Abstract
Low levels of oxygen (O2) occur naturally in developing embryos. Cells respond to their hypoxic microenvironment by stimulating several hypoxia-inducible factors (and other molecules that mediate O2 homeostasis), which then coordinate the development of the blood, vasculature, placenta, nervous system, and other organs. Furthermore, embryonic stem and progenitor cells frequently occupy hypoxic ‘niches’ and low O2 regulates their differentiation. Recent work has revealed an important link between factors involved in regulating stem/progenitor cell behaviour and hypoxia-inducible factors, which provides a molecular framework for hypoxic control of differentiation and cell fate. These findings have important implications for the development of therapies for tissue regeneration and disease.
Introduction
Joseph Priestley clearly demonstrated the importance of molecular oxygen (O2) for animal life in 1774 when he placed a burning candle in a bell jar alongside a mouse. O2 consumption by the candle had obvious deleterious effects on the unfortunate rodent, underscoring the potentially lethal outcome of exposure to low levels of O2 (hypoxia). Even moderate hypoxia elicits immediate, transient responses, which range from rapid changes in the carbohydrate metabolism of tissues to more permanent changes in local blood vessel networks. Most organisms, including bacteria, yeasts, invertebrates and vertebrates, require O2 for survival. O2 is the primary electron acceptor in many intracellular biochemical reactions and is harnessed by mitochondria to generate ATP via aerobic metabolism.
What constitutes physiologically ‘normoxic’ conditions for embryonic or adult cells varies widely, but largely falls in the 2–9% O2 (14.4–64.8 mm Hg) range (ambient air is 21% O2). However, there are some exceptions to this rule — for example, the thymus, kidney medulla, and bone marrow niches can exist at 1% O2 (7.2 mm Hg) or lower due to their atypical blood vessel networks. Homeostasis of O2 levels within an organism is maintained by multiple processes; many of these mechanisms have been characterized at the molecular level, including hypoxia-inducible transcription factors (HIFs), the environmental sensing mammalian target of rapamycin (mTOR), and the endoplasmic reticulum (ER) stress response 1–3.
Hypoxia is commonly associated with pathologies such as tissue ischaemia and inflammation, and occurs in solid tumours 4. However, hypoxic microenvironments also occur in both the developing embryo and adult, and often create specific ‘niches’ that regulate cellular differentiation 5,6. Of note, stem cells reside in niches, or specific anatomic locations, which modulate their activities during development and tissue maintenance or repair. A connection between mammalian embryogenesis and O2 levels was first appreciated in the 1970s, when Morriss and New demonstrated that successful development of the neural fold by ex utero mouse embryos was dependent on the creation of low O2 culture conditions 7. Since then, discrete molecular mechanisms through which O2 levels modulate embryonic development have been elucidated by the cloning and subsequent characterization of HIFs, dimeric transcription factors (see Boxes 1 and 2) that regulate many hypoxic responses in cells and tissues 1,8. Genetic analysis of HIF function in multiple species and the multiple developmental defects exhibited by HIF-deficient embryos have revealed the importance of O2 as a key regulator of ontogeny. In this Reviews, we discuss the role of O2 availability and HIFs in the regulation of development and stem cell behaviour. We also outline HIF-independent pathways that confer tolerance to hypoxia and also contribute to embryogenesis (e.g. mTOR).
Box 1 Hypoxia-inducible factors: subunit complexity
Hypoxia-inducible factors (HIFs) belong to a family of environmental sensors known as bHLH–PAS (basic Helix-Loop-Helix–per-Arnt-Sim) transcription factors 113, which regulate diverse biological processes. HIF-1 was cloned on the basis of its affinity for the hypoxia-response element (HRE, see Box 2) located within the enhancer region of the human erythropoietin gene 114,115. The HIF-1 heterodimer consists of an α (HIF-1α) and a β subunit (HIF-1β; also known as the aryl hydrocarbon receptor nuclear translocator (ARNT)).
Both HIF-1α and HIF-1β/ARNT are bHLH–PAS contain two PAS domains of 100–120 amino acids, designated PAS-A and PAS-B (see figure), which are necessary for heterodimerization and DNA binding. PAS domains can mediate environmental sensing through direct ligand binding or by interacting with other cofactors such as heat shock protein 90 (HSP90) 113. However, so far no ligands have been found to bind to the HIF PAS domains. HIF-1α contains two transactivation domains (TADs) bridged by an inhibitory domain.
Three genes — HIF-1α, HIF-2α and HIF-3α — encode mammalian HIF-α subunits. HIF-1α is ubiquitously expressed, whereas HIF-2α (also called EPAS (endothelial PAS protein), HLF (HIF-1α-like factor), and HRF (HIF-related factor)) and HIF-3α exhibit more restricted tissue distributions. HIF-2α is expressed primarily in the vasculature of the early developing embryo and subsequently in the lung, kidney interstitial cells, liver parenchyma, and neural crest cells 116–118HIF-3α mRNA and protein are primarily detected in the thymus, kidney, cerebellar Purkinje cells, and corneal epithelium of the eye 119,120. HIF-1β/ARNT is constitutively expressed and is largely insensitive to changes in O2 levels, whereas all three HIF-α subunits are acutely regulated by hypoxia (see Box 2). Two HIF-1β/ARNT homologues, ARNT2 and ARNT3 (also known as bMAL) have also been described; however, they largely participate in O2-independent pathways such as development of the hypothalamus and regulation of circadian clocks, respectively 121,122. ODD, oxygen-dependent degradation domain.
Box 2 Regulation of hypoxia-inducible factor by O2 deprivation
The genes that encode hypoxia-inducible factor (HIF)-α are transcribed and translated at a high rate, but HIF-α proteins are rapidly degraded in the presence of sufficient O2 levels. Under hypoxic conditions, the oxygen-dependent degradation domain (ODD), which comprises residues 403–602 of human HIF-1α (see Box 1), is hydroxylated on two conserved proline residues, P402 and P564 (see Box 1) 123–126 by a family of three HIF-specific prolyl hydroxylases, PHD1, PHD2, and PHD3 127–129. The residues that surround these two proline residues (30 amino acids each) are highly conserved between HIF-1α, HIF-2α, and HIF-3α. Hydroxylated HIF-α proteins are recognized by the von Hippel-Lindau (pVHL) tumour suppressor gene product (a component of a multisubunit ubiquitin-ligase complex), covalently tagged with polyubiquitin, and degraded by the 26S proteasome 130.
Under hypoxic conditions (3–5% O2), HIF-α ODD hydroxylation and interaction with pVHL is inhibited. HIF-α subunits therefore accumulate in the cytoplasm of O2-starved cells, and translocate to the nucleus, where they dimerize with HIF-1β/aryl hydrocarbon receptor nuclear translocator (ARNT) through their PER-ARNT-SIM (PAS) domains (see Box 1), and bind to HIF-response elements (which contain the core recognition sequence 5’-RCGTG-3’) located within the promoters, introns, and 3’ enhancers of a large number of O2-regulated target genes. During this process the C-terminal transcriptional activation domain (TAD) also interacts with coactivators such as p300/CBP (CREB-binding protein; CREB is CRE-response element binding protein)131 (see figure). This interaction is thought to be required for full HIF activity, but is also regulated by normoxic hydroxylation reactions; in this case at asparagine 803 (see Box 1)132. Asparagine hydroxylation is carried out by factor inhibiting HIF (FIH), and FIH activity is inhibited under hypoxic conditions in a manner that is reminiscent of the prolyl hydroxylases 133–135. HIF targets include members of stress-response gene families that mediate acute and chronic hypoxic adaptations, such as glucose transporters, glycolytic enzymes, angiogenic factors, haematopoietic growth factors, and molecules that affect cell growth, survival, and motility 4,130,136,137. This panel has been adapted from a review article written by Bruick (2003).
The effects of O2 on development
The development of oxygen delivery systems is directly dependent on subtle differences in tissue O2 levels, and ensures that resident cells maintain proper metabolic activity. The genetic regulation of these responses has been conserved throughout the animal kingdom 9. For example, the effects of O2 on mammalian cardiovascular components share certain features with the Drosophila melanogaster respiratory organ, the trachea, as described below.
O2 controls branching in tracheal development
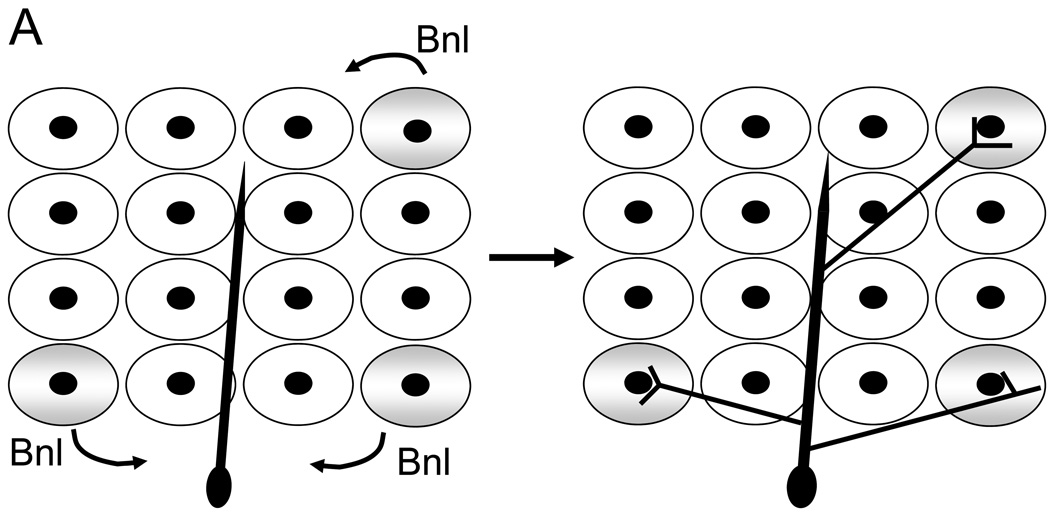
The D. melanogaster tracheal system consists of a tubular epithelial network that delivers O2 to internal tissues and develops by sequential sprouting of branches from epithelial sacs within larvae 10. Sprouting of the main tracheal branches is simple, stereotyped, and controlled by predetermined developmental cues that involve Branchless (a homologue of the human fibroblast growth factor [FGF]), Breathless (a homologue of the FGF receptor), and Pointed (an ETS transcription factor), but the pattern of terminal branching is highly complex and variable 11. Krasnow and colleagues have shown that ramification of fine terminal tracheal branches is in fact regulated by a local signal provided by O2-starved cells 12. This local signal is Branchless: O2 deprivation stimulates larval cells to secrete Branchless, which then functions as a chemoattractant to guide new terminal branches to the Branchless-expressing cells (see Figure 1A). Importantly, this change in airway branch patterning is the result of a ‘switch’ from developmental to physiological control of Branchless expression. Specifically, environmental cues, like O2 availability, “fine tune” Branchless expression to promote an optimal tracheal network that effectively delivers O2 to the organism.
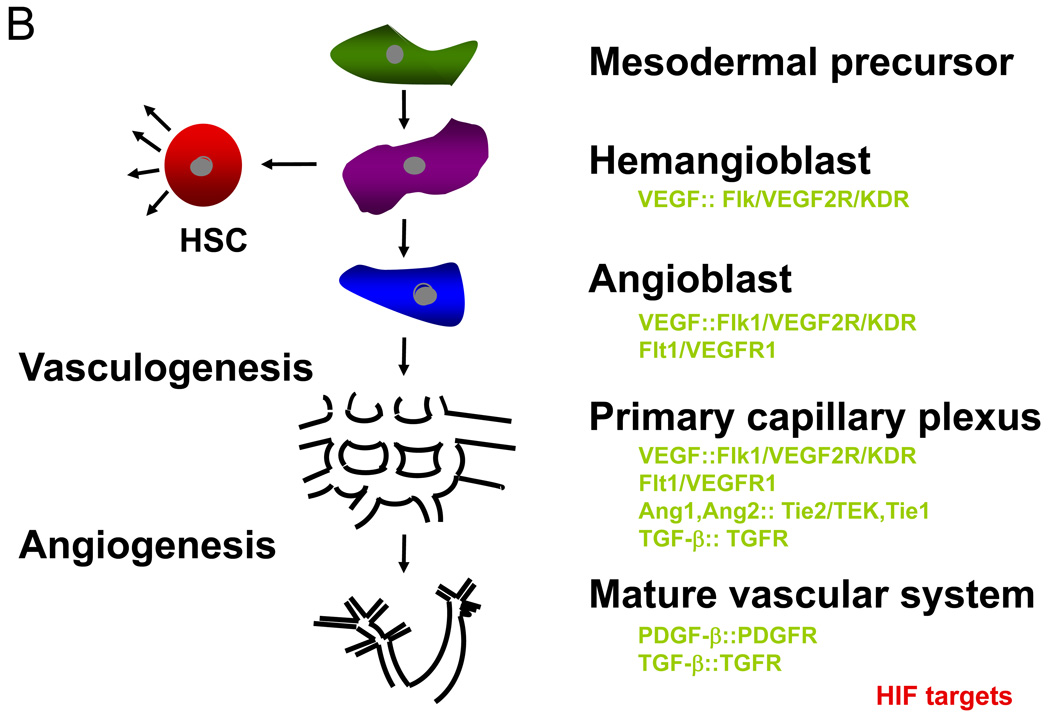
Figure 1. Branch morphogenesis during D. melanogaster tracheal development and mammalian blood vessel formation are regulated by O2 levels.
