Abstract
Differentiation of fibroblasts to myofibroblasts and collagen fibrillogenesis are two processes essential for normal cutaneous development and repair, but their misregulation also underlies skin-associated fibrosis. Periostin is a matricellular protein normally expressed in adult skin, but its role in skin organogenesis, incisional wound healing and skin pathology has yet to be investigated in any depth. Using C57/BL6 mouse skin as model, we first investigated periostin protein and mRNA spatiotemporal expression and distribution during development and after incisional wounding. Secondarily we assessed whether periostin is expressed in human skin pathologies, including keloid and hypertrophic scars, psoriasis and atopic dermatitis. During development, periostin is expressed in the dermis, basement membrane and hair follicles from embryonic through neonatal stages and in the dermis and hair follicle only in adult. In situ hybridization demonstrated that dermal fibroblasts and basal keratinocytes express periostin mRNA. After incisional wounding, periostin becomes re-expressed in the basement membrane within the dermal-epidermal junction at the wound edge re-establishing the embryonic deposition pattern present in the adult. Analysis of periostin expression in human pathologies demonstrated that it is over-expressed in keloid and hypertrophic scars, atopic dermatitis, but is largely absent from sites of inflammation and inflammatory conditions such as psoriasis. Furthermore, in vitro we demonstrated that periostin is a transforming growth factor beta 1 inducible gene in human dermal fibroblasts. We conclude that periostin is an important ECM component during development, in wound healing and is strongly associated with pathological skin remodeling.
Summary: Periostin is a fibrogenic protein that mediates fibroblast differentiation and extracellular matrix synthesis. Here, we show that periostin is dynamically and temporally expressed during skin development, is induced by TGF-β1 in vitro and is significantly upregulated during wound repair as well as cutaneous pathologies.
Keywords: Development, Fibrosis, Periostin, Skin, Wound healing, Pathology
Introduction
Originally identified as an 811-amino acid protein secreted by osteoblasts (Takeshita et al. 1993), periostin is an extracellular matrix (ECM) protein containing four domains with structural homology with the insect protein fasciclin-1 (Conway and Molkentin 2008). Periostin was recently classed as a matricellular protein (Norris et al. 2008a) (modulator of cell-matrix interactions and cell function (Bornstein and Sage 2002)) as a result of an explosion of research in the past few years that has shown that periostin is prominently expressed during ECM remodeling, including in heart after myocardial infarction (Kuhn et al. 2007; Shimazaki et al. 2008), asthma-associated sub-epithelial fibrosis in lungs (Takayama et al. 2006), and pulmonary vascular remodeling (Chen et al. 2006). Moreover, periostin is known to be a key regulator during cardiac development which is particularly evident in the atrioventricular valve where a lack of periostin inhibits differentiation of the cushion mesenchyme into myofibroblastic-valve tissue (Butcher et al. 2007; Lie-Venema et al. 2008; Norris et al. 2008b). Initial assessment of adult skin in periostin−/−mice highlighted significant abnormalities in collagen fibrillogenesis which manifest in increased stiffness and decreased elasticity in comparison with wild-type mice (Norris et al. 2007).Therefore, during development, it appears that periostin could be required to mediate differentiation of fibroblasts to myofibroblasts as well as collagen synthesis and assembly.
Although critically important during development, matricellular proteins are typically restricted to tissue remodeling and wound repair in the healthy adult (Hamilton 2008). Unlike many other members of the matricellular protein family, periostin is normally expressed in adult tissues, including skin where it localizes to dermal fibroblasts, keratinocytes and the basal lamina (Jackson-Boeters et al. 2009). Interestingly, we have previously shown that periostin is only present in the extracellular matrix under tissue remodeling conditions such as those associated with pathological insult (nevus) (Jackson-Boeters et al. 2009). To further investigate periostin expression during tissue remodeling, using a full-thickness excisional wound healing model in C57/BL6 mice, we observed periostin in the granulation tissue at 3 days, with protein levels peaking at 7 days and returning to basal levels at 28 days (Jackson-Boeters et al. 2009). Interestingly, maximal periostin expression was associated with the presence of myofibroblasts (Jackson-Boeters et al. 2009), providing further evidence that periostin likely mediates fibroblast to myofibroblast differentiation.
Having shown association of periostin with normal skin, wound repair and nevus (Jackson-Boeters et al. 2009), we hypothesize that periostin is an important mediator of skin development, healing and remodeling. The aim of this study was to examine the spatiotemporal expression patterns of periostin in murine skin during development and incisional wound healing and whether or not persistence of periostin is associated with human cutaneous pathologies. Here we demonstrate a dynamic pattern of expression in the dermis, hair follicles as well as deposition along the basement membrane (BM) within the dermal-epidermal junction (DEJ) during skin organogenesis. Moreover, the abundance of periostin in fibrotic scars and its relative absence in pre-lesional wounds suggest that it may be a key regulator of ECM synthesis and deposition in skin.
