Abstract
The histopathological characteristics of Alzheimer’s disease (AD) are amyloid-β (Aβ) containing plaques and neurofibrillary tangles (NFTs) as well as neuronal and synaptic loss. Until today, the underlying mechanisms of the interplay of plaques and tangles remained unresolved. There is increasing evidence that mitochondrial dysfunction might be a possible link, as revealed by studies in several APP and tau transgenic mouse models. Recently, we examined mitochondrial function in a novel triple transgenic mouse model (pR5/APP/PS2)—tripleAD mice—that combines both pathologic features of the disease in brain. Using comparative, quantitative proteomics (iTRAQ) and mass spectroscopy, we found a massive deregulation of 24 proteins, of which one third were mitochondrial proteins mainly related to complexes I and IV of the oxidative phosphorylation system (OXPHOS). Remarkably, deregulation of complex I was related to tau, whereas deregulation of complex IV was Aβ dependent, both at the protein and activity levels. The tripleAD mice showed synergistic effects of Aβ and tau already at the age of 8 months, resulting in a depolarized mitochondrial membrane potential. At 12 months, the strongest defects on OXPHOS, synthesis of ATP and reactive oxygen species, were exhibited in the tripleAD mice, again emphasizing synergistic, age-associated effects of Aβ and tau in impairing mitochondria. This review highlights the convergence of Aβ and tau on mitochondria and establishes a molecular link in AD pathology in vivo.
Keywords: Alzheimer's disease, Amyloid-β peptide, Energy metabolism, Mitochondria, Oxidative phosphorylation system (OXPHOS), Tau protein, Transgenic AD mouse models
Introduction
Alzheimer’s disease (AD) is the most common neurodegenerative disorder affecting around 15 million people worldwide. Because of the increase in life expectancy already for 2020, the number of cases will rise to about 30 million people worldwide. Although the hallmark lesions of the disease were described by Alois Alzheimer already in 1906—extracellular amyloid plaques mainly composed of Aβ and intracellular neurofibrillary tangles (NFTs) built up of hyperphosphorylated tau—the molecular mechanisms underlying the disease are still unknown. However, more recently, energy deficiency and mitochondrial dysfunction have been recognized as a prominent, early event in AD [1–11]. The successful development of single, double, and recently triple transgenic mouse models that mimic diverse aspects of the disease facilitated the investigation of pathogenic mechanisms in AD and assisted in an understanding of the interplay of Aβ and tau on bioenergetic processes in vivo [12, 13].
Pathophysiological Alterations in Transgenic AD Mouse Models
APP Transgenic Mice
In 1995, Games and co-workers established the first Aβ plaque-forming mouse model that expressed high levels of the disease-linked V717F mutant form of APP in the brain [14]. These PD-APP mice (PDGF-β promotor) showed many pathological features of AD, including extensive deposition of extracellular amyloid plaques, astrocytosis, and neuritic dystrophy [14]. Subsequently, several APP-based transgenic models have been developed [15–19] that were helpful in addressing aspects of Aβ toxicity and age-dependent cognitive decline as well as testing therapies like vaccination trials [20, 21]. Finally, the discovery of FAD mutations in the presenilin-encoding PSEN genes which affect APP processing opened the path for PS1 and PS2 transgenic mouse models, which were subsequently used to establish double transgenic APP/PSEN mouse models [12].
Tau Transgenic Mice
Also in 1995, Götz and colleagues established the first tau transgenic mouse model, expressing a wild-type form of the longest human brain tau isoform hTau40 (441 amino acids), using the hThy1 promoter for neuronal expression [22]. Despite the lack of NFT pathology, these mice modeled selected aspects of human AD, such as the somatodendritic localization of hyperphosphorylated tau and, therefore, represented an early “pre-NFTs” phenotype. Once the first pathogenic mutations were identified in the MAPT gene in a familial form of frontotemporal dementia (FTD), FTDP-17, in 1998, several groups expressed mutant forms of MAPT to achieve a more advanced pathology. For example, P301L tau expressing pR5 mice (longest four-repeat (4R2N) tau together with the P301L mutation) develop aggregated forms of hyperphosphorylated tau and NFTs [23–26]. Moreover, these mice showed age-related behavioral impairment in amygdala- and hippocampus-dependent tasks which could be correlated with the aggregation pattern of the transgene [27, 28].
Effects of Aβ and Tau on Tau Pathology and APPxTau Double Transgenic Mice
Conventional transgenic mouse models for the APP- and tau-related pathologies reproduce only some selected aspects of the human disease. Therefore, in 2001 two new approaches were pursued to allow studying the synergistic effects of both histopathological hallmarks. The group of Mike Hutton generated a double transgenic mouse model by crossing P301L mutant tau transgenic JNPL3 mice (shortest four-repeat (4R0N) tau together with the P301L mutation) with APPsw transgenic Tg2576 mice (KM670/671NL) [29]. The resulting TAPP mice showed detectable NFTs as early as 3 months of age in both the spinal cord and pons. These were consistently present and numerous as the mice aged, especially in limbic areas of (9–11 months old) female mice. Amyloid plaques were evident as early as 6 months of age, similar in morphology, distribution, and density to those in the parental Tg2576 strain. As an indication for the role of APP or Aβ on NFT formation, the double transgenic TAPP mice showed substantially enhanced tau pathology in the limbic system and olfactory cortex as compared to the single transgenic tau mice [29]. An interaction of Aβ and tau pathology was also shown by Götz and colleagues, by injecting synthetic Aβ1–42 fibrils into brains of P301L tau transgenic pR5 mice [24]. This led to a 5-fold increase in NFT pathology in 6-month-old mice already 18 days after injection. Data from our group could confirm a synergistic effect of Aβ and tau on mitochondrial function when cortical brain cells of P301L tau transgenic pR5 mice were treated with different Aβ1–42 conformations [30].