(A) Models for O2 sensing and patterning in D. melanogaster. Cells within a target tissue experiencing low O2 (blue), due to their distance from an existing tracheal branch (red), begin expressing Branchless (Bnl, the orthologue of mammalian FGF). Branchless expression increases in these O2-starved cells and the tracheal cells respond by sprouting terminal branches that grow toward each Bnl signalling centre. When the branch approaches the source, it starts to arborize (adapted from 12). (B) Model for vascular morphogenesis. Haemangioblasts are putative mesodermal progenitor cells giving rise to both haematopoietic stem cells (HSCs) and angioblasts, the forerunner of endothelial cells which line the vasculature. Vascular endothelial growth factor (VEGF) is required to generate haemangioblasts in the developing embryo. Vasculogenesis, the formation of a primary endothelial cell plexus, also depends on VEGF. Angiogenic remodelling into a mature vascular system (including arteries and veins), involves other important endothelial cell receptors and their ligands, such as Tie2, Tie1, angiopoietin-1 (Ang-1), angiopoietin-2 (Ang-2), and transforming growth factor-β (TGFβ)/TGF-receptor (TGFR) interactions. Of note, virtually all of these vasculogenic and angiogenic regulatory factors (VEGF, Tie2, angiopoietins, TGFβ, platelet-derived growth factor-β (PDGFβ), etc.) are regulated by both decreased O2 levels and the HIFs.
Mammalian cardiovascular morphogenesis is regulated by HIF
During mammalian vascular development (see Figure 1B), vascular endothelial growth factor (VEGF) is a dominant angiogenic growth factor produced by O2-starved cells 13,14. Like FGF, VEGF is used reiteratively during several steps of vertebrate vascular morphogenesis (as shown in Figure 1B), including vasculogenesis and angiogenesis 15,16. VEGF, FGF2, and many other angiogenic factors (such as transforming growth factor-β, platelet-derived growth factor-β, angiopoietin-1, and angiopoietin-2) are direct transcriptional targets of HIF 17–19.
Before the circulatory system is established, mammalian development occurs in a relatively O2 poor environment (3% O2) 20,21. It would seem logical that blood vessel patterning could be fine-tuned by local hypoxic microenvironments that are encountered during embryogenesis and organogenesis, in which existing vessels would sprout into regions containing O2-starved cells. This hypothesis was tested by generating HIF-1β (also known as aryl hydrocarbon receptor nuclear translocator [ARNT]) -deficient mice. These animals show lethality by embryonic day (E)10.5, due to vascular defects in the yolk sac, branchial arches, cranium, somites, and placenta 22,23. Reminiscent of the scenario in tracheal morphogenesis, the initial development of vascular beds is intact in Arnt−/− embryos, but vessel remodelling is subsequently compromised. Furthermore, ARNT -deficient embryos have decreased VEGF mRNA and protein levels, which implies that VEGF secretion is modulated by naturally low O2 microenvironments in the early conceptus 22–24. Consistent with these findings, Iyer et al. demonstrated that HIF-1α protein can be detected in E8–E18 mouse embryos 25; HIF-1α -deficient embryos show similar phenotypes to Arnt−/− mice, with defects in blood vessel formation and neural fold closure 25,26.
Endothelial cells share a spatial and functional relationship with haematopoietic stem cells (HSCs). Analysis of Arnt−/− embryos revealed decreased numbers of yolk sac haematopoietic progenitors 27. Early haematopoietic cell numbers are also substantially reduced in the aorta–gonad–mesonephros (AGM) domain of the embryo proper, and some of the vascular defects exhibited in the AGM domain probably occur as a consequence of decreased numbers of HSCs in Arnt−/− embryos 23. Strikingly, the haematopoietic phenotype of the yolk sac and haematopoietic/vascular abnormalities associated with the AGM domain are all attributable to VEGF deficiency 23,27, which underscores the importance of VEGF regulation by hypoxic foetal microenvironments.
Mammalian placentation
Placental development is also clearly influenced by O2 tension. Before E9.5, the murine embryo relies on glycolysis to supply ATP for metabolic demands. Establishment of the placental circulation by E10.5–E11.5 permits O2 and nutrient delivery to the rapidly growing foetus. Mice in which Arnt, Vhl (the gene that encodes von Hippel–Lindau, a protein involved in oxygen sensing and vasculogenesis), Phd2, or a combination of Hif-1α and Hif-2α have been deleted exhibit aberrant placental architecture owing to reduced labyrinthine layers and markedly fewer foetal blood vessels. These observations clearly validate the hypothesis that O2 levels regulate several steps of placentation 28–31.
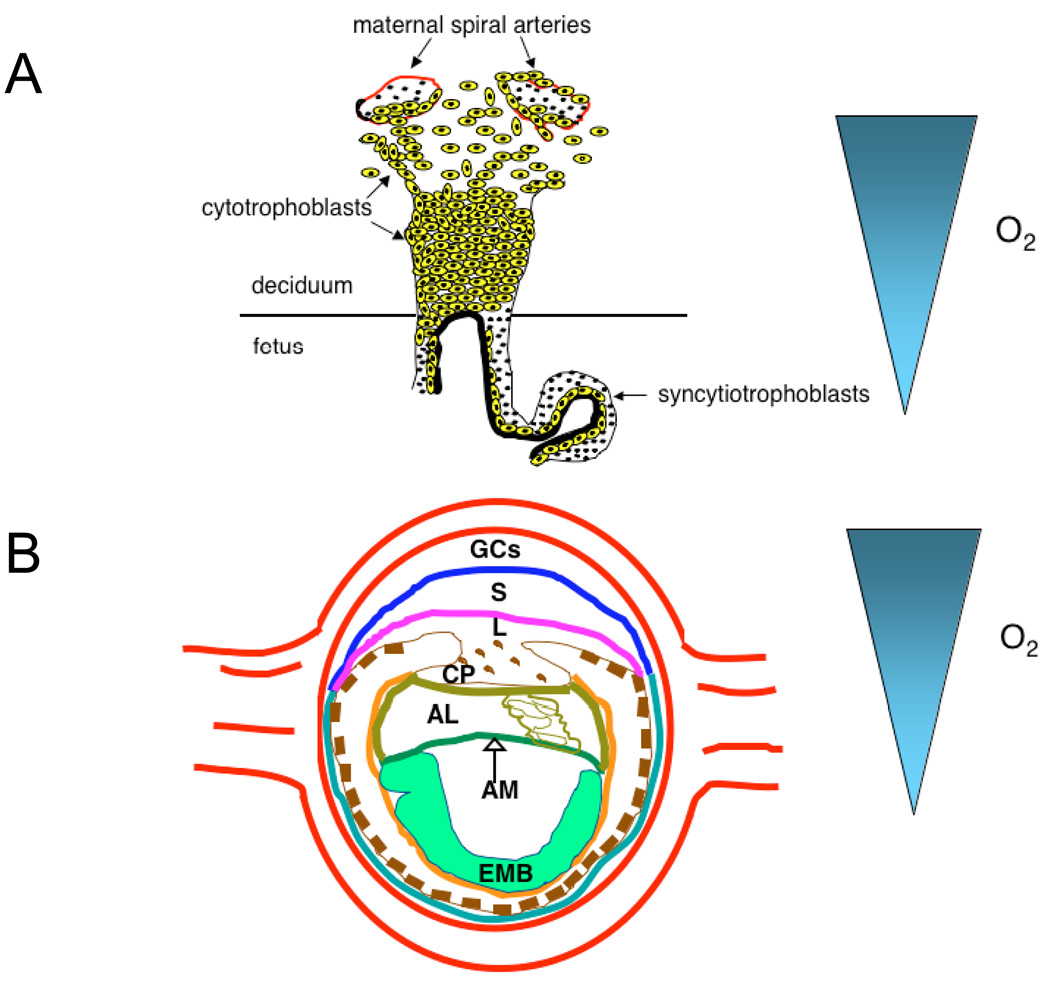
Similar results were obtained in experiments designed to investigate the role of O2 tension in controlling cell fate during human placental cytotrophoblast development 32,33. The uterine surface experiences low O2 levels (17.9 mm Hg, or 2.5% O2) during early pregnancy. Cytotrophoblasts from the embryo invade into maternal spiral arterioles, an event essential for the generation of an utero–placental circulation. After establishing connections with the maternal vasculature, placental O2 levels increase to a relatively rich 8.6% (60 mm Hg). Genbacev et al. demonstrated that human cytotrophoblasts proliferate in vitro under low O2 conditions but at higher O2 levels differentiate into a more invasive phenotype, mimicking the developmental transition they undergo as they invade the placental bed to establish the maternal–foetal circulation 32 (see Figure 2A). These results imply that O2 can directly influence mammalian cell-fate decisions.
Figure 2. Oxygen gradients are generated in developing human and mouse placentae.
(A) Diagrammatic representation of the differentiation pathway that cytotrophoblast stem cells undertake in vivo. These cells detach from the underlying uterine basement membrane and either fuse to form multinucleated syncytiotrophoblasts or columns of mononuclear cells that attach the conceptus to the uterine wall. A subset of these cells stops proliferating and differentiates into invasive cytotrophoblasts that breach and enlarge maternal blood vessels to generate an utero–placental circulation. The differentiation of proliferating cytotrophoblasts into invasive cytotrophoblasts is an O2-dependent process, with O2 levels increasing as cells migrate towards the maternal spiral arteries. (B) Placentation is regulated by changes in O2 availability. An E8.0 mouse embryo is shown to illustrate the O2 gradient generated during murine placentation. Similar to human placentae, the early murine placenta generates an O2 gradient where cells that migrate dorsally experience increasing O2 levels. Trophoblast stem cells adopt specific cell fates in the placenta when they encounter discrete O2 levels: low O2 enforces a spongiotrophoblast cell fate, whereas higher O2 levels enforce a giant cell fate.
Mammalian cardiovascular–pulmonary development
Although targeted mutation of the genes that encode HIF-1α or ARNT results in early (E9.5–10.5) embryonic lethality, HIF-2α−deficient mice survive until mid-late gestation or, in some cases, birth. These animals succumb to one (or more) of several cardiovascular and pulmonary phenotypes that are not shared with Hif-1α mutants (see below). This indicates that the two proteins probably regulate overlapping, but not identical, target genes.
Surprisingly, the phenotypes observed in Hif-2α−/− embryos vary markedly depending on the mouse strain used. In one background strain (C57/129SvJ), McKnight and colleagues described embryonic lethality as a result of bradycardia owing to catecholamine dysregulation, whereas Carmeliet et al. demonstrated a perinatal lung maturation defect in Swiss/129Sv animals owing to improper surfactant production by Hif-2α−/− type II pneumocytes 34,35. Hif-2α−/− embryos generated in another background (129Sv/Sv-CP) developed severe vascular defects in both the yolk sac and embryo proper 36. Finally, in yet another genetic background (129 x C57 F1 hybrid), Hif-2α−/− mice showed pathologies including retinopathy, hepatic steatosis, cardiac hypertrophy, and skeletal myopathy 37. Therefore, both HIF-1α–ARNT and HIF-2α–ARNT heterodimers regulate multiple, non-redundant developmental pathways. More recently, it was also shown that postnatal deletion of a conditional Hif-2α allele, but not Hif-1α, resulted in anaemia associated with decreased expression of erythropoietin 38,39.
