Abstract
Objectives
To describes a cadaveric temporal bone model of labyrinthitis ossificans and investigate the utility of intra-operative C-arm computed tomography (CBCT) in facilitating cochlear implantation.
Design
Cadaveric temporal bone study.
Methods
Five cadaveric heads had cement introduced into the 10 cochleas. CBCT and a conventional CT scans were compared to asses the extent of cochlear obliteration. The cement was drilled-out (under CBCT guidance, if required) and a cochlear implant electrode arrays (from 3 different manufacturers) inserted.
Results
CBCT images demonstrated temporal bone anatomy and the extent of cochlear obliteration as clearly as conventional CT in all cases. Intra-operative CBCT guided drilling and facilitated electrode placement in 2 out of 5 heads (3/10 ears). Streak-artifact from the electrodes of two devices partially obscured image clarity.
Conclusions
The obliterated cochlear model reproduced a disease-ossified cochlear both radiographically and surgically. CBCT is useful for intra-operative imaging, to facilitate electrode array placement in the obliterated or congenitally abnormal cochlea.
Introduction
Abnormal cochlear anatomy or ossification of the cochlea may present a significant challenge for the correct placement of cochlear implant electrode arrays in the scala tympani. Meningitis is an increasingly rare cause of acquired severe deafness in the developed world, but by causing inflammatory changes throughout the basal, middle and apical turns of the cochlea may cause fibrous obstruction or even ossification of the lumen of the cochlea. This typically involves the basal turn, although the whole cochlea may be affected. When the lumen of the scala tympani is obliterated, the electrode array may be inserted into the scala vestibuli. If both scala are obliterated, the ossified lumen can be drilled-out as far as the end of the basal turn of the cochlea. At this point the lumen becomes inaccessible beyond the central modiolus of the cochlea and cannot be drilled-out without inappropriate damage to the cochlear nerve at the spiral ganglion. If the electrode array will not pass beyond this point, a second cochleostomy can be drilled to open the middle turn of the cochlea and electrodes placed in both turns by using a device with double electrode arrays.
High-quality preoperative imaging is an important component of surgical planning for implantation of the post-meningitic cochlea. Both high-resolution CT and T2-weighted magnetic resonance (MR) imaging are valuable means of ascertaining the patency of the basal turn of the cochlea. High-resolution CT can detect established ossification of the inner ear, while MR imaging is a more sensitive test of cochlear obliteration and can also reveal fibrous obstruction. While such modalities are vital to diagnosis and planning, the value of such information is somewhat limited with regard to intraoperative guidance. First, the geometric accuracy of guidance based on preoperative images – even with state of the art navigation systems, immobilization, and geometric registration – is usually limited to around 2mm.1 In addition, the images do not indicate the position of the lumen in a severely ossified cochlea. Most importantly, preoperative images do not allow visualization of anatomical changes incurred in the course of the operation nor the verification of electrode placement. Translation of high-resolution CT and MR imaging into the operating room is a topic of considerable interest, challenged by issues of imaging time, radiation dose, compatibility with the surgical environment, and cost.2,3
Cone-beam computed tomography (CBCT) on a mobile C-arm presents a promising technology for intraoperative imaging that is finding application in a fairly broad range of interventions, ranging from spine surgery4,5 to brachytherapy,6 with guidance of ENT surgery arising as one of the most promising.7,8 It offers intraoperative volumetric images with sub-millimeter spatial resolution and soft-tissue visibility at low radiation dose and fairly rapid acquisition time.9 Spatial resolution is approximately 0.6 – 1.0 mm, depending on the choice of reconstruction filter and voxel size, and is nearly isotropic in three dimensions. Soft-tissue contrast (or contrast-to-noise ratio) in CBCT approaches that of diagnostic CT but is limited by a number of physical factors, including increased levels of x-ray scatter and reduced detector efficiency. Soft-tissue discrimination down to ∼20-50 HU contrast has been demonstrated – e.g., in application of CBCT for daily soft-tissue targeting in image-guided radiation therapy. Future improvements in both spatial resolution and soft-tissue contrast may be expected through improved detector technology and 3D image reconstruction techniques. The utility of CBCT has already been demonstrated in the temporal bone.8,10 Cone-beam computed tomography has previously been used in isolated temporal bones to demonstrate the proximity of implant electrodes to the modiolus, and the accuracy of the imaging data was confirmed histologically.11 The objectives of the study reported below were: i.) to develop a model of cochlear ossification in the temporal bone laboratory; and ii.) to test the clinical utility of intraoperative CBCT in assisting implantation of the partially obliterated cochlea with a cochlear implant electrode array. We compared image quality and metal streak artifacts associated with 3 commercially available implant designs, scanning each in situ (cadavers presenting the cochlear ossification model) using C-arm CBCT.
