Abstract
Cervical cancer is the second most common female tumor worldwide and its incidence is disproportionately high (>80%) in the developing world. In the U.S., where Pap tests have reduced the annual incidence to approximately 11,000 cervical cancers, more than 60% of cases occur in medically-underserved populations as part of a complex of diseases linked to poverty, race/ethnicity, and/or health disparities. Because carcinogenic human papillomavirus (HPV) infections cause virtually all cervical cancer, two new approaches for cervical cancer prevention have emerged: 1) HPV vaccination to prevent infections in younger women (≤18 years old) and 2) carcinogenic HPV detection in older women (≥30 years old). Together, HPV vaccination and testing, if used in an age-appropriate manner, have the potential to transform cervical cancer prevention particularly among underserved populations. Yet significant barriers of access, acceptability, and adoption to any cervical cancer prevention strategy remain. Without understanding and addressing these obstacles, these promising new tools for cervical cancer prevention may be futile. We share our experiences in the delivery of cervical cancer prevention strategies to U.S. populations experiencing high cervical cancer burden: African-American women in South Carolina, Alabama, Mississippi; Haitian immigrant women in Miami; Hispanic women in the U.S.-Mexico Border; Sioux/Native American women in the Northern Plains; white women in the Appalachia; and Vietnamese-American women in Pennsylvania and New Jersey. Our goal is to inform future research and outreach efforts to reduce the burden of cervical cancer in underserved populations.
I. State of the Union: Cervical Cancer in the U.S. in 2009
In 2008, cervical cancer was the 13th most common cancer in women living in the U.S. (1;2). Annual rates have declined by 75% or more over the past half century due to the introduction regular cervical cancer screening using cervical cytology (Pap smears). The decline of cervical cancer rates where successful cytology programs have been implemented must be considered one of the greatest successes in cancer prevention to date.
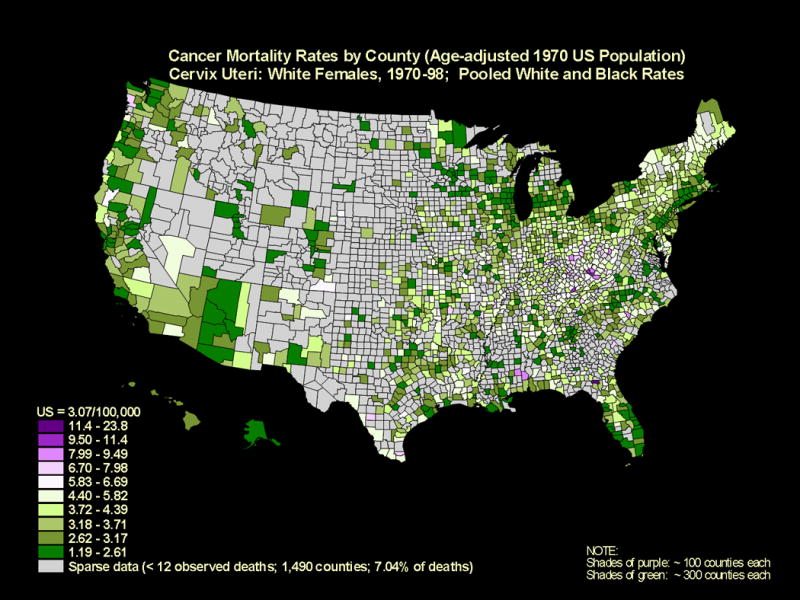
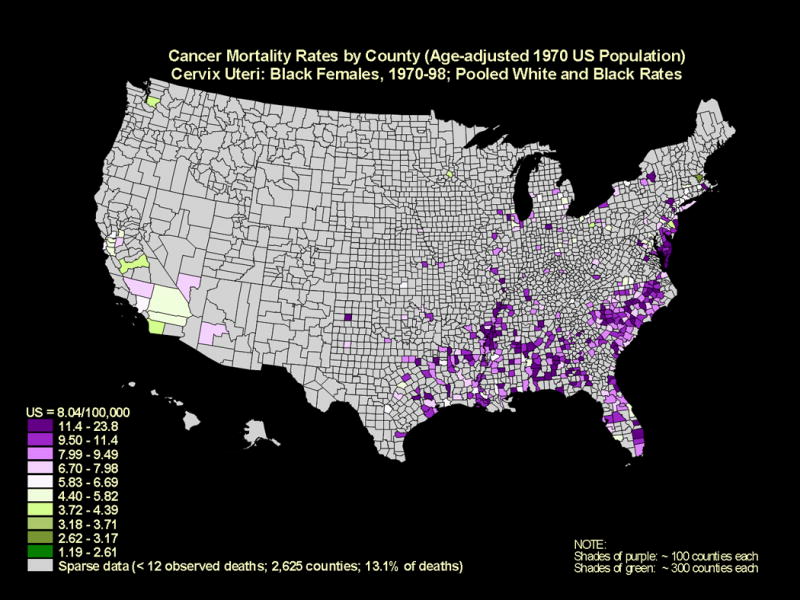
Yet, there is an unequal burden of cervical cancer. Globally, more than 80% of all 500,000 annual cases of cervical cancer occur in developing countries (3). In the U.S., with an annual incidence of approximately 11,000 cervical cancers (2), more than 60% of cases occur in small pockets of underserved, under-screened populations of women (4). Annual rates in these populations are 1.5-4 times higher than the national age-standardized rate of 8.4 per 100,000† and approach the rates of cervical cancer observed in much lower resource settings. Geographical cervical cancer mortality maps stratified by race, which tracks with disparities in incidence, are shown in Figures 1 and 2.
Figure 1.
Cancer mortality rates are shown by county (age-adjusted 1970 US population) for the cervix uteri in white females, 1970 through 1998. (Source: National Cancer Institute, 2001)
Figure 2.
Cancer mortality rates are shown by county (age-adjusted 1970 US population) for the cervix uteri in black females, 1970 through 1998. (Source: National Cancer Institute, 2001)
II. Human Papillomavirus (HPV) and the Etiology of Cervical Cancer
Cervical infections by ∼15 cancer-associated (carcinogenic or high-risk) HPV genotypes cause virtually all cervical cancer and its immediate precursors worldwide (3). A new paradigm of cervical carcinogenesis replaces an older pathology model of stepwise progression from low-grade to high-grade morphological changes and can now be summarized as four reliable measured stages: 1) HPV acquisition, 2) HPV persistence (vs. clearance), 3) progression of a persisting infection to cervical precancer, and 4) invasion (6).
Globally, HPV is the most common sexually transmitted infection. Most HPV infections, including carcinogenic HPV genotypes, are typically transient and resolve within 6-12 months, sometimes causing mild morphologic changes. In general, it can be said that carcinogenic HPV is the necessary but infrequent cause of cervical cancer. That cervical cancer is the 2nd most common cancer worldwide is to great extent a result of the nearly ubiquitous exposure to HPV after sexual debut. Women with persistent carcinogenic HPV infections are at risk of developing precancer (6), although not all persistent infections progress to precancer. If precancer is not detected and treated in a timely fashion, a significant proportion can invade (7).
