Abstract
Molecular cloning of the NIS gene in 1996 allowed examination of the molecular basis of congenital hypothyroidism due to iodide transport defect (ITD) many years after the first case was decribed by Federman et al. in 1958. Since 1997, when the first NIS mutation causing ITD was identified and characterized, 12 different NIS molecular defects have been described in 31 ITD patients. Interestingly, marked clinical heterogeneity between patients with the same NIS mutation and in patients with different mutations in the NIS gene without a clear genotype-phenotype correlation has been observed. The study of NIS mutations as the molecular basis of ITD has not only yielded extremely valuable structure/function information on NIS, but has also provided an important tool for preclinical diagnosis and genetic counseling of ITD patients.
Keywords: sodium iodide symporter (NIS), congenital hypothyroidism, iodide transport defect, NIS mutations
Introduction
The sodium iodide symporter (NIS), an intrinsic plasma membrane glycoprotein, represents a highly specialized transport system mediating the active uptake of iodide from the bloodstream into the thyroid gland as well as a number of non-thyroidal tissues, including mammary gland during lactation, cancerous breast tissue, stomach, salivary and lacrimal glands (Dai et al., 1996; Jhiang et al., 1998; Smanik et al., 1996; Spitzweg et al., 1998; Spitzweg et al., 1999b; Spitzweg et al., 2001b; Tazebay et al., 2000). In the thyroid, NIS provides iodide for the synthesis of the thyroid hormones, triiodothyronine (T3) and thyroxine (T4), the only iodine containing hormones in vertebrates, which play an important role in the metabolism, growth and maturation of a variety of organ systems, especially the nervous system (Carrasco, 1993). Cloning and molecular characterization of the NIS gene in 1996 (Dai et al., 1996; Smanik et al., 1996) allowed investigation of its expression and regulation in thyroidal and extrathyroidal tissues, its pathophysiological role in benign and malignant thyroid disease as well as its potential role in diagnosis and therapy of cancer outside the thyroid gland.
The sodium iodide symporter (NIS) – expression, regulation and clinical significance
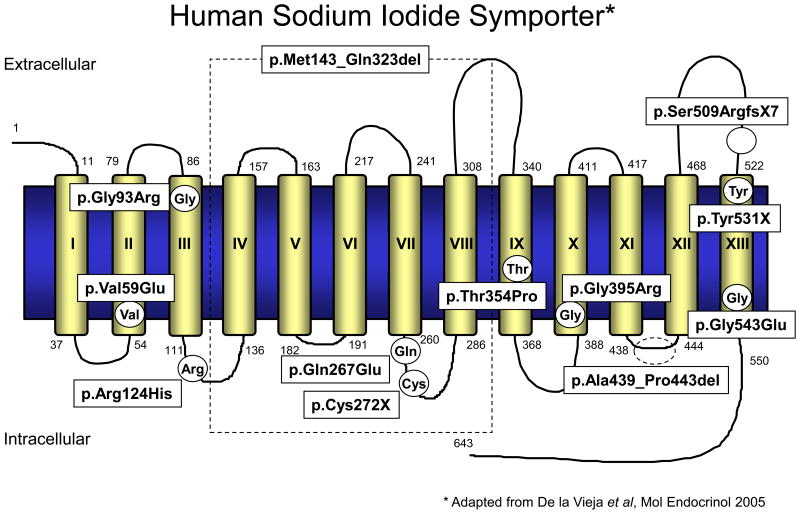
The human NIS gene (SLC5A5) is localized on chromosome 19p12-13.2 with an open reading frame of 1929 nucleotides encoding a glycoprotein of 643 amino acids with a molecular mass of approximately 70-90 kDa. The coding region of human NIS contains 15 exons interrupted by 14 introns and codes for a 3.9 kb mRNA (Smanik et al., 1997). NIS is a member of the SLC5A transporter family, which includes the high and low affinity Na+/glucose co-transporters (sodium/glucose co-transporters 1 and 2), the Na+/myoinositol transporter (SMIT), and the Na+/monocarboxylate transporter (SMCT) (De la Vieja et al., 2007a). The currently proposed secondary structure of NIS is an intrinsic membrane protein with 13 putative transmembrane domains, an extracellular amino-terminus and an intracellular carboxyl-terminus with three putative N-linked glycosylation sites at position 225, 485, and 497 (Levy et al., 1998a) (Fig. 1). NIS cotransports one iodide ion against its electrochemical gradient together with two sodium ions along their electrochemical gradient. This transmembrane sodium gradient, which serves as the driving force for the transport of iodide, is generated by the ouabain-sensitive Na+/K+-ATPase. NIS-mediated iodide transport is therefore inhibited by the Na+/K+-ATPase inhibitor ouabain, as well as by the competitive inhibitors thiocyanate and perchlorate (Carrasco, 1993). Although its main physiological function is to transport iodide across the basolateral membrane of thyroid follicular cells, NIS has also been demonstrated to transport other structurally similar anions like ClO3-, SCN-, SeCN-, NO3-, Br-, TcO4-, ReO4- as well as At- (Dohan et al., 2003; Van Sande et al., 2003; Willhauck et al., 2007; Willhauck et al., 2008c). Following this active transport across the basolateral membrane, iodide is translocated across the apical membrane into the thyroidal colloid by pendrin, the PDS gene (SLC26A4) product, which is a chloride/iodide transporter (Bizhanova et al., 2009) and probably other nonspecific ion channels (Spitzweg et al., 2002). Iodide is then attached to tyrosyl residues along the thyroglobulin (Tg) backbone within the colloid through a process termed organification, which is catalyzed by the thyroid-specific enzyme thyroid peroxidase (TPO). Thyroid hormones T3 and T4 are synthesized by coupling of two iodotyrosine residues and stored in the colloid until thyroglobulin is taken up by thyroid follicular cells. Thyroid hormone is released into the blood stream, when thyroid hormones are needed as signaled by pituitary TSH stimulation.
Figure 1.
Human NIS secondary structure model with all identified mutations causing iodide transport defect. For detailed characterization of mutations see table 1.
1 - 643 : amino acid residues
I – XIII : transmembrane domains
The main regulator of thyroidal iodide transport is pituitary-derived thyroid stimulating hormone (TSH), which interacts with the TSH receptor at the basolateral membrane of thyroidal cells and stimulates NIS gene and protein expression via the adenylate cyclase-cAMP mediated pathway (Dohan et al., 2003; Spitzweg et al., 2002). In addition, TSH plays a crucial role in the correct plasma membrane targeting of the NIS protein – an important prerequisite for its functional activity (Riedel et al., 2001).
Several inhibitors of NIS expression have been identified, including a panel of cytokines that are involved in the pathogenesis of autoimmune thyroid disease and the euthyroid sick syndrome, such as transforming growth factor (TGF)-β1 (Kawaguchi et al., 1997), tumor necrosis factor (TNF) α, interferon (IFN) γ, interleukin (IL)-1α, IL-1β and IL-6 (Ajjan et al., 1998a; Ajjan et al., 1998b; Caturegli et al., 2000; Pekary et al., 1998; Spitzweg et al., 1999a), as well as glucocorticoids and estradiol (Furlanetto et al., 1999; Spitzweg et al., 1999a). Interestingly, iodide itself downregulates NIS gene and protein expression, representing the molecular basis of the escape from the acute Wolff-Chaikoff effect (Eng et al., 1999; Spitzweg et al., 1999a; Uyttersprot et al., 1997; Wolff et al., 1948; Wolff et al., 1949). In contrast, lack of iodide induces NIS gene and protein expression, which was demonstrated in the fetal thyroid gland and on the fetal side of the placenta of iodine-deficient pregnant rats suggesting that adaptive mechanisms against iodine deficiency are already present in the fetal thyroid gland (Schröder-van der Elst et al., 2001).