Materials & methods
Animal and tissue preparation
Periostin mice (Snider et al. 2008) were maintained under specific-pathogen-free conditions in individual cages with a 12 h light/dark cycle. The animal use protocols were approved by the Institutional Animal Care and Use Committee’s at IUPUI and the University Council on Animal Care at the University of Western Ontario. Serological analyses were performed on the mice prior to experiments to test for the presence of blood borne pathogens or infection. Embryos were collected from timed matings at E13.5, 15.5, 17.5, P2, P9, P19 and P60 and processed for routine paraffin or cryosection sections (10 µm). Skin from postnatal animals was spread on X-ray film (to maintain flat orientation) prior to 4% paraformaldehyde fixation.
Wounding
Mice were anesthetized with 1.2% Avertin (125 mg/kg) and the back shaved and sterilized using a 70% alcohol swab. A full thickness incision wound (1 cm long) was cut along back skin below the shoulder blades to prevent self-licking. Ointment (100 mg pure white petrolatum jelly; Vaseline) was applied to the wound and changed every 2 days. The mice were caged individually with regular light/dark cycles. The animals were sacrificed at specified post-surgical time points and skin samples were collected as above for histological analysis.
Human tissue samples
Formalin fixed biopsy specimens were provided by Dr. Jeff Travers (Department of Dermatology, IUPUI). Four samples from patients (mean age, 33.0 years) with severe atopic dermatitis, five samples from patients of psoriasis (mean age, 39.7 years), three samples from patients with keloid scars (mean age, 28 years), 4 samples from patients with hypertrophic scars (mean age, 32.6 years), 2 samples from patients with chronic dermal inflammation (mean age, 64.5 years) and five samples from healthy individuals (mean age, 48.2 years) were included in this study. The fixed human skin samples were processed for routine paraffin section.
Histological staining
Masson’s trichrome staining was performed as previously described to visualize collagen (blue) and ECM (red) deposition(Liu et al. 2008).
Immunohistochemistry and in situ hybridization analysis
Periostin and Ki67 were detected on paraffin sections using the ABC kit (Vectorstain) following the manufacturer’s instructions. The antibodies were diluted 1:3000 for rabbit anti-periostin (Kruzynska-Frejtag et al. 2004), and 1:500 for mouse anti-Ki67 (DB). Signals were revealed by using DAB and hydrogen peroxide as the chromogen. Sections were counterstained with methyl green. As a negative control for periostin labeling specificity, corresponding tissues from age-matched periostin null mice was processed in parallel. The negative control for Ki67 was set by using normal rabbit or mouse serum respectively at 1:500 dilutions. In situ hybridization for periostin mRNA was performed on paraffin sections using S35-labeled anti-sense riboprobes (Lindsley et al. 2007) and the specificity was controlled by using corresponding sense probe.
Culture of human dermal fibroblasts
Human dermal fibroblasts were isolated using an explant technique as previously described (Chen et al. 2008). Cells were cultured in high glucose DMEM (Invitrogen, Burlington, Ontario). All media was supplemented with 10% FBS and 1% antibiotic/antimycotic solution. Cells were plated onto a six well plate at a density of 60,000 cells/well and were allowed to grow for 24 h at 37°C. Cells were then serum-starved for 24 h, pre-treated Transforming growth factor β1 (TGF- β) (R and D Systems, 4 ng/ml) for 6 h (for mRNA analysis) or 6 and 24 h (for protein analysis).
Taqman realtime polymerase chain reaction
Total RNA was isolated using Trizol reagent (Invitrogen). Total RNA (25 ng) was amplified using the TaqMan One Step RT-PCR Master Mix (4309169; Applied Biosystems Inc., Streetsville, ON, Canada). Reverse transcription and quantitative real-time PCR reactions were performed using the Prism 7900 HT Sequence Detector (Applied Biosystems Inc.). Samples were incubated at 48°C for 30 mins to make cDNA templates. The resulting cDNA was amplified for 40 cycles. Cycles alternated between 95°C for 15 s and 60°C for 1 min. Results were analysed using SDS v2.1 software (Applied Biosystems Inc.). The ΔΔCt method was used to calculate gene expression levels relative to GAPDH and normalized to control cells. Data were log-transformed prior to analysis by one-way analysis of variance and Tukey’s post-hoc test, using Graphpad Software v. 4 (Graphpad Software, La Jolla, CA, USA).
Western blotting
Western blotting was performed as previously described (Hamilton et al. 2007; Kokubu et al. 2009).
Results
Periostin exhibits a dynamic spatiotemporal expression pattern in skin development
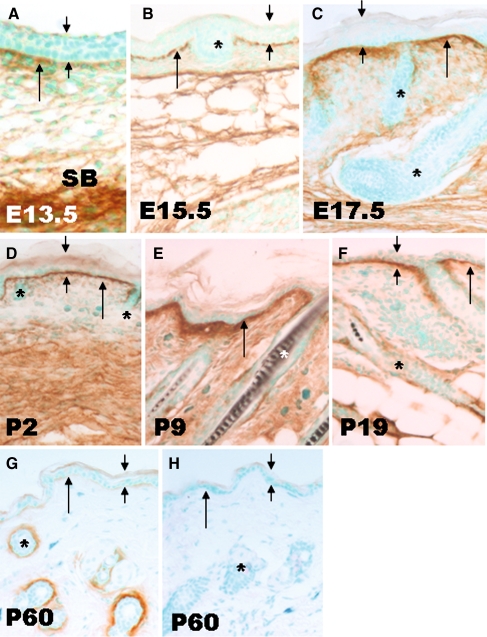
We examined periostin protein deposition in wild-type skin during embryonic development and at several postnatal stages. At E13.5 and E15.5, periostin was faintly detectable along the BM (Fig. 1a, b) at the dermal-epidermal junction (DEJ) and in the lower dermis. At E17.5, the deposition was more uniform and intense in both the DEJ and dermis (Fig. 1c) compared to earlier stages. The robust deposition along DEJ persisted to postnatal day 19 (Fig 1d, e, f). From P9 onwards, the ECM surrounding hair follicles labeled positively for periostin (Fig. 1e, f, g). In adult mice (P60 and older), hair follicles were intensively labeled, but the DEJ labeling and deep dermal staining was significantly reduced (Fig. 1g).
