Abstract
D5 dopamine receptor (D5R) deficient (D5-/-) mice have hypertension that is aggravated by an increase in sodium intake. The current experiments were designed to test the hypothesis that a dysregulation of renal sodium transporters is related to the salt sensitivity in D5-/- mice. D5R was expressed in the renal proximal tubule, thick ascending limb, distal convoluted tubule, and cortical and outer medullary collecting ducts in D5 +/+ mice. On a control Na+ diet, renal protein expressions of sodium-potassium-2 chloride cotransporter (NKCC2), sodium chloride cotransporter (NCC), α and γ subunits of the epithelial sodium channel (ENaC) were greater in D5-/- than in D5+/+ mice. Renal renin abundance and urine aldosterone levels were similar but renal angiotensin II receptor (AT1R) protein expression was increased in D5-/- mice. An elevated Na+ diet increased further the elevated blood pressure of D5-/- mice but did not affect the normal blood pressure of D5+/+ mice. The increased levels of NKCC2, NCC, α and γ subunits of ENaC persisted with the elevated Na+ diet and unaffected by chronic AT1R blockade (losartan) in D5-/- mice. The expressions of proximal sodium transporters, sodium hydrogen exchanger type 3 (NHE3) and sodium phosphate cotransporter type 2 (NaPi2) were increased by the elevated Na+ diet in D5-/- mice; the increased expression of NHE3 but not NaPi2 was abolished by AT1R blockade. Our findings suggest that the increased protein expression of sodium transporters/channels in distal nephron segments may be the direct consequence of the disruption of D5R, independent of the renin-angiotensin aldosterone system.
Keywords: dopamine, dopamine receptor, knockout mouse, hypertension, sodium excretion, sodium transporters, AT1R blockade
Introduction
Dopamine receptors include the D1-like (D1R and D5R) and D2-like (D2R, D3R, and D4R) receptor subfamilies. All dopamine receptor subtypes are expressed in the kidney (1, 2). Lack or dysfunction of one dopamine receptor gene may affect renal tubular sodium transport, which may result in sodium retention and contribute to the development of hypertension. However, the differential nephron segment expression of dopamine receptor subtypes suggests that they may regulate sodium transport and blood pressure via different mechanisms (3).
The locus of the human D5R gene, 4p15.1–16.1 and its pseudogenes, 1q21.1 and 2p11.1-p11.2 (4), have been linked to human essential hypertension (5-8). Several single nucleotide polymorphisms of the human D5R gene are associated with diminished function (9). Disruption of the D5R gene in mice increases blood pressure that is aggravated by an increase in sodium intake (10,11). It is possible that the salt sensitivity in D5-/- mice is caused by increased sodium transport in the kidney but the nephron segment(s) involved has not been established.
Active sodium transport along the nephron occurs mainly through transport proteins at the apical membrane: sodium-hydrogen exchanger isoform 3 (NHE3) and the type 2 sodium phosphate cotransporter (NaPi2) in the proximal tubule, the bumetanide-sensitive sodium-potassium-2 chloride cotransporter (BSC1 or NKCC2) and NHE3 in the thick ascending limb of loop of Henle, the thiazide-sensitive sodium-chloride cotransporter (TSC or NCC) in the distal convoluted tubule and the amiloride-sensitive epithelial sodium channel (ENaC, subunits α, β and γ) in the connecting tubule and collecting duct, as well as Na+K+ ATPase at the basolateral membrane in almost all nephron segments. However, it is not known if the D5R can regulate the expression of these proteins, in part, because the nephron segments in which the D5R is expressed in mice have not been determined (10-12). It is also not clear if the knockout of the D5R gene alters the renal expression of the different dopamine receptor subtypes. Therefore, the current experiments were designed to determine a novel renal mechanism of salt-sensitive hypertension by testing the hypothesis that a dysregulation of renal sodium transporters is related to the salt sensitivity of the blood pressure of D5-/- mice. We measured blood pressure, urinary sodium and aldosterone excretions, and renal protein expressions of dopamine receptors, the type 1 angiotensin II receptor (AT1R), renin and sodium transporters/channels in D5-/- mice and D5+/+ littermates on a control and an elevated sodium diet. In order to determine if the increased expression and activity of AT1R is responsible for any alteration in renal sodium transporters in D5-/- mice (13), we also measured renal sodium transporters/channels in mice chronically given the AT1R blocker losartan.
Methods
Generation of D5-/- mice
Heterozygous mice (D5 +/-) were generated as reported (10,11). The D5-/- and D5+/+ (6th generation in C57BL/6/Taconic) mice were identified by DNA genotyping. C57BL/6/Taconic mice were used instead of C57BL/6 Jackson mice because C57BL/6/Taconic mice, unlike C57BL/6 Jackson mice, do not develop elevated blood pressure with an increase in sodium intake (11, 14). The studies were conducted in accordance with NIH guidelines for the ethical treatment and handling of animals in research, and approved by the Institutional Animal Care and Use Committee, initially at Georgetown University, and subsequently at the Children's National Medical Center.
Sodium balance study in ration-fed mice
D5-/- and D5+/+ mice (4-6 months old, mixed gender, n=5) were housed in metabolic cages. The mice, for each 25 g of body weight, were ration-fed for 10 days with gelled control sodium diet (0.2-0.3% Na+) consisting of ground rodent chow (5 g) and melted agar (0.04 g) in 10 ml distilled water (15-17). Ration feeding of gelled food eliminates the possibility of food crumbs contaminating the urine collections. Blood pressures were measured in the aortae, via the femoral arteries of mice under anesthesia (pentobarbital, 50 mg/kg intravenously) (10,11,15). Urine and blood samples were analyzed for electrolytes (E4A Electrolyte system, Beckman, Fullerton, CA), creatinine (Creatinine Analyzer 2, Beckman), and aldosterone (radioimmunoassay, Diagnostic Products Corp., Los Angeles, CA).