In addition to its key role in thyroid physiology, NIS and NIS-mediated iodide accumulation in the thyroid gland represent crucial prerequisites for diagnostic thyroid scintigraphy, as well as therapeutic application of radioiodine in benign and malignant thyroid diseases (Spitzweg et al., 2001c). NIS therefore represents one of the oldest targets for molecular imaging and therapy. Functional NIS expression in differentiated thyroid carcinomas allows not only postoperative localization and ablation of the thyroid remnant as well metastases, but also provides the possibility of subsequent postablative 131I total body scanning that can diagnose local and metastatic residual and recurrent disease followed by 131I ablation, thereby improving the prognosis of thyroid cancer patients significantly and making thyroid cancer one of the most manageable cancers (Spitzweg et al., 2001c). Thyroidal NIS expression therefore provides the molecular basis for the most effective form of systemic anticancer radiotherapy available to the clinician today.
Based on its central role in the management of thyroid cancer, cloning of the NIS gene in 1996 was not only a breakthrough in the study of thyroidal iodide transport, but also paved the way for the characterization of NIS as novel reporter and therapy gene (Cengic et al., 2005; Dingli et al., 2003a; Dingli et al., 2003b; Dingli et al., 2004; Dohan et al., 2003; Dwyer et al., 2005a; Dwyer et al., 2005b; Dwyer et al., 2006a; Dwyer et al., 2006b; Klutz et al., 2009; Merron et al., 2007; Scholz et al., 2005; Spitzweg et al., 1999c; Spitzweg et al., 2000; Spitzweg et al., 2001a; Spitzweg et al., 2001c; Spitzweg et al., 2007; Willhauck et al., 2007; Willhauck et al., 2008a; Willhauck et al., 2008b; Willhauck et al., 2008c). The development of a promising cytoreductive gene therapy strategy based on NIS gene transfer in non-thyroidal tumors followed by radioiodine application, and the identification of endogenous NIS expression in breast cancer (Dohan et al., 2006; Kogai et al., 2000; Kogai et al., 2004; Kogai et al., 2005; Unterholzner et al., 2006; Willhauck et al., 2008b) suggests a potential role of NIS in diagnosis and therapy of extrathyroidal malignancies.
NIS as a target of genetic alterations causing iodide transport defect (ITD)
Iodide transport defect
Given its essential role in thyroid physiology, changes in NIS expression or function due to NIS gene mutations are expected to cause a broad spectrum of thyroid disorders. In particular, congenital goiter / hypothyroidism caused by defective iodide concentrating ability is suggestive of genetic alterations of the NIS gene. Diagnostic criteria for the syndrome termed ‘iodide transport defect’ (ITD) proposed by Stanbury et al. (Stanbury et al., 1983) are: 1) goiter with hypothyroidism or compensated hypothyroidism, 2) little if any uptake of radioiodine in the thyroid gland, 3) no concentration of iodide by salivary glands and stomach. Another critically important diagnostic clue derives from the response to high dose iodide therapy. Although patients with defective iodide transport respond to thyroid hormone replacement therapy, they should respond equally well to iodide alone with rapid restoration of the euthyroid state, which has been demonstrated in most of the described cases (Wolff, 1983). The age at which patients are first seen, as well as the age of earliest recognition of goiter and/or hypothyroidism, varies from the perinatal period to adulthood. The goiter of patients with ITD varies in size and may be either diffuse or nodular. Histological examination of surgical specimens of resected thyroid glands revealed nodular goiters with many, usually small nodules or adenomas with frequent polymorphism between nodules. There is usually evidence of extensive stimulation of the parenchyma with columnar cells, small follicles, scant colloid, and, often marked nuclear abnormalities (Wolff, 1983). Approximately 55 patients from 29 families that presented a phenotype compatible with iodide transport defect have been described in the literature since the first case was reported by Federman et al. in 1958 (Federman et al., 1958), although the nature of the defect was not clarified until two years later by Stanbury and Chapman (Stanbury et al., 1960). The molecular cloning of NIS in 1996 made it possible for the first time to examine the molecular basis of congenital hypothyroidism due to iodide transport defect defect (Pohlenz et al., 1999), which therefore represented the first group of thyroid diseases that is conclusively caused by a disorder of NIS.
High prevalence of p.Thr354Pro (T354P) NIS gene mutation in Japanese patients with iodide transport defect
Only 9 months after the cloning of NIS, a first homozygous missense mutation in the NIS gene was reported by Fujiwara et al. in a Japanese patient, born to consanguinous parents, who presented with congenital hypothyroidism caused by an iodide transport defect (Fujiwara et al., 1997). The diagnosis was made by a failure to concentrate radioiodine by the salivary gland (saliva/plasma 123I ratio was 1.6 (normal > 20)) and by clinical and biological response to potassium iodide treatment. A mild goiter with multiple nodules had developed at the age of 8 years resulting in a left thyroid lobectomy revealing follicular adenoma. Sequencing of cDNA obtained by reverse transcription of RNA extracted from the patient's thyroid revealed a single base alteration of adenine to cytosine in codon 354 of the NIS gene, which resulted in an exchange of threonine to proline (p.Thr354Pro (T354P)). The same mutation was also found in the patient's genomic DNA. In agreement with the autosomal recessive inheritance pattern of ITD, sequence analysis of the genomic DNA of family members showed that the unaffected parents and two siblings were heterozygous for the mutation, confirming the recessive nature of the inactivating mutation (Fujiwara et al., 1997). Transfection of HEK-293 cells with the mutated symporter revealed complete loss of iodide transport activity, confirming the pathogenic role of this mutation. According to the proposed protein structure, the mutated Thr354 residue lies in the midst of the putative ninth transmembrane segment of the NIS protein (Fig. 1). This transmembrane segment is conserved in the sodium/solute symporter family from mammals to bacteria and also corresponds to a ‘hot spot’ for the human Na+/glucose cotransporter (SGLT1) gene (Fujiwara et al., 1997).
Simultaneously, the same homozygous single base mutation in the ninth transmembrane domain of the NIS protein was identified in another Japanese patient with an iodide accumulation defect, born to consanguinous parents. In this patient a voluminous diffuse goiter was first noted at the age of 18 years. Thyroidal 131I uptake was shown to be low as were saliva/serum and gastric juice/serum 131I ratios, while analysis of 125I-iodinated amino acids revealed normal organification. The patient was euthyroid while consuming normal meals with relatively high iodine content, hypothyroid with iodine-poor meals for 3 weeks, and became euthyroid when large amounts of iodide were administered. At the age of 60 years the patient was reevaluated showing no developmental or intellectual problems, normal physical examination besides a diffusely enlarged thyroid gland in the presence of a euthyroid status. Histologically the thyroid specimen showed diffuse hyperplasia of thyrocytes (Matsuda et al., 1997). The patient's daughter was heterozygous for the mutation and revealed no thyroid abnormalities supporting the recessive nature of the inactivating mutation. COS-7 cells transfected with the mutant NIS cDNA showed markedly decreased iodide uptake confirming that this mutation was the direct cause of the iodide trapping disorder. In addition, Northern blot analysis revealed that NIS mRNA levels were markedly increased in the patient's thyroid gland as compared to normal thyroid tissue suggesting compensation by upregulation of NIS expression probably due to transcriptional autoregulation of NIS expression by iodide itself (Matsuda et al., 1997).
Since the original reports above, the p.Thr354Pro (T354P) mutation has been demonstrated in 14 Japanese patients with an iodide transport defect defect, demonstrating a high prevalence of the p.Thr354Pro (T354P) mutation in Japan (Fujiwara et al., 1997; Fujiwara et al., 1998; Kosugi et al., 1998a; Kosugi et al., 1998b; Matsuda et al., 1997; Tatsumi et al., 1998). In addition, Kosugi et al., who reported the homozygous p.Thr354Pro (T354P) NIS germline mutation in seven Japanese patients from five unrelated families, demonstrated by Western blot analysis that the NIS protein levels in the patients' thyroids were significantly increased. Further, by immunohistochemical staining it was convincingly demonstrated that the p.Thr354Pro (T354P) mutant NIS protein was overexpressed in the basolateral plasma membranes of pateints' thyrocytes confirming proper membrane targeting of the mutant NIS protein (Kosugi et al., 1998b).
