Abstract
Autoantibody tests have been used extensively in diagnosis and follow-up of patients in rheumatology clinics. Immunofluorescent antinuclear antibody test using HEp-2 cells is still considered the gold standard for screening of autoantibodies, and most of specific autoantibodies are currently tested by ELISA as a next step. Among the many autoantibody specificities described, some have been established as clinically useful diagnostic markers and are included in the classification criteria of diseases. Despite a long history of routine tests and attempts to standardize such assays, there are still limitations and problems that clinicians need to be aware of. Clinicians should be able to use autoantibody tests more efficiently and effectively with a basic knowledge on the significance of and potential problems in autoantibody tests.
Keywords: Antinuclear antibodies, Autoantibodies, SLE, Scleroderma, Polymyositis
Introduction
Autoimmune disease is defined as a condition with tissue destruction or organ malfunction caused by autoimmune mechanisms. It is often more generously interpreted as a disease accompanied by an autoimmune phenomenon, since the direct role of the autoimmune reaction in the disease pathogenesis is not always apparent. Systemic autoimmune diseases (as opposed to organ-specific autoimmune diseases) are characterized by the presence of non-organ-specific autoantibodies that target antigens that are present in virtually any type of cell. Clinically, they are characterized by the systemic involvement of autoimmune tissue destruction in various organs. Systemic autoimmune diseases, autoimmune rheumatic diseases, and systemic rheumatic diseases basically refer to the same category of diseases, and these terms are used interchangeably. Systemic lupus erythematosus (SLE), scleroderma (SSc), polymyositis/dermatomyositis (PM/DM), and rheumatoid arthritis (RA) are representative disorders in this category. Whether Sjögren’s syndrome (SjS) should be classified as organ-specific (salivary glands, lacrimal glands) or systemic is arguable.
The presence of autoantibodies that react with cellular constituents, including proteins and nucleic acids (dsDNA, RNA), can be screened for by observing mammalian cells on a slide [1]. The incubation of human sera with fixed cells is followed by fluorochrome (such as fluorescein isothiocyanate: FITC) conjugated antibodies against human IgG. Observing under a fluorescent microscope allows one to determine the patterns as well as titers of autoantibodies. This standard method of performing antinuclear antibody (ANA) tests by immunofluorescence has been used for over 40 years as a first-step screening test for autoimmune diseases and is still the standard method. Although the ANA test has a nearly 100% sensitivity for the diagnosis of SLE, it is not specific for this diagnosis and is frequently positive in other systemic autoimmune rheumatic diseases such as SSc, PM/DM, and SjS as well. ANA is also found in organ-specific autoimmune diseases, and in other nonautoimmune diseases such as viral infections [2]. In this review, we will focus on the clinical significance and interpretation of autoantibody data for clinicians, and provide a practical guide on the use of autoantibody tests for specific diseases.
Patterns of antinuclear antibodies (ANA)
Although it is usually called the ANA test, the same procedure also exhibits reactivity against all types of subcellular structures and cell organelles including cell surfaces, cytoplasm, nuclei, or nucleoli [1]. The antigens recognized are mainly proteins, protein macromolecular complexes, protein–nucleic acid complexes, and nucleic acids. In fact, most autoantibodies that are clinically useful target RNA–protein or DNA–protein complexes. The staining may be purely nucleolar, as seen in certain SSc patients, or purely cytoplasmic, as in anti-Jo-1 positive PM/DM patients. Thus, the ANA test is not just for “nuclear” staining.
The interpretation of most nuclear staining patterns is relatively straightforward, and they are usually reported as being nuclear, centromere, or nucleolar. Cytoplasmic staining may not be reported at all by some laboratories, and it is useful to know and distinguish whether it was read as negative or it was simply not reported by the technical staff at the laboratory. A positive nuclear staining result will usually come back with a more detailed staining pattern, such as speckled (Fig. 1a), homogeneous, or peripheral. A homogeneous/peripheral pattern reflects antibodies to histone/dsDNA/chromatin, whereas many other specificities found in systemic rheumatic diseases show speckled patterns of various sizes and densities (fine speckled, large speckled, etc.). Thus, while this information is somewhat useful, it is relatively subjective, and varies depending on the laboratory or individual; the pattern may also differ at different dilutions. A centromere pattern is usually reported as distinct pattern, but they can also be termed discrete speckled nuclear staining patterns (Fig. 1b). Unusual staining patterns in nuclei, such as those for the nuclear mitotic apparatus (NuMA) [3] (Fig. 1c), Cajal body (p80-coilin), or nuclear dots (Fig. 1d), may not be reported depending on the experience of the laboratory. The cytoplasmic staining shown by anti-Jo-1 (histidyl tRNA synthetase) antibodies in PM/DM or antimitochondrial antibodies in primary biliary cirrhosis (PBC) (Fig. 1d) is often either not reported or incorrectly interpreted. The staining from cytoplasmic dots called GW bodies (GWBs)/P-bodies (Fig. 1e) was ignored or unrecognized until recently [4], but this cytoplasmic structure has become a hot area of research in molecular and cellular biology following the discovery of the critical role it plays in the functions of siRNA and miRNA [5, 6]. Furthermore, anti-Su antibodies that recognize a component of GWB, Ago2 [7], are very common autoantibodies found in 10–20% of various systemic autoimmune diseases in American and Japanese populations [8] and ~25% of Mexican SLE patients (Vázquez-Del Mercado, manuscript in preparation). Thus, GWB staining from anti-Su antibodies is in fact a quite common staining pattern if it is recognized by an experienced laboratory [1]. Golgi patterns [9] may also be overlooked or not reported properly in routine screening tests [1]. Another example of unrecognized staining is a complete lack of reports of punctate nucleolar staining by anti-RNA polymerase I from hospital laboratories [10].
Fig. 1.
