Abstract
Background
Age-related cognitive decline begins in mid-life and continues with advancing age. Leukocyte telomere length (LTL) shortens with age, and inflammation and oxidative stress enhance this process. Shorter LTL is associated with dementia.
Methods
The relationship between cognitive function and LTL was investigated in a cross-sectional study of 382 women (mean age 50.6 years, range 19–78), not diagnosed with any form of dementia or cognitive impairment, from the TwinsUK cohort using 6 tests from the Cambridge Neuropsychological Test Automated Battery (CANTAB).
Results
After adjusting for age and estimated prior intellectual ability, we observed significant correlations of LTL with episodic memory and associated learning (PAL, p=0.032), recognition memory for non-verbal patterns (DMS, p=0.007), and working memory capacity (SSP, p=0.003). In pairs of twins discordant for LTL the twin with longer telomeres also had significantly better DMS (p<0.05) and SSP (p<0.013) scores than their co-twin with shorter telomeres. The correlations between these two scores and LTLwas significant both in women over the median mean age and in those below the median age, and remained significant after statistical adjustment for potential confounders.
Conclusions
Leukocyte telomere length correlates with a subset of measures of cognitive performance, suggesting that it might be a biomarker of cognitive aging in women before the onset of dementia.
Keywords: memory, telomere length, cognitive aging
1. Introduction
Age related cognitive decline begins in mid-life and continues with advancing age (Park et al 2002). However, there is high inter-individual variability associated with it (Foster 2006). The variability in memory function in the ageing population is likely due to both genetic and environmental factors. For example, measures of plasma cholesterol, inflammation and oxidative stress have all been negatively associated with memory and cognitive function in older individuals (Engelhart et al 2004; Rafnsson et al 2007; Droge et al 2007). The presence of hypertension and of type 2 diabetes (T2D) are also associated with poorer cognitive performance (e.g. Korczyn & Vakhapova 2007; Kumari & Marmot 2005). In contrast, higher levels of physical activity are associated with better cognitive function (Lautenschlager & Almeida 2006). Oxidative stress and inflammation offer possible common causes to ageing and age-related memory impairment, which may help explain why physical and cognitive capabilities are highly correlated in old age (Rafnsson et al 2007, Antsey et al 2005; Peila dn Launer 2006; Lau et al 2007). A possible index of the core concept of ‘common cause’ is leukocyte telomere length. Telomere length decreases with age and this is in large measure a record of the accruing burden of oxidative stress (von Zglinicki et al. 2000, von Zglinicki. 2002), and inflammation (Aviv 2006).
Shortened telomere length has been implicated in a spectrum of aging related diseases, including cardiovascular disease, (Benetos et al 2004; Fitzpatrick et al 2007; Brouilette et al 2007; van der Harst et al 2007) and other disorders, such as obesity (Valdes et al 2005), osteoarthritis (Zhai et al 2006), and osteoporosis (Valdes et al 2007). Further, individuals with dementia display shortened telomere length (Panossian et al 2003, Martin-Ruiz et al 2006; von Zglinicki et al 2000). Although a study of non-demented individuals aged 79 years (n=190) did not find any relationship between leukocyte telomere length and cognitive function (Harris et al 2006), the relationship between telomere length and cognitive performance in a sample representative of the general adult population has not been investigated. In the present study we examined whether LTL was associated with cognitive function in 382 healthy (non-demented or cognitively impaired) women.
2. Subjects and Methods
2.1 Study participants
Participants were 382 women (aged 19–78) from the TwinsUK cohort. All provided informed consent approved by The St Thomas’ Hospital Research Ethics Committee.
Cognitive function was assessed with the Cambridge Neuropsychological Test Automated Battery (CANTAB) (Robbins et al 1994).
The Cambridge Neuropsychological Test Automated Battery (CANTAB) is a series of computerised tests of cognition that run on a personal computer fitted with a touch sensitive screen. It has been standardised on a large sample of 787 normal elderly volunteers (Robbins et al., 1994) and test–retest reliability studies demonstrate correlations for individual test items range between .56 and .86 (Lowe & Rabbitt, 1998). Subjects were screened with a preliminary motor task to ascertain whether or not they were capable of performing the task and all of the participants passed. A practice run was not administered. Ten scores pertaining to six cognitive function measures were assessed in the original study (Singer et al 2006). For this study we have used only one score from each cognitive function test as follows:
Delayed Matching to Sample (DMS) test. This test assesses forced choice recognition memory for non-verbal patterns. Subjects are presented with a pattern in a box and then—either simultaneously (with no delay) or a 12 s delay—shown four boxes and instructed to choose the pattern that had previously appeared. The total number of patterns correctly remembered was used in the subsequent analysis.