III. New Prevention Tools Targeting HPV: Vaccination and Screening
Based on the nearly absolute etiologic link between carcinogenic HPV and cervical cancer, two new approaches for the cervical cancer prevention have emerged: 1) Primary prevention via HPV vaccination to prevent HPV infection; and 2) Secondary prevention via carcinogenic HPV detection for identifying and treating women with cervical precancerous lesions and early-stage cancers. Both technologies are highly efficacious when used in their respective target populations. HPV vaccines have shown better than 90% efficacy for preventing persistent HPV infections and precancerous lesions from the targeted types for up to 5 years in HPV-naïve women (8-10), but neither treat pre-existing HPV infections (8;9;11). Carcinogenic HPV DNA testing is more clinically sensitive than cytology for the detection of precancerous lesions and cancer in routine screening (12-22), including a demonstration that one-time HPV-based screening is superior to Pap smears and visual inspection with acetic acid for reducing cervical cancer mortality (23;24).
IV. Using HPV National History to Guide Prevention Strategies
An understanding of the natural history of HPV can then guide the use of these new tools to maximize the benefit and therefore cost-effectiveness of cervical cancer prevention. The peak prevalence of HPV in women is within 5-7 years of sexual debut; in the U.S. the median age is 17 years old (25). In contrast, the peak of screen-detected precancerous lesions is in women in their late 20's and early 30's (26), approximately 10-15 years after sexual debut, at ages when the prevalence of HPV has declined significantly. Thus, the implementation of new prevention technologies must be age-appropriate to maximize the health benefits. For cervical cancer prevention, the maximum benefits of HPV vaccination and HPV-based screening will be derived from women prior to and more than 10 years after sexual debut, respectively.
V. U.S. Populations with Excessive Burden of Cervical Cancer: A Bell Weather of Health Disparities
Cervical cancer occurs mainly in low-resource, underserved regions as part of a complex of diseases linked to poverty, race/ethnicity, and/or other health disparities (27). However, it is important to recognize that the only two significant determinants of cervical cancer incidence are persistent carcinogenic HPV infection and lack of access to screening. There is no evidence to date to suggest that any population defined by race or ethnicity is more susceptible than another to cervical cancer, once all confounding factors such as behaviors, access to screening, and timely follow-up of screen positives are taken into account.
It has been shown that women at high risk of cervical cancer either do not have adequate access to preventive services or choose not to utilize these services for a number of reasons ranging from measurable structural barriers (e.g., transportation) to subjective intrapersonal barriers (e.g., fear of results, mistrust of the health care system). Community-based interventions that account for cultural beliefs, attitudes and behaviors aiming at increasing knowledge about HPV and its link to cervical cancer, cervical cancer screening, sexual risk reduction, and HPV vaccination uptake may represent a promising approach to minimize current disparities that exist in high-risk populations. The following sections describe the cervical cancer burden and approaches used to address cervical cancer primary and/or secondary prevention among specific high-risk populations.
A. South Carolina: A Faith-Based Approach to Screening and HPV Vaccination (Brandt)
Cervical cancer incidence and mortality rates for South Carolina, Alabama, and Mississippi exceed the national rates, with African-American women accounting for much of this difference. Incidence rates for invasive cervical cancer were 9.3 for African Americans versus 6.9 for whites in South, Carolina, 11.1 and 8.0 in Alabama, and 9.4 and 6.4 respectively in Mississippi. Mortality rates were 5.5 versus 1.8 respectively in South Caroline, 6.8 versus 2.4 in Alabama, and 6.0 and 2.4 in Mississippi (28).
Cancer prevention and control research and programs have been successfully implemented in faith-based settings, particularly in African American churches. In general, there is an attitude among African American church members in which they embrace health and well being with the potential to intervene at multiple levels of change. Among African American adults, approximately 67% attend church at least monthly; therefore, the church emerges as an important setting for public health programming (29).
In South Carolina, statewide faith- and community-based approaches have been undertaken to address excessive cervical cancer incidence and mortality with a focus on prevention and control among African-American women (27;30). Prompted by advertisements for Gardasil®, the State Baptist Young Woman's Auxiliary (YWA) of the Woman's Baptist Education and Missionary Convention Health Ministry approached researchers at the University of South Carolina about a partnership to address cervical cancer among African-American women. Formative research was conducted, by partnering with five Baptist Education and Missionary Convention churches in a rural region of South Carolina to conduct 20 in-depth interviews and 10 focus groups with 116 African-Americans (mean age=38.8; 92% female), to explore the acceptance of and opportunities for promotion of the vaccine in faith-based settings using a community-based participatory approach. Preliminary results indicate that less than half (41%) correctly identified HPV as a main cause of cervical cancer, but 75% of participants had heard of HPV. Most participants (77%) favored a school requirement for HPV vaccination. Most participants felt that the church was an appropriate setting for HPV and HPV vaccine education, and the involvement of youth in such efforts was emphasized.
While most of the faith- and community-based partners were supportive of HPV vaccination, a few vocal opponents swayed opinions in faith-based settings and in public forums. Some who delivered educational programs or worked directly with the partnering churches were asked explicitly if they were paid by Merck (the maker of Gardasil®) to “push the vaccine.” To avoid “controversy” associated with Gardasil®, a balanced message of preventing cervical cancer through regular Pap tests, adherence to recommended follow-up care of abnormal results, and making informed decisions about Gardasil® was adopted. Information provided about Gardasil® was non-industry sponsored and none of the related activities have involved industry support. These decisions proved fruitful as even the most opposed to the vaccine joined forces to prevent cervical cancer using the agreed upon messages.
B. Alabama and Mississippi: Screening and Self-Collected Sampling for HPV Testing among African-American women (Scarinci, Partridge, Castle)
The Deep South Network for Cancer Control (DSN) was established to develop sustainable community infrastructure to promote cancer awareness among African-Americans residing in the Alabama Black Belt and the Mississippi Delta (31). The development and implementation of the program was based on principles of Community-Based Participatory Research (CBPR) and the Empowerment Model (32;33), in which volunteer community health workers (CHWs), investigators, and partners jointly developed the action plan to provide public education and promote screening for breast and cervical cancer. We have trained and retained over 500 CHWs who are “natural helpers” in their respective communities to provide cancer awareness messages and resources to their communities. We focused specifically on breast and cervical cancer screening because it has been shown that screening decreases mortality and, most importantly, we could provide screening and treatment through the National Breast and Cervical Cancer Early Detection Program (NBCCEDP) to uninsured women who were within 200% of poverty levels or worse. There has been a continuous increase in innovative cancer awareness activities within the communities with examples ranging from hat contests to cancer awareness walks, town hall meetings, fashion shows, local medial messages, and one-on-one messages (34).
