown | 2·35 ± 0·03 | 1·96 ± 0·02 | 1·22 ± 0·01 |
Values are means ± s.e.
* Indicates significant difference at P < 0·05, with an independent two-sample t-test.
Water-gap anatomy, morphology, origin and location differ among families as well as within the same family (Baskin et al., 2000). Moreover, anatomy and morphology of the seed (or fruit) coat in the water-gap region differ from those of the rest of the seed (or fruit) coat. Nine different water-gap types have been characterized in seven (excluding Geraniaceae) of the 17 angiosperm families with PY (Baskin and Baskin, 1998; Baskin et al., 2000; Baskin, 2003; Jayasuriya et al., 2007, 2008; Hu et al., 2008; Turner et al., 2009).
Among the six genera of Geraniaceae, viz. California, Erodium, Geranium, Hypseocharis, Monsonia and Pelargonium (Fiz et al., 2008), PY has been shown to occur in Erodium, Geranium and Pelargonium (Baskin and Baskin, 1974; Meisert, 2002). The recently recognized monotypic genus California (Aldasoro et al., 2002; Fiz et al., 2006) can also be considered as exhibiting PY, since Erodium macrophyllum (identified as California macrophyllum by Aldasoro et al., 2002) was reported to have PY (Gillespie and Andersen, 2005). Based on seed-coat anatomical studies on Hypseocharis remy (Boesewinkel, 1988) and Monsonia senagalensis (Narayana and Arora, 1963), it can be inferred that PY also occurs in Hypseocharis and Monsonia. Thus, it appears that all six genera of Geraniaceae have PY or (PY + PD). Further, we are not aware of a species in any of these six genera with PD, morphological dormancy, morphophysiological dormancy or with non-dormant seeds.
The water-impermeable cell layer in the seed coat of Geraniaceae is a continuous layer of palisade cells (except in the chalazal region) that is located below the outer polygonal and middle parenchyma layer(s) of the outer integument (Kay and Lees, 1913; Boesewinkel and Been, 1979; Schulz et al., 1991; Meisert et al., 1999). The palisade cells are strongly lignified and contain tannin depositions and calcium oxalate crystals (Schulz et al., 1991; Aedo et al., 1998a; Meisert et al., 2001). The gap between palisade cells of the chalaza is filled with a chalazal plug (= suberized stopper, sensu Boesewinkel and Been, 1979) that maintains seed-coat impermeability. When the palisade layer is damaged, e.g. by mechanical scarification, acid scarification, etc., seeds lose their impermeability and imbibe water (Nell et al., 1981, Schulz et al., 1991).
Baskin and Baskin (1974) suggested that the hilum is the water gap in Geranium carolinianum, but they did not document the presence of a water gap. Nell et al. (1981) compared the morphology of the hilum region of four cultivars of Pelargonium hortorum using scanning electron micrographs and concluded that there is no relationship between occlusion of the hilar fissure and seed germination. Meisert et al. (1999) suggested that the chalazal opening is the main pathway of water entry into water-permeable seeds of P. mollicomum. However, they described neither the initial water entrance into innately permeable seeds nor the water gap of impermeable seeds after they become non-dormant.
Using scanning electron micrographs, Schulz et al. (1991) compared the effect of acid scarification of the seed coat of water-permeable and water-impermeable varieties of P. zonale. These authors concluded that neither the light line in the palisade layer nor the palisade layer itself is responsible for water impermeability, but they did not study the effect of acid scarification on opening of the water gap. Gillespie and Andersen (2005) tested the effect of different treatments on seed germination of E. macrophyllum (= C. macrophyllum). Based on the high germination percentage after manual scarification, they suggested that germination of E. macrophyllum may be enhanced by physical abrasion to the seed coat when seeds drill themselves into the soil.
The primary study species Geranium carolinianum (Carolina geranium) is a herbaceous winter annual that belongs to the family Geraniaceae, subgenus Geranium, section Geranium (which consists of 339 species; Aedo et al., 1998b). Two varieties, G. carolinianum var. carolinianum and var. confertiflorum, have been identified in Kentucky, USA (Jones, 2005). This species is native to eastern North America but is widely distributed throughout the North American continent (Piper, 1906; Small, 1907; Aedo, 2000). Geranium carolinianum has long been considered a weed that grows in disturbed habitats such as roadsides, waste places, gardens, old fields, turfs and fallow and cultivated fields in the United States (Kay and Lees, 1907; Britton, 1918; Spencer, 1976; Haragan, 1991). Moreover, it has been reported to be a naturalized weed in China, Japan, northern Europe, South America and Taiwan (Peng, 1978; Xu and Aedo, 2008; Nishida and Yamashita, 2009).
Seed dormancy of G. carolinianum is caused by a water-impermeable seed coat. Freshly matured seeds also have shallow PD (i.e. small amount of embryo dormancy); thus, the seeds have (PY + PD) (Baskin and Baskin, 1974). In the Baskin and Baskin (1974) study, embryos became non-dormant during a short after-ripening period before the seeds became permeable; thus, seeds of G. carolinianum primarily have PY. In nature, seeds are dispersed in early summer and come out of PY by late summer due to dry and hot weather conditions and germinate in autumn when soil moisture becomes readily available.
Previous studies on Geraniaceae have considered water entry into innately permeable seeds only. Since breaking of seed dormancy is an ecologically significant event in the life history of a species, it is important to study the initial site of water entry into PY seeds after the breaking of dormancy. Therefore, the current study focused mainly on identifying and morphologically and anatomically characterizing the water gap in PY seeds of Geraniaceae, with special reference to G. carolinianum. In addition, morphological changes in the seed coat during seed burial and after heat treatment were studied.
MATERIALS AND METHODS
Seed collection and preparation
Stems of Geranium carolinianum L. bearing mature fruits were collected from plants growing on a railroad right-of-way in Rosemont, Lexington, KY, USA, in May 2009. They were covered with a net and allowed to dry for 3 d inside a non-heated greenhouse. Seeds released naturally were used in this study. The same procedure was followed in obtaining seeds of Geranium pusillum, G. molle and G. columbinum, which were collected, in June 2009, on the campus of the University of Kentucky, and Erodium cicutarium, which were collected at Spindletop Farm, Lexington. Subsequently, seeds were stored at room temperature (approx. 23 °C and 50–60 % RH, dry storage) until used.
Characteristics of the two seed types
Geranium carolinianum was observed to produce dark-brown and light-brown seeds. Length, width and mass of seeds of each colour type were measured. For measurement of length and width, 30 seeds from each colour type were randomly selected and measured using a dissecting microscope with a calibrated micrometer eyepiece. For measurement of mass, ten replicates of ten seeds each of the two colour morphology types were weighed to the nearest 0·0001 g.
To compare the anatomy of the micropylar and chalazal regions, and of the seed coat away from these two regions, 20-μm vibratome sections (longitudinal and transverse) of both dark-brown and light-brown seeds were taken using a VIBRATOME® 1500 sectioning system. Sections were observed under a light microscope (Olympus BX40) equipped with a digital camera (Olympus DP25) and micrographs taken and compared. During the rest of the study, either a dissecting microscope (ZEISS STEMI SVII) or the Olympus BX40 light microscope was used along with the Olympus DP25 digital camera for obtaining micrographs.
Ten replicates (each with ten seeds) of fresh mechanically scarified and non-scarified seeds of both dark-brown and light-brown colour types were germinated on moist sand at 20/10 °C (12 h/12 h) under a 14 h/10 h daily light/dark period. Photon irradiance during the light phase was approx. 40 µmol m−2 s−1, 400–700 nm, and the light source was cool white fluorescent tubes. The number of germinated seeds was counted daily for 10 d (but only final germination is shown in Results). Hereafter, fresh seeds were selected for study irrespective of their colour.