Paired associated learning (PAL). Six boxes appear and each opens in sequence showing up to 6 patterns. Subjects were then shown a pattern in the middle of the screen and asked to choose the box the pattern previously appeared in. The total number of errors was used as a score.
Pattern recognition memory (PRM). Twelve patterns appear one after the other, none of which can be given a simple verbal label. Then, two choice boxes appear and the subject is instructed to choose which of the patterns they had already seen.
Simple reaction time (RTI) test. This test evaluates psychomotor retardation. Reaction time is measured by asking the subject to touch the screen immediately after a spot appears in the centre of the screen.
Space Span (SSP) test. This test assesses working memory capacity. Many boxes appear and after presenting a sequence of opening boxes of different colours, the subject was asked to recall the order of the colours that appeared by pointing to the correct boxes in sequence. The total number of stages completed was used to score this test.
Spatial Working Memory (SWM) test. This is a self-ordered, searching task which is sensitive to fronto-subcortical dysfunction. Blue squares are hidden inside a subset of boxes on the left side of the screen. The subject is required to try to locate all the blue squares and transfer them to the right side of the screen without reopening a box that has previously been selected. The score recorded in this study is the total number of errors made.
The National Adult Reading Test (NART), is a measure of an individual’s ability to pronounce 50 irregular English words. It is widely used as a well-validated estimate of prior intellectual ability, as it is highly correlated with overall intelligence (Crawford et al 2001, McGurn et al 2004).
Current physical activity during leisure time was recorded on a 4 point scale; 1=inactive, 2=light, 3=moderate and 4=heavy. This classification system was significantly correlated with more detailed activity assessments reported in previous exercise research on a subset of these individuals several years earlier, based on the Allied Dunbar Health Survey (Cherkas et al 2008).
Blood was collected for determination of leukocyte telomere length (LTL) and fasting glucose. LTL was derived from the mean length of the terminal restriction fragments, measured by Southern blot analysis (Benetos et al 2001). The coefficient of variation of duplicates resolved on different gels and occasions was 1.48%. Fasting glucose was measured on Ektachem 700 multichannel analyzer, using an enzymatic colorimetric slide assay (Johnson and Johnson Clinical Diagnostic Systems, Amersham, UK). Diagnosis of T2D was based on history of hypoglycaemic treatment and/or confirmed fasting blood glucose >126 mg/dl (7.0 mmol/L). Blood pressure was measured with an Omron BP machine. Prior to measuring each study participant sat still for 3 minutes. Three readings were taken (one minute rest between readings). Diagnosis of hypertension was based on the presence of systolic blood pressure >140 mmHg and/or diastolic blood pressure >90 mmHg and/or the use of anti-hypertensive medications. Serum homocysteine levels and vitamin D levels were measured as described elsewhere (Richards et al 2007, Richards et al 2008).
Subjects completed a questionnaire detailing the age at which they finished education and their own and their partner’s occupation. The grouping of occupations was based on Goldthorpe & Hope (1974) and classifications based on the National Statistics Socio-Economic Classification (NS-SEC, 2002).
Subjects were then assigned to a socio-economic status (SES) I, II, IIIN, IIIM, IV or V (as used routinely in UK studies (Marmot, 2004)), based on the higher occupational level of either the twin or their partner, in accordance with the new UK NS-social-economic measure. According to this system, I = Professional Occupations, II = Managers & Administrators and Associate Professional & Technical Workers, IIIN = Clerical and Secretarial non-manual skilled occupations, IIIM = Crafts & manual related skilled occupations, IV = partly skilled occupations and V = unskilled occupations (Cherkas et al 2006).
2.2. Statistical Analysis
Using standard linear regression, leukocyte telomere length was regressed on age and NART and the Pearson’s correlation coefficients between the multiply-adjusted leukocyte telomere length and cognitive test variables were computed. Because twin-pair data are not independent observations, we examined the correlation between LTL versus the various factors using a linear mixed effects model which included twin-pair of origin as a random effect and any other independent variables as fixed effects. Analyses of covariance were used to compute the multiple adjusted leukocyte telomere length means of the top (T1) and bottom (T3) tertiles of the cognitive trait distributions.
In addition, correlation coefficients between all six CANTAB scores and LTL and various potential confounders were computed adjusting only for age. The potential confounders tested were: diastolic blood pressure, fasting serum glucose, body mass index, smoking status (never, ex-smoker, current), serum levels of homocysteine, serum concentration of vitamin D, levels of physical activity, age at which the individual finished education and socio-economic status.
We examined within twin pair CANTAB score differences for 40 pairs of twins discordant for telomere length (above vs below the median of age adjusted LTL). All such twins were non-identical. This mean and standard error of within pair CANTAB score difference were calculated and a paired t-test was carried to assess statistical significance. S-Plus 6.0 (Insightful Corp WA) was used for all analyses.