The overall conclusion from these studies is that ‘physiological hypoxia’ encountered in utero by developing embryos is essential for generating all components of an intact cardiovascular–pulmonary system. As observed in earlier studies of D. melanogaster, patterning and morphogenesis of the nutrient and O2 delivery system of mammals is itself modified by O2 availability. A ‘switch’ from purely developmental to physiological control of these processes is required to meet the metabolic needs of the rapidly growing conceptus. It is notable that mutations in the D. melanogaster gene Trachealess, which encodes a bHLH–PAS (for basic-Helix-Loop-Helix-Per-Arnt-Sim proteins, the original members of this transcription factor family) protein, cause defects in the tracheal O2 delivery system, which implies that bHLH–PAS factors have been evolutionarily conserved to regulate O2 delivery 40,41.
O2 and bone morphogenesis
Another intriguing case of O2 levels influencing development involves the growth plates of developing bones. Growth plates are constitutively avascular structures; therefore, low O2 partial pressures experienced by the cartilaginous microenvironment were assumed to affect chondrocyte phenotypes as they evolved from a proliferative to a terminally differentiated state 42. Growth plate chondrocytes progress through ordered phases of cell proliferation, differentiation and apoptosis 43. Proliferating chondrocytes synthesize collagen type II and then differentiate into postmitotic hypertrophic cells that express collagen type X and VEGF. Targeted deletion of Hif-1α in murine growth plate chondrocytes results in cell death owing to defects in HIF-1α-regulated growth arrest 44. Mice that lack HIF-1α in this chondrocyte population have markedly shorter limbs owing to increased apoptosis and a disorganized transition from hypertrophic chondrocytes to primary spongiosa. It has been postulated that physiological O2 gradients in the cartilaginous growth plate have a role in modulating chondrocyte proliferation, differentiation, and growth arrest via HIF activity [REF 44]. HIF-1α is also critical for earlier steps in bone formation, such as the generation of cartilaginous primordial limb bud mesenchyme and chondrogenesis 45.
Adipogenesis is modified by O2 levels
O2 concentrations are also important regulators of adipogenesis. As fatty acid metabolism requires mitochondrial respiration, hypoxia limits fatty acid usage and the need for additional adipose tissue. Presumably, hypoxia inhibits adipocyte development from mesenchymal precursors by attenuating the expression of peroxisome proliferative activated receptor γ (PPARγ), a nuclear hormone that regulates many adipocyte-specific genes and promotes differentiation of mesenchymal cells to adipocytes 46. Yun et al. have shown that fibroblasts from HIF-1α-deficient mouse embryos are refractory to the hypoxic inhibition of adipogenesis 47. HIF regulation of DEC1/Stra13 (Drosophila hairy/Enhancer of split transcription factor family member; also known as Stra13), a repressor of the PPARγ promoter, provides an underlying molecular mechanism for O2-mediated effects on adipogenesis. Low O2 activates HIF-1α–ARNT heterodimers, which upregulate Dec1 gene expression; DEC1, in turn, represses PPARγ transcription and inhibits the differentiation of preadipocytes into adipocytes. HIF-2α expression is induced during adipogenesis in vivo and in vitro, but plays a distinct role from HIF-1α 48. Therefore, O2 availability directly controls the development of adipose tissue via HIF.
Context-dependency of O2 on developmental programmes
It has become increasingly clear that O2 influences specific cell fates in several developmental processes; however, the effect of O2 levels on cell differentiation is context-dependent. For example, terminal differentiation of megakaryocytes into platelets is promoted by high O2 concentrations 49. By contrast, reducing O2 levels of cultured rat peripheral and central nervous system stem cells from 21% to 3–5% (physiological normoxia for these cells) promotes their differentiation into neurons with specific neurotransmitter phenotypes 50,51. As O2 can modulate cell fates in a concentration-dependent manner, it seems reasonable to consider O2 as a developmental morphogen that influences cell fate in a manner that is similar to the more traditionally recognized gradients of secreted growth factors. The precise mechanisms by which HIFs, and other O2-responsive transcriptional regulators (such as nuclear factor κB [NFκB] and activator protein-1 [AP1]), modulate differentiation in response to these gradients is a subject of ongoing research.
O2 levels influence stem cell phenotypes
Stem cells, as well as multipotent progenitor cells and germ cells, reside in complex microenvironments or “niches” 52. Several studies have revealed that O2 levels might profoundly influence stem cell niches, and can promote the differentiation of certain types stem or progenitor cells, while inhibiting the differentiation of others. These differing results have been demonstrated in experiments in which stem cell populations have been cultured under hypoxic conditions in vitro. For example, murine placental trophoblast stem cells adopt a spongiotrophoblast cell fate as opposed to a trophoblast giant cell fate when cultured at 3% O2 instead of 21% O2 28. Rat bone marrow mesenchymal stem cells exhibit enhanced colony-forming capability and increased proliferation at 5% O2 (as opposed to ambient air), similar to previous observations obtained by culturing embryonic haematopoietic progenitors 27,53. The rat mesenchymal stem cells cultured under low O2 also produce more osteocytes when subsequently implanted in vivo. Human cytotrophoblasts and murine trophoblast stem cells are excellent examples of how numerous cells transit through a natural O2 gradient as they migrate from one microenvironment to another, varying their spatial relationship with blood vessels 28,32 (see Figure 2B).
O2 levels regulate hematopoietic and embryonic stem cells
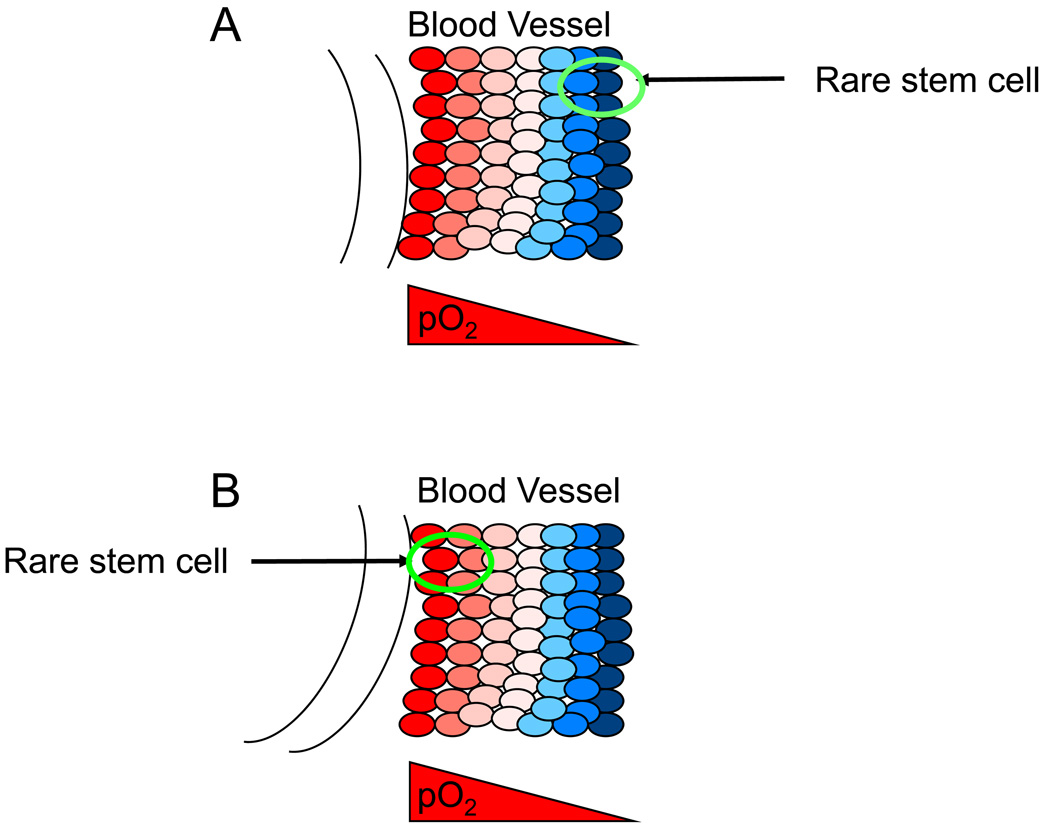
HSCs in adult mammals reside in the bone marrow. The partial pressure of O2 in human bone marrow is lower than in peripheral blood and the architecture of medullary sinuses and arterial blood flow patterns generate an O2 gradient. It has been proposed that HSCs and their proliferating progenitors are naturally distributed along this gradient, with the HSCs occupying the most hypoxic niches 54,55. Furthermore, Danet et al. demonstrated that bone marrow HSCs cultured at 1.5% O2 promoted their ability to engraft and repopulate the haematopoietic organ of immunocompromised recipient mice 56. Intriguingly, antibodies against markers that substantially enriched for HSCs during purification identify cells that are localized to the sinusoidal endothelium, as opposed to the more hypoxic endosteal lining 57. The precise location of bone marrow HSCs remains controversial; however, it is possible that different stem and progenitor cell populations require different O2 conditions, so that multiple niches characterized by different O2 levels might exist in bone marrow. In this regard, it is interesting to note that spermatogonial stem cells 58 and brain tumour stem cells 59 also seem to be associated with vascular niches. In summary, it seems that some stem cells occupy hypoxic niches (see Figure 3A), whereas others occupy relatively well-oxygenated perivascular microenvironments (see Figure 3B). Changes in O2 tension probably influence stem cell quiescence, proliferation and differentiation.
Figure 3. Distinct populations of stem cells occupy microenvironments that contain different O2 levels.
As described in the main text, some stem cells (such as those in the endosteal bone marrow compartment) occupy extremely low O2 microenvironments (less than 0.5% O2) as shown in (A). Other stem cells (as those described as perivascular SLAM+ (for Signalling Lymphocyte Activation Molecule) stem cells can occupy relatively well-oxygenated environments as they are in close proximity to blood vessel endothelial cells (B). However, it should be noted that although stem cells can be perivascular, the vessels might be associated with venous structures and therefore be relatively hypoxic.
Finally, embryonic stem (ES) cells also grow more efficiently under low O2 conditions, as opposed to ambient air supplemented with 5% CO2. It was previously noted that bovine blastocysts produced under reduced O2 tensions exhibited significantly more inner cell mass (ICM) cells than those maintained at higher O2 levels 60. The ICM and their ES cell counterparts are pluripotent. Roberts et al. demonstrated that human ES cells proliferate at similar rates when cultured at 3–5% O2 as they do under 21% O2 61. However, the appearance of differentiated regions in these ES cultures, as assessed by morphology and loss of stem cell markers like stage-specific embryonic antigen (SSEA) and OCT-4 (see below), was substantially reduced under hypoxic conditions. The authors concluded that hypoxic conditions are required to maintain the full pluripotency of mammalian ES cells.
O2, stem cells, and disease
O2 effects on the function of stem and progenitor cells might also be important in pathophysiological settings, as suggested by recent investigations of neuroblastoma 62. The sympathetic nervous system (SNS) develops from the neural crest and is composed of both neurons and neuroendocrine (chromaffin) cells. Neuroblastoma is a childhood cancer that originates from the developing SNS, and consists of tumour cells that exhibit several differentiation stages, with immature cells generating a more aggressive form of the disease. Some neuroblastomas contain both neuroblastic and neuroendocrine cell types, with spontaneous changes in cell differentiation status clearly affected by O2 availability within these tumours. Pahlman and colleagues showed that hypoxia (1–5% O2) induced the expression of markers associated with neural crest sympathetic progenitors, such as c-Kit and Notch, in cultured neuroblastoma cells, whereas it decreased the expression of SNS transcription factors HASH-1 and dHAND 62. Similar changes in gene expression were also noted in O2-starved regions of neuroblastoma xenografts grown in mice. Thus, hypoxia causes dedifferentiation of neuroblastoma cells both in vitro and in vivo and selects for cells with stem cell characteristics. Taken together, these findings implicate oxygenation levels as an important aspect of microenvironmental niches (along with stromal cell contacts, extracellular matrix proteins, growth factors, and temperature) that influence stem cell behaviour.
Hypoxic control of stem cell behaviour
Hypoxia clearly promotes the undifferentiated state in several stem cell and precursor cell populations, but the molecular mechanisms underlying these observations remained obscure until recently. A clear link has been demonstrated between hypoxia, HIFs and molecules that are crucial for the regulation of the differentiation of stem and/or progenitor cells, including Notch, β-catenin, OCT4, and c-MYC.