Dormancy breaking
Fresh seeds of G. carolinianum were incubated on wet filter papers in Petri dishes for 3 d and any innately permeable seeds discarded. Subsequently, fully filled, undamaged seeds were selected from non-imbibed seeds. This procedure was used whenever impermeable seeds were selected. Based on the results of preliminary studies, the selected seeds were made permeable by subjecting them to dry heat at 80 °C for 7 d.
Morphological changes during dormancy breaking
Using the dissecting microscope equipped with a digital camera, micrographs of seeds were taken before and after heat treatment to compare morphological changes that occur during breaking of dormancy.
Germination
Two hundred impermeable seeds were selected, and the outermost permeable cell layers (above the palisade layer) were removed carefully from 100 of them with the aid of a toothpick and a dissecting microscope. The other 100 seeds were left intact. The same procedure was repeated with permeable (heat-treated) seeds. Another set of 100 seeds was scarified mechanically with a razor blade at places on the seed coat away from micropylar and chalazal ends without damaging the embryo and was used as a control. All seeds were germinated as described above.
Imbibition
An imbibition test was carried out to compare water uptake in impermeable, permeable (heat-treated) and mechanically scarified seeds. Fifty seeds each of each group of seeds were used for the test. Each category was separated into ten replicates of five seeds each and weighed to the nearest 0·0001 g. Seeds of each replicate sample were placed on wet filter paper in Petri dishes. The seeds were blotted and weighed at 30-min intervals for 10 h; percentage mass increase (fresh mass basis) for each interval was calculated.
Dye tracking
Dye-tracking experiments were carried out to locate the site of water movement through the seed coat of permeable seeds (heat-treated) during imbibition. Concentrated solutions of both acid fuchsin and methylene blue dyes were used. After the dormancy-breaking treatment, 100 seeds of G. carolinianum were dipped in a concentrated acid fuchsin solution. Four seeds each were removed initially after 5 min and 15 min and then at 15-min intervals for 4 h and blotted with tissue papers. Seeds were cut longitudinally into two halves across the micropylar and chalazal regions, and the cut surfaces were observed under external artificial illumination using the light microscope. The pathway of the dye (marked in pink) was observed and micrographs taken. The same procedure was repeated with impermeable seeds.
Forty heat-treated seeds from which the outer water-permeable cell layers had been removed (for better visualization of the water gap) were immersed in a concentrated methylene blue dye solution. Four seeds each were removed initially after 10 min, and then at 30-min intervals for 4 h. The surface of the micropylar and chalazal regions (with and without removing the palisade layer) and cut-surfaces of seeds cut longitudinally into two halves through the chalazal and micropylar regions were observed and micrographs taken as described above. The pathway of methylene blue (marked in blue) was observed.
Blocking experiment
The outermost permeable cell layers in the micropylar and chalazal regions of heat-treated seeds were removed carefully from approx. 500 seeds with a toothpick. The micropylar region was blocked with Super Glue® (methyl 2-cyanoacrylate) in 100 seeds with a sharpened toothpick. One set of 100 seeds was blocked similarly at the chalazal region only and another 100 at both chalazal and micropylar regions. Ten replicates of ten seeds for each treatment were placed on wet sand in Petri dishes and incubated for 3 d under the same conditions used in germination experiments. The number of imbibed seeds (larger in size and lighter in colour) was counted at intervals of 24 h.
Light microscopy
Microtome sections
Microtome and hand sections were taken to study the anatomy of the water gap. Four fully matured seeds of G. carolinianum were scarified mechanically to facilitate wax infiltration and fixed in FAA solution for 7 d. Then, they were dehydrated in a series of tertiary-butanol (TBA) and embedded in paraffin wax. Subsequently, 12-μm longitudinal sections of the seeds were cut using a microtome (LEICA RM 2135). Sections were stained with 1 % safranin solution. The micropylar and chalazal regions and the seed coat away from micropyle and chalaza were observed and photomicrographed.
Free-hand sections
The outer permeable cell layers of three permeable seeds were removed. Sections were cut periclinally through the micropylar region, including the presumed water-gap and palisade cells away from presumed water gap. Sections were mounted on glass slides and micrographs taken.
Scanning electron microscopy
All of the seeds of Geraniaceae examined were made permeable by drying at 80 °C for 1 week. Three impermeable and three permeable (heat-treated) seeds, each with and without outer cell layers, were used to compare the morphological changes during the breaking of dormancy. To observe the morphological changes during early imbibition, six permeable seeds each with and without outer cell layers were immersed in water, and three seeds of each were removed from the water after 10 and 20 min of imbibition and blotted dry. The same procedure was followed with impermeable seeds. Two dislodged blisters were used to study the morphology of the lower surface of palisade cells of the presumed water gap. To compare the water gaps of E. cicutarium, G. columbinum, G. molle and G. pusillum, permeable seeds of each species from which the outer cell layers had been removed were immersed in water for 20 min and then blotted dry. All of the samples were mounted on scanning electron microscopy specimen stubs using double-sided carbon tapes. Then, the samples were sputter-coated with gold-palladium (15 nm), scanned with an S-3200 Hitachi scanning electron microscope at an acceleration voltage of 5·0 kV and micrographs taken and compared.
Effect of morphological changes of seed coat during burial on seed germination
To identify the morphological changes in the seed coat in vivo, seeds that had been buried at a depth of 2 cm in soil for 4 months (June to October) in an open area on the campus of University of Kentucky were exhumed, observed under a dissecting microscope and micrographs taken. Fifty seeds each with and without a change in colour near the micropylar region were germinated as described above (five replicates of ten seeds each for both types).
Statistical analysis
Percentage germination and imbibition data were normalized by arcsine-transformation prior to the analysis. Seed length, width and mass data of the two seed colour types and germination percentages data of the burial experiment were compared using an independent two-sample t-test (P < 0·05). All other germination and imbibition percentage data were analysed by one-way ANOVA, and Duncan's multiple range test was used to determine significant differences between each treatment (P < 0·05). All analyses were carried out using SAS® ver. 9·2 software.
RESULTS
Characteristics of the two seed types
The light-brown and dark-brown seeds differ significantly in width but not in mass and length (Table 1).
The two seed types did not differ in anatomy of the chalazal or micropylar regions or seed coat away from micropylar and chalazal regions. There was more thickening in radial walls of the outermost polygonal cell layer of the outer integument in light-brown seeds than in dark-brown seeds (images not shown).
After mechanical scarification, all seeds imbibed water within 24 h, but only a few seeds germinated at 7 d. Within the next 3 d, germination increased rapidly to 89·0 ± 5·3 % and 96·0 ± 1·7 % in scarified light-brown and dark-brown seeds, respectively. Non-scarified light-brown and dark-brown seeds germinated to only 3·0 ± 2·1 % and 2·0 ± 2·6 %, respectively, after 10 d of incubation. Germination differed significantly between scarified and non-scarified seeds, but not between scarified or between non-scarified seeds of the two seed colour types (P < 0·05).
