Abstract
Background
The combination of tenofovir and emtricitabine (TDF/FTC) shows promise as HIV pre-exposure prophylaxis (PrEP). We sought to forecast clinical, epidemiologic, and economic outcomes of PrEP, taking into account uncertainties regarding efficacy, risk of resistance and toxicity, behavioral disinhibition, and drug costs.
Methods
We adapted a computer simulation of HIV acquisition, detection, and care to model PrEP in high-risk (1.6% average annual HIV incidence) men who have sex with men (MSM) in the United States. Base case assumptions included: 50% PrEP efficacy and $753 monthly TDF/FTC costs. We used sensitivity analyses to examine the stability of results and to identify critical input parameters.
Results
In a cohort with mean age 34 years, PrEP reduced lifetime HIV infection risk from 44% to 25% and increased average life expectancy from 39.9 to 40.7 years (21.7 to 22.2 discounted, quality-adjusted life-years or QALYs). Discounted mean lifetime treatment costs increased from $81,100 to $232,700 per person, indicating an incremental cost-effectiveness ratio (ICER) of $298,000 per QALY gained. Markedly larger reductions in lifetime infection risk (from 43.5% to 5.8%) were observed assuming greater (90%) PrEP efficacy. More favorable ICERs were obtained by targeting younger, higher-incidence populations and with improvements in the efficacy and cost of PrEP.
Conclusions
PrEP could substantially reduce HIV transmission in high-risk populations in the United States. Although it is unlikely to confer sufficient benefits to justify current TDF/FTC costs, price reductions and/or increases in efficacy could make PrEP a cost-effective option in younger or higher-risk populations. Given recent disappointments in HIV prevention and vaccine development, further study of PrEP-based HIV prevention is warranted.
INTRODUCTION
Dramatic advances in HIV treatment in the United States over the last decade [1] contrast with the persistent frustrations surrounding HIV prevention efforts over the same period [2]. Recent setbacks include the halting of major microbicide and vaccine trials [3–6], discouraging results regarding the efficacy of female-initiated barrier methods in preventing heterosexual transmission [7, 8] and equivocal findings on the impact of male circumcision on HIV risk in MSM [9–12]. In the face of these disappointments, the combination of tenofovir and emtricitabine (TDF/FTC) shows promise as pre-exposure prophylaxis (PrEP) for persons at high risk of HIV infection [13–19].
Trials of TDF/FTC-based chemoprophylaxis in macaques report nearly 8-fold reductions in the risk of HIV infection [20]. In humans, recent observations in high-risk women suggest an efficacy estimate of 65% [21]. However, the long-term impact of PrEP on transmission, behavior, clinical outcomes, and cost has not been studied. Based on its current annual price of US$8,700 per person (when used for treatment), some observers conclude that TDF/FTC could not be cost-effective when used for PrEP, except in populations at highest HIV risk [22]. Others have noted that PrEP poses risks for additional drug toxicity, viral resistance, and behavioral disinhibition [23]. Our objective was to weigh these considerations and to provide practical guidance – to practitioners, payers and designers of upcoming clinical trials [24] – regarding the clinical, epidemiological, and economic circumstances under which PrEP might serve as a viable and appropriate use of prevention resources.
METHODS
Study design
We used a widely published computer simulation of HIV acquisition, detection and care [1, 25–27] to forecast outcomes of TDF/FTC-based PrEP delivered to a population of high-risk MSM (1.6% average annual HIV incidence) in the US. Outcomes included lifetime infection risk, life expectancy, quality-adjusted life expectancy and cost. Conforming with the recommendations of the US Panel on Cost-Effectiveness in Health and Medicine [28], we measured comparative value in 2006 US dollars per quality-adjusted life-year (QALY) gained, reporting all economic evaluation outcomes from the societal perspective using a 3% annual discount rate.
Disease model
The Cost-Effectiveness of Preventing AIDS Complications (CEPAC) Model (the Disease Model) – is a state-transition, Monte Carlo simulation of the natural history, clinical management, outcomes, and costs of HIV disease [25–27]. Natural history of illness in a patient is represented by a sequence of monthly transitions between “health states” describing current health (i.e., CD4 cell count, HIV RNA level, relevant history, quality of life, and resource use) and predicting further disease progression (i.e., immune system deterioration, opportunistic infections (OIs), therapeutic response, and medication resistance/toxicity). The model permits users to define both patient attributes (i.e., age, sex, CD4 count, HIV RNA, and other demographic/clinical attributes) and therapeutic alternatives (i.e., number, sequencing, and efficacy of antiretroviral therapy (ART) regimens). Treated patients receive up to six sequential regimens, with progressively diminishing HIV RNA suppressive efficacy (Table 1). ART failure is defined by either an observed increase in HIV RNA level or a decrease of 50% from peak CD4. Each patient’s clinical course is tracked from entry into the simulation until death. Large numbers of individual simulations are then aggregated to estimate survival, quality-adjusted survival, and costs for alternative PrEP strategies.
Table 1.