3. Results
Characteristics of the study participants are shown in table 1. Leukocyte telomere length was negatively associated with age (Pearson’s correlation coefficient r = −0.342 p<.001). In this cohort the mean extrapolated telomere length shortening rate was 18.2 base pairs/year (std. err. 2.6 base pairs) and appeared to be constant with age, with no significant difference in rates of loss between various age ranges (not shown).
Table 1.
Descriptive statistics of the study cohort
| Trait | Count or Percentage |
|---|---|
| Sample size | 382 |
| % Hypertension | 21.1% |
| % ex-smokers | 34.0% |
| % current smokers | 18.2% |
| % T2D | 0.8% |
| % Post-menopausal | 65.4% |
| mean | SD | (Q1–Q3) | |||
|---|---|---|---|---|---|
| LTL kb | 7.04 | 0.66 | (6.56–7.51) | ||
| Age years | 50.65 | 12.34 | (42.8 –60.1) | ||
| BMI (kg/m2) | 25.15 | 4.27 | (22.12–26.96) | ||
| Physical activity (1) | 2.6 | 0.6 | (2 – 3) | ||
| National average reading test (NART) |
113.7 | 7.9 | (109.0 – 119.8) | ||
| Finished education (years) | 17.6 | 4.9 | (15–18) | ||
| Socio economic status (2) | 2.3 | 1.1 | (2–3) | ||
| CANTAB scores (3) | Correlation with LTL adjusted for age + NART(4) |
||||
| Delayed Matching to Sample (DMS) | 17.2 | 1.9 | (16.0 – 19.0) | r= +0.14 | p<0.006 |
| Paired Associates Learning (PAL) | 23.8 | 26.8 | (9.0– 29.0) | r= −0.12 | p<0.013 |
| Pattern Recognition Memory (PRM) | 20.8 | 2.4 | (19.0– 23.0) | r= +0.04 | p<0.47 |
| Reaction Time Test (RTI) | 357.8 | 65.5 | (314.0 – 392) | r= −0.12 | p<0.032 |
| Space Span (SSP) | 5.5 | 1.2 | (5.0 – 7.0) | r= +0.15 | p<0.003 |
| Spatial Working Memory (SWM) | 30.7 | 19.7 | (15.0 – 46.0) | r= −0.08 | p<0.10 |
SD = standard deviation, Q1 – Q3 interquartile range, LTL= leukocyte telomere length;
Categories of physical activity 1=inactive, 2=light, 3=moderate, 4=heavy.
Categories of SES 1=I, 2=II, 3=IIIN, 4=IIIM, 5=IV&V
DMS=Delayed Matching to Sample, total number of patterns correctly remembered ; PAL= Paired Associates Learning, total number of errors made;PRM=Pattern Recognition Memory, total number of patterns correctly remembered.
RTI=Reaction Time Test simple reaction time in seconds; ; SSP= Space Span, total stages completed; SWM= Spatial Working Memory, total number of errors made;
Pearson’s correlation coefficient adjusted for age and NART.
The mean NART-estimated IQ score for all the women of Twins UK is 113.6 (SD =7.5) (n=1379) (range 85–129). The mean LTL for the 3200 women measured is 7.05 kilobases (SD=0.68), and their mean age is 48.6 (SD=13.6), with an annual rate of telomere loss of 19.1 base pairs/year. Therefore, the present report’s subsample of 382 women is representative of the whole cohort with regards to these traits.