Dormancy breaking
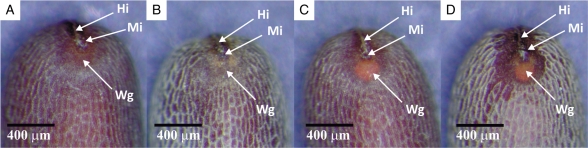
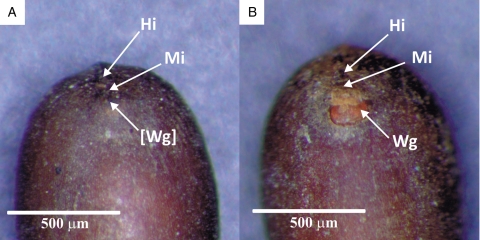
After the dry heat treatment (80 °C for 1 week), a brownish-orange circular area appeared near the micropylar region in all treated seeds (Fig. 1C, D). The colour of this area in fresh dormant seeds was similar to that of the seed coat (Fig. 1A, B).
Fig. 1.
The micropylar region of Geranium carolinianum: (A) dark-brown and (B) light-brown impermeable seeds with no colour change in the area adjacent to the micropyle; (C) dark-brown and (D) light-brown permeable seeds with colour change in the area adjacent to the micropyle after dry heat treatment at 80 °C for 1 week. Abbreviations: Hi, hilum; Wg, water gap; Mi, micropyle.
Imbibition
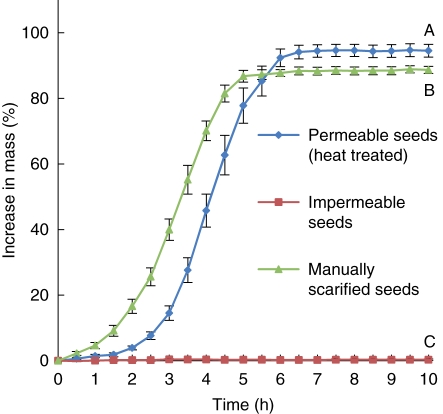
Imbibition in manually scarified seeds was faster than it was in heat-treated seeds. Manually scarified seeds and heat-treated seeds reached their maximum mass in 5·5 h and 6·5 h, respectively. After 10 h, the mass of manually scarified and heat-treated seeds had increased by 88·7 ± 1·1 % and 94·5 ± 1·9 %, respectively, whereas that of non-scarified seeds had increased by only 0·3 ± 0·2 % (P < 0·05; Fig. 2).
Fig. 2.
Percentage mass increase (mean ± s.e.) in permeable (heat-treated), manually scarified and impermeable seeds of G. carolinianum during 10 h of incubation at ambient room temperature. Different letters indicate significant differences between treatments (P < 0·05).
Germination
After 10 d of incubation on wet sand at 20/10 °C, heat-treated (outer cell layer intact), heat-treated (outer cell layers removed) and scarified seeds germinated to 100 %. Germination percentages of impermeable (outer cell layers intact) and impermeable (outer cell layers removed) were 0·0 % and 2·0 ± 0·0 %, respectively (P < 0·05).
Dye tracking
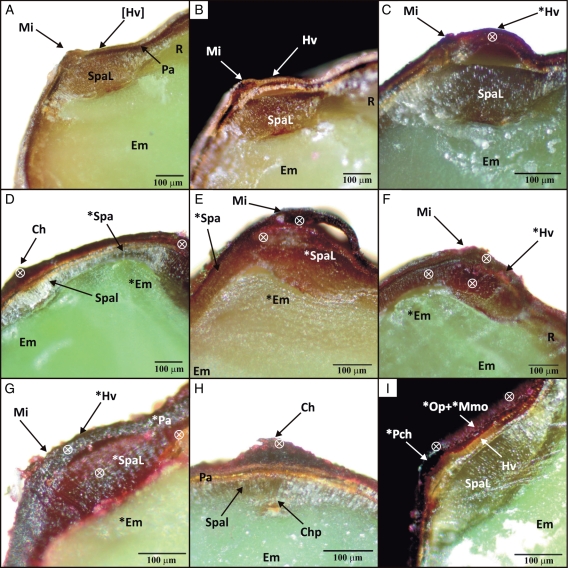
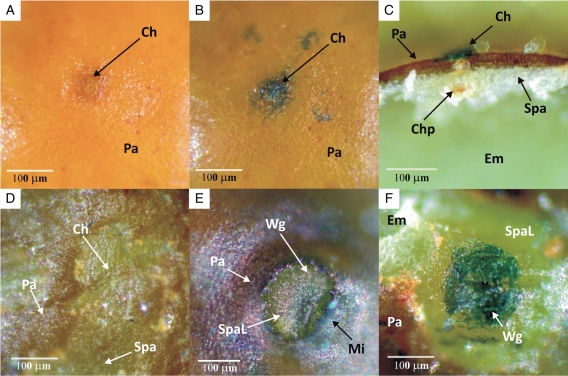
Within 5 min, the outermost layers [outer layer and middle layer(s) of the outer integument] of both impermeable and permeable seeds were stained pink (Fig. 3C, I). After 5 min, the palisade cells of the ‘hinged valve’ had stained pink in permeable seeds (Fig. 3B) and blue in the chalazal opening (Fig. 4B). The first appearance of dye below the palisade layer was observed after 10 min in the subpalisade cells of the micropylar region below the ‘hinged valve’ (Fig. 4E), and this stained area had increased in size after 30 min, indicating water movement between palisade and subpalisade layers (Fig. 4F). Thereafter, movement of the stain was slower than that of water, but the cells swelled due to imbibition. After 1·5 h, swelling of the embryo occurred first under the micropylar region towards the chalaza (Fig. 3D, E). At the same time, no staining or swelling occurred in the chalazal region (Fig. 3D, H). After 2 h, a pink colour was observed halfway through the subpalisade directly below the micropylar region and after 2·5 h in the subpalisade cells adjacent to the micropyle towards the chalaza (Fig. 3F, G). The nucellus and the whole micropylar region were stained after 4 h of incubation in dye, while the whole embryo had imbibed (Fig. 3G). Even after 24 h, the embryo remained unstained (figure not shown).
Fig. 3.
Longitudinal sections through micropylar and chalazal areas of seeds of G. carolinianum allowed to imbibe acid fuchsin for different periods of time: (A) micropylar region of an impermeable seed before imbibition; (B) micropylar region of a permeable seed before imbibition; (C), (E), (F) and (G) micropylar region of permeable seeds with opened water gap after 5 min, 2 h, 3·5 h and 4 h, respectively; (D) both chalazal and micropylar regions of a permeable seed after 1·5 h; (H) chalazal region of a permeable seed after 1·5 h; (I) micropylar region of an impermeable seed after 4 h. Abbreviations: Ch, chalaza; Chp, chalazal plug; Em, embryo; Hv, hinged valve (opened); [Hv], hinged valve (closed); Mi, micropyle; Mmo, multi-layered middle parenchyma cells; Op, outermost polygonal parenchyma cell layer; Pa, palisade cells; Pch, parenchyma cells of micropyle; R, radicle; Spa, subpalisade cells; SpaL, elongated subpalisade cells of micropylar region; Spal, elongated subpalisade cells of chalazal region. A cross in a circle indicates presence of acid fuchsin dye in different cell layers; an asterisk indicates regions which have imbibed.
Fig. 4.
Light micrographs of micropylar and chalazal regions of seeds of G. carolinianum allowed to imbibe methylene blue for different periods of time: (A) and (B) surface view of chalazal end of a seed before and 1·5 h after imbibition; (C) longitudinal section through chalazal region 1·5 h after imbibition; (D) chalazal region of a seed 1·5 h after imbibition (palisade layer removed to expose subpalisade layer); (E) micropylar region of a seed after 10 min imbibing the dye (hinged valve removed); (F) micropylar region of a seed after 30 min (palisade layer removed to expose elongated subpalisade layer). Abbreviations: Ch, chalaza; Chp, chalazal plug; Em, embryo; Mi, micropyle; Pa, palisade cells; Spa, subpalisade cells; SpaL, elongated subpalisade cells of micropylar region; Wg, water gap.