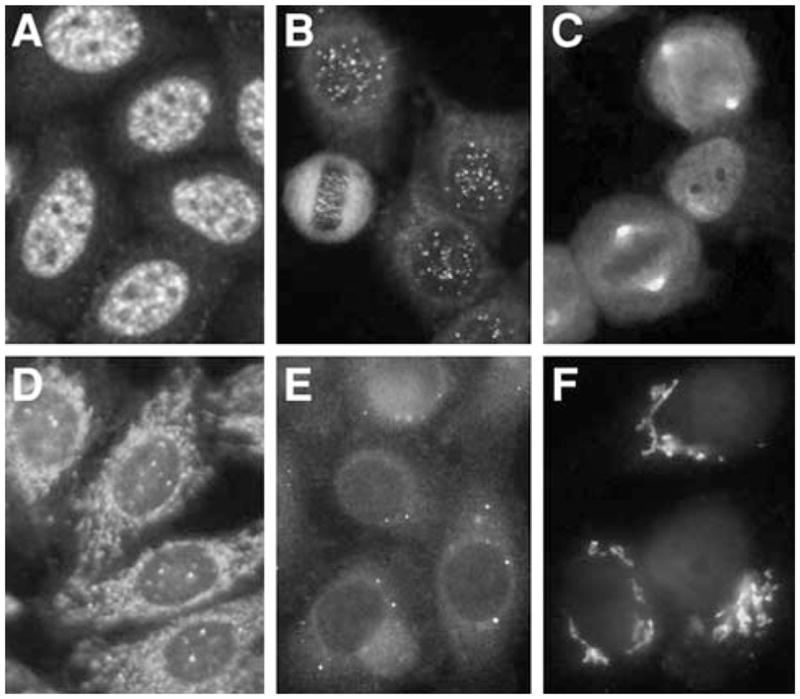
Immunofluorescent antinuclear antibodies in patients with rheumatic diseases. a Nuclear speckled pattern. b Centromere. c Mitotic spindle apparatus (NuMA). d Antimitochondria and nuclear dots. e GW body (GWB) staining by anti-Su/Ago2 antibodies. f Anti-Golgi antibodies
Any of the staining patterns described above are considered evidence of non-organ-specific autoimmunity. It is useful to know the significance of various staining patterns; however, in practice, it is equally important for clinicians to know what will be reported from their clinical laboratory, what may not be reported, and what may be incorrectly reported.
Titers of ANA
The introduction of human cancer cell lines (human laryngeal cancer cell line HEp-2 is the standard) as a substrate for the ANA test significantly increased the sensitivity in the detection of ANA in patients with systemic rheumatic diseases. However, a concomitant increase in positive results in healthy individuals decreased the specificity. One multicenter study reported that 31.7% of normal individuals were ANA positive at 1:40 dilution, which was decreased to 13.3% at 1:80 and 5.0% at 1:160 dilution. Since ~95% of SLE were still positive at 1:160 dilution, raising the negative cut-off titer from 1:40 to 1:160 may improve the distinction between a clinically significant ANA result and a positive ANA result occurring in a normal individual [2]. The frequency of positive ANA detection using this cut-off was 95% in SLE, 87% in SSc, 74% in SjS, and 14% in RA [2]. It is generally true that the prevalence of positive ANA among healthy individuals increases after switching the ANA substrate from frozen tissues to human cell lines; however, the reactivity is significantly affected by the cell substrate, fixation, secondary antibodies, buffer, fluorescent microscope, and other conditions. Thus, an appropriate cut-off should be defined by each laboratory [1].
Based on these observations, there is some truth in saying that higher titers of ANA are more clinically significant; ANA in healthy individual is generally in low titers. There is some evidence that clinical manifestations associated with certain autoantibodies are more evident among patients with high titers of that specificity. Sclerodactyly, Raynaud’s phenomenon and vascular disease associated with anticentromere antibodies are more common in patients with high titers of this specificity than in those with low titers [11]. Classic feature of mixed connective tissue disease (MCTD) is associated with very high titers of anti-U1RNP antibodies [12, 13]. However, it should be noted that higher titers of ANA do not always mean that the patient’s disease is more severe or active. Specificity of autoantibodies is one of the factors that correlate strongly with titers of ANA. Certain autoantibodies such as anti-dsDNA usually show relatively low titers in ANA, while others such as anti-U1RNP and centromere may show titers of 1:10,240 or even higher [11]. Individuals with high titers of these antibodies may lack serious organ involvement, being classified as having undifferentiated connective tissue disease (UCTD) or Raynaud’s disease, and may not require any medical treatment [14, 15]. On the other hand, patients with low titers of disease marker antibodies (see the next section) could have a typical disease requiring attention and follow-up.
Autoantibodies associated with a certain diagnosis (disease marker antibodies), particular symptoms, or disease activity
Several autoantibody specificities that are found almost exclusively in patients with a particular diagnosis and are useful for diagnosing or predicting the development of disease are called disease marker antibodies. Anti-Sm and anti-dsDNA antibodies are highly specific for the diagnosis of SLE and are included in the classification criteria for SLE by the American College of Rheumatology (ACR) [16]. ANA is also included among these criteria, and nearly all SLE patients are ANA positive; however, ANA is not specific for SLE. Antiphospholipid antibodies, listed under immunological disorders in the SLE criteria, are also common in SLE but can also be found in various systemic rheumatic diseases and often in patients with anti-phospholipid syndrome who do not fulfill the criteria for any rheumatic diseases. Antiribosomal P antibodies found in ~10% of patients and anti-PCNA (proliferating cell nuclear antigen) antibodies seen in ~2% of SLE patients are also considered to be disease-specific, but the supporting data are not as extensive as those for anti-dsDNA or anti-Sm [1, 17, 18]. An association between anti-ribosomal P antibodies and neuropsychiatric symptoms has been suggested for many years, but a recent meta-analysis indicated that anti-ribosomal P antibody testing has a negligible capacity to predict neuropsychiatric manifestations of SLE in individual cases [19].