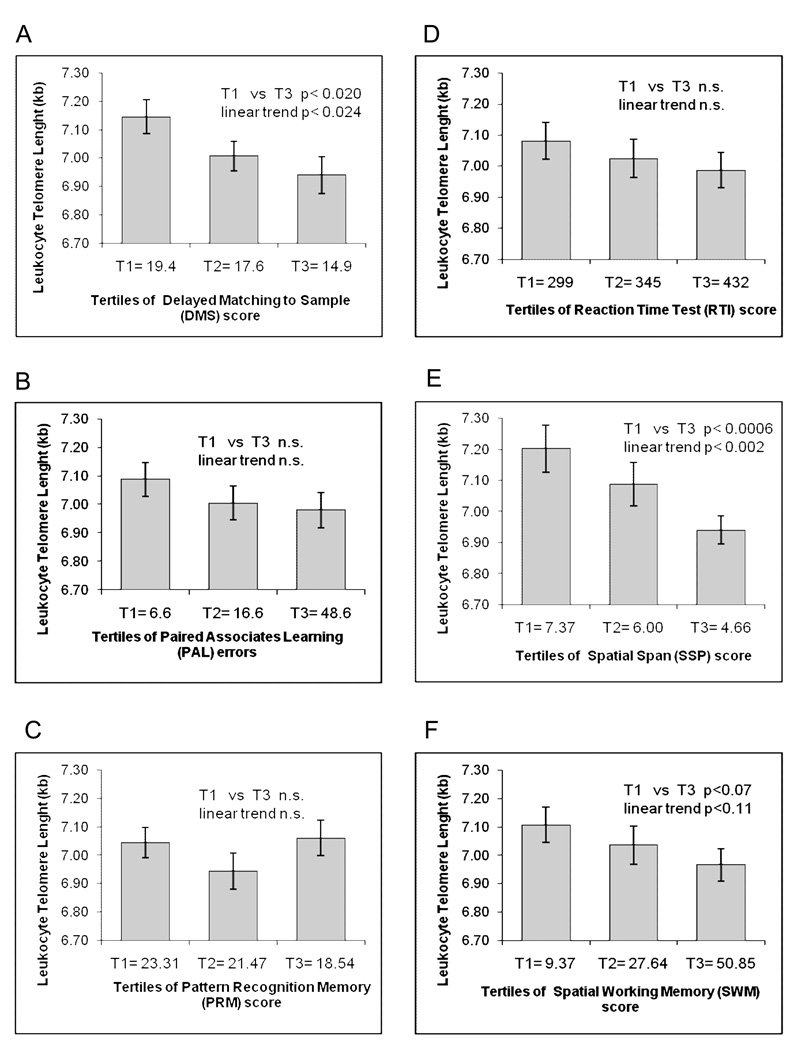
The estimate of prior intelligence used here, the NART score, was not significantly correlated with LTL (supplementary table 1). Five of the 6 CANTAB scores (all except RTI) were significantly correlated with the NART score, with better CANTAB subtest scores being associated with a higher NART score (supplementary table 1). We first assessed the correlation between the 6 cognitive performance measures and telomere length, adjusting for age and NART score. For clarity, we note that a higher PAL, SWM or RTI score indicated a worse performance whereas a higher PRM, DMS or SSP score indicated a better performance. Better performance on all 6 CANTAB scores was positively correlated with longer LTL (Table 1). The correlations were statistically significant for PAL, RTI, DMS and SSP, but not for PRM or SWM. For 5 of the 6 cognitive function scores studied, individuals in the highest performance tertiles had longer LTL than those with the lowest performance tertile. This was not observed for the PRM, which showed no significant association with leukocyte telomere length (Figure 1). However, the difference was statistically significant only for SSP and DMS. Women with the highest (T1) tertile of SSP had on average 304 bp leukocyte telomere length than those in the lowest (T3) tertile (p<0.001), while women in the highest tertile for DMS had 190 bp longer leukocyte telomere length than those in the worst tertile (p=0.020) (Figure 1).
Figure 1.
Adjusted mean leukocyte telomere length adjusted for tertiles of six CANTAB scores. For all CANTAB scores T1 indicates the tertile with the best performance T2 the middle tertile and T3 the tertile with the worst performance. For PAL, RTI and SWM the best tertile (T1) is the tertile with the lowest scores, for DMS, PRM and SSP the best tertile (T1) is the tertile with the highest value. The mean score value for each tertile is shown. Results are adjusted for NART score, and age.
Forty pairs of twins discordant for LTL were identified. The twins with the higher LTL had a better mean DMS score (17.6, SD=1.9) than their co-twins with lower LTL (16.6, SD=2.3) (p<0.05). Similarly women in the top half of LTL had a higher mean SSP score (5.7, SD=1.3) than their co-twin with low LTL (5.1, SD=1.1) (p<0.013). The difference in the other scores between twins discordant for telomere length were not statistically significant with p values>0.15.
Because twins share to a large extent the same environmental (education, socio-economic status) and genetic background these data suggested to us that the associations between SSP and LTL and between DMS and LTL were the most likely to be robust to confounding effects and thus they were further investigated.
We next explored the role of a number of potential confounders for the observed associations in the whole set of women with LTL and CANTAB data regardless of their discordant or concordant twin status (see supplementary table 1). In this sample of 382 women from the TwinsUK cohort none of these potential confounders is associated with both LTL and CANTAB scores—which would normally be required before a variable may be classed as a possible confounder—so, for the initial exploratory part of the analysis, these factors were not included. However, having observed a robust correlation between SSP and LTL, and between DMS and LTL, we investigated the effect of including the additional factors in the analysis. We further adjusted for those factors which have been previously implicated with either LTL (Richards et al 2008, Richards et al 2007, Aviv et al 2006) or with cognitive function, including BMI, smoking, menopausal status, presence of T2D, hypertension, level of education, socio-economic status, homocysteine and vitamin D serum levels, and levels of physical activity. The correlations with LTL remained statistically significant for both SSP and DMS (Table 2).