Not only synthetic Aβ induces an increase in tau pathology, as the injection of diluted brain extract from aged APP23 transgenic mice (expressing the KM670/671NL mutant APP) into the cerebellum of young B6/P301L tau transgenic mice (obtained through backcrossing the JNPL3 mice with C57BL/6J mice) also shows an effect [31]. This treatment leads to an induction of tau pathology at the injection site, but interestingly also in areas with a neuronal projection such as the entorhinal cortex and the amygdala. Additionally, in double transgenic APPxTau mice, a neurofibrillary pathology was induced in vivo [31]. Recently, the work from Clavaguera and colleagues showed that the injection of brain extracts from P301S mice (expressing the shortest four-repeat tau isoform bearing the P301S mutation) into brains of wild-type human tau transgenic ALZ17 mice caused the assembly of wild-type human tau into filaments [32]. In contrast, mouse lines that express wild-type tau (such as the ALZ17 strain) do normally not produce tau filaments nor do they show neurodegeneration [33, 34]. Interestingly, it seems that the tau pathology has been transmitted as a spreading of tau pathology was found from the site of injection to neighboring brain regions [32].
Triple Transgenic Mice
The fact that mutations in the PSEN1 and 2 genes affect APP processing was utilized to develop triple AD models that combine an enhanced Aβ and tau pathology in one model [35–37]. Moreover, recent studies found an active PS2-containing γ-secretase complex in mitochondria [38] and the knockout of PS2 impaired mitochondrial functionality by reducing mitochondrial membrane potential and lowering the basal respiratory rate [39]. The first triple AD mouse model (3xTg-AD) was generated 2003 by LaFerla and colleagues, harboring the PS1 M146V mutation and co-expressing mutant tau (P301L) as well as APPsw (KM670/671NL) [35]. This model, which exhibits plaques and tangles, shows behavioral and neuronal symptoms of AD including synaptic dysfunction and LTP deficits [40, 41]. Recently, another triple transgenic mouse model which co-expresses mutant tau (P301L), PS2 (N141I), and APPsw (KM670/671NL) was established, first termed TauPS2APP triple [37] and subsequently triple AD transgenic mice [10]. The tripleAD mice develop tau and amyloid deposits in an age-dependent manner. [37]. At the age of 4 months, tau accumulation was detected within the subiculum and the CA1 region, where most amyloid deposits were found. At 8 months, the number of amyloid plaques and intracellular tau deposits increased considerably within the subiculum and the CA1 region while rare amyloid plaques as well as few tau deposits appeared in the M1/M2 and somatosensory cortex. While tau phosphorylated at pT231 was already present at high levels in tripleAD mice at 4 months of age, but with almost unchanged levels over the next 12 months, tau phosphorylated at pS422 was barely detectable at 4 months, but levels increased roughly 10-fold at an age of 16 months. Of note, this suggests that accumulation of Aβ in tripleAD mice impacts on tau pathology by increasing the phosphorylation of tau at S422, but not at T231 [37]. In contrast, the 3xTg-AD model did not exhibit conformational changes of tau or immunoreactivity with phospho-specific tau markers before the age of 12 months [35, 40]. Finally, NFT pathology was reported in 16-month-old tripleAD mice and, relatively late, at the age of 18–24 months in the 3xTg-AD model [35, 41]. Furthermore, tripleAD mice show impaired spatial learning already at 4 months of age [37]. Similarly, cognitive impairment manifested in 3xTg-AD mice at 4–6 months of age [41, 42].
However, a molecular link between Aβ and tau protein in AD pathology was still missing in vivo. The tripleAD model is therefore particularly suited to study the relationship between Aβ and tau in an age-related way. Moreover, resulting from crossing PS2APP mice with P301Ltau transgenic pR5 mice, the tripleAD model offers the advantage of analyzing tau and Aβ pathology together and separately, while this is not possible in the 3xTg-AD mice generated by co-injection of DNA. It has been shown in the tripleAD model that Aβ accumulation leads to the development of tau phosphorylation at the specific AD-epitope Ser422 [37]. Although the mice do not develop extensive neuronal loss or pronounced cognitive deficits, the progression of biochemical changes and histopathological features is reminiscent of the pathogenic progress observed in AD. Consequently, this model may be very useful for assessing therapeutic interventions addressing amyloidoses and/or tau pathology. Importantly, behavioral deficits are present before the detection of any protein aggregates which is especially meaningful considering the paradigm of early mitochondrial dysfunction reported in AD [37].
Evidence for Mitochondrial Dysfunction in Transgenic Mouse Models
APP Transgenic Mice
Early energy dysfunction characterized by a decreased mitochondrial membrane potential, ATP level, and complex IV activity has been reported for 3- and 6-month-old APP transgenic mice (APP; Swedish (KM670/671NL) and London (V717I) mutation) [9]. These mice showed also increased levels of 4-hydroxynonenal, a marker of lipid oxidation, and reduced activity of Cu/Zn superoxide dismutase [43]. Interestingly, mitochondrial defects such as the decrease of complex IV activity in 3-month-old APP transgenic mice were already observed in the absence of plaques but in the presence of increased Aβ levels in brain [9, 44]. Furthermore, an age-dependent impairment of oxygen consumption such as a decrease of state 3 and uncoupled respiration were observed in APP transgenic mice compared to aged-matched controls [9, 45, 46]. In addition, APP/PS1 transgenic mice, which in contrast to APP transgenic mice exhibit Aβ plaques already at an age of 3 months, presented stronger reductions in mitochondrial membrane potential and ATP levels compared to aged-matched APP transgenic mice. Consequently, Aβ-dependent mitochondrial dysfunction starts already at a very young age and accelerates substantially with increasing age as does Aβ plaque load [7]. Moreover, a mitochondrial accumulation of Aβ has been shown in AD and APP transgenic mouse brain [3, 45, 47]. In transgenic APP mice expressing APP V717/F and the APPsw mutation, mitochondrial Aβ accumulation increased at around 4 months of age, well before the formation of plaques [45]. Taken together, these findings are in line with the recently proposed hypothesis of an intracellular Aβ toxicity cascade which suggests that the toxic Aβ species intervening in molecular and biochemical abnormalities may be intracellular oligomeric aggregates instead of extracellular, insoluble plaques [3, 48].