In one familial case, three affected siblings were heterozygous for the p.Thr354Pro (T354P) mutation. Because the p.Thr354Pro (T354P) mutation is a recessive mutation and causes iodide transport defect only in the homozygous or compound heterozygous state, another NIS gene mutation was hypothesized to be present in these patients. PCR-directed sequencing of the NIS gene indeed revealed a single T to A transversion at the second base of the 59th codon in exon 1, thereby replacing the normal Val with Glu (p.Val59Glu (V59E) (Fujiwara et al., 2000). The Val59 residue is located in the second transmembrane domain of the human NIS protein, which is highly homologous to the rat NIS protein in this region (Fig. 1). The Val59 residue of the NIS protein and the adjacent amino acid residues are well conserved among members of the sodium-dependent solute symporter family suggesting that this region around the 59th residue plays a functional role within the NIS protein (Fujiwara et al., 2000).
The study of the p.Thr354Pro (T354P) NIS mutation as one of the most prevalent mutations causing congenital iodide transport defect has yielded extremely valuable structure/function information on NIS. Carrasco's group demonstrated that p.Thr354Pro (T354P) protein is expressed and properly targeted to the plasma membrane of COS cells transfected with the p.Thr354Pro (T354P) mutant. Further it was shown by site-directed mutagenesis that lack of iodide transport activity of p.Thr354Pro (T354P) NIS is not due to a structural change in the α-helix induced by proline, but rather due to the absence of a hydroxyl group at the β-carbon of the amino acid residue at position 354. The hydroxyl side chain at position 354 could possibly play a role in the translocation of Na+, and together with the cluster of serine and threonine residues present in helix 9 these hydroxyl groups could be involved in the Na+ binding, translocation or coupling domains. The fact that Thr354 is conserved in several Na+-dependent cotransporters and has been postulated to be a ‘hot spot’ for mutations supports this notion (Levy et al., 1998b). In a more recent study by De la Vieja et al., it was further demonstrated that five β-OH group-containing residues (Thr-351, Ser-353, Thr-354, Ser-356 and Thr-357) and Asn-360, all of which putatively face the same side of the helix in transmembrane segment IX, plus Asp-369, located in the membrane/cytosol interface, play key roles in NIS function and seem to be involved in Na+ binding and /or the lining of the Na+ translocation (De la Vieja et al., 2007b).
Although all of these ITD patients were characterized by the same p.Thr354Pro (T354P) mutation, marked heterogeneity in clinical pictures, especially concerning goiter and hypothyroidism were noted among them. In the case series of Kosugi et al. (Kosugi et al., 1998b) no goiter was observed in two patients, while a diffuse goiter was present in all other cases. Two patients were euthyroid, while three patients had severe hypothyroidism observed in early childhood. In contrast to patients reported by other authors no nodular changes were observed in the thyroid glands of the patients reported by Kosugi et al. (Kosugi et al., 1998b).
Differences in the level of upregulation of NIS expression in patients' thyroids may be related to clinical heterogeneity in ITD patients. Further, the amount of iodine intake influences thyroid function in patients with ITD. During infancy this is dependent on the source of milk, with breast milk containing much larger amounts of iodide than artificial milk, which is even more evident in Japanese patients with large amounts of iodide intake, especially from seaweeds. Two patients in the series published by Kosugi et al. who were fed with artificial milk developed cretinism before 1 year of age (Kosugi et al., 1998b). Only a few of the reported patients had goiter at birth or shortly after birth, while goiter usually developed in later developmental stages. Goiter development is dependent on levels of TSH stimulation, i.e. degree of hypothyroidism, but also on intrathyroidal iodide concentration. Overall, the exact mechanisms explaining the clinical heterogeneity in patients with ITD due to the same NIS mutation have not been characterized in detail yet, and it is likely that additional, as yet unknown factors are involved.
Other well characterized NIS gene mutations causing iodide transport defect
Pohlenz et al. have reported three other NIS gene mutations. A patient of Mexican origin with congenital hypothyroidism resulting from iodide transport defect was shown to be heterozygous for two different mutations in the NIS gene (Pohlenz et al., 1998): a C to G transversion of nucleotide 1146 in exon 6, resulting in Gln 267 to Glu substitution (p.Gln267Glu (Q267E)); and a second C to G transversion of nucleotide 1940, producing a stop codon as well as a downstream cryptic 3′ splice acceptor site in exon 13, resulting in a 67 nucleotide deletion, frameshift, and premature stop (p.Tyr531X (Y531X); p.Ser509ArgfsX7 (Fs-515X)) (Pohlenz et al., 1998) (Fig. 1). The patient presented with a nodular goiter at the age of 12 years, after she had been initially diagnosed with congenital hypothyroidism due to athyreosis at birth, because her thyroid gland could not be visualized by pertechnetate scintigraphy. In the presence of a thyroidal radioiodine uptake below 1% and a low radioiodine saliva/plasma ratio of 2.5, the diagnosis was revised to be ITD at the age of 12 years (Pohlenz et al., 1998). The p.Gln267Glu (Q267E) mutation is located in the intracellular loop between transmembrane segments VII and VIII (De la Vieja et al., 2003) (Fig. 1). Thorough molecular analysis of the p.Gln267Glu (Q267E) NIS substitution by Carrasco's group revealed that the p.Gln267Glu (Q267E) NIS mutation decreases iodide transport activity by lowering the turnover number of the molecule, without affecting NIS expression or trafficking to the plasma membrane, and without altering the affinity of NIS for I- or Na+ (De la Vieja et al., 2003).
In a Brazilian kindred with congenital hypothyroidism, in which the index case was diagnosed at age 3 months with a large nodular goiter and low thyroidal and salivary gland radioiodine uptake, sequencing of the entire NIS cDNA derived from thyroidal mRNA revealed a substitution of the normal cytosine in nucleotide 1163 with an adenine, resulting in a stop at codon 272 (exon 6) (p.Cys272X (C272X)) and expression of a truncated, nonfunctional form of NIS (Pohlenz et al., 1997). This mutation is located in the 4th intracellular loop between transmembrane segments VII and VIII (Fig. 1).
Kosugi et al. have identified two further loss-of-function NIS mutations in three Japanese patients belonging to two families. One patient had a compound heterozygous mutation of p.Thr354Pro (T354P) / p.Gly93Arg (G93R) (Gly93→Arg [GGC→CGC]) and two siblings had a homozygous mutation of p.Gly543Glu (G543E) (Gly543→Glu [GGA→GAA]). p.Gly93Arg (G93R) is located in the third transmembrane segment and p.Gly543Glu (G543E) in the 13th transmembrane segment of the NIS protein (Fig. 1). These NIS mutants had minimal iodide uptake activity in transfected COS-7 cells. While the patient with p.Thr354Pro (T354P) / p.Gly93Arg (G93R) was diagnosed with a diffuse goiter at the age of 16 years, the patients with p.Gly543Glu (G543E) / p.Gly543Glu (G543E) showed severe hypothyroidism from early childhood (Kosugi et al., 1998a). In contrast to the p.Thr354Pro (T354P) and p.Gln267Glu (Q267E) NIS mutants that are correctly synthesized and targeted to the plasma membrane, the p.Gly543Glu (G543E) NIS matures only partially and is not targeted properly to the cell surface and retained intracellularly probably due to faulty folding. These findings indicate that the Gly543 residue plays a significant role in NIS maturation and trafficking (De la Vieja et al., 2005).