Blocking of presumed water gap
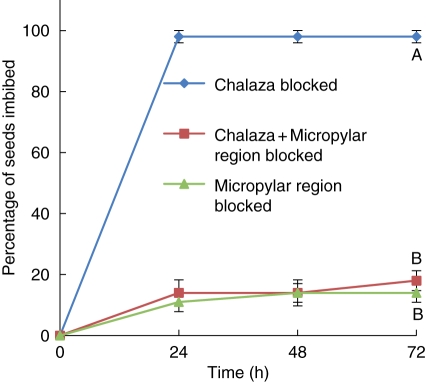
After 72 h of incubation, the percentage of imbibition in seeds with the chalaza blocked (98 ± 1·1 %) was significantly higher than that in seeds with the micropylar region blocked (14·0 ± 3·1 %) or with the micropylar region + chalaza blocked (18·0 ± 3·3 %; P < 0·05). No significant difference was observed between seeds in which the micropylar region or chalaza + micropylar region were blocked (P < 0·05; Fig. 5).
Fig. 5.
Percentage of G. carolinianum seeds which imbibed (mean ± s.e.) during 72 h of incubation at 20/10 °C with the chalaza blocked, the chalaza + micropylar region blocked or the micropylar region blocked. Different letters indicate significant differences between treatments (P < 0·05).
Light microscopy
The seed coat of G. carolinianum consists of two integuments. The outer integument contains an outermost polygonal cell layer with thickened radial walls, middle parenchyma cell layer(s) and a palisade layer. The inner integument consists of a subpalisade layer, a middle compressed layer and an innermost large cell layer. The nucellus is much compressed (not shown).
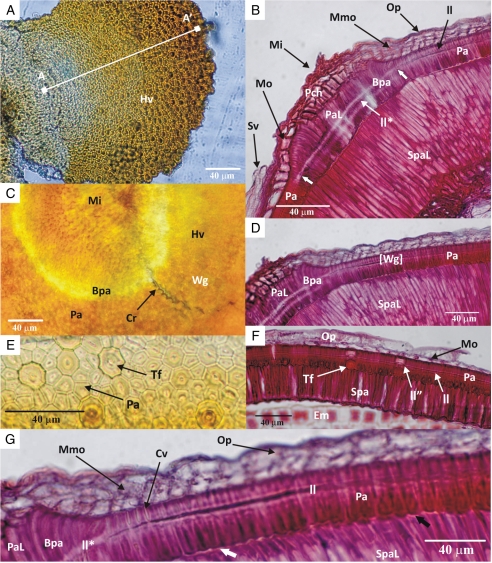
The palisade layer forms a continuous water-impermeable layer except in the chalazal region. Near the chalazal region of mature seeds, the chalazal plug originates from the nucleus and fills the gap between palisade cells. The palisade cells are polygonal (transverse section), and they can have from four to twelve sides. Normally, when compared with cells with four to six sides, cells with more than six sides are darker, larger and contain larger cell depositions at the base of the cell. These are called tanniferous cells (Fig. 6E, F). The light line in the tanniferous cells is slightly more raised than it is in normal palisade cells.
Fig. 6.
Light micrographs of G. carolinianum: (A) top view of hinged valve (the line A–A′ indicates the increasing size of the palisade cells); (B) longitudinal section through micropylar region of a dormant seed (the two short thick white arrows demarcate the widened light line in the micropylar region); (C) periclinal section through the water gap with the hinged valve; (D) longitudinal section of water gap; (E) periclinal section of the palisade layer away from the water gap; (F) longitudinal section of the seed coat away from the water gap; (G) close-up of a longitudinal section of a water gap (the short, thick white or black arrow indicates subpalisade cells with smooth or rough outer periclinal walls, respectively. Abbrevioations: Bpa, bent palisade cells; Cr, crack demarcates the margin of the water gap; Cv, cell lumen; Em, embryo; Hv, hinged valve; ll, light line; ll*, widened light line in the micropylar region; ll'', raised light line in the tanniferous cells; Mi, micropyle; Mmo, multi-layered middle parenchyma cells; Mo, single layer of middle parenchyma cells; Op, outermost polygonal parenchyma cell layer; Pa, palisade cells; PaL, elongated palisade cells of micropylar region; Pch, parenchyma cells of micropyle; Spa, subpalisade cells; SpaL, elongated subpalisade cells of micropylar region; Sv, seed-coat vascular tissue; Tf, tanniferous cells in palisade layer; Wg, water gap open; [Wg], water gap closed.
The anatomy of the seed coat in the micropylar region differs from that of the seed coat away from the micropyle. Near the micropylar region, the middle parenchyma cell layer of the outer integument is several layers thick, and the palisade cells are radially elongated. These elongated cells are lighter in colour than the palisade cells away from the micropylar region and have a wider light line (Fig. 6B, C). Elongated palisade cells form a dome-shaped structure by bending sideways, and the top of the dome is formed by parenchyma cells (Fig. 6B). Subpalisade cells in the micropylar region are multilayered, and the cells are elongated radially. Consequently, these subpalisade cells appear to be taller than other subpalisade cells away from the micropyle (Fig. 6B).
Palisade cells of the ‘hinged valve’ comprise elongated and bent palisade cells of the micropyle and non-elongated palisade cells of the micropylar region. These palisade cells differ from palisade cells away from the water-gap region due to the absence of tanniferous cells in this region. Near the micropyle, the width of palisade cells is less than that of normal palisade cells, and their size increases towards the radicle end of the seed. The inner periclinal cell wall of the palisade cells near the micropyle is flat and becomes convex towards the radicle. Also, cell lumens are visible above the light line of the palisade cells near the micropyle (Fig. 6G).
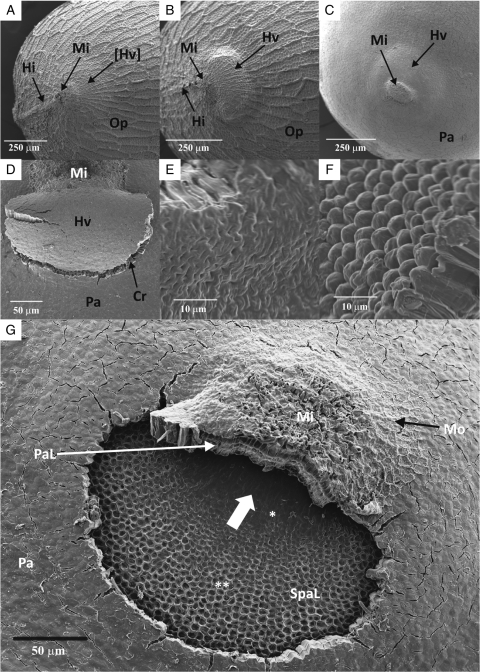
Electron microscopy
No apparent morphological differences were observed between dormant and non-dormant (heat-treated) seeds with or without outer cell layers (Fig. 7A, C). When permeable seeds started to imbibe water, a ‘clam shell’-shaped blister formed near the microplylar region towards the radicle end of the seed (Fig. 7B, D). The blister was formed by swelling of palisade cells in this region, and it remained hinged to elongated palisade cells of the micropyle (Fig. 7C). Apparently, the outer two cell layers also play a role in preventing the blister from detaching. After 20 min, the blister was detached from the imbibed seeds (without outer cell layers) revealing an opening (Fig. 7G). The cell size of the palisade and subpalisade layers of the water gap increased gradually – from the micropyle to the radicle end. Also, it was observed that the inner periclinal walls of palisade cells of the water gap were flat near the micropyle and became convex towards the radicle end (Fig. 7E, F). Accordingly, outer periclinal walls of subpalisade cells of the water gap are flat and concave (Fig. 7G).