Semi-quantitative immunoblotting
The kidneys, removed after euthanasia, were homogenized (15-17). Renal plasma membrane-enriched fractions were obtained by centrifugation of kidney homogenates at 17,000 × g, 30 min at 4°C (18); the 17,000 × g fraction resulted in the enrichment of Na+K+ ATPase α subunit by more than 60%, relative to whole kidney homogenates (data not shown). Equal amounts of protein were separated by 7.5 or 10% SDS PAGE and transferred onto nitrocellulose membranes (Bio-Rad, Hercules, CA). The membranes were probed with the indicated primary antibodies, and then exposed to horseradish peroxidase-conjugated secondary antibodies (Pierce, Rockford, IL). The primary antibodies were rabbit polyclonal antibodies against NHE3, NaPi2, NKCC2, NCC, ENaC (gifts from Dr. Mark A. Knepper, LKEM, NHLBI, NIH), D1-5R, actin (Sigma), and mouse monoclonal antibody to Na+K+ ATPase α subunit (Upstate Biotechnology, Lake Placid, NY). The specificities of these antibodies have been reported (16-26). The specificity of the polyclonal D5R antibody was initially determined by the ability to the antibody to detect human D5R protein heterologously expressed in HEK293 cells (positive control) without and with peptide-pre-adsorption (negative control) (12). In this report we show that the D5R antibody recognizes D5R in kidneys from mice with D5R gene (D5R +/+) but not in mice without the D5R gene (D5R -/-) (Figure 1). The chemiluminescent signals were quantified by the NIH Image J program and normalized against the corresponding actin bands.
Figure 1.
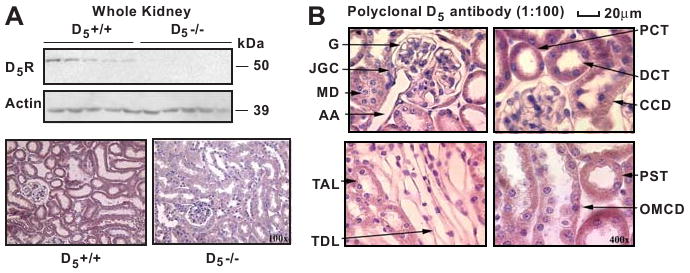
Renal D5R expression
Figure 1A: Immunoblots of D5R and actin in whole kidney homogenates from D5-/- (n=5) and D5+/+ mice (n=5, upper panel). A 55 kDa band is found in D5+/+ but not in D5-/- mice, confirming the disruption of the D5R gene and the specificity of the D5R antibody. Actin is present in both mouse strains. Renal immunocytochemistry of D5R (lower panel: 40×). Positive staining (purple) is observed in specific nephron segments in the D5+/+ but not in the D5-/- mice.
Figure 1B. Renal D5R expression in D5+/+ mice. The D5R staining (purple, 400×) is mainly located in the proximal convoluted tubule (PCT), distal convoluted tubule (DCT), and cortical collecting duct (CCD). There is also D5R immunostaining in the proximal straight tubule (PST), thick ascending limb of Henle (TAL), and outer medullary collecting duct (OMCD). Little or negative staining is found in the juxtaglomerular cell (JGC), afferent arteriole (AA), macula densa, glomerulus (G), and thin descending limb (TDL) of Henle.
Renal immunocytochemistry
In separate studies, kidneys from anesthetized D5+/+ and D5-/- mice were perfused with PBS (pH 7.4) and fixed with Histochoice (Amresco, Solon, OK). Three μm sections were cut from paraffin-embedded tissues for H&E staining and indirect immunoperoxidase labeling (12). Immunoreactivity was visualized with the VIP kit (Vector, Burlingame, CA) and bright-field microscopy, under the same conditions and magnifications (Eclipse E600, Nikon, Tokyo, Japan). The experiments were performed at least 3 times.
Chronic salt loading
In additional studies, D5+/+ and D5-/- mice were fed an elevated sodium (2-3% Na+) diet for 3 weeks. After euthanasia, the kidneys were harvested for immunoblotting, as above.
Telemetry
D5+/+ and D5-/- mice were fed a control sodium diet (0.2-0.3 %Na+) for 3-4 weeks, then switched to an elevated sodium (2-3% Na+)(27) diet for 2 weeks. The mice had free access to food and water. Blood pressures were monitored by telemetry, via pre-implanted transmitters in conscious mice (11, 15). Mice were acclimated to the apparatus and the environment for a total of 7 days before measurements were taken.
Losartan treatment
D5+/+ and D5-/- mice were fed with an elevated sodium (2-3% Na+) diet for 3 weeks. In the last 5-7 days of the study, the AT1R blocker, losartan, was injected intraperitoneally (20 mg (100 μl) /kg/day, × 5-7 days) (26). After euthanasia, the kidneys were harvested for immunoblotting, as above.
Statistical analysis
Data are expressed as mean ± SE and as percent of D5+/+ mice for immunoblotting. Unpaired Student's t-test was used for a two-group comparison and ANOVA, Holm-Sidak test for multi-group comparison. P<0.05 was considered significant.
Results
Distribution of D5R along the nephron
Whole kidney homogenates immunoblotted with the polyclonal antibody to D5R showed a 55 kDa band in D5+/+ but not in D5-/- mice. Strong immunostaining was observed in kidneys from D5+/+ mice but not in D5-/- mice (Figure 1A). No immunostaining was observed when the antibody was blocked by pre-adsorption with the immunizing peptide (not shown). These studies confirmed the specificity of the D5R antibody and the disruption of the D5R gene in D5-/- mice.