A Hutterite family living in central Cancada with extensive consanguinity – the largest ITD family reported to date – included 10 patients identified to carry a homozygous missense loss-of-function mutation of the NIS gene p.Gly395Arg (G395R) (Gly395→Arg [GGA→AGA]) that was localized in the 10th transmembrane segment of the NIS protein (Kosugi et al., 1999) (Fig. 1). The p.Gly395Arg (G395R) NIS mutant did not show iodide uptake activity in transfected COS-7 cells. Analyses by immunoblotting and immunohistochemistry suggested that p.Gly395Arg (G395R) mutant NIS protein was properly synthesized and targeted to the plasma membrane (Kosugi et al., 1999). These findings were confirmed by Dohan et al., who also demonstrated by site-directed mutagenesis that the presence of an uncharged amino acid residue with a small side chain at position 395 is a requirement for NIS function suggesting that glycine 395 is located in a tightly packed membrane-embedded region of NIS. The fact that turnover (Vmax) rather than substrate affinities (Km) was affected by the substitution suggested that the Na+/I- coupling reaction was affected probably due to a hampered conformational change involving transmembrane segment × (Dohan et al., 2002). Interestingly patients harboring the p.Gly395Arg (G395R) mutation did not reveal goiter development, but were all severely hypothyroid (Kosugi et al., 1999). This might be a specific characteristic of the p.Gly395Arg (G395R) mutation, but might also be due to early diagnosis in these patients, who were all diagnosed by neonatal screening and started on T4 treatment at the age of 3-14 days (Kosugi et al., 1999).
More recently, Kosugi et al. identified another and peculiar loss-of-function germline mutation consisting of a large 6192-bp deletion spanning from exon 3 to intron 7 and an inverted insertion of a 431-bp fragment spanning from exon 5 to intron 5 of the NIS gene in two siblings of Spanish origin with ITD (Kosugi et al., 2002b). One sibling had a goiter as a baby and had been treated with thyroid extract, but had severe mental retardation probably due to the delay of initiation and insufficiency of treatment. The other sibling was diagnosed with congenital hypothyroidism at birth and treatment was started immediately, and did not develop goiter or mental retardation. 131I thyroidal uptake and saliva/plasma ratios were low in both patients. Further, the patients remained euthyroid on long-term iodide therapy. The patients were homozygous for the mutation, while their mother was heterozygous. The mutation results in an in-frame 182-amino acid deletion from Met142 in the 4th transmembrane domain to Gln323 in the 4th extracellular loop (Fig. 1). The NIS mutant showed no iodide uptake activity when transfected into COS-7 cells confirming that the mutation was the direct cause of the ITD. Furthermore, it was demonstrated by immunocytochemical staining of transfected cells that the mutant NIS protein was synthesized but mostly accumulated in the cytoplasm and not properly expressed on the cell surface, suggesting impaired plasma membrane targeting (Kosugi et al., 2002b).
The first Italian case of congenital hypothyroidism due to a mutated NIS gene was described by Tonacchera et al. (Tonacchera et al., 2003). It was diagnosed in a 28-year old woman, born from consanguineous parents, with congenital hypothyroism which was first identified at the age of 3 months and treated with thyroxine replacement therapy. The patients had mental retardation, psychomotor defects and bilateral deafness. At the age of 28 years thyroid ultrasound revealed a slightly enlarged thyroid gland with bilateral hypoechoic nodules. A 131I scan showed no radioiodine accumulation in the thyroid gland, salivary glands, or stomach. In addition the saliva/plasma iodide ratio was low. Analysis of the patient's NIS gene revealed a 15 nucleotide deletion of the coding sequence (nt 1314-1328) and the insertion of 15 nt duplicating the first 15 nt of the adjacent intron. This resulted in a homozygous deletion of the five terminal amino acids of exon 11 located in the 6th intracellular loop of the NIS protein between transmembrane segments XI and XII (Fig. 1). Both parents were clinically euthyroid and were heterozygous for this insertion/deletion. COS-7 cells transfected with the mutant p.Ala439_Pro443del NIS construct were unable to concentrate 125I suggesting that the mutation was the direct cause of the ITD in this patient (Tonacchera et al., 2003).
The most recently discovered NIS mutation in ITD was reported by Szinnai et al. in a newborn with symptomatic congenital hypothyroidism and a larger goiter without radioiodine uptake in the thyroid gland (Szinnai et al., 2006). NIS gene sequencing identified a new homozygous mutation p.Arg124His (R124H) in exon 2 (a single G to A nucleotide change at base 718). Both parents were heterozygous for this mutation. This mutation was associated with absence of iodide uptake activity in vitro when transfected into COS-7 cells. Immunocytochemical staining documented correct targeting of the mutated protein to the plasma membrane. According to the current model of the secondary structure of NIS, the p.Arg124His (R124H) mutation is located within the intracellular loop between transmembrane segment III and IV (Fig. 1). The functional role of Arg124 and the adjacent Phe in the NIS protein are unknown, but are highly conserved in the sodium/solute symporter family from mammals to bacteria, and might therefore be involved in Na+ binding (Szinnai et al., 2006).
Genotype-phenotype correlations in iodide transport defect due to NIS mutations
Altogether, since cloning of the NIS gene in 1996, 31 ITD patients with identified NIS mutations have been reported. In these patients 12 different NIS molecular defects have been characterized: 10 mutations (p.Val59Glu (V59E), p.Gly93Arg (G93R), p.Gln267Glu (Q267E), p.Cys272X (C272X), p.Thr354Pro (T354P), p.Gly395Arg (G395R), p.Ser509ArgfsX7 (FS515X), p.Tyr531X (Y531X), p.Gly543Glu (G543E), p.Arg124His (R124H)) and two deletions (p.Met143_Gln323del (DelM143-Q323) and p.Ala439_Pro443del (DelA439-P443)) (Fig. 1) (Table 1). The clinical heterogeneity, in particular concerning onset of hypothyroidism and goiter development, in patients with homozygous p.Thr354Pro (T354P) mutation has already been described above. Hypothyroidism occurred during infancy in three patients, during childhood in four patients, while two siblings were euthyroid. In contrast, six of the 10 patients with p.Gly395Arg (G395R) experienced hypothyroidism during the neonatal period and four patients between the age of 1 and 3 months. Overall, the onset of hypothyroidism was detected during the neonatal period in patients with p.Arg124His (R124H), p.Gly395Arg (G395R), p.Gln267Glu (Q267E) / p.Tyr531X (Y531X), and p.Met143_Gln323del (DelM143-Q323), while it was detected during infancy in patients with p.Cys272X (C272X), p.Ala439_Pro443del (DelA439-P443) and p.Thr354Pro (T354P) and during childhood in patients with p.Gly543Glu (G543E), p.Val59Glu (V59E) / p.Thr354Pro (T354P), and p.Gly93Arg (G93R) / p.Thr354Pro (T354P) (Szinnai et al., 2006). Some degree of developmental delay was present in seven of 11 patients with onset of hypothyroidism during infancy and in four of six patients with onset of hypothyroidism during childhood. The age of onset of hypothyroidism was therefore, at least in part, genotype-specific and correlated with mean residual radioiodine uptake values in the thyroid gland (RIUT): patients with onset of hypothyroidism in the neonatal period showed significantly lower mean residual RIUT values compared with those with onset of hypothyroidism during infancy or childhood, which showed the highest levels of residual RIUT (Szinnai et al., 2006). This observation suggests that genotype-specific residual NIS activity in the thyroid gland may be a major determinant of the onset of hypothyroidism. No correlation with genotype or residual RIUT was observed for goiter onset and severity of hypothyroidism. Goiter was diagnosed at a median age of 11 years in 18 of 21 patients with NIS defects other than p.Gly395Arg (G395R), that did not result in goiter development in any of the 10 Gly395Arg (G395R) patients. Goiter occurred before onset of hypothyroidism, concomitantly with hypothyroidism or after onset of hypothyroidism despite replacement therapy (Szinnai et al., 2006).