Fig. 1.
Detection of periostin protein deposition in development by immunohistochemistry. At E13.5 (a), periostin labeling in dermal-epidermal junction (long arrow) was faint and uneven, in contrast the subcutaneous tissue (SB) was strongly labeled. As development advanced (b, c, d, e), periostin immunoreactivity within the DEJ increased, and was maintained within the dermis. The outline of HF (*) remained negative till P9 (e). Compared with P9, the labeling within the DEJ was reduced at P19 when HF(*) was faintly labeled. In adults (P60 days), periostin was faintly detectable within the DEJ, whilst the ECM surrounding hair follicles was strongly labeled (g). As a negative control, the periostin null skin (h) showed no labeling either in the DEJ or surrounding the HFs. All images were taken at same magnification. Scale bar = 200 µm
Comparison of periostin mRNA and protein localization
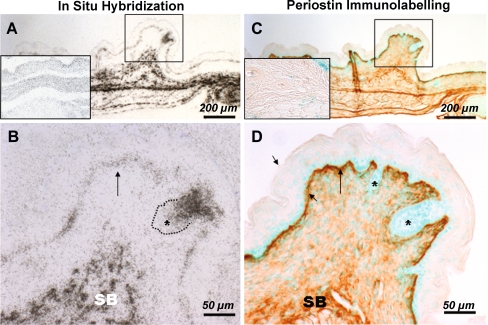
To assess what cell populations produce periostin, we utilized in situ hybridization. Skin was examined from wild-type mice at E17.5, when periostin protein is present in the dermis and DEJ. Basal keratinocytes above the DEJ contained mRNA for periostin (Fig. 2a), as did the dermal fibroblasts/mesenchymal cells within the upper and lower dermis. Using immunohistochemistry, periostin protein deposition closely correlated to the same area (Fig. 2b). However, in hair follicles, cells contained mRNA for periostin (Fig. 2c), but the protein was not present showing that these cells were not actively secreting periostin protein into the ECM (Fig. 2d).
Fig. 2.
Comparison of periostin mRNA and protein localization in embryonic skin. Adjacent sections from E17.5 wild type embryos were labeled for periostin transcript (a & b) by in situ hybridization or protein (c & d) by immunohistochemistry. Whilst the basal epidermis expressed relatively low levels of periostin transcript compared to subcutaneous or dermal tissues (SB), periostin protein deposition within the interfollicular dermal-epidermal junction (long arrow) is robustly labeled as in subcutaneous tissues. Note, the hair follicle, which is positively labeled for periostin message, its outlining at the dermal-epidermal junction was negative for periostin protein labeling. b & d were high-power images of boxed regions in a & b respectively. The images were taken at same magnification for a & c (bar = 200 um) and B & D (bar = 50 um). Inset in (a) is ribcage, an area where periostin is heavily expressed. Inset in (b) is periostin labeling of periostin knockout skin
Wounding restores embryonic periostin distribution pattern in adult skin
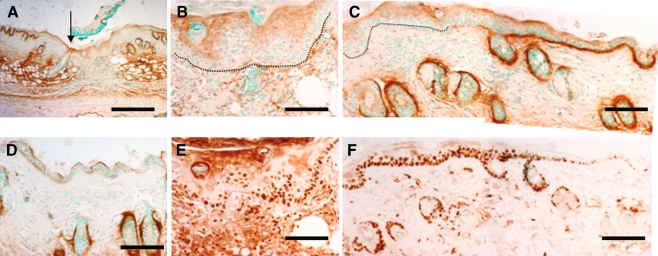
To assess patterns of periostin expression in wound repair, we induced the wound healing process using the established incisional wound model. At day 5, keratinocytes labeled positively for periostin protein, but not the granulation tissue in the wound (Fig 3a, b). Periostin protein was present in the DEJ in areas adjacent to the wound (Fig. 3c). In areas away from the wound site, periostin labeling is decreased suggesting that periostin is upregulated in the DEJ at the wound edges (Fig. 3d). As cell proliferation is a necessary event in re-epithelialization, we examined the expression of the cell proliferation marker Ki67 and periostin on adjacent sections. Significantly, the epithelium within the wound was positive for Ki67 labeling (Fig 3e), as was the tissue at the edge of the wound (Fig. 3f).
Fig. 3.