Methods and Materials
C-arm Cone Beam Computerised Tomography
The CBCT system is based upon a Siemens PowerMobil (Siemens Medical Solutions, Erlangen Germany) modified as described previously.4,7-9,12,13 The primary modifications include: replacement of the imaging chain with a flat-panel digital x-ray detector (PaxScan 4030CB, Varian Imaging Products, Palo Alto CA); modification of the x-ray tube and collimators to provide an expanded field of view; addition of x-ray filtration appropriate to CBCT; motorization of the C-arm orbit; a method for geometric calibration of the C-arm orbit; computer control of the x-ray generator, C-arm motion, and imaging systems; and 3D image reconstruction. All CBCT images used in this study were reconstructed from 200 projections acquired across ∼180°, with 0.2 mm isotropic voxel size, and a sharp reconstruction filter. The experimental setup is illustrated in Figure 1.
Figure 1.
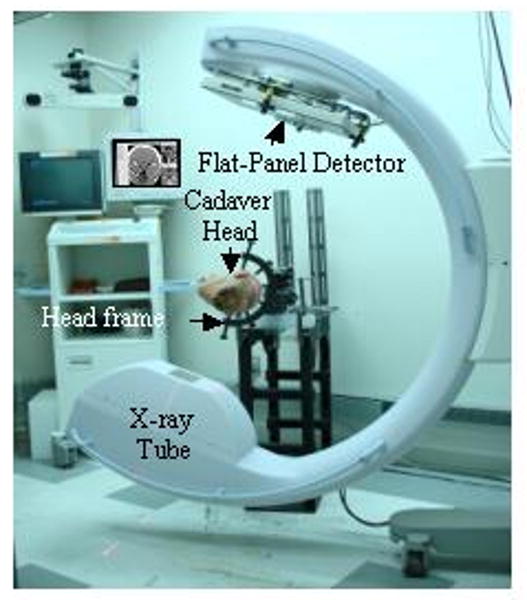
Illustration of the experimental set-up.
Cochlear Ossification Model
Five cadaveric heads were used for the study, each obtained from the Division of Anatomy (University of Toronto, Toronto ON) and used in accordance with the Anatomy Act of Ontario. No approval was required from the Institutional Review Board. Each head was scanned with C-arm CBCT (described below) and a diagnostic CT scanner (Discovery ST, GE Healthcare, Milwaukee, USA) to confirm the presence of normal temporal bone anatomy prior to surgical drilling.
Standard preparation for cochlear implant insertion was completed on each temporal bone with cortical mastoidectomy, posterior tympanotomy and cochleostomy (with a 1 mm diamond burr anterior-inferior to the round window niche). After suctioning fluid from the cochlea, 0.1-0.3 ml of Surgical Simplex® P (Stryker, MI, USA: REF 6191-0-001) was introduced to the cochlea with a 20-guage angiocath™ catheter. The bone cement was left to harden >15 minutes before moving the head. Following the preparation of both temporal bones, the heads were rescanned using the C-arm CBCT for visualization of the ossification model. If, after imaging, a cochlea was determined to be inadequately infused with cement, the ear was examined, re-drilled and infused with additional cement if necessary, then rescanned with CBCT. After adequate obliteration of each temporal bone, the heads were re-scanned on the diagnostic CT scanner for comparison with CBCT.