Table 1. Identified mutations of the sodium iodide symporter gene causing ITD.
| Mutation | Result | Reference |
|---|---|---|
| p.Thr354Pro (T354P) | Non-functioning NIS protein Impaired Na+ binding Proper membrane targeting |
(Fujiwara et al., 1997; Fujiwara et al., 1998; Kosugi et al., 1998a; Kosugi et al., 1998b; Matsuda et al., 1997; Tatsumi et al., 1998) |
| p.Gln267Glu (Q267E) | Non-functioning NIS protein Decrease of NIS turnover number Proper membrane targeting |
(Pohlenz et al., 1998) |
| p.Tyr531X (Y531X) p.Ser509ArgfsX7 (FS515X) |
67 nt. deletion, frameshift, premature stop, truncated NIS protein | (Pohlenz et al., 1998) |
| p.Cys272X (C272X) | Premature stop, truncated NIS protein | (Pohlenz et al., 1997) |
| p.Gly93Arg (G93R) | Non-functioning NIS protein | (Kosugi et al., 1998a) |
| p.Gly543Glu (G543E) | Non-functioning NIS protein Defective NIS maturation and membrane trafficking |
(Kosugi et al., 1998a) |
| p.Gly395Arg (G395R) | Non-functioning NIS protein Defective Na+/I-coupling Proper membrane targeting |
(Kosugi et al., 1999) |
| p.Val59Glu (V59E) | Non-functioning NIS protein | (Fujiwara et al., 2000) |
| p.Met143_Gln323del (DelM143-Q323) | Non-functioning NIS protein Impaired membrane trafficking |
(Kosugi et al., 2002a) |
| p.Ala439_Pro443del (DelA439-P443) | Non-functioning NIS protein | (Tonacchera et al., 2003) |
| p.Arg124His (R124H) | Non-functioning NIS protein Proper membrane targeting |
(Szinnai et al., 2006) |
It is important to note that normal neonatal TSH screening does not exclude ITD with onset of hypothyroidism in infancy or childhood. Therfore, ITD should be considered when hypothyroidism occurs after the neonatal period, and genetic studies in newborns of families with onset of hypothyroidism in infancy or childhood provide an invaluable tool for preclinical diagnosis of ITD thereby preventing developmental delay.
Conclusion
Iodide transport defect represents the first group of thyroid disease that has been demonstrated to be conclusively caused by a disorder of NIS. Isolation of the rat NIS cDNA and the subsequent elucidation of the exon-intron organization of the human NIS gene, allowed the analysis of the molecular basis of congenital hypothyroidism due to iodide transport defect. So far, 12 different NIS molecular defects have been identified in 31 ITD patients: 10 mutations (p.Val59Glu (V59E), p.Gly93Arg (G93R), p.Gln267Glu (Q267E), p.Cys272X (C272X), p.Thr354Pro (T354P), p.Gly395Arg (G395R), p.Ser509ArgfsX7 (FS515X), p.Tyr531X (Y531X), p.Gly543Glu (G543E), p.Arg124His (R124H)) and two deletions (p.Met143_Gln323del (DelM143-Q323) and p.Ala439_Pro443del (DelA439-P443)). The marked clinical heterogeneity in patients with the same NIS mutation as well as in patients with different NIS mutations without a clear genotype-phenotype correlation, in particular concerning onset and severity of hypothyroidism and goiter, suggests that additional unknown factors are involved in the clinical manifestion of iodide transport defect due to NIS mutations. The study of NIS mutations as molecular basis of ITD has yielded extremely valuable structure/function information on NIS, establishing the functional role of the hydroxyl group at the β-carbon of the residue at position 354, the ability of a charged or large side chain at position 395 to interfere with NIS activity, the significance of the Gln267 residue in maintaining the turnover number of NIS, and the role of the Gly543 residue in NIS maturation and trafficking. In addition, the identification of NIS mutations as underlying cause of ITD also provides an important tool for preclinical diagnosis and genetic counseling of ITD patients.
Acknowledgments
The authors are grateful to Prof. Dr. Peter Lohse, Department of Clinical Chemistry, Ludwig-Maximilians-University Munich, Germany for his generous assistance with the new nomenclature for gene mutations.
This work was supported by grants from the Deutsche Forschungsgemeinschaft, Bonn, Germany (SFB 824) and from the Wilhelm-Sander-Stiftung (2008.037.1) to C. Spitzweg, and by the Mayo Clinic Prostate Cancer SPORE grant from the NCI (CA91956) and the Minnesota Partnership for Biotechnology and Medical Genomics grant 08-06 to J.C. Morris.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ajjan RA, Kamaruddin NA, Crisp M, Watson PF, Ludgate M, Weetman AP. Regulation and tissue distribution of the human sodium iodide symporter gene. Clin Endocrinol. 1998a;49:517–523. doi: 10.1046/j.1365-2265.1998.00570.x. [DOI] [PubMed] [Google Scholar]
- Ajjan RA, Watson PF, Findlay C, Metcalfe RA, Crisp M, Ludgate M, Weetman AP. The sodium iodide symporter gene and its regulation by cytokines found in autoimmunity. J Endocrinol. 1998b;158:351–358. doi: 10.1677/joe.0.1580351. [DOI] [PubMed] [Google Scholar]
- Bizhanova A, Kopp P. Minireview: the sodium iodide symporter NIS and pendrin in iodide homeostasis of the thyroid. Endocrinology. 2009;150:1084–1090. doi: 10.1210/en.2008-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco N. Iodide transport in the thyroid gland. Biochem Biophys Acta. 1993;1154:65–82. doi: 10.1016/0304-4157(93)90017-i. [DOI] [PubMed] [Google Scholar]
- Caturegli P, Hejazi M, Suzuki K, Dohan O, Carrasco N, Kohn LD, Rose NR. Hypothyroidism in transgenic mice expressing IFN-g in the thyroid. Proc Natl Acad Sci USA. 2000;97:1719–1724. doi: 10.1073/pnas.020522597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cengic N, Baker CH, Schutz M, Göke B, Morris JC, Spitzweg C. A novel therapeutic strategy for medullary thyroid cancer based on radioiodine therapy following tissue-specific sodium iodide symporter gene expression. J Clin Endocrinol Metab. 2005;90:4457–4464. doi: 10.1210/jc.2004-2140. [DOI] [PubMed] [Google Scholar]
- Dai G, Levy O, Carrasco N. Cloning and characterization of the thyroid iodide transporter. Nature. 1996;379:458–460. doi: 10.1038/379458a0. [DOI] [PubMed] [Google Scholar]
- De la Vieja A, Ginter CS, Carrasco N. The Q267E mutation in the sodium/iodide symporter NIS) causes congenital ioddie transport defect (ITD) by decreasing NIS turnover. J Cell Sci. 2003;117:677–687. doi: 10.1242/jcs.00898. [DOI] [PubMed] [Google Scholar]