Antitopoisomerase I (topo I, Scl-70), found in 15–25% of patients, and anti-RNA polymerase III (RNAP III) antibodies, found in 20–25% of patients, are highly specific for SSc [20–23]. Anticentromere antibodies were classically described in association with a subset of SSc, CREST syndrome (calcinosis, Raynaud’s phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasias) [24]; however, this specificity is also common in limited SSc without typical features of CREST syndrome. It can also be found in other diagnoses [25, 26] and in UCTD [11, 14]. Anti-U3RNP (fibrillarin) and anti-Th are also considered SSc markers, although they may also be found in idiopathic interstitial lung disease (ILD) or primary pulmonary hypertension without apparent SSc at low frequency [27, 28]. Antitopo I, RNAP III, and U3RNP are associated with diffuse SSc, whereas anticentromere and Th are mainly found in limited SSc. Severe ILD is frequently found in antitopo I positive patients, while it is rare in anti-RNAP III positive cases. Anti-RNAP III is strongly associated with scleroderma renal crisis. Isolated pulmonary hypertension is more common in patients with anticentromere, U3RNP, and Th. Other SSc-related autoantibodies are also associated with unique clinical features [23, 29–31].
In PM/DM, the anti-Jo-1 antibodies found in ~20% of patients are classic disease marker antibodies, and are associated with a unique subset of PM/DM called anti-synthetase syndrome, characterized by symptoms such as myositis, ILD, arthritis, Raynaud’s phenomenon, and mechanic’s hand. Autoantibodies to other aminoacyl tRNA synthetases including PL-7 (threonyl), PL-12 (alanyl), EJ (glycyl), OJ (isoleucyl), and KS (asparaginyl) are also associated with similar clinical features [32, 33]. Although either myositis or ILD can precede the other symptoms [34], anti-PL-12 and anti-KS may be more frequent than the others in idiopathic ILD without myositis [35]. Antibodies to SRP (signal recognition particle) are also specific for PM and are associated with severe myositis that occurs without histopathological inflammation and is resistant to treatment [36]; however, the unavailability of an anti-SRP test limits its clinical utility.
Other types of autoantibodies are found in various systemic rheumatic diseases, but they are associated with certain clinical symptoms, regardless of the diagnosis [1]. Autoantibodies in this category include anti-Ro/SS-A and La/SS-B (associated with SjS), anti-U1RNP (associated with Raynaud’s phenomenon, swollen hands, leukopenia, overlapping features such as sclerodactyly or myositis) [12], and anti-Ku (associated with muscle involvement) [37].
Anti-dsDNA antibodies are the only autoantibodies that may be used to monitor the disease activity of SLE. High levels of anti-dsDNA antibodies, often with hypocomplementemia, correlate with clinical activity in a subset of SLE, such as patients with proliferative nephritis [17]. Anti-dsDNA antibodies have been tested using various types of assays, including the Farr assay, PEG (polyethylene glycol) assay, Crithidia lucilliae assay, and ELISA [38]. Each assay has its advantages and disadvantages. The Crithidia lucilliae assay and Farr assays are specific but not particularly sensitive, whereas an ELISA can give a higher number of false positives, in part because there can be ssDNA domains/epitopes in many preparations.
Availability of testing for disease-related autoantibodies
Different groups of autoantibodies are strongly associated with SLE, PM/DM, and SSc, as described above. In many cases, patients positive for these specificities have distinctive clinical characteristics. However, despite detailed descriptions of these autoantibodies in virtually all textbooks or review articles [18, 30, 32, 33, 39], many of these tests are still not commercially available for clinicians [1].
In SLE, three autoantibody tests—anti-Sm, dsDNA, and phospholipid—included in the classification criteria of SLE are commercially available in addition to standard immunofluorescent ANA. Anti-dsDNA antibodies are detected at some point during the course in ~70% of patients. Anti-Sm is found in ~15% of patients. Antiribosomal P, available as P-peptide ELISA, is found in ~10% of patients (Table 1).
Table 1.
Autoantibody tests that are widely available and help diagnosis
| Specificity | Frequency | Disease specificity | Currently tested by |
|---|---|---|---|
| SLE | |||
| dsDNA | 50–80% | High | ELISA, Crithidia |
| Sm | 15% | High | ELISA, (DID) |
| Ribosomal P | 10% | High | ELISA |
| Scleroderma | |||
| Topo I | 15% | High | ELISA, (DID) |
| Centromere | 25% | Moderate | IF |
| RNA polymerase III (RNAP III) | 20% | High | ELISA |
| PM/DM | |||
| Jo-1 (his-tRNA) | 20% | High | ELISA (DID) |
| RA | |||
| CCP | 70% | Moderate | ELISA |
| Rheumatoid factor (RF) | 70% | Low | Laser nephelometry, ELISA, Latex agglutination |
| Sjögren’s syndrome | |||
| Ro/SS-A | 70% | Low | ELISA, (DID) |
| La/SS-B | 40% | Moderate | ELISA, (DID) |
| MCTD (Mixed Connective Tissue Disease) | |||
| U1RNP | 100% | Low | ELISA (DID) |
ELISA enzyme-linked immunosorbent assay, DID double immunodiffusion
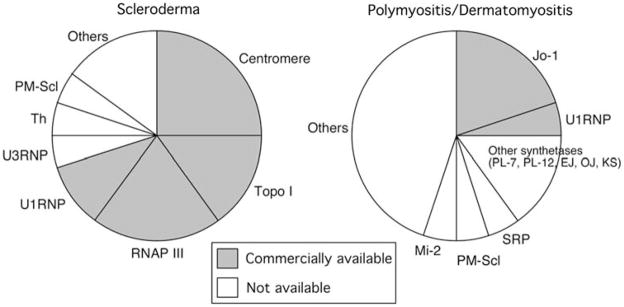
Among SSc-associated autoantibodies, only tests for anti-topo I (found in 15–25%, Fig. 2 left), anticentromere (20–25% by ANA), and anti-U1RNP (10%, frequency depends on whether MCTD is classified as a separate entity) were available until recently. Anti-RNAP III antibodies seen in ~20% of SSc patients have been described for 20 years [20–22]. However, anti-RNAP III has not become standard or received as much clinical appreciation as that anti-topo I despite its high specificity for SSc and tight link to scleroderma renal crisis [23, 30, 31]. This is mainly because anti-RNAP III can only be detected by immunoprecipitation, which has been performed at only a small number of institutes around the world. However, an anti-RNAP III ELISA kit [40] was approved by the Food and Drug Administration (FDA) of the United States in 2006 and has become widely available as a commercial test [10]. The high sensitivity and specificity of this ELISA were confirmed by several independent reports [10, 41–43]. With this addition, ~70% of SSc patients will have identifiable antibodies that should help with predicting prognosis and unique organ involvement. Tests for other autoantibodies including U3RNP (fibrillarin), Th, and PM-Scl are not commercially available.