The involvement of mitochondria in the pathogenic pathway of Aβ was confirmed by specific binding of Aβ and APP to mitochondrial proteins which causes energy impairment and cell physiology defects. Firstly, Aβ specifically binds to the mitochondrial Aβ-binding alcohol dehydrogenase (ABAD) [3], a mitochondrial matrix protein which is up-regulated in the temporal lobe of AD patients as well as in APP transgenic mice [49–51]. The Aβ–ABAD interaction caused elevated reactive oxygen species (ROS) production, cell death as well as spatial learning and memory deficits in 5-month-old APP/ABAD double transgenic mice. The investigation of the crystal structure of ABAD–Aβ demonstrated that the formation of the complex prevents the binding of NAD+ to ABAD, thereby changing mitochondrial membrane permeability [52] and reducing the activities of respiratory enzymes [3] which then may lead to mitochondrial failure. Secondly, mitochondrial Aβ may interact with cyclophilin D (CypD), an integral part of the mitochondrial permeability transition pore (mPTP) which potentiates free radical production, causes synaptic failure, and promotes opening of the mPTP leading to apoptosis [53]. Finally, the group of Anandatheerthavarada observed an accumulation of APP in mitochondrial membranes leading to mitochondrial dysfunction in neuronal cells of APP transgenic mice (Tg2576) [54, 55].
The development of sophisticated proteomic methods allowed the examination of synaptosomal fractions from APP transgenic mice (Tg2576) and revealed a massive neuronal decay and synapse loss as the final consequence from all pathological changes occurring in AD [56]. Additional studies revealed significant differences in mitochondrial hsp70 and protein subunit composition of respiratory chain complexes I and III in this transgenic mouse model [46].
Finally, the critical role of mitochondria in the early pathogenesis of AD may make them attractive as a preferential target for treatment strategies such as antioxidants. Transgenic mice modeling some pathological aspects are hence very valuable in monitoring therapeutic interventions at the mitochondrial level. In agreement, recent data suggest that natural plant antioxidants such as a standardized Ginkgo biloba extract or the green tea component epigallocatechin-3-gallate may be promising treatment strategies. In addition to their anti-oxidative properties, these compounds stabilize mitochondrial functions such as the mitochondrial membrane potential, ATP levels, and mitochondrial respiratory complexes [57–59]. Moreover, in APP transgenic mouse models, an anti-amyloidogenic effect of these compounds was reported by inhibiting amyloid fibril formation either by a direct interaction with Aβ [60, 61] or by activating the α-secretase pathway [62]. In view of the increasing interest in mitochondrial protection as a treatment strategy in dementia, the findings of a substantial protection of mitochondria by natural antioxidants against Aβ-induced dysfunction deserves further attention.
Tau Transgenic Mice
Mitochondrial defects in AD are in agreement with the axon transport failure hypothesis. Hyperphosphorylated tau may block the transport of mitochondria leading to energy deprivation and oxidative stress at the synapse as well as to neurodegeneration [63–66]. Transgenic pR5 mice overexpressing the P301L mutant human tau protein exhibit an accumulation of hyperphosphorylated tau and develop NFTs [24]. A mass-spectrometric analysis of the brain proteins from these mice revealed deregulation of mitochondrial respiratory chain complex components (including complex V), antioxidant enzymes, and synaptic proteins [67]. Functional analysis showed mitochondrial dysfunction in pR5 mice together with a reduced complex I activity and, with age, impaired mitochondrial respiration and ATP synthesis. Mitochondrial dysfunction was associated with higher levels of ROS in aged pR5 mice. Increased tau pathology as in aged homozygous pR5 mice revealed modified lipid peroxidation levels and up-regulation of antioxidant enzymes in response to oxidative stress [67]. These findings demonstrated for the first time that not only the Aβ but also the tau pathology acts on the enzyme metabolism of the brain and the oxidative conditions in AD. One mechanism proposed is that tau accumulation could have direct repercussions on the mitochondria as the accumulation of increasingly insoluble ATP synthase α-chain together with NFTs has been shown in AD brains [68]. Interestingly, the deleterious effect of tau on mitochondria may be reciprocal as mitochondrial stress led to tau hyperphosphorylation in a mouse model lacking the detoxifying enzyme superoxide dismutase 2 (Sod2−/−) [69]. Furthermore, the inhibition of complex I with annonacin led beside a concentration-dependent decrease of ATP levels to a redistribution of tau from the axons to the cell body as well as a retrograde transport of mitochondria and finally to cell death [70].
Triple Transgenic Mice
Although Aβ and tau pathologies are both common features in AD, it is still inexplicable how they relate to each other. However, a close relationship between mitochondrial failure and Aβ on the one hand and tau on the other hand has been demonstrated. Therefore, could mitochondria be the point of convergence of the two unquestionable pathologic hallmarks of the disease? Aβ aggregates and hyperphosphorylated tau may block the transport of mitochondria leading to bioenergetic defects and cell death [65, 71]. Moreover, elevated tau may inhibit the transport of APP into axons and dendrites, causing impaired axonal transport suggesting a linkage between tau and APP [63, 64]. The development of triple transgenic mouse models combining Aβ and tau pathologies in recent years was helpful in investigating the precise impact of both lesions on the mitochondrial respiratory machinery and energy homeostasis in vivo. Data from our group indicate that mitochondria of tau transgenic pR5 mice show increased vulnerability towards an Aβ insult in vitro [30, 67], suggesting a synergistic action of tau and Aβ pathology on mitochondria. The Aβ insult caused an increased reduction of mitochondrial membrane potential in cerebral cells of pR5 mice [67]. Furthermore, incubation of isolated mitochondria from P301L mice with either oligomeric or fibrillar Aβ1–42 preparations resulted in a reduction of state 3 respiration and respiratory control ratio as well as uncoupled respiration. Interestingly, aging particularly increased the sensitivity of mitochondria to oligomeric Aβ1–42 insult, demonstrating that oligomeric as well as fibrillar Aβ1–42 are both toxic but exert different degrees of toxicity [30].