Fig. 7.
Scanning electron micrographs of Geranium carolinianum seeds: (A) micropylar area of a dormant seed; (B) micropylar area of a non-dormant (heat-treated) seed immersed in water for 10 min; (C) micropylar area of a non-dormant seed without outer permeable cell layers; (D) raised hinged valve of a non-dormant seed without outer permeable cell layers (soaked in water for 10 min); (E) relatively smooth inner periclinal cell walls of water-gap palisade cells near the micropyle; (F) convex-shaped inner periclinal cell walls of water-gap palisade cells near radicle end; (G) water-gap opening of a non-dormant seed without outer permeable cell layers and hinged valve dislodged (soaked in water for 20 min). Abbreviations: Cr, crack demarcates the margin of the water gap; Hi, hilum; Hv, hinged valve (opened); [Hv], hinged valve (closed); Mi, micropyle; Mo, single layer of middle parenchyma cells; Op, outermost polygonal parenchyma cell layer; Pa, palisade cells; PaL, elongated palisade cells of the micropyle; SpaL, elongated subpalisade cells; *, subpalisade cells with smooth outer periclinal cell wall; **, subpalisade cells with concave outer periclinal cell wall. The large white short arrow in (G) indicates the region of initial water uptake.
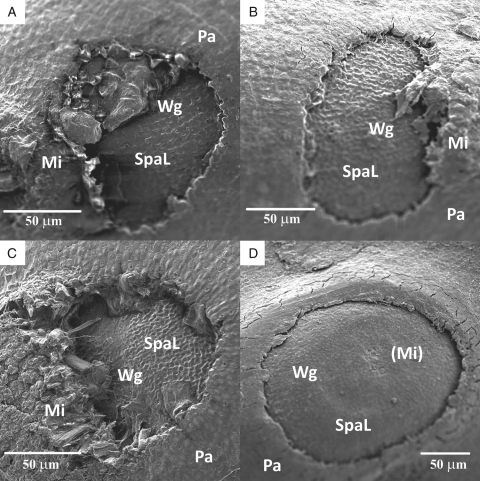
The water gap of G. columbinum, G. molle and G. pusillum seeds is similar in shape and location to that of G. carolinianum (Fig. 8A–C). However, the water-gap morphology of E. cicutarium differs from that of the Geranium spp. in that the whole micropylar region, including the micropyle, is removed during blister formation (Fig. 8D).
Fig. 8.
Scanning electron micrographs of water gaps of Geraniaceae species: (A) G. molle; (B) G. pusillum; (C) G. columbinum; (D) E. cicutarium. Mi, Micropyle; (Mi) location of the micropyle before it is dislodged due to imbibition. Abbreviations: Pa, palisade cells; SpaL, elongated subpalisade cells of the micropylar region; Wg, water gap.
Effect of morphological changes of seed coat during burial on seed germination
During burial, the outer permeable cell layers were damaged or lost. A change of colour near the micropylar region was observed in some seeds (Fig. 9B) similar to that in seeds after heat treatment. Seeds with a change in colour germinated to a significantly higher percentage (100 %) than those without a colour change (20 ± 3·16 %; P < 0·05).
Fig. 9.
Micropylar region of G. carolinianum seeds exhumed after 4 months of burial in soil at 2 cm depth: (A) impermeable seed; (B) permeable seed. Abbreviations: Hi, hilum; Mi, micropyle; Wg, water gap open; [Wg], water gap closed.
DISCUSSION
The two seed-coat colours in G. carolinianum can be attributed to different degrees of radial wall thickening in the outermost polygonal parenchyma cell layer of the outer integument. This cell layer along with the middle cell layer(s) is responsible for the reticulate appearance on the surface of seeds of Geraniaceae (Boesewinkel and Been, 1979; Schulz et al., 1991; Aedo et al., 1998a). Seeds with additional cell wall thickening in the outermost polygonal cell layer are lighter in colour, while those with less thickening are darker in colour. Due to the higher degree of thickening, the seed width of light-coloured seeds is significantly higher than that of darker seeds. However, the two seed types do not differ significantly in length, mass or seed-coat anatomy.
Even though all of the fresh scarified seeds of both seed types imbibed water within 24 h, the percentage germination remained very low within the first 7 d. However, it reached a mean of 92 % germination by the end of 10 d. This slow rate of germination indicates the presence of some non-deep PD in the embryo. Thus, the seeds have (PY + PD). PD of intact seeds was lost within 2 months, and then scarified seeds germinated rapidly. Loss of PD in Geraniaceae during dry storage has been reported by Baskin and Baskin (1974) and Van Assche and Vandelook (2006).
According to Meisert (2002), some physically dormant species of Geraniaceae have an innately permeable seed fraction. This fraction is <3 % in G. carolinianum. The permeable seed fraction of G. carolinianum seems to comprise three categories; viz. immature seeds, seeds with open chalaza and seeds with cracked seed coats. Seeds with PY acquire impermeability during maturation drying in the last stages of seed development (Egley, 1976; Egley et al., 1983; Baskin and Baskin, 1998; Li et al., 1999a; Jayasuriya et al., 2007; N. S. Gama-Arachchige et al., unpubl. res.). If seeds are shed prematurely before development of PY, they may not become dormant due to lack of depositions such as lignin, wax, phenolic compounds, etc. that make them impermeable (Werker, 1997). The width of the chalazal opening plays a role in impermeable and permeable seed fractions in Geraniaceae. Water-permeable seeds form a wider opening than impermeable seeds in the chalazal slit (Meisert et al., 1999). Cracks in the seed coat may also be responsible for the occurrence of an innately permeable seed fraction (Ma et al., 2004). Cracks in both permeable and impermeable seeds of G. carolinianum were observed in scanning electron micrographs. If the cracks have reached the subpalisade layer, seeds may have become permeable.
The outermost cell layers of the outer integument are not responsible for seed-coat dormancy due to the lack of mechanical barriers and presence of stomata. However, as observed by Boesewinkel and Been (1979) in seeds of Geranium pratense, wax deposited on cell walls may act as a partial barrier to water. Apparently, when buried in soil these cell layers can be removed easily due to abrasion caused by soil particles and microbial and chemical breakdown. In the present study, removal of these outer cell layers in impermeable seeds did not break PY. Also, in dye-tracking experiments these cell layers were stained by dye in both permeable and impermeable seeds, indicating their permeability. Therefore, as reported in other species of Geraniaceae, PY of G. carolinianum is maintained by an impermeable palisade layer. The palisade layer is yellowish brown in vibratome sections, due to lignin and tannin depositions, while the other entire cell layers in the seed coat are colourless.
Impermeable seeds subjected to dry heat (80 °C) for 1 week became permeable and could be identified by their brownish orange colour in the area of the water gap. This colour change is due to detachment of the palisade layer from the subpalisade layer in the water gap. We suggest that when the two layers separate, calcium oxalate crystals are shattered and cause the incident light to scatter, resulting in lightening of colour. The colour change was also observed in fresh seeds when pressure was applied on the micropylar area. Formation of a blister of a lighter colour in the chalazal region after application of a small force was reported in Sida spinosa (Egley and Paul, 1981). Therefore, the colour change in the water gap of G. carolinianum during heat treatment may not be due to a chemical change. Heat-treated seeds with a colour change germinated to 100 %, while none of the non-treated seeds germinated. Thus, the colour change in the water-gap region after heat treatment is related to breaking of dormancy in G. carolinianum seeds.