- De la Vieja A, Ginter CS, Carrasco N. Molecular analysis of a congenital iodide transport defect: G543E impairs maturation and trafficking of the Na+/I- symporter. Mol Endocrinol. 2005;19:2847–2858. doi: 10.1210/me.2005-0162. [DOI] [PubMed] [Google Scholar]
- De la Vieja A, Reed MD, Ginter CS, Carrasco N. Amino acid residues in transmembrane segment IX of the Na/I symporter play a role in its Na dependence and are critical for trasnport activity. J Biol Chem. 2007a;282:25290–25298. doi: 10.1074/jbc.M700147200. [DOI] [PubMed] [Google Scholar]
- De la Vieja A, Reed MD, Ginter CS, Carrasco N. Amino acid residues in transmembrane segment IX of the Na+/I- symporter play a role in its Na+ dependence and are critical for transport activity. J Biol Chem. 2007b;282:25290–25298. doi: 10.1074/jbc.M700147200. [DOI] [PubMed] [Google Scholar]
- Dingli D, Diaz RM, Bergert ER, O'Connor MK, Morris JC, Russell SJ. Genetically targeted radiotherapy for multiple myeloma. Blood. 2003a;102:489–496. doi: 10.1182/blood-2002-11-3390. [DOI] [PubMed] [Google Scholar]
- Dingli D, Russell SJ, Morris JC. In vivo imaging and tumor therapy with the sodium iodide symporter. J Cell Biochem. 2003b;90:1079–1086. doi: 10.1002/jcb.10714. [DOI] [PubMed] [Google Scholar]
- Dingli D, Peng KW, Harvey ME, Greipp PR, O'Connor MK, Cattaneo R, Morris JC, Russell SJ. Image-guided radiovirotherapy for multiple myeloma using a recombinant measles virus expressing the thyroidal sodium iodide symporter. Blood. 2004;103:1641–1646. doi: 10.1182/blood-2003-07-2233. [DOI] [PubMed] [Google Scholar]
- Dohan O, Gavrielides MV, Ginter CS, Amzel LM, Carrasco N. Na+/I- symporter activity requires a small and uncharged amino acid residue at position 395. Mol Endocrinol. 2002;16:1893–1902. doi: 10.1210/me.2002-0071. [DOI] [PubMed] [Google Scholar]
- Dohan O, De la Vieja A, Paroder V, Riedel C, Artani M, Reed M, Ginter CS, Carrasco N. The sodium/Iodide symporter (NIS): Characterization, regulation, and medical significance. Endocrine Rev. 2003;24:48–77. doi: 10.1210/er.2001-0029. [DOI] [PubMed] [Google Scholar]
- Dohan O, De la Vieja A, Carrasco N. Hydrocortisone and purinergic signaling stimulate NIS-mediated iodide transport in breast cancer cells. Mol Endocrinol. 2006;20:1121–1137. doi: 10.1210/me.2005-0376. [DOI] [PubMed] [Google Scholar]
- Dwyer RM, Bergert ER, O'Connor MK, Gendler SJ, Morris JC. In vivo radioiodide imaging and treatment of breast cancer xenografts after MUC1-driven expression of the sodium iodide symporter. Clin Cancer Res. 2005a;11:1483–1489. doi: 10.1158/1078-0432.CCR-04-1636. [DOI] [PubMed] [Google Scholar]
- Dwyer RM, Schatz SM, Bergert ER, Myers RM, Harvey ME, Classic KL, Blanco MC, Frisk CS, Marler RJ, Davis BJ, O'Connor MK, Russell SJ, Morris JC. A preclinical large animal model of adenovirus-mediated expression of the sodium-iodide symporter for radioiodie imaging and therapy of locally recurrent prostate cancer. Mol Ther. 2005b;12:835–841. doi: 10.1016/j.ymthe.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Dwyer RM, Bergert ER, O'Connor MK, Gendler SJ, Morris JC. Sodium iodide symporter-mediated radioiodide imaging and therapy of ovarian tumor xenografts in mice. Gene Ther. 2006a;13:60–66. doi: 10.1038/sj.gt.3302599. [DOI] [PubMed] [Google Scholar]
- Dwyer RM, Bergert ER, O'Connor MK, Gendler SJ, Morris JC. Adenovirus-mediated and targeted expression of the sodium-iodide symporter permits in vivo radioiodie imaging and therapy of pancreatic tumors. Hum Gene Ther. 2006b;17:661–668. doi: 10.1089/hum.2006.17.661. [DOI] [PubMed] [Google Scholar]
- Eng PHK, Cardona GR, Fang SL, Previti M, Alex S, Carrasco N, Chin WW, Braverman LE. Escape from the acute Wolff-Chaikoff effect is associated with a decrease in thyroid sodium/iodide symporter messenger ribonucleic acid and protein. Endocrinology. 1999;140:3404–3410. doi: 10.1210/endo.140.8.6893. [DOI] [PubMed] [Google Scholar]
- Federman D, Robbins J, Rall JE. Some observations on cretinism and its treatment. N Engl J Med. 1958;259:610–613. doi: 10.1056/NEJM195809252591302. [DOI] [PubMed] [Google Scholar]
- Fujiwara H, Tatsumi KI, Miki K, Harada T, Miyai K, Takai Si, Amino N. Congenital hypothyroidism caused by a mutation in the Na+/I- symporter. Nature Genet. 1997;16:124–125. doi: 10.1038/ng0697-124. [DOI] [PubMed] [Google Scholar]
- Fujiwara H, Tatsumi KI, Miki K, Harada T, Okada S, Nose O, Kodama S, Amino N. Recurrent T354P mutation of the Na+/I- symporter in patients with iodide transport defect. J Clin Endocrinol Metab. 1998;83:2940–2943. doi: 10.1210/jcem.83.8.5029. [DOI] [PubMed] [Google Scholar]
- Fujiwara H, Tatsumi K, Tanaka S, Kimura M, Nose O, Amino N. A novel V59E missense mutation in the sodium iodide symporter gene in a familiy with iodide transport defect. Thyroid. 2000;10:471–474. doi: 10.1089/thy.2000.10.471. [DOI] [PubMed] [Google Scholar]
- Furlanetto TW, Nguyen LQ, Jameson JL. Estradiol increases proliferation and down-regulates the sodium/iodide symporter gene in FRTL-5 cells. Endocrinology. 1999;140:5705–5711. doi: 10.1210/endo.140.12.7197. [DOI] [PubMed] [Google Scholar]
- Jhiang SM, Cho JY, Ryu KY, De Young BR, Smanik PA, McGaughy VR, Fischer AH, Mazzaferri EL. An immunohistochemical study of Na+/I- symporter in human thyroid tissues and salivary gland tissues. Endocrinology. 1998;139:4416–4419. doi: 10.1210/endo.139.10.6329. [DOI] [PubMed] [Google Scholar]
- Kawaguchi A, Ikeda M, Endo T, Kogai T, Miyazaki A, Onaya T. Transforming growth factor-β1 suppresses thyrotropin-induced Na+/I- symporter messenger RNA and protein levels in FRTL-5 rat thyroid cells. Thyroid. 1997;7:789–794. doi: 10.1089/thy.1997.7.789. [DOI] [PubMed] [Google Scholar]
- Klutz K, Russ V, Willhauck MJ, Wunderlich N, Zach C, Gildehaus FJ, Göke B, Wagner E, Ogris M, Spitzweg C. Targeted radioiodine therapy of neuroblastoma tumors following systemic non-viral delivery of the sodium iodide symporter (NIS) gene. Clin Cancer Res. 2009 doi: 10.1158/1078-0432.CCR-09-0851. in press. [DOI] [PubMed] [Google Scholar]
- Kogai T, Schultz J, Johnson L, Huang M, Brent G. Retinoic acid induces sodium/iodide symporter gene expression and radioiodine uptake in the MCF-7 breast cancer cell line. Proc Natl Acad Sci USA. 2000;97:8519–8524. doi: 10.1073/pnas.140217197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogai T, Kanamoto Y, Che LH, Taki K, Moatamed F, Schultz JJ, Brent GA. Systemic retinoic acid treatment induces sodium/iodide symporter expression and radioiodine uptake in mouse breast cancer models. Cancer Res. 2004;64:415–422. doi: 10.1158/0008-5472.can-03-2285. [DOI] [PubMed] [Google Scholar]