HIFs affect stem and progenitor cell differentiation
Optimal in vitro culture conditions for maintaining precursor cells in the desired state of differentiation probably reflect the physiological O2 levels that these cells encounter in either the embryo or the adult. The fact that the developmental state of multiple stem or progenitor cell populations is influenced by oxygenation levels strongly implicates O2-sensitive intracellular pathways, such as HIF-dependent pathways, in the regulation of cell fate. Genetic studies in mice have confirmed this idea in vivo 27,28,45.
Placentas from HIF-deficient mice (that lack both the HIF-1α and HIF-2α subunits or the ARNT subunit) exhibit aberrant cellular architecture owing to reduced labyrinthine and spongiotrophoblast layers and increased numbers of trophoblast giant cells 28,29. These in vivo findings are consistent with a role for hypoxia (via HIF) in promoting the in vitro differentiation of trophoblast stem cells into spongiotrophoblasts as opposed to giant cells 28. As noted above for human cytotrophoblasts, murine trophoblast stem cells migrate through a natural O2 gradient as they transit from the O2-lacking region (in the chorionic plate) to the relatively O2-rich region that surrounds maternal spiral arteries (Figure 2B). We have shown that these stem cells adopt the spongiotrophoblast fate at low O2 concentrations (3%, close to their ‘natural’ oxygenation levels) and the giant cell fate at higher O2 concentrations 28,29.
Similarly, the in vivo yolk sac haematopoietic progenitor phenotype of decreased cell numbers exhibited by Arnt−/− embryos can be recreated by three-dimensional embryoid bodies derived from Arnt−/− ES cells 27. Of note, wild-type embryoid bodies grown at 3% O2 generate significantly more erythroid and myeloid progenitors than those cultured at 21% O2. Therefore, ‘physiological hypoxia’ encountered by embryos is important for the proliferation and/or survival of haematopoietic precursors during development.
Endothelial and haematopoietic cells emerge simultaneously in both the yolk sac blood islands and regions surrounding the dorsal aorta during organogenesis, which suggests they probably arise from a common mesodermal precursor known as the haemangioblast. The abundance of haemangioblasts within the early embryo also appears to be regulated by O2 availability 63. Haemangioblast proliferation within embryoid bodies is enhanced by hypoxia, which implies that the vascular and haematopoietic defects seen in HIF-deficient embryos are partly the result of depletion of a common progenitor pool.
O2 availability regulates Notch activity
Notch signalling has been evolutionarily conserved to maintain stem or progenitor cell fates in multicellular organisms 64,65; myogenic, haematopoietic, and neuronal precursor cell differentiation is inhibited by members of the Notch family 66–69. Notch mediates cell-cell signalling between adjacent cells that express Notch receptors (Notch 1–4) and Notch ligands (Delta, Serrate, and Lag-2). When activated by ligand binding, Notch receptors undergo a series of proteolytic cleavages to liberate the Notch intracellular domain (ICD), which translocates to the nucleus and interacts with the DNA-binding protein CSL (C-promoter-binding factor/Suppressor-of-Hairless/Lag1) and coactivators such as CBP/p300 and Mastermind to activate targets such as Hes and Hey. Hes and Hey, in turn, negatively regulate the expression or activity of differentiation factors like Mash, MyoD, and Neurogenin 70,71.
Some hypoxic effects on progenitor cells correlate with the effects of Notch signalling in these cells. Gustaffson et al. have shown that hypoxia directly influences Notch activity 72. Hypoxia (1% O2), via the accumulation of HIF-1α, blocks the myogenic differentiation of C2C12 myoblast cells and the neuronal differentiation of P19 embryonic carcinoma cells. Reduced O2 levels also inhibit the maturation of primary satellite cells obtained from muscle and neural stem cells derived from embryonic rat cortex. These effects are abrogated in the presence of γ-secretase inhibitors, which inhibit Notch signalling by blocking endomembranous Notch proteolysis. Hypoxia induces the expression of the Notch transcriptional targets Hes1 and Hey2. Hes1 levels are also elevated by hypoxia mimetics that stabilize HIF-1α. These results imply that HIF-1α directly mediates the hypoxic effects on Notch activity; indeed, HIF-1α has been shown to physically associate with Notch ICD, promoting its stability 72. The authors propose a model in which HIF-1α interacts with Notch–CSL transcriptional complexes at Notch-responsive promoters in hypoxic cells to control the differentiation status of myogenic and neuronal precursors (see Figure 4A). HIF-1α also regulates the expression of the APH-1A gene, which encodes a component of the γ-secretase complex; this finding suggests a potential additional mechanism whereby hypoxia augments Notch signalling 73. In this case, Notch ICD levels increase due to enhanced γ-secretase-mediated proteolysis. Both mechanisms result in elevated Notch ICD in hypoxic cells.
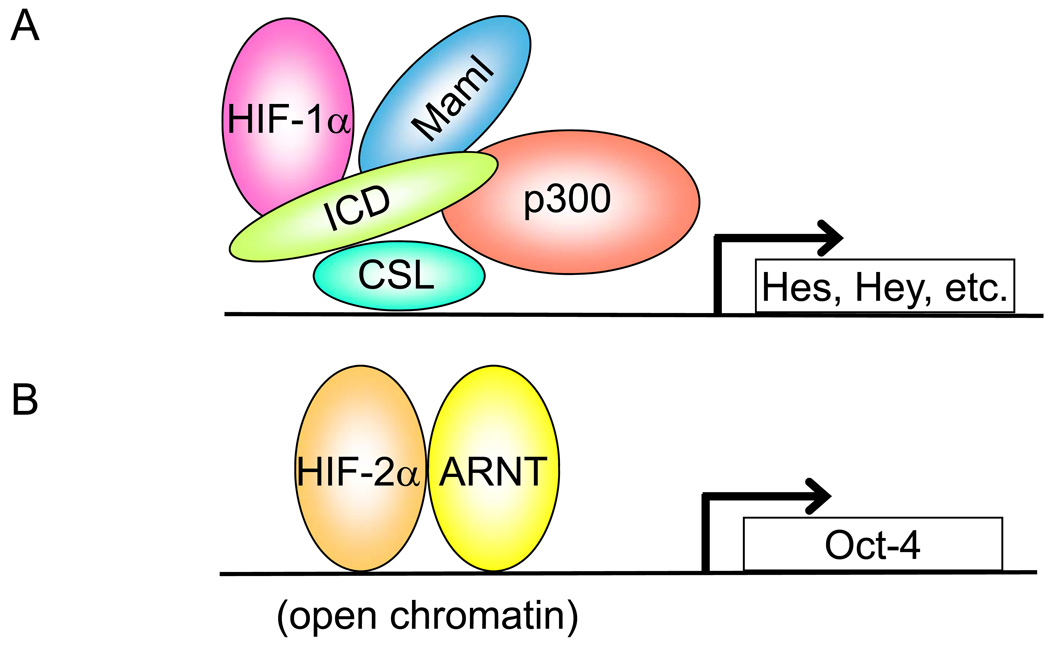
Figure 4. Models depicting O2 availability and transcriptional activity.
(A) Under hypoxic conditions, hypoxia-inducible factor-1α (HIF-1α) typically interacts with HIF-1β (also known as aryl hydrocarbon receptor nuclear translocator (ARNT)) to stimulate target genes such as vascular endothelial growth factor (VEGF), fibroblast growth factor 2 (FGF-2), and platelet-derived growth factor-β (PDGF- β). HIF-1α can also interact with the intracellular domain (ICD) of Notch in the nucleus at Notch-responsive promoters. In the nucleus, Notch interacts with the CSL (C-promoter-binding factor/Suppressor-of-Hairless/Lag1) DNA-binding protein and coactivators such as CBP/p300 and Mastermind (Mam1) to activate target genes such as Hes and Hey. It is currently not known if the initial HIF-1α–Notch interaction occurs outside or within the nucleus. Furthermore, the actual relationship between components of the Notch complex at promoters is unclear. HIF-1α could directly interact with ICD, an unidentified ‘bridging’ protein, or with Maml 138. (B) In cells where the Oct-4 locus is accessible as a result of open chromatin, its transcription is induced directly by HIF-2α–ARNT dimers in response to hypoxia.
Hypoxia modulates Wnt activity
The Wnt signalling pathway is another important regulator of stem cell function in D. melanogaster, Caenorhabditis elegans and mammals. Hypoxia downregulates β-catenin (via its interaction with HIF-1α), which is stabilized in response to Wnt signalling and forms an active transcriptional complex with lymphoid enhancer factor/T-cell factor-4 (LEF/TCF4). Kaidi and colleagues demonstrated that HIF-1α competes with TCF4 for direct binding to β-catenin, resulting in hypoxia-mediated cell-cycle arrest and inhibition of transcriptional activity 74. Intriguingly, β-catenin can also promote HIF-1α-mediated transcriptional activity, which might help cells to adapt to severe hypoxia 74. It will be interesting to determine the degree to which these interactions affect specific stem-cell functions.
OCT4 regulation by O2 levels
A third molecular pathway underpinning hypoxic control of stem-cell behaviour involves the POU-domain transcription factor OCT4 (also known as OCT3/4 or Pou5F1), which is directly activated by HIF-2α 75. OCT4 is essential for maintaining the undifferentiated state of ES cells, ICM, the embryonic epiblast, and primordial germ cells (PGCs) 76,77. Oct4 expression is tightly controlled during embryogenesis and adult life: Oct4 downregulation is required for differentiation of the trophectoderm lineage and subsequent gastrulation by the epiblast; however, Oct4 expression is maintained in PGCs. In the adult, Oct4 is exclusively expressed in germ cells, and had been detected in stem-cell populations such as bone marrow multipotent adult progenitors, haematopoietic stem cells, and stem cells that reside in epidermal basal layers 78,79. More recent studies demonstrate that OCT4 is essential for germ-cell maintenance, but dispensable for somatic stem-cell self-renewal 80,81. The importance of strictly maintaining Oct4 expression levels has been demonstrated both in vitro and in vivo: even a two-fold change in OCT4 protein abundance can cause ES cells to lose pluripotency 82, and ectopic Oct4 expression promotes epithelial dysplasia in transgenic mice 83.
We have shown that HIF-2α, but not HIF-1α, binds to the Oct4 promoter and induces Oct4 expression in hypoxic cells if the Oct-4 locus is in an ‘open’ configuration and not embedded in heterochromatin 75,84. There are several putative HREs in the promoter region of Oct4 that are conserved between mice and humans 85. Deletion of these HREs abrogates hypoxic induction of the Oct4 promoter in transient transfection assays, indicating that they are functional 75. Furthermore, chromatin immunoprecipitation (ChIP) assays demonstrated that endogenous HIF-2α occupies the Oct-4 HREs in O2-starved cells. By generating mice with an expanded region of HIF-2α expression, we found that early embryos exhibited elevated OCT4 levels and severe developmental patterning defects. HIF-2α-overexpressing ES cells also generated, in an OCT4-dependent manner, large subcutaneous teratomas characterized by altered cellular differentiation 75. Of note, Hif-2α−/− embryos display a striking reduction in the number of PGCs, which require Oct4 expression for survival and/or maintenance 75. Taken together, the data identify HIF-2α as an upstream regulator of Oct4 expression (see Figure 4B), and indicate a potential novel pathway in which hypoxia directly influences stem-cell function.
Can O2 levels influence adult cell ‘reprogramming’?
The opposing effects of HIF-1α and HIF-2α on the activity of c-MYC have implications for stem-cell function. HIF-1α inhibits c-MYC activity 86,87, whereas HIF-2α has been shown to promote c-MYC-dependent proliferation 88. The fact that HIF-2α enhances the levels of OCT4 and c-MYC activity is particularly intriguing, as these two factors can directly regulate murine ES cell identity. Several reports 89–92 demonstrate that enforced expression of OCT4, c-MYC and two other transcription factors, Krüppel-like factor 4 (KLF4), and SOX2 in differentiated murine fibroblasts alters DNA methylation, chromatin structure, and gene expression, giving rise to cells that are functionally indistinguishable from bona fide murine ES cells. These remarkable results provide strong support for the idea that ‘stemness’ can be conferred on more differentiated cells. Moreover, they indicate a potential mechanism whereby hypoxia can regulate stem-cell function by modulating the expression or activity of OCT4, c-MYC, and possibly other proteins in a HIF-dependent manner.