D5R was expressed in the proximal convoluted and straight tubules (including brush border membranes), thick ascending limb of Henle, distal convoluted tubule, and cortical and outer medullary collecting ducts. Minimal staining was observed in the juxtaglomerular cell, glomerulus, macula densa, afferent arteriole, and thin descending limb of Henle (Figure 1B).
Effect of the disruption of D5R gene (D5-/-) on some physiological parameters in mice fed a control sodium diet
All physiological data are summarized in Table1. We have reported that the blood pressures are higher in D5-/- mice than D5+/+ littermates when measured by telemetry in the conscious state or when measured from the aorta via the femoral artery in the anesthetized state (10,11). The systolic and diastolic blood pressures were measured under anesthesia in this group of mice in the current studies. The higher blood pressure in D5-/- compared to D5+/+ mice was confirmed (Table 1). There were no differences in heart rate, body weight, and food/water intake between the two groups. Serum Na+, K+, Cl- and creatinine concentrations and creatinine clearance were also similar in the two mouse strains. No differences were found in daily urinary sodium and water excretion between the two mouse strains (only the data on the last day of the balance study are shown in Table 1). The higher blood pressure in D5-/- mice than in D5+/+ littermates was not associated with a greater urinary sodium, suggesting that a state of sodium retention may have already been achieved by the time the variables were measured. Mice retain sodium for several hours and then achieve sodium balance by the first or second day after a high salt load (28, 29).
Table 1.
Physiological data of D5-/- and D5+/+ mice ration-fed a control sodium diet
| Mouse group | D5+/+ | D5-/- |
|---|---|---|
| SBP(mmHg) | 93± 2 | 122± 5* |
| DBP(mmHg) | 78 ± 3 | 101± 2* |
| Heart rate (beats/min) | 347 ± 8 | 328 ± 14 |
| Body weight (g) | 26± 0.9 | 25 ± 0.2 |
| Serum Na+ (mmol/l) | 152± 1 | 148 ± 2 |
| Serum K+ (mmol/l) | 4.3±0.4 | 4.5 ±0.8 |
| Serum Cl- (mmol/l) | 124±0.4 | 126 ± 2.9 |
| Serum creatinine (μmol/l) | 26.4±3.5 | 31.7±6.2 |
| Gelled-food intake (g/day) | 14.7± 0.2 | 13.2 ±0.7 |
| Water intake (ml/g/day) | 0.39±0.01 | 0.35±0.02 |
| Urine output (ml/day) | 2.9± 0.3 | 3.1 ± 0.9 |
| Na+ excretion (mmol/day) | 0.31 ± 0.03 | 0.29± 0.07 |
| Creatinine clearance (ml/day) | 159± 33 | 120 ± 32 |
Data are M ± SE.
P<0.05, vs. D5+/+ mice (4-6 months old, mixed gender, n=5/group), Student's t-test,. SBP: systolic blood pressure, DBP: Diastolic blood pressure, MAP: Mean arterial blood pressure. BPs were measured under pentobarbital anesthesia.
Effect of the disruption of D5R gene (D5-/-) on the expression of renal sodium transporter, channels, and pump in mice fed a control sodium diet
The protein expressions of renal sodium transporters, channels, and pump in D5-/- and D5+/+ mice are shown in Figures 2A, B &C. NHE3 expression did not differ between D5-/- and D5+/+ mice while NaPi2 protein was decreased in D5-/- relative to D5+/+ mice. Expressions of NKCC2, a sodium transporter in the thick ascending limb of Henle, and NCC, a sodium transporter in the distal convoluted tubule, were increased in D5-/- mice relative to D5+/+ mice (Figure 2), although the nephron segment distribution of NCC (Figure 3) was not altered in D5-/- mice. The α and γ subunits of ENaC were also increased in D5-/- mice but no differences were found in the expression of the β subunit of ENaC, and the α subunit of Na+K+ ATPase (Figures 2 B & C) between the two groups.
Figure 2.
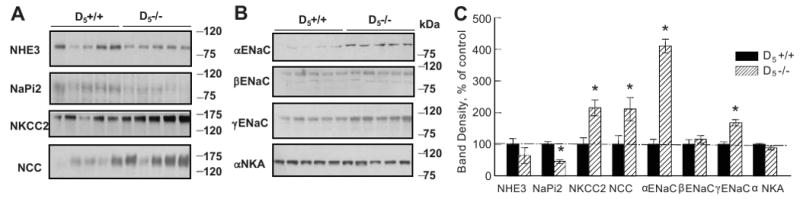
Immunoblotting of whole kidney homogenates for sodium transporters, channels, and pump in D5-/- and D5+/+ mice fed a control sodium diet.
Figure 2A. Immunoblots of NHE3, NaPi2, NKCC2 and NCC in D5-/- (n=5) and D5+/+ mice (n=5).
Figure 2B. Immunoblots of the α, β, and γ subunits of ENaC and α subunit of Na+K+ ATPase (αNKA) in D5-/- (n=5) and D5+/+ mice (n=5).
Figure 2C. Densitometric analysis of the immunoblots. The protein expressions of NKCC2, NCC, αENaC and γENaC are increased and the protein expression of NaPi2 is decreased, while the protein expression of NHE3 and Na+K+ ATPase α subunit (αNKA) is not altered in D5-/- mice relative to D5R+/+ mice. *P<0.05, Student's t-test.
Figure 3.