- Kogai T, Kanamoto Y, Li AI, Che LH, Ohashi E, Taki K, Chandraratna RA, Saito T, Brent GA. Differential regulation of sodium/iodide symporter gene expression by nuclear receptor ligands in MCF-7 breast cancer cells. Endocrinology. 2005;146:3059–3069. doi: 10.1210/en.2004-1334. [DOI] [PubMed] [Google Scholar]
- Kosugi S, Inoue S, Matsuda A, Jhiang SM. Novel, missense and loss-of-function mutations in the sodium/iodide symporter gene causing iodide transport defect in three Japanese patients. J Clin Endocrinol Metab. 1998a;83:3373–3376. doi: 10.1210/jcem.83.9.5245. [DOI] [PubMed] [Google Scholar]
- Kosugi S, Sato Y, Matsuda A, Ohyama Y, Fujieda K, Hiroaki I, Kameya T, Isozaki O, Jhiang SM. High prevalence of T354P sodium/iodide symporter gene mutation in Japanese patients with iodide transport defect who have heterogeneous clinical pictures. J Clin Endocrinol Metab. 1998b;83:4123–4129. doi: 10.1210/jcem.83.11.5229. [DOI] [PubMed] [Google Scholar]
- Kosugi S, Bhayana S, Dean HJ. A novel mutation in the sodium/iodide symporter gene in the largest family with iodide transport defect. J Clin Endocrinol Metab. 1999;84:3248–3253. doi: 10.1210/jcem.84.9.5971. [DOI] [PubMed] [Google Scholar]
- Kosugi S, Okamoto H, Tamada A, Sanchez-Franco F. A novel peculiar mutation in the sodium/iodide symporter gene in Spanish siblings with iodide transport defect. J Clin Endocrinol Metab. 2002a;87:3830–3836. doi: 10.1210/jcem.87.8.8767. [DOI] [PubMed] [Google Scholar]
- Kosugi S, Okamoto H, Tamada A, Sanchez-Franco F. A novel peculiar mutation in the sodium/iodide symporter gene in Spanisch siblings with iodide transport defect. J Clin Endocrinol Metab. 2002b;87:3830–3836. doi: 10.1210/jcem.87.8.8767. [DOI] [PubMed] [Google Scholar]
- Levy O, De la Vieja A, Carrasco N. The Na+/I- symporter (NIS): Recent advances. J Bioenerget Biomembr. 1998a;30:195–206. doi: 10.1023/a:1020577426732. [DOI] [PubMed] [Google Scholar]
- Levy O, Ginter CS, De la Vieja A, Levy D, Carrasco N. Identification of a structural requirement for thyroid Na+/I- symporter (NIS) function from analysis of a mutation that causes human congenital hypothyroidism. FEBS Letters. 1998b;429:36–40. doi: 10.1016/s0014-5793(98)00522-5. [DOI] [PubMed] [Google Scholar]
- Matsuda A, Kosugi S. A homozygous missense mutation of the sodium/iodide symporter gene causing iodide transport defect. J Clin Endocrinol Metab. 1997;82:3966–3971. doi: 10.1210/jcem.82.12.4425. [DOI] [PubMed] [Google Scholar]
- Merron A, Peerlinck I, Martin-Duque P, Burnet J, Quintanilla M, Mather S, Hingorani M, Harrington KJ, Vassaux G. SPECT/CT imaging of oncolytic adenovirus propagation in tumours in vivo using the Na/I symporter as a reporter gene. Gene Ther. 2007;14:1731–1738. doi: 10.1038/sj.gt.3303043. [DOI] [PubMed] [Google Scholar]
- Pekary AE, Hershman JM. Tumor necrosis factor, ceramide, transforming growth factor-beta1, and aging reduce Na+/I- symporter messenger ribonucleic acid levels in FRTL-5 cells. Endocrinology. 1998;139:703–712. doi: 10.1210/endo.139.2.5760. [DOI] [PubMed] [Google Scholar]
- Pohlenz J, Mederios-Neto G, Gross JL, Silveiro SP, Knobel M, Refetoff S. Hypothyroidism in a Brazilian kindred due to iodide trapping defect caused by a homozygous mutation in the sodium/iodide symporter gene. Biochem Biophys Res Commun. 1997;240:488–491. doi: 10.1006/bbrc.1997.7594. [DOI] [PubMed] [Google Scholar]
- Pohlenz J, Rosenthal IM, Weiss RE, Jhiang SM, Burant C, Refetoff S. Congenital hypothyroidism due to mutations in the sodium/iodide symporter. Identification of a nonsense mutation producing a downstream cryptic 3′ splice site. J Clin Invest. 1998;101:1028–1035. doi: 10.1172/JCI1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlenz J, Refetoff S. Mutations in the sodium/iodide symporter (NIS) gene as a cause for iodide transport defects and congenital hypothyroidism. Biochimie. 1999;81:469–476. doi: 10.1016/s0300-9084(99)80097-2. [DOI] [PubMed] [Google Scholar]
- Riedel C, Levy O, Carrasco N. Post-transcriptional regulation of the sodium/iodide symporter (NIS) by thyrotropin. J Biol Chem. 2001;276:21458–21463. doi: 10.1074/jbc.M100561200. [DOI] [PubMed] [Google Scholar]
- Scholz IV, Cengic N, Baker CH, Harrington KJ, Maletz K, Bergert ER, Vile R, Göke B, Morris JC, Spitzweg C. Radioiodine therapy of colon cancer following tissue-specific sodium iodidie symporter gene transfer. Gene Ther. 2005;12:272–280. doi: 10.1038/sj.gt.3302410. [DOI] [PubMed] [Google Scholar]
- Schröder-van der Elst JP, van der Heide D, Kastelijn J, Rousset B, Obregon MJ. The expression of the sodium/iodide symporter is up-regulated in the thyroid of fetuses of iodine-deficient rats. Endocrinology. 2001;142:3736–3741. doi: 10.1210/endo.142.9.8377. [DOI] [PubMed] [Google Scholar]
- Smanik PA, Liu Q, Furminger TL, Ryu K, Xing S, Mazzaferri EL, Jhiang SM. Cloning of the human sodium iodide symporter. Biochem Biophys Res Commun. 1996;226:339–345. doi: 10.1006/bbrc.1996.1358. [DOI] [PubMed] [Google Scholar]
- Smanik PA, Ryu KY, Theil KS, Mazzaferri EL, Jhiang SM. Expression, exon-intron organization, and chromosome mapping of the human sodium iodide symporter. Endocrinology. 1997;138:3555–3558. doi: 10.1210/endo.138.8.5262. [DOI] [PubMed] [Google Scholar]
- Spitzweg C, Joba W, Eisenmenger W, Heufelder AE. Analysis of human sodium iodide symporter gene expression in extrathyroidal tissues and cloning of its complementary deoxyribonucleic acids from salivary gland, mammary gland, and gastric mucosa. J Clin Endocrinol Metab. 1998;83:1746–1751. doi: 10.1210/jcem.83.5.4839. [DOI] [PubMed] [Google Scholar]
- Spitzweg C, Joba W, Morris JC, Heufelder AE. Regulation of sodium-iodide symporter gene expression in FRTL-5 rat thyroid cells. Thyroid. 1999a;9:821–830. doi: 10.1089/thy.1999.9.821. [DOI] [PubMed] [Google Scholar]
- Spitzweg C, Joba W, Schriever K, Goellner JR, Morris JC, Heufelder AE. Analysis of human sodium iodide symporter immunoreactivity in human exocrine glands. J Clin Endocrinol Metab. 1999b;84:4178–4184. doi: 10.1210/jcem.84.11.6117. [DOI] [PubMed] [Google Scholar]