Additional mechanisms regulating embryogenesis
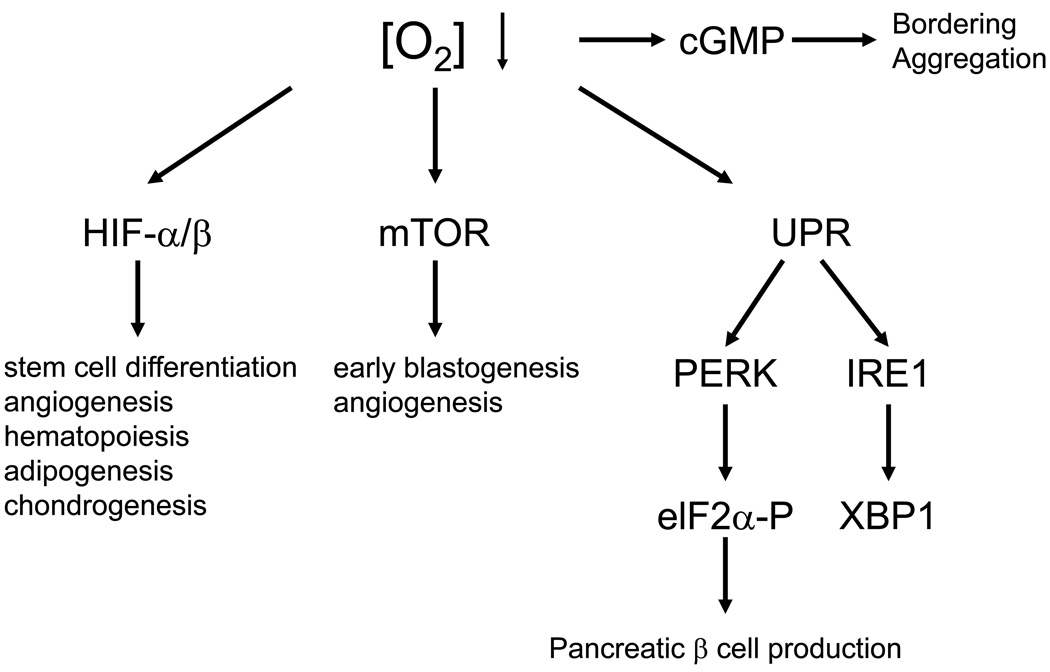
While HIFs regulate a critical transcriptional response to low O2, other pathways (as shown in Figure 5) also provide important hypoxic adaptations. In mammals, hypoxia results in both acute and more chronic responses. Rapid and reversible effects on cellular metabolism, cell mass, ion channel activity, and protein synthesis affect the balance between energy supply and demand in the face of reduced capacity for oxidative metabolism 93. Although HIFs promote cellular survival and vascular remodelling during chronic hypoxia, several HIF-independent pathways, engaged by acute hypoxia, are critical for ATP conservation by limiting energy-dependent processes such as cell division, ribosome biogenesis, mRNA translation, and ion flux. These have now been described, albeit to a limited degree, and include the “mammalian target of rapamycin” (mTOR) energy sensing 94, the “unfolded protein response” (UPR) 95, and soluble guanylate cyclase 96 pathways. O2 sensation by the developing nervous system, while poorly understood, also ultimately impacts behavioural responses in adult animals 96. In C. elegans this involves neural cGMP-gated channels and a soluble guanylate cyclase homologue, GCY-35 that directly binds O2 via a haem domain. In this section, we discuss the regulation of mTOR and UPR pathways and their possible contribution to embryonic development.
Figure 5. Multiple pathways responding to changes in O2 availability affect developmental processes as well as social behaviour.
As described in the text, HIFs regulate many aspects of cardiovascular morphogenesis and stem and/or progenitor cell maintenance. Mutagenesis of mammalian target of rapamycin (mTOR) and its associated proteins, such as raptor, rictor, and mLST8, has revealed an important role for mTORC1 and mTORC2 during embryonic development. However, whether mTOR is responding to hypoxia in embryonic microenvironments to regulate development remains to be determined. The “unfolded protein response” (UPR)-regulated kinase PERK (and its substrate eIF2α) is necessary for pancreatic β cell production during development or shortly after birth. Inositol-requiring-1 (IRE1) is another ER-associated UPR effector that activates X box-binding protein 1 (XBP-1), promoting the transcription of ER chaperone genes such as BiP and c/EBP-homologous protein (CHOP) 139. Finally, cyclic guanosine monophosphate (cGMP) regulation promotes neuronal activity and social feeding behaviour in C. elegans, allowing them to avoid O2 levels outside the range of 5–12% O2. This leads to specific appearances of nematode colonies, causing “bordering” or “aggregation”.
mTOR regulates early development
mTOR is a highly conserved serine/threonine kinase that integrates multiple environmental cues to regulate metabolism, mRNA translation, cell survival, and actin organization in response to O2, nutrient, hormone, and growth factor availability 94. mTOR exists in two complexes; mTOR complex 1 (mTORC1), which also contains raptor, mLST8, and other associated proteins. mTORC1phosphorylates initiation factor 4E binding protein-1 (4E–BP1) and p70 ribosomal protein S6 kinase (p70S6K), resulting in decreased cellular protein synthesis, growth and proliferation, to conserve ATP97. By contrast, the second complex, mTORC2, contains mTOR, mLST8, and rictor. mTORC2 phosphorylates and activates the kinase AKT/protein kinase B (PKB), which regulates cell proliferation, survival and metabolism 98. By regulating the actin cytoskeleton, mTORC2 also controls cell shape and motility.
Germline disruption of mTOR in mice causes embryonic lethality during blastocyst implantation 99–101. Explanted mTOR-null blastocysts appear normal, but the ICM and trophoblast giant cells fail to expand during culture. Explanted raptor-null blastocysts exhibit similar phenotypes: they stop growing by day 4 and by day 7, most cells detach and presumably die 101. By contrast, explanted mLST8-null blastocysts exhibit no obvious phenotypes and grow normally. However, mLST8−/− embryos die in vivo by day 10.5 of gestation due to cardiovascular defects. Although they exhibit a beating heart, the cardiac wall is slightly thinner and they are visibly smaller than wild type or heterozygous embryos 101. Defective vascular development was observed in these embryos, wherein many blood vessels, particularly in the head, were dilated. The phenotype of mLST8−/− mice is similar to the phenotype of embryos that lacking phosphoinositol 3-kinase (PI3K; p110α) and the endothelial kinase 2-receptor (TIE2) 102,103. When TIE2 is stimulated by its ligand angiopoietin-1, it signals through PI3K to regulate vascular development. Results from analysing mLST8−/− embryos suggest that mLST8 participates in TIE2-mediated endothelial cell signal transduction.
Rictor-deficient embryos look very similar to mLST8-deficient concepti101, which suggests that mLST8 is necessary to maintain rictor/mTOR interactions but not raptor/mTOR interactions. Furthermore, both mLST8 and rictor (but not raptor) are required for phosphorylation of AKT and protein kinase Cα (PKCα), but not p70S6K. These results demonstrate that mTORC1 functions in early development and becomes essential by gestational day 5.5–6.5 shortly after implantation (i.e. the egg cylinder stage). By contrast, mTORC2 is necessary for later vascular development, possibly due to its role in TIE2-mediated endothelial cell signal transduction, or effects on endothelial cell cytoskeletal function. mTOR activity is clearly inhibited by O2 deprivation 104. Furthermore, HIF-α protein expression is dependent on mTOR in some cellular contexts 105,106. However, it remains to be determined if mTOR modulation by low O2 levels or a connection between mTOR and HIF in the developing conceptus promote normal development.
Hypoxic regulation of the endoplasmic reticulum (ER)
Nascent proteins enter the ER, which serves as a critical site for protein folding, disulfide bond formation, and glycosylation before peptides become secreted or translocated to the plasma membrane. ER “stress” occurs during variations in new protein accumulation or overabundance of unfolded proteins 107. To alleviate ER stress, a series of cell defence mechanisms are activated, collectively known as the “ER stress response”, the “integrated stress response”, or UPR 107. These mechanisms include the phosphorylation of eukaryotic initiation factor-2α (eIF2α) by the ER pancreatic eIF2-α kinase (PERK), which results in reduced protein translation to prevent further accumulation of unfolded polypeptides. Hypoxia activates PERK (by unknown mechanisms), resulting in increased eIF2α phosphorylation and decreased rates of translation initiation 108.
In contrast to mTORC1 inhibition in response to chronic hypoxia, eIF2α phosphorylation is transient and decreases after reaching a plateau at 2 hours 108. Studies of PERK-deficient mice, and mice with a mutation in the eIF2α PERK phosphorylation site (S51A) reveal that eIF2α phosphorylation is connected to glucose metabolism 109,110. PERK-deficient mice are viable but develop marked hyperglycemia at 4 weeks of age, while the eIF2α−S51A mutant mice appear normal at birth but die of severe hypoglycemia within 18 hours. Both mutant strains exhibit defects in pancreatic β-cell development. These defects are apparent in eIF2α−S51A embryos but only become apparent in PERK-deficient animals several weeks after birth. Such differences indicate that more than one kinase phosphorylates eIF2α in the β−cells of the pancreas. PERK is specifically required in the insulin-secreting pancreating β cells during the foetal and neonatal period to ensure β cell proliferation and differentiation 111. In conclusion, pathways that involve the O2 sensitive ER clearly regulate pancreatic morphogenesis. However, a link between O2 availability, the ER, and development of the pancreas remains to be established.
Conclusions and future directions
As stated above, changes in O2 availability occur naturally during embryonic development. O2-starved cells respond to their microenvironment by stimulating several adaptive responses that include HIFs, mTOR, ER-associated kinases, and soluble guanylate cyclases (see Figure 5). Findings from in vitro and in vivo models (invertebrate and vertebrate) discussed herein convincingly demonstrate that molecular O2 is not simply a fuel to maintain cellular bioenergetics and metabolism, but is also an essential signal that regulates cell fate. As stated, physiological ‘normoxia’ is usually much lower than ambient air. Although most cells are maintained in culture conditions at 21% O2, this is unlikely to be optimal for maintaining their normal proliferative or developmental state. Derivation of novel stem and undifferentiated cell populations should therefore be enhanced by culture in the 3–5% O2 range.
Genetic dissection of the HIFs in mammals is extensive and convincingly demonstrates that O2 levels and gradients play a significant role in developmental processes, including but not limited to angiogenesis, haematopoiesis, placentation, cardiogenesis, bone formation, and adipogenesis. While the role of mTOR and mTOR-associated proteins (raptor, rictor, and mLST8) are critical for embryonic development, their connection to changes in embryonic O2 availability is not conclusive at this time. However, given the similarity of phenotypes between HIF-deficient and mTORC2-deficient embryos 22,26,101, it seems plausible that mTOR is sensing O2 in the early embryo to also regulate cardiovascular differentiation. By contrast, O2 sensation by soluble guanylate cyclases appears to regulate neuronal function as opposed to neuronal development in nematodes. It remains to be determined if O2 sensation by cyclic GMP pathways regulate neuronal development in mammals.
Continued analysis of the role of hypoxia in embryonic development in general, and stem and/ or progenitor cell behaviour in particular should reveal additional interactions between O2-sensitive regulators (like HIFs) and essential pathways that control differentiation. Furthermore, as HIFs are clearly active at the microenvironmental O2 concentrations in which stem/progenitor cell populations commonly reside, it is important to determine if other developmental regulators, such as Fox-family members or ephrins, intersect with pathways that mediate O2 homeostasis. It should be emphasized that many HIF-independent, O2-regulated mechanisms (such as those involving mTOR and ER stress responsive kinases) that promote tolerance to hypoxia are also likely to control multipotency and differentiation and further investigation is required to address this.
In addition to regulating normal stem cell function, hypoxia might also modify the behaviour of so-called ‘cancer stem cells’ and their progeny. If so, inhibiting HIF activity could reduce Notch or Oct-4 levels below a threshold required to maintain stem cell identity, and thereby promote tumour dormancy 112. Hopefully, the recent discovery that hypoxia regulates factors that are crucial for stem/progenitor cell maintenance in normal development and tumour progression will guide the development of novel therapeutics for both tissue regeneration and cancer treatment.
Supplementary Material
GLOSSARY
- Adipogenesis
differentiation of lipid producing and storage cells known as “adipocytes”.
- Angiogenesis
remodeling of blood vessels into the large and small vessels typical of mature networks containing arteries, capillaries and veins.
- Arborize
to develop many branching parts or formations.
- Bradycardia
a slowing of heart rate, usually measured as fewer than 60 beats per minute in humans.