The most compelling data regarding the impact of the program on breast and cervical cancer disparities is based on data obtained through the Alabama NBCCEDP in our targeted counties in Alabama among women between the ages of 50 and 64. In 2004-2005, only 2% of eligible white women and 6% of eligible African-American women obtained a mammogram through this program. In 2007-2008, this percentage was 8% and 29% respectively. That is, an increase of 6% among whites and 23.6% increase among African Americans. With regard to Pap smear in the Alabama targeted counties, 0.8% of eligible white women between the ages of 50 and 64 obtained a Pap smear in 2004-2005 as compared to 3.6% in 2007-2008. For African Americans, Pap utilization increased from 2% in 2004-2005 to 9.2% in 2007-2008.
Although the data above show an increase in breast and cervical cancer screening among African-American women in the targeted counties, there is still a hard-to-reach group, which, despite our efforts, has not engaged in these behaviors. As such, alternative approaches to cytology screening must be considered since a large percentage of eligible women were not going to the clinics to obtain their Pap smear. Through the community-based participatory process in our targeted communities, DSN and the U.S. National Cancer Institute have partnered to explore the acceptability of self-collected sampling for HPV testing among African-American women in three of our targeted counties in the Mississippi Delta. Preliminary data among African-American women who reported not having a Pap smear in the past three years indicate that almost 60% agreed to participate, and among those more than 90% mailed their self-collected material for analysis.
Throughout this experience we have learned two major lessons: 1) “Built it and they will come” may not make for a successful strategy because women may not come. Unscreened women have reported that they are embarrassed, and they do not want others in their communities to know that they are not taking care of their health. In order to recruit participants for the self-collected sampling HPV testing, we have used door-to-door canvassing. However, in order for this approach to be successful it was necessary to build trust and credibility in the community by promoting screening so when women were approached individually they were more receptive to the message while keeping their privacy; 2) Credibility and trust take time and effort.
C. Haitian Immigrants Living in Miami, FL (Little Haiti): Self-Collected Sampling for HPV Testing and Understanding of Feminine Hygiene Practices (Kobetz)
In Miami, Florida, cervical cancer incidence is highest among Haitian women, particularly those who are recent immigrants, and reside in Little Haiti, a large ethnic enclave located just northeast of the City center. Between 2004-2007, the estimated incidence of disease for Little Haiti was 38/100,000 (35).
To address this disparity, community leaders from Little Haiti and academic investigators from University of Miami created a campus-community collaborative known as Patne en Akyson (Partners in Action). This Partnership aims to improve cancer outcomes among Haitian women through CBPR (36-38). Currently, the partnership oversees five complementary research initiatives that address the excess cervical cancer burden in Little Haiti. The success of such research can be primarily attributed to our reliance on CHWs to recruit participants and collect study data (37). The CHWs are women of Haitian descent, who speak English and Haitian Kreyol fluently, and are employed by a community-based organization located in Little Haiti. The affiliation between the CHWs and this organization is critical to the success of Partnership research initiatives. By providing our studies with a “community home,” we are able to dissuade widespread distrust of research in Little Haiti associated with the misidentification of Haitian ancestry as a risk factor for HIV/AIDS (39;40).
The advisory board, which is comprised primarily of community members, drives the Partnership's research agenda. To date, we have:
Documented the prevalence of lifetime and routine Pap test use in Little Haiti. Among nearly 1,000 survey respondents, one third had never had a Pap test, and less than half of women with prior screening experience reported having a Pap test in the past three years (36);
Identified primary barriers to screening, including language difficulties, limited access to care, and socio-cultural concerns about modesty;
Examined the acceptability of self-sampling as an alternative to Pap test, and found that nearly 90% of participants with a history of having had a Pap test (n=189) prefer self-sampling;
Conducted formative research to understand the potential influence of a culturally-bounded, feminine hygiene practice on HPV susceptibility.
As is often true for CBPR initiatives, this agenda is born out of lessons learned and significant compromise (38;41). The academic investigators, in particular, must continually relinquish their assumptions about how data should be collected, by whom, and in what context (37). All partners must invest considerable time to establish and maintain trust, as well as, foster mutual respect for each other, despite occasionally divergent ideas about the research process. Doing so is essential for effectively engaging residents in research, and for collecting quality data that may advance community health and social change in Little Haiti and other similarly disenfranchised communities.
D. U.S.-Mexico Border Region: HPV Vaccination, Screening, and Follow-Up among Hispanic Women (Garcia)
Cervical cancer incidence and mortality rates for the United States-Mexico border region exceed those of the rest of the nation, with Hispanic women accounting largely for this differential (42-44). Age-adjusted cervical cancer rates for Hispanic women living in border counties are twice those of their non-Hispanic peers in the same communities (13.9/100,000 vs. 7.0/100,000), and significantly higher than for other Hispanics in the U.S. (13.2/100,000)(45). Hispanic border residents are more likely to present with squamous disease (11.0 compared to 9.7 for Hispanics in non-border states) and late stage disease with earlier age at presentation.
Factors associated with non-adherence to cervical cancer screening in this population include low income, lack of health insurance, limited access to health care services, lack of clinician recommendation, length of residency in the U.S., limited English language proficiency, acculturation and lack of awareness (46-52). Most of these are not easily addressed through standard public health strategies. Interventions that promote patient awareness of cancer screening and cervical cancer, those that facilitate access to health care services, and clinician recommendations may have a positive effect in Hispanic border populations. For example, women for whom both breast and cervical cancer screenings were recommended were more likely (odds ratio [OR] = 7.7) to get a mammogram than women told only to get a mammogram (OR = 2.4) or when no recommendation was made (OR = 1.0, reference). They were also more likely to get a Pap smear within the last 5 years (OR = 14) than women told only to get a Pap smear (OR = 2.3) or when no recommendation was made (OR = 1.0, reference).
In particular, awareness promoting cancer education interventions especially when delivered by CHWs (promotoras) may be particularly useful among isolated, low educated, acculturated women of Mexican origin in this area (53;54). The addition of molecular based HPV screening technologies to the screening paradigm whether clinician or patient collected has been demonstrated to be feasible in this population (55). Although self collection promises to overcome important cultural, geographic and access barriers, its use will still be hindered by the same cost issues faced by cytology-based screening programs.
The Pima County Cervical Cancer Prevention Partnership (PCCCPP) funded by the CDC under the REACH U.S. initiative attempts to foster a sustainable systemic response to cervical cancer prevention. PCCCPP, which includes school districts, community health centers, county government and community-based organizations, has grown out of a community-based participatory process, and has as its mission to increase awareness and knowledge of cervical cancer screening, prevention, and management, as well as to facilitate access to diagnostic and treatment services for women throughout Pima county. In its first 18 months PCCCPP has developed and disseminated a CHW training program, which has trained nearly 75 promotoras (all Mexican origin women) and reached more than 2,000 individuals through small group sessions and home visits. Current efforts are focused on developing school-based parent education interventions to facilitate HPV vaccination decision making, and developing navigation strategies for women with abnormal screenings who are concurrently at higher risk of cancer and being lost to clinical follow up.
Although publicly funded initiatives like the NBCCEDP have increased screening coverage in this area, the diagnostic and therapeutic follow up of these patients is highly variable and dependent on state specific Medicaid residency requirements which in Border States may exclude from coverage recent and/or illegal immigrants. This is particularly problematic given that foreign-born women living in the U.S. have significantly increased mortality compared to their U.S.-born counterparts (56). Additionally, although many of the screening related determinants (age, access, acculturation, clinician recommendation, etc.) likely apply to primary prevention, little is known about the knowledge, acceptability and uptake of the HPV vaccine in border communities (57). Despite distribution of vaccine through the CDC's Vaccine for Children program, the major public health concern is to ensure that the populations with the greatest burden of risk and disease (in this case the children of immigrants) are able to access these services without compromising their own or their parents legal status in the country.
E. Sioux/American Native Americans of the Northern Plains: Screening and Vaccination (Bell)
Cervical cancer is one of many diseases that disproportionately impact the Native American population in the Northern Plains. The Aberdeen Area of the Indian Health Service, which encompasses North Dakota, Nebraska, Iowa, and South Dakota, has a cervical cancer mortality rate of 11.5/100,000 and age-adjusted mortality rate of 4.9/100,000 for the 1994-1998 time periods (58).
Increased cervical cancer incidence in the Native American population is due to many factors including behaviors (e.g., more sexual partners resulting in greater exposure to HPV) geographical issues (e.g., very rural population with poor access to care), and economic issues (59). The unemployment rate is approximately 50%, the median household income is $20,089, 58% of the households have an annual income less than $25,000) and 68.7% of families live below the poverty level. The climate is harsh ranging from severe heat in the summer (110°F) to extreme cold in the winter (-30°F). Housing is of poor quality, often without running water or adequate heating (4).
Our effort to reach this population has spanned eight years. In our initial project, we found 21.5% and 14.2% of 287 Native American patients tested positive for any HPV and carcinogenic HPV infection, respectively. Among HPV-positive women, 41% presented with multiple HPV genotypes and 48.7% were infected with HPV16 and/or HPV18 (60). Collaborations between the state NBCCEDP program and IHS service units have begun, increasing the number of Native American women who participate in the state NBCCDEP program from 79 Native American women participated in NBCCDEP in 2000 (7% of the Pap tests performed) to 481 (18%) in 2008.
Because of the increased awareness of the problem of cervical cancer by the tribes, the tribal health board has made HPV vaccination a priority. The vaccination rates for pediatric vaccines are high among the Native American population. However, the adolescent patient is often difficult to access. To address this issue, the tribal school system has paired with the medical community to aid in school based HPV vaccination programs. To aid with diagnosis, treatment, and follow-up of cervical dysplasia and cancer, telemedicine programs are being developed to connect cancer specialists to the Native American patient remotely.
Despite making some progress with cervical cancer screening and awareness, there are persistent problems which need to be addressed. According to a recent cost model, the Indian Health Service (IHS) appropriated funding provides only 55% of the necessary federal funding to assure mainstream personal health care. It is not uncommon for the service units to “run out of funds” by the fourth fiscal quarter (1). Most of the health care budget is spent on acute care and much less on prevention. Physician recruitment and retention continues to be problematic. The lack of continuity of care is a consistent problem, as locum tenens often staff the clinic. In the Lakota language, there is a not word for cancer, and according to Lakota beliefs, the act of looking for a bad thing such as cancer, will cause it to happen. Only when we address the cultural and socioeconomic issues of this population, will we make an impact on its cervical cancer problem.
F. Kentucky: Follow-up to Abnormal Pap Tests among Appalachian Women (Dignan)
Patient Navigation for Cervical Cancer in Appalachia was established to provide public health departments with additional support needed to increase adherence to recommendations for follow-up care for women with abnormal Pap tests. Cervical cancer incidence (11.4/100,000 during 2001-5) and mortality rates (3.1/100,000) for Appalachia have been elevated for decades (5;61). Guided by Social Cognitive Theory, the project was initiated by recruiting and training of local women to work in county health departments and provide patient navigation to overcome barriers to obtaining follow-up care. The primary barriers described by women are uncertainty regarding the follow-up care they are to receive and logistics. To address uncertainty regarding follow-up care, patient navigators are prepared to provide information and describe step-by-step what women are likely to experience with follow-up medical procedures. Similarly, patient navigators are equipped to address logistical concerns, including helping women schedule appointments, arrange for transportation, and obtain other services needed follow-up care.
Patient navigators are working in 10 county health departments in Appalachian Kentucky, and thus far 130 women, aged 18 and older with abnormal Pap smear results have been referred for follow-up and are enrolled in the project. Of those enrolled, 31% have been told that they need a repeat Pap smear, 21% have been referred for additional follow-up at the health department, and 53% have been referred to a provider outside the health department. When asked about barriers to obtaining follow-up, concerns about health insurance (29%), child care (10%), out of pocket costs (9%), and fear of what may be found (4%) were most commonly mentioned. On the other hand, when asked about needs related to obtaining follow-up, women responded that having knowledge that cancer ‘runs in my family so I need to resolve’ the abnormal Pap smear result (22%), having support from family/friends (11%), being able to cope with financial issues (7%), and having someone to accompany them were important to obtaining follow-up.
Among the most common are health service access barriers, culturally related fear and fatalism, a lack of confidence in cancer screening, and limited awareness of variation in successful treatment. For many Appalachians, cancer is believed to be one disease that is universally fatal and therefore early detection through screening provides little if any added value to the life of the patient or their family. These beliefs added to access barriers are clearly associated with low rates of screening and low rates of obtaining recommended diagnostic procedures.
There is a long tradition of negative experiences with cancer among Appalachian women that manifests as avoidance behaviors in seeking screening and follow up because of fear of a cancer diagnosis. The avoidance is usually seen as ‘passive refusal,’ but also occurs in an active form as refusal to obtain services even when offered. This problem is compounded by poverty, which influences many parts of life and is associated with lack of transportation, child care, and exclusive reliance on public health departments and other safety net health care providers to seek cancer screening.
G. Vietnamese-Americans Living along the Eastern Seaboard: Compliance with Screening (Ma)
Among U.S. racial/ethnic groups, Vietnamese-American women have the highest incidence rate of invasive cervical cancer. The incidence rate for cervical cancer among Vietnamese women (43/100,000), is five times higher than that of White women (8.5/100,000) (62). Vietnamese and other Asian Americans experience a dynamic state of acculturation. They acculturate at the same rate as other groups, but unlike other groups, tend to maintain strong cultural and linguistic links with their respective traditional societies. These behaviors tend to affect lifestyles as well as health care beliefs and practices. This may partially explain why some Asian subgroups lag behind other racial/ethnic groups in seeking and obtaining getting basic screening tests such as Pap smear.