Following review of the radio-images, each cochleostomy was re-drilled and the lumen of the cochlear drilled-out for cochlear implantation. Intra-operative C-arm CBCT images were taken during the procedure and reconstructed in 3D to guide drilling if required. The images were reviewed on a 3D visualization workstation before further drilling. There was no limit to the number of scans that could be requested. After the drilling was completed, an electrode array was introduced and the temporal bone was re-scanned with the electrode in position to determine the position of the array. The surgeon completed a questionnaire after each procedure to describe the level of ossification, insertion depth, extent to which intra-operative scanning was required and how helpful the scans were.
Cochlear Implant Electrode Arrays
Cochlear implant electrode arrays from three manufacturers were used in this study: Implant #1, Nucleus Contour 24 electrode (Nucleus, Cochlear Corporation, Lane Cove, New South Wales, Australia); Implant #2, Maestro (MED-EL, Innsbruck, Austria) and Implant #3, HiFocus 1j electrode, (Advanced Bionics, Valencia, California, USA). The three arrays were expected to present different levels of metal artifact with CBCT, owing to differences in design and material composition. The relative proportion of platinum and iridium differs in each array. The Advanced Bionics implant also contains titanium. To examine the severity of image artifacts, each implant was fully inserted into the cochlea of a single specimen (following cochleostomy but prior to ossification) and scanned using the C-arm CBCT. 3D CBCT images were reconstructed and image quality evaluated for the magnitude and perceived significance of the artifact.
Results
Assessment of cement-ossification model
As shown in Figure 2, CBCT provided high-resolution images of the cochlea, which clearly allowed assessment of the extent of cochlear obliteration. There was no significant difference in extent of obliteration as shown on CBCT as compared with conventional diagnostic CT. Structures such as the cochlear and ossicles could be qualitatively identified in CBCT images. Four of the 5 heads (7 of the 10 cochleas) demonstrated ‘ossification’ following the first introduction of the bone cement. The remaining 3 cochleas appeared radiographically as non-ossified and were re-explored by dissection. Two required further cement, which yielded a successfully ossified model. The third cochlea appeared ossified in dissection, although this was not apparent in CT or CBCT images.
Figure 2.
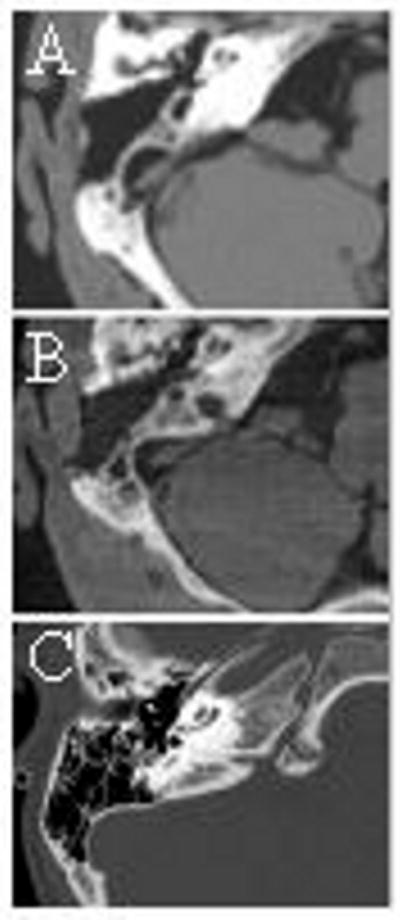
Illustration of an ossified cochlea as seen in (a) diagnostic CT and (b) intraoperative CBCT (c) clinical diagnostic CT of ossified cochlea (post meningitis).