Wounding re-establishes embryonic pattern of perostin deposition in adult skin. Panels a–d, g and h were stained for periostin protein. Panel a was low power magnification of a day 5 wound, the arrow marks the left margin of wound. Panels b and c were magnified regions to the right for that arrow in a. Periostin deposition was absent in DEJ underneath the nascent epidermis (underlined in b) and the thickened epidermis immediately adjacent to the wound (underlined in c). Robust periostin protein deposition was detected along the DEJ in regions distal to wound-edge (c). Panel d is a region far distal to the wound, showing minimal periostin protein along the DEJ but abundant periostin deposition in the dermis (*). Panels e and f were stained for Ki67 to label proliferating cells. The proliferating cells were present within the thickened and nascent epidermis, in a region devoid of periostin protein deposition. Scale bar = 100 µm in panel A and 200 µm in the rest panels
Periostin immunoreactivity is elevated in hypertrophic and keloid scars, atopic dermatitis, but is reduced in chronically inflamed pre-lesional skin and psoriasis
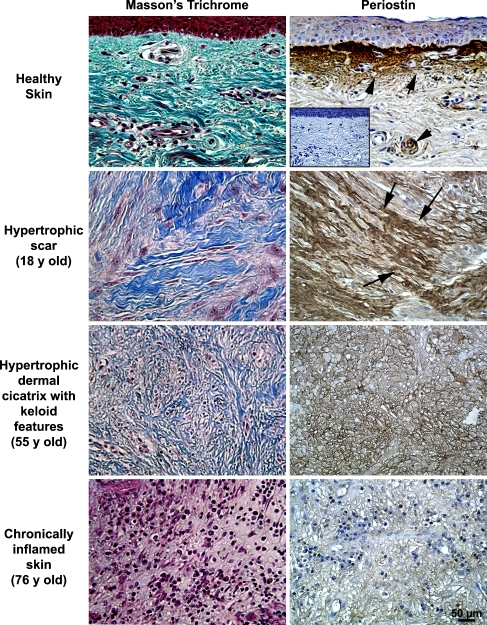
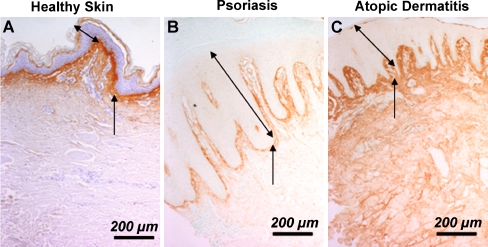
As periostin is implicated in fibrosis, we investigated whether periostin levels were altered in several types of skin lesions. Using immunohistochemistry, we examined spatial deposition of periostin in healthy skin, keloid scars, hypertrophic scars, chronically inflamed skin, atopic dermatitis and psoriasis lesions. Periostin protein levels were highest in keloid and hypertrophic scars, but were significantly reduced in chronically inflamed tissue (Fig. 4). In hypertrophic scars, periostin immunoreactivity was associated with large collagen bundles (shown by Masson’s trichrome), but in hypertrophic scars, periostin was present mainly surrounding cells (Fig. 4). The lack of periostin in chronically inflamed tissue correlated with an increase in inflammatory cell infiltration (Fig. 4) as we have previously reported in murine wound repair(Jackson-Boeters et al. 2009). In healthy and psoriatic skin periostin protein was predominantly deposited along the DEJ, but in skin from atopic dermatitis, periostin was detected throughout the entire dermis (Fig. 5).
Fig. 4.
Immunohistochemical detection of periostin in hypertrophic, keloid and prelesional skin pathologies. Healthy (n = 6), hypertrophic and keloid scars (n = 5) and chronically inflamed tissue (n = 3) were harvested during routine surgeries and were labeled with antibodies to periostin. In healthy skin, periostin immunoreactivity (brown staining) is evident in keratinocytes, dermal fibroblasts and the basal lamina. Periostin immunoreactivity was highest in hypertrophic and keloid scars, where it often localized with collagen (arrows). Periostin labeling was significantly reduced in chronically inflamed pre-lesional skin, strikingly absent from the basal lamina. In hypertrophic scars, periostin was associated with large collagen bundles, but in keloid scars, periostin localized to acellular node-like structures in the deep dermis. In chronically inflamed tissue, periostin was largely absent from the ECM particularly in areas associated with high inflammatory infiltration. Inset shows primary delete control
Fig. 5.
Immunohistochemical detection of periostin in human skin samples. Periostin was detected in healthy skin (a), skin of psoriasis (b) and skin of atopic dermatitis (c) skin biopsy samples. In healthy skin (a) and skin of psoriasis (b) patients, the periostin protein is predominantly deposited along the DEJ. However, in skin from atopic dermatitis (c) patients, the deposition is enhanced and more widely distributed with whole the dermis labeled positive. Note the epidermal thickness is significantly increased in both types of skin disease compared to healthy skin. The two-headed arrows denote the epidermal thickness and the arrows point to the DEJ. Bar = 200 µm
Periostin is a TGF-β inducible gene in human dermal fibroblasts
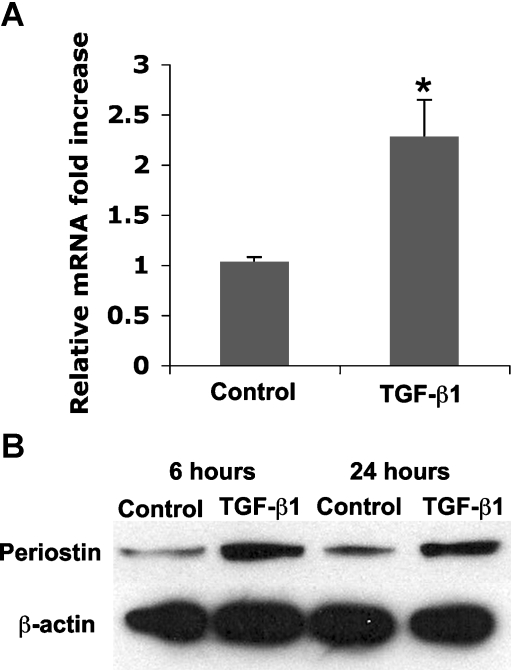
Fibrosis is often associated with elevated TGF-β levels and periostin is known to be regulated by TGF-β in osteoblasts (Horiuchi et al. 1999), periodontal ligament fibroblasts (Rios et al. 2008) and cardiac valve precursor cells (Norris et al. 2009). We therefore assessed whether periostin is regulated by TGF-β in human dermal fibroblasts. Human dermal fibroblasts were cultured for 6 and 24 h in the presence of 10 µM TGF-β. Periostin mRNA levels were elevated 2-fold 6 h post stimulation (Fig. 6a), and protein levels were elevated at 6 and 24 h post stimulation (Fig. 6b).
Fig. 6.
Influence of TGF-β1 on periostin expression in dermal fibroblasts. a Realtime PCR with specific primers to periostin were used to quantify mRNA levels 6 h post stimulation, p < 0.05. Alterations in mRNA levels were assessed at the protein levels using Western blotting with antibodies specific to periostin, with β-actin as a control. Note that periostin protein levels still remain elevated in comparison with controls at 24 h post stimulation
Discussion
Skin, the largest organ of the body, provides a physical and immunological barrier against pathogens, regulates body temperature and water loss, and mediates endocrine and sensory functions (Boulais and Misery 2008; Loewenthal 1963; Loewenthal 1964). The composition of the ECM is an important determinant of skin homeostasis, and alterations of the ECM constituents can result in abnormal tissue function (Li et al. 2007; Shibusawa et al. 2008). We have recently confirmed that periostin is expressed in human and murine skin (Jackson-Boeters et al. 2009), and periostin knockout mice display a reduction in dermal thickness (Norris et al. 2007). As these findings suggest that periostin is required for normal skin development and homeostasis, we first investigated the spatiotemporal expression of periostin during development and after incisional wound healing.
Using immunohistochemistry, we identified that periostin localizes to the dermis, basement membrane and hair follicles during embryonic development, but is downregulated in all structures except hair follicles in postnatal development. This suggests that periostin may mediate initial development of the dermis and basement membrane. As highlighted, periostin has already been implicated in fibroblast to myofibroblast differentiation in cardiac development (Norris et al. 2009), but our data also implicates periostin in basement membrane and basal keratinocyte development. Periostin has been heavily implicated in epithelial to mesenchymal transition (Kanno et al. 2008; Ruan et al. 2009; Yan and Shao 2006), but our studies suggests that it may influence epithelial behaviour without inducing fibroblast differentiation. It will be of great interest to assess the influence of periostin on keratinocyte behaviour in vitro, as well as assessing where periostin localizes within the basement membrane in vivo.
As periostin is heavily implicated in ECM remodeling, we investigated the expression of periostin after incisional wounding. Periostin was re-expressed in the BM at the wound edges, which correlated with increased keratinocyte proliferation. As keratinocyte migration is required for re-epithelialization of the wound, this suggests that periostin may trigger this process. Alternatively, as matricellular proteins are known to stimulate an intermediate state of cell adhesion associated with increased migration, periostin may act in this capacity stimulating keratinocyte migration into the wound area. Previous studies have shown that periostin is a potent inducer of cell migration in C3H10T1/2 cells (Lindner et al. 2005), epithelial ovarian carcinoma (Gillan et al. 2002), and vascular smooth muscle cells (Li et al. 2009). Interestingly, periostin is not upregulated in the dermis after incisional wounding, particularly when compared to excisional wounding where it is abundant at 3 and 7 days (Jackson-Boeters et al. 2009). This suggests that periostin expression may not be required unless severe trauma occurs to the dermis. Future studies will focus on whether the incisional and excisional wound repair process is altered in periostin null mice.