Table 2.
Correlation between multiply adjusted leukocyte telomere length and Delayed Match to Sample (DMS) and Space Span (SSP) scores stratified by median age.
| CANTAB test | Age <50.6 (1) (n=192) |
Age >=50.6 (1) (n=190) |
Total sample multiply adjusted(1) (n=382) |
|||
|---|---|---|---|---|---|---|
| DMS | r= 0.21 | p<0.002 | r= 0.14 | p<0.035 | r= 0.16 | p<0.002 |
| SSP | r= 0.18 | p<0.026 | r= 0.13 | p<0.048 | r= 0.16 | p<0.002 |
Pearson’s correlation coefficient and corresponding p-value adjusted for age, NART, non-independence between twins, hypertension, type 2 diabetes, SES, education, physical exercise, smoking, BMI, menopausal status, serum vitamin D, and homocysteine levels.
Because age is a major determinant of both telomere length and cognitive functions we also explored whether the association between LTL and SSP and DMS remained statistically significant stratifying by median age. The results (Table 2) indicate that a similar correlation between these two CANTAB scores and telomere length is found among the younger half as among the older half of the study cohort, and although the correlations appear slightly stronger in the younger half the difference is not statistically significant.
4. Discussion
The central finding of this work was that cognitive performance, notably SSP and DMS, correlated significantly with LTL among healthy (non-demented) women within a broad age-range. LTL accounted for up to 2.3% of the variance in cognitive ability. Previous work found that telomere length was predictive of better outcomes and better cognitive performance among stroke survivors and among demented subjects (Martin-Ruiz et al 2006, Honig et al 2006), suggesting that oxidative stress and inflammation might be the common thread linking LTL shortening with cognitive decline and dementia.
LTL has been previously noted to be heritable (familial) based on twin and family studies (Jeanclos et al 2000; Nawrot et al 2004). Therefore, genetics could be a confounder of the relationship observed. From a previous study – of which the current sample is a subgroup – we estimated heritability to be around 36% (95% CI 18–48%) (Andrew et al, 2006). Our discordant twin analysis confirmed that the two cognitive scores significantly associated with telomere length were also significantly different between twins from the same pair discordant for telomere length. Such results results confirm that the associations observed between memory scores and telomere lengths are robust to age and some possible confounding factors.
Indices of oxidative damage and inflammation increase during normal brain aging concomitantly with decline in cognitive and motor performance; these changes take place even in the absence of neurodegenerative diseases (Rafnsson et al 2007, Peila and Launer 2006, Lau et al 2007. Leukocyte telomere length is associated with indices of oxidative stress (Demissie et al 2006; Epel et al 2004) and inflammation (Fitzpatrick et al 2007, Bekaert et al 2007) and in this same cohort, shorter telomeres are associated with higher levels of c-reactive protein, a marker of inflammation (Valdes et al 2007). Oxidative stress enhances telomere erosion (Saretzki & Von Zglinicki 2002) with each cell division, while inflammation entails increased leukocyte turnover, which would further heighten telomere erosion. Leukocyte telomere length might hence register, at least in part, the cumulative burden of oxidative stress and inflammation during the individual’s lifetime.
We note some potential study limitations. The sample consisted of twins rather than singletons, but participants of the TwinsUK cohort are comparable to age-matched population singletons in terms of disease-related and lifestyle characteristics, (Andrew et al 2001). Twins are also related and not independent observations, which could bias the levels of significance, but we have adjusted for this using robust regression methods and, the discordant twin analysis confirmed results from the whole cohort. Another limitation is a relatively modest sample size (n=382) although this sample is larger than any previous study investigating the relationship between leukocyte telomere length and cognitive function in a healthy population. Finally, our results are derived from cross-sectional and not longitudinal observations, thus no direct inference about the role that shorter telomeres may have on cognitive decline can be made.
Our data support the hypothesis that oxidative stress and inflammation and perhaps other factors that contribute to cognitive performance also influence leukocyte telomere length and the differences in telomere length relating to cognitive performance can be detected before the onset of any signs of dementia, as all the women in our sample were healthy. Thus, telomere length may represent a potential biomarker to help identify individuals at risk of cognitive decline although this is an avenue that would need to be explored in a prospective study setting.
Supplementary Material
Acknowledgments
We thank Pauline Rook who assisted in twin recruitment, Raj Gill and Janine McLaney for administering CANTAB and Dr Joanne Iddon and colleagues at CeNes for advice. This work was supported by the Wellcome Trust (AM Valdes, TD Spector and LF Cherkas) and by NIH grants to A. Aviv AG021593 and AG020132. The UK Medical Research Council and the University of Edinburgh provide core funding for the MRC Centre for Cognitive Ageing and Cognitive Epidemiology, of which Ian Deary is Director.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement
All authors report no conflicts of interest.