In all other cases there was good correlation between radiographic and surgical observation of the degree of obstruction. The basal turn of the scala tympani was completely ‘ossified’ in three cases and partially (basal 3-4 mm) in seven. One cochlea appeared over-staged by imaging (partial verses full ossification) and two under-staged.
Bone cement obliteration provided a realistic model of the ossified cochlea surgically as well as radiographically (Fig 2), presenting a drilling task comparable to real disease (Fig 3).
Figure 3.
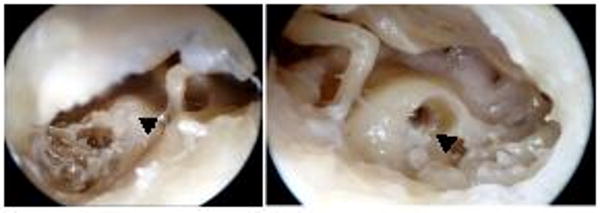
Photographs illustrating the cement-ossified cochlea (A) posterior tympanotomy (left ear) showing Simplex B cement filling the cochleostomy (arrow). (B) right cochleostomy after drill out of cement-ossification. A rim of residual cement is still present (arrow).
Intra-operative CBCT
Full insertion of the electrode array was achieved in 4 out of 5 heads (8 of the 10 ossified cochleas). Intra-operative CBCT was only required in one of these cases and facilitated full insertion. ‘Ossification’ beyond the basal turn prevented full insertion in two cochleas, despite assistance from CBCT imaging. A middle turn cochleostomy for a double array technique was necessary in these cases. Scala vestibuli insertion was used in two of the three cochleas with complete scala tympani obstruction.
CBCT images taken after implantation clearly demonstrated the position of the electrode array within the cochlea and showed successful insertion without folding of the array. Radiological assessment of insertion depth correlated with the surgeons' perception of the electrode position.
Figure 4 illustrates the image quality in a region of interest about the cochlea for the three implants considered. Both Implant #1 (Nucleus Contour) and Implant #2 (Med El Maestro) exhibited significant metal streak artifacts, which diminished image quality, limiting interpretation. Implant #3 (Advanced Bionics HiFocus 1j) demonstrated significantly reduced streak artifacts, allowing fairly clear visualization of adjacent structures. Figure 5 shows a surface rendering of Implant #3, illustrating that the full length of the electrode can be well visualized with reformatting.
Figure 4.
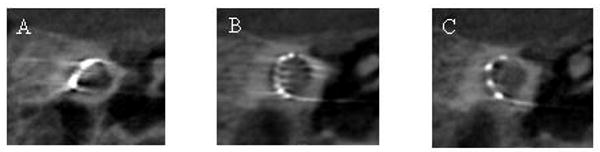
CBCT of an implanted cochlea to demonstrate metal streak artifact. (A) Implant # (Nucleus Contour, Cochlea); (B) Implant #2 (Maestro, MED-EL) and (C) Implant #3 (HiFocus 1j, Advanced Bionics).
Figure 5.
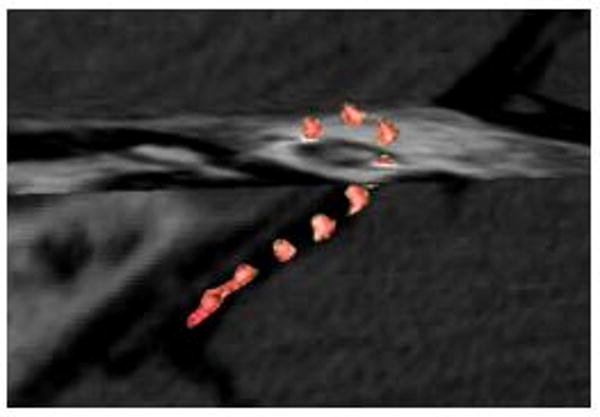
Reconstructed CBCT of Implant #3 (HiFocus 1j, Advanced Bionics) to demonstrate surface rendering in the context of CBCT cut-planes, illustrating the full electrode length.
Discussion
Ossification of the cochlea can provide a surgical challenge. When ossification is well established, the cochlear lumen can be difficult to identify. Even in partial obstruction, removal of soft bone to provide an adequate lumen for the electrode array can be challenging. The relative rarity of this presentation, especially with declining rates of meningitis, limits the opportunity to gain surgical experience or training in the techniques required. However, second-side electrode placement for post-meningitic patients is becoming more desirable, transiently increasing the number of ossified cochleas requiring implants.14 We have developed a realistic cadaveric model of cochlear ossification using bone cement, which we have found to be very beneficial in surgical training.
This model produces a heterogeneous ‘ossification’ pattern ranging from just the basal 3-4 mm of the scala tympani to the entire scala tympani being obliterated. This pattern of ossification is similar to that seen in labyrinthitis ossificans.15 Otosclerosis also causes ossification of the cochlea, which predominates in the first part of the basal turn. Significant apical and middle-turn ossification is uncommon in the absence of basal turn ossification.15 This rarer pattern of obstruction was not produced by our model, but could be achieved by cementing the cochlear from an apical or middle fossa cochleostomy. The model is suitable for training scala vestibuli insertion and use of the double array, which are important techniques in implantation of the ossified cochlea.16,17
Imaging with CBCT and diagnostic CT provided good assessment of the completeness of obliteration in the model in 4 out of 5 heads (9 out of 10 cochleas). The single exception was understaged in both CT and CBCT. In a study by Young et al in 2000,18 assessing the pre-operative evaluation of ossified cochleas, 20 high resolution CT scans obtained post cochlear implantation were reviewed and compared to surgical findings and the pre-operative CT scan. Ninety percent of patients required drilling of the ossified bone within the basal turn at surgery. High-resolution pre-operative CT scans predicted ossification within the basal turn in 45% of cases (50% sensitivity). Five of 6 cases without radiographic evidence of ossification had positive findings at surgery. Interestingly, this study found that lateral semicircular canal ossification was a more sensitive measure for predicting cochlear ossification. Immediate pre- or intra-operative CBCT would appear to be an appropriate means to detect progression of ossification, which may continue to develop after diagnostic imaging has been obtained. As mentioned above, CBCT demonstrates sub-mm spatial resolution and soft-tissue contrast visibility, but its performance does not match that of diagnostic CT due to a variety physical limitations (e.g., x-ray scatter and detector efficiency). Of course, the CBCT system described here is clearly not intended as a replacement of preoperative/diagnostic imaging, and its imaging performance appears suitable to tasks of image guidance, as demonstrated here and in previous work.
We have found intra-operative CBCT to be valuable in guiding drilling and electrode insertion in the more obstructed cochlea models. We acknowledge that the questionnaire used in this study was subjective, but still felt it an appropriate, valuable means to evaluate the surgeons' perspective of utility of the imaging tool. While helpful in the simulation setting, we envisage that this could be of great benefit surgically when electrode insertion is more difficult – in congenitally abnormal cochlea as well as the ossified cochlea. With the C-arm CBCT system, intraoperative scanning provided detailed information regarding electrode position and allowed an opportunity to re-position the electrode if required. Surgical navigation systems have been described for electrode placement in the ossified cochlea19 but do not offer the advantage of intra-operative CBCT in showing surgical changes in anatomy.