To address the contribution of Aβ and tau pathologies in vivo, a new tripleAD (pR5/APP/PS2) mouse was generated [37]. Using combinatorial transgenesis, quantitative proteomics, and functional assays, our findings support first of all that Aβ and tau act synergistically in amplifying mitochondrial respiratory deficits, mainly of complex I and IV activities. Thereby, hyperphosphorylated tau may drive a vicious cycle within the Aβ cascade. Remarkably, deregulation of complex I was related to tau, whereas deregulation of complex IV was Aβ dependent both at the protein and activity levels. The synergistic effects of Aβ and tau led already at the age of 8 months to a depolarized mitochondrial membrane potential in the tripleAD mice. Additionally, we found that age-related oxidative stress at 12 months of age may exaggerate the dysfunctional energy homeostasis and synthesis of ATP and, in turn, take part in the vicious cycle that finally leads to cell death (Fig. 1) [10]. Our data complement those obtained in another triple transgenic mouse model 3xTg-AD (P301Ltau/APP/PS1) [35]. Yao and colleagues described age-related bioenergetic deficits in female 3xTg-AD mice aged from 3 to 12 months [72]. They found a decreased activity of regulatory enzymes of the OXPHOS (pyruvate dehydrogenase (PDH) and cytochrome c oxidase (COX)), increased oxidative stress, and lipid peroxidation. Most of the effects on mitochondria were seen at the age of 9 months, whereas mitochondrial respiration was significantly decreased with 12 months of age. Importantly, mitochondrial bioenergetic deficits precede the development of AD pathology in the 3xTg-AD mice.
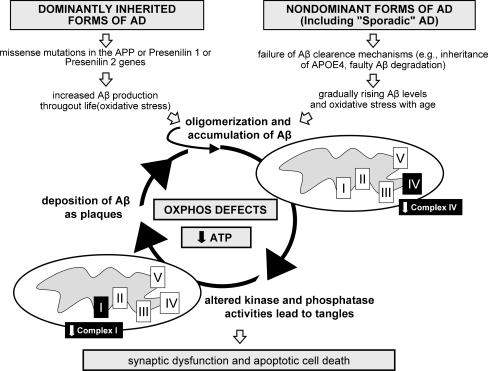
Fig. 1.
The vicious cycle of bioenergetic defects in AD. Tau and Aβ, the two major histopathological hallmarks of AD, act synergistically on mitochondria inducing amplified oxidative phosphorylation system (OXPHOS) deficiencies thereby triggering a vicious cycle. Notably, deregulation of mitochondrial complex IV was shown to be Aβ dependent, while deregulation of complex I was tau dependent, both at the protein and activity levels. The strong decrease of the mitochondrial respiratory capacity and the drop of ATP production associated with oxidative stress may finally lead to the synaptic loss and neuronal death that characterizes AD
Conclusion
In conclusion, we discussed in this review the key role of the vital organelle, mitochondria, in the pathogenesis of AD. Specifically, mitochondrial dysfunction integrates the two indisputable hallmarks of AD, plaques and NFTs, which act independently as well as synergistically. Consequently, besides the treatment and/or removal of both Aβ and tau pathology, strategies to protect cells at the mitochondrial level by stabilizing or restoring mitochondrial function or by interfering with the energy metabolism appear to be promising in treating or preventing AD. Moreover, the better understanding of the biochemical pathways by which mitochondria-protecting drugs act may not only optimize our therapeutic options but also clarify the role of mitochondria in the pathogenesis of AD. Transgenic mice and particularly triple transgenic models combining both pathologies may be very valuable in monitoring therapeutic interventions at the mitochondrial level. Eventually, this may prevent the progression of Aβ deposits and tau hyperphosphorylation at early stages of the disease.