References
- Andrew T, Aviv A, Falchi M, Surdulescu GL, Gardner JP, Lu X, Kimura M, Kato BS, Valdes AM, Spector TD. Mapping genetic loci that determine leukocyte telomere length in a large sample of unselected female sibling pairs. Am. J. Human Genet. 2006;78:480–486. doi: 10.1086/500052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew T, Hart DJ, Snieder H, de LM, Spector TD, MacGregor AJ. Are twins and singletons comparable? A study of disease-related and lifestyle characteristics in adult women. Twin Res. 2001;4:464–477. doi: 10.1375/1369052012803. [DOI] [PubMed] [Google Scholar]
- Anstey KJ, Dear K, Christensen H, Jorm AF. Biomarkers, health, lifestyle, and demographic variables as correlates of reaction time performance in early, middle, and late adulthood. Q J Exp Psychol A. 2005;58(1):5–21. doi: 10.1080/02724980443000232. [DOI] [PubMed] [Google Scholar]
- Aviv A, Valdes A, Gardner JP, Swaminathan R, Kimura M, Spector TD. Menopause modifies the association of leukocyte telomere length with insulin resistance and inflammation. J Clin Endocrinol Metab. 2006;91(2):635–640. doi: 10.1210/jc.2005-1814. [DOI] [PubMed] [Google Scholar]
- Aviv A. Telomeres and human somatic fitness. J Gerontol. Med Sci. 2006;61A:871–873. doi: 10.1093/gerona/61.8.871. [DOI] [PubMed] [Google Scholar]
- Bekaert S, De Meyer T, Rietzschel ER, De Buyzere ML, De Bacquer D, Langlois M, Segers P, Cooman L, Van Damme P, Cassiman P, Van Criekinge W, Verdonck P, De Backer GG, Gillebert TC, Van Oostveldt P, Van Oostveldt P on behalf of the Asklepios investigators. Telomere length and cardiovascular risk factors in middle-aged population free of overt cardiovascular disease. Aging Cell. 2007;6:639–674. doi: 10.1111/j.1474-9726.2007.00321.x. [DOI] [PubMed] [Google Scholar]
- Benetos A, Gardner JP, Zureik M, Labat C, Xiaobin L, Adamopoulos C, Temmar M, Bean KE, Aviv A. Short telomeres are associated with increased carotid artery atherosclerosis in hypertensive subjects. Hypertension. 2004;43:182–185. doi: 10.1161/01.HYP.0000113081.42868.f4. [DOI] [PubMed] [Google Scholar]
- Benetos A, Okuda K, Lajemi M, Kimura M, Thomas F, Skurnick J, Labat C, Bean K, Aviv A. Telomere length as an indicator of biological aging: the gender effect and relation with pulse pressure and pulse wave velocity. Hypertension. 2001;37(2 Part 2):381–385. doi: 10.1161/01.hyp.37.2.381. [DOI] [PubMed] [Google Scholar]
- Brouilette SW, Moore JS, McMahon AD, Thompson JR, Ford I, Shepherd J, Packard CJ, Samani NJ West of Scotland Coronary Prevention Study Group. Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: a nested case-control study. Lancet. 2007;369(9556):107–114. doi: 10.1016/S0140-6736(07)60071-3. [DOI] [PubMed] [Google Scholar]
- Cherkas LF, Hunkin JL, Kato BS, Richards JB, Gardner JP, Surdulescu GL, Kimura M, Lu X, Spector TD, Aviv A. The association between physical activity in leisure time and leukocyte telomere length. Arch Intern Med. 2008;168(2):154–158. doi: 10.1001/archinternmed.2007.39. [DOI] [PubMed] [Google Scholar]
- Cherkas LF, Aviv A, Valdes AM, Hunkin JL, Gardner JP, Surdulescu GL, Kimura M, Spector TD. The effects of social status on biological ageing as measured by white cell telomere length. Aging Cell. 2006;5(5):361–365. doi: 10.1111/j.1474-9726.2006.00222.x. [DOI] [PubMed] [Google Scholar]
- Crawford JR, Deary IJ, Starr J, Whalley LJ. The NART as an index of prior intellectual functioning: a retrospective validity study covering a 66 year interval. Psychological Medicine. 2001;31:451–458. doi: 10.1017/s0033291701003634. [DOI] [PubMed] [Google Scholar]