Post-operative imaging is widely used to confirm electrode placement, typically with plain radiographs. Though not routinely used in all centers,20 it is highly advisable in the ossified or congenitally abnormal cochlea due to the increased chance of poor placement. Interpretation of electrode position can be difficult with 2D radiographs, especially in bilateral implantation where the implants overlie each other on lateral views. The advantage of CBCT over a conventional x-ray radiograph is the increased 3D volumetric information provided. The radiation dose is significantly less than diagnostic CT (in the order of 3-10 mGy, compared to 50-100 mGy).9 In addition, CBCT is acquired over only ∼180° (with the x-ray tube orbiting posterior to the head) so that radiation-sensitive organs, including the eyes, receive even lower doses. Finally, intraoperative CBCT can be performed with a relatively short acquisition time, with volumetric images available within ∼1 minute. The ability to visualize implant placement intraoperatively, in a manner consistent with time and radiation dose constraints, is particularly valuable in the ossified or congenitally abnormal cochlea.
The pattern of metal streak artifact in CT and CBCT images depended strongly on the design of cochlear implant used. This is due to the different metallic components in each case. Specifically, Implant #3 (HiFocus 1j, Advanced Bionics) incorporated titanium components that were significantly more CT-friendly than heavier metals incorporated in other implants. The streak artifact is primarily a result of “beam-hardening” (i.e., attenuation such that the mean energy of the x-ray beam is increased in a manner not accounted by the reconstruction algorithm) and very low x-ray transmission through such dense materials (for which the detector records a very low signal and the 3D reconstruction algorithm backprojects a very high attenuation value) – each resulting in white streaks through the electrodes.
Conclusion
This novel cochlear ossification model provides realistic simulation of disease and is a valid simulation for research and surgical training. In addition, in the cadaveric setting, we have demonstrated the usefulness of intra-operative CBCT in the surgical management and verification of implant placement for such cases. It is envisaged that in the future intra-operative CBCT may be of clinical benefit in implantation of the ossified or congenitally abnormal cochlea. However, in order to prove this hypothesis, further studies, including a randomized controlled trial, comparing cochlear implant surgery with and without CBCT will be needed.
Acknowledgments
This work was supported in part by a grant from the National Institutes of Health (NIH R01-CA-127944-02) and by the Princess Margaret Hospital Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Labadie RF, Shah RJ, Harris SS, et al. Submillimetric target-registration error using a novel, non-invasive fiducial system for image-guided otologic surgery. Comput Aided Surg. 2004;9(4):145–153. doi: 10.3109/10929080500066922. [DOI] [PubMed] [Google Scholar]
- 2.Cartellieri M, Vorbeck F. Endoscopic sinus surgery using intraoperative computed tomography imaging for updating a three-dimensional navigation system. Laryngoscope. 2000;110(2 Pt 1):292–296. doi: 10.1097/00005537-200002010-00020. [DOI] [PubMed] [Google Scholar]
- 3.Fried MP, Hsu L, Topulos GP, et al. Image-guided surgery in a new magnetic resonance suite: preclinical considerations. Laryngoscope. 1996;106(4):411–417. doi: 10.1097/00005537-199604000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Siewerdsen JH, Moseley DJ, Burch S, et al. Volume CT with a Flat-Panel Detector on a Mobile C-Arm: Pre-Clinical Investigation in Guidance of Minimally Invasive Surgery. Med Phys. 2005;32(1):241–254. doi: 10.1118/1.1836331. [DOI] [PubMed] [Google Scholar]