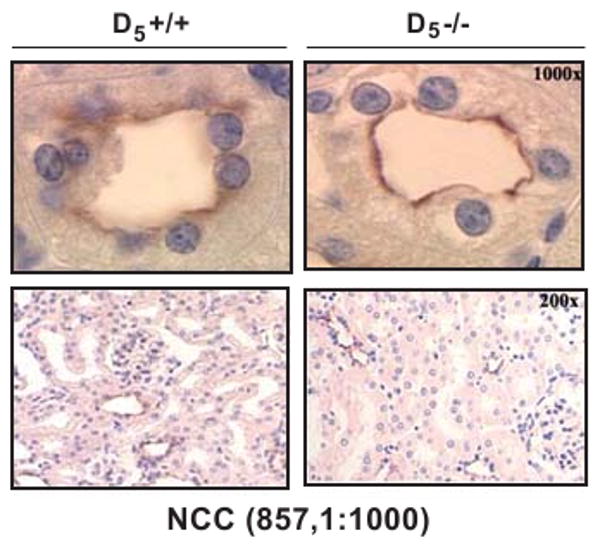
Renal immunostaining of NCC in D5-/- and D5+/+ mice. NCC protein (purple) is expressed in the luminal membrane of the distal convoluted tubule; the expression is greater in D5-/- than in D5+/+ mice (upper panel, 1000×). Nephron segment distribution of NCC (lower panel 200×) is similar in D5-/- and D5+/+ mice.
Effect of the disruption of D5R gene (D5-/-) on renal dopamine receptors, AT1R, and renin in mice fed a control sodium diet
The renal protein expression of all D2-like dopamine receptors was similar in D5-/- and D5+/+ mice, but the renal protein expression of D1R was lower in D5-/- than in D5+/+ mice (Figure 4A), indicating that there was no compensatory upregulation of the other dopamine receptor subtypes in the kidney as a consequence of the deletion of the D5R gene.
Figure 4.
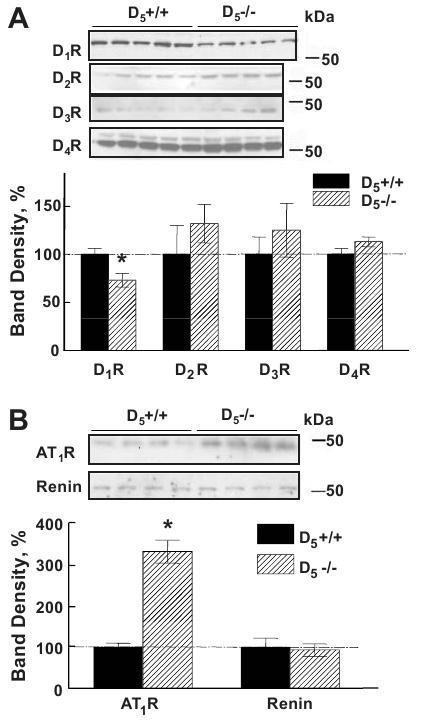
Immunoblotting of dopamine receptor subtypes, renin, and AT1R in D5+/+ and D5-/- mice fed a control sodium diet.
Figure 4A. Immunoblots in whole kidney homogenates from D5+/+ (n=5) and D5-/- mice (n=4). The protein expression of D1R is decreased but not the expression of D2R, D3R, and D4R in D5-/- mice. *P<0.05, Student's t-test.
Figure 4B. Immunoblotting in whole kidney homogenates for AT1R, and renin in D5-/- and D5+/+ mice (n=4). Relative to D5+/+ mice, the protein expression of AT1R is increased in D5-/- mice, but the renin protein abundance is similar in the two mouse strains. *P<0.05, Student's t-test.
Renal AT1R protein was increased in D5-/- mice, in agreement with our previous report (13, 26) but renal renin protein was similar in D5-/- and D5+/+ mice (Figure 4B). The specificity of the antibodies against AT1R (AT1R, N-10, Santa Cruz, CA) and renin (Innovative Research, Southfield, MI) were verified (data not shown) prior to these experiments. Urinary aldosterone excretion was also similar in the two mouse strains (D5-/-: 2.9±1.1, D5+/+: 2.5±0.7, ng/mg of creatinine, n=6/group), close to the values reported in the literature (30, 31). We have also reported that the levels of renal and plasma catecholamines are similar in D5-/- and D5+/+ mice (10, 26).
Effect of the disruption of D5R gene (D5-/-) in mice fed an elevated sodium diet
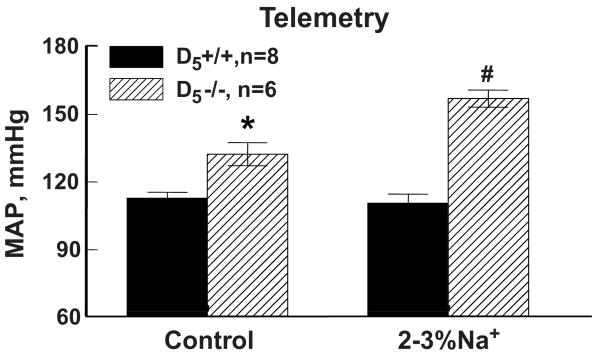
Figure 5 shows the mean arterial blood pressure in D5-/- and D5+/+ mice. The data included some of the mice reported in our previous publication (11). On the control sodium diet, blood pressure was higher in D5-/- mice than in D5+/+ littermates. The elevated sodium diet increased blood pressure further in D5-/- mice but did not affect the normal blood pressure of D5+/+ mice, confirming the salt sensitivity of blood pressure of D5-/- mice (11).
Figure 5.
Effect of an elevated sodium diet on blood pressure in D5+/+ (n=8) and D5-/- mice (n=6). Blood pressure (average shown for each diet) was measured by telemetry in conscious mice. The mean arterial blood pressure (MAP) was higher in D5-/- than D5+/+ mice fed a control sodium diet (2 weeks). MAP was increased further by an elevated sodium diet (2 weeks) in D5-/- mice. P<0.05, * vs. D5+/+; # vs. others, ANOVA, Holm-Sidak test.