Acknowledgments
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Gibson GE, Huang HM. Oxidative processes in the brain and non-neuronal tissues as biomarkers of Alzheimer's disease. Front Biosci. 2002;7:d1007–d1015. doi: 10.2741/gibson. [DOI] [PubMed] [Google Scholar]
- 2.Blass JP. Cerebrometabolic abnormalities in Alzheimer's disease. Neurol Res. 2003;25(6):556–566. doi: 10.1179/016164103101201995. [DOI] [PubMed] [Google Scholar]
- 3.Lustbader JW, Cirilli M, Lin C, Xu HW, Takuma K, Wang N, Caspersen C, Chen X, Pollak S, Chaney M, Trinchese F, Liu S, Gunn-Moore F, Lue LF, Walker DG, Kuppusamy P, Zewier ZL, Arancio O, Stern D, Yan SS, Wu H. ABAD directly links Abeta to mitochondrial toxicity in Alzheimer's disease. Science. 2004;304(5669):448–452. doi: 10.1126/science.1091230. [DOI] [PubMed] [Google Scholar]
- 4.Moreira PI, Santos MS, Oliveira CR. Alzheimer's disease: a lesson from mitochondrial dysfunction. Antioxid Redox Signal. 2007;9(10):1621–1630. doi: 10.1089/ars.2007.1703. [DOI] [PubMed] [Google Scholar]
- 5.Moreira PI, Santos MS, Oliveira CR, Shenk JC, Nunomura A, Smith MA, Zhu X, Perry G. Alzheimer disease and the role of free radicals in the pathogenesis of the disease. CNS Neurol Disord Drug Targets. 2008;7(1):3–10. doi: 10.2174/187152708783885156. [DOI] [PubMed] [Google Scholar]
- 6.Berchtold NC, Cribbs DH, Coleman PD, Rogers J, Head E, Kim R, Beach T, Miller C, Troncoso J, Trojanowski JQ, Zielke HR, Cotman CW. Gene expression changes in the course of normal brain aging are sexually dimorphic. Proc Natl Acad Sci U S A. 2008;105(40):15605–15610. doi: 10.1073/pnas.0806883105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eckert A, Hauptmann S, Scherping I, Rhein V, Muller-Spahn F, Götz J, Muller WE. Soluble beta-amyloid leads to mitochondrial defects in amyloid precursor protein and tau transgenic mice. Neurodegener Dis. 2008;5(3-4):157–159. doi: 10.1159/000113689. [DOI] [PubMed] [Google Scholar]
- 8.Su B, Wang X, Nunomura A, Moreira PI, Lee HG, Perry G, Smith MA, Zhu X. Oxidative stress signaling in Alzheimer's disease. Curr Alzheimer Res. 2008;5(6):525–532. doi: 10.2174/156720508786898451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hauptmann S, Scherping I, Drose S, Brandt U, Schulz KL, Jendrach M, Leuner K, Eckert A, Muller WE. Mitochondrial dysfunction: an early event in Alzheimer pathology accumulates with age in AD transgenic mice. Neurobiol Aging. 2009;30(10):1574–1586. doi: 10.1016/j.neurobiolaging.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 10.Rhein V, Song X, Wiesner A, Ittner LM, Baysang G, Meier F, Ozmen L, Bluethmann H, Dröse S, Brandt U, Savaskan E, Muller-Spahn F, Czech C, Götz J, Eckert A. Amyloid-beta and tau synergistically impair the oxidative phosphorylation system in triple transgenic Alzheimer’s disease mice. Proc Natl Acad Sci U S A. 2009;106(47):20057–20062. doi: 10.1073/pnas.0905529106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rhein V, Baysang G, Rao S, Meier F, Bonert A, Muller-Spahn F, Eckert A. Amyloid-beta leads to impaired cellular respiration, energy production and mitochondrial electron chain complex activities in human neuroblastoma cells. Cell Mol Neurobiol. 2009;29(6-7):1063–1071. doi: 10.1007/s10571-009-9398-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGowan E, Eriksen J, Hutton M. A decade of modeling Alzheimer's disease in transgenic mice. Trends Genet. 2006;22(5):281–289. doi: 10.1016/j.tig.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 13.Van Dam D, De Deyn PP. Drug discovery in dementia: the role of rodent models. Nat Rev Drug Discov. 2006;5(11):956–970. doi: 10.1038/nrd2075. [DOI] [PubMed] [Google Scholar]
- 14.Games D, Adams D, Alessandrini R, Barbour R, Berthelette P, Blackwell C, Carr T, Clemens J, Donaldson T, Gillespie F, et al. Alzheimer-type neuropathology in transgenic mice overexpressing V717F beta-amyloid precursor protein. Nature. 1995;373(6514):523–527. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- 15.Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274(5284):99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 16.Sturchler-Pierrat C, Abramowski D, Duke M, Wiederhold KH, Mistl C, Rothacher S, Ledermann B, Burki K, Frey P, Paganetti PA, Waridel C, Calhoun ME, Jucker M, Probst A, Staufenbiel M, Sommer B. Two amyloid precursor protein transgenic mouse models with Alzheimer disease-like pathology. Proc Natl Acad Sci U S A. 1997;94(24):13287–13292. doi: 10.1073/pnas.94.24.13287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stalder M, Phinney A, Probst A, Sommer B, Staufenbiel M, Jucker M. Association of microglia with amyloid plaques in brains of APP23 transgenic mice. Am J Pathol. 1999;154(6):1673–1684. doi: 10.1016/S0002-9440(10)65423-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janus C, Pearson J, Mclaurin J, Mathews PM, Jiang Y, Schmidt SD, Chishti MA, Horne P, Heslin D, French J, Mount HT, Nixon RA, Mercken M, Bergeron C, Fraser PE, St George-Hyslop P, Westaway D. A beta peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer's disease. Nature. 2000;408(6815):979–982. doi: 10.1038/35050110. [DOI] [PubMed] [Google Scholar]