- 5.Burch S, Bogaards A, Siewerdsen J, et al. Photodynamic therapy for the treatment of metastatic lesions in bone: studies in rat and porcine models. J Biomed Opt. 2005;10(3):034011. doi: 10.1117/1.1921887. [DOI] [PubMed] [Google Scholar]
- 6.Vicini FA, Jaffray DA, Horwitz EM, et al. Implementation of 3D-virtual brachytherapy in the management of breast cancer: a description of a new method of interstitial brachytherapy. Int J Radiat Oncol Biol Phys. 1998;40(3):629–35. doi: 10.1016/s0360-3016(97)00499-9. 1. [DOI] [PubMed] [Google Scholar]
- 7.Rafferty MA, Siewerdsen JH, Chan Y, et al. Investigation of C-arm cone-beam CT-guided surgery of the frontal recess. Laryngoscope. 2005;115(12):2138–43. doi: 10.1097/01.mlg.0000180759.52082.45. [DOI] [PubMed] [Google Scholar]
- 8.Rafferty MA, Siewerdsen JH, Chan Y, et al. Intraoperative cone-beam CT for guidance of temporal bone surgery. Otolaryngol Head Neck Surg. 2006;134(5):801–8. doi: 10.1016/j.otohns.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 9.Daly MJ, Siewerdsen JH, Moseley DJ, et al. Intraoperative cone-beam CT for guidance of head and neck surgery: Assessment of dose and image quality using a C-arm prototype. Med Phys. 2006;33(10):3767–80. doi: 10.1118/1.2349687. [DOI] [PubMed] [Google Scholar]
- 10.Peltonen LI, Aarnisalo AA, Kortesniemi MK, et al. Limited cone-beam computed tomography imaging of the middle ear: a comparison with multislice helical computed tomography. Acta Radiol. 2007;48(2):207–12. doi: 10.1080/02841850601080465. [DOI] [PubMed] [Google Scholar]
- 11.Husstedt HW, Aschendorff A, Richter B, et al. Nondestructive three-dimensional analysis of electrode to modiolus proximity. Otol Neurotol. 2002;23(1):49–52. doi: 10.1097/00129492-200201000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Bachar G, Siewerdsen JH, Daly MJ, et al. Image quality and localization accuracy in C-arm tomosynthesis-guided head and neck surgery. Med Phys. 2007;34(12):4664–77. doi: 10.1118/1.2799492. [DOI] [PubMed] [Google Scholar]
- 13.Siewerdsen JH, Jaffray DA, Edmundson GK, et al. Flat panel cone-beam CT: A novel imaging technology for image-guided procedures. Proc SPIE Medical Imaging Visualization Display and Image Guided Procedures. 2001;4319:435–444. [Google Scholar]
- 14.Scherf F, van Deun L, van Wieringen A, et al. Hearing benefits of second-side cochlear implantation in two groups of children. Int J Pediatr Otorhinolaryngol. 2007;71(12):1855–63. doi: 10.1016/j.ijporl.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 15.Green JD, Jr, Marion MS, Hinojosa R. Labyrinthitis ossificans: histopathologic consideration for cochlear implantation. Otolaryngol Head Neck Surg. 1991;104(3):320–6. doi: 10.1177/019459989110400306. [DOI] [PubMed] [Google Scholar]
- 16.Berrettini S, Forli F, Neri E, et al. Scala vestibuli cochlear implantation in patients with partially ossified cochleas. J Laryngol Otol. 2002;116(11):946–50. doi: 10.1258/00222150260369516. [DOI] [PubMed] [Google Scholar]
- 17.Millar DA, Hillman TA, Shelton C. Implantation of the ossified cochlea: management with the split electrode array. Laryngoscope. 2005;115(12):2155–60. doi: 10.1097/01.MLG.0000181494.21654.5E. [DOI] [PubMed] [Google Scholar]
- 18.Young NM, Hughes CA, Byrd SE, et al. Postmeningitic ossification in pediatric cochlear implantation. Otolaryngol Head Neck Surg. 2000;122(2):183–8. doi: 10.1016/S0194-5998(00)70236-1. [DOI] [PubMed] [Google Scholar]
- 19.Raine CH, Strachan DR, Gopichandran T. How we do it: using a surgical navigation system in the management of the ossified cochlea. Cochlear Implants Int. 2003;4(2):96–101. doi: 10.1179/cim.2003.4.2.96. [DOI] [PubMed] [Google Scholar]
- 20.Barker EV, Pringle M. Survey of Prophylactic Antibiotic Use Amongst UK Cochlear Implant Surgeons 2008. Cochlear Implants Int. doi: 10.1179/cim.2008.9.2.82. in press. [DOI] [PubMed] [Google Scholar]