- Cancer stem cells
cancer-initiating cells capable of generating distinct cell types.
- Cardiac hypertrophy
overgrowth of organ size via increased cell size rather than cell number.
- Catecholamine dysregulation
mice lacking HIF-2α die by in utero due to decreased catecholamine (e.g. L-3, 4-dihydroxyphenylalanine [L-DOPA]) production by the organ of Zucker-Kandl (02) chromaffin cells. Catecholamines are required for normal cardiovascular function.
- Conceptus
an embryo or fetus.
- Cytotrophoblasts
outer cells of the developing embryo that adhere to the endometrium.
- Embryoid body
a three dimensional structure consisting of differentiated derivatives of embryonic stem cells.
- ETS
the founding member of a family of oncogenes and proto-oncogenes. ETS refers “E26 specific”.
- Hepatic steatosis
lipid accumulation in the liver.
- Hypoxia
decreased O2 levels relative to normal, which is 1%–9% for most mammalian cell types.
- Inner cell mass
early cells in the embryo that generate all lineages of the mature organism but do not give rise to the placenta.
- Ischaemia
a pathologic condition resulting from blood vessel occlusion involving oxygen, nutrient, and growth factor deprivation. This condition usually also leads to decreased tissue pH levels.
- Niche
the natural, anatomic environment that supports stem cell behaviour.
- Normoxia
although frequently defined in the literature as 21% O2, physiologic normoxia is actually in the 2–9% O2 range for most adult cells in vivo.
- Ontogeny
development of the fetus during embryogenesis.
- Physiological hypoxia
the natural low O2 encountered by cells within the developing embryo, in particular prior to establishment of the utero-placental network.
- Pluripotent
capable of differentiating into cell lineages of the developing organism.
- Ramification
the process of dividing or spreading into branches.
- Retinopathy
abnormal increase in retinal vascular networks.
- Skeletal myopathy
any disease of muscle tissues, such as muscular dystrophy.
- Somites
primordial tissue generating the vertebrae, dermis, and muscles.
- Vasculogenesis
the formation of nascent blood vessels from newly generated endothelial cells.
REFERENCES
- 1.Semenza GL. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol. 1999;15:551–578. doi: 10.1146/annurev.cellbio.15.1.551. [DOI] [PubMed] [Google Scholar]
- 2.Liu L, Simon MC. Regulation of transcription and translation by hypoxia. Cancer Biol Ther. 2004;3:492–497. doi: 10.4161/cbt.3.6.1010. [DOI] [PubMed] [Google Scholar]
- 3.Wouters BG, van den Beucken T, Magagnin MG, Koritzinsky M, Fels D, Koumenis C. Control of the hypoxic response through regulation of mRNA translation. Semin Cell Dev Biol. 2005;16:487–501. doi: 10.1016/j.semcdb.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Semenza GL. Hypoxia-inducible factor 1: oxygen homeostasis and disease pathophysiology. Trends Mol Med. 2001;7:345–350. doi: 10.1016/s1471-4914(01)02090-1. [DOI] [PubMed] [Google Scholar]
- 5.Maltepe E, Simon MC. Oxygen, genes, and development: an analysis of the role of hypoxic gene regulation during murine vascular development. J Mol Med. 1998;76:391–401. doi: 10.1007/s001090050231. [DOI] [PubMed] [Google Scholar]
- 6.Simon MC, Ramirez-Bergeron D, Mack F, Hu CJ, Pan Y, Mansfield K. Hypoxia, HIFs, and cardiovascular development. Cold Spring Harb Symp Quant Biol. 2003;67:127–132. doi: 10.1101/sqb.2002.67.127. [DOI] [PubMed] [Google Scholar]
- 7.Morriss GM, New DA. Effect of oxygen concentration on morphogenesis of cranial neural folds and neural crest in cultured rat embryos. J Embryol Exp Morphol. 1979;54:17–35. [PubMed] [Google Scholar]
- 8.Bruick RK. Oxygen sensing in the hypoxic response pathway: regulation of the hypoxia-inducible transcription factor. Genes Dev. 2003;17:2614–2623. doi: 10.1101/gad.1145503. [DOI] [PubMed] [Google Scholar]
- 9.Guillemin K, Krasnow MA. The hypoxic response: huffing and HIFing. Cell. 1997;89:9–12. doi: 10.1016/s0092-8674(00)80176-2. [DOI] [PubMed] [Google Scholar]
- 10.Manning G, Krasnow MA. Development of the Drosophila tracheal system. Cold Spring Harbor Laboratory Press; In the Development of Drosophila melanogaster. 1993:609–686. [Google Scholar]
- 11.Samakovlis C, Hacohen N, Manning G, Sutherland DC, Guillemin K, Krasnow MA. Development of the Drosophila tracheal system occurs by a series of morphologically distinct but genetically coupled branching events. Development. 1996;122:1395–1407. doi: 10.1242/dev.122.5.1395. [DOI] [PubMed] [Google Scholar]
- 12. Jarecki J, Johnson E, Krasnow MA. Oxygen regulation of airway branching in Drosophila is mediated by branchless FGF. Cell. 1999;99:211–220. doi: 10.1016/s0092-8674(00)81652-9.This article elegantly describes a role for O2 starved cells in branching morphogenesis of the tracheal system so that deprived cells become fully oxygenated
- 13. Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–845. doi: 10.1038/359843a0.This is the first paper indicating that VEGF likely responds to O2 deprivation to increase vascular density as a hypoxic adaptation
- 14.Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 16.Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 17.Wood SM, Gleadle JM, Pugh CW, Hankinson O, Ratcliffe PJ. The role of aryl hydrocarbon receptor nuclear translocator (ARNT) in hypoxia induction of gene expression. J. Biol. Chem. 1996;271:15117–15123. doi: 10.1074/jbc.271.25.15117. [DOI] [PubMed] [Google Scholar]
- 18.Hirota K, Semenza GL. Regulation of angiogenesis by hypoxia-inducible factor 1. Crit Rev Oncol Hematol. 2006;59:15–26. doi: 10.1016/j.critrevonc.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 19.Manalo DJ, Rowan A, Lavoie T, Natarajan L, Kelly BD, Ye SQ, Garcia JG, Semenza GL. Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood. 2005;105:659–669. doi: 10.1182/blood-2004-07-2958. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell JA, Yochim JM. Intrauterine oxygen tension during the estrous cycle in the rat: its relation to uterine respiration and vascular activity. Endocrinology. 1968;83:701–705. doi: 10.1210/endo-83-4-701. [DOI] [PubMed] [Google Scholar]
- 21.Rodesch F, Simon P, Donner C, Jauniaux E. Oxygen measurements in endometrial and trophoblastic tissues during early pregnancy. Obstet Gynecol. 1992;80:283–285. [PubMed] [Google Scholar]
- 22. Maltepe E, Schmidt JV, Baunoch D, Bradfield CA, Simon MC. Abnormal angiogenesis and responses to glucose and oxygen deprivation in mice lacking the protein ARNT. Nature. 1997;386:403–407. doi: 10.1038/386403a0.This paper shows that an environmental sensing bHLH-PAS protein regulates blood vessel morphogenesis in the developing consensus
- 23.Ramirez-Bergeron DL, Runge A, Adelman DM, Gohil M, Simon MC. HIF-dependent hematopoietic factors regulate the development of the embryonic vasculature. Dev Cell. 2006;11:81–92. doi: 10.1016/j.devcel.2006.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kozak KR, Abbott B, Hankinson O. ARNT-deficient mice and placental differentiation. Dev Biol. 1997;191:297–305. doi: 10.1006/dbio.1997.8758. [DOI] [PubMed] [Google Scholar]
- 25. Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY, Semenza GL. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev. 1998;12:149–162. doi: 10.1101/gad.12.2.149.This article along with reference #26 shows that the HIF pathway senses the low O2 environment of the developing embryo to promote embryogenesis and angiogenesis
- 26. Ryan HE, Lo J, Johnson RS. HIF-1alpha is required for solid tumor formation and embryonic vascularization. EMBO J. 1998;17:3005–3015. doi: 10.1093/emboj/17.11.3005.This article along with reference #25 shows that the HIF pathway senses the low O2 environment of the developing embryo to promote embryogenesis and angiogenesis
- 27.Adelman DM, Maltepe E, Simon MC. Multilineage embryonic hematopoiesis requires hypoxic ARNT activity. Genes Dev. 1999;13:2478–2483. doi: 10.1101/gad.13.19.2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adelman DM, Gertsenstein M, Nagy A, Simon MC, Maltepe E. Placental cell fates are regulated in vivo by HIF-mediated hypoxia responses. Genes Dev. 2000;14:3191–3203. doi: 10.1101/gad.853700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cowden Dahl KD, Fryer BH, Mack FA, Compernolle V, Maltepe E, Adelman DM, Carmeliet P, Simon MC. Hypoxia-inducible factors 1alpha and 2alpha regulate trophoblast differentiation. Mol Cell Biol. 2005;25:10479–10491. doi: 10.1128/MCB.25.23.10479-10491.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gnarra JR, Ward JM, Porter FD, Wagner JR, Devor DE, Grinberg A, Emmert-Buck MR, Westphal H, Klausner RD, Linehan WM. Defective placental vasculogenesis causes embryonic lethality in VHL-deficient mice. Proc Natl Acad Sci U S A. 1997;94:9102–9107. doi: 10.1073/pnas.94.17.9102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takeda K, Ho VC, Takeda H, Duan LJ, Nagy A, Fong GH. Placental but not heart defects are associated with elevated hypoxia-inducible factor alpha levels in mice lacking prolyl hydroxylase domain protein 2. Mol Cell Biol. 2006;26:8336–8346. doi: 10.1128/MCB.00425-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Genbacev O, Zhou Y, Ludlow JW, Fisher SJ. Regulation of human placental development by oxygen tension. Science. 1997;277:1669–1672. doi: 10.1126/science.277.5332.1669.This article was the first to demonstrate that progenitor cell proliferation and differentiation is regulated in response to changes in O2 availability
- 33.Caniggia I, Mostachfi H, Winter J, Gassmann M, Lye SJ, Kuliszewski M, Post M. Hypoxia-inducible factor-1 mediates the biological effects of oxygen on human trophoblast differentiation through TGFbeta(3) [see comments] J Clin Invest. 2000;105:577–587. doi: 10.1172/JCI8316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tian H, Hammer RE, Matsumoto AM, Russell DW, McKnight SL. The hypoxia-responsive transcription factor EPAS1 is essential for catecholamine homeostasis and protection against heart failure during embryonic development. Genes Dev. 1998;12:3320–3324. doi: 10.1101/gad.12.21.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Compernolle V, Brusselmans K, Acker T, Hoet P, Tjwa M, Beck H, Plaisance S, Dor Y, Keshet E, Lupu F, Nemery B, Dewerchin M, Van Veldhoven P, Plate K, Moons L, Collen D, Carmeliet P. Loss of HIF-2alpha and inhibition of VEGF impair fetal lung maturation, whereas treatment with VEGF prevents fatal respiratory distress in premature mice. Nat Med. 2002;8:702–710. doi: 10.1038/nm721. [DOI] [PubMed] [Google Scholar]
- 36.Peng J, Zhang L, Drysdale L, Fong GH. The transcription factor EPAS-1/hypoxia-inducible factor 2alpha plays an important role in vascular remodeling. Proc. Natl. Acad. Sci. USA. 2000;97:8386–8391. doi: 10.1073/pnas.140087397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scortegagna M, Ding K, Oktay Y, Gaur A, Thurmond F, Yang L-J, Marck BT, Matsumoto AM, Shelton JM, Richardson JA, Bennett MJ, Garcia JA. Multiple organ pathology, metabolic abnormalities and impaired homeostasis of reactive oxygen species in Epas1−/− mice. Nature Genetics. 2003;35:331–340. doi: 10.1038/ng1266. [DOI] [PubMed] [Google Scholar]
