Abstract
We are in the midst of a dire, unprecedented, and global epidemic of obesity and secondary sequelae, most prominently diabetes and hyperlipidemia. Underlying this epidemic is the most hated of cells, adipocytes, and their inherent dynamic ability to expand and renew. This capacity highlights a heretofore undefined stem compartment. Recent in vivo studies, relying upon lineage tracing and flow cytometry methods, have begun to unravel the identity of adipose stem cells, their niche, and the dynamism central to adipose expansion. Thus, the field is moving in a direction that may allow us to manipulate adipose stem cells to beneficial therapeutic ends.
Traditionally fueling strong emotions related to the aesthetics of body image, adipocytes are now at the center of a medical-sociological crisis that has changed the landscape of public health. Indeed, obesity is central to the explosion of medical costs that have triggered the national debate on health care and is a major culprit behind the alarming fact that the life expectancy of Americans is diminishing for the first time in generations (Olshansky et al., 2005). Estimates indicate that obesity is already responsible for >300,000 deaths each year in the United States alone, triggering the largest growth in mortality over the past decade (Allison et al., 1999). Moreover, the increase in obesity and diabetes (a.k.a. “diabesity”) is even greater in Southeast Asia, underscoring the global nature of this epidemic, with estimates between 1.1 to 1.7 billion people affected (Hossain et al., 2007).
Yet, there may be some shelter from this storm. Recent insights into adipocyte biology—particularly the isolation of the white adipose stem cell and identification of its niche—have altered our understanding of adipose biology (Rodeheffer et al., 2008; Tang et al., 2008). This work indicates that the recruitment of stem cells into the adipose lineage is a regulated process, one that occurs throughout life and may contribute to the obesigenic phenotype. These findings raise the possibility of unraveling the mechanisms and molecules that control these processes and the potential of manipulating them to treat a variety of serious medical conditions.
The Skinny on Fat: Location, Location, Location
Adipose tissues regulate reproduction and lifespan and provide thermal and traumatic protection, but are most notable for their vital role in metabolism (Gregoire et al., 1998; MacDougald and Mandrup, 2002; Spiegelman and Flier, 2001). In contrast to the traditional view of adipocytes as a passive storage site, adipose tissues are the largest endocrine organ, actively controlling metabolism by secreting lipids, hormones, and other factors (Nawrocki and Scherer, 2004; Waki and Tontonoz, 2007). The disease states—insulin resistance, hyperlipidemia, cardiovascular disease, diabetes, etc.—that accompany both excess (obesity) and deficient (lipodystrophy) fat stores highlight the central role of adipose tissues.
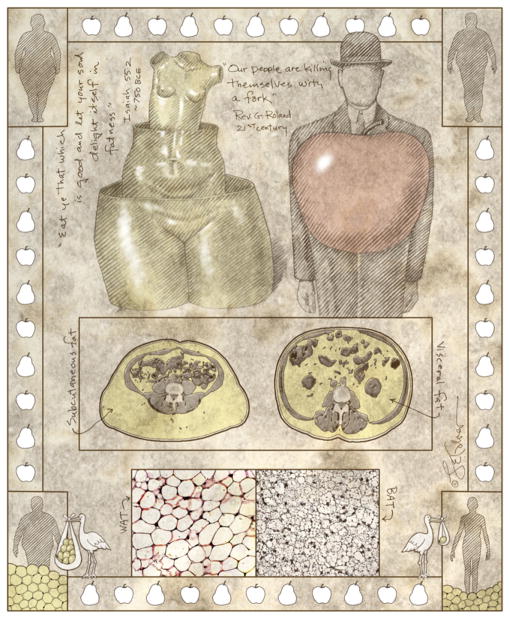
Adipocytes are of two broad functional and histological lineages: brown adipose tissues (BAT), which dissipate energy, and white adipose tissues (WAT), which store energy (Figure 1) (Gregoire et al., 1998; MacDougald and Mandrup, 2002; Spiegelman and Flier, 2001). Brown adipocytes are primarily thermogenic, converting nutrients into heat. Thermogenesis is accomplished through the abundant number of mitochondria in BAT (which are responsible for its signature brown color) and the expression of uncoupling protein 1 (UCP-1), which uncouples the proton electrochemical gradient from the generation of ATP (Cannon and Nedergaard, 2004). Although relatively scarce in adult humans, BAT may be more prevalent than previously appreciated, and its ability to burn calories may enable it to be manipulated for novel antiobesity strategies (Cypess et al., 2009; van Marken Lichtenbelt et al., 2009; Virtanen et al., 2009). For example, stimuli such as cold exposure and β3 adrenergic drugs stimulate the formation of brown adipocytes within white adipose depots; it is unclear whether these nascent brown adipocytes somehow derive from white adipocytes, from white adipose stem cells, or from an as yet unidentified brown adipose stem cell (Granneman and Whitty, 1991; Loncar et al., 1988; Rohlfs et al., 1995). BMP7 may also play a related role in regulating the BAT compartment (Tseng et al., 2008). Studies indicate that during murine development, at least some BAT derives from a muscle-related En1, Myf5 lineage (Atit et al., 2006; Seale et al., 2008). Yet this does not appear to be the case during the formation of BAT that, under the influence of external stimuli, is induced within WAT tissue (Seale et al., 2008). Therefore, it may ultimately be possible to reduce obesity and diabetes by converting WAT, or the newly identified white adipose stem cells (see below), to BAT (Elabd et al., 2009; Orci et al., 2004).
Figure 1. The Skinny on Fat: Location, Location, Location.
In ancient times, the ability to become fat was associated with health, wealth, and good fortune, but modern lifestyles have led to an unprecedented medical-sociological-financial crisis, with obesity at epidemic proportions. This is due to expansion of white adipose tissues (WAT), but there is also brown adipose tissue (BAT) lineage. White adipose tissues are primarily distributed subcutaneously (pear or female pattern), fat that appears to be metabolically protective, or intra-abdomimably (apple or male pattern), fat that is associated with enormous health risks. Adipose depots are remarkably plastic, and recent evidence indicates that new fat cells derive from a stem cell source (depicted as a stork in bottom outside panels).
White adipocytes comprise the vast majority of adipocytes. In times of caloric abundance, these cells store excess calories as triglycerides, and in times of caloric need, release fuel (as fatty acids and glycerol) for use by other organs. WAT is itself subdivided into subcutaneous and visceral (intra-abdominal) depots (Figure 1). Importantly, visceral depots are much more strongly associated with metabolic complications—markedly increasing the risk of hyperlipidemia, diabetes and cardiovascular disease—while subcutaneous beds appear protective against these sequelae (Gesta et al., 2007; Shi and Clegg, 2009). Paralleling these distinctions, the two types of WAT, defined only by location, also differ in morphology and function (Gesta et al., 2006; Tchernof et al., 2006; Tchkonia et al., 2007). The mechanisms and developmental cues that account for these unique characteristics are unknown, however, and work in this area has been hindered by a lack of reagents to enable researchers to track and manipulate the different lineages in vivo.
Sex steroids are the best-characterized endogenous stimuli that differentially affect the development, distribution and function of subcutaneous and visceral WAT depots (Shi and Clegg, 2009). Although men, on average, have less total body fat, they have more visceral adipose tissue, whereas women have more total and subcutaneous adipose tissue (Grauer et al., 1984). The “female” or “pear” pattern of fat distribution develops during puberty in an estrogen-dependent manner (de Ridder et al., 1990). Further, estrogen treatment of males increases subcutaneous adipose accrual relative to visceral adipose tissue (Elbers et al., 1999). After menopause, as estrogen levels decline, women develop increased visceral adiposity; however, those who receive estrogen replacement therapy do not (Gambacciani et al., 1997; Munoz et al., 2002). Therefore, gonadal or “sex” steroids are endogenous regulators of fat distribution, potentially through mechanisms involving adipose progenitors. Indeed, the many differences between WAT depots may be intrinsic to the depots and the stem cells located therein: indirect evidence indicates that subcutaneous depots contain a greater number of adipose progenitors, and these cells have a higher proliferative and adipogenic potential than those located intra-abdominally (Gesta et al., 2007; Joe et al., 2009; Tran et al., 2008). This enhanced ability to generate adipocytes may be protective against metabolic dysfunction, while the hypertrophic response characteristic of visceral depots may be maladaptive (Joe et al., 2009).