The increased protein levels of renal distal sodium transporters, channels, and pump in the D5-/- mice fed the control sodium diet, including NKCC2, NCC and α and γ subunits of ENaC persisted in the D5-/- mice fed the elevated sodium diet. In addition, NHE3, NaPi2 and the α subunit Na+K+ ATPase were also increased in D5-/- mice, relative to D5+/+ mice. The β subunit of ENaC was similar in the two mouse strains (Table 2).
Table 2.
Results of the immunoblotting of Na+ transporters/channels in mice fed an elevated sodium diet
| Mouse group | D5+/+ (n=3) | D5-/-(n=3) |
|---|---|---|
| NHE3 | 100 ± 6 | 287 ± 62* |
| NaPi2 | 100 ± 9 | 177 ± 19* |
| NKCC2 | 100 ± 3 | 174 ± 15* |
| NCC | 100 ±39 | 705 ± 141* |
| αENaC | 100 ± 7 | 227 ±13* |
| βENaC | 100 ± 27 | 94 ± 5 |
| γENaC | 100 ± 5 | 454 ± 110* |
| αNKA | 100 ±7 | 322 ± 27* |
Data are M ± SE, % of D5+/+ mice, corrected by actin.
P<0.05, vs. D5+/+, Student's t-test αNKA= Na+K+ ATPase, α subunit
Effects of losartan on renal sodium transporters in D5-/- and D5+/+ mice
To determine if the increased AT1R expression in the kidney contributed to the alterations in sodium transporters, channels, and pump in D5-/- mice, we studied mice treated with the AT1R blocker losartan, and fed the elevated sodium diet (Figure 6A, B&C). We have reported that renal AT1R is increased (13, 26) and losartan treatment normalized the high blood pressure of D5-/- mice (26).
Figure 6.
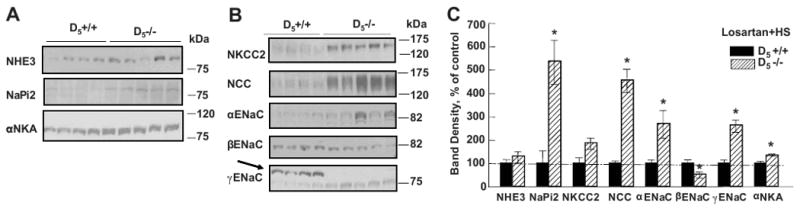
Renal sodium transporters, channels, and pump in D5+/+ (n=4) and D5-/- mice (n=5) fed an elevated sodium (HS) diet and treated with an AT1R blocker (Losartan)
Figure 6A. Immunoblots of the proximal sodium transporters and Na+K+ ATPase α subunit (αNKA).
Figure 6B. Immunoblots of the renal distal sodium transporters. Arrow points to 90 kDa γENaC which is probably an inactive form (21).
Figure 6C. Densitometric analysis of the immunoblots. NaPi2 and Na+K+ ATPase α subunit (αNKA) are increased in D5-/- relative to D5+/+ littermates. D5-/- mice continued to have increased renal expression of NKCC2, NCC and α and γ subunits of ENaC, despite losartan treatment. *P<0.05, Student's t-test.
The increased protein expression of the distal sodium transporters (NKCC2, NCC, α and γ subunits of ENaC) (Figure 6B and 6C) in D5-/- mice, on the control and elevated sodium diets, respectively, persisted in the losartan-treated D5-/- mice. There were 2 bands for γENaC, a higher band (90kDa), and a lower band (75kDa), which is believed to be the active form of γENaC, following the proteolysis or deglycosylation of the larger, inactive form (90kDa) (21). The higher band was absent and the density of the lower band was increased in D5-/- mice, relative to their D5+/+ littermates. We do not know the reason for the absence of the higher band in the D5-/- mice but it is possible that all the “inactive” forms are converted to the “active” forms in these mice. Losartan abrogated the increased expression of NHE3 but not the increased expression of NaPi2 and Na+K+ ATPase α subunit observed in D5-/- mice (Figure 6, Table 2); losartan also decreased the expression of the β subunit of ENaC.
Discussion
Under normal circumstances, an increase in blood pressure activates renal mechanisms to increase sodium excretion to maintain a normal blood pressure (pressure-natriuresis phenomenon) (32). In the current report, the sodium intake and urinary sodium excretion on the control sodium diet are similar in D5-/- mice and D5+/+ littermates, indicating the achievement of sodium balance. However, this was achieved at the expense of higher blood pressure in the D5-/- mice, indicating a blunting of the pressure-natriuresis relationship with the disruption of the D5R gene.
The natriuresis associated with increased sodium intake and blood pressure, thought to be an example of aldosterone escape, has been reported to caused by a decrease in the protein abundance/activity of NCC in the renal distal convoluted tubule (25). It is possible that the impaired pressure-natriuresis mechanism in D5-/- mice may be due to increased renal expression of NCC. In the current study, D5-/- mice not only have increased protein expression of NCC, but also one of the sodium transporters in the thick ascending limb (NKCC2) and the α and γ subunits of ENaC in the collecting duct. The increase in renal sodium transporters with the elevated sodium diet in D5-/- mice is not in agreement with the effect of high salt intake in normotensive rats (27,33). There may be species and strain differences because an elevated sodium diet for one week does not affect NHE3 or NKCC2 expression in SJL mice (unpublished data), similar to that reported in normotensive rats; these transporters are decreased by the elevated sodium diet in wild-type C57BL/6 mice, the genetic background of D5-/- mice. NCC expression, which is decreased by an elevated sodium diet in the rat in one study (27) but not in another (33), is also decreased by the elevated sodium diet in SJL mice but not in C57BL/6 wild-type mice (unpublished data). In contrast, the elevated sodium diet in C57BL/6 mice with deleted D5R increases NCC expression (Table 2). The disruption of the D5R gene in mice results in the up-regulation of renal sodium transporters/channels in all of the distal nephron segments. The increased expression of these distal tubule sodium transporters/channels in D5-/- mice may have attenuated the magnitude of the natriuresis that would have normally accompanied the increase in systemic blood pressure.
Using a specific antibody against D5R, we find D5R expression in the thick ascending limb, distal convoluted tubule, as well as the cortical and outer medullary collecting ducts, the nephron segments that express the sodium transporter/channels that are increased in D5-/- mice fed either control or elevated sodium diet. These data suggest that the D5R may have a direct inhibitory effect on the expression of renal sodium transporters and channels in the distal nephron segments. An effect of D5R on distal sodium transporter and channel expression could also be indirect, as a result of increased AT1R expression. D5R promotes the degradation of the AT1R (26) and renal AT1R expression is increased in D5-/- mice (13, 26), confirmed in the current report. Both NCC and αENaC are regulated by AT1R (34, 35). The protein abundance of NCC and αENaC is decreased in AT1A null mice (34) and in rats treated with AT1R antagonists but increased in rats treated with angiotensin II (35).
Renal renin abundance and urinary aldosterone levels are not increased in D5-/- mice. That the D5R may not regulate renin and indirectly, aldosterone secretion, is in keeping with the absence of D5R immunostaining in the juxtaglomerular apparatus, including the juxtaglomerular cell, and macula densa. These data suggest that the D5R, unlike the D1R (1-3, 36), may not regulate the secretion/release of renin. High salt intake suppresses renin secretion in kidney. Renin secretion is probably suppressed by salt intake to a similar extent in both D5+/+ and D5-/- mice since there is no difference in renal renin level between the mouse strains placed on high salt diet (26). It is, therefore, unlikely that alterations in angiotensin II levels are responsible for the differential expression of distal sodium transporters in D5-/- and D5+/+ mice. Nevertheless, we determined whether the increased expression and activity of the AT1R is responsible for the increased expression of distal tubule sodium transporters/channel subunits in D5-/- mice by blocking AT1R function with losartan. In spite of AT1R blockade, the increase in renal distal sodium transporters/channel subunits observed in D5-/- mice fed the elevated sodium diet persisted, suggesting that the increased expression of distal renal sodium transporters/channel subunits in D5-/- mice is independent of the AT1R. The increase in NHE3, but not NaPi2, is abolished by AT1R blockade, suggesting an additional effect of AT1R on NHE3 expression in D5-/- mice. The absence of D5R seems to enhance the renal tubular effects of AT1R when sodium intake is increased because losartan treatment abolishes not only the increase in NHE3 expression, but also the salt-sensitivity in D5-/- mice (26). Endothelin is probably not involved in the hypertension of D5-/- mice because endothelin A and B receptor antagonists, BQ610 (37) and BQ788 (38), respectively, do not affect the arterial blood pressure of D5-/- or D5+/+ mice (10). The role of increased sympathetic tone (10) and reactive oxygen species production (11) in the increased expression of renal distal tubule transporters in D5-/- mice was not evaluated (3).
In summary, relative to D5+/+ littermates, D5-/- mice have higher blood pressure and greater renal protein expressions of NKCC2, NCC, and α and γ subunits of ENaC on control and elevated sodium diet which is not abrogated by AT1R blockade. The expression of proximal sodium transporters, NHE3 and NaPi2, is increased only on elevated sodium diet and the increase in NHE3 but not NaPi2 is abolished by AT1R blockade in D5-/- mice. We suggest that the increased renal protein expression of these transporters/channels may play an important role in the pathogenesis of salt-sensitive hypertension that occurs with the disruption of the D5R gene in mice.
Perspectives
Several single nucleotide polymorphisms of the human D5R gene are associated with diminished function of the receptor (9). The increased renal distal sodium transporters and channels (NKCC2, NCC and ENaC) suggest that inhibitors of distal sodium transport may have a greater anti-hypertensive effect in humans with impaired function of the D5R
Acknowledgments
We would like to thank Dr. Mark A. Knepper from LKEM, NHLBI, National Institutes of Health, for providing the antibodies against the sodium transporters/channels.
Sources of funding: This work was supported in part by grants from the National Institutes of Health, HL68686 HL23081, HL074940, HL092196, and DK39308.
Footnotes
Conflicts: None
References
- 1.Jose PA, Eisner GM, Felder RA. Renal dopamine receptors in health and hypertension. Pharmacol Ther. 1998;80:149–182. doi: 10.1016/s0163-7258(98)00027-8. [DOI] [PubMed] [Google Scholar]
- 2.Hussain T, Lokhandwala MF. Renal dopamine receptor function in hypertension. Hypertension. 1998;32:187–197. doi: 10.1161/01.hyp.32.2.187. [DOI] [PubMed] [Google Scholar]
- 3.Wang X, Villar VA, Armando I, Eisner GM, Felder RA, Jose PA. Dopamine, kidney, and hypertension: studies in dopamine receptor knockout mice. Pediatr Nephrol. 2008;23:2131–2146. doi: 10.1007/s00467-008-0901-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grandy DK, Allen LJ, Zhang Y, Magenis RE, Civelli O. Chromosomal localization of three human D5 dopamine receptor genes. Genomics. 1992;13:968–973. doi: 10.1016/0888-7543(92)90009-h. [DOI] [PubMed] [Google Scholar]
- 5.Allayee H, de Bruin TW, Dominguez KM, Cheng LS, Ipp E, Cantor RM, Krass KL, Keulen ET, Aouizerat BE, Lusis AJ, Rotter JI. Genome scan for blood pressure in Dutch dyslipidemic families reveals linkage to a locus on chromosome 4p. Hypertension. 2001;38:773–778. doi: 10.1161/hy1001.092617. [DOI] [PubMed] [Google Scholar]
- 6.Casari G, Barlassina C, Cusi D, Zagato L, Muirhead R, Righetti M, Nembri P, Amar K, Gatti M, Macciardi F, Binelli G, Bianchi G. Association of the α-adducin locus with essential hypertension. Hypertension. 1995;25:320–326. doi: 10.1161/01.hyp.25.3.320. [DOI] [PubMed] [Google Scholar]
- 7.Cohn DH, Shohat T, Yahav M, Ilan T, Rechavi G, King L, Shohat M. A locus for an autosomal dominant form of progressive renal failure and hypertension at chromosome 1q21. Am J Hum Genet. 2000;67:647–651. doi: 10.1086/303051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper RS, Luke A, Zhu X, Kan D, Adeyemo A, Rotimi C, Bouzekri N, Ward R, Rorimi C. Genome scan among Nigerians linking blood pressure to chromosomes 2, 3, and 19. Hypertension. 2002;40:629–633. doi: 10.1161/01.hyp.0000035708.02789.39. [DOI] [PubMed] [Google Scholar]
- 9.Cravchik A, Gejman PV. Functional analysis of the human D5 dopamine receptor missense and nonsense variants: differences in dopamine binding affinities. Pharmacogenetics. 1999;9:199–206. [PubMed] [Google Scholar]
- 10.Hollon TR, Bek MJ, Lachowicz JE, Ariano MA, Mezey E, Ramachandran R, Wersinger SR, Soares-da-Silva P, Liu ZF, Grinberg A, Drago J, Young WS, III, Westphal H, Jose PA, Sibley DR. Mice lacking D5 dopamine receptors have increased sympathetic tone and are hypertensive. J Neurosci. 2002;22:10801–10810. doi: 10.1523/JNEUROSCI.22-24-10801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang Z, Asico LD, Yu P, Wang Z, Jones JE, Escano CS, Wang X, Quinn MT, Sibley DR, Romero GG, Felder RA, Jose PA. D5 dopamine receptor regulation of reactive oxygen species production, NADPH oxidase, and blood pressure. Am J Physiol Regul Integr Comp Physiol. 2006;290:R96–R104. doi: 10.1152/ajpregu.00434.2005. [DOI] [PubMed] [Google Scholar]
- 12.Zheng S, Yu P, Zeng C, Wang Z, Yang Z, Andrews PM, Felder RA, Jose PA. Gα12- and Gα13-protein subunit linkage of D5 dopamine receptors in the nephron. Hypertension. 2003;41:604–610. doi: 10.1161/01.HYP.0000057422.75590.D7. [DOI] [PubMed] [Google Scholar]
- 13.Zeng C, Yang Z, Wang Z, Jones J, Wang X, Altea J, Mangrum AJ, Hopfer U, Sibley DR, Eisner GM, Felder RA, Jose PA. Interaction of angiotensin II type 1 and D5 dopamine receptors in renal proximal tubule cells. Hypertension. 2005;45:804–810. doi: 10.1161/01.HYP.0000155212.33212.99. [DOI] [PubMed] [Google Scholar]
- 14.Sugiyama F, Churchill GA, Higgins DC, Johns C, Makaritsis KP, Gavras H, Paigen B. Concordance of murine quantitative trait loci for salt-induced hypertension with rat and human loci. Genomics. 2001;71:70–77. doi: 10.1006/geno.2000.6401. [DOI] [PubMed] [Google Scholar]
- 15.Bek MJ, Wang X, Asico LD, Jones JE, Zheng S, Li X, Eisner GM, Grandy DK, Carey RM, Soares-da-Silva P, Jose PA. Angiotensin-II type 1 receptor-mediated hypertension in D4 dopamine receptor-deficient mice. Hypertension. 2006;47:288–295. doi: 10.1161/01.HYP.0000198427.96225.36. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez-Llama P, Andrews P, Ecelbarger CA, Nielsen S, Knepper MA. Concentrating defect in experimental nephrotic syndrome: Altered expression of aquaporins and thick ascending limb Na+ transporters. Kidney Int. 1998;54:170–179. doi: 10.1046/j.1523-1755.1998.00984.x. [DOI] [PubMed] [Google Scholar]
- 17.Kim GH, Martin SW, Fernandez-Llama P, Masilamani S, Packer RK, Knepper MA. Long-term regulation of sodium-dependent cotransporters and ENaC in rat kidney: response to altered acid-base intake. Am J Physiol Renal Physiol. 2000;279:F459–F467. doi: 10.1152/ajprenal.2000.279.3.F459. [DOI] [PubMed] [Google Scholar]
- 18.Tiwari S, Nordquist L, Halagappa VK, Ecelbarger CA. Trafficking of ENaC subunits in response to acute insulin in mouse kidney. Am J Physiol Renal Physiol. 2007;293:F178–185. doi: 10.1152/ajprenal.00447.2006. [DOI] [PubMed] [Google Scholar]
- 19.Kim GH, Ecelbarger CA, Mitchell C, Packer RK, Wade JB, Knepper MA. Vasopressin increases Na-K-2Cl cotransporter expression in thick ascending limb of Henle's loop. Am J Physiol. 1999;276:F96–F103. doi: 10.1152/ajprenal.1999.276.1.F96. [DOI] [PubMed] [Google Scholar]