- 38.Gruber M, Hu CJ, Johnson RS, Brown EJ, Keith B, Simon MC. Acute postnatal ablation of Hif-2alpha results in anemia. Proc Natl Acad Sci U S A. 2007;104:2301–2306. doi: 10.1073/pnas.0608382104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rankin EB, Biju MP, Liu Q, Unger TL, Rha J, Johnson RS, Simon MC, Keith B, Haase VH. Hypoxia-inducible factor-2 (HIF-2) regulates hepatic erythropoietin in vivo. J Clin Invest. 2007;117:1068–1077. doi: 10.1172/JCI30117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Isaac DD, Andrew DJ. Tubulogenesis in Drosophila: a requirement for the trachealess gene product. Genes & Dev. 1996;10:103–117. doi: 10.1101/gad.10.1.103. [DOI] [PubMed] [Google Scholar]
- 41.Wilk R, Weizman I, Shilo B-Z. trachealess encodes a bHLH-PAS protein that is an inducer of tracheal cell fates in Drosophila. Genes & Dev. 1996;10:93–102. doi: 10.1101/gad.10.1.93. [DOI] [PubMed] [Google Scholar]
- 42.Rajpurohit R, Koch CJ, Tao Z, Teixeira CM, Shapiro IM. Adaptation of chondrocytes to low oxygen tension: relationship between hypoxia and cellular metabolism. J Cell Physiol. 1996;168:424–432. doi: 10.1002/(SICI)1097-4652(199608)168:2<424::AID-JCP21>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 43.Erlebacher A, Filvaroff EH, Gitelman SE, Derynck R. Toward a molecular understanding of skeletal development. Cell. 1995;80:371–378. doi: 10.1016/0092-8674(95)90487-5. [DOI] [PubMed] [Google Scholar]
- 44. Schipani E, Ryan HE, Didrickson S, Kobayashi T, Knight M, Johnson RS. Hypoxia in cartilage: HIF-1alpha is essential for chondrocyte growth arrest and survival. Genes Dev. 2001;15:2865–2876. doi: 10.1101/gad.934301.This is among the first of many articles using a conditional allele of Hif-1α to show that hypoxic microenvironments specifically regulate developing progenitor cells during organ formation
- 45.Provot S, Zinyk D, Gunes Y, Kathri R, Le Q, Kronenberg HM, Johnson RS, Longaker MT, Giaccia AJ, Schipani E. Hif-1alpha regulates differentiation of limb bud mesenchyme and joint development. J Cell Biol. 2007;177:451–464. doi: 10.1083/jcb.200612023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tontonoz P, Hu E, Devine J, Beale EG, Spiegelman BM. PPAR gamma 2 regulates adipose expression of the phosphoenolpyruvate carboxykinase gene. Mol Cell Biol. 1995;15:351–357. doi: 10.1128/mcb.15.1.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yun Z, Maecker HL, Johnson RS, Giaccia AJ. Inhibition of PPAR gamma 2 gene expression by the HIF-1-regulated gene DEC1/Stra13: a mechanism for regulation of adipogenesis by hypoxia. Dev Cell. 2002;2:331–341. doi: 10.1016/s1534-5807(02)00131-4. [DOI] [PubMed] [Google Scholar]
- 48.Shimba S, Wada T, Hara S, Tezuka M. EPAS1 promotes adipose differentiation in 3T3-L1 cells. J Biol Chem. 2004;279:40946–40953. doi: 10.1074/jbc.M400840200. [DOI] [PubMed] [Google Scholar]
- 49.Mostafa SM, Papoutsakis ET, Miller WM. Oxygen tension has significant effects on human megakaryocye maturation. Exp. Hematology. 2000;28:1498. doi: 10.1016/s0301-472x(99)00150-2. [DOI] [PubMed] [Google Scholar]
- 50.Morrison SJ, Csete M, Groves AK, Melega W, Wold B, Anderson DJ. Culture in reduced levels of oxygen promotes clonogenic sympathoadrenal differentiation by isolated neural crest stem cells. J Neurosci. 2000;20:7370–7376. doi: 10.1523/JNEUROSCI.20-19-07370.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Studer L, Csete M, Lee SH, Kabbani N, Walikonis J, Wold B, McKay R. Enhanced proliferation, survival, and dopaminergic differentiation of CNS precursors in lowered oxygen. J Neurosci. 2000;20:7377–7383. doi: 10.1523/JNEUROSCI.20-19-07377.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spradling A, Drummond-Barbosa D, Kai T. Stem cells find their niche. Nature. 2001;414:98–104. doi: 10.1038/35102160. [DOI] [PubMed] [Google Scholar]
- 53.Lennon DP, Edmison JM, Caplan AI. Cultivation of rat marrow-derived mesenchymal stem cells in reduced oxygen tension: effects on in vitro and in vivo osteochondrogenesis. J Cell Physiol. 2001;187:345–355. doi: 10.1002/jcp.1081. [DOI] [PubMed] [Google Scholar]
- 54.Cipolleschi MG, Dello Sbarba P, Olivotto M. The role of hypoxia in the maintenance of hematopoietic stem cells. Blood. 1993;82:2031–2037. [PubMed] [Google Scholar]
- 55.Parmar K, Mauch P, Vergilio JA, Sackstein R, Down JD. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc Natl Acad Sci U S A. 2007;104:5431–5436. doi: 10.1073/pnas.0701152104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Danet GH, Pan Y, Luongo JL, Bonnet DA, Simon MC. Expansion of human SCID-repopulating cells under hypoxic conditions. J. Clin. Invest. 2003;112:126–135. doi: 10.1172/JCI17669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kiel MJ, Yilmaz OH, Iwashita T, Terhorst C, Morrison SJ. SLAM Family Receptors Distinguish Hematopoietic Stem and Progenitor Cells and Reveal Endothelial Niches for Stem Cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026.This was the first manuscript to suggest that stem and progenitor cells are associated with endothelial microenvironments
- 58.Yoshida S, Sukeno M, Nabeshima Y. A vasculature-associated niche for undifferentiated spermatogonia in the mouse testis. Science. 2007;317:1722–1726. doi: 10.1126/science.1144885. [DOI] [PubMed] [Google Scholar]
- 59.Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, Oh EY, Gaber MW, Finklestein D, Allen M, Frank A, Bayazitov IT, Zakharenko SS, Gajjar A, Davidoff A, Gilbertson RJ. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 60.Harvey AJ, Kind KL, Pantaleon M, Armstrong DT, Thompson JG. Oxygen-regulated gene expression in bovine blastocysts. Biol Reprod. 2004;71:1108–1119. doi: 10.1095/biolreprod.104.028639. [DOI] [PubMed] [Google Scholar]
- 61.Ezashi T, Das P, Roberts RM. Low O2 tensions and the prevention of differentiation of hES cells. Proc Natl Acad Sci U S A. 2005;102:4783–4788. doi: 10.1073/pnas.0501283102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jogi A, Ora I, Nilsson H, Lindeheim A, Makino Y, Poellinger L, Axelson H, Pahlman S. Hypoxia alters gene expression in human neuroblastoma cells toward an immature and neural crest-like phenotype. Proc Natl Acad Sci U S A. 2002;99:7021–7026. doi: 10.1073/pnas.102660199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ramirez-Bergeron DL, Runge A, Dahl KD, Fehling HJ, Keller G, Simon MC. Hypoxia affects mesoderm and enhances hemangioblast specification during early development. Development. 2004;131:4623–4634. doi: 10.1242/dev.01310. [DOI] [PubMed] [Google Scholar]
- 64.Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 65.Hansson EM, Lendahl U, Chapman G. Notch signaling in development and disease. Semin Cancer Biol. 2004;14:320–328. doi: 10.1016/j.semcancer.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 66.Nofziger D, Miyamoto A, Lyons KM, Weinmaster G. Notch signaling imposes two distinct blocks in the differentiation of C2C12 myoblasts. Development. 1999;126:1689–1702. doi: 10.1242/dev.126.8.1689. [DOI] [PubMed] [Google Scholar]
- 67.Dahlqvist C, Blokzijl A, Chapman G, Falk A, Dannaeus K, Ibanez CF, Lendahl U. Functional Notch signaling is required for BMP4-induced inhibition of myogenic differentiation. Development. 2003;130:6089–6099. doi: 10.1242/dev.00834. [DOI] [PubMed] [Google Scholar]
- 68.Varnum-Finney B, Xu L, Brashem-Stein C, Nourigat C, Flowers D, Bakkour S, Pear WS, Bernstein ID. Pluripotent, cytokine-dependent, hematopoietic stem cells are immortalized by constitutive Notch1 signaling. Nat Med. 2000;6:1278–1281. doi: 10.1038/81390. [DOI] [PubMed] [Google Scholar]
- 69.de la Pompa JL, Wakeham A, Correia KM, Samper E, Brown S, Aguilera RJ, Nakano T, Honjo T, Mak TW, Rossant J, Conlon RA. Conservation of the Notch signalling pathway in mammalian neurogenesis. Development. 1997;124:1139–1148. doi: 10.1242/dev.124.6.1139. [DOI] [PubMed] [Google Scholar]
- 70.Cornell RA, Eisen JS. Delta/Notch signaling promotes formation of zebrafish neural crest by repressing Neurogenin 1 function. Development. 2002;129:2639–2648. doi: 10.1242/dev.129.11.2639. [DOI] [PubMed] [Google Scholar]
- 71.Kopan R, Nye JS, Weintraub H. The intracellular domain of mouse Notch: a constitutively activated repressor of myogenesis directed at the basic helix-loop-helix region of MyoD. Development. 1994;120:2385–2396. doi: 10.1242/dev.120.9.2385. [DOI] [PubMed] [Google Scholar]
- 72. Gustafsson MV, Zheng X, Pereira T, Gradin K, Jin S, Lundkvist J, Ruas JL, Poellinger L, Lendahl U, Bondesson M. Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev Cell. 2005;9:617–628. doi: 10.1016/j.devcel.2005.09.010.The article was among the first to link the HIF and a stem cell pathway involving Notch
- 73.Wang R, Zhang YW, Zhang X, Liu R, Zhang X, Hong S, Xia K, Xia J, Zhang Z, Xu H. Transcriptional regulation of APH-1A and increased gamma-secretase cleavage of APP and Notch by HIF-1 and hypoxia. Faseb J. 2006;20:1275–1277. doi: 10.1096/fj.06-5839fje. [DOI] [PubMed] [Google Scholar]
- 74.Kaidi A, Williams AC, Paraskeva C. Interaction between beta-catenin and HIF-1 promotes cellular adaptation to hypoxia. Nat Cell Biol. 2007;9:210–217. doi: 10.1038/ncb1534. [DOI] [PubMed] [Google Scholar]
- 75. Covello KL, Kehler J, Yu H, Gordan JD, Arsham AM, Hu CJ, Labosky PA, Simon MC, Keith B. HIF-2alpha regulates Oct-4: effects of hypoxia on stem cell function, embryonic development, and tumor growth. Genes Dev. 2006;20:557–570. doi: 10.1101/gad.1399906.This first article connects the pluripotent Oct-4 transcription factor to changes in O2 availability
- 76.Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, Chambers I, Scholer H, Smith A. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95:379–391. doi: 10.1016/s0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- 77.Scholer HR, Ruppert S, Suzuki N, Chowdhury K, Gruss P. New type of POU domain in germ line-specific protein Oct-4. Nature. 1990;344:435–439. doi: 10.1038/344435a0. [DOI] [PubMed] [Google Scholar]
- 78.Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 79.Tai MH, Chang CC, Kiupel M, Webster JD, Olson LK, Trosko JE. Oct4 expression in adult human stem cells: evidence in support of the stem cell theory of carcinogenesis. Carcinogenesis. 2005;26:495–502. doi: 10.1093/carcin/bgh321. [DOI] [PubMed] [Google Scholar]
- 80.Kehler J, Tolkunova E, Koschorz B, Pesce M, Gentile L, Boiani M, Lomeli H, Nagy A, McLaughlin KJ, Scholer HR, Tomilin A. Oct4 is required for primordial germ cell survival. EMBO Rep. 2004;5:1078–1083. doi: 10.1038/sj.embor.7400279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lengner CJ, Camargo FD, Hochedlinger K, Welstead GG, Zaidi S, Gokhale S, Scholer HR, Tomilin A, Jaenisch R. Oct4 expression is not required for mouse somatic stem cell self-renewal. Cell Stem Cell. 2007;1:403–415. doi: 10.1016/j.stem.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Niwa H, Miyazaki J, Smith AG. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet. 2000;24:372–376. doi: 10.1038/74199. [DOI] [PubMed] [Google Scholar]
- 83.Hochedlinger K, Yamada Y, Beard C, Jaenisch R. Ectopic expression of Oct-4 blocks progenitor-cell differentiation and causes dysplasia in epithelial tissues. Cell. 2005;121:465–477. doi: 10.1016/j.cell.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 84.Hu C-J, Wang L-Y, Chodosh LA, Keith B, Simon MC. Differential Roles of Hypoxia-Inducible Factor 1alpha (HIF-1alpha) and HIF-2alpha in Hypoxic Gene Regulation. Mol Biol Cell. 2003;23:9361–9374. doi: 10.1128/MCB.23.24.9361-9374.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nordhoff V, Hubner K, Bauer A, Orlova I, Malapetsa A, Scholer HR. Comparative analysis of human, bovine, and murine Oct-4 upstream promoter sequences. Mamm Genome. 2001;12:309–317. doi: 10.1007/s003350010279. [DOI] [PubMed] [Google Scholar]