- Spitzweg C, Zhang S, Bergert ER, Castro MR, McIver BD, Heufelder AE, Tindall DJ, Young CYF, Morris JC. Prostate-specific antigen (PSA) promoter-driven androgen-inducible expression of sodium iodide symporter in prostate cancer cell lines. Cancer Res. 1999c;59:2136–2141. [PubMed] [Google Scholar]
- Spitzweg C, O'Connor MK, Bergert ER, Tindall DJ, Young CYF, Morris JC. Treatment of prostate cancer by radioiodine therapy after tissue-specific expression of the sodium iodide symporter. Cancer Res. 2000;60:6526–6530. [PubMed] [Google Scholar]
- Spitzweg C, Dietz AB, O'Connor MK, Bergert ER, Tindall DJ, Young CYF, Morris JC. In vivo sodium iodide symporter gene therapy of prostate cancer. Gene Ther. 2001a;8:1524–1531. doi: 10.1038/sj.gt.3301558. [DOI] [PubMed] [Google Scholar]
- Spitzweg C, Dutton CM, Castro MR, Bergert ER, Goellner JR, Heufelder AE, Morris JC. Expression of the sodium iodide symporter in human kidney. Kidney Int. 2001b;59:1013–1023. doi: 10.1046/j.1523-1755.2001.0590031013.x. [DOI] [PubMed] [Google Scholar]
- Spitzweg C, Harrington KJ, Pinke LA, Vile RG, Morris JC. The sodium iodide symporter and its potential in cancer therapy. J Clin Endocrinol Metab. 2001c;86:3327–3335. doi: 10.1210/jcem.86.7.7641. [DOI] [PubMed] [Google Scholar]
- Spitzweg C, Morris JC. The sodium iodide symporter: its pathophysiological and therapeutic implications. Clin Endocrinol. 2002;57:559–574. doi: 10.1046/j.1365-2265.2002.01640.x. [DOI] [PubMed] [Google Scholar]
- Spitzweg C, Baker CH, Bergert ER, O'Connor MK, Morris JC. Image-guided radioiodide therapy of medullary thyroid cancer following carcinoembryonic antigen (CEA)-promoter-targeted sodium iodide symporter (NIS) gene expression. Hum Gene Ther. 2007;18:916–924. doi: 10.1089/hum.2007.081. [DOI] [PubMed] [Google Scholar]
- Stanbury JB, Chapman EM. Congenital hypothyroidism with goiter: absence of an iodide-concentrating mechanism. Lancet. 1960;1:1162–1165. doi: 10.1016/s0140-6736(60)91042-4. [DOI] [PubMed] [Google Scholar]
- Stanbury JB, Dumont JE. Familial goiter and related disorders The metabolic basis of inherited disease. McGraw-Hill; New York: 1983. pp. 231–269. [Google Scholar]
- Szinnai G, Kosugi S, Derrien C, Lucidarme N, David V, Czernichow P, Polak M. Extending the clinical heterogeneity of iodide transport defect (ITD): a novel mutation R124H of the sodium/iodide symporter gene and review of genotype-phenotype correlations in ITD. J Clin Endocrinol Metab. 2006;91:1199–1204. doi: 10.1210/jc.2005-1832. [DOI] [PubMed] [Google Scholar]
- Tatsumi KI, Miyai K, Amino N. Genetic basis of congenital hypothyroidism: abnormalities in the TSHbgene, the PIT1 gene, and the NIS gene. Clin Chem Lab Med. 1998;36:659–662. doi: 10.1515/CCLM.1998.117. [DOI] [PubMed] [Google Scholar]
- Tazebay UH, Wapnir IL, Levy O, Dohan O, Zuckier LS, Zhao QH, Deng HF, Amenta PS, Fineberg S, Pestell RG, Carrasco N. The mammary gland iodide transporter is expressed during lactation and in breast cancer. Nat Med. 2000;6:871–878. doi: 10.1038/78630. [DOI] [PubMed] [Google Scholar]
- Tonacchera M, Agretti P, De Marco G, Elisei R, Perri A, Ambrogini E, De Servi M, Cecarelli C, Viacava P, Refetoff S, Panunzi C, Bitti MLM, Vitti P, Chiovato L, Pinchera A. Congenital hypothyroidism due to a new deletion in the sodium/iodide symporter protein. Clin Endocrinol. 2003;59:500–506. doi: 10.1046/j.1365-2265.2003.01877.x. [DOI] [PubMed] [Google Scholar]
- Unterholzner S, Willhauck MJ, Cengic N, Schütz M, Göke B, Morris JC, Spitzweg C. Dexamethasone stimulation of retinoic acid-induced sodium iodide symporter expression and cytotoxicity of 131-I in breast cancer cells. J Clin Endocrinol Metab. 2006;91:69–78. doi: 10.1210/jc.2005-0779. [DOI] [PubMed] [Google Scholar]
- Uyttersprot N, Pelgrims N, Carrasco N, Gervy C, Maenhaut C, Dumont JE, Miot F. Moderate doses of iodide in vivo inhibit cell proliferation and the expression of thyroperoxidase and Na+/I- symporter mRNAs in dog thyroid. Mol Cell Endocrinol. 1997;131:195–203. doi: 10.1016/s0303-7207(97)00108-1. [DOI] [PubMed] [Google Scholar]
- Van Sande J, Massart C, Beauwens R, Schoutens A, Costagliola S, Dumonat JE, Wolff J. Anion selectivity by the sodium iodide symporter. Endocrinology. 2003;144:247–252. doi: 10.1210/en.2002-220744. [DOI] [PubMed] [Google Scholar]
- Willhauck MJ, Sharif Samani B, Gildehaus FJ, Wolf I, Senekowitsch-Schmidtke R, Stark HJ, Göke B, Morris JC, Spitzweg C. Application of 188Re as an alternative radionuclide for treatment of prostate cancer following tumor-specific sodium iodide symporter (NIS) gene expression. J Clin Endocrinol Metab. 2007;92:4451–4458. doi: 10.1210/jc.2007-0402. [DOI] [PubMed] [Google Scholar]
- Willhauck MJ, Sharif-Samani B, Cengic N, Mohr L, Geissler M, Göke B, Morris JC, Spitzweg C. Alpha fetoprotein promoter-targeted sodium iodide symporter gene therapy f hepatocellular carcinoma. Gene Ther. 2008a;15:214–223. doi: 10.1038/sj.gt.3303057. [DOI] [PubMed] [Google Scholar]
- Willhauck MJ, Sharif-Samani B, Senekowitsch-Schmidtke R, Wunderlich N, Göke B, Morris JC, Spitzweg C. Functional sodium iodide symporter expression in breast cancer xenografts in vivo after systemic treatment with retinoic acid and dexamethasone. Breast Cancer Res Treat. 2008b;109:263–272. doi: 10.1007/s10549-007-9646-0. [DOI] [PubMed] [Google Scholar]
- Willhauck MJ, Sharif-Samani B, Wunderlich N, Wolf I, Senekowitsch-Schmidtke R, Meyer GJ, Knapp WH, Göke B, Morris JC, Spitzweg C. The potential of astatine-211 for NIS-mediated radionuclide in prostate cancer. Eur J Nucl Med Mol Imaging. 2008c;35:1272–1281. doi: 10.1007/s00259-008-0775-4. [DOI] [PubMed] [Google Scholar]
- Wolff J, Chaikoff IL. Plasma inorganic iodide as a homeostatic regulator of thyroid function. J Biol Chem. 1948;174:555–564. [PubMed] [Google Scholar]
- Wolff J, Chaikoff IL, Goldberg RC, Meier JR. The temporary nature of the inhibitory action of excess iodide on organic iodine synthesis in the normal thyroid. Endocrinology. 1949;45:504–513. doi: 10.1210/endo-45-5-504. [DOI] [PubMed] [Google Scholar]
- Wolff J. Congenital goiter with defective iodide transport. Endocrine Rev. 1983;4:240–254. doi: 10.1210/edrv-4-3-240. [DOI] [PubMed] [Google Scholar]