After 4 months of burial in soil, the germination of seeds with a colour change in the water-gap area was significantly higher (100 %) than those without visible colour change (20 ± 3·2 %). These results are similar to those of heat-treated and non-treated seeds. The colour change in the water gap begins at the micropyle and gradually spreads toward the radicle end of the seed. The initial stages of colour change are not visible even under a dissecting microscope. This possibly explains why a considerable amount of germination in seeds without a visible colour change was obtained.
The micropyle, lumens (located above the light line) and cracks in ‘hinged valve’ palisade cells near the micropyle may play an important role in imbibition. When the palisade cells of the ‘hinged valve’ imbibe water, they immediately start to swell, forming a blister in the micropylar region. When the blister erupts, it exposes an opening (water gap) near the micropyle towards the radicle end of the seed. In heat-treated seeds, the outer permeable cell layers prevent dislodgement of the blister, but in buried seeds the blister may be dislodged due to previous erosion of these outer cell layers.
Dye-tracking showed that water enters through the water gap and not through the chalaza, since the subpalisade layer was stained first below the water gap and not in the chalazal region. Moreover, the embryo imbibed first near the water gap after 1·5 h, while it remained non-imbibed near the chalaza. Swelling of palisade and subpalisade cells and of the embryo started first near the micropyle and then extended towards the chalaza. This pattern of swelling confirms that movement of water after opening of the water gap is radial and is directed towards the chalaza. Once cells near the chalaza are swollen, more water can be imbibed through the chalazal opening. Movement of the dye lagged behind that of water. This may be due to the larger size of dye molecules and lack of affinity of the dye for cell walls, thus slowing the movement of the dye. Moreover, the embryo was not stained by acid fuchsin or by methylene blue even after 24 h of incubation. These results agree with those obtained for Dodonaea petiolaris (Turner et al., 2009).
The idea that initial water entry occurs first through the water gap near the micropyle and not through the chalazal opening was also supported by blocking experiments. Within the first 24 h of incubation, the percentage imbibition of seeds with the micropylar region sealed was significantly lower than that of those with the chalaza sealed. Even after 72 h, the difference remained the same. Therefore, water should first enter through the water gap to initiate imbibition. The uptake of some water even after blocking the water-gap region may be due to imperfect binding of the glue. As previously reported by Egley and Paul (1981) and Turner et al. (2009), incomplete blocking is a common problem associated with sealants in blocking experiments. The role of the chalazal opening in initial water uptake can be disregarded since there was no significant difference in percentage imbibition of seeds with the chalaza blocked and with both the chalaza and micropylar regions blocked. Blocking of the water gap only (i.e. without also blocking the micropyle) was not successful due to their closeness to each other. Therefore, the role of the micropyle in initial water uptake is still unresolved.
Meisert et al. (1999) documented the chalazal slit as the site of water entry into innately permeable seeds of Geraniaceae. In that study, the authors compared the chalazal anatomy of water-permeable and impermeable seeds. The permeable seed fraction of Pelargonium mollicomum and seeds of the permeable species, Erodium manescavii, form a wide chalazal opening that maintains permeability, while the impermeable seed fraction of P. mollicomum and seeds of the PY species P. candicans form a narrow opening through which water does not pass. Meisert et al. (1999) used OsO4 to visualize the entry of water into innately permeable P. mollicomum seeds and concluded that the chalazal opening was the main site of water entry into the seed. However, they documented neither the location of initial movement of water through innately permeable seeds nor the water gap of impermeable seeds after breaking PY in Geraniaceae. The present study clearly shows that the initial water uptake in PY seeds after breaking of dormancy takes place through the water gap near the micropyle and not via the chalazal opening.
Baskin and Baskin (1974) suggested that the hilum functions as a ‘hygroscopic valve’ that is involved in initial water entry into the seed, but based on the reports of Boesewinkel and Been (1979), they subsequently reported the chalaza as the water gap in Geraniaceae (Baskin and Baskin, 1998; Baskin et al., 2000). Nell et al. (1981) compared the morphology of the hilum region of four cultivars of Pelargonium hortorum using scanning electron microscope photographs and concluded that there is no relationship between occlusion of the hilar fissure and seed germination. The hilar slit acts as the water gap in certain species like Cercis canadensis (Jones and Geneve, 1995; Geneve, 2009), Cuscuta australis (Jayasuriya et al., 2008) and Sophora alopecuroides (Hu et al., 2008). However, unlike the hilar fissure in these three species, the hilar fissure in Geraniaceae is located in the outer permeable layers of the seed coat and thus cannot act as a water gap.
Gillespie and Andersen (2005) suggested that physical abrasions may play a role in the breaking of PY in E. macrophyllum (= C. macrophyllum). This would occur when seeds are drilled into soil by coiling and uncoiling of their awns in response to changing relative humidity levels. However, Baskin and Baskin (2000) argue that it is rather unrealistic to consider that abrasive action, which is an ever-present probability in the physical environment, has a significant effect on breaking dormancy in nature. Such a mechanism would not allow for control of timing of dormancy break, which is a critical event in the adaptation of plants to their environment.
Schulz et al. (1991) compared scanning electron microscope photographs of the seed coat of water-permeable and impermeable varieties of Pelargonium zonale before and after scarification with conc. H2SO4. They concluded that the palisade layer no longer acts as a water-impermeable barrier after the seeds are acid scarified. However, they did not show the effect of H2SO4 on either the chalazal or micropylar region, and therefore the effect of acid scarification on opening of the water gap is not clear.
Germination of physically dormant seeds occurs only after a plug or lid that closes the discontinuity (‘water gap’) in the water-impermeable layer(s) is dislodged or disrupted, thereby creating an opening for entrance of water to the embryo (Baskin et al., 2000). Nine types of water gaps in seven angiosperm families (excluding Geraniaceae) have been characterized previously: (1) a bulge gap adjacent to the micropyle in Convolvulaceae (Jayasuriya et al., 2007); (2) a carpellary micropyle in Anacardiaceae (Li et al., 1999b); (3) a chalazal blister gap in Malvaceae–Malveae (La Croix and Staniforth, 1964; Egley and Paul, 1981); (4) a chalazal opening in Malvaceae–Gossypieae (Christiansen and Moore, 1959; Simpson et al., 1940) and Hibisceae (Serrato-Valenti et al., 1992; Poljakoff-Mayber et al., 1994); (5) a gap adjacent to the hilum in Sapindaceae (Turner et al., 2009); (6) a hilar slit in Convolvulaceae (Jayasuriya et al., 2008), Fabaceae–Caesalpinoideae (Jones and Geneve, 1995; Geneve, 2009) and Faboideae (Hu et al., 2008); (7) a lens gap in Fabaceae–Caesalpinoideae (Lersten et al., 1992), Mimosoideae (Dell, 1980; Hanna, 1984; Morrison et al., 1998) and Faboideae (Morrison et al., 1998); (8) a protruberance in Nelumbonaceae (Ohga, 1926); and (9) a raphal scar in Cannaceae (Grootjen and Bouman, 1988; Graven et al., 1997; Mass-Van De Kamer and Mass, 2008).