Fig. 2.
Prevalence of autoantibodies associated with scleroderma and polymyositis/dermatomyositis (PM/DM). Commercially available tests (shaded) and other disease-related autoantibodies are indicated
In PM/DM, anti-Jo-1, found in ~20% of patients, is the only commercially available test for myositis-specific antibodies (Fig. 2 right). Anti-U1RNP, which is not specific for PM/DM, is found in ~5% of patients. While anti-Jo-1 is the most common specificity found in PM/DM, antibodies to other tRNA synthetases, such as PL-7 (threonyl), PL-12 (alanyl), EJ (glycyl), and OJ (isoleucyl) are well described [32, 33, 39]. Typically, an individual patient is positive for only one of these antibodies, so many individuals with anti-tRNA synthetase antibodies (associated with the “antisynthetase autoantibody syndrome”) go undetected and may be clinically misclassified. Other clinically significant autoantibody tests, such as reactivity to SRP [36], PM-Scl, are also unavailable. Unlike the pattern in SSc, in which three major autoantibodies are detected in ~20% each, most PM/DM-related autoantibodies are each found in only 1–5% of patients. This makes the development of commercial tests useful for many patients difficult from a financial viewpoint.
Specialized autoantibody tests at research laboratories
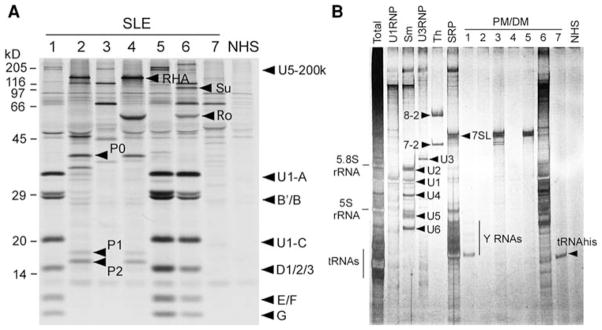
Most of the disease-related autoantibodies that are unavailable commercially, as described above, can be identified at research laboratory level by a combination of 35S-methionine-labeled protein immunoprecipitation and analysis of immunoprecipitated RNAs by silver staining (Fig. 3) [18, 44]. Many autoantibody specificities can be confirmed definitively based on protein analysis alone when they have a characteristic set of proteins such as snRNPs or ribosomal P proteins (Fig. 3a). Identification of a single protein is more difficult unless it has a unique migration pattern, and may require additional tests for conclusive identification [45]. Others, such as anti-Th, U3RNP, and SRP, may require confirmation by immunoprecipitation of the associated RNA component (Fig. 3b). However, only a few institutes in the United States have the ability to analyze all of these autoantibodies in systemic rheumatic diseases using a combination of various techniques. The characterization of autoantibodies in samples from Mexican patients using a combination of these specialized immunological assays is shown as an example of the systematic analysis of sera from a group of patients (Fig. 3). As discussed above, some autoantibodies related to SSc or PM/DM are still not widely available. Considering the high disease specificity and unique clinical association, it will be necessary to make reliable assays for these autoantibodies available for clinicians.
Fig. 3.
Characterization of various autoantibodies by immunoprecipitation. a Protein analysis by immunoprecipitation. 35S-methionine-labeled K562 cell lysate was immunoprecipitated by sera from Mexican patients with SLE. Immunoprecipitated proteins were fractionated by SDS-PAGE followed by autoradiography. b Analysis of RNA components immunoprecipitated by sera from Mexican patients with PM/DM. K562 cell lysate was immunoprecipitated by sera from patients with PM/DM (lanes 1–9) or control (NHS). Nucleic acid components were extracted using phenol/chloroform and analyzed by urea-PAGE followed by silver staining
Autoantibody testing for clinicians
ANA by immunofluorescence has been used for more than 40 years as a screening method for autoimmunity and is still the standard method. Several different types of alternative assays based on ELISA or multiplex beads assay have been developed in an attempt to replace immunofluorescent ANA. However, serious concerns have been raised about these new assays among rheumatologists [46]. The ACR has established an ANA Task Force to address these concerns and has been tracking members’ concerns on their website. The Autoantibody Standardization Committee in Rheumatic and Related Disorders (http://www.AutoAb.org) organized a Study Group at the 2008 Annual ACR Scientific Meeting that included a presentation entitled “Inaccurate results for ANA” referring to ANA screening using these new, unconventional tests. Based on the prevalence of specific autoantibodies in patients with rheumatic diseases, it is apparent that using mixtures of several recombinant or purified autoantigens in these assays will not cover all the reactivities of immunofluorescent ANA-positive patients. In addition, even if the target antigens of a particular patient’s autoantibodies are included, recombinant or denatured proteins used in the assay may be poorly recognized by human autoantibodies, similar to the false negatives found in a specific autoantibody ELISA [1]. Although these new assays can be cost efficient and are somewhat comparable to immunofluorescent ANA, to avoid confusion during interpretation by the clinician, laboratory results derived from these assays should be reported along with exactly the type of assay used; it should not be reported as an “ANA screening test.” Immunofluorescence ANA is still the gold standard for the screening of ANA [1].
When an ANA result comes back positive, clinicians must decide what assay to order next to confirm or follow-up on the initial finding. A list of potential diagnoses for the patient, clinical symptoms, and laboratory findings will all help to make this decision. If the immunofluorescent ANA pattern is read properly, this information should also help determine the next step. ANA staining patterns and corresponding common autoantibodies for different diagnoses are summarized in Table 2. Although the ANA patterns are helpful for narrowing down the tests for specific autoantibodies when performed and interpreted correctly, several potential pitfalls should be noted. First, interpretation is somewhat subjective, and weaker staining may not be reported. Since our eyes compare the fluorescent intensity of the area of interest to its surrounding area (e.g., nucleoli vs. nuclei, nuclei vs. cytoplasm), only the stronger staining may be reported [1, 10]. For example, if strong nucleolar staining accompanies weaker nuclear staining, as in certain anti-topo I-positive sera, it may be reported as nucleolar staining only. Similarly, weak nuclear staining may be reported as negative in the presence of strong cytoplasmic staining. Second, the report on the staining pattern may not always accurately reflect the known cell biological location of the target antigen. Ro/SS-A antigens are present in both nuclei and cytoplasm; however, anti-Ro/SS-A has been historically linked with ANA-negative lupus, and the staining pattern from anti-Ro/SS-A has been controversial; nuclear staining are reported by many, while some have reported cytoplasmic staining.
Table 2.
Common specificities of autoantibodies based on the ANA pattern and diagnoses
| ANA pattern | Clinical diagnosis |
|||
|---|---|---|---|---|
| SLE | SSc | PM/DM | ||
| Nuclear | Homogeneous/peripheral | dsDNA histones | ||
| Speckled | Sm, U1RNP, Ro/SS-Aa, La/SS-B, RNA helicase A (RHA) | Topo I (Scl-70), RNAP III, U1RNP | U1RNP, Mi-2, Ku | |
| Discrete speckled | Centromere | |||
| Nucleolar | (Ribosomal P)b | U3RNP, Th, PM-Scl, (Topo I)c | PM-Scl | |
| Cytoplasmic | Diffuse | Ribosomal P | Jo-1 (histidyl tRNA synthetase), Other ARSd, SRP | |
| GWBs | Su/Ago2 | Su/Ago2 | Su/Ago2 | |
Some sera show only cytoplasmic staining or negative ANA
Always with cytoplasmic, which may not be reported
With nuclear staining
ARS: aminoacyl tRNA synthetases, PL-7 (threonyl), PL-12 (alanyl), EJ (glycyl), OJ (Isoleucyl), KS (asparaginyl)
Bold commercially available, nonbold research level, underlined disease marker antibodies
Autoantibody testing in patients with a specific diagnosis
SLE
If SLE is clinically suspected, three specific autoantibodies listed in the ACR SLE classification criteria—anti-dsDNA, phospholipids, and Sm—should be tested for. Presence of active nephritis is often accompanied by anti-dsDNA antibodies, and some patients with a history of thrombosis or recurrent spontaneous abortions may have anti-phospholipid antibodies; however, many unsuspected patients will also be found to be positive. This is mainly due to the relatively low sensitivity of autoantibodies when used to detect their associated clinical symptoms, as shown in a recent meta-analysis [19]. In some cases, this may be explained by the observation that autoantibodies are often produced prior to clinical manifestation [14, 47]. There are reports of the association of anti-Sm with certain clinical manifestations; however, there is no way to predict or rule out the presence of anti-Sm. Antiribosomal P antibodies are associated with neuropsychiatric symptoms of SLE in some studies, although the specificity does not appear to be high [19]. Specific assays for anti-Ro/SS-A, La/SS-B, and U1RNP may be ordered as well.
SSc
Autoantibodies to RNAP I always coexist with anti-RNAP III and RNAP I localizes to the nucleoli; however, none of the anti-RNAP I/III-positive SSc sera were reported to be nucleolar staining positive from a hospital laboratory in one study; all sera were reported to be nuclear speckled that reflected RNAP III staining [10]. Thus, despite earlier reports describing nucleolar staining by anti-RNAP I antibodies in SSc [48], a report of nucleolar staining should not be expected all the time [10]. It is reasonable to recommend that all SSc patients with nuclear speckled and nucleolar patterns should be tested for anti-topo I and RNAP III [1]. Patients with anticentromere patterns may be excluded, because patients with SSc seldom have more than one SSc-related autoantibody, and it is unlikely that they also have anti-topo I or anti-RNAP III. Nucleolar staining by anti-U3RNP, Th, and PM-Scl is usually reported as such from a hospital laboratory in our experience [10]. Anti-topo I may be reported as nucleolar instead of nuclear, or nuclear plus nucleolar, due to strong nucleolar staining by some sera. Inclusion of anti-topo I, RNAP III, and centromere will be considered in the classification criteria of SSc in the future.
PM/DM
The targets of many myositis-specific autoantibodies such as anti-Jo-1 and other tRNA synthetases and SRP are cytoplasmic antigens [32, 33, 39]. Unfortunately, they are often not reported in clinical practice. Anti-Jo-1 for cytoplasmic and anti-U1RNP for nuclear speckled are the only widely available tests for myositis-related autoantibodies. Nuclear speckled patterns with overlapping features of PM/DM and SLE or SSc may be similar in patients with anti-U1RNP (MCTD) and anti-Ku-positive myositis patients.
RA
Although anti-CCP antibodies were first described just ten years ago, and the anti-CCP ELISA test has only been widely available for a few years, it has rapidly become a standard test in clinical practice. The frequencies of anti-CCP and rheumatoid factor (RF) are both ~70%; however, anti-CCP is much more specific for RA. Nevertheless, anti-CCP may be positive in up to 10–20% of other rheumatic diseases and associated with chronic arthritis or Jaccoud’s-type arthritis in SLE [49]. Anti-CCP in nonrheumatic disease patients is less extensively studied, and one may find unexpected positives in infections, such as in patients with pulmonary tuberculosis [50]. Anti-CCP always needs to be interpreted carefully with other clinical and laboratory features in the individual patient, particularly in non-RA patients.