- Demissie S, Levy D, Benjamin EJ, Cupples A, Gardner JP, Herbert A, Kimura M, Larson MG, Meigs JB, Keaney JF, Aviv A. Insulin resistance, oxidative stress, hypertension, and leukocyte telomere length in men from the framingham heart study. Aging Cel. 2006;5:325–330. doi: 10.1111/j.1474-9726.2006.00224.x. [DOI] [PubMed] [Google Scholar]
- Droge W, Schipper HM. Oxidative stress and aberrant signalling in aging and cognitive decline. Aging Cell. 2007;6(3):361–370. doi: 10.1111/j.1474-9726.2007.00294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhart MJ, Geerlings MI, Meijer J, Kiliaan A, Ruitenberg A, van Swieten JC, Stijnen T, Hofman A, Witteman JC, Breteler MM. Inflammatory proteins in plasma and the risk of dementia: the Rotterdam study. Arch Neurol. 2004;61(5):668–672. doi: 10.1001/archneur.61.5.668. [DOI] [PubMed] [Google Scholar]
- Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci USA. 2004;101:17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick AL, Kronmal RA, Gardner JP, et al. Leukocyte Telomere Length and Cardiovascular Disease in the Cardiovascular Health Study. Am J Epidem. 2007;165:14–21. doi: 10.1093/aje/kwj346. [DOI] [PubMed] [Google Scholar]
- Foster TC. Biological markers of age-related memory deficits: treatment of senescent physiology. CNS Drugs. 2006;20(2):153–166. doi: 10.2165/00023210-200620020-00006. [DOI] [PubMed] [Google Scholar]
- Goldthorpe JH, Hope K. The Social Grading of Occupations: A New Approach and Scale. Oxford: Clarendon Press; 1974. [Google Scholar]
- Harris SE, Deary IJ, MacIntyre A, Lamb KJ, Rhadakrishnan K, Starr JM, Whalley LJ, Shiels P. The association between telomere length, physical health, cognitive ageing, and mortality in non-demented older people. Neuroscience Letters. 2006;406:260–264. doi: 10.1016/j.neulet.2006.07.055. [DOI] [PubMed] [Google Scholar]
- Honig LS, Schupf N, Lee JH, Tang MX, Mayeux R. Shorter telomeres are associated with mortality in those with APOE epsilon4 and dementia. Ann Neurol. 2006;60:181–187. doi: 10.1002/ana.20894. [DOI] [PubMed] [Google Scholar]
- Jeanclos E, Schork NJ, Kyvik KO, Kimura M, Skurnick JH, Aviv A. Telomere length inversely correlates with pulse pressure and is highly familial. Hypertension. 2000;36:195–200. doi: 10.1161/01.hyp.36.2.195. [DOI] [PubMed] [Google Scholar]
- Korczyn AD, Vakhapova V. The prevention of the dementia epidemic. J Neurol Sci. 2007;257(1–2):2–4. doi: 10.1016/j.jns.2007.01.081. [DOI] [PubMed] [Google Scholar]
- Kumari M, Marmot M. Diabetes and cognitive function in a middle-aged cohort: findings from the Whitehall II study. Neurology. 2005;65(10):1597–1603. doi: 10.1212/01.wnl.0000184521.80820.e4. [DOI] [PubMed] [Google Scholar]
- Lau FC, Shukitt-Hale B, Joseph JA. Nutritional intervention in brain aging: reducing the effects of inflammation and oxidative stress. Subcell Biochem. 2007;42:299–318. [PubMed] [Google Scholar]
- Lautenschlager NT, Almeida OP. Physical activity and cognition in old age. Curr Opin Psychiatry. 2006;19(2):190–193. doi: 10.1097/01.yco.0000214347.38787.37. [DOI] [PubMed] [Google Scholar]
- Marmot M. Status Syndrome: How Your Social Standing Directly Affects Your Health and Life Expectancy. London: Bloomsbury Publishing Plc.; 2004. [Google Scholar]
- Martin-Ruiz C, Dickinson HO, Keys B, Rowan E, Kenny RA, Von Zglinicki T. Telomere length predicts poststroke mortality, dementia, and cognitive decline. Ann Neurol. 2006;60(2):174–180. doi: 10.1002/ana.20869. [DOI] [PubMed] [Google Scholar]
- McGurn B, Starr JM, Topfer JA, Pattie A, Whiteman MC, Lemmon HA, Whalley LJ, Deary IJ. Pronunciation of irregular words is preserved in dementia, validating premorbid IQ estimation. Neurology. 2004;62:1184–1186. doi: 10.1212/01.wnl.0000103169.80910.8b. [DOI] [PubMed] [Google Scholar]
- National Statistics (UK) Household level NS-SEC. 2002 http://www.statistics.gov.uk/methods_quality/ns_sec/household_level.asp.