Considering the rapid growth of the U.S. Vietnamese population, prevention of cervical cancer in this high risk community through screening and early detection becomes a critical public health issue. Vietnamese-American women encounter substantial healthcare system as well as cultural, educational, and linguistic barriers that prevent them from obtaining screening services (62-66). Nearly two-thirds (60%) of Vietnamese women in our catchment service area in the eastern region of the U.S. are also medically underserved or uninsured and have low incomes.
Building on our previous CBPR research (67;68), we are conducting a large-scale, community-based participatory group randomized intervention trial to increase cervical cancer screening and reduce health system access barriers among medically underserved and low income Vietnamese women in the eastern region of the U.S. (PA, NJ). Preliminary data from 1,020 eligible participants indicate the following characteristics of the participants: the majority-(74.0%) were married; nearly half had completed high school; less than 10 percent had college education, the majority had annual household incomes <$20,000; and overwhelming majority (97%) spoke Vietnamese at home and spoke and wrote English poorly (90%). The baseline data indicated low ever-screened percentages for cervical cancer. Few women had perceived themselves to be at risk for cervical cancer (<10%) (3% to 7%). Although most women perceived that there are benefits of obtaining a Pap test (52% to 73%), various barriers to screening were reported by a considerable percentage of women, including language difficulties, financial barriers (cost), lack of time, and lack of knowledge about what will be done during a Pap test (35% to 50%). Although no final outcome data are available at this time, we observed a significant increase (0% to 74 %) in Pap testing among non-compliant Vietnamese women in the intervention group at 12 month follow-up visit.
We have learned two major lessons through our research experience in working with the Vietnamese community: (1) Effort to reduce health disparities is not limited to health care professionals. Engaging Asian community organizations within the targeted populations from program concept and content to implementation are critical elements in building links, trust, and respect for long-term partnerships. Our engagement of community gatekeepers and organizations fostered broader community interest and participation in our cancer control intervention. The strength of this partnership determines the quality of our research outcomes; and (2) Comprehensive intervention strategies addressing a broad range of identified barriers and cultural beliefs at individual and system levels enhanced the participation and utilization of beneficial education and screening.
VI. Conclusions
The development and availability of the new technology for cervical cancer prevention and control provide a golden opportunity to address cervical cancer disparities in the U.S. Because these new cervical cancer tools are robust, a few age-appropriate interventions in those currently not receiving the “standard of care” could quickly reduce the endemicity of the key intermediate steps in the pathway leading to cervical cancer and thereby have a long-lasting impact (69). However, the public health benefits of old or new tools will only be attained if they are deliverable to, and accepted and adopted by, at-risk populations. Only by intervening in these underserved populations, rather than doing more in the majority of the population who are already at a vastly reduced risk of cervical cancer, will there be a substantial decrease in the burden of cervical cancer in the U.S.
We have collectively shown that community involvement (through outreach or CBPR) and culturally-relevant strategies are promising approaches to the elimination of cervical cancer disparities in the United States across diverse populations. We also learned that the development and implementation of these efforts take time and effort. Formative assessments in collaboration with targeted communities not only provide relevant surface and deep structure information to be used in the intervention development; they provide both the academicians and the community the opportunity to establish trust and “train” each other on their specific skills and talents. Further, most of the lessons learned in the implementation of these programs are very similar independent of the racial/ethnic population, which begs the question on whether we should focus on similarities rather than differences across sub-populations. Structural, and even intrapersonal, barriers are very similar across these populations. With regard to structural barriers most are related to health care access and environmental factors.
It has been argued that most differences across racial/ethnic groups rely on the intrapersonal factors, particularly as they relate to attitudes and beliefs. Interestingly, most of intrapersonal barriers encountered in our programs are very similar (e.g., fear of results and fatalism). These findings suggest that most of the cultural, ethnic, and racial differences across sub-populations are not factors associated with cervical cancer prevention and control behavior (e.g., screening and HPV vaccination). These differences will be critical for HOW we address these barriers and motivators to successfully intervene in these populations, taking into account their cultural background as well as the needs and assets within these communities.
Another common thread of our experiences is the socio-ecological perspective we have used in establishing our programs including public policy, communities, institutions and organizations, social networks (e.g., families, religious organizations), and individuals as well as the importance of a consistent message in all these intervention levels. Although some of our programs focused more in one component of the socio-ecological model than others, the involvement of different segments of society (in addition to the health care system) has been shown to be critical to the achieved success. For instance, involvement of the faith-based community if conducted in a culturally-relevant manner can have a great impact not only in promotion of screening and/or HPV vaccination but also in change of attitudes and beliefs of the community and, potentially, public policy. Although some studies have shown that religious beliefs, particularly among Muslim and Hindu/Sikh can served as barriers to acceptance and uptake of HPV and other vaccinations, these issues have not emerged in our work (70-75). Perhaps such difference in findings is due to our community-based, participatory approach with these faith-based organizations and members of the target community and the fact that we have worked mostly with Christian churches.
Although most of our research programs discussed above has not focused on public policy, this represents a great opportunity given that most of us have already engaged constituents. Once provided with adequate training and support, the engaged community can influence policy, particularly related to the delivery of evidence-based, culturally-relevant, age-appropriate cost-effective methods of cervical cancer prevention and control. Individuals from these communities can influence legislative changes at the national level such as implementation of a targeted or universal HPV vaccination programs like the one developed in Australia for women between the ages of 12 and 26 years of age primarily through school-based clinics and catch-up among girls not in school through general practitioners (76-78). The preliminary results from Australia are very encouraging with an achieved coverage of 70% or more among the school cohorts during the first year (76). Whether the ideal approach to reach underserved populations is targeted or universal HPV vaccination is an unanswered question.
Although well-planned and participatory, such implementation was not without important challenges that were overcome and we can learn from: the importance of focusing on the “community benefit from the reduction of cervical cancer rates with less focus on the reduction of sexually transmitted diseases (e.g., genital warts); opposition from some religious schools; assurance that there is sufficient vaccine supply, anti-vaccination organized groups and inaccurate information through the media, particularly regarding side effects (77).
In summary, we are on the verge of a seachange in cervical cancer prevention. Yet, without the commitment to, and concomitant investment in, understanding and overcoming the psychosocial, institutional, and access barriers that perpetuate health disparities, these revolutionary technological developments will go for naught. Here, we provide an interim report of our successes and remaining challenges in reducing the disproportionate burden of cervical cancer, an almost entirely preventable malignancy, among medically-underserved women living in the U.S. Looking to the future, addressing cervical cancer health disparities will provide the impetus for addressing the conjoining health disparities.
Acknowledgments
We acknowledge the support of Tameka Walls, Vanessa Olivio, Kerrygrace Morrissey, and Shelly Niwa of Westat Corporation (Rockville, MD).