- 86.Koshiji M, Kageyama Y, Pete EA, Horikawa I, Barrett JC, Huang LE. HIF-1alpha induces cell cycle arrest by functionally counteracting Myc. Embo J. 2004;23:1949–1956. doi: 10.1038/sj.emboj.7600196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang H, Gao P, Fukuda R, Kumar G, Krishnamachary B, Zeller KI, Dang CV, Semenza GL. HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of C-MYC activity. Cancer Cell. 2007;11:407–420. doi: 10.1016/j.ccr.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 88.Gordan JD, Bertout JA, Hu CJ, Diehl JA, Simon MC. HIF-2alpha promotes hypoxic cell proliferation by enhancing c-myc transcriptional activity. Cancer Cell. 2007;11:335–347. doi: 10.1016/j.ccr.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024.The paper is the first in a series showing that a relatively small number of factors can reprogram fibroblasts into pluripotent cells
- 90.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 91.Maherali N, Sridharan R, Xie W, Utikal J, Eminli S, Arnold K, Stadtfeld M, Yachechko R, Tchieu J, Jaenisch R, Plath K, Hochedlinger K. Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell. 2007;1:55–70. doi: 10.1016/j.stem.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 92.Wernig M, Meissner A, Foreman R, Brambrink T, Ku M, Hochedlinger K, Bernstein BE, Jaenisch R. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318–324. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
- 93. Hochachka PW, Buck LT, Doll CJ, Land SC. Unifying theory of hypoxia tolerance: molecular/metabolic defense and rescue mechanisms for surviving oxygen lack. Proc Natl Acad Sci U S A. 1996;93:9493–9498. doi: 10.1073/pnas.93.18.9493.Among the first articles to show that O2 deprivation is tolerated by altered intracellular metabolic pathways to conserve ATP
- 94.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 95.Koumenis C, Wouters BG. "Translating" tumor hypoxia: unfolded protein response (UPR)-dependent and UPR-independent pathways. Mol Cancer Res. 2006;4:423–436. doi: 10.1158/1541-7786.MCR-06-0150. [DOI] [PubMed] [Google Scholar]
- 96.Gray JM, Karow DS, Lu H, Chang AJ, Chang JS, Ellis RE, Marletta MA, Bargmann CI. Oxygen sensation and social feeding mediated by a C. elegans guanylate cyclase homologue. Nature. 2004;430:317–322. doi: 10.1038/nature02714. [DOI] [PubMed] [Google Scholar]
- 97.Guertin DA, Sabatini DM. An expanding role for mTOR in cancer. Trends Mol Med. 2005;11:353–361. doi: 10.1016/j.molmed.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 98.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 99.Gangloff YG, Mueller M, Dann SG, Svoboda P, Sticker M, Spetz JF, Um SH, Brown EJ, Cereghini S, Thomas G, Kozma SC. Disruption of the mouse mTOR gene leads to early postimplantation lethality and prohibits embryonic stem cell development. Mol Cell Biol. 2004;24:9508–9516. doi: 10.1128/MCB.24.21.9508-9516.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Murakami M, Ichisaka T, Maeda M, Oshiro N, Hara K, Edenhofer F, Kiyama H, Yonezawa K, Yamanaka S. mTOR is essential for growth and proliferation in early mouse embryos and embryonic stem cells. Mol Cell Biol. 2004;24:6710–6718. doi: 10.1128/MCB.24.15.6710-6718.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Guertin DA, Stevens DM, Thoreen CC, Burds AA, Kalaany NY, Moffat J, Brown M, Fitzgerald KJ, Sabatini DM. Ablation in Mice of the mTORC Components raptor, rictor, or mLST8 Reveals that mTORC2 Is Required for Signaling to Akt-FOXO and PKCalpha, but Not S6K1. Dev Cell. 2006;11:859–871. doi: 10.1016/j.devcel.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 102.Bi L, Okabe I, Bernard DJ, Wynshaw-Boris A, Nussbaum RL. Proliferative defect and embryonic lethality in mice homozygous for a deletion in the p110alpha subunit of phosphoinositide 3-kinase. J Biol Chem. 1999;274:10963–10968. doi: 10.1074/jbc.274.16.10963. [DOI] [PubMed] [Google Scholar]
- 103.Lelievre E, Bourbon PM, Duan LJ, Nussbaum RL, Fong GH. Deficiency in the p110alpha subunit of PI3K results in diminished Tie2 expression and Tie2(−/−)-like vascular defects in mice. Blood. 2005;105:3935–3938. doi: 10.1182/blood-2004-10-3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Arsham AM, Howell JJ, Simon MC. A novel hypoxia-inducible factor-independent hypoxic response regulating mammalian target of rapamycin and its targets. J Biol Chem. 2003;278:29655–29660. doi: 10.1074/jbc.M212770200. [DOI] [PubMed] [Google Scholar]
- 105.Hudson CC, Liu M, Chiang GG, Otterness DM, Loomis DC, Kaper F, Giaccia AJ, Abraham RT. Regulation of hypoxia-inducible factor 1alpha expression and function by the mammalian target of rapamycin. Mol Cell Biol. 2002;22:7004–7014. doi: 10.1128/MCB.22.20.7004-7014.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhong H, Chiles K, Feldser D, Laughner E, Hanrahan C, Georgescu MM, Simons JW, Semenza GL. Modulation of hypoxia-inducible factor 1alpha expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: implications for tumor angiogenesis and therapeutics. Cancer Res. 2000;60:1541–1545. [PubMed] [Google Scholar]
- 107.Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- 108.Koumenis C, Naczki C, Koritzinsky M, Rastani S, Diehl A, Sonenberg N, Koromilas A, Wouters BG. Regulation of protein synthesis by hypoxia via activation of the endoplasmic reticulum kinase PERK and phosphorylation of the translation initiation factor eIF2alpha. Mol Cell Biol. 2002;22:7405–7416. doi: 10.1128/MCB.22.21.7405-7416.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Harding HP, Zeng H, Zhang Y, Jungries R, Chung P, Plesken H, Sabatini DD, Ron D. Diabetes mellitus and exocrine pancreatic dysfunction in perk−/− mice reveals a role for translational control in secretory cell survival. Mol Cell. 2001;7:1153–1163. doi: 10.1016/s1097-2765(01)00264-7. [DOI] [PubMed] [Google Scholar]
- 110.Scheuner D, Song B, McEwen E, Liu C, Laybutt R, Gillespie P, Saunders T, Bonner-Weir S, Kaufman RJ. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol Cell. 2001;7:1165–1176. doi: 10.1016/s1097-2765(01)00265-9. [DOI] [PubMed] [Google Scholar]
- 111.Zhang W, Feng D, Li Y, Iida K, McGrath B, Cavener DR. PERK EIF2AK3 control of pancreatic beta cell differentiation and proliferation is required for postnatal glucose homeostasis. Cell Metab. 2006;4:491–497. doi: 10.1016/j.cmet.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 112.Keith B, Simon MC. Hypoxia-inducible factors, stem cells, and cancer. Cell. 2007;129:465–472. doi: 10.1016/j.cell.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gu YZ, Hogenesch JB, Bradfield CA. The PAS superfamily: sensors of environmental and developmental signals. Annu Rev Pharmacol Toxicol. 2000;40:519–561. doi: 10.1146/annurev.pharmtox.40.1.519. [DOI] [PubMed] [Google Scholar]
- 114.Wang GL, Semenza GL. Purification and characterization of hypoxia-inducible factor 1. J. Biol. Chem. 1995;270:1230–1237. doi: 10.1074/jbc.270.3.1230. [DOI] [PubMed] [Google Scholar]
- 115.Wang GL, Jiang B-H, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jain S, Maltepe E, Lu MM, Simon C, Bradfield CA. Expression of ARNT, ARNT2, HIF1alpha, HIF2alpha and Ah receptor mRNAs in the developing mouse. Mech Dev. 1998;73:117–123. doi: 10.1016/s0925-4773(98)00038-0. [DOI] [PubMed] [Google Scholar]
- 117.Tian H, McKnight SL, Russell DW. Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev. 1997;11:72–82. doi: 10.1101/gad.11.1.72. [DOI] [PubMed] [Google Scholar]
- 118.Wiesener MS, Jurgensen JS, Rosenberger C, Scholze CK, Horstrup JH, Warnecke C, Mandriota S, Bechmann I, Frei UA, Pugh CW, Ratcliffe PJ, Bachmann S, Maxwell PH, Eckardt KU. Widespread hypoxia-inducible expression of HIF-2alpha in distinct cell populations of different organs. Faseb J. 2003;17:271–273. doi: 10.1096/fj.02-0445fje. [DOI] [PubMed] [Google Scholar]
- 119.Gu YZ, Moran SM, Hogenesch JB, Wartman L, Bradfield CA. Molecular characterization and chromosomal localization of a third alpha-class hypoxia inducible factor subunit, HIF3alpha. Gene Expr. 1998;7:205–213. [PMC free article] [PubMed] [Google Scholar]
- 120.Makino Y, Cao R, Svensson K, Bertilsson G, Asman M, Tanaka H, Cao Y, Berkenstam A, Poellinger L. Inhibitory PAS domain protein is a negative regulator of hypoxia-inducible gene expression. Nature. 2001;414:550–554. doi: 10.1038/35107085. [DOI] [PubMed] [Google Scholar]
- 121.Keith B, Adelman DM, Simon MC. Targeted mutation of the murine arylhydrocarbon receptor nuclear translocator 2 (Arnt2) gene reveals partial redundancy with Arnt. Proc. Natl. Acad. Sci. USA. 2001;98:6692–6697. doi: 10.1073/pnas.121494298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bunger MK, Wilsbacher LD, Moran SM, Clendenin C, Radcliffe LA, Hogenesch JB, Simon MC, Takahashi JS, Bradfield CA. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell. 2000;103:1009–1017. doi: 10.1016/s0092-8674(00)00205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG., Jr HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 124.Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 125.Yu F, White SB, Zhao Q, Lee FS. HIF-1alpha binding to VHL is regulated by stimulus-sensitive proline hydroxylation. Proc. Natl. Acad. Sci. USA. 2001;98:9630–9635. doi: 10.1073/pnas.181341498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Masson N, William C, Maxwell PH, Pugh CW, Ratcliffe PJ. Independent function of two destruction domains in hypoxia-inducible factor-alpha chains activated by prolyl hydroxylation. Embo J. 2001;20:5197–5206. doi: 10.1093/emboj/20.18.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O’Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, Tian YM, Masson N, Hamilton DL, Jaakkola P, Barstead R, Hodgkin J, Maxwell PH, Pugh CW, Schofield CJ, Ratcliffe PJ. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- 128.Bruick RK, McKnight SL. A conserved family of prolyl-4-hydroxylases that modify HIF. Science. 2001;294:1337–1340. doi: 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- 129.Ivan M, Haberberger T, Gervasi DC, Michelson KS, Gunzler V, Kondo K, Yang H, Sorokina I, Conaway RC, Conaway JW, Kaelin WG., Jr Biochemical purification and pharmacological inhibition of a mammalian prolyl hydroxylase acting on hypoxia-inducible factor. Proc Natl Acad Sci U S A. 2002;99:13459–13464. doi: 10.1073/pnas.192342099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Schofield CJ, Ratcliffe PJ. Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol. 2004;5:343–354. doi: 10.1038/nrm1366. [DOI] [PubMed] [Google Scholar]
- 131.Ema M, Hirota K, Mimura J, Abe H, Yodoi J, Sogawa K, Poellinger L, Fujii-Kuriyama Y. Molecular mechanisms of transcription activation by HLF and HIF1alpha in response to hypoxia: their stabilization and redox signal-induced interaction with CBP/p300. Embo J. 1999;18:1905–1914. doi: 10.1093/emboj/18.7.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lando D, Peet DJ, Gorman JJ, Whelan DA, Whitelaw ML, Bruick RK. FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes