Abstract
Podocytes play a key role in maintaining and modulating the filtration barrier of the glomerulus. Because of their location, podocytes are exposed to mechanical strain in the form of fluid flow shear stress (FFSS). Several human diseases are characterized by glomerular hyperfiltration, such as diabetes mellitus and hypertension. The response of podocytes to FFSS at physiological or pathological levels is not known. We exposed cultured podocytes to FFSS, and studied changes in actin cytoskeleton, prostaglandin E2 (PGE2) production and expression of cyclooxygenase-1 and–2 (COX-1, COX-2). FFSS caused a reduction in transversal F-actin stress filaments and the appearance of cortical actin network in the early recovery period. Cells exhibited a pattern similar to control state by 24 h following FFSS without significant loss of podocytes or apoptosis. FFSS caused increased levels of PGE2 as early as 30 min after onset of shear stress, levels that increased over time. PGE2 production by podocytes at post-2 h and post-24 h was also significantly increased compared to control cells (p < 0.039 and 0.012, respectively). Intracellular PGE2 synthesis and expression of COX-2 was increased at post-2 h following FFSS. The expression of COX-1 mRNA was unchanged. We conclude that podocytes are sensitive and responsive to FFSS, exhibiting morphological and physiological changes. We believe that PGE2 plays an important role in mechanoperception in podocytes.
Keywords: Prostaglandin E2, Cyclooxygenase, Actin, Mechanical strain, Shear stress
Introduction
The glomerular filtration barrier is made up of fenestrated endothelial cells, glomerular basement membrane (GBM), and podocytes through which ultrafiltrate passes from capillary into the urinary space (Rodewald and Karnovsky 1974). Podocytes are believed to play an important role in modulating ultrafiltration, stabilizing glomerular capillaries and in preventing loss of proteins into the urine. Podocytes under normal conditions are exposed to mechanical forces arising from both glomerular capillary pressure and glomerular ultrafiltration (Endlich and Endlich, 2006). Podocytes possess a dynamic actin cytoskeleton and are thus uniquely situated to provide immediate response to mechanical strain in the glomerulus. Podocytes have been shown to respond to mechanical strain (Durvasula et al. 2004; Endlich et al. 2001; Friedrich et al. 2006). Response of podocytes to mechanical forces arising from capillary distention due to glomerular capillary hydrostatic pressure has been studied using the in vitro model of substrate stretch. Podocytes respond to substrate stretch with an increase in radial stress filaments and actin-rich centers, as well as narrowing of the cell bodies and elongation of cytoplasmic processes (Durvasula et al. 2004; Endlich et al. 2001). In addition to mechanical strain resulting from intracapillary hydrostatic pressure, podocytes are exposed to fluid flow shear stress (FFSS) resulting from flow of ultrafiltrate through the filtration slits and over the apical surface of podocytes. Podocytes in culture respond to FFSS with a reduction in transversal F-actin stress filaments, formation of a cortical actin network and disruption of the cell monolayer (Friedrich et al. 2006).
Several pathways have been shown to be involved in sensing and responding to mechanical strain in other cell types. Examples of such mediators implicated in early response to mechanical strain include nitric oxide (NO) (Klein-Nulend et al. 1995; Johnson et al. 1996; Zaman et al. 1999; McAllister et al. 2000), Ca2+ (Jorgensen et al. 1997; You et al. 2002; Jorgensen et al. 2002; Jorgensen et al. 2003; You et al. 2001) and PGE2 (Wadhwa et al. 2002; Jee et al. 1985; Klein-Nulend et al. 1997; Forwood 1996; Ajubi et al. 1996, 1997). The signaling events involved in mechanoperception and response to FFSS in podocytes are not known.
Eicosanoids of the cyclooxygenase (COX) pathway such as PGE2 are synthesized by the glomerulus and act in an autocrine fashion to modulate glomerular function (Breyer et al. 1996). PGE2 regulates glomerular filtration rate (GFR) and can modify the permselectivity of the glomerular filtration barrier (Breyer and Breyer 2000; Schlondorff 1986; Kömhoff et al. 1997; McCarthy and Sharma 2002; Sharma et al. 2006). Podocytes express both COX-1 and COX-2 (Kömhoff et al. 1997; Cheng et al. 2007). We hypothesize that PGE2 plays a role in the response of cultured podocytes to mechanical strain in the form of FFSS. In our current study, we confirmed that podocytes are sensitive to FFSS by demonstrating changes in podocyte cell morphology and F-actin cytoskeleton and showed that PGE2 plays a role in early response to FFSS.
Material and methods
Podocyte culture
The well-characterized conditionally immortalized mouse podocyte line (a kind gift from Dr. Peter Mundel) with thermosensitive tsA58 mutant T-antigen was used in these studies (Mundel et al. 1997; Shankland et al. 2007). We followed the culture protocol as outlined in literature and did not study cultured cells beyond 15 passages (Shankland et al. 2007). In brief, podocytes were maintained in RPMI 1640 with L-glutamine supplemented with 10% fetal bovine serum, 100 units/ml penicillin and 0.1 mg/ml streptomycin (Invitrogen, Carlsbad, CA). To propagate podocytes, cells were grown on collagen coated (BD Biosciences, San Diego, CA) tissue culture flasks or glass slides under permissive conditions, i.e. at 33°C with 10 units/ml of mouse γ-interferon (Cell Sciences, Norwood, MA). To induce differentiation, cells were grown under non-permissive conditions, i.e. at 37°C without mouse γ-interferon. Podocytes were grown under non-permissive conditions on 25 × 75 × 1 mm glass slides (Fischer Scientific, Pittsburg, PA) with 3 glass slides in each culture dish containing 12 ml of culture media. Differentiated podocytes on day 14 were used for FFSS experiments.
Application of fluid flow shear stress
FFSS was applied to differentiated podocytes using a FlexCell Streamer Gold apparatus (Flexcell International, Hillsborough, NC). The apparatus was sterilized with 300 ml of 70% ethanol for 30 min, and checked for leaks. This was followed by two wash steps with 300–400 ml of sterilized PBS for 5 min each. The PBS was then replaced by 350 ml of media. The flow device chamber was then moved into a sterile hood and using sterilized forceps, glass slides with podocytes were placed in each one of the slots of the flow device chamber (Fig. 1). All six slots were filled to ensure consistent flow. The chamber was then replaced in the incubator at 37°C with 5% CO2. The computer was programmed to apply FFSS at a pre-determined level (dynes/cm2) for a pre-determined period of time according to experimental design. We applied FFSS (1–16 dynes/cm2) for 30–120 min to podocytes on a FlexCell Streamer Gold in our pilot studies, and chose to use 2 dynes/cm2 (or 75 ml/min) for 2 h as our FFSS model based on these preliminary studies. A 2 h of FFSS was selected based on our earlier published experience with osteocyte cell line (Zhang et al. 2006; Cheng et al. 2001). In some experiments a 3-way stop-cock was attached to collect 1–1.5 ml of media during FFSS. Control podocytes were grown on glass slides and placed in the same hood and incubator as experimental cells but were not exposed to FFSS. At the end of FFSS treatment, the fluid device chamber was moved to the sterile hood and was opened to remove the slides for processing. The glass slides were then placed back in the original medium after completion of FFSS, and podocytes were allowed to recover for 24 h (Fig. 1). The study samples obtained prior to onset of FFFSS was termed pre-FFSS, 2 h and 24 h following cessation of FFSS as post-2 h and post-24 h respectively.
Fig. 1.
The figure on the left shows the arrangement of apparatus used to apply fluid flow shear stress (FFSS) to podocytes. It shows the ‘fluid device chamber’ in which six glass slides containing podocytes are placed for FFSS studies, and the 3-way stop cock attachment to collect media during application of FFSS. The figure on the right provides the experiment design for collection of culture media at 3 different time points
Assessment of morphology and actin cytoskeleton
Podocytes were washed twice with PBS, fixed with 2% glutaraldehyde for 10 min and then stained with 0.1% crystal violet for 20 min for assessment of cell morphology. In separate experiments, immunostaining for F-actin was performed after washing with PBS and fixing with 4% paraformaldehyde, using phalloidin tagged to Alexa Fluor 568 dye (Invitrogen, Carlsbad, CA), which binds to F-actin filaments but not to monomeric G-actin. The images were taken on fluorescence microscope Olympus BX60 (Hamburg, Germany). Finally, podocytes were incubated with PGE2 (0–20 µM for 2 and 24 h) and stained with crystal violet for cell morphology and phalloidin for F-actin. The images were obtained from 10–20 random fields by an unbiased observer. We also incubated podocytes with indomethacin prior to (2.5 µM × 1 h) and during (2.5 µM) FFSS, and stained for F-actin with phalloidin and cell morphology with crystal violet.
TUNEL assay for apoptosis
Podocytes exposed to FFSS and control podocytes were assessed for apoptosis by TUNEL assay using a commercial kit (Cell Death Detection Kit, Roche, Tucson, AZ). Cells were studied at post-2 h and post-24 h FFSS along with time-paired controls. The TUNEL assay was performed directly on podocytes on glass slides and on cells following cytospin preparation.
Media collection and preparation of cell lysate
We have previously used a dye diffusion assay to determine that the equilibration time, i.e. the time taken for dye placed at the inlet side of the slide chamber to become fully mixed within the circulating media is approximately 2 min at a flow rate of 16 dynes/cm2 and 5–6 min at a flow rate of 2 dynes/cm2. We collected 1.0–1.5 ml aliquots of medium from the continuously circulating 350 ml medium during application of FFSS at 0, 30 and 120 min using a 3-way stop-cock (Fig. 1a). We also collected an aliquot of 1 ml media from 12 ml of culture media immediately before application of FFSS (pre-FFSS that had been changed 24 h prior to application of FFSS. Once the glass slides were returned to their original culture dish after FFSS, we collected an aliquot of 1 ml 2 h after cessation of FFSS (post-2 h FFSS) and the remaining 10 ml 24 h after cessation of FFSS (post-24 h FFSS, Fig. 1b). Culture media were collected from control podocytes at same time points. Because PGE2 concentration was very low given the large volume (350 ml) of medium used during FFSS, it was concentrated using a PGE2 affinity column (#414018, Cayman Chemical, Ann Arbor, MI). We used a 7-fold concentration, i.e. 840 μl of medium was run over the affinity column and PGE2 was eluted out in 120 μl in our column extraction method. Thus we could express the measured PGE2 as an absolute amount of PGE2 and as change from baseline/control as we had kept the media volume fixed at 12 ml for culture dishes containing 3 slides, 350 ml for FlexCell Streamer Gold containing 6 slides, and PGE2 affinity column extraction fixed at a 7-fold concentration change.
Podocytes were washed with PBS following application of FFSS and lysed with 100 μl of 1% Triton in PBS for measurement of intracellular PGE2. Measured PGE2 was corrected for DNA content of lysate, and intracellular PGE2 was expressed as pg PGE2/μg DNA.
Measurement of PGE2
PGE2 was measured using PGE2 EIA kit (#514010, Cayman Chemical) in (a) medium obtained from the FlexCell apparatus during FFSS, (b) culture medium after application of FFSS, and (c) cell lysate. This kit measures free PGE2 in media and cell lysate and has a very low cross-reactivity (0.02%) with the major PGE2 metabolite (13,14-dihydro-15-keto PGE2). PGE2 accumulates in culture media and undergoes non-enzymatic oxidation while intracellular PGE2 undergoes enzymatic degradation as per manufacturer. To further confirm this we analyzed the media for 13,14-dihydro-15-keto PGE2 using PGE2 Metabolite EIA kit (#514531, Cayman Chemical) that in turn has very low cross-reactivity (<0.01%) with PGE2. The detection limit of the PGE2 and PGE2 Metabolite EIA kits are 15 pg/ml and 2 pg/ml respectively. PGE2 and PGE2 metabolite levels were measured for each condition from 5 separate experiments.
mRNA extraction and RT-PCR
Total RNA was isolated from podocytes by homogenization in 1 ml Trizol Reagent (Life Technologies, Gaithersburg, MD) following the manufacturer’s protocol. Isolated total RNA was subjected to DNase digestion and cleanup using RNeasy Mini Kit (Qiagen, Valencia, CA) per manufacturer’s instructions. RNA 100 ng was reverse transcribed with 50 µM Oligo (dT)20 using SuperScript III First-Strand Synthesis SuperMix (Invitrogen, Carlsbad, CA). PCR reactions were carried out by standard technique using 2 µl of the RT reaction (20 ng cDNA), and 22 U/ml complexed recombinant Taq DNA Polymerase, Pyrococcus species GB-D thermostable polymerase, and Platinum Taq Antibody; 66 mM Tris-SO4 (pH 8.9); 19.8 mM (NH4)2SO4; 2.4 mM MgSO4; 220 µM dNTPs; and stabilizers in Platinum PCR SuperMix High Fidelity (Invitrogen) under the following conditions: denaturation at 94°C for 30 s, annealing at 52°C, and extension at 72°C for 30 s (35 cycles) after the initial denaturation at 94°C for 2 min and with a final extension at 72°C. The amplification products of 10 µl of each PCR reaction were separated on a 2% NuSeive 3:1 agarose gel, stained with SYBR safe DNA gel stain, and visualized by ultraviolet irradiation. The primer sequences used were: COX-1—forward 5′-GGT CCT GCT CGC AGA TCC TG-3’, reverse 5’-AGG ACC CAT CTT TCC AGA GG-3’, product size 580 bp; COX-2—forward 5’-CTG TAC AAG CAG TGG CAA-3’, reverse 5’-TTA CAG CTC AGT TGA ACG CCT-3’, product size 530 bp; β-Actin—forward 5'-ACC AAC TGG GAC GAC ATG GAG-3', reverse 5'-GTC AGG ATC TTC ATG AGG TAG TC-3', product size 380 bp.
Statistics
Data were analyzed with repeated measures ANOVA analyses using the SPSS 16.0 statistical software for group and time comparison. A p value <0.05 was considered significant.
Results
Fluid flow shear stress alters actin cytoskeleton of podocytes
Podocytes were exposed to FFSS at 2 dynes/cm2 for 2 h, and F-actin filaments stained at mid-point (60 min), at end of FFSS (120 min) and in the recovery period of post-2 h and post-24 h following cessation of mechanical strain. FFSS caused a reduction in transversal F-actin stress filaments and the appearance of a cortical actin network at mid-point (60 min), at end of FFSS (120 min) and in the early recovery period (post-2 h) following FFSS (Fig. 2). The F-actin filaments resumed a pattern that resembles control state by 24 h following FFSS (post-24 h). In order to further examine morphological changes that occur due to FFSS, podocytes were stained using crystal violet. Podocytes exhibited indistinct cellular margins and blunted cytoplasmic processes at 2 h post-FFSS compared to control cells (Fig. 3). The cells regained distinct cellular margins and elongated, elaborate processes by 24 h following cessation of FFSS, an appearance that closely resembled that of control cells. Our results indicate that FFSS causes alteration in podocyte morphology characterized by reorganization of F-actin filaments, and the observed changes were largely reversed by 24 h post-FFSS. We further incubated podocytes with indomethacin prior to (2.5 µM × 1 h) and during (2.5 µM) application of FFSS, and stained for F-actin and cell morphology. As done with FFSS without indomethacin, the glass slides were placed back in the original medium containing indomethacin after completion of FFSS, and podocytes were allowed to recover for 24 h (Fig. 1). We found inhibition of COX prevented changes in both actin cytoskeleton and cellular morphology caused by FFSS, Figs. 2 and 3.
Fig. 2.
The figure shows immunostaining for F-actin in control podocytes, and in podocytes exposed to FFSS at end of FFSS (120 min), during early recovery period following FFSS at 2 h (post-2 h FFSS) and 24 h (post-24 h FFSS) in the left column (400X). The right column shows podocytes pre-treated with indomethacin (2.5 μM for 1 h) prior to application of FFSS during early recovery period at 2 h (post-2 h FFSS) and 24 h (post-24 h FFSS) in the presence of indomethacin (200X). Indomethacin prevented FFSS-induced changes in F-actin
Fig. 3.
The figure shows control podocytes, and podocytes exposed to FFSS (upper panel) stained with crystal violet for cellular morphology during early recovery period following FFSS at 2 h (post-2 h FFSS) and 24 h (post-24 h FFSS). Podocytes show an indistinct cellular margin and blunted cytoplasmic processes compared to control cells at post-2 h following application of FFSS, changes that are resolving by post-24 h FFSS. The lower panel shows podocytes pre-treated with indomethacin (2.5 μM for 1 h) prior to application of FFSS during early recovery period at 2 h (post-2 h FFSS) and 24 h (post-24 h FFSS). Indomethacin prevented FFSS-induced changes in cell morphology in podocytes
Fluid flow shear stress is not associated with cell loss or apoptosis
Application of FFSS of 2 dynes/cm2 for 2 h did not result in significant cell loss (post-24 h FFSS 2.31 ± 0.45 × 105 cells/slide vs control 2.51 ± 0.55 × 105 cells/slide, p = 0.41). We conclude that cell loss following application of FFSS in these studies was minimal (8%). We then measured total DNA in cell lysate as an indirect estimate of cell number and again found no difference between experimental and control (FFSS 39.0 ± 5.4 µg DNA/slide post-2 h and 39.1 ± 8.3 µg DNA/slide post-24 h vs control 43.8 ± 6.2 µg DNA/slide, p = 0.08 and p = 0.18, respectively), FFSS did not affect apoptosis as measured by the TUNEL assay at either time point studied. When we studied cells grown on glass slides we found no difference in percentage of apoptotic podocytes in experimental and paired control groups (FFSS post-2 h 6.2 ± 5.3 vs control 3.7 ± 2.8, p = 0.10, and FFSS post-24 h 7.6 ± 3.8 vs control 6.3 ± 4.1, p = 0.38). We repeat the TUNEL assay on cytospin preparations of podocytes and again did not find significant difference in percentage of apoptotic podocytes (FFSS post-2 h 3.7 ± 2.1 vs control 2.9 ± 2.2, p = 0.31, and FFSS post-24 h 3.4 ± 1.9 vs control 3.8 ± 3.2, p = 0.70). We conclude that FFSS at 2 dynes/cm2 for 2 h is not associated with significant cell loss or apoptosis.
Fluid flow shear stress increases PGE2 in podocytes
We determined the effect of ongoing FFSS on podocyte secretion of PGE2 by studying aliquots of medium obtained before and during FFSS. FFSS caused significantly increased PGE2 levels in the bathing medium by 30 min after onset of mechanical strain and PGE2 levels progressively increased over time. PGE2 level at onset of FFSS was below the detection limit of the EIA kit. There was a significant change over time for measured PGE2 from baseline at 120 min (p < 0.05, Fig. 4). We conclude that FFSS leads to increased synthesis of PGE2 by cultured podocytes.
Fig. 4.
Prostaglandin E2 in media collected at time 0 min, 30 min and 120 min during application of fluid flow shear stress (FFSS) at 2 dynes/cm2 are shown as bar figure with ±1 SE and the absolute difference in prostaglandin E2 from 5 separate experiments are shown as a line figure (* denotes p < 0.05)
Podocytes continue to produce PGE2 during recovery following fluid flow shear stress
In order to determine the pattern of PGE2 secretion following cessation of FFSS, aliquots of medium were analyzed 2 and 24 h following treatment. Control consisted of aliquots obtained from culture medium of untreated podocytes (i.e. no FFSS) at 2 and 24 h. There was no difference in the amount of PGE2 in the culture media in the pre-FFSS samples between control podocytes (3,911 ± 1,567 pg) and podocytes that were subjected to FFSS (4,847 ± 2,361 pg, p = 0.48). PGE2 levels in the medium at both time points was significantly increased compared to control (post-2 h FFSS 6,340 ± 2,078 pg vs control 3,767 ± 1,056 pg, p = 0.039, and post-24 h FFSS 6,980 ± 2,606 pg vs control 2,756 ± 820 pg, p = 0.012, respectively). There was a trend for effect of group (p = 0.059) and time (p = 0.088), and there was significant interaction for group by time (p < 0.001).
When analyzed separately, PGE2 levels in the control group fell over time probably due to ongoing nonenzymatic degradation, though the levels were not significantly different from those at baseline (post-2 h p = 0.87 and post-24 h p = 0.10). Conversely, PGE2 levels following FFSS increased over time, and were significantly different at both post-2 h (p = 0.01) and post-24 h (p = 0.002) compared to baseline. The pattern of change was similar in all experiments studied (Fig. 5).
Fig. 5.
Prostaglandin E2 in culture media prior to FFSS, and following recovery at 2 and 24 h post application of FFSS from control and experimental podocytes are shown as bar figure with ±1 SE, and the absolute difference in prostaglandin E2 from 5 separate control and FFSS experiments are shown as line figures (* denotes p < 0.05)
The rate of PGE2 synthesis was increased following FFSS. The rate of PGE2 production between cessations of FFSS to post-2 h sample was significantly different from the rate of PGE2 production seen during the same time period for control podocytes (0 h to post-2 h FFSS 746.0 ± 281.3 pg/hr vs control–72.0 ± 305.4 pg/hr, (p = 0.03). Likewise, the rate of PGE2 synthesis from cessation of FFSS to post-24 h sample was significantly increased compared to the same period for control (0 h to post-24 h FFSS 88.9 ± 36.2 pg/hr vs control −48.1 ± 32.9 pg/hr, p = 0.007).
Secreted PGE2 does not undergo enzymatic degradation
We analyzed the media for PGE2 metabolite 13,14-dihydro-15-keto PGE2. There was no difference in the amount of PGE2 metabolite in the culture media between podocytes subjected to FFSS and control at any time point studied (pre-FFSS 531.2 ± 151.9 pg vs control 632.7 ± 210.4 pg, p = 0.41; post-2 h FFSS 559.9 ± 346.7 pg vs control 555.3 ± 171.1 pg, p = 0.98; post-24 h FFSS 646.9 ± 183.4 pg vs control 578.3 ± 152.2 pg, p = 0.54). Additionally, there was no effect of group (p = 0.93), time (p = 0.68) or group by time (p = 0.42). We conclude that secreted PGE2 found in culture medium does not undergo enzymatic degradation.
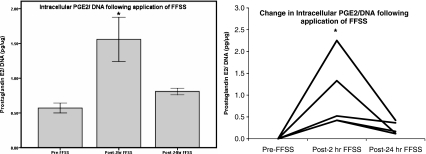
Intracellular levels of PGE2 increases in podocytes
We measured intracellular PGE2 in cell lysates and normalized to DNA levels. Intracellular PGE2 levels were significantly elevated following FFSS compared to control (i.e. pre-FFSS levels, 0.57 ± 0.16 pgPGE2/μgDNA) at post-2 h (1.56 ± 0.71 pgPGE2/μgDNA, p = 0.02), Fig. 6. Intracellular PGE2 levels were near baseline by post-24 h of FFSS (0.81 ± 0.11 pgPGE2/μgDNA, p = 0.69 compared to control). Unlike extracellular PGE2 intracellular PGE2 is subject to enzymatic degradation.
Fig. 6.
The measured intracellular PGE2/DNA and the change in PGE2/DNA from control podocytes following FFSS from 5 separate experiments are shown as a bar figure with ±1 SE and as a line figure respectively (* denotes p < 0.05)
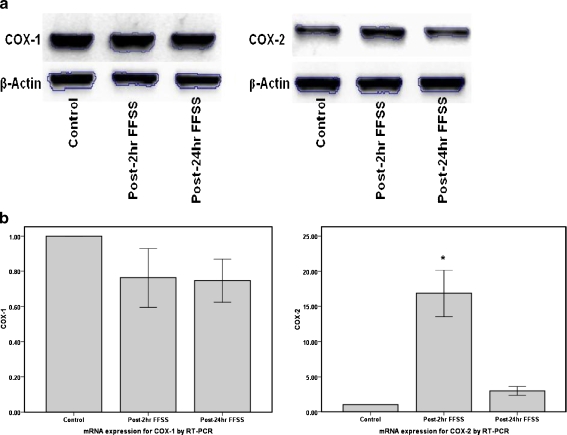
Fluid flow shear stress increases expression of COX-2
Increased intra–and extracellular levels of PGE2 may be due to increased expression of cyclooxygenase. RNA was extracted from podocytes at post-2 h or post-24 h following cessation of FFSS and expression of COX-1 and COX-2 were examined using reverse transcriptase PCR. COX-2 expression (n = 6) was significantly increased at post-2 h (16.84 ± 9.99, p < 0.001) and returned to near control levels by post-24 h (3.01 ± 1.87, p = 0.47) following FFSS, Fig. 7. FFSS did not affect COX-1 expression (n = 3). COX-1 expression was 0.76 ± 0.29 (p = 0.21) at post-2 h and 0.75 ± 0.21 (p = 0.18) at post-24 h following FFSS.
Fig. 7.
The figure shows the gene expression for COX-1 and COX-2 in podocytes following application of FFSS. a The gel figure shows an increase in COX-2 gene expression at post-2 h following application of FFSS that begins to resolve at post-24 h FFSS. COX-1 expression is unchanged with FFSS. b The bar graph denotes the change in gene expression for COX-1 (n = 3) and COX-2 (n = 6) on RT-PCR at post-2 h and post-24 h FFSS (* denotes p < 0.05)
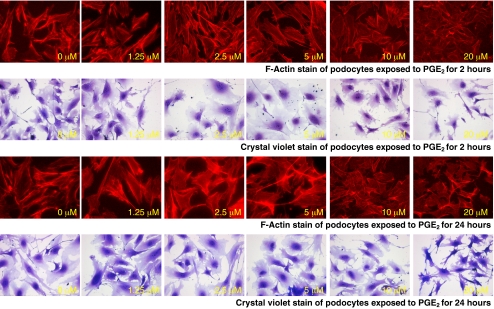
Exogenous PGE2 causes reorganization of actin cytoskeleton
It is possible that morphological changes seen in podocytes exposed to FFSS are related to some event other than the increase in PGE2 synthesis. To better understand if this is the case, we incubated podocytes with PGE2 (0–20 µM/ml) for 2 and 24 h. PGE2 (5 μM for 2 h) showed changes in F-actin stress filament in F-actin compared to control, Fig. 8. Changes in F-actin were marked at 10 μM and 20 μM, and persisted at 24 h. We then examined effect of PGE2 on cell morphology of experimental and control cells using crystal violet staining. PGE2 (5 μM for 2 h) caused blunting of cytoplasmic processes compared to control, Fig. 8. As before, morphological changes were marked at 10 μM and 20 μM, and persisted at 24 h. We could reproduce part of changes observed in podocytes with FFSS with direct exposure to PGE2. We conclude that PGE2 plays a role in the cytoskeletal changes in FFSS.
Fig. 8.
The figure shows changes in F-actin on phalloidin staining and cell morphology on crystal violet staining in podocytes following continuous treatment with PGE2 from 0–1.25–2.5–5–10–20 µM (from left to right) without FFSS for 2 h (upper two panels) and 24 h (lower two panels). The early discernable changes start to appear at 5 μM and are marked at 10 μM and 20 μM at both 2 h and 24 h
Discussion
We have demonstrated that podocytes in culture are responsive to mechanical strain in the form of FFSS. We have shown that podocytes respond by alterations in actin structure, in gene expression and eicosanoid synthesis. The location of podocytes in the glomerulus is such that under normal conditions it is exposed to mechanical forces arising from both intracapillary pressure and flow of filtrate over the cell surface (Endlich and Endlich 2006). Micropuncture studies in animals indicate that the transmural hydrostatic pressure gradient of the glomerular capillary is 40–50 mmHg (Arendshorst and Navare 1993). To ensure structural stability, these outwardly directed expansile forces from glomerular capillary pressure must be balanced by inwardly directed forces on the wall. The podocytes are believed to provide this counteracting tone by virtue of their contractile actin cytoskeleton. The effect of substrate stretch on podocytes and other cell types has been studied using instruments that cause biaxial elongation of cultured cells. Podocytes are mechanosensitive to substrate stretch, and exhibit an increase in radial stress filaments and actin-rich centers, and narrowing of the cell bodies with sharp and elongated cytoplasmic processes (Durvasula et al. 2004; Endlich et al. 2001; Martineau et al. 2004).
Flow of ultrafiltrate through the filtration slits and over the apical surface of podocytes exerts FFSS on podocytes. The shear stress to which podocytes are exposed in vivo has not been directly measured. The effect of FFSS on several non-podocyte cell types, most notably endothelial cells and osteocytes, has been studied using instruments that modulate flow of medium over the surface of cultured cells (Klein-Nulend et al. 1995; Johnson et al. 1996; Zaman et al. 1999; McAllister et al. 2000; Jorgensen et al. 1997; You et al. 2002; Jorgensen et al. 2002, 2003; You et al. 2001; Wadhwa et al. 2002; Jee et al. 1985; Klein-Nulend et al. 1997; Forwood 1996; Ajubi et al. 1996, 1997). In designing the present studies we turned to reports of the effect of FFSS on osteocytes as a starting point. Osteocytes share similarities with podocytes in that both cells types are terminally differentiated and possess elaborate cytoplasmic extensions. We have been among those investigators who propose that osteocytes sense and respond to mechanical strain in their environment (Cherian et al. 2005; Zhang et al. 2006; Cheng et al. 2001). There are numerous reports describing the effect of FFSS on osteocytes (Klein-Nulend et al. 1995). We and others have commonly used FFSS ranging from 4 to 16 dynes/cm2 for 1–2 h in in vitro studies to examine responsiveness of osteocytes to mechanical strain (Ajubi et al. 1997; Cherian et al. 2005; Zhang et al. 2006; Cheng et al. 2001; Bakker et al. 2006). We used FFSS at 2 dynes/cm2 for 2 h, and studied post-2 h and post-24 h FFSS time points based on results from our pilot studies with podocytes and our previous studies with osteocytes (Zhang et al. 2006; Cheng et al. 2001).
There are few previous reports of the effect of FFSS on podocytes. Investigators have used FFSS ranging from 0.015 to 649 dynes/cm2 (Friedrich et al. 2006; Dandapani et al. 2007; Huang and Miller 2007). Friedrich et al (2006) used a mathematical model to estimate that mouse podocytes are exposed to in vivo FFSS of 0.3 dyne/cm2. They demonstrated a reduction in transversal F-actin stress filaments, formation of a cortical actin network and disruption of the cell monolayer. We corroborated these results, and demonstrated a reduction in transversal F-actin stress filaments and development of a cortical actin network after 60 and 120 min of FFSS (data not shown). In contrast to the findings of these investigators, we did not see disruption of the cell monolayer or significant loss of cells with FFSS at 2 dynes/cm2. Explanations for these differences may include the shorter length of time that we exposed cells to FFSS (2 h vs 20 h). In order to substantiate our theory that PGE2 was responsible for changes seen following FFSS, we exposed normal podocytes to exogenous PGE2. We were able to reproduce the actin cytoskeleton and morphological changes that we had seen with FFSS.
We found the FFSS-induced changes in actin cytoskeleton persisted for at least 2 h following termination of mechanical strain, and actin had nearly resumed its baseline organization by 24 h following FFSS. In other words, the change in the podocyte actin cytoskeleton caused by FFSS was at least partially reversible. Our findings of morphological changes in cell shape seen with crystal violet staining though qualitative in nature support our results of F-actin staining. It is possible that the changes described above could be due to cell injury rather than response to FFSS. We do not believe that cell injury played a role in our reported findings for the following reasons: (a) we did not find a significant difference in apoptosis between experimental and control podocytes, (b) the severity of mechanical strain was much lower than those used in osteocytes, and (c) only living cells can resume near normal morphology by 24 h after FFSS. These observations lead us to conclude that podocyte injury is not significant in our FFSS model.
Eicosanoids are biologically active fatty acids derived from oxygenation of arachidonic acid. Prostaglandins are eicosanoids synthesized by cyclooxygenase (COX) and function as autocoids (Creminon et al. 1995; FitzGerald 2002). Constitutive COX-1 and inducible COX-2 metabolize arachidonic acid to PGG2 and PGH2. PGH2 is subsequently metabolized to PGE2, PGI2, PGD2, PGF2α or thromboxane A2 (TXA2) (Creminon et al. 1995; FitzGerald 2002). PGE2 is the major product of renal arachidonic acid metabolism under physiological conditions. PGE2 interacts with E-prostanoid (EP) receptors, four of which have been cloned and characterized and are designated EP1 to EP4 (Narumiya et al. 1999). EP1 is coupled to Ca2+ mobilization, EP2 and EP4 are linked to Gs protein and EP3 is coupled to Gi protein (Narumiya et al. 1999; Sugimoto et al. 1994). Mouse podocytes express EP1 and EP4, and EP4 has been postulated to be functionally significant (Bek et al. 1999).
We have demonstrated for the first time the role of PGE2 in podocytes following application of FFSS. We find the response of podocytes to FFSS is similar to that described by us and others in osteocytes [20,32]. Osteocytes exhibit increased PGE2 synthesis as an early response to FFSS (Ajubi et al. 1997; Cherian et al. 2005; Cheng et al. 2001; Bakker et al. 2006; Bakker et al. 2005). This increase is associated with increased COX-2 expression and translocation of connexin 43 hemichannels (Ajubi et al. 1997; Cherian et al. 2005; Cheng et al. 2001; Bakker et al. 2005, 2006). These studies demonstrate that PGE2 plays an important role in mechanoperception, though the signaling pathways involved in mechanotransduction have yet to be described fully. Possible candidate pathways in osteocytes include the non-canonical Wnt/β-catenin pathway (Robinson et al. 2006).
Increased PGE2 synthesis appears to be an early response to FFSS, detectable by 30 min of FFSS and continuing as long as 120 min of exposure. In addition, we found that podocytes continued to secrete PGE2 at 2 h following discontinuation of FFSS, and that synthesis declined by 24 h after cessation of FFSS. We observed an increase in intracellular PGE2 as well as an increase in COX-2 gene expression during the same time period. Interestingly, our results are very similar to those seen in osteocytes (Cherian et al. 2005; Cheng et al. 2001). Increased PGE2 synthesis does not appear to be a general response to mechanical strain, as neither we nor other investigators found significantly increased levels following substrate stretch in podocytes (Martineau et al. 2004; Srivastava et al. 2008). We conclude that increased PGE2 synthesis is a specific early response of podocytes to FFSS. There is little data on dose response for PGE2 on actin cytoskeleton in podocytes; hence we performed a dose response evaluation for PGE2 from 1.25 to 20 μM for 2 h and 24 h without addition of another stimulus. We have found 5 μM PGE2 without FFSS to be an optimal dose in our studies with osteocytes (unpublished observation). One group found no change with 1 μM PGE2 for 90 min until the cells were primed with a stretch stimulus (not FFSS) (Martineau et al. 2004). Thus we are not completely surprised with the discrepancy between PGE2 levels observed in our FFSS experiments versus exogenous concentration of PGE2 without FFSS needed to show similar changes in podocyte.
Endogenous PGE2 plays a role in regulating renal blood flow and glomerular filtration rate directly by its effect on vascular smooth muscle, causing arteriolar vasodilatation of the afferent arteriole (Arendshorst and Navare 1993; Edwards 1985; Chatziantoniou and Arendshorst 1992). It is postulated that PGE2 attenuates vasoconstriction resulting from activation of the renin-angiotensin-aldosterone system and sympathetic nervous system, and the response to PGE2 is dependent on baseline renal vascular tone ( De Forrest et al. 1980; Conte et al. 1992). Additionally, we have shown that PGE2 acts on the glomerular permeability barrier independently of any effect on vascular tone (McCarthy and Sharma 2002). We hypothesize that podocytes respond to alterations in FFSS with an increase in PGE2, permitting ultrafiltration to be more tightly regulated through changes in both hemodynamic tone and barrier properties.
Increased PGE2 synthesis occurs in models of hyperfiltration such as the 5/6 nephrectomy (Stahl et al. 1986; Griffin et al. 1992; Nath et al. 1987; Wang et al. 1998). Additionally, we have shown that PGE2 increases albumin permeability in isolated rat glomeruli in an in vitro assay, an effect that can be abrogated by COX inhibition (McCarthy and Sharma 2002; Sharma et al. 2006). Thus PGE2 appears to be important in modifying permselectivity of the glomerular filtration barrier. In clinical practice, use of nonsteroidal anti-inflammatory agents (NSAIDs) can reduce proteinuria (Vriesendorp et al. 1986; Golbetz et al. 1989; Vriesendorp et al. 1985). Vriesendorp et al. (1986) found that NSAIDs that reduce renal PGE2 excretion also decrease proteinuria, whereas sulindac which does not influence PGE2 synthesis had no impact on protein excretion. Several studies have shown that use of chronic inhibition of COX slows progression of renal injury in animal models and human disease (Velosa et al. 1985; Wang et al. 2000; Nakayama et al. 2007). There are compelling data to suggest that PGE2 plays a role in progression of renal dysfunction in diseases characterized by hyperfiltration.
In summary, we provide compelling evidence that podocytes are sensitive and responsive to FFSS. FFSS causes disruption of the actin cytoskeleton which is reversible following discontinuation of mechanical strain. FFSS causes increased expression of COX-2 and production of PGE2 in cultured podocytes. We hypothesize that PGE2 plays a role in mechanoperception in podocytes. Further study is required to define the events of mechanotransduction that result in altered podocyte structure. We believe that examination of cellular events caused by FFSS is crucial to understanding changes that occur in diseases characterized by glomerular hyperfiltration, and may allow us to design effective therapeutic strategies in future.
Acknowledgement
This work was supported in part by The Sam and Helen Kaplan Research Fund in Pediatric Nephrology, Marion Merrell Dow Foundation Clinical Scholar Award and The Norman S. Coplon Extramural Research Grant to TS and AR046798 to MLJ and LFB. We are indebted to Ashley Sherman, MA for statistical assistance.
Disclosure None of the authors of this work have financial interests to disclose.
References
- Ajubi NE, Klein-Nulend J, Nijweide PJ, et al. Pulsating fluid flow increases prostaglandin production by cultured chicken osteocytes—a cytoskeleton—dependent process. Biochem Biophy Res Comm. 1996;225:62–68. doi: 10.1006/bbrc.1996.1131. [DOI] [PubMed] [Google Scholar]
- Ajubi NE, Lkein-Nulend J, Alblas MJ, et al. Signal transduction pathways involved in fluid flow-induced PGE2 production by cultured osteocytes. Am J Physiology. 1997;276:E171–E178. doi: 10.1152/ajpendo.1999.276.1.E171. [DOI] [PubMed] [Google Scholar]
- Arendshorst WJ, Navare LG. (1993) Renal circulation and glomerular hemodynamics. In: Schrier RW, Gottschalk CW (eds). Diseases of the kidney, 6th edn. Little Brown and Company, pp 59–106
- Bakker AD, Klein-Nulend J, Tanck E, et al. Additive effects of estrogen and mechanical stress on nitric oxide and prostaglandin E2 production by bone cells from osteoporotic donors. Osteoporos Int. 2005;16:983–989. doi: 10.1007/s00198-004-1785-0. [DOI] [PubMed] [Google Scholar]
- Bakker AD, Klein-Nulend J, Tanck E, et al. Different responsiveness to mechanical stress of bone cells from osteoporotic versus osteoarthritic donors. Osteoporos Int. 2006;17:827–833. doi: 10.1007/s00198-006-0072-7. [DOI] [PubMed] [Google Scholar]
- Bek M, Nüsing R, Kowark P, et al. Characterization of prostanoid receptors in podocytes. J Am Soc Nephrol. 1999;10:2084–2093. doi: 10.1681/ASN.V10102084. [DOI] [PubMed] [Google Scholar]
- Breyer MD, Breyer RM. Prostaglandin receptors: their role in regulating renal function. Curr Opin Nephrol Hypertens. 2000;9:23–29. doi: 10.1097/00041552-200001000-00005. [DOI] [PubMed] [Google Scholar]
- Breyer MD, Jacobson HR, Breyer RM. Functional and molecular aspects of renal prostaglandin receptors. J Am Soc Nephrol. 1996;7:8–17. doi: 10.1681/ASN.V718. [DOI] [PubMed] [Google Scholar]
- Chatziantoniou C, Arendshorst WJ. Prostaglandin interactions with angiotensin, norepinephrine, and thromboxane in rat renal vasculature. Am J Physiol. 1992;262(1 Pt 2):F68–F76. doi: 10.1152/ajprenal.1992.262.1.F68. [DOI] [PubMed] [Google Scholar]
- Cheng B, Kato Y, Zhao S, et al. PGE(2) is essential for gap junction-mediated intercellular communication between osteocyte-like MLO-Y4 cells in response to mechanical strain. Endocrinology. 2001;142:3464–3473. doi: 10.1210/en.142.8.3464. [DOI] [PubMed] [Google Scholar]
- Cheng H, Wang S, Jo YI, et al. Overexpression of cyclooxygenase-2 predisposes to podocyte injury. J Am Soc Nephrol. 2007;18:551–559. doi: 10.1681/ASN.2006090990. [DOI] [PubMed] [Google Scholar]
- Cherian PP, Wang X, Gu S, et al. Mechanical strain opens connexin 43 hemichannels in osteocytes: a novel mechanism for the release of prostaglandin. Molec Biol Cell. 2005;16:3100–3106. doi: 10.1091/mbc.E04-10-0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte G, Cianciaruso B, Nicola L, et al. Inhibition of urea tubular reabsorption by PGE1 infusion in man. Nephron. 1992;60:42–48. doi: 10.1159/000186703. [DOI] [PubMed] [Google Scholar]
- Créminon C, Habib A, Maclouf J, et al. Differential measurement of constitutive (COX-1) and inducible (COX-2) cyclooxygenase expression in human umbilical vein endothelial cells using specific immunometric enzyme immunoassays. Biochim Biophys Acta. 1995;1254:341–348. doi: 10.1016/0005-2760(94)00197-7. [DOI] [PubMed] [Google Scholar]
- Dandapani SV, Sugimoto H, Matthews BD, et al. (2007) Alpha-actinin-4 is required for normal podocyte adhesion. J Biol Chem. 2007;282:467–477. doi: 10.1074/jbc.M605024200. [DOI] [PubMed] [Google Scholar]
- Forrest JM, Davis JO, Freeman RH, et al. Effects of indomethacin and meclofenamate on renin release and renal hemodynamic function during chronic sodium depletion in conscious dogs. Circ Res. 1980;47:99–107. doi: 10.1161/01.res.47.1.99. [DOI] [PubMed] [Google Scholar]
- Durvasula RV, Petermann AT, Hiromura K, et al. Activation of a local tissue angiotensin system in podocytes by mechanical strain. Kidney Int. 2004;65:30–39. doi: 10.1111/j.1523-1755.2004.00362.x. [DOI] [PubMed] [Google Scholar]
- Edwards RM. Effects of prostaglandins on vasoconstrictor action in isolated renal arterioles. Am J Physiol. 1985;248:F779–F784. doi: 10.1152/ajprenal.1985.248.6.F779. [DOI] [PubMed] [Google Scholar]
- Endlich N, Endlich K. Stretch, tension and adhesion—adaptive mechanisms of the actin cytoskeleton in podocytes. Eur J Cell Biol. 2006;85:229–234. doi: 10.1016/j.ejcb.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Endlich N, Kress KR, Reiser J, et al. Podocytes respond to mechanical stress in vitro. J Am Soc Nephrol. 2001;12:413–422. doi: 10.1681/ASN.V123413. [DOI] [PubMed] [Google Scholar]
- FitzGerald GA. The choreography of cyclooxygenases in the kidney. J Clin Invest. 2002;110:33–34. doi: 10.1172/JCI16044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forwood MR. Inducible cyclo-oxygenase (COX-2) mediates the induction of bone formation by mechanical loading in vivo. J Bone Miner Res. 1996;11:1688–1693. doi: 10.1002/jbmr.5650111112. [DOI] [PubMed] [Google Scholar]
- Friedrich C, Endlich N, Kriz W, Endlich K. Podocytes are sensitive to fluid shear stress in vitro. Am J Physiol Renal Physiol. 2006;291:F856–F865. doi: 10.1152/ajprenal.00196.2005. [DOI] [PubMed] [Google Scholar]
- Golbetz H, Black V, Shemesh O, Myers BD. Mechanism of the antiproteinuric effect of indomethacin in nephrotic humans. Am J Physiol. 1989;256:F44–F51. doi: 10.1152/ajprenal.1989.256.1.F44. [DOI] [PubMed] [Google Scholar]
- Griffin KA, Bidani AK, Picken M, et al. Prostaglandins do not mediate impaired autoregulation or increased renin secretion in remnant rat kidneys. Am J Physiol. 1992;263:F1057–F1062. doi: 10.1152/ajprenal.1992.263.6.F1057. [DOI] [PubMed] [Google Scholar]
- Huang C, Miller RT. Shear force induces c-src phosphorylation and phospholipase D activation in differentiated podocytes. J Am Soc Nephrol. 2007;18:209A. doi: 10.1681/ASN.2007070792. [DOI] [Google Scholar]
- Jee WSS, Ueno K, Deng YP, Woodbury DM. The effects of prostaglandin E2 in growing rats: increased metaphyseal hard tissue and corticoendosteal bone formation. Calcif Tissue Int. 1985;37:148–157. doi: 10.1007/BF02554834. [DOI] [PubMed] [Google Scholar]
- Johnson DL, McAllister TN, Frangos JA. Fluid flow stimulates rapid and continuous release of nitric oxide in osteoblasts. Am J Physiol. 1996;271:E205–E208. doi: 10.1152/ajpendo.1996.271.1.E205. [DOI] [PubMed] [Google Scholar]
- Jorgensen NR, Geist ST, Civitelli R, Steinberg TH. ATP-and gap junction-dependent intracellular calcium signaling in osteoblastic cells. J Cell Biol. 1997;139:497–506. doi: 10.1083/jcb.139.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen NR, Henriksen Z, Sorensen OH, et al. Intracellular calcium signaling occurs between human osteoblasts and osteoclasts and requires activation of osteoclast P2X7 receptors. J Biol Chem. 2002;277:7574–7580. doi: 10.1074/jbc.M104608200. [DOI] [PubMed] [Google Scholar]
- Jorgensen NR, Teilmann SC, Henriksen Z, et al. Activation of L-type calcium channels is required for gap junction-mediated intracellular calcium signaling in osteoblastic cells. J Biol Chem. 2003;278:4082–4086. doi: 10.1074/jbc.M205880200. [DOI] [PubMed] [Google Scholar]
- Klein-Nulend J, Semeins CM, Ajubi NE, et al. Pulsating fluid flow increases nitric oxide (NO) synthesis by osteocytes but not periosteal fibroblasts—correlations with prostaglandin upregulation. Biochem Biophys Res Commun. 1995;217:640–648. doi: 10.1006/bbrc.1995.2822. [DOI] [PubMed] [Google Scholar]
- Klein-Nulend J, Burger EH, Semeins CM, et al. Pulsating fluid flow stimulates prostaglandin release and inducible prostaglandin G/H synthase mRNA expression in primary mouse bone cells. J Bone Miner Res. 1997;12:45–51. doi: 10.1359/jbmr.1997.12.1.45. [DOI] [PubMed] [Google Scholar]
- Kömhoff M, Grone HJ, Klein T, Seyberth HW, et al. Localization of cyclooxygenase-1 and–2 in adult and fetal human kidney: implication for renal function. Am J Physiol. 1997;272(4 Pt 2):F460–F468. doi: 10.1152/ajprenal.1997.272.4.F460. [DOI] [PubMed] [Google Scholar]
- Martineau LC, McVeigh LI, Jasmin BJ, Kennedy CR. p38 MAP kinase mediates mechanically induced COX-2 and PG EP4 receptor expression in podocytes: implications for the actin cytoskeleton. Am J Physiol Renal Physiol. 2004;286:F693–F701. doi: 10.1152/ajprenal.00331.2003. [DOI] [PubMed] [Google Scholar]
- McAllister TN, Du T, Frangos JA. Fluid shear stress stimulates prostaglandin and nitric oxide release in bone marrow-derived preosteoclast-like cells. Biochem Biophys Res Commun. 2000;270:643–648. doi: 10.1006/bbrc.2000.2467. [DOI] [PubMed] [Google Scholar]
- McCarthy ET, Sharma M. Indomethacin protects permeability barrier from focal segmental glomerulosclerosis serum. Kidney Int. 2002;61:534–541. doi: 10.1046/j.1523-1755.2002.00172.x. [DOI] [PubMed] [Google Scholar]
- Mundel P, Heid HW, Mundel TM, et al. Synaptopodin: an actin-associated protein in telencephalic dendrites and renal podocytes. J Cell Biol. 1997;139:193–204. doi: 10.1083/jcb.139.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama Y, Nonoguchi H, Inoue T, et al. Survival analysis of renal function on combination therapy with prostaglandin and angiotensin converting enzyme inhibitor (PAC) for chronic kidney disease. J Am Soc Nephrol. 2007;18:379A. doi: 10.1681/ASN.2006101097. [DOI] [PubMed] [Google Scholar]
- Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev. 1999;79:1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- Nath KA, Chmielewski DH, Hostetter TH. Regulatory role of prostanoids in glomerular microcirculation of remnant nephrons. Am J Physiol. 1987;252:F829–F837. doi: 10.1152/ajprenal.1987.252.5.F829. [DOI] [PubMed] [Google Scholar]
- Robinson JA, Chatterjee-Kishore M, Yaworsky PJ, et al. Wnt/beta-catenin signaling is a normal physiological response to mechanical loading in bone. J Biol Chem. 2006;281:31720–31728. doi: 10.1074/jbc.M602308200. [DOI] [PubMed] [Google Scholar]
- Rodewald R, Karnovsky MJ. Porous substructure of the glomerular slit diaphragm in the rat and mouse. J Cell Biol. 1974;60:423–433. doi: 10.1083/jcb.60.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlondorff D. Renal prostaglandin synthesis. Sites of production and specific actions of prostaglandins. Am J Med. 1986;81:1–11. doi: 10.1016/0002-9343(86)90903-4. [DOI] [PubMed] [Google Scholar]
- Shankland SJ, Pippin JW, Reiser J, Mundel P. Podocytes in culture: past, present, and future. Kidney Int. 2007;72:26–36. doi: 10.1038/sj.ki.5002291. [DOI] [PubMed] [Google Scholar]
- Sharma M, McCarthy ET, Sharma R, et al. Arachidonic acid metabolites mediate the radiation-induced increase in glomerular albumin permeability. Exp Biol Med. 2006;231:99–106. doi: 10.1177/153537020623100112. [DOI] [PubMed] [Google Scholar]
- Srivastava T, Cudmore PA, Sharma R et al. (2008) Fluid flow shear stress (FF) but not substrate stretch (SS) increases podocyte release of prostaglandin E2 (PGE2). EPAS2008:3803.1.
- Stahl RA, Kudelka S, Paravicini M, Schollmeyer P. Prostaglandin and thromboxane formation in glomeruli from rats with reduced renal mass. Nephron. 1986;42:252–257. doi: 10.1159/000183676. [DOI] [PubMed] [Google Scholar]
- Sugimoto Y, Namba T, Shigemoto R, et al. Distinct cellular localization of mRNAs for three subtypes of prostaglandin E receptor in kidney. Am J Physiol. 1994;266:F823–F828. doi: 10.1152/ajprenal.1994.266.5.F823. [DOI] [PubMed] [Google Scholar]
- Velosa JA, Torres VE, Donadio JV, Jr, et al. Treatment of severe nephrotic syndrome with meclofenamate: an uncontrolled pilot study. Mayo Clin Proc. 1985;60:586–592. doi: 10.1016/s0025-6196(12)60980-x. [DOI] [PubMed] [Google Scholar]
- Vriesendorp R, Donker AJ, Zeeuw D, et al. Antiproteinuric effect of naproxen and indomethacin. A double-blind crossover study. Am J Nephrol. 1985;5:236–242. doi: 10.1159/000166941. [DOI] [PubMed] [Google Scholar]
- Vriesendorp R, Donker AJ, Zeeuw D, et al. Effects of nonsteroidal anti-inflammatory drugs on proteinuria. Am J Med. 1986;81:84–94. doi: 10.1016/0002-9343(86)90910-1. [DOI] [PubMed] [Google Scholar]
- Wadhwa S, Godwin SL, Peterson DR, et al. Fluid flow induction of cyclo-oxygenase 2 gene expression in osteoblasts is dependent on an extracellular signal regulated kinase signaling pathway. J Bone Miner Res. 2002;17:266–274. doi: 10.1359/jbmr.2002.17.2.266. [DOI] [PubMed] [Google Scholar]
- Wang JL, Cheng HF, Zhang MZ, et al. Selective increase of cyclooxygenase-2 expression in a model of renal ablation. Am J Physiol. 1998;275:F613–F622. doi: 10.1152/ajprenal.1998.275.4.F613. [DOI] [PubMed] [Google Scholar]
- Wang JL, Cheng HF, Shappell S, Harris RC. A selective cyclooxygenase-2 inhibitor decreases proteinuria and retards progressive renal injury in rats. Kidney Int. 2000;57:2334–2342. doi: 10.1046/j.1523-1755.2000.00093.x. [DOI] [PubMed] [Google Scholar]
- You J, Reilly GC, Zhen X, et al. Osteopontin gene regulation by oscillatory fluid flow via intracellular calcium mobilization and activation of mitogen-activated protein kinase in MC3T3-E1 osteoblasts. J Biol Chem. 2001;276:13365–13371. doi: 10.1074/jbc.M009846200. [DOI] [PubMed] [Google Scholar]
- You J, Jacobs CR, Steinberg TH, Donahue HJ. P2Y purinoceptors are responsible for oscillatory fluid flow-induced intracellular calcium mobilization in osteoblastic cells. J Biol Chem. 2002;277:48724–48729. doi: 10.1074/jbc.M209245200. [DOI] [PubMed] [Google Scholar]
- Zaman G, Pitsillides AA, Rawlinson SC, et al. Mechanical strain stimulates nitric oxide production by rapid activation of endothelial nitric oxide synthase in osteocytes. J Bone Miner Res. 1999;14:1123–1131. doi: 10.1359/jbmr.1999.14.7.1123. [DOI] [PubMed] [Google Scholar]
- Zhang K, Barragan-Adjemian C, Ye L, et al. E11/gp38 selective expression in osteocytes: regulation by mechanical strain and role in dendrite elongation. Mol Cell Biol. 2006;26:4539–4552. doi: 10.1128/MCB.02120-05. [DOI] [PMC free article] [PubMed] [Google Scholar]