Sjögren’s syndrome
Anti-Ro/SS-A (60kD) and anti-La/SS-B are detected in ~70% and ~40%, respectively, by standard tests [1]. These antibodies are included in the European Criteria [51] and should be tested for when the presence of SjS is suspected. Anti-Ro52 [52] and anti-NA14 [53] can be found in patients with primary SjS without anti-Ro/SS-A or La/SS-B and may become useful in the future.
Conclusion
Clinically useful information on common autoantibodies in rheumatic diseases was summarized, emphasizing potential problems and pitfalls. Unfortunately there is a discrepancy between the autoantibody tests described in textbooks or review articles and their availability in clinical practice. Also, despite the long history of performing autoantibody assays using standard methods, there are still many limitations and pitfalls that clinicians should be aware of. Clinicians should be able to use autoantibody tests more efficiently and properly, and have a basic knowledge of their significance and potential problems.
Acknowledgments
We thank Dr. Luis E. C. Andrade (Escola Paulista de Medicina, São Paulo, Brazil) for his critical reading of the manuscript. This study was supported by CONACyT grant 51353, Universidad de Guadalajara agreement 25473, to MVDM. MS is supported by the Lupus Foundation of America, Inc. EKLC is supported in part by NIH AI47859.
Contributor Information
Minoru Satoh, Email: Minoru.Satoh@medicine.ufl.edu, Division of Rheumatology and Clinical Immunology, Department of Medicine, and Department of Pathology, Immunology, and Laboratory Medicine, University of Florida, P.O. Box 100221, Gainesville, FL 32610-0221, USA.
Monica Vázquez-Del Mercado, Instituto de Investigación en Reumatología y del Sistema Músculo Esquelético Sierra Mojada 950, Planta Baja, CP 44240 Edificio P Ala Oriente, Mexico. Centro Universitario de Ciencias de la Salud, Universidad de Guadalajara, Guadalajara, Jalisco, Mexico. Divisiòn de Medicina Interna, Reumatòloga, Departamento de Reumatologìa, Hospital Civil Juan I, Mechaca, Guadalajara, Jalisco, Mexico.
Edward K. L. Chan, Department of Oral Biology, University of Florida, Gainesville, FL 32610-0424, USA
References
- 1.Satoh M, Chan EKL, Sobel ES, Kimpel DL, Yamasaki Y, Narain S, et al. Clinical implication of autoantibodies in patients with systemic rheumatic diseases. Exp Rev Clin Immunol. 2007;3:721–38. doi: 10.1586/1744666X.3.5.721. [DOI] [PubMed] [Google Scholar]
- 2.Tan EM, Feltkamp TE, Smolen JS, Butcher B, Dawkins R, Fritzler MJ, et al. Range of antinuclear antibodies in “healthy” individuals. Arthritis Rheum. 1997;40:1601–11. doi: 10.1002/art.1780400909. [DOI] [PubMed] [Google Scholar]
- 3.Andrade LE, Chan EK, Peebles CL, Tan EM. Two major autoantigen-antibody systems of the mitotic spindle apparatus. Arthritis Rheum. 1996;39:1643–53. doi: 10.1002/art.1780391006. [DOI] [PubMed] [Google Scholar]
- 4.Eystathioy T, Chan EK, Tenenbaum SA, Keene JD, Griffith K, Fritzler MJ. A phosphorylated cytoplasmic autoantigen, GW182, associates with a unique population of human mRNAs within novel cytoplasmic speckles. Mol Biol Cell. 2002;13:1338–51. doi: 10.1091/mbc.01-11-0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jakymiw A, Lian S, Eystathioy T, Li S, Satoh M, Hamel JC, et al. Disruption of GW bodies impairs mammalian RNA interference. Nat Cell Biol. 2005;7:1267–74. doi: 10.1038/ncb1334. [DOI] [PubMed] [Google Scholar]
- 6.Jakymiw A, Pauley KM, Li S, Ikeda K, Lian S, Eystathioy T, et al. The role of GW/P-bodies in RNA processing and silencing. J Cell Sci. 2007;120:1317–23. doi: 10.1242/jcs.03429. [DOI] [PubMed] [Google Scholar]
- 7.Jakymiw A, Ikeda K, Fritzler MJ, Reeves WH, Satoh M, Chan EK. Autoimmune targeting of key components of RNA interference. Arthritis Res Ther. 2006;8:R87. doi: 10.1186/ar1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Satoh M, Langdon JJ, Chou CH, McCauliffe DP, Treadwell EL, Ogasawara T, et al. Characterization of the Su antigen, a macromolecular complex of 100/102 and 200 kDa proteins recognized by autoantibodies in systemic rheumatic diseases. Clin Immunol Immunopathol. 1994;73:132–41. doi: 10.1006/clin.1994.1179. [DOI] [PubMed] [Google Scholar]
- 9.Nozawa K, Fritzler MJ, Chan EK. Unique and shared features of Golgi complex autoantigens. Autoimmun Rev. 2005;4:35–41. doi: 10.1016/j.autrev.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 10.Yamasaki Y, Honkanen-Scott M, Hernandez L, Ikeda K, Barker T, Bubb MR, et al. Nucleolar staining cannot be used as a screening test for the scleroderma marker anti-RNA polymerase I/III antibodies. Arthritis Rheum. 2006;54:3051–6. doi: 10.1002/art.22043. [DOI] [PubMed] [Google Scholar]
- 11.Buchanan RR, Riglar AG. The titre of anti-centromere antibodies: its relationship to Raynaud’s phenomenon and vascular occlusion. Br J Rheumatol. 1989;28:221–6. doi: 10.1093/rheumatology/28.3.221. [DOI] [PubMed] [Google Scholar]
- 12.Sharp GC, Irvin WS, May CM, Holman HR, McDuffie FC, Hess EV, et al. Association of autoantibodies to ribonucleoprotein and Sm antigens with mixed connective-tissue disease, systemic lupus erythematosus and other rheumatic diseases. N Engl J Med. 1976;295:1149–54. doi: 10.1056/NEJM197611182952101. [DOI] [PubMed] [Google Scholar]
- 13.Hoffman RW, Maldonado ME. Immune pathogenesis of mixed connective tissue disease: a short analytical review. Clin Immunol. 2008;128:8–17. doi: 10.1016/j.clim.2008.03.461. [DOI] [PubMed] [Google Scholar]
- 14.Bodolay E, Csiki Z, Szekanecz Z, Ben T, Kiss E, Zeher M, et al. Five-year follow-up of 665 Hungarian patients with undifferentiated connective tissue disease (UCTD) Clin Exp Rheumatol. 2003;21:313–20. [PubMed] [Google Scholar]
- 15.Mosca M, Tani C, Bombardieri S. Undifferentiated connective tissue diseases (UCTD): a new frontier for rheumatology. Best Pract Res Clin Rheumatol. 2007;21:1011–23. doi: 10.1016/j.berh.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 16.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 17.Reeves WH, Satoh M, Richards HB. Origins of antinuclear antibodies. In: Lahita RG, editor. Systemic lupus erythematosus. 4. San Diego: Academic Press; 2004. pp. 401–31. [Google Scholar]
- 18.Reeves WH, Narain S, Satoh M. Autoantibodies in systemic lupus erythematosus. In: Koopman WJ, Moreland LW, editors. Arthritis and allied conditions. A textbook of rheumatology. 15. Philadelphia: Lippincott Williams and Wilkins; 2005. pp. 1497–521. [Google Scholar]
- 19.Karassa FB, Afeltra A, Ambrozic A, Chang DM, De Keyser F, Doria A, et al. Accuracy of anti-ribosomal P protein antibody testing for the diagnosis of neuropsychiatric systemic lupus erythematosus: an international meta-analysis. Arthritis Rheum. 2006;54:312–24. doi: 10.1002/art.21539. [DOI] [PubMed] [Google Scholar]
- 20.Okano Y, Steen VD, Medsger TAJ. Autoantibody reactive with RNA polymerase III in systemic sclerosis. Ann Intern Med. 1993;119:1005–13. doi: 10.7326/0003-4819-119-10-199311150-00007. [DOI] [PubMed] [Google Scholar]
- 21.Kuwana M, Kaburaki J, Mimori T, Tojo T, Homma M. Auto-antibody reactive with three classes of RNA polymerases in sera from patients with systemic sclerosis. J Clin Invest. 1993;91:1399–404. doi: 10.1172/JCI116343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Satoh M, Ajmani AK, Ogasawara T, Langdon JJ, Hirakata M, Wang J, et al. Autoantibodies to RNA polymerase II are common in systemic lupus erythematosus and overlap syndrome. Specific recognition of the phosphorylated (IIO) form by a subset of human sera. J Clin Invest. 1994;94:1981–9. doi: 10.1172/JCI117550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steen VD. The many faces of scleroderma. Rheum Dis Clin North Am. 2008;34:1–15. doi: 10.1016/j.rdc.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 24.Fritzler MJ, Kinsella TD. The CREST syndrome: a distinct serologic entity with anticentromere antibodies. Am J Med. 1980;69:520–6. doi: 10.1016/0002-9343(80)90462-3. [DOI] [PubMed] [Google Scholar]
- 25.Gelber AC, Pillemer SR, Baum BJ, Wigley FM, Hummers LK, Morris S, et al. Distinct recognition of antibodies to centromere proteins in primary Sjogren’s syndrome compared with limited scleroderma. Ann Rheum Dis. 2006;65:1028–32. doi: 10.1136/ard.2005.046003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayashi N, Koshiba M, Nishimura K, Sugiyama D, Nakamura T, Morinobu S, et al. Prevalence of disease-specific antinuclear antibodies in general population: estimates from annual physical examinations of residents of a small town over a 5-year period. Mod Rheumatol. 2008;18:153–60. doi: 10.1007/s10165-008-0028-1. [DOI] [PubMed] [Google Scholar]
- 27.Kuwana M, Kimura K, Hirakata M, Kawakami Y, Ikeda Y. Differences in autoantibody response to Th/To between systemic sclerosis and other autoimmune diseases. Ann Rheum Dis. 2002;61:842–6. doi: 10.1136/ard.61.9.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fischer A, Pfalzgraf FJ, Feghali-Bostwick CA, Wright TM, Curran-Everett D, West SG, et al. Anti-Th/To-positivity in a cohort of patients with idiopathic pulmonary fibrosis. J Rheumatol. 2006;33:1600–5. [PubMed] [Google Scholar]
- 29.Okano Y. Antinuclear antibody in systemic sclerosis (scleroderma) Rheum Dis Clin North Am. 1996;22:709–35. doi: 10.1016/s0889-857x(05)70297-0. [DOI] [PubMed] [Google Scholar]
- 30.Medsger TAJ. Systemic sclerosis (scleroderma): clinical aspects. In: Koopman WJ, editor. Arthritis and allied conditions. A textbook of rheumatology. 14. Philadelphia: Lippincott Williams and Wilkins; 2001. pp. 1590–624. [Google Scholar]
- 31.Steen VD. Autoantibodies in systemic sclerosis. Semin Arthritis Rheum. 2005;35:35–42. doi: 10.1016/j.semarthrit.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 32.Hirakata M. Humoral aspects of polymyositis/dermatomyositis. Mod Rheumatol. 2000;10:199–206. doi: 10.3109/s101650070002. [DOI] [PubMed] [Google Scholar]
- 33.Mimori T, Imura Y, Nakashima R, Yoshifuji H. Autoantibodies in idiopathic inflammatory myopathy: an update on clinical and pathophysiological significance. Curr Opin Rheumatol. 2007;19:523–9. doi: 10.1097/BOR.0b013e3282f01a8c. [DOI] [PubMed] [Google Scholar]
- 34.Yoshida S, Akizuki M, Mimori T, Yamagata H, Inada S, Homma M. The precipitating antibody to an acidic nuclear protein antigen, the Jo-1, in connective tissue diseases. Arthritis Rheum. 1983;26:604–11. doi: 10.1002/art.1780260505. [DOI] [PubMed] [Google Scholar]