Financial Support: Dr. Castle was supported by the intramural program of the NIH/NCI and the NCI Center for Reducing Cancer Health Disparities. The following grants supported this work: Drs. Scarinci and Partridge, NCI (U01 CA114619); Dr. Kobetz, NCI (R21CA-11981), the American Cancer Society (ACS) (MRSGT-07-159-01-CPHPS), the Dr. John T. Macdonald Foundation (98218), the Women's Fund of Miami-Dade County (M0600284); Dr. Bell, ACS, and NCI (U01CA86098-04); Dr. Dignan, NCI (R01 CA120606); Dr. Ma, NCI (R01 CA111570; U01 CA114582); Dr. Brandt, NCI (U01 CA114601; U01CA114601-03S4); Dr. Garcia, Centers for Disease Control and Prevention (1 U58 DP001044-01).
Footnotes
This rate was based on cases diagnosed in 2001-2005 from 17 SEER geographic areas (5).
Reference List
- 1.Jemal A, Thun MJ, Ries LA, Howe HL, Weir HK, Center MM, et al. Annual report to the nation on the status of cancer, 1975-2005, featuring trends in lung cancer, tobacco use, and tobacco control. J Natl Cancer Inst. 2008;100:1672–94. doi: 10.1093/jnci/djn389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 3.Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370:890–907. doi: 10.1016/S0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention: Cervical Cancer. 2009.
- 5.Surveillance, Epidemiology and End Results (SEER) Program. 2009.
- 6.Wright TC, Jr, Schiffman M. Adding a test for human papillomavirus DNA to cervical-cancer screening. N Engl J Med. 2003;348:489–90. doi: 10.1056/NEJMp020178. [DOI] [PubMed] [Google Scholar]
- 7.McCredie MR, Sharples KJ, Paul C, Baranyai J, Medley G, Jones RW, et al. Natural history of cervical neoplasia and risk of invasive cancer in women with cervical intraepithelial neoplasia 3: a retrospective cohort study. Lancet Oncol. 2008;9:425–34. doi: 10.1016/S1470-2045(08)70103-7. [DOI] [PubMed] [Google Scholar]
- 8.Garland SM, Hernandez-Avila M, Wheeler CM, Perez G, Harper DM, Leodolter S, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356:1928–43. doi: 10.1056/NEJMoa061760. [DOI] [PubMed] [Google Scholar]
- 9.Future II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356:1915–27. doi: 10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- 10.Paavonen J, Jenkins D, Bosch FX, Naud P, Salmeron J, Wheeler CM, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007;369:2161–70. doi: 10.1016/S0140-6736(07)60946-5. [DOI] [PubMed] [Google Scholar]
- 11.Hildesheim A, Herrero R, Wacholder S, Rodriguez AC, Solomon D, Bratti MC, et al. Effect of human papillomavirus 16/18 L1 viruslike particle vaccine among young women with preexisting infection: a randomized trial. JAMA. 2007;298:743–53. doi: 10.1001/jama.298.7.743. [DOI] [PubMed] [Google Scholar]
- 12.Cuzick J, Szarewski A, Cubie H, Hulman G, Kitchener H, Luesley D, et al. Management of women who test positive for high-risk types of human papillomavirus: the HART study. Lancet. 2003;362:1871–6. doi: 10.1016/S0140-6736(03)14955-0. [DOI] [PubMed] [Google Scholar]
- 13.Cuzick J, Clavel C, Petry KU, Meijer CJ, Hoyer H, Ratnam S, et al. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer. 2006;119:1095–101. doi: 10.1002/ijc.21955. [DOI] [PubMed] [Google Scholar]
- 14.Arbyn M, Sasieni P, Meijer CJ, Clavel C, Koliopoulos G, Dillner J. Chapter 9: Clinical applications of HPV testing: A summary of meta-analyses. Vaccine. 2006;24 3:S78–89. doi: 10.1016/j.vaccine.2006.05.117. [DOI] [PubMed] [Google Scholar]
- 15.Ronco G, Giorgi-Rossi P, Carozzi F, Dalla PP, Del Mistro A, De Marco L, et al. Human papillomavirus testing and liquid-based cytology in primary screening of women younger than 35 years: results at recruitment for a randomised controlled trial. Lancet Oncol. 2006;7:547–55. doi: 10.1016/S1470-2045(06)70731-8. [DOI] [PubMed] [Google Scholar]
- 16.Ronco G, Segnan N, Giorgi-Rossi P, Zappa M, Casadei GP, Carozzi F, et al. Human papillomavirus testing and liquid-based cytology: results at recruitment from the new technologies for cervical cancer randomized controlled trial. J Natl Cancer Inst. 2006;98:765–74. doi: 10.1093/jnci/djj209. [DOI] [PubMed] [Google Scholar]
- 17.Sherman ME, Lorincz AT, Scott DR, Wacholder S, Castle PE, Glass AG, et al. Baseline cytology, human papillomavirus testing, and risk for cervical neoplasia: a 10-year cohort analysis. J Natl Cancer Inst. 2003;95:46–52. doi: 10.1093/jnci/95.1.46. [DOI] [PubMed] [Google Scholar]
- 18.Kjaer S, Hogdall E, Frederiksen K, Munk C, van den BA, Svare E, et al. The absolute risk of cervical abnormalities in high-risk human papillomavirus-positive, cytologically normal women over a 10-year period. Cancer Res. 2006;66:10630–6. doi: 10.1158/0008-5472.CAN-06-1057. [DOI] [PubMed] [Google Scholar]
- 19.Bulkmans NW, Berkhof J, Rozendaal L, van Kemenade FJ, Boeke AJ, Bulk S, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year follow-up of a randomised controlled implementation trial. Lancet. 2007;370:1764–72. doi: 10.1016/S0140-6736(07)61450-0. [DOI] [PubMed] [Google Scholar]
- 20.Mayrand MH, Duarte-Franco E, Rodrigues I, Walter SD, Hanley J, Ferenczy A, et al. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007;357:1579–88. doi: 10.1056/NEJMoa071430. [DOI] [PubMed] [Google Scholar]
- 21.Naucler P, Ryd W, Tornberg S, Strand A, Wadell G, Elfgren K, et al. Human papillomavirus and Papanicolaou tests to screen for cervical cancer. N Engl J Med. 2007;357:1589–97. doi: 10.1056/NEJMoa073204. [DOI] [PubMed] [Google Scholar]
- 22.Ronco G, Giorgi-Rossi P, Carozzi F, Confortini M, Dalla PP, Del MA, et al. Results at recruitment from a randomized controlled trial comparing human papillomavirus testing alone with conventional cytology as the primary cervical cancer screening test. J Natl Cancer Inst. 2008;100:492–501. doi: 10.1093/jnci/djn065. [DOI] [PubMed] [Google Scholar]
- 23.Sankaranarayanan R, Nene BM, Shastri SS, Jayant K, Muwonge R, Budukh AM, et al. HPV screening for cervical cancer in rural India. N Engl J Med. 2009;360:1385–94. doi: 10.1056/NEJMoa0808516. [DOI] [PubMed] [Google Scholar]
- 24.Schiffman M, Wacholder S. From India to the world--a better way to prevent cervical cancer. N Engl J Med. 2009;360:1453–5. doi: 10.1056/NEJMe0901167. [DOI] [PubMed] [Google Scholar]
- 25.Mosher WD, Chandra A, Jones J. Sexual behavior and selected health measures: men and women 15-44 years of age, United States, 2002. Adv Data. 2005:1–55. [PubMed] [Google Scholar]
- 26.Castle PE, Fetterman B, Akhtar I, Husain M, Gold MA, Guido R, et al. Age-appropriate use of human papillomavirus vaccines in the U.S. Gynecol Oncol. 2009;114:365–9. doi: 10.1016/j.ygyno.2009.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Freeman HP, Wingrove BK. Excess cervical cancer mortality: A marker for low access to health care in poor communities. National Institutes of Health; 2007. 05-5282. [Google Scholar]
- 28.United States Cancer Statistics. 2009.
- 29.Matthews AK, Berrios N, Darnell JS, Calhoun E. A qualitative evaluation of a faith-based breast and cervical cancer screening intervention for African American women. Health Educ Behav. 2006;33:643–63. doi: 10.1177/1090198106288498. [DOI] [PubMed] [Google Scholar]
- 30.Brandt HM, Modayil MV, Hurley D, Pirisi-Creek LA, Johnson MG, Davis J, et al. Cervical cancer disparities in South Carolina: an update of early detection, special programs, descriptive epidemiology, and emerging directions. JSC Med Assoc. 2006;102:223–30. [PubMed] [Google Scholar]
- 31.Partridge EE, Fouad N, Hinton A, Hardy M, Liscovicz N, White-Johnson F, et al. The deep South network for cancer control: eliminating cancer disparities through community-academic collaboration. Fam Community Health. 2005;28:6–19. doi: 10.1097/00003727-200501000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Freire P. Pedagogia do oprimido. Rio de Janeiro, Brazil: SIGLO XXI EDITORES, S. A. DE C. V; 1970. [Google Scholar]
- 33.Wallerstein N, Duran B. In: The conceptual, historical, and practice roots of community based participatory research and related participatory traditions. Minkler M, Wallerstein N, editors. San Francisco: Jossey-Bass; 2003. pp. 27–52. [Google Scholar]
- 34.Lisovicz N, Johnson RE, Higginbotham J, Downey JA, Hardy CM, Fouad MN, et al. The Deep South Network for cancer control. Building a community infrastructure to reduce cancer health disparities. Cancer. 2006;107:1971–9. doi: 10.1002/cncr.22151. [DOI] [PubMed] [Google Scholar]
- 35.Florida Cancer Data System (FCDS) Cervical cancer incidence and state of diagnosis among women in Miami-Dade County. Miami: University of Miami, Miller School of Medicine, Florida Cancer Data System; 2004. [Google Scholar]
- 36.Kobetz E, Menard J, Barton B, Pierre L, Diem J, Auguste PD. Patne en Aksyon: Addressing Cancer Disparities through Research and Social Action. Am J Public Health. 2009;99:1163–65. doi: 10.2105/AJPH.2008.142794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kobetz E, Menard J, Diem J, Barton B, Blanco J, Pierre L, Etienne M, Auguste PD, Brewster C. Community-Based Participatory Research in Little Haiti: Challenges and Lessons Learned. Progress in Community Health Partnerships: Research, Education and Action. 2009;3:133–7. doi: 10.1353/cpr.0.0072. [DOI] [PubMed] [Google Scholar]
- 38.Israel B, Schultz A, Parker E, Becker A, Allen A, Guzman R. Developing and Following Community Based Research Principles. San Francisco, CA: Jossey-Bass; 2003. [Google Scholar]
- 39.Stepick A. Pride Against Prejudice: Haitians in the United States. Boston: Allyn & Bacon; 1998. [Google Scholar]
- 40.Farmer P. AIDS and Accusation: Haiti and the Geography of Blame. Berkeley, CA: University of California Press; 1992. [Google Scholar]
- 41.Community-Based Participatory Research for Health. San Francisco: Jossey-Bass; 2003. [Google Scholar]
- 42.U.S. Census 2000. 2000.
- 43.U.S.-Mexico Border Health Commission. Healthy Border 2010: An agenda for improving health on the United States-Mexico border. 2003 [Google Scholar]
- 44.Williams MA, Mokry B, Risser D. Cervical Cancer in Texas 2006. Austin, TX: Texas Department of State Health Services; 2006. [Google Scholar]
- 45.Coughlin SS, Richards TB, Nasseri K, Weiss NS, Wiggins CL, Saraiya M, et al. Cervical cancer incidence in the United States in the US-Mexico border region, 1998-2003. Cancer. 2008;113:2964–73. doi: 10.1002/cncr.23748. [DOI] [PubMed] [Google Scholar]
- 46.Suarez L, Pulley L. Comparing acculturation scales and their relationship to cancer screening among older Mexican-American women. J Natl Cancer Inst Monogr. 1995:41–7. [PubMed] [Google Scholar]
- 47.Fernandez-Esquer ME, Espinoza P, Ramirez AG, McAlister AL. Repeated Pap smear screening among Mexican-American women. Health Educ Res. 2003;18:477–87. doi: 10.1093/her/cyf037. [DOI] [PubMed] [Google Scholar]
- 48.Fernandez-Esquer ME, Cardenas-Turanzas M. Cervical cancer screening among Latinas recently immigrated to the United States. Prev Med. 2004;38:529–35. doi: 10.1016/j.ypmed.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 49.Leybas-Amedia V, Nuno T, Garcia F. Effect of acculturation and income on Hispanic women's health. J Health Care Poor Underserved. 2005;16:128–41. doi: 10.1353/hpu.2005.0127. [DOI] [PubMed] [Google Scholar]
- 50.Buki LP, Jamison J, Anderson CJ, Cuadra AM. Differences in predictors of cervical and breast cancer screening by screening need in uninsured Latino women. Cancer. 2007;110:1578–85. doi: 10.1002/cncr.22929. [DOI] [PubMed] [Google Scholar]
- 51.Adams EK, Breen N, Joski PJ. Impact of the National Breast and Cervical Cancer Early Detection Program on mammography and Pap test utilization among white, Hispanic, and African American women: 1996-2000. Cancer. 2007;109:348–58. doi: 10.1002/cncr.22353. [DOI] [PubMed] [Google Scholar]
- 52.Wallace D, Hunter J, Papenfuss M, de Zapien JG, Denman C, Giuliano AR. Pap smear screening among women >/=40 years residing at the United States-Mexico border. Health Care Women Int. 2007;28:799–816. doi: 10.1080/07399330701563111. [DOI] [PubMed] [Google Scholar]
- 53.Larkey L. Las mujeres saludables: reaching Latinas for breast, cervical and colorectal cancer prevention and screening. J Community Health. 2006;31:69–77. doi: 10.1007/s10900-005-8190-2. [DOI] [PubMed] [Google Scholar]