- 19.Mucke L, Masliah E, Yu GQ, Mallory M, Rockenstein EM, Tatsuno G, Hu K, Kholodenko D, Johnson-Wood K, McConlogue L. High-level neuronal expression of abeta 1-42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J Neurosci. 2000;20(11):4050–4058. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Götz J. Tau and transgenic animal models. Brain Res Brain Res Rev. 2001;35(3):266–286. doi: 10.1016/S0165-0173(01)00055-8. [DOI] [PubMed] [Google Scholar]
- 21.Götz J, Streffer JR, David D, Schild A, Hoerndli F, Pennanen L, Kurosinski P, Chen F. Transgenic animal models of Alzheimer's disease and related disorders: histopathology, behavior and therapy. Mol Psychiatry. 2004;9(7):664–683. doi: 10.1038/sj.mp.4001508. [DOI] [PubMed] [Google Scholar]
- 22.Götz J, Probst A, Spillantini MG, Schafer T, Jakes R, Burki K, Goedert M. Somatodendritic localization and hyperphosphorylation of tau protein in transgenic mice expressing the longest human brain tau isoform. EMBO J. 1995;14(7):1304–1313. doi: 10.1002/j.1460-2075.1995.tb07116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Götz J, Chen F, Barmettler R, Nitsch RM. Tau filament formation in transgenic mice expressing P301L tau. J Biol Chem. 2001;276(1):529–534. doi: 10.1074/jbc.M006531200. [DOI] [PubMed] [Google Scholar]
- 24.Götz J, Chen F, Van Dorpe J, Nitsch RM. Formation of neurofibrillary tangles in P301l tau transgenic mice induced by Abeta 42 fibrils. Science. 2001;293(5534):1491–1495. doi: 10.1126/science.1062097. [DOI] [PubMed] [Google Scholar]
- 25.Götz J, Tolnay M, Barmettler R, Chen F, Probst A, Nitsch RM. Oligodendroglial tau filament formation in transgenic mice expressing G272V tau. Eur J NeuroSci. 2001;13(11):2131–2140. doi: 10.1046/j.0953-816x.2001.01604.x. [DOI] [PubMed] [Google Scholar]
- 26.Deters N, Ittner LM, Götz J. Divergent phosphorylation pattern of tau in P301L tau transgenic mice. Eur J NeuroSci. 2008;28(1):137–147. doi: 10.1111/j.1460-9568.2008.06318.x. [DOI] [PubMed] [Google Scholar]
- 27.Pennanen L, Welzl H, D'adamo P, Nitsch RM, Götz J. Accelerated extinction of conditioned taste aversion in P301L tau transgenic mice. Neurobiol Dis. 2004;15(3):500–509. doi: 10.1016/j.nbd.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 28.Pennanen L, Wolfer DP, Nitsch RM, Götz J. Impaired spatial reference memory and increased exploratory behavior in P301L tau transgenic mice. Genes Brain Behav. 2006;5(5):369–379. doi: 10.1111/j.1601-183X.2005.00165.x. [DOI] [PubMed] [Google Scholar]
- 29.Lewis J, Dickson DW, Lin WL, Chisholm L, Corral A, Jones G, Yen SH, Sahara N, Skipper L, Yager D, Eckman C, Hardy J, Hutton M, Mcgowan E. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science. 2001;293(5534):1487–1491. doi: 10.1126/science.1058189. [DOI] [PubMed] [Google Scholar]
- 30.Eckert A, Hauptmann S, Scherping I, Meinhardt J, Rhein V, Drose S, Brandt U, Fandrich M, Muller WE, Götz J. Oligomeric and fibrillar species of beta-amyloid (A beta 42) both impair mitochondrial function in P301L tau transgenic mice. J Mol Med. 2008;86(11):1255–1267. doi: 10.1007/s00109-008-0391-6. [DOI] [PubMed] [Google Scholar]
- 31.Bolmont T, Clavaguera F, Meyer-Luehmann M, Herzig MC, Radde R, Staufenbiel M, Lewis J, Hutton M, Tolnay M, Jucker M. Induction of tau pathology by intracerebral infusion of amyloid-beta-containing brain extract and by amyloid-beta deposition in APP x Tau transgenic mice. Am J Pathol. 2007;171(6):2012–2020. doi: 10.2353/ajpath.2007.070403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clavaguera F, Bolmont T, Crowther RA, Abramowski D, Frank S, Probst A, Fraser G, Stalder AK, Beibel M, Staufenbiel M, Jucker M, Goedert M, Tolnay M. Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol. 2009;11(7):909–913. doi: 10.1038/ncb1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Probst A, Götz J, Wiederhold KH, Tolnay M, Mistl C, Jaton AL, Hong M, Ishihara T, Lee VM, Trojanowski JQ, Jakes R, Crowther RA, Spillantini MG, Burki K, Goedert M. Axonopathy and amyotrophy in mice transgenic for human four-repeat tau protein. Acta Neuropathol. 2000;99(5):469–481. doi: 10.1007/s004010051148. [DOI] [PubMed] [Google Scholar]
- 34.Frank S, Clavaguera F, Tolnay M. Tauopathy models and human neuropathology: similarities and differences. Acta Neuropathol. 2008;115(1):39–53. doi: 10.1007/s00401-007-0291-9. [DOI] [PubMed] [Google Scholar]
- 35.Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, Laferla FM. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39(3):409–421. doi: 10.1016/S0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 36.Boutajangout A, Authelet M, Blanchard V, Touchet N, Tremp G, Pradier L, Brion JP. Characterisation of cytoskeletal abnormalities in mice transgenic for wild-type human tau and familial Alzheimer's disease mutants of APP and presenilin-1. Neurobiol Dis. 2004;15(1):47–60. doi: 10.1016/j.nbd.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 37.Grueninger F, Bohrmann B, Czech C, Ballard TM, Frey JR, Weidensteiner C, Von Kienlin M, Ozmen L (2010) Phosphorylation of Tau at S422 is enhanced by Abeta in TauPS2APP triple transgenic mice. Neurobiol Dis, 37(2), 294–306. [DOI] [PubMed]
- 38.Hansson CA, Frykman S, Farmery MR, Tjernberg LO, Nilsberth C, Pursglove SE, Ito A, Winblad B, Cowburn RF, Thyberg J, Ankarcrona M. Nicastrin, presenilin, APH-1, and PEN-2 form active gamma-secretase complexes in mitochondria. J Biol Chem. 2004;279(49):51654–51660. doi: 10.1074/jbc.M404500200. [DOI] [PubMed] [Google Scholar]