Adipose tissues are highly dynamic, expanding and shrinking in response to various homeostatic, pharmacologic, and dietary stimuli (Figure 1) (Gesta et al., 2007; Shi and Clegg, 2009; Spalding et al., 2008; Spiegelman and Flier, 2001). This growth pattern is due to both adipocyte hypertrophy and hyperplasia, the latter highlighting the potential presence of a stem compartment (Hirsch and Batchelor, 1976; Salans et al., 1973). As early as the 1950s, there was evidence stemming from radio-labeled nucleotides administered to rodents that brown and white adipocytes have a defined lifespan, renew, and that adipose tissues contain proliferative and label-retaining cells, presaging current molecular analyses of stem compartments (Hellman and Hellerstrom, 1961; Hollenberg and Vost, 1969). Human radioactive labeling studies performed in the wake of increased atmospheric nuclear emissions during the middle part of the 20th century showed that human adipocytes also turn over continuously and at a rate much greater than previously believed (Spalding et al., 2008). These observations suggested the existence of an adipogenic stem cell, and the behavior of adipose tissue in pathological conditions further supported this possibility. For example, adipose tissues regenerate following lipectomy or liposuction, and adipose tissue is hyperplastic in obese subjects (Mauer et al., 2001). In fact, adipocyte number can increase during obesity formation, despite the higher rate of apoptotic death in this setting (Strissel et al., 2007). Even in the lean state, formation of new adipocytes appears to be a lifelong regulated process. Despite this wealth of indirect evidence, however, no direct proof in vivo of a physiologically relevant adipogenic stem cell existed prior to recent studies highlighted below.
Fat Times at Ridgemont High: White Adipose Stem Cells Move to the Head of the Class
Until recently, the majority of studies on adipocyte precursors and adipogenic differentiation had been performed in vitro and primarily using cultured cell lines (MacDougald and Mandrup, 2002). While this work provided valuable clues and insights, most notably unraveling the adipogenic transcription cascade, such in vitro studies do not allow the examination of many developmental questions that are vital to understand if we are to have a clear picture of adipocyte biology. For example, the lack of in vivo lineage analyses and stem cell marker delineation had, until now, hindered identification of the adipose stem cell and the characteristics of its niche, thereby precluding an understanding of how developmental, physiological, and pharmacological stimuli regulate the growth of adipose tissue.
Recent papers from Friedman’s group (Rodeheffer et al., 2008) and our group (Tang et al., 2008) shed light on the molecular signature of white adipose stem cells and the niche from which they derive (Figure 2). The reports use cell surface markers or lineage tracing to identify and prospectively isolate the stem cells and to demonstrate the ability of these newly identified cells to self-renew and, following transplantation, to form functional adipose depots. Moreover, these in vivo studies show that white adipose stem cells reside within the mural cell compartment of blood vessels that supply adipose depots (Figure 2). These papers employ a range of new in vivo tools and novel methodologies to confirm long-suspected but unproven notions about adipose stem cells.
Figure 2. Adipose Stem Cells Reside in a Vascular Niche.
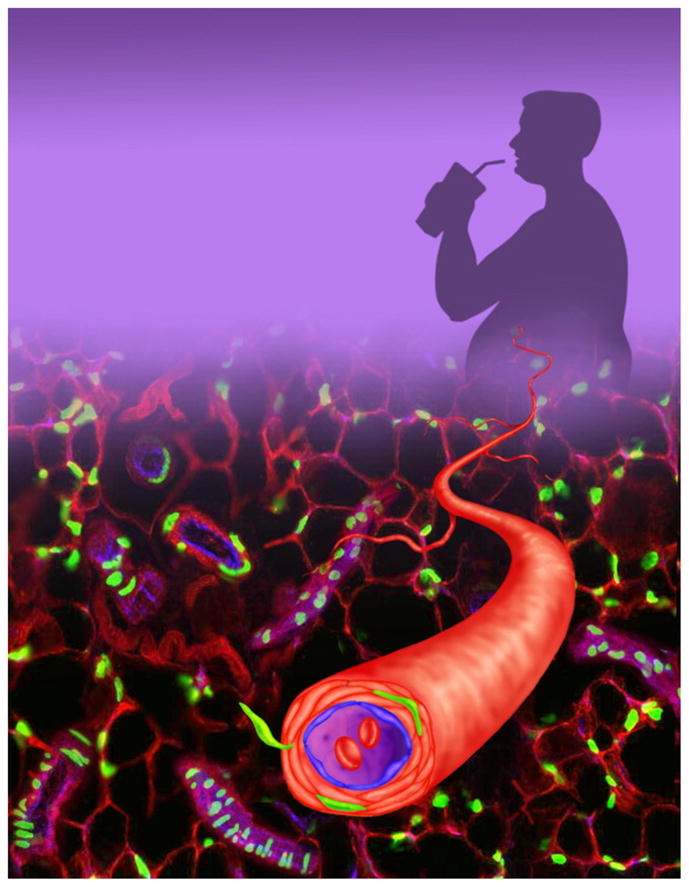
White adipose stem cells, marked green, reside in a vascular niche embedded within the wall of blood vessels that supply fat tissues. This location is well suited to receive environmental stimuli such as nutritional cues, indicated by a supersized soda, that can trigger the cells to leave their niche and mature into fat cells.
Two important features help define stem cells: self-renewal and differentiation into cells of the tissues in which they reside. For example, neural stem cells reside within neural tissue, muscle stem cells reside within muscle, and breast stem cells reside within the breast. Lineage studies reveal that these tissue-resident stem cells are not pluripotent but rather differentiate into a limited set of cell types. While these cells are critical for normal tissue biology, finding the needle (e.g., stem cell) in the proverbial haystack (e.g., tissue of interest) is often a significant challenge. One widely implemented strategy is to dissociate the relevant tissue, sort the cells based on cell-surface markers using fluorescence-activated cell sorting (FACS), and then to functionally test the isolated fractions in cell culture and after transplantation (Morrison et al., 1999; Shackleton et al., 2006; Spangrude et al., 1988; Stingl et al., 2006). An advantage of this methodology is that related or overlapping sets of the identified cell-surface markers may also be present on the analogous human stem cell population. What’s more, the identified cell surface markers are easily communicated to, and disseminated within, the scientific community where they can be rapidly applied in a variety of experimental settings. A drawback of such techniques, however, is that they can alter cellular phenotype and that they disrupt the normal tissue architecture and, therefore, place cells in proximity with nonphysiological neighbors (Javazon et al., 2004). Moreover, while flow cytometry studies can enrich for stem cells, the sorted samples are typically heterogeneous. Nevertheless, such studies have been quite influential and succeeded in defining several stem cell populations: notable examples include hematopoeitic stem cells, neural stem cells, and breast epithelial stem cells (Morrison et al., 1999; Shackleton et al., 2006; Spangrude et al., 1988; Stingl et al., 2006).
Tissue dissociation studies implicated the adipose stromalvascular (SV) compartment as the site of origin of adipose stem cells (Hollenberg and Vost, 1969; Rodeheffer et al., 2008; Tang et al., 2008). The SV fraction is operationally defined as the heterogeneous mixture of cells that is isolated by enzymatic dissociation and density separation, a procedure designed to remove the assortment of cells that reside in the depot from surrounding adipocytes, which float. These SV cells are a potentially rich resource to examine a variety of questions relevant to adipogenesis, as well as to regenerative medicine. For within this complex set of cells are adipose-derived stem cells (ADSCs), and also strikingly similar mesenchymal stem cells (MSCs), that can be induced into a variety of cell types, including bone, cartilage, fat, muscle, and perhaps even endothelial cells and neurons (Crisan et al., 2008; Planat-Benard et al., 2004; Yamamoto et al., 2007; Zuk et al., 2002). Of note, the identity and lineage of both ADSCs and MSCs is controversial (Bianco et al., 2008). Nonetheless, while possibly a subset of the aforementioned ADSCs and MSCs populations, the SV fraction also contains bona fide adipose stem cells, possibly a subset of the aforementioned ADSC and MSC populations. When such cells are isolated from BAT, they form brown-like adipocytes that express UCP-1 (Tseng et al., 2008), while those isolated from WAT form adipocytes with cognate white adipocyte characteristics (Rodeheffer et al., 2008; Tang et al., 2008). The SV compartment has therefore attracted a great deal of attention, and there have been several attempts, using both rodent and human adipose tissues, to identify relevant markers to distinguish the cell types in this microenvironment (Gronthos et al., 2001; Yoshimura et al., 2006; Zannettino et al., 2008). In that regard, the cell-surface markers Sca-1 and CD-34 provided some refinement to this complex mixture.
The work of Rodeheffer and colleagues significantly advanced the ability to isolate cells of interest from the SV fraction. They employed an elegant FACS-based protocol to isolate a subpopulation of cells able to generate adipocytes following transplantation into, and restore adipose function to, a lipodystrophic mouse model that has a markedly reduced amount of adipose tissue (Rodeheffer et al., 2008). They reasoned that the lack of adipose tissue in these mice would lead to a strong endogenous adipogenic stimulus. As a donor, Rodeheffer and colleagues exploited a mouse strain that selectively expresses luciferase, driven by a leptin bacterial artificial chromosome, in mature adipocytes. This approach allowed them to visualize adipose development in a noninvasive manner by imaging luciferase activity in live animals. Notably, although several of the different FACS-isolated subpopulations had multilineage and adipogenic potential in vitro, only one fraction—Lin−, CD29+, CD34+, Sca1+, CD24+—formed adipose depots after transplantation into the ill-formed perigonadal depots of lipodystrophic mice. Two weeks after the procedure, luciferase expression was observed at the transplant site. And, these incipient adipose depots approximated the size of wild-type depots twelve weeks following transplant. This newly formed depot corrected hyperinsulinemia and hyperglycemia (e.g., diabetes), two metabolic hallmarks of lipodystrophy. More than 50% of AIDS patients on anti-HIV medications suffer from lipodystrophy as a side effect, underscoring the prevalence of this syndrome and the potential therapeutic implications of this finding (Grinspoon and Carr, 2005).
Using a different experimental strategy, our group independently identified white adipose stem cells expressing roughly the same set of cell-surface proteins (Tang et al., 2008). Lineage analysis, a powerful experimental strategy in which the introduction of molecular markers into progenitor populations allows for the delineation of stem cell behavior in vivo, was used in this study. For over a century, lineage studies have been used to define the fate of a field of progenitors or stem cells (Stern and Fraser, 2001). Indeed, lineage studies have been instrumental in a vast number of important advances in developmental biology, spanning the discovery of neural induction in Spemann’s era to current studies unraveling the generation of neuronal diversity (Graff, 1997; Livet et al., 2007; Stern and Fraser, 2001). Lineage studies have historically involved several basic strategies. In one, classically employed in amphibian embryos, developing cells are labeled with vital dyes that are used to track cells over time and space. Direct observation, based upon pigmentation or morphology, has also been exploited; potentially the pinnacle of these studies was the nearly complete definition of the C. elegans lineage. A related approach involved the transplantation of donor tissues or cells into host organisms, often generating interspecies chimeras, with the transplanted cells being discriminated from host tissues microscopically (e.g., by cell size), by heterochromatin structure, or by altered pigmentation (Stern and Fraser, 2001). Unfortunately, many of these approaches are restricted in mammals because in utero development prevents precise spatial and temporal localization, for example, of the transplants. To overcome these limitations, scientists employed viral marking or, more recently, markers that are engineered into the mouse genome (Cepko et al., 2000; Gu et al., 2002; Tang et al., 2008). Such modern genetic lineage tracing has provided tremendous insight for pancreas development, neural stem cells, muscle stem cells, and epidermal stem cells (Gu et al., 2002; Horsley et al., 2006; Lepper et al., 2009; Livet et al., 2007).
The lineage tracing experiments that led to the identification of the adipose stem cell relied upon an extensive series of previous studies that had unraveled the molecular underpinnings of adipogenesis. “Adipogenesis” refers to the differentiation of cultured preadipocytes into mature adipocytes. The most widely used model of adipogenesis is the in vitro differentiation of the 3T3-L1 “preadipocyte” cell line. This system was used to characterize an important adipogenic transcriptional hierarchy involving C/EBPs, SREBP, and PPARγ (Gregoire et al., 1998; MacDougald and Mandrup, 2002; Spiegelman and Flier, 2001), and microRNAs have more recently been implicated in this pathway (Sun et al., 2009; Wang et al., 2008). PPARγ, a nuclear hormone receptor, is a central regulator of fat biology (Gregoire et al., 1998; MacDougald and Mandrup, 2002; Spiegelman and Flier, 2001). It is expressed in the adipose anlagen by embryonic day 13.5, long before adipocytes are formed (Kliewer et al., 1994). PPARγ is necessary and sufficient for adipogenesis in vitro, and the necessity almost certainly translates for fat formation in vivo as well (Gregoire et al., 1998; MacDougald and Mandrup, 2002; Spiegelman and Flier, 2001). Conventional homozygous mutants lacking PPARγ die early in utero, precluding characterization of adipose formation in the null mutants, but a variety of further studies—tetraploid rescues, chimeric analyses, mutant ESC, and mutant MEF studies, as well as conditional and hypomorphic alleles—all point to a requirement for PPARγ in fat development (Barak et al., 1999; Duan et al., 2007; Koutnikova et al., 2003; Kubota et al., 1999; Rosen et al., 1999). It is also worth noting that PPARγ is expressed at low levels within the aforementioned SV fraction, the very compartment that contains adipose stem cells (Tontonoz and Spiegelman, 2008).
Based upon this work, lineage analyses were performed on mice in which PPARγ-expressing cells were marked (Tang et al., 2008). These studies relied upon mice in which the doxycycline (Dox) repressible transcription factor tTA (“Tet-off”) was placed under control of the PPARγ locus, and these mice were crossed to two lines that express different markers in a tTA-dependent fashion: one that expresses an indelible lacZ reporter and one that expresses a proliferation-sensitive GFP reporter. The mice resulting from these crosses were used in combination with BrdU-labeling strategies to examine adipose stem cell behavior. Using this “Adipo-Trak” system, we found that PPARγ-expressing cells display the properties expected of adipose stem cells. These cells are present prior to birth and behave as an amplifying and self-replenishing population that contributes to the adipocytes present in the white adipocyte lineage. Moreover, this same population was strongly adipogenic in vitro and formed ectopic adipose depots even after transplantation into wild-type mice. Molecular, expression-profiling, and FACS studies were used to further elucidate the molecular characteristics of these cells, and many of the findings are similar to those of Rodeheffer and colleagues.
A Bloody Niche
Stem cells reside in a specialized environment, a niche, that controls many aspects of their behavior—quiescence, proliferation, and differentiation (Jones and Wagers, 2008; Li and Xie, 2005). Therefore, niche identification and characterization are significant and dynamic areas of stem cell biology with many recent revelations. Immunohistochemical methods, combined with GFP marking, showed that adipose stem cells are found in the wall of blood vessels that supply white adipose depots but are absent from blood vessels that supply other tissues (Tang et al., 2008). Utilizing the GFP marker, we characterized a microanatomic location of adipocyte stem cells and demonstrated that many coexpress three mural cell markers (SMA, PDGFRβ, and NG2), arguing that adipose stem cells constitute a subset of mural cells embedded in the wall of blood vessels present within adipose tissue (Figure 2). PDGFRβ cell-surface expression is characteristic of the mural population, comprised of vascular smooth muscle cells and pericytes, and is required for their formation (Jain, 2003). Notably, lineage tracing with a PDGFRβ-cre strain and cell sorting based upon PDGFRβ expression followed by in vitro or in vivo adipogenesis assays were all consistent with the idea that a subset of mural cells are indeed adipose stem cells.
The idea that the mural compartment is a reservoir for adipose stem cells reverberated with studies done more than 25 years ago involving fat depot transplantation and fat depot thermal lesions (Iyama et al., 1979; Napolitano, 1963; Richardson et al., 1982). These experiments indicated that an uncommon subset of adipose depot resident cells were near endothelial cells, possessed long cytoplasmic processes (pericyte-like attributes), and contained multiple, small lipid droplets. The emerging biology of ADSCs and MSCs provide indirect support for the possibility that the adipose stem cell resides in the mural compartment (Lin et al., 2008; Traktuev et al., 2008). Indeed, pericytes in a variety of tissues have been proposed as an MSC repository (Crisan et al., 2008). How the GFP marked cells (Tang et al., 2008) relate to this pool is unknown, although it seems plausible that they represent a subset of this population.