- 20.Kim GH, Masilamani S, Turner R, Mitchell C, Wade JB, Knepper MA. The thiazide-sensitive Na-Cl cotransporter is an aldosterone-induced protein. Proc Natl Acad Sci USA. 1998;95:14552–14557. doi: 10.1073/pnas.95.24.14552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masilamani S, Kim GH, Mitchell C, Wade JB, Knepper MA. Aldosterone-mediated regulation of ENaC α, β, and γ subunit proteins in rat kidney. J Clin Invest. 1999;104:R19–R23. doi: 10.1172/JCI7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu J, Li XX, Albrecht FE, Hopfer U, Carey RM, Jose PA. Dopamine1 receptor, GSα, and Na+-H+ exchanger interactions in the kidney in hypertension. Hypertension. 2000;36:395–399. doi: 10.1161/01.hyp.36.3.395. [DOI] [PubMed] [Google Scholar]
- 23.Li XX, Bek M, Asico LD, Yang Z, Grandy DK, Goldstein DS, Rubinstein M, Eisner GM, Jose PA. Adrenergic and endothelin B receptor-dependent hypertension in dopamine receptor type-2 knockout mice. Hypertension. 2001;38:303–308. doi: 10.1161/01.hyp.38.3.303. [DOI] [PubMed] [Google Scholar]
- 24.Ladines CA, Zeng C, Asico LD, Sun X, Pocchiari F, Semeraro C, Pisegna J, Wank S, Yamaguchi I, Eisner GM, Jose PA. Impaired renal D1-like and D2-like dopamine receptor interaction in the spontaneously hypertensive rat. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1071–1078. doi: 10.1152/ajpregu.2001.281.4.R1071. [DOI] [PubMed] [Google Scholar]
- 25.Wang XY, Masilamani S, Nielsen J, Kwon TH, Brooks HL, Nielsen S, Knepper MA. The renal thiazide-sensitive Na-Cl cotransporter as mediator of the aldosterone-escape phenomenon. J Clin Invest. 2001;108:215–222. doi: 10.1172/JCI10366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H, Armando I, Yu P, Escano C, Mueller SC, Asico L, Pascua A, Lu Q, Wang X, Villar VA, Jones JE, Wang Z, Periasamy A, Lau YS, Soares-da-Silva P, Creswell K, Guillemette G, Sibley DR, Eisner G, Gildea JJ, Felder RA, Jose PA. Dopamine 5 receptor mediates Ang II type 1 receptor degradation via an ubiquitin-proteasome pathway in mice and human cells. J Clin Invest. 2008;118:2180–2189. doi: 10.1172/JCI33637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang LE, Sandberg MB, Can AD, Pihakaski-Maunsbach K, McDonough AA. Effects of dietary salt on renal Na+ transporter subcellular distribution, abundance, and phosphorylation status. Am J Physiol Renal Physiol. 2008;295:F1003–1016. doi: 10.1152/ajprenal.90235.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oliverio MI, Best CF, Smithies O, Coffman TM. Regulation of sodium balance and blood pressure by the AT1A receptor for angiotensin II. Hypertension. 2000;35:550–554. doi: 10.1161/01.hyp.35.2.550. [DOI] [PubMed] [Google Scholar]
- 29.Mattson DL, Krauski KR. Chronic sodium balance and blood pressure response to captopril in conscious mice. Hypertension. 1998;32:923–928. doi: 10.1161/01.hyp.32.5.923. [DOI] [PubMed] [Google Scholar]
- 30.Ge Y, Bagnall A, Stricklett PK, Webb D, Kotelevtsev Y, Kohan DE. Combined knockout of collecting duct endothelin A and B receptors causes hypertension and sodium retention. Am J Physiol Renal Physiol. 2008;295:F1635–1640. doi: 10.1152/ajprenal.90279.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crowley SD, Gurley SB, Oliverio MI, Pazmino AK, Griffiths R, Flannery PJ, Spurney RF, Kim HS, Smithies O, Le TH, Coffman TM. Distinct roles for the kidney and systemic tissues in blood pressure regulation by the renin-angiotensin system. J Clin Invest. 2005;115:1092–1099. doi: 10.1172/JCI200523378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Granger JP, Alexander BT, Llinas M. Mechanisms of pressure natriuresis. Curr Hypertens Rep. 2002;4:152–159. doi: 10.1007/s11906-002-0040-3. [DOI] [PubMed] [Google Scholar]
- 33.Frindt G, Palmer LG. Surface expression of sodium channels and transporters in rat kidney: effects of dietary sodium. Am J Physiol Renal Physiol. 2009;297:F1249–1255. doi: 10.1152/ajprenal.00401.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brooks HL, Allred AJ, Beutler KT, Coffman TM, Knepper MA. Targeted proteomic profiling of renal Na+ transporter and channel abundances in angiotensin II type 1a receptor knockout mice. Hypertension. 2002;39:470–473. doi: 10.1161/hy02t2.102959. [DOI] [PubMed] [Google Scholar]
- 35.Beutler KT, Masilamani S, Turban S, Nielsen J, Brooks HL, Ageloff S, Fenton RA, Packer RK, Knepper MA. Long-term regulation of ENaC expression in kidney by angiotensin II. Hypertension. 2003;41:1143–1150. doi: 10.1161/01.HYP.0000066129.12106.E2. [DOI] [PubMed] [Google Scholar]
- 36.Charpentier S, Jarvie KR, Severynse DM, Caron MG, Tiberi M. Silencing of the constitutive activity of the dopamine D1B receptor. Reciprocal mutations between D1 receptor subtypes delineate residues underlying activation properties. J Biol Chem. 1996;271:28071–28076. doi: 10.1074/jbc.271.45.28071. [DOI] [PubMed] [Google Scholar]
- 37.Strachan FE, Spratt JC, Wilkinson IB, Johnston NR, Gray GA, Webb DJ. Systemic blockade of the endothelin-B receptor increases peripheral vascular resistance in healthy men. Hypertension. 1999;33:581–585. doi: 10.1161/01.hyp.33.1.581. [DOI] [PubMed] [Google Scholar]
- 38.Allcock GH, Warner TD, Vane JR. Roles of endothelin receptors in the regional and systemic vascular responses to ET-1 in the anaesthetized ganglion-blocked rat: use of selective antagonists. Br J Pharmacol. 1995;116:2482–2486. doi: 10.1111/j.1476-5381.1995.tb15099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]