Abstract
Sixty Crossfire (Stryker Orthopaedics, Mahwah, NJ) liners were consecutively revised after an average of 2.9 years (range: 0.01 – 8.0 years) for reasons unrelated to wear or mechanical performance of the polyethylene. Femoral head penetration was measured directly from 42 retrievals implanted for over 1 year. Penetration rate results (0.04 mm/y, on average; range: 0.00-0.13 mm/y) confirmed decreasing wear rates with longer in vivo times. Overall, we observed oxidation levels at the bearing surface of the 60 liners (0.5, on average; range: 0.1-1.7) comparable to those of non-implanted liners (0.5, on average; range: 0.3-1.1) and preservation of mechanical properties. We also measured elevated oxidation of the rim (3.4, on average; range: 0.2-8.8) that was correlated with implantation time. Rim surface damage, however, was observed in only 3/60 (5%) cases. Retrieval analysis of the three rim-damaged liners did not reveal an association between surface damage and the reasons for revision.
Keywords: Mechanical properties, oxidation, wear, hip arthroplasty, highly crosslinked ultra-high molecular weight polyethylene
Introduction
Highly crosslinked polyethylene materials are now approaching one decade of clinical use in hip arthroplasty [1]. Radiographic-based clinical studies have continued to report encouraging results for annealed [2-6] and remelted [7-11] highly crosslinked polyethylene liners. However, other than quantifying the radiographic penetration rate and incidence of osteolysis, these clinical studies provide little information about the wear and damage mechanisms associated with this new family of orthopedic biomaterials. Nearly ten years after their clinical introduction, the retrieval experience with remelted and annealed highly crosslinked polyethylene materials continues to be limited to isolated case studies [12-14] and series with relatively short implantation times [15-20].
Recent retrieval studies have reported high levels of oxidation in retrieved annealed liners [16, 17, 20], however, these observations have thus far been generally limited to the rim face, or the unworn regions of the articulating surface. In a series of 12 explanted liners, Currier and colleagues also observed six cases of rim fatigue damage or delamination [16]. However, 8/12 (67%) of the liners in Currier's study had evidence of impingement or dislocation, and consequently the clinical significance of rim damage under these circumstances remains unclear. On one hand, none of the annealed retrievals reported in the literature to date have been explanted due to rim oxidation or rim fatigue [16, 17, 20]. In addition, the femoral head penetration rate of annealed polyethylene in multiple clinical studies has been superior to conventional polyethylene [2-6]. On the other hand, the observations of rim oxidation raise questions about the long-term viability of the rim and liner locking mechanisms.
We have followed the clinical performance of annealed highly crosslinked polyethylene in a high volume urban hospital within the framework of our multicenter prospective retrieval program that was initiated in 2000 [19, 20]. Previously, we have observed that the bearing surface of annealed (Crossfire) liners appeared to be partially protected from in vivo oxidation, when compared with the rim [19, 20]. Because of the short-term follow-up in our previous retrieval studies, which were also limited to a single liner design, it was not known whether our earlier findings could be extrapolated to longer-term implantation of the same design, or to Crossfire liners of differing designs. Furthermore, the effect of in vivo oxidation, if any, on the polyethylene around locking mechanisms has not yet been explored. Thus, in the current study, we tested the hypothesis that, independent of liner design among the two designs examined, the femoral head and metal shell would reduce in vivo oxidation of the bearing surface and locking mechanisms, respectively. In addition, we also sought correlations between oxidation levels and penetration at the bearing surface as well as fatigue damage and cracking of the rim to test the hypothesized association between in vivo oxidation and clinical damage.
Materials and Methods
Implant Information
Sixty acetabular liners fabricated from annealed, highly cross-linked Crossfire™ polyethylene (Stryker Orthopedics, Mahwah, NJ) were retrieved during consecutive revision surgeries at a single, high-volume urban surgical center, in collaboration with a regional retrieval center. The index procedures were performed between May 1999 and November 2007, and the 60 retrieved liners were implanted between 0 and 8 years (average ± SD: 2.9 ± 2.1 y). The implants were collected and analyzed continuously throughout an eight-year period. Explanted liners were cleaned using institutional procedures and expeditiously stored in a subzero freezer to minimize ex vivo oxidative changes, as described previously [19]. Data from four new, never implanted liners served as controls.
The Crossfire liners were produced in the Omnifit (Series II; n=31) and Trident (n=29) designs (Table 1). The locking mechanisms differ between the Omnifit and Trident liner designs. In the Omnifit design, which was the first to be introduced clinically with Crossfire in the fall of 1998, the liner is retained in the shell by an equatorial locking wire that fits within a groove in the backside of the liner. For the Trident design, the backside of the liner is captured in the shell by interference of an equatorial polymer bead and a groove in the metal shell. Omnifit and Trident liners were both stored in a polymeric barrier blister package with a metallic foil barrier cover, which was filled with nitrogen to minimize oxidation during shelf storage [21]. The shelf life was traceable by the manufacturer from the lot codes in 49/60 (82%) retrieved liners. The average shelf life of the retrievals was 0.5±0.7 years.
Table 1.
Summary of Clinical and Implant Data for 60 Crossfire Retrievals (Omnifit Series II and Trident designs). N/A* refers to liners not measurable due to iatrogenic damage.
| Study # | Gender | Age | Weight (lbs) |
BMI (kg/m2) |
UCLA Activity Score |
Previous Revision |
Revision Reason | Implantatio n Time (y) |
Head Size (mm) |
Head Material |
Shell Size (mm) |
Liner Design |
Liner Shelf Life (y) |
Liner Thickness (mm) |
Penetration Rate (mm/y) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 24 | M | 64 | 190 | 26 | 4 | 0 | Dislocation | 0.0 | 28 | CoCr Alloy | 66 | Omnifit | 0.08 | 14.0 | N/A |
| 31 | M | 46 | 247 | 45 | 3 | 1 | Recurrent Dislocation | 0.3 | 28 | CoCr Alloy | 52 | Omnifit | 0.13 | 9.1 | N/A |
| 60 | M | 50 | 193 | 28 | 3 | 1 | Loosening | 0.8 | 28 | Zirconia | 52 | Omnifit | N/A | 12.1 | N/A |
| 71 | F | 53 | 160 | 26 | 4 | 1 | Dislocation | 0.9 | 28 | Zirconia | 50 | Omnifit | 0.09 | 8.5 | N/A |
| 73 | M | 36 | 205 | 29 | 4 | 1 | Dislocation | 0.5 | 28 | Zirconia | 50 | Omnifit | 0.10 | 8.7 | N/A |
| 168 | M | 80 | 140 | 23 | N/A | 0 | Malalignment | 0.0 | 28 | CoCr Alloy | 50 | Trident | 0.59 | 9.3 | N/A |
| 184 | M | 55 | 260 | 34 | 4 | 1 | Loosening | 0.3 | 32 | CoCr Alloy | 60 | Trident | 0.15 | 10.6 | N/A |
| 206 | F | 67 | 160 | 26 | 2 | 0 | Recurrent Dislocation | 0.7 | 28 | CoCr Alloy | 52 | Omnifit | N/A | 8.9 | N/A |
| 222 | M | 68 | 250 | 34 | 4 | 0 | Loosening | 1.6 | 28 | Zirconia | 56 | Omnifit | 0.25 | 10.8 | 0.02 |
| 250 | F | 60 | 160 | 28 | 7 | 0 | Cup Malpositioning | 3.9 | 28 | Zirconia | 48 | Omnifit | 0.17 | 7.5 | N/A* |
| 265 | M | 74 | 154 | 26 | 9 | 0 | Leg Length Discrepancy | 0.8 | 28 | Alumina | 56 | Trident | 0.22 | 11.1 | N/A |
| 279 | F | 81 | 165 | 27 | 2 | 1 | Loosening and Infection of bone | 1.4 | 32 | CoCr Alloy | 62 | Trident | 0.68 | 12.1 | 0.06 |
| 291 | F | 51 | 149 | 29 | 4 | 1 | Femoral Loosening | 2.7 | 28 | CoCr Alloy | 52 | Omnifit | 0.10 | 8.8 | 0.05 |
| 292 | M | 80 | 110 | 16 | 5 | 1 | Loosening | 1.1 | 32 | CoCr Alloy | 60 | Trident | 0.36 | 10.7 | 0.03 |
| 297 | F | 46 | 148 | 25 | 2 | 0 | Acetabular Loosening | 1.6 | 28 | CoCr Alloy | 52 | Omnifit | 0.23 | 11.0 | 0.09 |
| 342 | M | 42 | 172 | 27 | 5 | 1 | Loosening and Infection | 1.4 | 32 | CoCr Alloy | 65 | Trident | 1.54 | 10.6 | 0.09 |
| 343 | F | 61 | 127 | 27 | 3 | 0 | Acetabular Loosening | 3.1 | 28 | CoCr Alloy | 46 | Omnifit | 2.42 | 7.2 | 0.06 |
| 345 | F | 78 | 210 | 33 | 4 | 0 | Instability | 3.3 | 28 | CoCr Alloy | 62 | Omnifit | 0.08 | 13.9 | 0.05 |
| 346 | F | 56 | 138 | 26 | 8 | 1 | Loosening | 4.8 | 28 | CoCr Alloy | 52 | Omnifit | 0.17 | 8.8 | 0.04 |
| 356 | F | 64 | 212 | 32 | 6 | 0 | Loosening | 3.4 | 28 | Zirconia | 52 | Omnifit | 0.45 | 9.1 | 0.04 |
| 369 | M | 59 | 230 | 30 | 8 | 2 | Instability | 1.6 | 32 | CoCr Alloy | 58 | Trident | 0.22 | 9.6 | 0.09 |
| 384 | M | 75 | 168 | N/A | 4 | 0 | Instability | 4.0 | 28 | Zirconia | 58 | Omnifit | 0.16 | 12.9 | 0.02 |
| 387 | M | 72 | 190 | N/A | 8 | 0 | Bony impingement | 3.3 | 28 | CoCr Alloy | 54 | Omnifit | N/A | 11.1 | 0.07 |
| 390 | M | 69 | 222 | 31 | 4 | 0 | Subsidence of Femoral Component | 4.6 | 28 | Zirconia | 54 | Omnifit | N/A | 10.8 | 0.02 |
| 399 | F | 29 | 150 | N/A | 4 | 1 | Loosening and instability | 5.0 | 28 | CoCr Alloy | 56 | Omnifit | 0.06 | 11.3 | 0.03 |
| 414 | F | 80 | 190 | 30 | N/A | 0 | Instability and Loosening | 0.6 | 32 | CoCr Alloy | 54 | Trident | 0.34 | 7.5 | N/A |
| 438 | M | 74 | 180 | 30 | 6 | 0 | Malalignment, Instability | 2.4 | 32 | CoCr Alloy | 58 | Trident | 0.84 | 10.9 | 0.01 |
| 453 | F | 74 | 162 | 29 | 3 | 0 | Loosening | 1.0 | 32 | CoCr Alloy | 52 | Trident | 0.08 | 7.2 | N/A* |
| 457 | F | 80 | 150 | 26 | N/A | 0 | Loosening | 0.8 | 32 | CoCr Allov | 52 | Trident | 0.55 | 7.8 | N/A |
| 458 | M | 60 | 200 | 30 | 6 | 1 | Loosening | 6.0 | 28 | CoCr Alloy | 60 | Omnifit | 0.06 | 13.8 | 0.01 |
| 474 | F | 57 | 153 | 23 | 2 | 0 | Infection | 1.1 | 36 | CoCr Alloy | 54 | Trident | 0.29 | 5.2 | 0.07 |
| 479 | F | 73 | 160 | 27 | N/A | N/A | Loosening | 3.7 | 28 | CoCr Alloy | 52 | Omnifit | N/A | 9.0 | 0.02 |
| 488 | F | 66 | 188 | 37 | 6 | 0 | Loosening | 5.2 | 28 | CoCr Alloy | 42 | Omnifit | 0.41 | 7.5 | 0.04 |
| 514 | F | 57 | 179 | 28 | N/A | 1 | Loosening | 2.9 | 32 | Alumina | 46 | Trident | 0.06 | 7.6 | 0.02 |
| 515 | F | 53 | 135 | 23 | 3 | 0 | Loosening | 5.2 | 28 | Zirconia | 48 | Omnifit | 0.06 | 7.4 | 0.01 |
| 522 | M | 62 | 200 | 29 | 7 | 0 | Loosening | 3.9 | 32 | CoCr Alloy | 54 | Trident | N/A | 9.1 | 0.01 |
| 541 | F | 78 | 107 | 22 | 3 | 0 | Loosening | 2.2 | 28 | CoCr Alloy | 50 | Trident | 0.92 | 9.6 | 0.03 |
| 549 | M | 53 | 198 | 28 | 5 | 1 | Infection | 1.2 | 32 | CoCr Alloy | 52 | Omnifit | 0.71 | 6.8 | 0.05 |
| 564 | F | 72 | 114 | 20 | 4 | 1 | Recurrent dislocation | 3.7 | 32 | CoCr Alloy | N/A | Omnifit | 1.61 | 7.5 | 0.03 |
| 568 | F | 66 | 216 | 37 | 5 | 1 | Dislocation | 0.7 | 36 | CoCr Alloy | 56 | Trident | 0.93 | 7.0 | N/A |
| 575 | F | 74 | 135 | 23 | 6 | 0 | Loosening of Acetabular Component | 1.5 | 36 | CoCr Alloy | 56 | Trident | 0.18 | 7.4 | 0.01 |
| 577 | F | 71 | 126 | 20 | 5 | 1 | Recurrent Dislocations | 3.2 | 32 | CoCr Alloy | 52 | Trident | 0.48 | 7.3 | 0.10 |
| 578 | F | 60 | 225 | 34 | 3 | 1 | Loosening | 4.6 | 32 | CoCr Alloy | 60 | Trident | 0.61 | 11.2 | 0.02 |
| 614 | M | 73 | 200 | 29 | 6 | 0 | Loosening (Femoral) | 1.6 | 32 | Alumina | 56 | Trident | 0.42 | 9.1 | 0.00 |
| 619 | M | 46 | 285 | 40 | N/A | 0 | Infection | 5.3 | 32 | CoCr Alloy | 54 | Trident | N/A | 9.5 | 0.03 |
| 664 | F | 61 | 135 | 23 | 2 | 0 | Loosening | 2.3 | 32 | CoCr Alloy | 52 | Trident | 0.49 | 7.3 | 0.03 |
| 674 | F | 49 | 135 | 23 | 4 | 1 | Instability | 2.9 | 36 | CoCr Alloy | 58 | Trident | 0.28 | 8.8 | 0.04 |
| 702 | M | 55 | 220 | 29 | 6 | 0 | Loosening | 2.7 | 32 | CoCr Alloy | 54 | Trident | 0.11 | 11.2 | 0.03 |
| 706 | M | 67 | 140 | 27 | 6 | 3 | Infection | 1.6 | 32 | CoCr Alloy | 64 | Omnifit | 3.39 | 12.3 | N/A* |
| 716A | F | 77 | 180 | 31 | 2 | 0 | Infection | 6.6 | 28 | Alumina | 52 | Omnifit | N/A | 9.1 | N/A* |
| 716B | F | 77 | 180 | 31 | 2 | 1 | Infection | 0.1 | 28 | CoCr Alloy | 52 | Omnifit | 3.4 | 9.5 | N/A |
| 729 | M | 58 | 255 | 37 | 6 | 0 | Loosening | 5.9 | 32 | Alumina | 50 | Trident | 0.1 | 9.4 | 0.01 |
| 735 | M | 63 | 214 | 30 | 7 | 1 | Fracture of ceramic head | 8.0 | 28 | Zirconia | 54 | Omnifit | 0.1 | 11.3 | 0.13 |
| 736 | F | 66 | 200 | 29 | 4 | 0 | luxation, Impingement, Cup Malposition | 5.2 | 28 | CoCr Alloy | 52 | Trident | 0.2 | 9.6 | 0.01 |
| 743A | M | 60 | 190 | 25 | 2 | 1 | Dislocation, migrated cup | 3.1 | 32 | CoCr Alloy | 58 | Omnifit | 0.8 | 10.8 | 0.01 |
| 760 | M | 75 | 169 | 27 | 5 | 1 | Periprosthetic femoral fracture | 4.2 | 36 | CoCr Alloy | N/A | Trident | 0.9 | 10.9 | N/A* |
| 775 | F | 66 | 142 | 26 | 7 | 2 | Loosening (Femoral) | 7.4 | 28 | CoCr Alloy | 50 | Omnifit | 0.1 | 9.0 | 0.01 |
| 795 | M | 66 | 175 | 28 | 7 | 0 | Femoral and Acetabular Loosening | 5.4 | 28 | Alumina | 52 | Trident | N/A | 9.5 | 0.01 |
| 798 | M | 56 | 272 | 38 | 7 | 0 | Fracture of ceramic of head | 7.4 | 28 | Zirconia | 56 | Omnifit | N/A | 10.7 | 0.04 |
| 805 | M | 48 | 200 | 30 | 7 | 1 | Loosening | 4.6 | 28 | Alumina | 64 | Trident | N/A | 14.7 | 0.00 |
Retrieved Omnifit liners had inner diameters of either 28 mm or 32 mm, whereas with the retrieved Trident liners, the inner diameter ranged from 28 to 36 mm (Table 1). The head material was a cobalt chrome alloy in 70% (42/60) of the cases, a ceramic in 30% (18/60) of the cases (zirconia in 18% (11/60) of the cases; alumina in 12% (7/60) of the cases; Table 1). The measured thickness of the liners, in unworn locations, ranged from 5.2 to 14.7 mm. The outer diameter of the acetabular shells varied between 42 to 66 mm (Table 1).
Clinical information
Clinical details for the 60 liners are summarized in Table 1; for completeness these data include the 12 retrieved Omnifit liners reported in our previous study [19]. Fifty-two percent (31/60) of the patients were females, and their average age was 63y (range: 29 to 81y). 47% (28/60) of the revised components were used in patients that had a history of previous revision surgeries. The implants in this study were revised for reasons unrelated to wear, and included aseptic loosening, n=28; instability and recurrent dislocation, n=16; infection, n=8; malalignment, n=3; leg length discrepancy, n=1; bony impingement, n=1; fracture of ceramic head, n=2; and periprosthetic femoral fracture, n=1. None of the revisions were due to osteolysis. In addition, preliminary statistical analysis (contingency tables) confirmed Omnifit and Trident retrievals showed no significant differences concerning the distributions of their respective reasons for revision.
Patient activity level was assessed in 90% (54/60) of the patients using the UCLA activity scale ranging from 1 to 10. Patients were asked in a questionnaire to assess their activity level prior to the onset of symptoms leading to revision surgery. The average patient activity score was 5 (range: 2 to 9), corresponding to occasional participation in moderate activities, such as unlimited housework or shopping (Table 1).
Small Punch Testing Method
Mechanical properties, including ultimate load (strength), was characterized using the standard small punch test (ASTM F2183), using the same sampling protocol as our previous retrieval studies [19], and briefly summarized again herein. In both worn and unworn locations, small punch specimens were sampled near the surface (0 to 0.5 mm) and below the surface (1.5 to 2 mm) of the retrieved liners. The superior and inferior locations were identified by examining the articulating surface under a stereomicroscope for the presence of contact damage, such as the presence of scratches and the removal of machining marks, as well as by inspecting the back surface of the liners for impressions made by screw holes. For each retrieved liner, up to 8 specimens (depending on material availability) were tested, giving a total of 466 small punch tests performed on the 60 retrievals. Data from 28 tests performed on four control liners were also available from the previous study [19].
Oxidation Index Testing Methods
Thin sections (200 μm) created using a microtome were also prepared in worn and unworn locations and boiled in heptane for 6 hours to remove absorbed lipids [22]. The oxidation index through the thickness of the liners was then analyzed following ASTM 2102. Regions of interest from the sections of the acetabular liners included the rim, bearing, and backside surfaces in both the superior and inferior regions of the component.
Wear Assessment
The thickness of the liners was measured in the loaded and unloaded regions using a calibrated digital micrometer. Femoral head penetration into the liner was calculated by subtracting the thickness of the liner in worn and unworn regions. During the 12 months following implantation, femoral head penetration in Crossfire inserts is a combination of creep and a comparatively lower contribution of wear [2]. After the first year of implantation, the contribution of creep to penetration decreases substantially, and changes in head penetration in Crossfire are considered to be primarily due to polyethylene wear [2-6]. Consequently, an average femoral head penetration rate was calculated only for liners that were implanted for longer than a year, by dividing the measured head penetration by the whole in vivo period, as described previously [23]. There were 42 liners with in vivo durations of greater than one year that were amenable to analysis of head penetration using this method.
Statistical Analysis
Student t-tests, Wilcoxon tests, or Spearman's correlations in the case of non-normal distributions, served to assess differences in mechanical, oxidation and linear penetration rate results between designs (level of significance p<0.05), as well as between retrievals corresponding to a same design but implanted for different periods. In this sense, retrievals were classified into three categories according to their implantation time: less than 1 year, one to five years, and five or more years. Regional differences in the ultimate mechanical properties, maximum oxidation index, and linear penetration rates were evaluated with paired t-tests. In addition, linear models with implantation time and other covariates (patient factors, femoral head material, etc.) were used to examine the potential significance of patient and implant factors on oxidation and penetration. All statistical tests were performed using JMP software (SAS Institute, Cary, NC)
Rim Damage Assessment
Liners were inspected using optical microscopy for evidence of rim damage, subsurface fatigue, and cracking consistent with the descriptions provided by Currier et al. [16]. During inspection, only three liners showed evidence of damage at the rim. To better understand the clinical mechanisms responsible for rim damage in Crossfire liners, we analyzed these three patients as individual case studies, and synthesized clinical data, with observations of rim damage, oxidation, bearing surface penetration, and mechanical properties.
Results
Overall Assessment of Crossfire Mechanical Behavior, Oxidation, and Wear
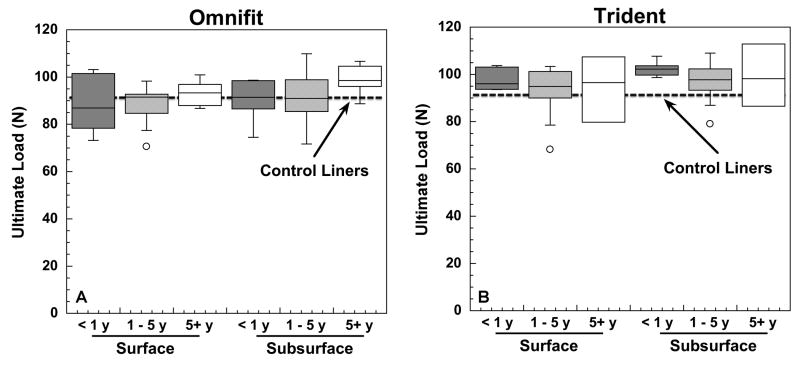
The mechanical behavior of Crossfire at the bearing surface was found to be insensitive to implantation time as well as to liner design for the two designs under consideration (Fig. 1A-B). Average ultimate loads near the bearing surface ranged from 70.6 to 103.2 N (mean ± SD: 89.7 ± 8.2 N), and from 68.2 to 107.4 N (mean ± SD: 94.5 ± 8.8 N), for Omnifit and Trident designs, respectively. Similarly, average subsurface mechanical properties were in the range from 71.7 to 109.8 N (mean ± SD: 93.0 ± 9.6 N), and from 79.0 to 112.9 N (mean ± SD: 98.7 ± 7.2 N), again for Omnifit and Trident liners, respectively. In contrast, the ultimate load of the unimplanted, control liners ranged between 76.6 and 97.9 N (mean ± SD: 90.1±8.8 N) near the surface and between 67.5 and 100.05 N (mean ± SD: 91.1±15.8) at the subsurface region. We found no statistical association between implantation time and the ultimate strength of the polyethylene near the surface or at subsurface locations of the explants (p>0.05). Trident liners showed comparable ultimate strength to Omnifit retrievals.
Figure 1.
A-B. Ultimate load results by sampling region (surface and subsurface) for retrieved Omnifit, (1A), and Trident, (1B), liners. Retrievals were classified into three categories (less than 1 year, one to five years, and five or plus year) according to their corresponding implantation time.
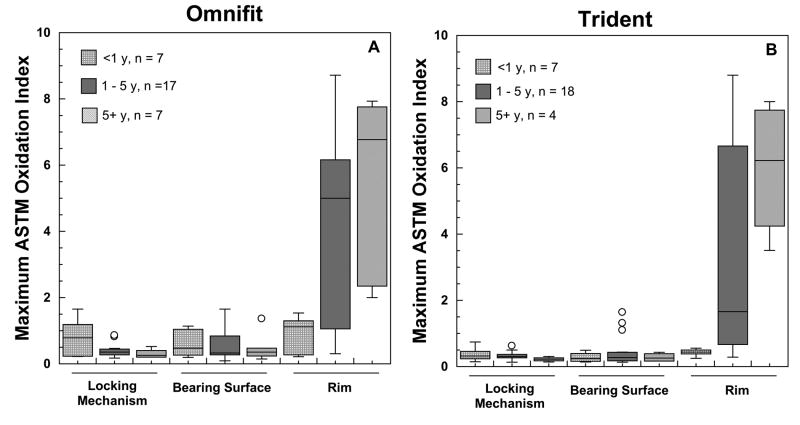
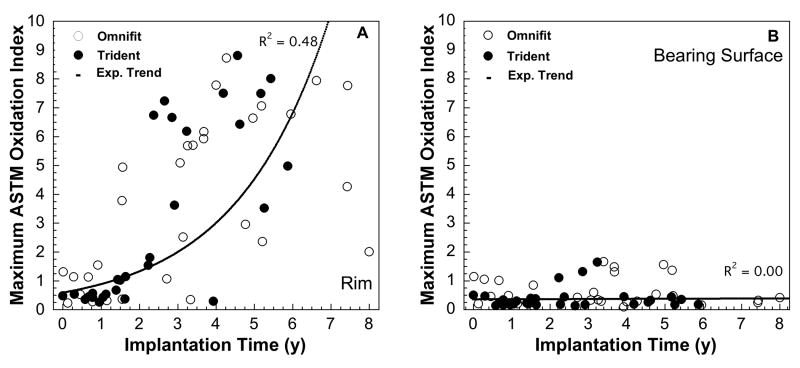
Significant regional variation was observed in the magnitude of oxidation in the retrieved Crossfire liners (Fig. 2A-B), with the highest oxidation index located at the rim face (range: 0.2-8.8; mean±SD: 3.4±2.9). In contrast, the oxidation values at the bearing surface (range: 0.1-1.7; mean±SD: 0.5±0.4), around the locking mechanism (range:0.1-1.7; mean±SD: 0.4±0.3), and at the back surface (range:0.1-1.3; mean±SD: 0.4±0.2) of the liners were substantially lower than at the rim (Fig. 2A-B). Rim oxidation significantly increased with implantation time (Spearman's Correlation ρ =0.71, P <0.0001); a positive trend was not detected at the bearing surface (P = 0.83), backside (ρ =−0.28; P = 0.03) or the locking mechanism (ρ =−0.31; P = 0.02) of the liners. The progression of in vivo oxidation with implantation time at the rim and the articulating surface of Omnifit and Trident retrievals is depicted in Figures 3A-B. The temporal evolution of oxidation in Crossfire retrievals was consistent with an exponential growth trend with time in vivo at the rim (R2 = 0.48; p < 0.0001), but not at the articulating surface (R2 = 0.00; p = 0.85). Retrieved Crossfire liners implanted for less than five years had oxidation indices close to the oxidation levels of the control liners (range: 0.3-1.1; mean±SD: 0.5±0.4) in the locking mechanism (range: 0.1-1.7: mean±SD: 0.4±0.3) and bearing surface regions (range: 0.1-1.7: mean±SD: 0.5±0.5), as well as in the rim region for liners implanted for less than 1 year (range: 0.2-1.5: mean±SD: 0.7±0.4). In general, and despite the shorter duration of Trident liners, the regional variations of oxidation were comparable for both Omnifit and Trident liners (Fig. 2A-B). Polyethylene in the vicinity of the Trident locking mechanism had less oxidation and less variability in oxidation than in the Omnifit liner design for retrieved liners implanted for less than five years (p = 0.02, Fig. 2A-B).
Figure 2.
A-B. Maximum oxidation index measured at three different regions in the retrieved Omnifit, (2A), and Trident, (2B), liners. The average oxidation level of control, never-implanted,Crossfire liners is shown for the sake of comparison.
Figure 3.
A-B. The progression of maximum in vivo oxidation with implantation time at the rim, (3A), and at the articulating surface, (3B) for retrieved Omnifit and Trident liners. The evolution of oxidation in Crossfire retrievals was consistent with an exponential growth trend with time in vivo at the rim (R2 = 0.48; p < 0.0001), but not at the articulating surface (R2 = 0.00; p = 0.85).
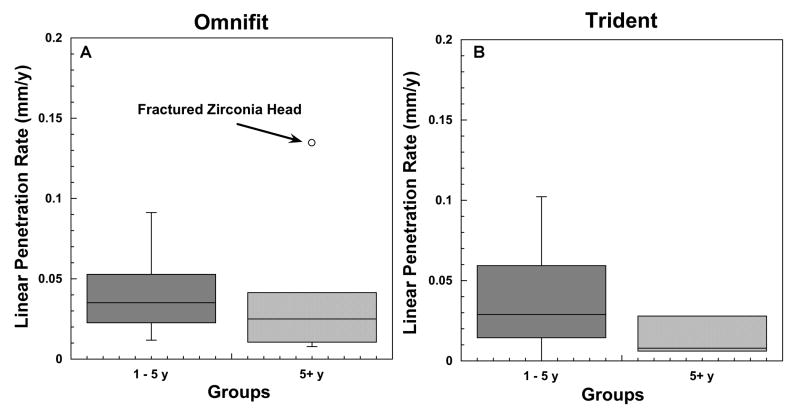
Retrieved Omnifit and Trident liners amenable to penetration measurements (n= 21 and n=21, respectively) exhibited similar, relatively low linear penetration rates during the first eight years of implantation (Fig. 4A-B, Table 1). The linear penetration rates fell in the range of 0.01 to 0.09 mm/y (mean ± SD: 0.04±0.02 mm/y) for Omnifit liners (n=15), and in the range from 0.00 to 0.10 mm/y (mean ± SD: 0.04±0.03 mm/y) for Trident liners (n=17) implanted for five years or less. All retrieved liners implanted for more than five years exhibited lower average linear penetration rates than explants revised before that period (mean ± SD: 0.02±0.01 mm/y for 4 Omnifit liners amenable to measure; 0.01±0.01 mm/y for 4 Trident liners), the only exceptions being two Omnifit liners revised due to fractured zirconia femoral heads (0.13 and 0.04 mm/y). Moreover, there was a negative correlation between linear penetration rate and implantation time (Spearman's Correlation; ρ = −0.35, P = 0.02), confirming the decreasing trend of the penetration rate with time. Using analysis of covariance, general linear models for penetration rate including in vivo time, femoral head size, and femoral head material as covariates also revealed a significant influence of implantation time (P=0.002; Power = 90%) and head material (P=0.03; Power=65%), but not for head size (P=0.2; Power = 23%). Overall, implants with alumina ceramic heads (n=5; 1 liner not amenable to measure due to iatrogenic damage) showed a trend of lower linear penetration rates (0.01±0.01 mm/y) in comparison with acetabular liners bearing against zirconia ceramic (0.04±0.04 mm/y; n=7; including 2 liners revised due to fractured ceramic head) and cobalt chrome alloy heads (0.04±0.03mm/y; n=30). In addition, the average penetration rates were identical for the three head sizes available for analysis (0.04±0.03 mm/y; n=22, 17 and 3 for 28, 32 and 36 mm heads, respectively). Finally, we observed no significant difference in the penetration rates of the two liner designs (p=0.23), and no significant influences of shell size on penetration rate.
Figure 4.
A-B. Linear penetration measured in the retrieved Omnifit, (4A), and Trident, (4B), liners (n=42).
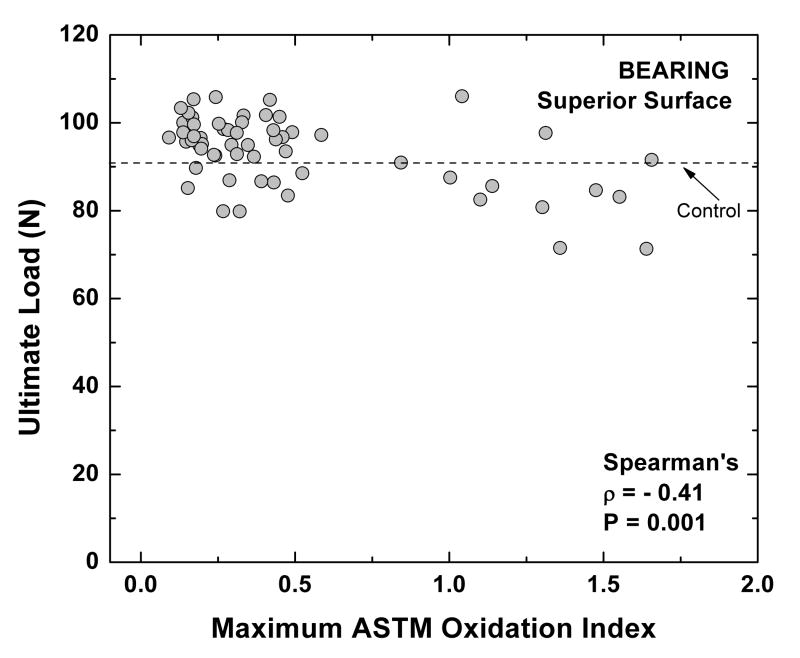
A significant correlation was observed between the maximum oxidation near the superior (worn) surface and the ultimate load of polyethylene near the superior bearing surface (Spearman's Correlation: ρ = −0.41, P = 0.001) in the retrieved Crossfire liners (Fig. 5). In spite of the aforementioned correlation, the penetration rate of the liners was not correlated with the ultimate load of polyethylene near the worn surface (P = 0.64). Likewise, no significant correlation was found between maximum oxidation near the worn surface and penetration rate (P=0.95). The variability of the previous three variables (maximum oxidation index near the worn surface, ultimate load near the superior surface, and linear penetration rate), as well as their average values and standard deviations, can be seen in Table 2.
Figure 5.
Correlation between ultimate load and maximum ASTM oxidation index at the superior surface of 60 retrieved Crossfire liners.
Table 2.
Variability registered for the linear penetration rate, as well as the maximum oxidation index and ultimate load of polyethylene at the superior (worn) surface of the Crossfire liners.
| Parameter | Mean ± SD | Percentile | Maximum | ||||
|---|---|---|---|---|---|---|---|
| 10 % | 25% | 50% | 75% | 90% | |||
| Max OI at the worn surface | 0.5 ± 0.4 | 0.2 | 0.2 | 0.3 | 0.5 | 1.3 | 1.7 |
| Ultimate Load at the worn surface (N) | 93.6 ± 8.0 | 82.3 | 87.4 | 95.4 | 98.8 | 102.4 | 106.0 |
| Linear penetration rate (mm/y)* | 0.04 ± 0.03 | 0.01 | 0.01 | 0.03 | 0.05 | 0.09 | 0.13 |
For liners implanted more than 1 year (n = 42)
Case Studies of Rim Damage
Three of the 60 consecutively retrieved liners (5%) exhibited fatigue-related surface damage at the rim, unrelated to extraction from the shell during revision surgery. The three liners with rim surface damage were revised between 3.1 and 4.6 years in vivo. None of these liners were revised due to wear, osteolysis, rim oxidation or rim damage. The outcomes of these three patients, summarized briefly as case studies, are provided below.
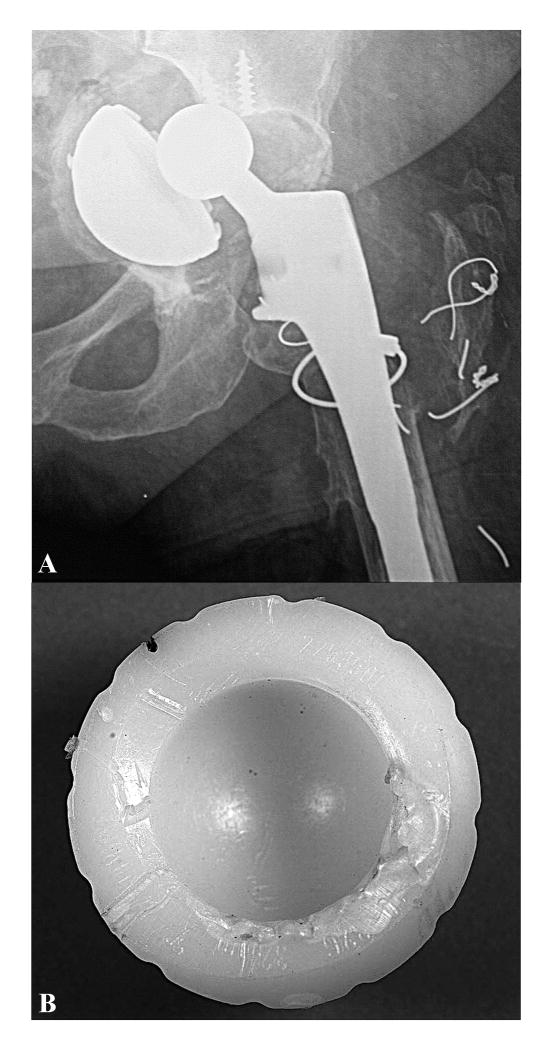
Case Study 1 (Retrieval 343). This 61-year-old female patient was implanted with a 28-mm diameter CoCr alloy femoral head and a Crossfire Omnifit liner in 2001. The patient's body mass index (BMI) was 27 kg/m2, and according to the UCLA activity level questionnaire, she was mostly inactive, corresponding to a maximum UCLA score of 3 at any point after surgery. Her activity score was 2 within 3 months prior to surgery. The shell was initially well-positioned (Fig. 6A), but became dislodged from her acetabulum following a fall and oriented vertically with respect to her pelvis (Fig. 6B). The patient experienced groin and buttocks pain after the fall for 7 months until her revision surgery in 2004. Following 3.1 years of implantation, the liner rim showed localized plastic deformation and whitening, consistent with subsurface “white band” and localized oxidation index values of 5.0 (Fig. 6C). The average penetration rate for this liner was 0.06 mm/y, and the maximum oxidation index at the bearing surface was 0.5. The ultimate strength of the liner near the bearing surface was 82.4 ± 3.6 N. The mechanism of rim damage appeared to be contact with the femoral head when the liner was oriented vertically. Thus, the rim damage in this case was judged to be unrelated to the reason for revision, which was precipitated by traumatic loosening of the shell.
Figure 6.
A-C. Anterior-posterior radiographs for case study 1, a Crossfire liner revised after 3.1 years. 1.5 months postoperative radiograph, (6A), and 3 years postoperative, (6B). After implantation, the liner rim, (6C), showed localized plastic deformation and whitening (oxidation index ~ 5.0 at the rim).
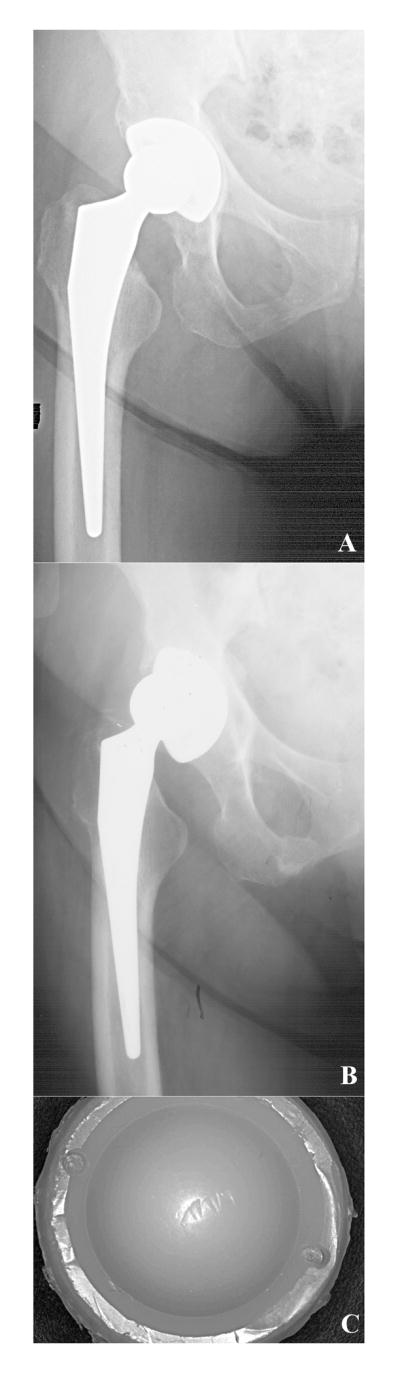
Case study 2 (Retrieval 345). This 78-year-old female patient was implanted with a 28-mm diameter CoCr alloy femoral head and a Crossfire Omnifit liner in 2001. The patient's body mass index (BMI) was 33 kg/m2, and she was mildly active, corresponding to a maximum UCLA score of 4 at any point after surgery. Her activity score was 2 within 3 months of her revision surgery. The shell was initially well-positioned (Fig. 7A-B), but the patient suffered from chronic instability and dislocated between 5 and 10 times before her revision surgery in 2004. After 3.3 years in vivo, the liner rim showed a 90° arc of delamination (Fig. 7C). The average penetration rate for this liner was 0.05 mm/y, and the maximum oxidation index at the bearing surface was 0.5. The surface ultimate load of the Crossfire bearing was 87.3 ± 18.1 N. Undamaged regions of the rim did not show evidence of delamination or white banding, and their associated maximum oxidation index was 0.3. The rim damage was likely produced during the chronic dislocations of the femoral head over the rim. Therefore, the rim damage in this case was generated secondary to the joint instability, which necessitated revision.
Figure 7.
A-C. Anterior-posterior radiographs for case study 2, a Crossfire liner revised after 3.3 years. 2.5 months postoperative radiograph, (7A), and 1.6 years postoperative, (7B). After implantation, the liner rim, (7C), showed a 90° arc of delamination, probably due to chronic dislocations of the femoral head over the rim.
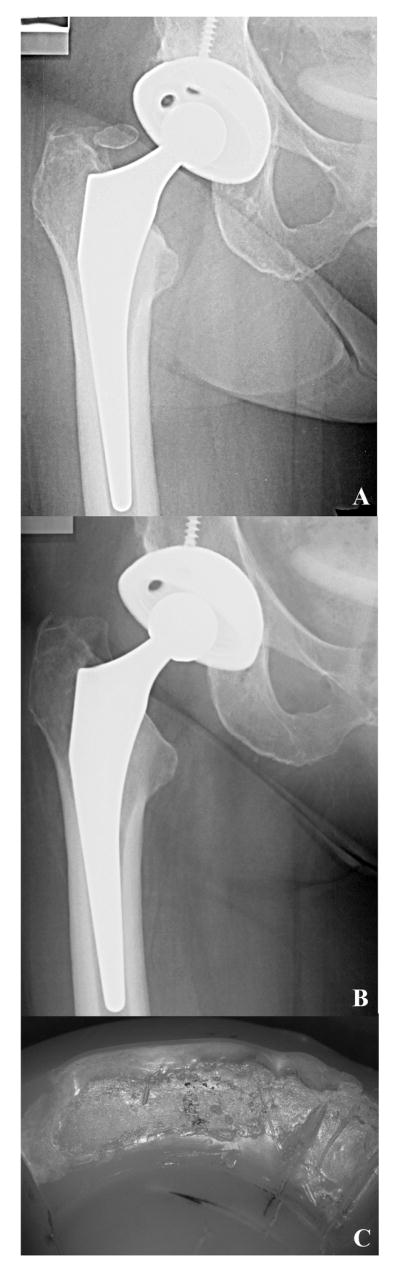
Case study 3 (Retrieval 578). This 60-year-old female patient was implanted with a 32-mm diameter CoCr alloy femoral head and a Crossfire Trident liner during revision surgery of primary left total hip arthroplasty in 2001. She weighed 225 lb, corresponding to a BMI of 34 kg/m2 (clinically obese), and she was mostly inactive (maximum UCLA score of 3). After 4.6 years in situ, the second implant had to be re-revised due to fracture of the medial wall of the pelvis (Fig. 8A). Analysis of the radiographs did demonstrate complete protrusio of the acetabular component. The average penetration rate for this liner was 0.02 mm/y, and the maximum oxidation index at the bearing surface was 0.3. The surface ultimate load of the Crossfire bearing was 90.0 ± 4.1 N. The liner rim showed a nearly 180° arc of surface damage and delamination, where the local peak oxidation was 6.4 (Fig. 8B). Neither delamination nor white banding was found in non-impinged areas of the rim. Abnormal loading of the femoral head likely caused the rim damage after fracture of the pelvis and dislocation of the implant.
Figure 8.
A-B. Anterior-posterior radiograph, (8A), for case study 3, a Crossfire liner revised after 4.6 years in situ. This Crossfire liner had to be re-revised due to fracture of the medial wall of the pelvis. After implantation, the liner rim, (8B), showed a nearly 180° arc of delamination, probably due to dislocation of the femoral head secondary to fracture of the pelvis.
Discussion
The retrieval results were consistent with our previous short-term retrieval findings [19, 20] and demonstrated low femoral head penetration and preservation of mechanical properties at the bearing surface of annealed highly crosslinked acetabular liners up to 8 and 6 years in vivo for the Omnifit and Trident designs, respectively. However, we also measured elevated rim oxidation, and these findings were likewise independent of design. In vivo oxidation occurs predominantly at the rim of acetabular liners because of its greater exposure to molecular oxygen in the in vivo environment [20]. Our retrieval data continue to support the hypothesis that the femoral head and metal shell reduce the in vivo oxidation of the annealed polyethylene liner at the articulating and backside surfaces. The in vivo oxidation mechanism is most likely fueled by molecular oxygen dissolved in the body fluids and tissues, as opposed to reactive oxygen species, such as superoxide radicals that have a short half life and would be unlikely to diffuse below the polyethylene surface.
Rim surface damage was observed in 3/60 (5%) consecutively revised annealed liners since 2000. Detailed analysis of the clinical circumstances associated with these revisions did not reveal an association between the rim damage and the reasons for revision, which were due to traumatic mechanical loosening of the acetabular shell; chronic instability and recurrent dislocations; and pelvic fracture, respectively. In all three cases, the clinical circumstances for these patients would have necessitated revision, regardless of the type of polyethylene liner.
Our research, as well as the results from a previous study [16], has shown that surface damage can occur at the rim as a consequence of elevated in vivo oxidation and relatively unusual clinical circumstances. However, the incidence of rim damage in the present study (3/60, 5%) was also lower than that reported previously by Currier et al. (6/12, 50%) [16]. Currier et al. also reported that 8/12 (67%) of their retrievals were removed due to dislocation or instability, whereas in our study, 16/60 (27%) were revised for similar reasons. The differences between both studies in the incidences of instability as well as rim damage are attributed to study design and potential sampling bias inherent in Currier's retrieval collection methodology. The retrieval method employed by Currier did not reflect a consecutive series obtained from a single institution, but rather a collection of individual cases with unknown selection criteria.
The penetration rate for the retrieved Trident and Omnifit liners was 0.04 mm/y, and was within the range of findings from short- and intermediate-term implantation for Crossfire [2-5]. At two years of follow-up, the average in vivo penetration rate, determined from radiographs, has been reported to range between 0.01 and 0.12 mm/y [2, 4, 5]. A five-year study by D'Antonio et al [3] found the average linear penetration rate of Crossfire in 56 patients to be 0.06±0.02 mm/y. A recent RSA study of 10 patients by Röhrl and coworkers [6] found an average wear rate of less than 0.006 mm per year in Crossfire cemented all-polyethylene cups after 6 years. In each of these radiographic studies, the penetration rate of Crossfire was found to be less than the conventional polyethylene control [2-6]. Thus, the head penetration determined from our retrievals, based on direct measurements with 42 liners with over 1 year of implantation time, was found to be generally consistent with clinical studies documenting relatively lower penetration rates for Crossfire as compared to conventional polyethylene.
Femoral head penetration data, for both retrieval analysis and radiographic wear assessments, are generally limited by the combined contribution of creep and wear. Despite this limitation, femoral head penetration after an early “bedding in” phase is widely used to characterize wear in clinical studies of conventional and highly crosslinked polyethylene acetabular liners [2-6]. Published radiographic wear data for Crossfire [2-6] indicates that the initial bedding in phase occurs within the first year of implantation. Thus, by restricting our attention in this study to penetration rates after one year of implantation, we have limited the contribution of creep to our measurements. It must be emphasized that the bedding in phase for head penetration may be material dependent, and one year may not be sufficient for creep to subside in other highly crosslinked polyethylene formulations. To facilitate the future comparison of our data with other studies with different selection criteria, we have reported the penetration values for individual retrievals in Table 1.
With regard to the influence of different femoral head materials on the penetration rate of Crossfire liners, we detected lower penetration rates for implants with alumina ceramic compared to those with zirconia ceramic and cobalt chrome heads. However, based upon post-hoc analysis of our data, we did not have sufficient power with only 5 alumina ceramic femoral heads to confirm significantly lower penetration rates with respect to zirconia ceramic and CoCr alloy heads, if it exists (general linear models: 65% power). There appears to be a lack of consensus on the theoretical advantages of ceramic over cobalt chrome heads in the literature [1]. The highest penetration rate, and an outlier in our study (0.13 mm/y, Fig. 4A), was observed in the case of a fractured zirconia head after 8 years in vivo. In this case, the surface damage was also generated by articulation of the exposed trunnion, as well as third-body fragments of the fractured ceramic head, with the Crossfire liner prior to revision. Additional retrievals will need to be analyzed to more definitively explore the hypothesis that ceramic femoral heads decrease the wear of hip arthroplasties incorporating Crossfire.
We observed greater variation in the oxidation near the locking mechanisms of Omnifit liners when compared with Trident liners. This may reflect a combination of differences in liner-shell conformity of the two designs, as well as differences in the proximity of the locking mechanisms to the equator of the shell, where the access to oxygen containing fluids would be greater for the Omnifit as compared with the Trident design. As there have been no instances of Omnifit liner disassociation to date at our multicenter retrieval program, the clinical significance of these findings near the locking mechanism remains unclear at the present time.
The results of this study are limited to a single, high volume surgical center and may not necessarily be generalized to the overall surgeon or patient population. Between 1999 and 2006, over 5,900 Crossfire liners have been implanted by the high volume surgical center participating in the present study. Our collaborative and ongoing retrieval program has enabled consecutive surveillance of Crossfire explants over a continuous 9-year time period, and thus provides crucial insight into the frequency and prevalence of failure modes for annealed polyethylene liners in two contemporary acetabular shell designs. The three most common reasons for revision: loosening, instability, and infection, did not differ between acetabular component designs and were the same as those reported for the general hip arthroplasty population based on the Nationwide Inpatient Sample for the United States during 2005 [24]. Furthermore, the observations of elevated rim oxidation with Crossfire have not been associated with clinically significant sequellae to date.
Acknowledgments
Supported by NIH Grant R01 AR47904 and by a research grant from Stryker Orthopedics.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kurtz SM. The UHMWPE Handbook: Ultra-High Molecular Weight Polyethylene in Total Joint Replacement. New York: Academic Press; 2004. [Google Scholar]
- 2.Martell JM, Verner JJ, Incavo SJ. Clinical performance of a highly cross-linked polyethylene at two years in total hip arthroplasty: a randomized prospective trial. J Arthroplasty. 2003;18(7 Suppl 1):55–59. doi: 10.1016/s0883-5403(03)00341-3. [DOI] [PubMed] [Google Scholar]
- 3.D'Antonio JA, Manley MT, Capello WN, Bierbaum BE, Ramakrishnan R, Naughton M, Sutton K. Five-year experience with Crossfire highly cross-linked polyethylene. Clin Orthop Relat Res. 2005;441:143–150. doi: 10.1097/00003086-200512000-00024. [DOI] [PubMed] [Google Scholar]
- 4.Krushell RJ, Fingeroth RH, Cushing MC. Early femoral head penetration of a highly crosslinked polyethylene liner vs a conventional polyethlene liner. J Arthroplasty. 2005;20(7 Suppl):73–76. doi: 10.1016/j.arth.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 5.Rohrl S, Nivbrant B, Mingguo L, Hewitt B. In vivo wear and migration of highly cross-linked polyethylene cups a radiostereometry analysis study. J Arthroplasty. 2005;20(4):409–413. doi: 10.1016/j.arth.2004.09.040. [DOI] [PubMed] [Google Scholar]
- 6.Rohrl SM, Li MG, Nilsson KG, Nivbrant B. Very low wear of non-remelted highly cross-linked polyethylene cups: an RSA study lasting up to 6 years. Acta orthopaedica. 2007;78(6):739–745. doi: 10.1080/17453670710014509. [DOI] [PubMed] [Google Scholar]
- 7.Dorr LD, Wan Z, Shahrdar C, Sirianni L, Boutary M, Yun A. Clinical performance of a Durasul highly cross-linked polyethylene acetabular liner for total hip arthroplasty at five years. J Bone Joint Surg Am. 2005;87(8):1816–1821. doi: 10.2106/JBJS.D.01915. [DOI] [PubMed] [Google Scholar]
- 8.Manning DW, Chiang PP, Martell JM, Galante JO, Harris WH. In vivo comparative wear study of traditional and highly cross-linked polyethylene in total hip arthroplasty. J Arthroplasty. 2005;20(7):880–886. doi: 10.1016/j.arth.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 9.Bragdon CR, Barrett S, Martell JM, Greene ME, Malchau H, Harris WH. Steady-state penetration rates of electron beam-irradiated, highly cross-linked polyethylene at an average 45-month follow-up. J Arthroplasty. 2006;21(7):935–943. doi: 10.1016/j.arth.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 10.Geller JA, Malchau H, Bragdon C, Greene M, Harris WH, Freiberg AA. Large diameter femoral heads on highly cross-linked polyethylene: minimum 3-year results. Clin Orthop Relat Res. 2006;447:53–59. doi: 10.1097/01.blo.0000218742.61624.80. [DOI] [PubMed] [Google Scholar]
- 11.Triclot P, Grosjean G, El Masri F, Courpied JP, Hamadouche M. A comparison of the penetration rate of two polyethylene acetabular liners of different levels of cross-linking: A PROSPECTIVE RANDOMISED TRIAL. J Bone Joint Surg Br. 2007;89(11):1439–1445. doi: 10.1302/0301-620X.89B11.19543. [DOI] [PubMed] [Google Scholar]
- 12.Furmanski J, Gupta S, Chawan A, Kohm A, Lannutti J, Jewett B, Pruitt LA, Ries MD. Aspherical femoral head with highly cross-linked ultra-high molecular weight polyethylene surface cracking. A case report. J Bone Joint Surg Am. 2007;89(10):2266–2270. doi: 10.2106/JBJS.F.00428. [DOI] [PubMed] [Google Scholar]
- 13.Halley D, Glassman A, Crowninshield RD. Recurrent dislocation after revision total hip replacement with a large prosthetic femoral head. A case report. J Bone Joint Surg Am. 2004;86-A(4):4827–830. doi: 10.2106/00004623-200404000-00025. [DOI] [PubMed] [Google Scholar]
- 14.Bradford L, Kurland R, Sankaran M, Kim H, Pruitt LA, Ries MD. Early failure due to osteolysis associated with contemporary highly cross-linked ultra-high molecular weight polyethylene. A case report. J Bone Joint Surg Am. 2004;86-A(5):1051–1056. doi: 10.2106/00004623-200405000-00026. [DOI] [PubMed] [Google Scholar]
- 15.Bradford L, Baker DA, Graham J, Chawan A, Ries MD, Pruitt LA. Wear and surface cracking in early retrieved highly cross-linked polyethylene acetabular liners. J Bone Joint Surg Am. 2004;86-A(6):1271–1282. doi: 10.2106/00004623-200406000-00021. [DOI] [PubMed] [Google Scholar]
- 16.Currier BH, Currier JH, Mayor MB, Lyford KA, Collier JP, Van Citters DW. Evaluation of oxidation and fatigue damage of retrieved crossfire polyethylene acetabular cups. J Bone Joint Surg Am. 2007;89(9):2023–2029. doi: 10.2106/JBJS.F.00336. [DOI] [PubMed] [Google Scholar]
- 17.Wannomae KK, Bhattacharyya S, Freiberg A, Estok D, Harris WH, Muratoglu O. In vivo oxidation of retrieved cross-linked ultra-high-molecular-weight polyethylene acetabular components with residual free radicals. J Arthroplasty. 2006;21(7):1005–1011. doi: 10.1016/j.arth.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 18.Muratoglu OK, Greenbaum ES, Bragdon CR, Jasty M, Freiberg AA, Harris WH. Surface analysis of early retrieved acetabular polyethylene liners: a comparison of conventional and highly crosslinked polyethylenes. J Arthroplasty. 2004;19(1):68–77. doi: 10.1016/j.arth.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Kurtz SM, Hozack W, Turner J, Purtill J, MacDonald D, Sharkey P, Parvizi J, Manley M, Rothman R. Mechanical properties of retrieved highly cross-linked Crossfire liners after short-term implantation. J Arthroplasty. 2005;20(7):840–849. doi: 10.1016/j.arth.2005.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurtz SM, Hozack WJ, Purtill JJ, Marcolongo M, Kraay MJ, Goldberg VM, Sharkey PF, Parvizi J, Rimnac CM, Edidin AA. Significance of in vivo degradation for polyethylene in total hip arthroplasty. Clin Orthop Relat Res. 2006;453:47–57. doi: 10.1097/01.blo.0000246547.18187.0b. [DOI] [PubMed] [Google Scholar]
- 21.Kurtz SM, Manley M, Wang A, Taylor S, Dumbleton J. Comparison of the properties of annealed crosslinked (Crossfire) and conventional polyethylene as hip bearing materials. Bull Hosp Jt Dis. 2002-2003;61(1-2):17–26. [PubMed] [Google Scholar]
- 22.Sun DC, Halleck A, Schmidig G, Wang A, Stark C, Dumbleton JH. Fourier transform infrared (FTIR) oxidation analysis of UHMWPE implants: Possible contamination from synovial fluid and serum. In: Gsell RA, Stein HL, Ploskonka JJ, editors. Characterization and properties of ultra-high molecular weight polyethylene. West Conshohoken: American Society for Testing and Materials; 1998. pp. 39–45. [Google Scholar]
- 23.Gomez-Barrena E, Li S, Furman BS, Masri BA, Wright TM, Salvati EA. Role of polyethylene oxidation and consolidation defects in cup performance. Clin Orthop. 1998;352(352):105–117. [PubMed] [Google Scholar]
- 24.Bozic K, Kurtz SM, Lau E, Ong K, Vail T, Berry D. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91(1):128–33. doi: 10.2106/JBJS.H.00155. [DOI] [PubMed] [Google Scholar]