To further investigate a blood vessel niche of the adipose stem cells, a procedure was developed to isolate the SV particulate (SVP) (Tang et al., 2008). This method was designed to maintain the native SV structure in which a niche is located while removing adipocytes that obscure visualization of precursor location. Analysis of these purified SVPs showed mural marker-expressing stem cells beautifully arrayed around vessels. The stem cells present in freshly isolated SVP vessels did not contain lipid droplets, whereas organotypic cultures of SVPs led to formation of lipid-laden GFP+ adipocytes along the blood vessels, indicating that the vessel-associated and lineage-marked stem cells were indeed adipogenic. Thus, this ex vivo experimental system preserves niche microanatomy and allows the assessment and manipulation of stem cells within this critical and privileged environment.
It is also important to underscore that to be defined as a niche, the microenvironment must provide regulatory inputs to the resident stem cell beyond simply representing a physical location. And our studies have yet to establish this key requirement. But the results discussed above do build upon a diverse series of reports implicating the blood vessel as a potentially important niche for a variety of progenitors, including hematopoietic and neural stem cells, that appear to be in direct contact with blood vessels (Hooper et al., 2009; Shen et al., 2008; Tang et al., 2008; Tavazoie et al., 2008). The identification of the vasculature as an adipose stem cell niche also supports earlier studies indicating that adipocytes form in intimate juxtaposition with blood vessels and that adipogenesis and angiogenesis are tightly orchestrated (Crandall et al., 1997; Kolonin et al., 2004; Tang et al., 2008). For example, the number of adipose stem cells present within a depot correlates with vascular density. Light and electron microscopic studies (some described above) performed as early as the 1960s suggested that adipose stem cells reside in or near the blood vessel. Some micrographs from these studies created the impression that developing fat cells were pulling away from, or even emerging from, the vasculature (Iyama et al., 1979; Napolitano, 1963; Richardson et al., 1982). During development, adipose depot vasculogenesis correlates temporally and spatially with adipocyte formation, and angiogenesis similarly correlates with the expansion of the adipocyte compartment observed in obesity (Cao, 2007; Crandall et al., 1997; Fukumura et al., 2003; Neels et al., 2004).
Classical morphological studies suggest that an early event in adipocyte development is the formation of a capillary network in loose regions of mesenchymal or connective tissue. During development, the appearance of vascular structures often precedes slightly the formation of fat cells in the primitive fat anlagen, after which adipocytes appear to sprout along the vasculature (Iyama et al., 1979). Several studies indicate that blood vessel development may promote recruitment of adipose stem cells, may stimulate adipogenesis, and may induce the morphogenic movements required to correctly localize adipocytes (Cao, 2007; Crandall et al., 1997; Fukumura et al., 2003; Neels et al., 2004). Notably, this relationship may reflect a reciprocal process as adipose lineage cells secrete a variety of angiogenic regulators, including vascular endothelial growth factor (VEGF), plasminogen activator inhibitor-1, fibroblast growth factor 2, and many matrix metalloproteinases (Cao, 2007). In vitro “mixing” studies aggregating adipose depot resident stem cells with endothelial cells indicate that the stem cells stimulate the ability of endothelial cells to form vessels (Traktuev et al., 2009). Nishimura et al. observed so called “adipogenic/angiogenic cell clusters” in which adipogenesis and angiogenesis occur in close proximity. Notably, these clusters increase significantly in genetically obese db/db mice (Nishimura et al., 2007), indicating that both compartments are stimulated to expand in obesity (see “From Stem to Stern” below).
Since the vasculature may supply important cues for adipocyte stem cells—controlling the balance between quiescence, proliferation, and differentiation—antiangiogenic factors seem a plausible approach for treating obesity. Indeed a spectrum of angiogenic inhibitors reversibly reduced murine obesity, although these studies suffer from possible confounds of an anorexic effect (Rupnick et al., 2002). Kolonin and colleagues identified a peptide motif that specifically targets white adipose depot endothelium. They then developed a proapoptotic peptide linked to this motif that, when administered to genetically obese and diabetic ob/ob mice, rapidly mitigated obesity and improved metabolic profiles (Kolonin et al., 2004). Similarly, treating obese db/db mice with an anti-VEGF antibody reduced adipose depot size and diminished the number of small, apparently nascent, adipocytes, as well as the aforementioned adipogenic/angiogenic clusters (Nishimura et al., 2007). Thus, inhibiting angiogenesis in adipose tissues may prove effective in ameliorating obesity and its sequelae.
From Stem to Stern: Stem Cells and the Obesigenic Phenotype
Surplus caloric intake and limited energy expenditure are common features of modern life and pose a great challenge for metabolic homeostasis. In large part, adipose tissues provide an evolutionarily programmed adaptation to this challenge, sequestering fatty acids, toxic to most tissue, within adipocytes (Gregoire et al., 1998; MacDougald and Mandrup, 2002; Spiegelman and Flier, 2001). Adipose tissues have an extraordinary ability to expand, perhaps more than any other tissue (Gesta et al., 2007; Hossain et al., 2007; Olshansky et al., 2005; Spiegelman and Flier, 2001). In principal, this expansion could involve several mechanisms with adipocyte hyperplasia and adipocyte hypertrophy primarily considered. The relative contributions of these two processes depend upon genetic factors, diet, and the particular depot (inguinal, perigonadal, etc.) under investigation. The presence of a hyperplastic response indicates the involvement of the stem cell compartment, but this interpretation does not imply that stem cell recruitment is the driving force for expansion. What is quite clear is that adipocyte hypertrophy is common in the development of obesity; indeed, individual adipocytes have a remarkable capacity to expand with estimates of a 2- to 3-fold increased volume (Hirsch and Batchelor, 1976; Salans et al., 1973). Yet once the stimulus (e.g., caloric intake greater than expenditure) is prolonged, the hypertrophic response may contribute to metabolic dysregulation. Since new adipocytes are thought protective against metabolic dysfunction, it is plausible that these maladaptive responses could be reversed by recruiting additional cells from a stem compartment.
Following the identification of the Lin−, CD29+, CD34+, Sca1+, CD24+ adipose stem cells, Rodeheffer and colleagues also probed the role of these cells in obesity and uncovered intriguing clues (Rodeheffer et al., 2008). In this case, they transplanted the Lin−, CD29+, CD34+, Sca1+, CD24+ population derived from adipocyte-luciferase mice into adipose depots of wild-type mice, rather than those with a paucity of fat as described above. However, no adipocyte formation was detected when the wild-type recipients were fed a normal mouse diet (<10% fat). In contrast, feeding the recipient mice a high-fat diet induced formation of adipocytes, scored by expression of luciferase, indicating that, similar to animals with lipodystrophy, obese animals contain physiological (or pathophysiological) signals that stimulate adipose stem cells.
Weighing into the fray, Joe et al. studied a Lin−, Sca1+, CD24+ population present in the adipose SV fraction in control and high-fat diet conditions (Joe et al., 2009). Although this Lin−, Sca1+, CD24+ contingent may be relatively heterogeneous, Joe and colleagues hypothesized that aspects of biological responses provoked therein might reflect the behavior of the included adipose stem cell subpopulation. In support of this notion, several studies report that isolated SV fraction cells display adipogenic behavior in vitro similar to that observed in vivo (Scime et al., 2005; Sengenes et al., 2005). Employing high-fat feeding, BrdU labeling, and flow cytometry, Joe and colleagues observed that the dietary stimulus increased proliferation of the Lin−, Sca1+, CD24+ fraction (Joe et al., 2009).
Additional lines of evidence support the idea that dietary cues can recruit stem cells into the adipogenic lineage. Bone marrow is a rich resource of a variety of stem cell populations, including hematopoeitic stem cells and multipotent MSCs. Bone marrow also contains adipocytes—and perhaps adipocyte stem cells—and the number of marrow resident adipocytes increases with age as well as in various pathophysiological conditions (e.g., osteoporosis). Crossno et al. transplanted labeled bone marrow into unlabeled hosts and observed labeled cells with characteristics of adipocytes, although these cells exhibited a multilocular rather than a prototypical unilocular appearance, in host adipose cells. Interestingly, this process was stimulated by high-fat diet (Crossno et al., 2006). However, a closely related follow-up report, albeit with some experimental distinctions, seems at odds with these data (Koh et al., 2007). Thus, several lines of evidence indicate that adipose stem cells play a role in obesity formation and are regulated by diet and obesity. Future experiments, for example, ablating adipose stem cells or altering their biology with pharmacologic or genetic means, are now required to rigorously assess their role in a variety of physiological and disease-related settings.
Adipose Stem Cells: Potential Therapeutic Applications
The identification of adipose stem cells and the tools to analyze them will enable investigators to explore and manipulate these cells in a variety of clinically relevant situations (Figure 3). Adipose tissue is an abundant source of stem cells (see below) that can be used for various regenerative approaches, but such methods have been reviewed in detail recently and will therefore not be discussed here (Gurdon and Melton, 2008; Hansson et al., 2009). The treatment of lipodystrophy, described above, will require inducing the stem cells to form adipose tissue, their natural path. Another promising application is the treatment of women who have undergone lumpectomy for breast cancers. Currently available reconstructive surgery using synthetic materials is often unsatisfactory, and moving fat depots, or mature adipocytes, from other locations is currently untenable. Transplanted adipose stem cells may overcome the problems, as they recruit a vascular supply and have a natural texture (Rodeheffer et al., 2008; Tang et al., 2008; Yoshimura et al., 2008). This strategy could also be used to correct other anatomical defects, for example in reconstructive surgery following trauma, as well as for wound healing, as adipose stem cells appear to stimulate this process (Lu et al., 2008; Nambu et al., 2007). Related applications for adipose stem cells are also widely desired for cosmetic purposes, and a large “body sculpting” industry is dedicated to finding available sources (Matsumoto et al., 2006). In essence, one could remove unwanted fat and then isolate and transplant the stem cells into a desired location. Such an approach might produce thinner hips and bigger lips—a surefire Hollywood blockbuster.
Figure 3. Adipose Stem Cells Have Clinical Promise.
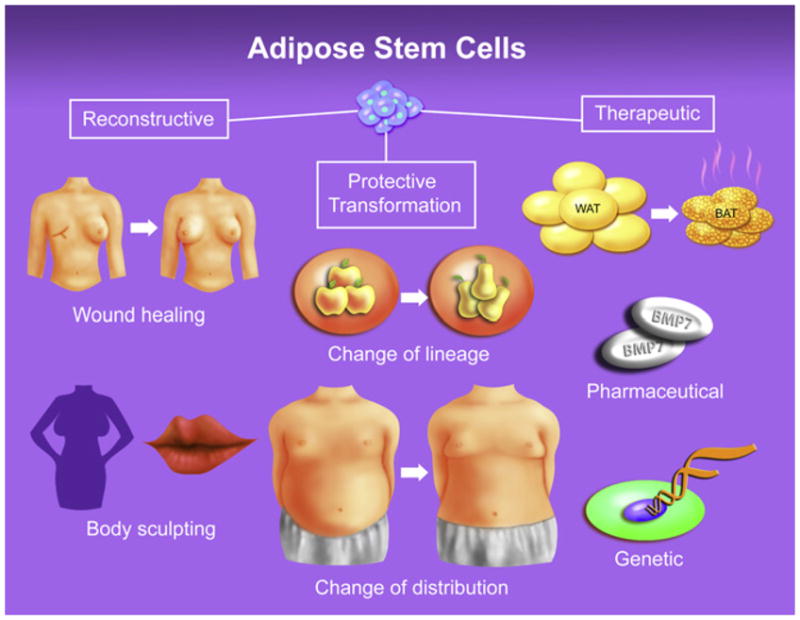
Adipose stem cells have a variety of clinical applications: reconstructive applications, protective transformations, and therapeutic applications. Reconstructive applications broadly encompass surgical, genetic or traumatic defects as well as purely cosmetic purposes. Protective transformations denote induction of a heightened subcutaneous, metabolically protective, adipose signature (pear fat), preferentially over a visceral one (apple fat); this latter type is strongly associated with disease risk. These protections, which do not reduce total fat mass, either change the phenotype of extant depots or change where the fat is located, diverting fat storage to subcutaneous depots. Therapeutic refers to molding the white, energy storing, adipose lineage to a brown-like, energy burning, adipocyte fate, thereby reducing obesity and metabolic dysfunction. These approaches exploit the proclivity of adipose lineage cells to form adipocytes, a feature that paradoxically may be exploited to cure obesity and diabetes.
In addition to reconstructive, regenerative, or reprogramming applications, adipose stem cells might also be directed toward the very diseases in which they are implicated. Subcutaneous WAT can counteract or reduce metabolic dysfunction, so isolating, expanding, and reimplanting subcutaneous adipose stem cells might be used to reduce blood glucose, cholesterol levels, and even cancer risk. The isolated stem cells might be manipulated to enhance their capacity to produce subcutaneous adipocytes or a heightened subcutaneous adipocyte signature, perhaps by treating them with small molecules; estrogenic signals are one potential avenue. An alternate strategy might be to induce white adipose stem cells into a brown-like phenotype, enhancing energy dissipation following reimplantation. Thermogenic (cold exposure), pharmacologic (β3 adrenergic agonists, BMP7), or genetic (PDRM16) stimuli might be advantageous in this regard (Cannon and Nedergaard, 2004; Granneman and Whitty, 1991; Seale et al., 2008; Tseng et al., 2008). The existence of an adipose stem cell niche in the blood vessel wall suggests that they are well situated to receive blood-borne cues or stimuli, such as small molecules or even gene therapy.
Regardless of the therapeutic application, adipose stem cells should be relatively easy to obtain, as humans harbor a striking amount of adipose tissue, which is a plentiful source of stem cells. According to the Center for Disease Control, the average American man or woman has more than 50 pounds of fat, and even a lean woman is more than 15% adipose tissue (the current average range for an American woman is 32%–42%). Thus, at 120 pounds (picture an Olympian), a woman may harbor more than a dozen pounds of fat from which to procure stem cells. On the other end of the spectrum, an obese man (current average range for American men is 23%–31% body fat) may contain hundreds of pounds of adipose tissue. What’s more, plastic surgeons routinely perform liposuction, removing and discarding unwanted fat along with the embedded stem cells. Thus the procedures for adipose stem cell isolation are already well established, minimally invasive, and safe.
Future Basic Science Directions
The insights and tools provided by the reports reviewed above will now enable investigators to address a variety of fundamental questions; the answers to many have important therapeutic implications. Do adipose stem cells arise in situ in the vessel, or do they form elsewhere and migrate to the vessel wall? What are the niche-derived signals that control adipose stem cell biology and how might we harness the signals for desired therapeutic outcomes? Are the white adipose stem cells important in homeostasis and maintenance or just in response to high-fat diet? Since the stem cells appear to inhabit a therapeutically accessible niche, do current treatments for diabetes, for example, thiazolidinediones that activate the stem cells in vitro, alter their biology in vivo? Although we are in the midst of a dire and intertwined epidemic of diabesity, the answers to these questions and the recently described discoveries provide a hopeful light within this roiling tempest.
“eat ye that which is good and let your soul delight itself in fatness.”
Isaiah 55:2, ~750 BCE
“Our people are killing themselves with a fork.”
Rev. G. Roland, 21st century
Acknowledgments
We wish to thank Drs. Debbie Clegg, William Dauer, Jeffrey Friedman, Jeffrey Kreuger, Jaemyoung Suh, and Michelle Tallquist, as well as former and current members of the Graff lab who have very much enlightened, informed, and refined our notions. Lewis Calver and the UTSW Biomedical Communications Graduate Program, which he directs, (Figure 1) and Roshanak Mehdibeigi (Figures 2 and 3) graciously produced all artwork. We also thank the NIDDK and NIH for support (DK066556, DK064261). J.G. is a founder of Reata Pharmaceuticals. We apologize for the inability to cite a vast number of papers that have paved the way for the studies reviewed above and for many of the notions put forth, but space limits preclude us from doing so.
References
- Allison DB, Fontaine KR, Manson JE, Stevens J, VanItallie TB. Annual deaths attributable to obesity in the United States. JAMA. 1999;282:1530–1538. doi: 10.1001/jama.282.16.1530. [DOI] [PubMed] [Google Scholar]
- Atit R, Sgaier SK, Mohamed OA, Taketo MM, Dufort D, Joyner AL, Niswander L, Conlon RA. Beta-catenin activation is necessary and sufficient to specify the dorsal dermal fate in the mouse. Dev Biol. 2006;296:164–176. doi: 10.1016/j.ydbio.2006.04.449. [DOI] [PubMed] [Google Scholar]
- Barak Y, Nelson MC, Ong ES, Jones YZ, Ruiz-Lozano P, Chien KR, Koder A, Evans RM. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol Cell. 1999;4:585–595. doi: 10.1016/s1097-2765(00)80209-9. [DOI] [PubMed] [Google Scholar]
- Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and Assays. Cell Stem Cell. 2008;2:313–319. doi: 10.1016/j.stem.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- Cao Y. Angiogenesis modulates adipogenesis and obesity. J Clin Invest. 2007;117:2362–2368. doi: 10.1172/JCI32239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepko CL, Ryder E, Austin C, Golden J, Fields-Berry S, Lin J. Lineage analysis with retroviral vectors. Methods Enzymol. 2000;327:118–145. doi: 10.1016/s0076-6879(00)27272-8. [DOI] [PubMed] [Google Scholar]
- Crandall DL, Hausman GJ, Kral JG. A review of the microcirculation of adipose tissue: anatomic, metabolic, and angiogenic perspectives. Microcirculation. 1997;4:211–232. doi: 10.3109/10739689709146786. [DOI] [PubMed] [Google Scholar]
- Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Crossno JT, Jr, Majka SM, Grazia T, Gill RG, Klemm DJ. Rosiglitazone promotes development of a novel adipocyte population from bone marrow-derived circulating progenitor cells. J Clin Invest. 2006;116:3220–3228. doi: 10.1172/JCI28510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ridder CM, Bruning PF, Zonderland ML, Thijssen JH, Bonfrer JM, Blankenstein MA, Huisveld IA, Erich WB. Body fat mass, body fat distribution, and plasma hormones in early puberty in females. J Clin Endocrinol Metab. 1990;70:888–893. doi: 10.1210/jcem-70-4-888. [DOI] [PubMed] [Google Scholar]
- Duan SZ, Ivashchenko CY, Whitesall SE, D’Alecy LG, Duquaine DC, Brosius FC, 3rd, Gonzalez FJ, Vinson C, Pierre MA, Milstone DS, Mortensen RM. Hypotension, lipodystrophy, and insulin resistance in generalized PPARgamma-deficient mice rescued from embryonic lethality. J Clin Invest. 2007;117:812–822. doi: 10.1172/JCI28859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elabd C, Chiellini C, Carmona M, Galitzky J, Cochet O, Petersen R, Penicaud L, Kristiansen K, Bouloumie A, Casteilla L, et al. Human multipotent adipose-derived stem cells differentiate into functional brown adipocytes. Stem Cells. 2009 doi: 10.1002/stem.200.. in print. Published online August 20, 2009. [DOI] [PubMed] [Google Scholar]
- Elbers JM, Asscheman H, Seidell JC, Gooren LJ. Effects of sex steroid hormones on regional fat depots as assessed by magnetic resonance imaging in transsexuals. Am J Physiol. 1999;276:E317–E325. doi: 10.1152/ajpendo.1999.276.2.E317. [DOI] [PubMed] [Google Scholar]
- Fukumura D, Ushiyama A, Duda DG, Xu L, Tam J, Krishna V, Chatterjee K, Garkavtsev I, Jain RK. Paracrine regulation of angiogenesis and adipocyte differentiation during in vivo adipogenesis. Circ Res. 2003;93:e88–e97. doi: 10.1161/01.RES.0000099243.20096.FA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambacciani M, Ciaponi M, Cappagli B, Piaggesi L, De Simone L, Orlandi R, Genazzani AR. Body weight, body fat distribution, and hormonal replacement therapy in early postmenopausal women. J Clin Endocrinol Metab. 1997;82:414–417. doi: 10.1210/jcem.82.2.3735. [DOI] [PubMed] [Google Scholar]
- Gesta S, Bluher M, Yamamoto Y, Norris AW, Berndt J, Kralisch S, Boucher J, Lewis C, Kahn CR. Evidence for a role of developmental genes in the origin of obesity and body fat distribution. Proc Natl Acad Sci USA. 2006;103:6676–6681. doi: 10.1073/pnas.0601752103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell. 2007;131:242–256. doi: 10.1016/j.cell.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Graff JM. Embryonic patterning: to BMP or not to BMP, that is the question. Cell. 1997;89:171–174. doi: 10.1016/s0092-8674(00)80196-8. [DOI] [PubMed] [Google Scholar]
- Granneman JG, Whitty CJ. CGP 12177A modulates brown fat adenylate cyclase activity by interacting with two distinct receptor sites. J Pharmacol Exp Ther. 1991;256:421–425. [PubMed] [Google Scholar]
- Grauer WO, Moss AA, Cann CE, Goldberg HI. Quantification of body fat distribution in the abdomen using computed tomography. Am J Clin Nutr. 1984;39:631–637. doi: 10.1093/ajcn/39.4.631. [DOI] [PubMed] [Google Scholar]
- Gregoire FM, Smas CM, Sul HS. Understanding adipocyte differentiation. Physiol Rev. 1998;78:783–809. doi: 10.1152/physrev.1998.78.3.783. [DOI] [PubMed] [Google Scholar]
- Grinspoon S, Carr A. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N Engl J Med. 2005;352:48–62. doi: 10.1056/NEJMra041811. [DOI] [PubMed] [Google Scholar]
- Gronthos S, Franklin DM, Leddy HA, Robey PG, Storms RW, Gimble JM. Surface protein characterization of human adipose tissue-derived stromal cells. J Cell Physiol. 2001;189:54–63. doi: 10.1002/jcp.1138. [DOI] [PubMed] [Google Scholar]
- Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. 2002;129:2447–2457. doi: 10.1242/dev.129.10.2447. [DOI] [PubMed] [Google Scholar]
- Gurdon JB, Melton DA. Nuclear reprogramming in cells. Science. 2008;322:1811–1815. doi: 10.1126/science.1160810. [DOI] [PubMed] [Google Scholar]
- Hansson EM, Lindsay ME, Chien KR. Regeneration next: toward heart stem cell therapeutics. Cell Stem Cell. 2009;5:364–377. doi: 10.1016/j.stem.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Hellman B, Hellerstrom C. Cell renewal in the white and brown fat tissue of the rat. Acta Pathol Microbiol Scand. 1961;51:347–353. doi: 10.1111/j.1699-0463.1961.tb00373.x. [DOI] [PubMed] [Google Scholar]
- Hirsch J, Batchelor B. Adipose tissue cellularity in human obesity. Clin Endocrinol Metab. 1976;5:299–311. doi: 10.1016/s0300-595x(76)80023-0. [DOI] [PubMed] [Google Scholar]
- Hollenberg CH, Vost A. Regulation of DNA synthesis in fat cells and stromal elements from rat adipose tissue. J Clin Invest. 1969;47:2485–2498. doi: 10.1172/JCI105930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper AT, Butler JM, Nolan DJ, Kranz A, Iida K, Kobayashi M, Kopp HG, Shido K, Petit I, Yanger K, et al. Engraftment and reconstitution of hematopoiesis is dependent on VEGFR2-mediated regeneration of sinusoidal endothelial cells. Cell Stem Cell. 2009;4:263–274. doi: 10.1016/j.stem.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsley V, O’Carroll D, Tooze R, Ohinata Y, Saitou M, Obukhanych T, Nussenzweig M, Tarakhovsky A, Fuchs E. Blimp1 defines a progenitor population that governs cellular input to the sebaceous gland. Cell. 2006;126:597–609. doi: 10.1016/j.cell.2006.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain P, Kawar B, El Nahas M. Obesity and diabetes in the developing world–a growing challenge. N Engl J Med. 2007;356:213–215. doi: 10.1056/NEJMp068177. [DOI] [PubMed] [Google Scholar]
- Iyama K, Ohzono K, Usuku G. Electron microscopical studies on the genesis of white adipocytes: differentiation of immature pericytes into adipocytes in transplanted preadipose tissue. Virchows Arch B Cell Pathol Incl Mol Pathol. 1979;31:143–155. doi: 10.1007/BF02889932. [DOI] [PubMed] [Google Scholar]
- Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9:685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- Javazon EH, Beggs KJ, Flake AW. Mesenchymal stem cells: paradoxes of passaging. Exp Hematol. 2004;32:414–425. doi: 10.1016/j.exphem.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Joe AW, Yi L, Even Y, Vogl AW, Rossi FM. Depot-specific differences in Adipogenic progenitor abundance and proliferative response to high fat diet. Stem Cells. 2009;27:2563–2570. doi: 10.1002/stem.190. [DOI] [PubMed] [Google Scholar]
- Jones DL, Wagers AJ. No place like home: anatomy and function of the stem cell niche. Nat Rev Mol Cell Biol. 2008;9:11–21. doi: 10.1038/nrm2319. [DOI] [PubMed] [Google Scholar]
- Kliewer SA, Forman BM, Blumberg B, Ong ES, Borgmeyer U, Mangelsdorf DJ, Umesono K, Evans RM. Differential expression and activation of a family of murine peroxisome proliferator-activated receptors. Proc Natl Acad Sci USA. 1994;91:7355–7359. doi: 10.1073/pnas.91.15.7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh YJ, Kang S, Lee HJ, Choi TS, Lee HS, Cho CH, Koh GY. Bone marrow-derived circulating progenitor cells fail to transdifferentiate into adipocytes in adult adipose tissues in mice. J Clin Invest. 2007;117:3684–3695. doi: 10.1172/JCI32504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolonin MG, Saha PK, Chan L, Pasqualini R, Arap W. Reversal of obesity by targeted ablation of adipose tissue. Nat Med. 2004;10:625–632. doi: 10.1038/nm1048. [DOI] [PubMed] [Google Scholar]
- Koutnikova H, Cock TA, Watanabe M, Houten SM, Champy MF, Dierich A, Auwerx J. Compensation by the muscle limits the metabolic consequences of lipodystrophy in PPAR gamma hypomorphic mice. Proc Natl Acad Sci USA. 2003;100:14457–14462. doi: 10.1073/pnas.2336090100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota N, Terauchi Y, Miki H, Tamemoto H, Yamauchi T, Komeda K, Satoh S, Nakano R, Ishii C, Sugiyama T, et al. PPAR gamma mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Mol Cell. 1999;4:597–609. doi: 10.1016/s1097-2765(00)80210-5. [DOI] [PubMed] [Google Scholar]
- Lepper C, Conway SJ, Fan CM. Adult satellite cells and embryonic muscle progenitors have distinct genetic requirements. Nature. 2009;460:627–631. doi: 10.1038/nature08209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Xie T. Stem cell niche: structure and function. Annu Rev Cell Dev Biol. 2005;21:605–631. doi: 10.1146/annurev.cellbio.21.012704.131525. [DOI] [PubMed] [Google Scholar]
- Lin G, Garcia M, Ning H, Banie L, Guo YL, Lue TF, Lin CS. Defining stem and progenitor cells within adipose tissue. Stem Cells Dev. 2008;17:1053–1063. doi: 10.1089/scd.2008.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livet J, Weissman TA, Kang H, Draft RW, Lu J, Bennis RA, Sanes JR, Lichtman JW. Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature. 2007;450:56–62. doi: 10.1038/nature06293. [DOI] [PubMed] [Google Scholar]
- Loncar D, Afzelius BA, Cannon B. Epididymal white adipose tissue after cold stress in rats. I. Nonmitochondrial changes. J Ultrastruct Mol Struct Res. 1988;101:109–122. doi: 10.1016/0889-1605(88)90001-8. [DOI] [PubMed] [Google Scholar]
- Lu F, Mizuno H, Uysal CA, Cai X, Ogawa R, Hyakusoku H. Improved viability of random pattern skin flaps through the use of adipose-derived stem cells. Plast Reconstr Surg. 2008;121:50–58. doi: 10.1097/01.prs.0000293876.10700.b8. [DOI] [PubMed] [Google Scholar]
- MacDougald OA, Mandrup S. Adipogenesis: forces that tip the scales. Trends Endocrinol Metab. 2002;13:5–11. doi: 10.1016/s1043-2760(01)00517-3. [DOI] [PubMed] [Google Scholar]
- Matsumoto D, Sato K, Gonda K, Takaki Y, Shigeura T, Sato T, Aiba-Kojima E, Iizuka F, Inoue K, Suga H, Yoshimura K. Cell-assisted lipotransfer: supportive use of human adipose-derived cells for soft tissue augmentation with lipoinjection. Tissue Eng. 2006;12:3375–3382. doi: 10.1089/ten.2006.12.3375. [DOI] [PubMed] [Google Scholar]
- Mauer MM, Harris RB, Bartness TJ. The regulation of total body fat: lessons learned from lipectomy studies. Neurosci Biobehav Rev. 2001;25:15–28. doi: 10.1016/s0149-7634(00)00047-6. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, White PM, Zock C, Anderson DJ. Prospective identification, isolation by flow cytometry, and in vivo self-renewal of multipotent mammalian neural crest stem cells. Cell. 1999;96:737–749. doi: 10.1016/s0092-8674(00)80583-8. [DOI] [PubMed] [Google Scholar]
- Munoz J, Derstine A, Gower BA. Fat distribution and insulin sensitivity in postmenopausal women: influence of hormone replacement. Obes Res. 2002;10:424–431. doi: 10.1038/oby.2002.59. [DOI] [PubMed] [Google Scholar]
- Nambu M, Ishihara M, Nakamura S, Mizuno H, Yanagibayashi S, Kanatani Y, Hattori H, Takase B, Ishizuka T, Kishimoto S, et al. Enhanced healing of mitomycin C-treated wounds in rats using inbred adipose tissue-derived stromal cells within an atelocollagen matrix. Wound Repair Regen. 2007;15:505–510. doi: 10.1111/j.1524-475X.2007.00258.x. [DOI] [PubMed] [Google Scholar]
- Napolitano L. The Differentiation of White Adipose Cells. An Electron Microscope Study. J Cell Biol. 1963;18:663–679. doi: 10.1083/jcb.18.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrocki AR, Scherer PE. The delicate balance between fat and muscle: adipokines in metabolic disease and musculoskeletal inflammation. Curr Opin Pharmacol. 2004;4:281–289. doi: 10.1016/j.coph.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Neels JG, Thinnes T, Loskutoff DJ. Angiogenesis in an in vivo model of adipose tissue development. FASEB J. 2004;18:983–985. doi: 10.1096/fj.03-1101fje. [DOI] [PubMed] [Google Scholar]
- Nishimura S, Manabe I, Nagasaki M, Hosoya Y, Yamashita H, Fujita H, Ohsugi M, Tobe K, Kadowaki T, Nagai R, Sugiura S. Adipogenesis in obesity requires close interplay between differentiating adipocytes, stromal cells, and blood vessels. Diabetes. 2007;56:1517–1526. doi: 10.2337/db06-1749. [DOI] [PubMed] [Google Scholar]
- Olshansky SJ, Passaro DJ, Hershow RC, Layden J, Carnes BA, Brody J, Hayflick L, Butler RN, Allison DB, Ludwig DS. A potential decline in life expectancy in the United States in the 21st century. N Engl J Med. 2005;352:1138–1145. doi: 10.1056/NEJMsr043743. [DOI] [PubMed] [Google Scholar]
- Orci L, Cook WS, Ravazzola M, Wang MY, Park BH, Montesano R, Unger RH. Rapid transformation of white adipocytes into fat-oxidizing machines. Proc Natl Acad Sci USA. 2004;101:2058–2063. doi: 10.1073/pnas.0308258100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planat-Benard V, Silvestre JS, Cousin B, Andre M, Nibbelink M, Tamarat R, Clergue M, Manneville C, Saillan-Barreau C, Duriez M, et al. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation. 2004;109:656–663. doi: 10.1161/01.CIR.0000114522.38265.61. [DOI] [PubMed] [Google Scholar]
- Richardson RL, Hausman GJ, Campion DR. Response of pericytes to thermal lesion in the inguinal fat pad of 10-day-old rats. Acta Anat (Basel) 1982;114:41–57. doi: 10.1159/000145577. [DOI] [PubMed] [Google Scholar]
- Rodeheffer MS, Birsoy K, Friedman JM. Identification of white adipocyte progenitor cells in vivo. Cell. 2008;135:240–249. doi: 10.1016/j.cell.2008.09.036. [DOI] [PubMed] [Google Scholar]
- Rohlfs EM, Daniel KW, Premont RT, Kozak LP, Collins S. Regulation of the uncoupling protein gene (Ucp) by beta 1, beta 2, and beta 3-adrenergic receptor subtypes in immortalized brown adipose cell lines. J Biol Chem. 1995;270:10723–10732. doi: 10.1074/jbc.270.18.10723. [DOI] [PubMed] [Google Scholar]
- Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, Spiegelman BM, Mortensen RM. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell. 1999;4:611–617. doi: 10.1016/s1097-2765(00)80211-7. [DOI] [PubMed] [Google Scholar]
- Rupnick MA, Panigrahy D, Zhang CY, Dallabrida SM, Lowell BB, Langer R, Folkman MJ. Adipose tissue mass can be regulated through the vasculature. Proc Natl Acad Sci USA. 2002;99:10730–10735. doi: 10.1073/pnas.162349799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salans LB, Cushman SW, Weismann RE. Studies of human adipose tissue. Adipose cell size and number in nonobese and obese patients. J Clin Invest. 1973;52:929–941. doi: 10.1172/JCI107258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scime A, Grenier G, Huh MS, Gillespie MA, Bevilacqua L, Harper ME, Rudnicki MA. Rb and p107 regulate preadipocyte differentiation into white versus brown fat through repression of PGC-1alpha. Cell Metab. 2005;2:283–295. doi: 10.1016/j.cmet.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scime A, Devarakonda S, Conroe HM, Erdjument-Bromage H, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–967. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengenes C, Lolmede K, Zakaroff-Girard A, Busse R, Bouloumie A. Preadipocytes in the human subcutaneous adipose tissue display distinct features from the adult mesenchymal and hematopoietic stem cells. J Cell Physiol. 2005;205:114–122. doi: 10.1002/jcp.20381. [DOI] [PubMed] [Google Scholar]
- Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, Wu L, Lindeman GJ, Visvader JE. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–88. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- Shen Q, Wang Y, Kokovay E, Lin G, Chuang SM, Goderie SK, Roysam B, Temple S. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3:289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Clegg DJ. Sex differences in the regulation of body weight. Physiol Behav. 2009;97:199–204. doi: 10.1016/j.physbeh.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, Blomqvist L, Hoffstedt J, Naslund E, Britton T, et al. Dynamics of fat cell turnover in humans. Nature. 2008;453:783–787. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell. 2001;104:531–543. doi: 10.1016/s0092-8674(01)00240-9. [DOI] [PubMed] [Google Scholar]
- Stern CD, Fraser SE. Tracing the lineage of tracing cell lineages. Nat Cell Biol. 2001;3:E216–E218. doi: 10.1038/ncb0901-e216. [DOI] [PubMed] [Google Scholar]
- Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D, Li HI, Eaves CJ. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439:993–997. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- Strissel KJ, Stancheva Z, Miyoshi H, Perfield JW, 2nd, DeFuria J, Jick Z, Greenberg AS, Obin MS. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes. 2007;56:2910–2918. doi: 10.2337/db07-0767. [DOI] [PubMed] [Google Scholar]
- Sun T, Fu M, Bookout AL, Kliewer SA, Mangelsdorf DJ. MicroRNA let-7 regulates 3T3–L1 adipogenesis. Mol Endocrinol. 2009;23:925–931. doi: 10.1210/me.2008-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Zeve D, Suh JM, Bosnakovski D, Kyba M, Hammer RE, Tallquist MD, Graff JM. White fat progenitor cells reside in the adipose vasculature. Science. 2008;322:583–586. doi: 10.1126/science.1156232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B, Garcia-Verdugo JM, Doetsch F. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3:279–288. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchernof A, Belanger C, Morisset AS, Richard C, Mailloux J, Laberge P, Dupont P. Regional differences in adipose tissue metabolism in women: minor effect of obesity and body fat distribution. Diabetes. 2006;55:1353–1360. doi: 10.2337/db05-1439. [DOI] [PubMed] [Google Scholar]
- Tchkonia T, Lenburg M, Thomou T, Giorgadze N, Frampton G, Pirtskhalava T, Cartwright A, Cartwright M, Flanagan J, Karagiannides I, et al. Identification of depot-specific human fat cell progenitors through distinct expression profiles and developmental gene patterns. Am J Physiol Endocrinol Metab. 2007;292:E298–E307. doi: 10.1152/ajpendo.00202.2006. [DOI] [PubMed] [Google Scholar]
- Tontonoz P, Spiegelman BM. Fat and beyond: the diverse biology of PPARgamma. Annu Rev Biochem. 2008;77:289–312. doi: 10.1146/annurev.biochem.77.061307.091829. [DOI] [PubMed] [Google Scholar]
- Traktuev DO, Merfeld-Clauss S, Li J, Kolonin M, Arap W, Pasqualini R, Johnstone BH, March KL. A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ Res. 2008;102:77–85. doi: 10.1161/CIRCRESAHA.107.159475. [DOI] [PubMed] [Google Scholar]
- Traktuev DO, Prater DN, Merfeld-Clauss S, Sanjeevaiah AR, Saadatzadeh MR, Murphy M, Johnstone BH, Ingram DA, March KL. Robust functional vascular network formation in vivo by cooperation of adipose progenitor and endothelial cells. Circ Res. 2009;104:1410–1420. doi: 10.1161/CIRCRESAHA.108.190926. [DOI] [PubMed] [Google Scholar]
- Tran TT, Yamamoto Y, Gesta S, Kahn CR. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab. 2008;7:410–420. doi: 10.1016/j.cmet.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng YH, Kokkotou E, Schulz TJ, Huang TL, Winnay JN, Taniguchi CM, Tran TT, Suzuki R, Espinoza DO, Yamamoto Y, et al. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature. 2008;454:1000–1004. doi: 10.1038/nature07221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerback S, Nuutila P. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- Waki H, Tontonoz P. Endocrine functions of adipose tissue. Annu Rev Pathol. 2007;2:31–56. doi: 10.1146/annurev.pathol.2.010506.091859. [DOI] [PubMed] [Google Scholar]
- Wang Q, Li YC, Wang J, Kong J, Qi Y, Quigg RJ, Li X. miR-17–92 cluster accelerates adipocyte differentiation by negatively regulating tumor-suppressor Rb2/p130. Proc Natl Acad Sci USA. 2008;105:2889–2894. doi: 10.1073/pnas.0800178105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto N, Akamatsu H, Hasegawa S, Yamada T, Nakata S, Ohkuma M, Miyachi E, Marunouchi T, Matsunaga K. Isolation of multipotent stem cells from mouse adipose tissue. J Dermatol Sci. 2007;48:43–52. doi: 10.1016/j.jdermsci.2007.05.015. [DOI] [PubMed] [Google Scholar]
- Yoshimura K, Sato K, Aoi N, Kurita M, Hirohi T, Harii K. Cell-assisted lipotransfer for cosmetic breast augmentation: supportive use of adipose-derived stem/stromal cells. Aesthetic Plast Surg. 2008;32:48–55. doi: 10.1007/s00266-007-9019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura K, Shigeura T, Matsumoto D, Sato T, Takaki Y, Aiba-Kojima E, Sato K, Inoue K, Nagase T, Koshima I, Gonda K. Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. J Cell Physiol. 2006;208:64–76. doi: 10.1002/jcp.20636. [DOI] [PubMed] [Google Scholar]
- Zannettino AC, Paton S, Arthur A, Khor F, Itescu S, Gimble JM, Gronthos S. Multipotential human adipose-derived stromal stem cells exhibit a perivascular phenotype in vitro and in vivo. J Cell Physiol. 2008;214:413–421. doi: 10.1002/jcp.21210. [DOI] [PubMed] [Google Scholar]
- Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]