Even though the location of the water gap of G. carolinianum seeds shows some similarity to that of Ipomoea lacunosa (Jayasuriya et al., 2007), they differ in that two openings are formed in I. lacunosa. Moreover, blister formation in G. carolinianum is similar to that of Abutilon theophrasti (La Croix and Staniforth, 1964), Albizia lophantha (Dell, 1980), Sida spinosa (Egley and Paul, 1981, 1982; Egley et al., 1986), Acacia kempeana (Hanna, 1984), Canna tuerckheimii (Grootjen and Bouman, 1988), I. lacunosa (Jayasuriya et al., 2007) and Dodonaea petiolaris (Turner et al., 2009). However, the water gap in G. carolianum differs from all other characterized blister-forming water-gap types in anatomy, morphology or location. Therefore, the ‘hinged valve gap’ adjacent to the micropyle in Geraniaceae is the tenth water-gap type to be characterized.
Based on scanning electron micrographs, the morphology of the water gaps of G. columbinum, G. molle and G. pusillum are similar in shape and location, and one half of the micropyle is retained when the blister is dislodged from the seed coat in all three species. In E. cicutarium, the whole micropylar region, including the micropyle, is removed when the blister is dislodged from the seed coat, and thus the water gap differs from that in these three Geranium spp. Therefore, as documented in Convolvulaceae, Fabaceae and Malvaceae, Geraniaceae also may have more than one type of water gap. Further studies on water gaps of all six genera (California, Erodium, Hypseocharis, Geranium, Monsonia and Pelargonium) of Geraniaceae are required to determine whether differences exist in the ‘hinged valve gap’ in terms of anatomy and morphology within the family.
Opening of the water gap is essential for germination of G. carolinianum. During the initial stages of imbibition in permeable seeds (after PY is broken), eruption of the special structure, ‘hinged valve’, adjacent to the micropyle opens the water gap in Geraniaceae. In G. carolinianum, morphology of the water gap of seeds made permeable by exposing them to natural conditions was observed to be similar to that of heat-treated seeds. Therefore, as predicted by Baskin and Baskin (1974), dry hot weather conditions in summer can be an environmental factor affecting the dormancy breaking of G. carolinianum under natural conditions. The present study reveals that the ‘hinged valve’ gap, which is adjacent to the micropyle, functions as the water gap in seeds of G. carolinianum after PY is broken.
ACKNOWLEDGEMENTS
We would like to express our sincere gratitude to Dr Sharyn E. Perry, Department of Plant and Soil Sciences, University of Kentucky, for allowing us to use the microtome and tissue-sectioning equipment; Ms Sharon T. Kester, Department of Horticulture, University of Kentucky, for helping with sectioning and staining of tissues; and Mr Larry Rice, Electron Microscopy Centre, University of Kentucky, for providing technical assistance with scanning electron microscopy imaging.
LITERATURE CITED
- Aedo C. The genus Geranium L. (Geraniaceae) in North America. I. annual species. Anales Jardín Botánico de Madrid. 2000;58:39–82. [Google Scholar]
- Aedo C, Aldasoro JJ, Navarro C. Taxonomic revision of Geranium sections Batrachioidea and Divaricata (Geraniaceae) Annals of the Missouri Botanical Garden. 1998a;85:594–630. [Google Scholar]
- Aedo C, Garmendia FM, Pando F. World checklist of Geranium L. (Geraniaceae) Anales Jardín Botánico de Madrid. 1998b;56:211–252. [Google Scholar]
- Aldasoro JJ, Navarro C, Vargas P. California, a new genus of Geraniaceae endemic to the southwest of North America. Anales Jardín Botánico de Madrid. 2002;59:209–216. [Google Scholar]
- Baskin CC. Breaking physical dormancy in seeds – focussing on the lens. New Phytologist. 2003;158:229–232. [Google Scholar]
- Baskin CC, Baskin JM. Seeds: ecology, biogeography, and evolution of dormancy and germination. San Diego, CA: Academic Press; 1998. [Google Scholar]
- Baskin JM, Baskin CC. Some eco-physiological aspects of seed dormancy in Geranium carolinianum L. from central Tennessee. Oecologia. 1974;16:209–219. doi: 10.1007/BF00345883. [DOI] [PubMed] [Google Scholar]
- Baskin JM, Baskin CC. Evolutionary considerations of claims for physical dormancy-break by microbial action and abrasion by soil particles. Seed Science Research. 2000;10:409–413. [Google Scholar]
- Baskin JM, Baskin CC, Li X. Taxonomy, anatomy and evolution of physical dormancy in seeds. Plant Species Biology. 2000;15:139–152. [Google Scholar]
- Baskin JM, Baskin CC, Dixon KW. Physical dormancy in the endemic Australian genus Stylobasium, a first report for the family Surianaceae (Fabales) Seed Science Research. 2006;16:229–232. [Google Scholar]
- Boesewinkel FD. The seed structure and taxonomic relationships of Hypseocharis remy. Acta Botanica Neerlandica. 1988;37:111–120. [Google Scholar]
- Boesewinkel FD, Been W. Development of ovule and testa of Geranium pratense L. and some other representatives of the Geraniaceae. Acta Botanica Neerlandica. 1979;28:335–348. [Google Scholar]
- Britton NL. Flora of Bermuda. New York, NY: Charles Scribner's Sons; 1918. [Google Scholar]
- Christiansen MN, Moore RP. Seed coat structural differences that influence water uptake and seed quality in hard seed cotton. Agronomy Journal. 1959;51:582–584. [Google Scholar]
- Dell B. Structure and function of the strophiolar plug in seeds of Albizia lophantha. American Journal of Botany. 1980;67:556–563. [Google Scholar]
- Egley GH. Germination of developing prickly sida seeds. Weed Science. 1976;24:239–243. [Google Scholar]
- Egley GH, Paul RN. Morphological observations on the early imbibition of water by Sida spinosa (Malvaceae) seed. American Journal of Botany. 1981;68:1056–1065. [Google Scholar]
- Egley GH, Paul RN. Development, structure and function of subpalisade cells in water impermeable Sida spinosa seeds. American Journal of Botany. 1982;69:1402–1409. [Google Scholar]
- Egley GH, Paul RN, Lax AR. Seed coat imposed dormancy – histochemistry of the region controlling onset of water entry into Sida spinosa seeds. Physiologia Plantarum. 1986;67:320–327. [Google Scholar]
- Egley GH, Paul RN, Vaughn KC, Duke SO. Role of peroxidase in the development of water-impermeable seed coats in Sida spinosa L. Planta. 1983;157:224–232. doi: 10.1007/BF00405186. [DOI] [PubMed] [Google Scholar]
- Fiz O, Vargas P, Alarcon ML, Aldasoro JJ. Phylogenetic relationships and evolution in Erodium (Geraniaceae) based on trnL-trnF sequences. Systematic Botany. 2006;31:739–763. [Google Scholar]
- Fiz O, Vargas P, Alarcon M, Aedo C, Garcia JL, Aldasoro JJ. Phylogeny and historical biogeography of Geraniaceae in relation to climate changes and pollination ecology. Systematic Botany. 2008;33:326–342. [Google Scholar]
- Geneve RL. Physical seed dormancy in selected caesalpinioid legumes from eastern North America. Propagation of Ornamental Plants. 2009;9:129–134. [Google Scholar]
- Gillespie IG, Andersen HM. Seed germination of Erodium macrophyllum (Geraniaceae) Madroño. 2005;52:123–124. [Google Scholar]