- Nawrot TS, Staessen JA, Gardner JP, Aviv A. Telomere length and possible link to X chromosome. Lancet. 2004;363:507–510. doi: 10.1016/S0140-6736(04)15535-9. [DOI] [PubMed] [Google Scholar]
- Panossian LA, Porter VR, Valenzuela HF, Zhu X, Reback E, Masterman D, Cummings JL, Effros RB. Telomere shortening in T cells correlates with Alzheimer's disease status. Neurobiol Aging. 2003;24(1):77–84. doi: 10.1016/s0197-4580(02)00043-x. [DOI] [PubMed] [Google Scholar]
- Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, Smith PK. Models of visuospatial and verbal memory across the adult life span. Psychol Aging. 2002;17(2):299–320. [PubMed] [Google Scholar]
- Peila R, Launer LJ. Inflammation and dementia: epidemiologic evidence. Acta Neurol Scand Suppl. 2006;185:102–106. doi: 10.1111/j.1600-0404.2006.00693.x. 2006. [DOI] [PubMed] [Google Scholar]
- Rafnsson SB, Deary IJ, Smith FB, Whiteman MC, Rumley A, Lowe GD, Fowkes FG. Cognitive decline and markers of inflammation and hemostasis: the Edinburgh Artery Study. J Am Geriatr Soc. 2007;55(5):700–707. doi: 10.1111/j.1532-5415.2007.01158.x. [DOI] [PubMed] [Google Scholar]
- Richards JB, Valdes AM, Gardner JP, Kato BS, Siva A, Kimura M, Lu X, Brown MJ, Aviv A, Spector TD. Homocysteine levels and leukocyte telomere length. Atherosclerosis. 2008 Feb 14; doi: 10.1016/j.atherosclerosis.2007.12.035. [DOI] [PubMed] [Google Scholar]
- Richards JB, Valdes AM, Gardner JP, Paximadas D, Kimura M, Nessa A, Lu X, Surdulescu GL, Swaminathan R, Spector TD, Aviv A. Higher serum vitamin D concentrations are associated with longer leukocyte telomere length in women. Am J Clin Nutr. 2007 Nov;86(5):1420–1425. doi: 10.1093/ajcn/86.5.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, James M, Owen AM, Sahakian BJ, McInnes L, Rabbitt P. Cambridge Neuropsychological Test Automated Battery (CANTAB): a factor analytic study of a large sample of normal elderly volunteers. Dementia. 1994;5(5):266–281. doi: 10.1159/000106735. [DOI] [PubMed] [Google Scholar]
- Saretzki G, Von Zglinicki T. Replicative ageing, telomeres, and oxidative stress. Ann N Y Acad Sci. 2002;959:24–29. doi: 10.1111/j.1749-6632.2002.tb02079.x. [DOI] [PubMed] [Google Scholar]
- Singer JJ, MacGregor AJ, Cherkas LF, Spector TD. Genetic influences on cognitive function using The Cambridge Neuropshychological Test Automated Battery. Intelligence. 2006;34:421–428. [Google Scholar]
- Valdes AM, Andrew T, Gardner JP, Kimura M, Oelsner E, Cherkas LF, Aviv A, Spector TD. Obesity and cigarette smoking are associated with short telomeres in women. The Lancet. 2005;366:662–664. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- Valdes AM, Richards JB, Gardner JP, Swaminathan R, Kimura M, Xiaobin L, Aviv A, Spector TD. Telomere length in leukocytes correlates with bone mineral density and is shorter in women with osteoporosis. Osteoporos Int. 2007;18(9):1203–1210. doi: 10.1007/s00198-007-0357-5. [DOI] [PubMed] [Google Scholar]
- van der Harst P, van der Steege G, de Boer RA, Voors AA, Hal AS, Mulder MJ, Van Gilst WH, van Veldhuissen DJ. Telomere length of circulating leukocytes is decreased in patients with chronic heart failure. J Am Coll Cardiol. 2007;49:1459–1464. doi: 10.1016/j.jacc.2007.01.027. [DOI] [PubMed] [Google Scholar]
- von Zglinicki T, Serra V, Lorenz M, Saretzki G, Lenzen-Grossimlighaus R, Gessner R, Risch A, Steinhagen-Thiessen E. Short telomeres in patients with vascular dementia: an indicator of low antioxidative capacity and a possible risk factor? Lab Invest. 2000;80(11):1739–1747. doi: 10.1038/labinvest.3780184. [DOI] [PubMed] [Google Scholar]
- von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci. 2002;27(7):339–344. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- Zhai G, Aviv A, Hunter DJ, Hart DJ, Gardner JP, Kimura M, Lu X, Valdes AM, Spector TD. Reduction of leucocyte telomere length in radiographic hand osteoarthritis: a population-based study. Ann Rheum Dis. 2006;65(11):1444–1448. doi: 10.1136/ard.2006.056903. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.