Input Data Values for Analysis of PrEP
| Variable | Baseline Value |
Range Evaluated |
Source |
|---|---|---|---|
| Initial cohort | |||
| Mean age (SD) | 34 (9.4) | 20 – 34 | [40] |
| % male | 100 | --- | Assumption |
| Average HIV incidence (per 100 person-years) | 1.6 | 0.1 – 3.1 | [29] |
| Incidence distribution by age (per 100 PY) | [29] | ||
| < 18 years | 2.1 | 0.1 – 4.1 | |
| 18 – 25 years | 2.1 | 0.1 – 4.1 | |
| 26 – 30 years | 1.9 | 0.1 – 3.8 | |
| 31 – 35 years | 1.8 | 0.1 – 3.6 | |
| 36 – 40 years | 1.1 | 0.07 – 2.1 | |
| 41 – 45 years | 0.8 | 0.05 – 1.7 | |
| > 45 years | 1.5 | 0.09 – 2.9 | |
| Viral load distribution if infected (%) | [30] | ||
| > 30,000 copies/ml | 25.73 | --- | |
| 10,001 – 30,000 copies/ml | 25.02 | --- | |
| 3,001 – 10,000 copies/ml | 25.21 | --- | |
| 500 – 3,000 copies/ml | 16.33 | --- | |
| < 500 copies/ml | 7.71 | ||
| PrEP efficacya (%) | 50 | 10 – 90 | [31] |
| Efficacy of antiretroviral therapy without history of PrEP: % HIV RNA suppressed to <400 copies/ml (increase in CD4 cells/µl)b | ||||
|---|---|---|---|---|
| % HIV RNA suppressed to <400 copies/mlc |
Increase in CD4 cells/µl c |
Source | ||
| 1. TDF/FTC + EFV | 81 | 190 | [32] | |
| 2. ATV/r + 2NRTIs | 70 | 110 | [33] | |
| 3. LPV/r + 2NRTIs | 58 | 121 | [33] | |
| 4. RAL + OBR | 65 (24 weeks) | 102 (24 weeks) | [34] | |
| 5. 50% ENF + OBR; 50% MVC + OBR +/− ENFd |
40 (24 weeks) | 117 (24 weeks) | [35, 36] | |
| 6. OBR | 12 | 45 | [35] | |
| Monthly Costs (2006 US$) |
Baseline Value |
Range evaluated |
Source | |
| Antiretroviral therapy | [37] | |||
| TDF/FTC + EFV | 1,139 | --- | ||
| ATV/r + 2NRTIs | 1,741 | --- | ||
| LPV/r + 2NRTIs | 1,748 | --- | ||
| RAL + OBR | 2,209 | --- | ||
| 50% ENF + OBR; 50% MVC + OBR +/− ENF | 3,338 | --- | ||
| OBR | 1,549 | --- | ||
| PrEP (TDF/FTC) | 753 | 101 – 753 | [37–39] | |
PrEP = pre-exposure prophylaxis; SD = standard deviation; TDF = tenofovir; FTC = emtricitabine; EFV = efavirenz; ATV/r = ritonavir-boosted atazanavir; LPV/r = ritonavir-boosted lopinavir; RAL = raltegravir; OBR = optimized background regimen; ENF = enfuvirtide; MVC = maraviroc
PrEP efficacy is modeled as a net percent reduction in the monthly incidence of HIV infection, accounting for both the chemoprophylactic effect of PrEP and the potential offset of behavioral disinhibition.
Values reported in the table reflect values found in the original source papers. In some instances, results reported by authors on an “intention-to-treat” basis were adjusted to reflect an “as-treated” basis before being used in the model.
Values are reported at 48 weeks unless otherwise specified.
Values reported for this regimen are weighted averages of the values found in the two original source papers.
Population model
Entry into the Disease Model is regulated by a population-level simulation that captures the incidence of HIV infection and the mechanics of HIV counseling, testing, and referral [26, 27]. Users can specify variables governing the provision, performance, and cost of pre- and post-test counseling services and PrEP. The Population Model has the flexibility to capture a range of program intensities, from no intervention, to basic risk counseling, to more elaborate behavioral and chemoprophylactic prevention. For patients receiving PrEP, the model tracks changes in risk-taking behavior, toxicity, occurrence of drug resistance in individuals who become HIV-infected and are subsequently treated with ART, quality of life, and cost of HIV-related care.
The Population Model also captures clinical and economic outcomes associated with HIV transmission, detection, and referral activities. Using methods described elsewhere [26, 27], the model estimates lifetime infection risk under alternative PrEP scenarios. The Population Model conveys information to the Disease Model on HIV infection status, whether and when HIV detection, follow-up, and linkage to care occur, and whether an infected person previously received TDF/FTC-based PrEP. The Disease Model then combines this information with its own output on the timing of AIDS-defining complications to establish whether, when, and how an individual case of HIV infection will be treated.
Input data: Target populations
We defined a high-risk target population, using age and annual HIV incidence data obtained from men who have sex with men (MSM) in the HIVNET Vaccine Preparedness Study (Table 1) [29]. The base case population had mean age 34 years (SD = 9.4 years), reflecting the expected enrollment of a US prevention trial amongst high-risk MSM [29, 40, 41]. We used HIVNET data to produce age-specific HIV incidence estimates (Table 1). In sensitivity analysis, we considered mean ages as low as 20 years (SD = 2) and population average annual HIV incidences ranging from 0.1% to 3.1%.
In the absence of published data on HIV screening activities in the target population, we assumed that individuals receive annual HIV tests. Given reports suggesting average infection-to-detection times greater than five years [42], this assumption introduced a deliberately conservative analytic bias against PrEP. In sensitivity analysis, we considered average frequencies ranging from monthly to every 3 years to never. We varied these frequencies depending on whether the individual was receiving PrEP.
Input data: Impact of PrEP
PrEP efficacy
We modeled PrEP efficacy as a percent reduction in HIV incidence. Our baseline efficacy assumption (50%) corresponds to the value used by Desai [31], reflects observed rates of protection in macaque-based studies of TDF/FTC-based chemoprophylaxis [20], and matches the efficacy for which current human trials are powered [24]. It is conservative compared with the 65% point estimate reported by the only study in humans to date [21]. Recognizing the uncertainty surrounding this estimate, the possibility that newer agents might exhibit greater preventive properties, and the potential offsetting influence of imperfect adherence and other behavioral factors, we examined efficacy values ranging from 10% to 90% in sensitivity analysis.
Resistance
Resistance to emtricitabine via the M184V mutation occurs rapidly in patients with detectable HIV RNA on this drug; tenofovir resistance with the K65R mutation is less common [32, 43, 44]. Although data are not yet available on resistance to either drug after PrEP failure, limited evidence from animal studies suggest that substantial rates of resistance are possible [16, 45]. We modeled PrEP-related resistance conservatively: First, we assumed resistance in all HIV-infected patients with a PrEP history. Second, we assumed that clinicians would eliminate the initial efavirenz-based regimen for patients with a history of PrEP, because of the low resistance threshold of efavirenz (especially when combined with potential NRTI resistance). Third, because these are generally used in the first line of therapy [46], we assumed an absolute 5% decrease in rates of virologic suppression for all lines of ART in patients infected after PrEP. Finally, we examined a wide range of alternative assumptions in sensitivity analyses, varying the decrease in suppression on all lines of therapy from 0% to 15%.
Toxicity
The incidence of adverse effects with TDF/FTC when used in HIV-infected patients is low [23, 47] but it is not zero [48, 49]. Studies addressing long-term safety in HIV-negative subjects are emerging [21, 50]. We conducted extensive sensitivity analysis on toxicity-related reductions in both quality of life and survival. Specifically, we considered scenarios ranging from no TDF-related toxicity to the case where 10% of all patients initiating PrEP suffer chronic renal disease resulting in a permanent decrement of 10% in quality of life and where an additional 1% of patients suffer a TDF-toxicity-related death shortly following PrEP initiation. This “extreme toxicity scenario” greatly exceeds the morbidity and mortality effects implied by recent reports of TDF toxicity [51].
Behavioral disinhibition
HIV risk reduction creates the potential for behavioral disinhibition [52]. However, there is little evidence linking HIV prevention to increased risk-taking and even less evidence to suggest that the magnitude of any risk compensation is sufficient to offset the preventive effect [53]. Recognizing that the issue remains to be resolved empirically for the particular case of PrEP, we considered a broad range of behavioral assumptions. In sensitivity analysis, the potential effects of disinhibition were modeled as a percent reduction in PrEP efficacy. Thus, the “PrEP efficacy” parameter described above should be understood as a “net” value that includes synergistic effects (e.g., increased condom use; adoption of adult male circumcision), unfavorable behavioral offsets (e.g., reduced condom use; increased partnerships), and imperfect adherence and take-up.
Cost
In the base case, we used the $724 current average monthly wholesale price of TDF/FTC (300/200 mg per day), adjusted to reflect rebates to Medicaid programs and retail pharmacy dispensing fees [37]. This value reflects the conservative assumption that prescriptions will be filled and costs incurred, even if pills are not consumed. We considered costs as low as 10% of this baseline value ($72) in sensitivity analysis. This permitted us to capture emerging evidence on the favorable outcomes and possible cost reductions associated with less frequent or lower dosing of TDF/FTC [16, 54, 55]. We further assumed that individuals receiving PrEP received quarterly laboratory monitoring (complete blood counts, comprehensive metabolic panels, and chemistry panels), semi-annual physical examinations, and annual full lipid panels; these added $28 to the monthly cost of PrEP (Table 1). Greater detail on cost data used in the analysis is provided elsewhere [27, 56].
RESULTS
Base case
In a high-risk population with mean age 34 years and an average annual HIV incidence of 1.6%, current practices of HIV prevention and care produced a lifetime HIV infection risk of 44% and survival of 39.9 years. Discounted survival for the entire population totaled 21.7 QALYs, at an average discounted lifetime cost of $81,100 (Table 2). Introduction of TDF/FTC-based PrEP with 50% efficacy reduced lifetime infection risk to 25% and increased survival to 40.7 years. Discounted quality-adjusted survival rose to 22.2 QALYs and discounted lifetime costs increased to $232,700 per person, suggesting an incremental cost-effectiveness ratio (ICER) of $298,000 per QALY gained.
Table 2.
Base Case Results and Selected Scenario Analyses
| Undiscounted Results | Discounted Results | ||||
|---|---|---|---|---|---|
| Lifetime Infection Risk (%) |
Life-Years | Cost ($) | QALYs | $/QALY | |
| Base case | |||||
| no PrEP | 44 | 39.9 | 81,100 | 21.7 | |
| PrEP | 25 | 40.7 | 232,700 | 22.2 | 298,000 |
| PrEP efficacya increased to 90% | |||||
| no PrEP | 44 | 39.9 | 81,100 | 21.7 | |
| PrEP | 6 | 42.5 | 215,400 | 23.0 | 107,000 |
| Base HIV incidence increased to 3.1% | |||||
| no PrEP | 66 | 37.8 | 136,700 | 20.7 | |
| PrEP | 44 | 38.9 | 250,800 | 21.4 | 150,000 |
| PrEP cost reduced 50% | |||||
| no PrEP | 44 | 39.9 | 81,100 | 21.7 | |
| PrEP | 25 | 40.7 | 139,300 | 22.2 | 114,000 |
| No routine HIV screening in the “no PrEP” scenario | |||||
| no PrEP | 44 | 35.4 | 16,800 | 20.2 | |
| PrEP | 25 | 40.7 | 232,700 | 22.2 | 109,000 |
| ART after PrEP including EFV | |||||
| no PrEP | 44 | 39.9 | 81,100 | 21.7 | |
| PrEP | 25 | 41.2 | 231,500 | 22.4 | 234,000 |
| PrEP extreme toxicity scenario b | |||||
| no PrEP | 44 | 39.9 | 81,100 | 21.7 | |
| PrEP | 25 | 40.7 | 232,700 | 21.8 | 1,546,000 c |
| Mean target population age decreased to 20 years (SD = 2) | |||||
| no PrEP | 55 | 49.5 | 116,900 | 24.1 | |
| PrEP | 25 | 51.2 | 270,600 | 24.9 | 189,000 |
QALY = quality-adjusted life-year; $/QALY = incremental cost-effectiveness ratio measured in 2006 US dollars per quality-adjusted life-year gained; ART = antiretroviral therapy; EFV = efavirenz.
PrEP efficacy is modeled as a net percent reduction in the monthly incidence of HIV infection, accounting for both the chemoprophylactic effect of PrEP and the potential offset of behavioral disinhibition.
The “extreme toxicity scenario” assumes that 10% of all patients initiatiating PrEP suffer chronic renal disease resulting in a permanent decrement of 10% in their quality of life and where an additional 1% of patients suffer a TDF-toxicity-related death shortly following PrEP initiation.
A dominated strategy has a higher cost and an equal or lower quality-adjusted life expectancy than some combination of other strategies.
Sensitivity analysis
Table 2 presents results from alternative illustrative scenarios. Lifetime infection risk decreased and the cost-effectiveness of PrEP improved when we assumed greater PrEP efficacy. We obtained more favorable ICERs by assuming either a younger or a riskier target population, reduced PrEP costs, and reduced rates of HIV case identification for persons not receiving PrEP. For example, when we assumed that PrEP prevented 90% of new HIV infections, lifetime infection risk fell to 6%, survival increased to 42.5 years, and the ICER improved to $107,000/QALY. Less favorable ICERs resulted when PrEP reduced the number of available ART regimens or increased adverse events (result not shown).
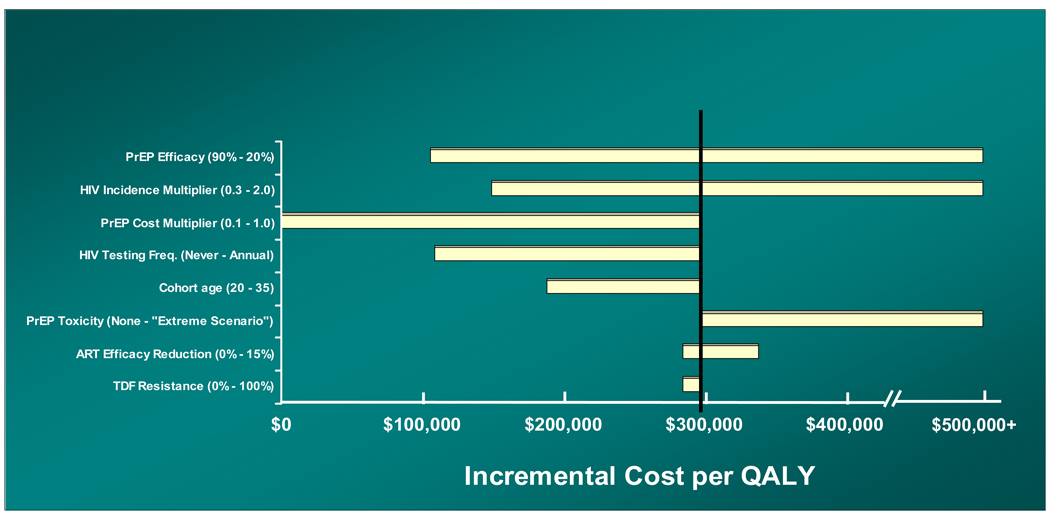
One-way sensitivity analyses (Figure 1) highlighted six parameters whose uncertainty over plausible ranges of variation produced sizeable changes in cost-effectiveness. Five of these parameters – PrEP efficacy, HIV incidence in the target population, cost of PrEP, rate of HIV case detection in persons not receiving PrEP, and age of the target population – could adopt values that materially improved the ICER. For example, reducing the rate of HIV screening among persons not receiving PrEP from “annual” to “never” reduced the ICER to $109,000/QALY. Assumptions regarding lost ART efficacy and the risk of TDF resistance in breakthrough HIV infections had little impact.
FIGURE 1. One-Way Sensitivity Analyses.
A “tornado diagram” summarizes the results of a series of 1-way sensitivity analyses on the incremental cost-effectiveness of PrEP. Each horizontal bar represents the full range of costeffectiveness ratios produced by varying a given model parameter across its entire plausible range, as described in the Methods section. The bar denoting the “HIV testing frequency” variable, for example, summarizes the results we obtained using all of the following frequencies: never; once every 10 years; once every 5 years; once every 3 years; once every 2 years; and annually. The vertical line denotes the base case incremental cost-effectiveness estimate ($298,000/QALY). Note: The horizontal axis should be understood to extend beyond $500,000/QALY and to include instances where the PrEP intervention is dominated (i.e., it costs more and confers fewer QALYs than its comparator). PrEP = pre-exposure prophylaxis; QALY = quality-adjusted life-year; ART = antiretroviral therapy; TDF = tenofovir.
Guidance for the prospective evaluation of PrEP
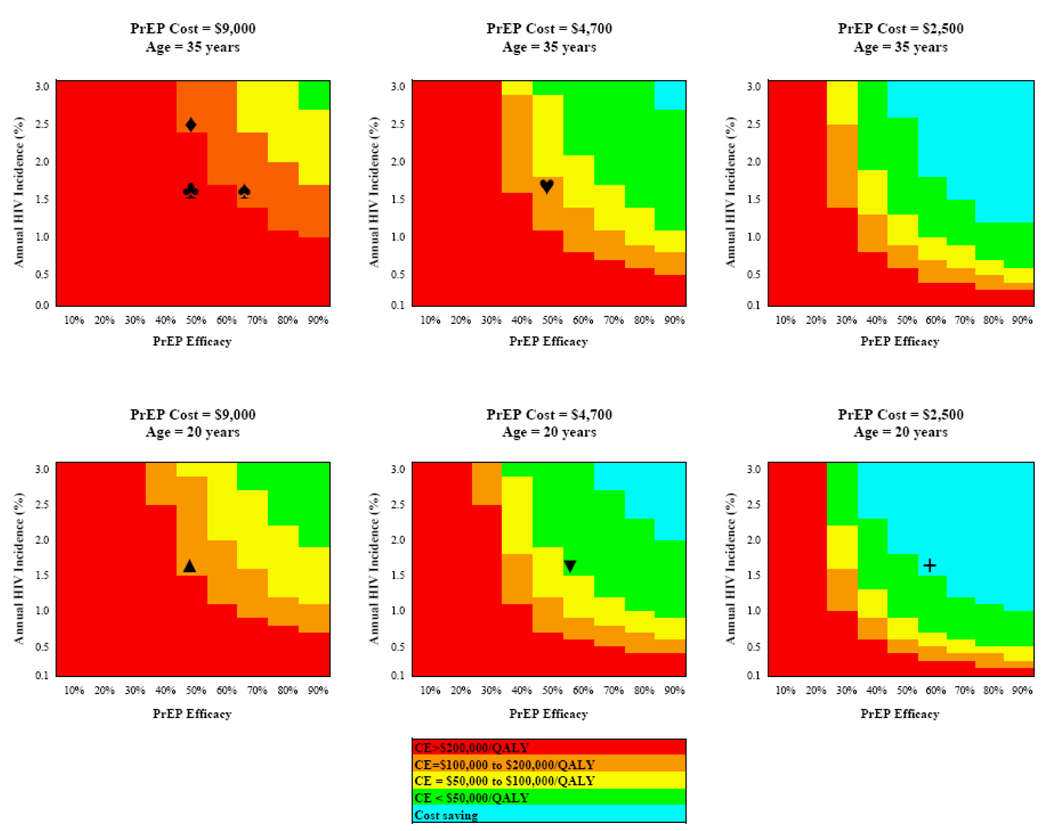
Figure 2 depicts the cost-effectiveness of PrEP as a function of four influential parameters identified via the one-way sensitivity analyses: PrEP efficacy, PrEP cost, and the age and HIV incidence in the target population. In the base case (50% PrEP efficacy, $9,000 annual cost, target population 34 years and 1.6% average annual HIV incidence, ♣), the intervention is deemed cost-effective only if society’s willingness to purchase an additional QALY of health for its citizens (i.e., the cost-effectiveness threshold) exceeds $200,000 [57, 58]. ICERs in the $100,00–$200,000/QALY range for PrEP can be achieved via any one of the following changes: increase efficacy above 70% (♠); target a population with annual incidence greater than 2.4% (♦); reduce price to $4,700/year (♥); or target a population with average age 20 years (▲). Simultaneous changes in these parameters could produce even lower ICERs. For example, a 60%-effective program costing $4,700/year and targeted to a 20-year-old population with annual HIV incidence 1.5% would have an ICER of $50,000/QALY (▼). Further reduction in the price of the same intervention to $2,500/year would be cost-saving (+).
FIGURE 2. Multi-Way Sensitivity Analyses.
The figure reports ranges of incremental cost-effectiveness for PrEP as a function of the four influential parameters identified via the one-way sensitivity analyses in Figure 1: PrEP efficacy, PrEP cost, and the age and HIV incidence in the target population. In each of the six panels, the horizontal axis denotes PrEP efficacy (measured as a % reduction in infections) and the vertical axis denotes the average annual HIV incidence in the target population. The top three panels consider a target population with mean age 34 years; the lower three panels consider a younger target cohort (mean age = 20 years). Moving from left to right, the three columns of panels consider decreasing annual PrEP costs, ranging from $9,000 to $2,000. The shading denotes the resultant ICER, ranging from >$200,000/QALY through cost-saving.
DISCUSSION
This analysis suggests that TDF/FTC-based PrEP could substantially reduce the lifetime risk of HIV infection in persons at high risk in the United States. Given the persistent disappointments of HIV prevention interventions over the last decade [2–8], this finding alone justifies continued study of PrEP-based approaches. While there is no consensus defining acceptable value for money, ICERs are often placed in context by comparisons with interventions that are widely recommended and generally viewed as non-controversial, such as colorectal cancer screening, home dialysis, and cholesterol-lowering drugs for men with cardiovascular risk factors [59, 60]. Based on the current treatment cost of TDF/FTC and conservative estimates of efficacy, we estimate that PrEP has an ICER of $298,000/QALY gained, making it an unattractive intervention, from a US-based cost-effectiveness perspective, compared to most (but not all) observers’ thresholds [57, 58].
This assessment hinges on several key parameter assumptions that, taken collectively, have important policy relevance. The PrEP efficacy parameter establishes a quantitative benchmark by which to judge newer ART agents that may exhibit HIV prevention properties. It suggests that small improvements in efficacy over that which has already been observed in preliminary studies would significantly improve the attractiveness of TDF/FTC-based PrEP but would not be sufficient to meet most standards of cost-effectiveness in the US [57]. It also provides a framework by which to weigh the transmission benefits of PrEP-based prevention and the additional, synergistic benefits of other HIV prevention strategies (e.g., increased condom use and and adoption of adult male circumcision) against the potential offsetting impact of behavioral disinhibition. The cost parameter indicates the potential of price reductions to greatly improve the attractiveness of PrEP. These reductions might be achieved either via lower pricing of ART when used for preventive purposes or if clinical evidence suggests a lower effective dose to achieve adequate HIV prevention. The age and HIV incidence parameters indicate that the attractiveness of PrEP would increase if it were targeted to populations at even greater risk (e.g., younger, HIV-discordant MSM). The final parameter – frequency of HIV testing in persons not receiving PrEP (Figure 1) – highlights the strong interdependence of HIV prevention and care. PrEP-based prevention is most attractive where there is poor identification and linkage of infected persons to lifesaving care. High rates of HIV testing in the principal target population groups may reduce the attractiveness of PrEP. At a minimum, they serve as a reminder that while prevention and treatment activities may not be substitutes, the costs and benefits of one can only be evaluated in the context of the other.
Although we adopted highly pessimistic base case assumptions, our findings do not identify resistance in breakthrough infections to be a critical driver of the attractiveness of PrEP. The expanding array of increasingly effective ART options dampens the potential harm to patients with resistant infection caused by the loss of an entire line of therapy and potential reduction in the efficacy of the remaining regimens. When risks and benefits are measured using a common metric (i.e., their impact on quality-adjusted survival) across the entire at-risk population, the preventive benefits of PrEP outweigh the risk of resistance.
This analysis has several limitations. First, we rely on data obtained from preliminary studies. Our intent is to suggest standards of evidence for further data collection. Until confirmatory trials can verify the plausibility of the critical input value assumptions (notably PrEP efficacy and required dosage), our policy conclusions should be interpreted with caution. Second, the usual parameters by which cost-effectiveness is judged may be different in persons at the very highest risk of infection, whose willingness to pay may differ from society’s. Anecdotal evidence suggests that TDF/FTC is already being used off-label by high-risk HIV-uninfected MSM [61, 62]. Third, by ignoring the secondary transmissions averted when a primary case of HIV infection is prevented, we understated the transmission benefits of PrEP. This might be an important omission in identifiable, small networks of sexually active MSM, given the possible adverse impact of transmitted resistance from failed PrEP subjects to their partners before being on suppressive ART [63, 64]. Fourth, we do not consider the possibility of optimizing the time on PrEP as a function of patient age and risk behavior. Here again, we may have overstated the costs of PrEP by pursuing an expensive strategy of HIV prophylaxis in patients who are at lower risk as they age. We do not tackle the challenges of using TDF/FTC in patients potentially requiring this treatment intervention for hepatitis B co-infection. Positive testing for hepatitis B surface antibody (acquired either through natural immunity or vaccination) might be an appropriate eligibility criterion for PrEP use. We have not addressed important ethical and financial considerations of priority setting. Even if the cost-effectiveness of PrEP can be established, questions of who should receive PrEP and who should pay for it – over what duration and at what frequency – remain to be addressed. Finally, our analysis focuses on the United States, ignoring the potential impact of PrEP globally. With an estimated 2.5 million people newly infected with HIV in 2007 [65], chemoprophylaxis with antiretroviral agents represents a promising new approach to containing the epidemic.
Current approaches to HIV chemoprophylaxis can substantially reduce the lifetime risk of HIV infection in the US. With improvements in efficacy, targeting, or pricing, such approaches may also be cost-effective by current US standards. The significance of this analysis lies not only in its relevance to TDF/FTC-based PrEP but also in the establishment of performance benchmarks for future generations of antiretroviral agents, many of which are likely to display chemoprophylactic properties. Given the many disappointments of HIV prevention efforts in recent years, greater focus on PrEP-based approaches is warranted.
ACKNOWLEDGMENT
Financial support
This research was supported by grants from the National Institute of Mental Health (R01MH65869), the National Institute of Allergy and Infectious Diseases (K24AI062476, K25AI50436, R37AI42006, P30AI42851), the National Institute on Drug Abuse (K01DA17179, R01DA015612), and the Doris Duke Charitable Foundation (Clinical Scientist Development Award). None of these funding sources played any role whatsoever in the design, analysis, or interpretation of the study or in the decision to submit the manuscript for publication.
Footnotes
Potential conflicts of interest
With the exception of Dr. Sax, none of the authors report any association that might pose a conflict of interest (e.g., pharmaceutical stock ownership, consultancy, advisory board membership, relevant patents, or research funding). Dr. Sax serves as a Consultant to Abbott, BMS, Gilead, GSK, Merck, and Tibotec. He receives honoraria for teaching from Abbott, BMS, Gilead, Merck, Tibotec. He receives grant support from Merck.
REFERENCES
- 1.Walensky RP, Paltiel AD, Losina E, et al. Three million years of life saved: The survival benefits of AIDS treatment in the United States. J Infect Dis. 2006 Jul 1;194(1):11–19. doi: 10.1086/505147. [DOI] [PubMed] [Google Scholar]
- 2.The PLoS Medicine Editors. HIV treatment proceeds as prevention research confounds. PLoS Med. 2007 Dec;4(12):e347. doi: 10.1371/journal.pmed.0040347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doncel G, van Damme L. Update on the CONRAD cellulose sulfate trial [Abstract 106LB]. 14th Conference on Retroviruses and Opportunistic Infections; Los Angeles, CA. 2007. [Google Scholar]
- 4.Ramjee G, Govinden R, Morar NS, Mbewu A. South Africa's experience of the closure of the cellulose sulphate microbicide trial. PLoS Med. 2007 Jul;4(7):e235. doi: 10.1371/journal.pmed.0040235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. [cited February 1 2008];Cellulose sulfate microbicide trial stopped. 2007 January 31; Available from: http://www.who.int/mediacentre/news/statements/2007/s01/en/index.html.
- 6.Robertson M, Mehrota D, Fitzgerald D, et al. Efficacy results from the STEP study (Merck V520 protocol 023/HVTN 502): A phase II test-of-concept trial of the MRKAd5 HIV-1 Gag/Pol/Nef trivalent vaccine [abstract 88LB]. 15th Conference on Retroviruses and Opportunistic Infections; Boston, MA. 2008. [Google Scholar]
- 7.Padian NS, van der Straten A, Ramjee G, et al. Diaphragm and lubricant gel for prevention of HIV acquisition in southern African women: a randomised controlled trial. Lancet. 2007 Jul 21;370(9583):251–261. doi: 10.1016/S0140-6736(07)60950-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Family Health International. [cited May 15 2008];Phase 3 trial in Nigeria evaluating the effectiveness of SAVVY gel in preventing HIV infection in women will close. 2006 August 28; Available from: http://www.fhi.org/en/AboutFHI/Media/Releases/Phase3SAVVY082806.
- 9.Millett GA, Flores SA, Marks G, Reed JB, Herbst JH. Circumcision status and risk of HIV and sexually transmitted infections among men who have sex with men: a meta-analysis. JAMA. 2008 Oct 8;300(14):1674–1684. doi: 10.1001/jama.300.14.1674. [DOI] [PubMed] [Google Scholar]
- 10.Auvert B, Taljaard D, Lagarde E, Sobngwi-Tambekou J, Sitta R, Puren A. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 Trial. PLoS Med. 2005 Nov;2(11):e298. doi: 10.1371/journal.pmed.0020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bailey RC, Moses S, Parker CB, et al. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet. 2007 Feb 24;369(9562):643–656. doi: 10.1016/S0140-6736(07)60312-2. [DOI] [PubMed] [Google Scholar]
- 12.Gray RH, Kigozi G, Serwadda D, et al. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet. 2007 Feb 24;369(9562):657–666. doi: 10.1016/S0140-6736(07)60313-4. [DOI] [PubMed] [Google Scholar]
- 13.Choopanya K, Martin M, Vanichseni S, et al. Enrollment, risk behavior, and adherence of injecting drug users in an HIV prevention trial in Bangkok [Abstract 568]. 15th Conference on Retroviruses and Opportunistic Infections; Boston, MA. 2008. [Google Scholar]
- 14.Atchison R, Peterson L, Leigler T, Cates W, Grant R. No evidence of drug-resistance mutations in a seroconverter exposed to tenofovir disoproxil fumarate chemoprophylaxis in Africa [Abstract 570]. 15th Conference on Retroviruses and Opportunistic Infections; Boston, MA. 2008. [Google Scholar]
- 15.McConnell J, Grant R, Goicochea P, et al. iPrEx: A timely intervention unfolds in a population of eminent risk of HIV infection [Abstract 569]. 15th Conference on Retroviruses and Opportunistic Infections; Boston, MA. 2008. [Google Scholar]
- 16.Frenkel L, Kuller L, Capalungan J, et al. Immunization by mucosal exposure to SIV or HIV-2 during chemoprophylaxis with tenofovir [Abstract 494]. 15th Conference on Retroviruses and Opportunistic Infections; Boston, MA. 2008. [Google Scholar]
- 17.Denton PW, Estes JD, Sun Z, et al. Antiretroviral pre-exposure prophylaxis prevents vaginal transmission of HIV-1 in humanized BLT mice. PLoS Med. 2008 Jan 15;5(1):e16. doi: 10.1371/journal.pmed.0050016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grant RM, Buchbinder S, Cates W, Jr, et al. AIDS. Promote HIV chemoprophylaxis research, don't prevent it. Science. 2005 Sep 30;309(5744):2170–2171. doi: 10.1126/science.1116204. [DOI] [PubMed] [Google Scholar]
- 19.Youle M, Wainberg MA. Pre-exposure chemoprophylaxis (PREP) as an HIV prevention strategy. J Int Assoc Physicians AIDS Care (Chic Ill) 2003 Jul–Sep;2(3):102–105. doi: 10.1177/154510970300200302. [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Lerma JG, Otten RA, Qari SH, et al. Prevention of rectal SHIV transmission in macaques by daily or intermittent prophylaxis with emtricitabine and tenofovir. PLoS Med. 2008 Feb 5;5(2):e28. doi: 10.1371/journal.pmed.0050028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peterson L, Taylor D, Roddy R, et al. Tenofovir disoproxil fumarate for prevention of HIV infection in women: a phase 2, double-blind, randomized, placebo-controlled trial. PLoS Clin Trials. 2007;2(5):e27. doi: 10.1371/journal.pctr.0020027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hill A, Youle M, Boucher C. Cost-effectiveness analysis of ART for pre-exposure prophylaxis [Abstract 901]. 13th Conference on Retroviruses and Opportunistic Infections; Denver, CO. 2006. [Google Scholar]
- 23.Paxton LA, Hope T, Jaffe HW. Pre-exposure prophylaxis for HIV infection: what if it works? Lancet. 2007 Jul 7;370(9581):89–93. doi: 10.1016/S0140-6736(07)61053-8. [DOI] [PubMed] [Google Scholar]
- 24.Cohen MS, Kashuba AD. Antiretroviral therapy for prevention of HIV infection: New clues from an animal model. PLoS Med. 2008 Feb 5;5(2):e30. doi: 10.1371/journal.pmed.0050030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Freedberg KA, Losina E, Weinstein MC, et al. The cost effectiveness of combination antiretroviral therapy for HIV disease. N Engl J Med. 2001 Mar 15;344(11):824–831. doi: 10.1056/NEJM200103153441108. [DOI] [PubMed] [Google Scholar]
- 26.Paltiel AD, Weinstein MC, Kimmel AD, et al. Expanded screening for HIV in the United States--an analysis of cost-effectiveness. N Engl J Med. 2005 Feb 10;352(6):586–595. doi: 10.1056/NEJMsa042088. [DOI] [PubMed] [Google Scholar]
- 27.Paltiel AD, Walensky RP, Schackman BR, et al. Expanded HIV screening in the United States: effect on clinical outcomes, HIV transmission, and costs. Ann Intern Med. 2006 Dec 5;145(11):797–806. doi: 10.7326/0003-4819-145-11-200612050-00004. [DOI] [PubMed] [Google Scholar]
- 28.Gold M, Siegel JE, Russel L, Weinstein MC, editors. Cost-effectiveness in health and medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 29.Seage GR, 3rd, Holte SE, Metzger D, et al. Are US populations appropriate for trials of human immunodeficiency virus vaccine? The HIVNET Vaccine Preparedness Study. Am J Epidemiol. 2001 Apr 1;153(7):619–627. doi: 10.1093/aje/153.7.619. [DOI] [PubMed] [Google Scholar]
- 30.Multicenter AIDS Cohort Study (MACS) Public Dataset: Release PO4. Springfield, VA: National Technical Information Service; 1995. [Google Scholar]
- 31.Desai K, McGreevey WP, Ackers ML, Hall HI, Hu DJ. Modeling the potential impact of HIV chemoprophylaxis strategies among men who have sex with men in the United States: HIV infections prevented and cost-effectiveness [Abstract THAD0101]. XVI International AIDS Conference; Toronto, Canada. 2006. [DOI] [PubMed] [Google Scholar]
- 32.Gallant JE, DeJesus E, Arribas JR, et al. Tenofovir DF, emtricitabine, and efavirenz vs. zidovudine, lamivudine, and efavirenz for HIV. N Engl J Med. 2006 Jan 19;354(3):251–260. doi: 10.1056/NEJMoa051871. [DOI] [PubMed] [Google Scholar]
- 33.Johnson M, Grinsztejn B, Rodriguez C, et al. Atazanavir plus ritonavir or saquinavir, and lopinavir/ritonavir in patients experiencing multiple virological failures. AIDS. 2005 Apr 29;19(7):685–694. doi: 10.1097/01.aids.0000166091.39317.99. [DOI] [PubMed] [Google Scholar]
- 34.Grinsztejn B, Nguyen BY, Katlama C, et al. Safety and efficacy of the HIV-1 integrase inhibitor raltegravir (MK-0518) in treatment-experienced patients with multidrug-resistant virus: a phase II randomised controlled trial. Lancet. 2007 Apr 14;369(9569):1261–1269. doi: 10.1016/S0140-6736(07)60597-2. [DOI] [PubMed] [Google Scholar]
- 35.Nelson M, Arasteh K, Clotet B, et al. Durable efficacy of enfuvirtide over 48 weeks in heavily treatment-experienced HIV-1-infected patients in the T-20 versus optimized background regimen only 1 and 2 clinical trials. J Acquir Immune Defic Syndr. 2005 Dec 1;40(4):404–412. doi: 10.1097/01.qai.0000185314.56556.c3. [DOI] [PubMed] [Google Scholar]
- 36.Lalezari J, Goodrich J, DeJesus E, et al. Efficacy and safety of maraviroc plus optimized background therapy in viremic ART-experienced patients infected with CCR5-tropic HIV-1: 24-week results of a phase 2b/3 study in the US and Canada [abstract 104bLB]. 14th Conference on Retroviruses and Opportunistic Infections; Los Angeles, CA. 2007. [Google Scholar]
- 37.Red Book. Montvale, NJ: Thomson PDR; 2006. [Google Scholar]
- 38.Centers for Medicare and Medicaid Services. [cited March 5 2008];Medicare Physician Fee Schedule. 2006 Available from:
- 39.Centers for Medicare and Medicaid Services. [cited 27 July 2007];Clinical laboratory fee schedule. 2006 Available from: http://www.cms.hhs.gov/ClinicalLabFeeSched/01_overview.asp.
- 40.Koblin B, Chesney M, Coates T. Effects of a behavioural intervention to reduce acquisition of HIV infection among men who have sex with men: the EXPLORE randomised controlled study. Lancet. 2004 Jul 3–9;364(9428):41–50. doi: 10.1016/S0140-6736(04)16588-4. [DOI] [PubMed] [Google Scholar]
- 41.Flynn NM, Forthal DN, Harro CD, Judson FN, Mayer KH, Para MF. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J Infect Dis. 2005 Mar 1;191(5):654–665. doi: 10.1086/428404. [DOI] [PubMed] [Google Scholar]
- 42.Dybul M, Bolan R, Condoluci D, et al. Evaluation of initial CD4+ T cell counts in individuals with newly diagnosed human immunodeficiency virus infection, by sex and race, in urban settings. J Infect Dis. 2002 Jun 15;185(12):1818–1821. doi: 10.1086/340650. [DOI] [PubMed] [Google Scholar]
- 43.Saag MS, Cahn P, Raffi F, et al. Efficacy and safety of emtricitabine vs stavudine in combination therapy in antiretroviral-naive patients: a randomized trial. JAMA. 2004 Jul 14;292(2):180–189. doi: 10.1001/jama.292.2.180. [DOI] [PubMed] [Google Scholar]
- 44.Johnson MA, Gathe JC, Jr, Podzamczer D, et al. A once-daily lopinavir/ritonavir-based regimen provides noninferior antiviral activity compared with a twice-daily regimen. J Acquir Immune Defic Syndr. 2006 Oct 1;43(2):153–160. doi: 10.1097/01.qai.0000242449.67155.1a. [DOI] [PubMed] [Google Scholar]
- 45.Booth CL, Geretti AM. Prevalence and determinants of transmitted antiretroviral drug resistance in HIV-1 infection. J Antimicrob Chemother. 2007 Jun;59(6):1047–1056. doi: 10.1093/jac/dkm082. [DOI] [PubMed] [Google Scholar]
- 46.DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents - A Working Group of the Office of AIDS Research Advisory Council (OARAC) [cited May 14 2008];Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. 2008 January 29; Available from: http://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL.pdf.