- Graven P, DeKoster CG, Boon JJ, Bouman F. Functional aspects of mature seed coat of the Cannaceae. Plant Systematics and Evolution. 1997;205:223–240. [Google Scholar]
- Grootjen CJ, Bouman F. Seed structure in Cannaceae – taxonomic and ecological implications. Annals of Botany. 1988;61:363–371. [Google Scholar]
- Hanna PJ. Anatomical features of the seed coat of Acacia kempeana (Mueller) which relate to increased germination rate induced by heat-treatment. New Phytologist. 1984;96:23–29. [Google Scholar]
- Haragan PD. Weeds of Kentucky and adjacent states. Lexington, KY: University Press of Kentucky; 1991. [Google Scholar]
- Horn JW. The morphology and relationships of the Sphaerosepalaceae (Malvales) Botanical Journal of the Linnean Society. 2004;144:1–40. [Google Scholar]
- Hu XW, Wang YR, Wu YP, Nan ZB, Baskin CC. Role of the lens in physical dormancy in seeds of Sophora alopecuroides L. (Fabaceae) from north-west China. Australian Journal of Agricultural Research. 2008;59:491–497. [Google Scholar]
- Jayasuriya KMGG, Baskin JM, Geneve RL, Baskin CC. Morphology and anatomy of physical dormancy in Ipomoea lacunosa: identification of the water gap in seeds of Convolvulaceae (Solanales) Annals of Botany. 2007;100:13–22. doi: 10.1093/aob/mcm070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayasuriya KMGG, Baskin JM, Geneve RL, Baskin CC, Chien CT. Physical dormancy in seeds of the holoparasitic angiosperm Cuscuta australis (Convolvulaceae, Cuscuteae): dormancy-breaking requirements, anatomy of the water gap and sensitivity cycling. Annals of Botany. 2008;102:39–48. doi: 10.1093/aob/mcn064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RL. Plant life of Kentucky: an illustrated guide to the vascular flora. Lexington, KY: University Press of Kentucky; 2005. [Google Scholar]
- Jones RO, Geneve RL. Seed-coat structure related to germination in eastern redbud (Cercis canadensis L.) Journal of the American Society for Horticultural Science. 1995;120:123–127. [Google Scholar]
- Kay GF, Lees JH. The weed flora of Iowa. Des Moines, IA: Robert Henderson State Printer; 1913. Geologic Survey Bulletin No. 4. [Google Scholar]
- La Croix LJ, Staniforth DW. Seed dormancy in velvetleaf. Weeds. 1964;12:171–174. [Google Scholar]
- Lersten NR, Gunn CR, Brubaker CL. Comparative morphology of the lens on legume (Fabaceae) seeds, with emphasis on species in subfamilies Caesalpinoideae and Mimosoideae. Washington, DC: US Department of Agriculture; 1992. United States Department of Agriculture Technical Bulletin No. 1791. [Google Scholar]
- Li XJ, Baskin JM, Baskin CC. Comparative morphology and physiology of fruit and seed development in the two shrubs Rhus aromatica and R. glabra (Anacardiaceae) American Journal of Botany. 1999a;86:1217–1225. [PubMed] [Google Scholar]
- Li XJ, Baskin JM, Baskin CC. Anatomy of two mechanisms of breaking physical dormancy by experimental treatments in seeds of two North American Rhus species (Anacardiaceae) American Journal of Botany. 1999b;86:1505–1511. [PubMed] [Google Scholar]
- Ma F, Cholewa E, Mohomad T, Peterson CA, Gigen M. Cracks in the palisade cuticle of soybean seeds correlate with their permeablity to water. Annals of Botany. 2004;94:213–228. doi: 10.1093/aob/mch133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mass-Van De Kamer H, Maas PJM. The Cannaceae of the world. Blumea. 2008;53:247–318. [Google Scholar]
- Meisert A. Physical dormancy in Geraniaceae seeds. Seed Science Research. 2002;12:121–128. [Google Scholar]
- Meisert A, Schulz D, Lehmann H. Structural features underlying hardseededness in Geraniaceae. Plant Biology. 1999;1:311–314. [Google Scholar]
- Meisert A, Schulz D, Lehmann H. The ultrastructure and development of the light line in the Geraniaceae seed coat. Plant Biology. 2001;3:351–356. [Google Scholar]
- Morrison DA, McClay K, Porter C, Rish S. The role of the lens in controlling heat-induced breakdown of testa-imposed dormancy in native Australian legumes. Annals of Botany. 1998;82:35–40. [Google Scholar]
- Nandi OI. Ovule and seed anatomy of Cistaceae and related Malvanae. Plant Systematics and Evolution. 1998;209:239–264. [Google Scholar]
- Narayana HS, Arora PK. The embryology of Monsonia senegalensis Guill. & Perr. The American Midland Naturalist. 1963;70:309–318. [Google Scholar]
- Nell TA, Marsh PM, Cantliffe DJ. Seed dormancy and germination of geranium (Pelargonium hortorum Bailey) Journal of the American Society for Horticultural Science. 1981;106:509–513. [Google Scholar]
- Nishida T, Yamashita N. Developing a pre-entry weed risk assessment system for use in Japan. Biological Invasions. 2009;11:1319–1333. [Google Scholar]
- Ohga I. On the structure of some ancient, but still viable fruits of Indian lotus, with special reference to their prolonged dormancy. Japanese Journal of Botany. 1926;3:1–19. [Google Scholar]
- Peng CI. Some new records for the flora of Taiwan. Botanical Bulletin of Academia Sinica. 1978;19:83–86. [Google Scholar]
- Poljakoff-Mayber A, Somers GF, Werker E, Gallagher JL. Seeds of Kosteletzkya virginica (Malvaceae): their structure, germination, and salt tolerance. II. Germination and salt tolerence. American Journal of Botany. 1994;81:54–59. [Google Scholar]
- Piper CV. Flora of the state of Washington. Vol. XI. Washington, DC: Smithsonian Institution; 1906. p. 379. Contributions from the United States National Herbarium. [Google Scholar]
- Schulz D, Bachthaler E, Kunz U. Seed coat structure of Pelargonium zonale. Gartenbauwissenschaft. 1991;56:118–126. [Google Scholar]
- Serrato-Valenti G, Cornara L, Lotito S, Quagliotti L. Seed coat structure and histochemistry of Abelmoschus esculentus. Chalazal region and water entry. Annals of Botany. 1992;69:313–321. [Google Scholar]
- Simpson DM. Anatomical structure of the cottonseed coat as related to problems of germination. Washington, DC: US Department of Agriculture; 1940. United States Department of Agriculture Technical Bulletin No.734. [Google Scholar]
- Small JK. North American flora. Bronx Park, NY: New York Botanical Garden; 1907. [Google Scholar]
- Spencer ER. All about weeds. New York, NY: Dover Publications; 1976. [Google Scholar]
- Turner SR, Cook A, Baskin JM, et al. Identification and characterization of the water gap in the physically dormant seeds of Dodonaea petiolaris: a first report for Sapindaceae. Annals of Botany. 2009;104:833–844. doi: 10.1093/aob/mcp171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Assche JA, Vandelook FEA. Germination ecology of eleven species of Geraniaeae and Malvaceae, with special reference to the effect of drying seeds. Seed Science Research. 2006;16:283–290. [Google Scholar]
- Werker E. Seed antomy. Berlin: Gebrüder Borntraeger; 1997. [Google Scholar]
- Xu L, Aedo C. Wu ZY, Raven PH, Hong DY, editors. Geraniaceae. Flora of China. 2008;Vol. 11:8–31. Oxalidaceae through Aceraceae Beijing: Science Press/St Louis, MO: Missouri Botanical Garden Press. [Google Scholar]