- 35.Hirakata M, Suwa A, Takada T, Sato S, Nagai S, Genth E, et al. Clinical and immunogenetic features of patients with autoantibodies to asparaginyl-transfer RNA synthetase. Arthritis Rheum. 2007;56:1295–303. doi: 10.1002/art.22506. [DOI] [PubMed] [Google Scholar]
- 36.Takada T, Hirakata M, Suwa A, Kaneko Y, Kuwana M, Ishihara T, et al. Clinical and histopathological features of myopathies in Japanese patients with anti-SRP autoantibodies. Mod Rheumatol. 2008 doi: 10.3109/s10165-009-0165-1. in press. [DOI] [PubMed] [Google Scholar]
- 37.Cavazzana I, Ceribelli A, Quinzanini M, Scarsi M, Airo P, Cattaneo R, et al. Prevalence and clinical associations of anti-Ku antibodies in systemic autoimmune diseases. Lupus. 2008;17:727–32. doi: 10.1177/0961203308089442. [DOI] [PubMed] [Google Scholar]
- 38.Rubin RL. Enzyme-linked immunosorbent assay for anti-DNA and antihistone antibodies including anti-(H2A-H2B) In: Rose NR, de Macario EC, Fahey JL, Friedman H, Penn GM, editors. Manual of clinical laboratory immunology. 4. Washington, DC: American Society for Microbiology; 1992. pp. 735–40. [Google Scholar]
- 39.Targoff IN. Laboratory testing in the diagnosis and management of idiopathic inflammatory myopathies. Rheum Dis Clin North Am. 2002;28:859–90. viii. doi: 10.1016/s0889-857x(02)00032-7. [DOI] [PubMed] [Google Scholar]
- 40.Kuwana M, Okano Y, Pandey JP, Silver RM, Fertig N, Medsger TA., Jr Enzyme-linked immunosorbent assay for detection of anti-RNA polymerase III antibody: analytical accuracy and clinical associations in systemic sclerosis. Arthritis Rheum. 2005;52:2425–32. doi: 10.1002/art.21232. [DOI] [PubMed] [Google Scholar]
- 41.Kuwana M, Kimura K, Kawakami Y. Identification of an immunodominant epitope on RNA polymerase III recognized by systemic sclerosis sera: application to enzyme-linked immunosorbent assay. Arthritis Rheum. 2002;46:2742–7. doi: 10.1002/art.10521. [DOI] [PubMed] [Google Scholar]
- 42.Santiago M, Baron M, Hudson M, Burlingame RW, Fritzler MJ. Antibodies to RNA polymerase III in systemic sclerosis detected by ELISA. J Rheumatol. 2007;34:1528–34. [PubMed] [Google Scholar]
- 43.Parker JC, Burlingame RW, Webb TT, Bunn CC. Anti-RNA polymerase III antibodies in patients with systemic sclerosis detected by indirect immunofluorescence and ELISA. Rheumatology (Oxf) 2008;47:976–9. doi: 10.1093/rheumatology/ken201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reeves WH, Satoh M, Lyons R, Nichols C, Narain S. Detection of autoantibodies against proteins and ribonucleoproteins by double immunodiffusion, immunoprecipitation, and western blotting. In: Rose NR, Hamilton RG, Detrick B, Reeves WH, editors. Manual of molecular and clinical laboratory immunology. 7. Washington, DC: American Society of Microbiology Press; 2006. pp. 1007–18. [Google Scholar]
- 45.Yamasaki Y, Narain S, Yoshida H, Hernandez L, Barker T, Hahn PC, et al. Autoantibodies to RNA helicase A: a new serological marker of early lupus. Arthritis Rheum. 2007;56:596–604. doi: 10.1002/art.22329. [DOI] [PubMed] [Google Scholar]
- 46.Bonilla E, Francis L, Allam F, Ogrinc M, Neupane H, Phillips PE, et al. Immunofluorescence microscopy is superior to fluorescent beads for detection of antinuclear antibody reactivity in systemic lupus erythematosus patients. Clin Immunol. 2007;124:18–21. doi: 10.1016/j.clim.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349:1526–33. doi: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]
- 48.Reimer G, Rose KM, Scheer U, Tan EM. Autoantibody to RNA polymerase I in scleroderma sera. J Clin Invest. 1987;79:65–72. doi: 10.1172/JCI112809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takasaki Y, Yamanaka K, Takasaki C, Matsushita M, Yamada H, Nawata M, et al. Anticyclic citrullinated peptide antibodies in patients with mixed connective tissue disease. Mod Rheumatol. 2004;14:367–75. doi: 10.1007/s10165-004-0325-2. [DOI] [PubMed] [Google Scholar]
- 50.Kakumanu P, Yamagata H, Sobel ES, Reeves WH, Chan EKL, Satoh M. Patients with pulmonary tuberculosis are frequently positive for anti-cyclic citrullinated peptide antibodies but also react with unmodified arginine-containing peptide. Arthritis Rheum. 2008;58:1576–81. doi: 10.1002/art.23514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, et al. Classification criteria for Sjogren’s syndrome: a revised version of the European criteria proposed by the American–European Consensus Group. Ann Rheum Dis. 2002;61:554–8. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McCauliffe DP, Wang L, Satoh M, Reeves WH, Small D. A recombinant 52 kD Ro (SS-A) ELISA detects autoantibodies in Sjogren’s syndrome sera that go undetected by conventional serologic assays. J Rheumatol. 1997;24:860–6. [PubMed] [Google Scholar]
- 53.Nozawa K, Ikeda K, Satoh M, Reeves WH, Stewart CA, Li YC, et al. Autoantibody to nuclear antigen NA14 is an independent marker primarily for Sjögren’s syndrome. Front Biosci. 2009;14:3733–9. doi: 10.2741/3484. [DOI] [PMC free article] [PubMed] [Google Scholar]