- 39.Behbahani H, Shabalina IG, Wiehager B, Concha H, Hultenby K, Petrovic N, Nedergaard J, Winblad B, Cowburn RF, Ankarcrona M. Differential role of Presenilin-1 and -2 on mitochondrial membrane potential and oxygen consumption in mouse embryonic fibroblasts. J Neurosci Res. 2006;84(4):891–902. doi: 10.1002/jnr.20990. [DOI] [PubMed] [Google Scholar]
- 40.Oddo S, Caccamo A, Kitazawa M, Tseng BP, Laferla FM. Amyloid deposition precedes tangle formation in a triple transgenic model of Alzheimer's disease. Neurobiol Aging. 2003;24(8):1063–1070. doi: 10.1016/j.neurobiolaging.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 41.Gimenez-Llort L, Blazquez G, Canete T, Johansson B, Oddo S, Tobena A, Laferla FM, Fernandez-Teruel A. Modeling behavioral and neuronal symptoms of Alzheimer's disease in mice: a role for intraneuronal amyloid. Neurosci Biobehav Rev. 2007;31(1):125–147. doi: 10.1016/j.neubiorev.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 42.Billings LM, Oddo S, Green KN, McGaugh JL, Laferla FM. Intraneuronal Aβ causes the onset of early Alzheimer's disease-related cognitive deficits in transgenic mice. Neuron. 2005;45:675–688. doi: 10.1016/j.neuron.2005.01.040. [DOI] [PubMed] [Google Scholar]
- 43.Schuessel K, Schafer S, Bayer TA, Czech C, Pradier L, Muller-Spahn F, Muller WE, Eckert A. Impaired Cu/Zn-SOD activity contributes to increased oxidative damage in APP transgenic mice. Neurobiol Dis. 2005;18(1):89–99. doi: 10.1016/j.nbd.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 44.Keil U, Bonert A, Marques CA, Scherping I, Weyermann J, Strosznajder JB, Muller-Spahn F, Haass C, Czech C, Pradier L, Muller WE, Eckert A. Amyloid beta-induced changes in nitric oxide production and mitochondrial activity lead to apoptosis. J Biol Chem. 2004;279(48):50310–50320. doi: 10.1074/jbc.M405600200. [DOI] [PubMed] [Google Scholar]
- 45.Caspersen C, Wang N, Yao J, Sosunov A, Chen X, Lustbader JW, Xu HW, Stern D, Mckhann G, Yan SD. Mitochondrial Abeta: a potential focal point for neuronal metabolic dysfunction in Alzheimer's disease. FASEB J. 2005;19(14):2040–2041. doi: 10.1096/fj.05-3735fje. [DOI] [PubMed] [Google Scholar]
- 46.Gillardon F, Rist W, Kussmaul L, Vogel J, Berg M, Danzer K, Kraut N, Hengerer B. Proteomic and functional alterations in brain mitochondria from Tg2576 mice occur before amyloid plaque deposition. Proteomics. 2007;7(4):605–616. doi: 10.1002/pmic.200600728. [DOI] [PubMed] [Google Scholar]
- 47.Manczak M, Anekonda TS, Henson E, Park BS, Quinn J, Reddy PH. Mitochondria are a direct site of A beta accumulation in Alzheimer's disease neurons: implications for free radical generation and oxidative damage in disease progression. Hum Mol Genet. 2006;15(9):1437–1449. doi: 10.1093/hmg/ddl066. [DOI] [PubMed] [Google Scholar]
- 48.Fernandez-Vizarra P, Fernandez AP, Castro-Blanco S, Serrano J, Bentura ML, Martinez-Murillo R, Martinez A, Rodrigo J. Intra- and extracellular Abeta and PHF in clinically evaluated cases of Alzheimer's disease. Histol Histopathol. 2004;19(3):823–844. doi: 10.14670/HH-19.823. [DOI] [PubMed] [Google Scholar]
- 49.Yan SD, Fu J, Soto C, Chen X, Zhu H, Al-Mohanna F, Collison K, Zhu A, Stern E, Saido T, Tohyama M, Ogawa S, Roher A, Stern D. An intracellular protein that binds amyloid-beta peptide and mediates neurotoxicity in Alzheimer's disease. Nature. 1997;389(6652):689–695. doi: 10.1038/39522. [DOI] [PubMed] [Google Scholar]
- 50.He XY, Wen GY, Merz G, Lin D, Yang YZ, Mehta P, Schulz H, Yang SY. Abundant type 10 17 beta-hydroxysteroid dehydrogenase in the hippocampus of mouse Alzheimer's disease model. Brain Res Mol Brain Res. 2002;99(1):46–53. doi: 10.1016/S0169-328X(02)00102-X. [DOI] [PubMed] [Google Scholar]
- 51.Wen GY, Yang SY, Kaczmarski W, He XY, Pappas KS. Presence of hydroxysteroid dehydrogenase type 10 in amyloid plaques (APs) of Hsiao's APP-Sw transgenic mouse brains, but absence in APs of Alzheimer's disease brains. Brain Res. 2002;954(1):115–122. doi: 10.1016/S0006-8993(02)03354-1. [DOI] [PubMed] [Google Scholar]
- 52.Aleardi AM, Benard G, Augereau O, Malgat M, Talbot JC, Mazat JP, Letellier T, Dachary-Prigent J, Solaini GC, Rossignol R. Gradual alteration of mitochondrial structure and function by beta-amyloids: importance of membrane viscosity changes, energy deprivation, reactive oxygen species production, and cytochrome c release. J Bioenerg Biomembr. 2005;37(4):207–225. doi: 10.1007/s10863-005-6631-3. [DOI] [PubMed] [Google Scholar]
- 53.Du H, Guo L, Fang F, Chen D, Sosunov AA, Mckhann GM, Yan Y, Wang C, Zhang H, Molkentin JD, Gunn-Moore FJ, Vonsattel JP, Arancio O, Chen JX, Yan SD. Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer's disease. Nat Med. 2008;14(10):1097–1105. doi: 10.1038/nm.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Anandatheerthavarada HK, Biswas G, Robin MA, Avadhani NG. Mitochondrial targeting and a novel transmembrane arrest of Alzheimer's amyloid precursor protein impairs mitochondrial function in neuronal cells. J Cell Biol. 2003;161(1):41–54. doi: 10.1083/jcb.200207030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Anandatheerthavarada HK, Devi L. Amyloid precursor protein and mitochondrial dysfunction in Alzheimer's disease. Neuroscientist. 2007;13(6):626–638. doi: 10.1177/1073858407303536. [DOI] [PubMed] [Google Scholar]