- 47.Madruga JVR, Cassetti I, Koenig E, et al. Six year safety and efficacy of tenofovir DF (TDF) in combination with lamivudine (3TC) and efavirenz (EFV) in antiretroviral-naïve patients [Abstract WEPEB030]. 4th International AIDS Society Conference on HIV Pathogenesis, Treatment and Prevention; Sydney, Australia. 2007. [Google Scholar]
- 48.Coca S, Perazella MA. Rapid communication: acute renal failure associated with tenofovir: evidence of drug-induced nephrotoxicity. Am J Med Sci. 2002 Dec;324(6):342–344. doi: 10.1097/00000441-200212000-00011. [DOI] [PubMed] [Google Scholar]
- 49.Krishnan M, Nair R, Haas M, Atta MG. Acute renal failure in an HIV-positive 50-year-old man. Am J Kidney Dis. 2000 Nov;36(5):1075–1078. doi: 10.1053/ajkd.2000.19114. [DOI] [PubMed] [Google Scholar]
- 50.Mayer KH, Mimiaga MJ, Cohen D, et al. Tenofovir DF plus lamivudine or emtricitabine for nonoccupational postexposure prophylaxis (NPEP) in a Boston community health center. J Acquir Immune Defic Syndr. 2008 Apr 1;47(4):494–499. doi: 10.1097/QAI.0b013e318162afcb. [DOI] [PubMed] [Google Scholar]
- 51.Horberg M, Tang B, Towner W, et al. Effect of tenofovir on renal function in patients using HAART [Abstract 975]. 15th Conference on Retroviruses and Opportunistic Infections; Boston, MA. 2008. [Google Scholar]
- 52.Philipson T, Posner RA. Private choices and public health: The AIDS epidemic in an economic perspective. Cambridge, MA: Harvard University Press; 1993. [Google Scholar]
- 53.Crepaz N, Hart TA, Marks G. Highly active antiretroviral therapy and sexual risk behavior: a meta-analytic review. JAMA. 2004 Jul 14;292(2):224–236. doi: 10.1001/jama.292.2.224. [DOI] [PubMed] [Google Scholar]
- 54.Garcia-Lerma J, Otten R, Qari S, et al. Prevention of rectal SHIV transmission in macaques by tenofovir/FTC combination [Abstract 32LB]. 13th Conference on Retroviruses and Opportunistic Infections; Denver, CO. 2006. [Google Scholar]
- 55.Garcia-Lerma JG, Otten RA, Cong ME, et al. Intermittent antiretroviral prophylaxis with tenofovir and emtricitabine (FTC) protects macaques against repeated rectal SHIV exposures. 16th International HIV Drug Resistance Workshop; Barbados, West Indies. 2007. [Google Scholar]
- 56.Schackman BR, Gebo KA, Walensky RP, et al. The lifetime cost of current human immunodeficiency virus care in the United States. Med Care. 2006 Nov;44(11):990–997. doi: 10.1097/01.mlr.0000228021.89490.2a. [DOI] [PubMed] [Google Scholar]
- 57.Hirth RA, Chernew ME, Miller E, Fendrick AM, Weissert WG. Willingness to pay for a quality-adjusted life year: in search of a standard. Med Decis Making. 2000 Jul–Sep;20(3):332–342. doi: 10.1177/0272989X0002000310. [DOI] [PubMed] [Google Scholar]
- 58.Ubel PA, Hirth RA, Chernew ME, Fendrick AM. What is the price of life and why doesn't it increase at the rate of inflation? Arch Intern Med. 2003 Jul 28;163(14):1637–1641. doi: 10.1001/archinte.163.14.1637. [DOI] [PubMed] [Google Scholar]
- 59.Center for the evaluation of value and risk in health. [cited July 3 2008];The cost-effectiveness analysis registry. Available from: https://research.tufts-nemc.org/cear/docs/phaseIIpreferenceweights.pdf.
- 60.Tengs TO, Adams ME, Pliskin JS, et al. Five-hundred life-saving interventions and their cost-effectiveness. Risk Anal. 1995 Jun;15(3):369–390. doi: 10.1111/j.1539-6924.1995.tb00330.x. [DOI] [PubMed] [Google Scholar]
- 61.Cohen J. Protect or Disinhibit. New York: Times Magazine; 2006. Jan 22, [Google Scholar]
- 62.Hilton H. Self-medicating with AIDS drugs. TIME. 2008 January 28; [Google Scholar]
- 63.Smith DM, Drumright LN, Frost SD, et al. Characteristics of recently HIV-infected men who use the internet to find male sex partners and sexual practices with those partners. J Acquir Immune Defic Syndr. 2006 Dec 15;43(5):582–587. doi: 10.1097/01.qai.0000243100.49899.2a. [DOI] [PubMed] [Google Scholar]
- 64.Hightow LB, Leone PA, Macdonald PD, McCoy SI, Sampson LA, Kaplan AH. Men who have sex with men and women: a unique risk group for HIV transmission on North Carolina College campuses. Sex Transm Dis. 2006 Oct;33(10):585–593. doi: 10.1097/01.olq.0000216031.93089.68. [DOI] [PubMed] [Google Scholar]
- 65.UNAIDS/WHO. [cited April 1 2008];UNAIDS/WHO AIDS epidemic update. 2007 December; Available from: http://data.unaids.org/pub/EPISlides/2007/2007_epiupdate_en.pdf.