- 54.Fernandez-Esquer ME, Espinoza P, Torres I, Ramirez AG, McAlister AL. A su salud: a quasi-experimental study among Mexican American women. Am J Health Behav. 2003;27:536–45. doi: 10.5993/ajhb.27.5.5. [DOI] [PubMed] [Google Scholar]
- 55.Garcia F, Barker B, Santos C, Brown EM, Nuno T, Giuliano A, et al. Cross-sectional study of patient- and physician-collected cervical cytology and human papillomavirus. Obstet Gynecol. 2003;102:266–72. doi: 10.1016/s0029-7844(03)00517-9. [DOI] [PubMed] [Google Scholar]
- 56.Seeff LC, McKenna MT. Cervical cancer mortality among foreign-born women living in the United States, 1985 to 1996. Cancer Detect Prev. 2003;27:203–8. doi: 10.1016/s0361-090x(03)00062-x. [DOI] [PubMed] [Google Scholar]
- 57.Vanslyke JG, Baum J, Plaza V, Otero M, Wheeler C, Helitzer DL. HPV and cervical cancer testing and prevention: knowledge, beliefs, and attitudes among Hispanic women. Qual Health Res. 2008;18:584–96. doi: 10.1177/1049732308315734. [DOI] [PubMed] [Google Scholar]
- 58.Lehman R. Invasive Cervical Cancer among American Indians in the Northern Plains, 1994-1998: Incidence, Mortality, and Missed Opportunities. Indian Health Service; Jun 13, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Network for Cancer Control Research Among Native American and Alaska Natives. Indian Health Service; 2008. [Google Scholar]
- 60.Bell MC, Schmidt-Grimminger D, Patrick S, Ryschon T, Linz L, Chauhan SC. There is a high prevalence of human papillomavirus infection in American Indian women of the Northern Plains. Gynecol Oncol. 2007;107:236–41. doi: 10.1016/j.ygyno.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kentucky Cancer Registry. 2009.
- 62.Miller BA, Kolonel LN, Bernstein L, Young JL, Jr, Swanson GM, West D, et al. Racial/ethnic patterns of cancer in the United States 1988-1992. NIH; 1996. NIH Pub. No. 96-4104. [Google Scholar]
- 63.Shive SE, Ma GX, Tan Y, Toubbeh J, Parameswaran L, Halowich J. Health information and cancer screening differences among Asian Americans. CA J Health Promotion. 2006;4:1–11. [Google Scholar]
- 64.Shive SE, Ma GX, Tan Y, Toubbeh J, Parameswaran L, Halowich J. Racial differences in preventive and complementary health behaviors and attitudes. J Health Disparities Res Pract. 2006;1:75–92. [Google Scholar]
- 65.Sadler GR, Dong HS, Ko CM, Luu TT, Nguyen HP. Vietnamese American women: breast cancer knowledge, attitudes, and screening adherence. Am J Health Promot. 2001;15:211–4. ii. doi: 10.4278/0890-1171-15.4.211. [DOI] [PubMed] [Google Scholar]
- 66.McPhee SJ, Stewart S, Brock KC, Bird JA, Jenkins CN, Pham GQ. Factors associated with breast and cervical cancer screening practices among Vietnamese American women. Cancer Detect Prev. 1997;21:510–21. [PubMed] [Google Scholar]
- 67.Ma GX, Tan Y, Toubbeh JI, Edwards RL, Shive SE, Siu P, et al. Asian Tobacco Education and Cancer Awareness Research Special Population Network. A model for reducing Asian American cancer health disparities. Cancer. 2006;107:1995–2005. doi: 10.1002/cncr.22150. [DOI] [PubMed] [Google Scholar]
- 68.Fang CY, Ma GX, Tan Y, Chi N. A multifaceted intervention to increase cervical cancer screening among underserved Korean women. Cancer Epidemiol Biomarkers Prev. 2007;16:1298–302. doi: 10.1158/1055-9965.EPI-07-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schiffman M, Castle PE. The Promise of Global Cervical-Cancer Prevention. N Engl J Med. 2005;353:2101–4. doi: 10.1056/NEJMp058171. [DOI] [PubMed] [Google Scholar]
- 70.Marlow LAV, Waller J, Evans REC, Wardle J. Predictors of interest in HPV vaccination: A study of British adolescents. Vaccine. 2009;27:2483–2488. doi: 10.1016/j.vaccine.2009.02.057. [DOI] [PubMed] [Google Scholar]
- 71.Santry C. Muslim girls may miss out on cervical cancer immunisation. Health Serv J. 2008 Sep 4;:4. [PubMed] [Google Scholar]
- 72.Warraich HJ. Religious opposition to polio vaccination. Emerg Infect Dis. 2009;15(6):978. doi: 10.3201/eid1506.090087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Daniels NA, Juarbe T, Moreno-John G, Pérez-Stable EJ. Effectiveness of adult vaccination programs in faith-based organizations. Ethn Dis. 2007 Winter;17(1 Suppl 1):S15–22. [PubMed] [Google Scholar]
- 74.Shafi S, Rashid H, Ali K, El Bashir H, Haworth E, Memish ZA, et al. Influenza vaccine uptake among British Muslims attending Hajj, 2005 and 2006. BMJ. 2006;333(7580):1220. doi: 10.1136/bmj.39051.734329.3A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Omer SB, Pan WK, Halsey NA, Stokley S, Moulton LH, Navar AM, et al. Nonmedical exemptions to school immunization requirements: secular trends and association of state policies with pertussis incidence. JAMA. 2006;296(14):1757–63. doi: 10.1001/jama.296.14.1757. [DOI] [PubMed] [Google Scholar]
- 76.Brotherton JM, Deeks SL, Campbell-Lloyd S, Misrachi A, Passaris I, Peterson K, et al. Interim estimates of human papillomavirus vaccination coverage in the school-based program in Australia. Commun Dis Intell. 2008;32(4):457–61. doi: 10.33321/cdi.2008.32.45. [DOI] [PubMed] [Google Scholar]
- 77.Watson M, Shaw D, Molchanoff L, McInnes C. Challenges, lessons learned and results following the implementation of a human papilloma virus school vaccination program in South Australia. Aust N Z J Public Health. 2009;3(4):365–70. doi: 10.1111/j.1753-6405.2009.00409.x. [DOI] [PubMed] [Google Scholar]
- 78.Weisberg E, Bateson D, McCaffery K, Skinner SR. HPV vaccination catch up program - utilisation by young Australian women. Aust Fam Physician. 2009;38(1-2):72–6. [PubMed] [Google Scholar]