During wound repair and certain pathologies, changes occur in the composition of the matricellular ECM to provide cell–matrix signals and misregulation of the changes can result in development of various pathologies (Midwood, Valenick et al. 2004; Berk, Fujiwara et al. 2007; Darby and Hewitson 2007). Periostin is prominently expressed during pathological ECM remodeling, including in heart tissue after myocardial infarction (Kuhn et al. 2007; Shimazaki et al. 2008), asthma-associated sub-epithelial fibrosis in lungs (Takayama et al. 2006), and pulmonary vascular remodeling (Chen et al. 2006). We have now confirmed that periostin protein is abundant in fibrotic scars, but not in chronically inflammed skin. Interestingly we have previously shown in excisional wounds in mice that periostin expression is associated with myofibroblasts, but not CD68 positive inflammatory cells (Jackson-Boeters et al. 2009). It is of particular interest that both keloid and hypertrophic scars contain abundant periostin because the structure of the scars is very different: thick collagen bundles are more abundant in hypertrophic scars often forming acellular nodes in the deep dermis (Figs. 4, 5) In contrast, the centre of the keloid lesion has relatively few cells. It will be of significant interest to assess the cellular source of periostin in each scar type using ISH.
We have also validated previous microarray data that reported periostin expression levels (along with 17 other genes including the matricellular protein tenascin-C) are all elevated in atopic dermatitis relative to psoriasis (Nomura et al. 2003). Though acquired periostin deposition has been noted in bronchial asthma (Takayama et al. 2006), the elevated expression levels and altered presentation patterns of periostin within atopic dermatitis are not sufficient to suggest a causal relationship between elevated levels of periostin and atopic dermatitis. Given that we have shown that periostin is a TGF-β1-responsive gene in dermal fibroblasts (Fig. 6), the elevation of periostin expression in atopic dermatitis may simply reflect disturbance of TGF-β signaling that is thought to suppress inflammatory responses in the skin (Sumiyoshi et al. 2002). Nevertheless, periostin expression may serve as a valuable diagnosis marker to differentiate atopic dermatitis from other skin lesions.
In summary, our study has established periostin as a novel dynamic ECM component associated with skin morphogenesis, homeostasis and wound healing. Our data suggests that periostin may exert effects over cells in the epidermis, dermis as well as within the hair follicles at different stages of development. The re-establishment of periostin expression in the basement membrane during wounding further suggests that periostin is an important regulator of keratinocyte biology. While the exact functions of periostin in development of mouse skin and the mechanism governing the dynamic protein deposition remains elusive, the periostin null mice will be of great value to validate the postulated functions of periostin during organogenesis, wound healing and development.
Acknowledgments
We thank Prof. Jeffery B. Travers (Departments of Dermatology and Pediatrics, IUPUI) for providing the fixed skin biopsy specimens and Linda Jackson-Boeters (Department of Pathology, UWO) for advice on histological analysis. This work was supported, in part, by the National Institutes of Health (S.J.C), the IU Department of Pediatrics (Cardiology) and Riley Children’s Foundation (H-M.Z), Natural Sciences and Engineering Research Council (C.E) and the Canadian Institutes of Health Research IMHA operating grants (D.W.H).
Conflict of interest None
Abbreviations
- BM
Basement membrane
- DEJ
Dermal-epidermal junction
- E
Embryonic
- ECM
Extracellular matrix
- HF
Hair follicle
- ISH
In situ hybridization
- P
Postnatal
- SB
Subcutaneous tissue
- TGF-β
Transforming growth factor beta
Contributor Information
Douglas W. Hamilton, Email: Douglas.Hamilton@schulich.uwo.ca
Simon J. Conway, Email: siconway@iupui.edu
References
- Berk BC, Fujiwara K, Lehoux S. ECM remodeling in hypertensive heart disease. J Clin Invest. 2007;117(3):568–575. doi: 10.1172/JCI31044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein P, Sage EH. Matricellular proteins: extracellular modulators of cell function. Curr Opin Cell Biol. 2002;14:608–616. doi: 10.1016/S0955-0674(02)00361-7. [DOI] [PubMed] [Google Scholar]
- Boulais N, Misery L. The epidermis: a sensory tissue. Eur J Dermatol. 2008;18:119–127. doi: 10.1684/ejd.2008.0348. [DOI] [PubMed] [Google Scholar]
- Butcher JT, Norris RA, Hoffman S, Mjaatvedt CH, Markwald RR. Periostin promotes atrioventricular mesenchyme matrix invasion and remodeling mediated by integrin signaling through Rho/PI 3-kinase. Dev Biol. 2007;302:256–266. doi: 10.1016/j.ydbio.2006.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YF, Feng JA, Li P, Xing D, Ambalavanan N, Oparil S. Atrial natriuretic peptide-dependent modulation of hypoxia-induced pulmonary vascular remodeling. Life Sci. 2006;79:1357–1365. doi: 10.1016/j.lfs.2006.03.051. [DOI] [PubMed] [Google Scholar]
- Chen Y, Leask A, Abraham DJ, Pala D, Shiwen X, Khan K, Liu S, Carter DE, Wilcox-Adelman S, Goetinck P, et al. Heparan sulfate-dependent ERK activation contributes to the overexpression of fibrotic proteins and enhanced contraction by scleroderma fibroblasts. Arthritis Rheum. 2008;58:577–585. doi: 10.1002/art.23146. [DOI] [PubMed] [Google Scholar]
- Conway SJ, Molkentin JD. Periostin as a heterofunctional regulator of cardiac development and disease. Curr Genomics. 2008;9:548–555. doi: 10.2174/138920208786847917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darby IA, Hewitson TD. Fibroblast differentiation in wound healing and fibrosis. Int Rev Cytol. 2007;257:143–179. doi: 10.1016/S0074-7696(07)57004-X. [DOI] [PubMed] [Google Scholar]
- Gillan L, Matei D, Fishman DA, Gerbin CS, Karlan BY, Chang DD. Periostin secreted by epithelial ovarian carcinoma is a ligand for alpha(V)beta(3) and alpha(V)beta(5) integrins and promotes cell motility. Cancer Res. 2002;62:5358–5364. [PubMed] [Google Scholar]
- Hamilton DW. Functional role of periostin in development and wound repair: implications for connective tissue disease. J Cell Commun Signal. 2008;2:9–17. doi: 10.1007/s12079-008-0023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton DW, Chehroudi B, Brunette DM. Comparative response of epithelial cells and osteoblasts to microfabricated tapered pit topographies in vitro and in vivo. Biomaterials. 2007;28:2281–2293. doi: 10.1016/j.biomaterials.2007.01.026. [DOI] [PubMed] [Google Scholar]
- Horiuchi K, Amizuka N, Takeshita S, Takamatsu H, Katsuura M, Ozawa H, Toyama Y, Bonewald LF, Kudo A. Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta. J Bone Miner Res. 1999;14:1239–1249. doi: 10.1359/jbmr.1999.14.7.1239. [DOI] [PubMed] [Google Scholar]
- Jackson-Boeters L, Wen W, Hamilton DW. Periostin localizes to cells in normal skin, but is associated with the extracellular matrix during wound repair. J Cell Commun Signal. 2009;3:125–133. doi: 10.1007/s12079-009-0057-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno A, Satoh K, Masamune A, Hirota M, Kimura K, Umino J, Hamada S, Satoh A, Egawa S, Motoi F, et al. Periostin, secreted from stromal cells, has biphasic effect on cell migration and correlates with the epithelial to mesenchymal transition of human pancreatic cancer cells. Int J Cancer. 2008;122:2707–2718. doi: 10.1002/ijc.23332. [DOI] [PubMed] [Google Scholar]
- Kokubu E, Hamilton DW, Inoue T, Brunette DM. Modulation of human gingival fibroblast adhesion, morphology, tyrosine phosphorylation, and ERK 1/2 localization on polished, grooved and SLA substratum topographies. J Biomed Mater Res A. 2009;91:663–670. doi: 10.1002/jbm.a.32273. [DOI] [PubMed] [Google Scholar]
- Kruzynska-Frejtag A, Wang J, Maeda M, Rogers R, Krug E, Hoffman S, Markwald RR, Conway SJ. Periostin is expressed within the developing teeth at the sites of epithelial-mesenchymal interaction. Dev Dyn. 2004;229(4):857–868. doi: 10.1002/dvdy.10453. [DOI] [PubMed] [Google Scholar]
- Kuhn B, Monte F, Hajjar RJ, Chang YS, Lebeche D, Arab S, Keating MT. Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair. Nat Med. 2007;13:962–969. doi: 10.1038/nm1619. [DOI] [PubMed] [Google Scholar]
- Li J, Chen J, Kirsner R. Pathophysiology of acute wound healing. Clin Dermatol. 2007;25:9–18. doi: 10.1016/j.clindermatol.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Li G, Jin R, Norris RA, Zhang L, Yu S, Wu F, Markwald RR, Nanda A, Conway SJ, Smyth SS et al (2009) Periostin mediates vascular smooth muscle cell migration through the integrins alphanubeta3 and alphanubeta5 and focal adhesion kinase (FAK) pathway. Atherosclerosis [DOI] [PMC free article] [PubMed]
- Lie-Venema H, Eralp I, Markwald RR, van den Akker NM, Wijffels MC, Kolditz DP, van der Laarse A, Schalij MJ, Poelmann RE, Bogers AJ et al (2008) Periostin expression by epicardium-derived cells is involved in the development of the atrioventricular valves and fibrous heart skeleton. Differentiation [DOI] [PubMed]
- Lindner V, Wang Q, Conley BA, Friesel RE, Vary CP. Vascular injury induces expression of periostin: implications for vascular cell differentiation and migration. Arterioscler Thromb Vasc Biol. 2005;25:77–83. doi: 10.1161/01.ATV.0000149141.81230.c6. [DOI] [PubMed] [Google Scholar]
- Lindsley A, Snider P, Zhou H, Rogers R, Wang J, Olaopa M, Kruzynska-Frejtag A, Koushik SV, Lilly B, Burch JB, Firulli AB, Conway SJ. Identification and characterization of a novel Schwann and outflow tract endocardial cushion lineage-restricted periostin enhancer. Dev Biol. 2007;307(2):340–355. doi: 10.1016/j.ydbio.2007.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Kapoor M, Shi-wen X, Kennedy L, Denton CP, Glogauer M, Abraham DJ, Leask A. Role of Rac1 in a bleomycin-induced scleroderma model using fibroblast-specific Rac1-knockout mice. Arthritis Rheum. 2008;58:2189–2195. doi: 10.1002/art.23595. [DOI] [PubMed] [Google Scholar]
- Loewenthal LJ. Anatomy and histochemistry of Skin L961. Dermatologica. 1963;126:380–387. doi: 10.1159/000254939. [DOI] [PubMed] [Google Scholar]
- Loewenthal LJ. Anatomy and histochemistry of Skin 1962. Dermatologica. 1964;129:507–515. doi: 10.1159/000254672. [DOI] [PubMed] [Google Scholar]
- Midwood KS, Williams LV, Schwarzbauer JE. Tissue repair and the dynamics of the extracellular matrix. Int J Biochem Cell Biol. 2004;36(6):1031–1037. doi: 10.1016/j.biocel.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Nomura I, Gao B, Boguniewicz M, Darst MA, Travers JB, Leung DY. Distinct patterns of gene expression in the skin lesions of atopic dermatitis and psoriasis: a gene microarray analysis. J Allergy Clin Immunol. 2003;112:1195–1202. doi: 10.1016/j.jaci.2003.08.049. [DOI] [PubMed] [Google Scholar]
- Norris RA, Damon B, Mironov V, Kasyanov V, Ramamurthi A, Moreno-Rodriguez R, Trusk T, Potts JD, Goodwin RL, Davis J, et al. Periostin regulates collagen fibrillogenesis and the biomechanical properties of connective tissues. J Cell Biochem. 2007;101:695–711. doi: 10.1002/jcb.21224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris RA, Borg TK, Butcher JT, Baudino TA, Banerjee I, Markwald RR. Neonatal and adult cardiovascular pathophysiological remodeling and repair: developmental role of periostin. Ann N Y Acad Sci. 2008;1123:30–40. doi: 10.1196/annals.1420.005. [DOI] [PubMed] [Google Scholar]
- Norris RA, Moreno-Rodriguez RA, Sugi Y, Hoffman S, Amos J, Hart MM, Potts JD, Goodwin RL, Markwald RR. Periostin regulates atrioventricular valve maturation. Dev Biol. 2008;316:200–213. doi: 10.1016/j.ydbio.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris RA, Potts JD, Yost MJ, Junor L, Brooks T, Tan H, Hoffman S, Hart MM, Kern MJ, Damon B et al (2009) Periostin promotes a fibroblastic lineage pathway in atrioventricular valve progenitor cells. Dev Dyn [DOI] [PMC free article] [PubMed]
- Rios HF, Ma D, Xie Y, Giannobile WV, Bonewald LF, Conway SJ, Feng JQ. Periostin is essential for the integrity and function of the periodontal ligament during occlusal loading in mice. J Periodontol. 2008;79:1480–1490. doi: 10.1902/jop.2008.070624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan K, Bao S, Ouyang G (2009) The multifaceted role of periostin in tumorigenesis. Cell Mol Life Sci [DOI] [PMC free article] [PubMed]
- Shibusawa Y, Negishi I, Tabata Y, Ishikawa O. Mouse model of dermal fibrosis induced by one-time injection of bleomycin-poly(L-lactic acid) microspheres. Rheumatology (Oxford) 2008;47:454–457. doi: 10.1093/rheumatology/ken058. [DOI] [PubMed] [Google Scholar]
- Shimazaki M, Nakamura K, Kii I, Kashima T, Amizuka N, Li M, Saito M, Fukuda K, Nishiyama T, Kitajima S et al (2008) Periostin is essential for cardiac healing after acute myocardial infarction. J Exp Med [DOI] [PMC free article] [PubMed]
- Snider P, Hinton RB, Moreno-Rodriguez RA, Wang J, Rogers R, Lindsley A, Li F, Ingram DA, Menick D, Field L, et al. Periostin is required for maturation and extracellular matrix stabilization of noncardiomyocyte lineages of the heart. Circ Res. 2008;102:752–760. doi: 10.1161/CIRCRESAHA.107.159517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumiyoshi K, Nakao A, Ushio H, Mitsuishi K, Okumura K, Tsuboi R, Ra C, Ogawa H. Transforming growth factor-beta1 suppresses atopic dermatitis-like skin lesions in NC/Nga mice. Clin Exp Allergy. 2002;32:309–314. doi: 10.1046/j.1365-2222.2002.01221.x. [DOI] [PubMed] [Google Scholar]
- Takayama G, Arima K, Kanaji T, Toda S, Tanaka H, Shoji S, McKenzie AN, Nagai H, Hotokebuchi T, Izuhara K. Periostin: a novel component of subepithelial fibrosis of bronchial asthma downstream of IL-4 and IL-13 signals. J Allergy Clin Immunol. 2006;118:98–104. doi: 10.1016/j.jaci.2006.02.046. [DOI] [PubMed] [Google Scholar]
- Takeshita S, Kikuno R, Tezuka K, Amann E. Osteoblast-specific factor 2: cloning of a putative bone adhesion protein with homology with the insect protein fasciclin I. Biochem J. 1993;294(Pt 1):271–278. doi: 10.1042/bj2940271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W, Shao R. Transduction of a mesenchyme-specific gene periostin into 293T cells induces cell invasive activity through epithelial-mesenchymal transformation. J Biol Chem. 2006;281:19700–19708. doi: 10.1074/jbc.M601856200. [DOI] [PubMed] [Google Scholar]