- 56.Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002;298(5594):789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 57.Eckert A, Keil U, Kressmann S, Schindowski K, Leutner S, Leutz S, Muller WE. Effects of EGb 761 Ginkgo biloba extract on mitochondrial function and oxidative stress. Pharmacopsychiatry. 2003;36(Suppl 1):S15–S23. doi: 10.1055/s-2003-40449. [DOI] [PubMed] [Google Scholar]
- 58.Eckert A, Keil U, Scherping I, Hauptmann S, Muller WE. Stabilization of mitochondrial membrane potential and improvement of neuronal energy metabolism by Ginkgo biloba extract EGb 761. Ann N Y Acad Sci. 2005;1056:474–485. doi: 10.1196/annals.1352.023. [DOI] [PubMed] [Google Scholar]
- 59.Abdel-Kader R, Hauptmann S, Keil U, Scherping I, Leuner K, Eckert A, Muller WE. Stabilization of mitochondrial function by Ginkgo biloba extract (EGb 761) Pharmacol Res. 2007;56(6):493–502. doi: 10.1016/j.phrs.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 60.Bastianetto S, Ramassamy C, Dore S, Christen Y, Poirier J, Quirion R. The Ginkgo biloba extract (EGb 761) protects hippocampal neurons against cell death induced by beta-amyloid. Eur J NeuroSci. 2000;12(6):1882–1890. doi: 10.1046/j.1460-9568.2000.00069.x. [DOI] [PubMed] [Google Scholar]
- 61.Yao Z, Drieu K, Papadopoulos V. The Ginkgo biloba extract EGb 761 rescues the PC12 neuronal cells from beta-amyloid-induced cell death by inhibiting the formation of beta-amyloid-derived diffusible neurotoxic ligands. Brain Res. 2001;889(1–2):181–190. doi: 10.1016/S0006-8993(00)03131-0. [DOI] [PubMed] [Google Scholar]
- 62.Colciaghi F, Borroni B, Zimmermann M, Bellone C, Longhi A, Padovani A, Cattabeni F, Christen Y, Di Luca M. Amyloid precursor protein metabolism is regulated toward alpha-secretase pathway by Ginkgo biloba extracts. Neurobiol Dis. 2004;16(2):454–460. doi: 10.1016/j.nbd.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 63.Ebneth A, Drewes G, Mandelkow EM, Mandelkow E. Phosphorylation of MAP2c and MAP4 by MARK kinases leads to the destabilization of microtubules in cells. Cell Motil Cytoskeleton. 1999;44(3):209–224. doi: 10.1002/(SICI)1097-0169(199911)44:3<209::AID-CM6>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 64.Stamer K, Vogel R, Thies E, Mandelkow E, Mandelkow EM. Tau blocks traffic of organelles, neurofilaments, and APP vesicles in neurons and enhances oxidative stress. J Cell Biol. 2002;156(6):1051–1063. doi: 10.1083/jcb.200108057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thies E, Mandelkow EM. Missorting of tau in neurons causes degeneration of synapses that can be rescued by the kinase MARK2/Par-1. J Neurosci. 2007;27(11):2896–2907. doi: 10.1523/JNEUROSCI.4674-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ittner LM, Fath T, Ke YD, Bi M, van Eersel J, Li KM, Gunning P, Götz J. Parkinsonism and impaired axonal transport in a mouse model of frontotemporal dementia. Proc Natl Acad Sci U S A. 2008;105(41):15997–16002. doi: 10.1073/pnas.0808084105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.David DC, Hauptmann S, Scherping I, Schuessel K, Keil U, Rizzu P, Ravid R, Drose S, Brandt U, Muller WE, Eckert A, Götz J. Proteomic and functional analyses reveal a mitochondrial dysfunction in P301L tau transgenic mice. J Biol Chem. 2005;280(25):23802–23814. doi: 10.1074/jbc.M500356200. [DOI] [PubMed] [Google Scholar]
- 68.Sergeant N, Wattez A, Galvan-Valencia M, Ghestem A, David JP, Lemoine J, Sautiere PE, Dachary J, Mazat JP, Michalski JC, Velours J, Mena-Lopez R, Delacourte A. Association of ATP synthase alpha-chain with neurofibrillary degeneration in Alzheimer's disease. Neuroscience. 2003;117(2):293–303. doi: 10.1016/S0306-4522(02)00747-9. [DOI] [PubMed] [Google Scholar]
- 69.Melov S, Adlard PA, Morten K, Johnson F, Golden TR, Hinerfeld D, Schilling B, Mavros C, Masters CL, Volitakis I, Li QX, Laughton K, Hubbard A, Cherny RA, Gibson B, Bush AI. Mitochondrial oxidative stress causes hyperphosphorylation of tau. PLoS ONE. 2007;2(6):e536. doi: 10.1371/journal.pone.0000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Escobar-Khondiker M, Höllerhage M, Muriel MP, Champy P, Bach A, Depienne C, Respondek G, Yamada ES, Lannuzel A, Yagi T, Hirsch EC, Oertel WH, Jacob R, Michel PP, Ruberg M, Höglinger GU. Annonacin, a natural mitochondrial complex I inhibitor, causes tau pathology in cultured neurons. J Neurosci. 2007;27(29):7827–7837. doi: 10.1523/JNEUROSCI.1644-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Götz J, Ittner LM, Kins S. Do axonal defects in tau and amyloid precursor protein transgenic animals model axonopathy in Alzheimer's disease? J Neurochem. 2006;98(4):993–1006. doi: 10.1111/j.1471-4159.2006.03955.x. [DOI] [PubMed] [Google Scholar]
- 72.Yao J, Irwin RW, Zhao L, Nilsen J, Hamilton RT, Brinton RD. Mitochondrial bioenergetic deficit precedes Alzheimer's pathology in female mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A. 2009;106(34):14670–14675. doi: 10.1073/pnas.0903563106. [DOI] [PMC free article] [PubMed] [Google Scholar]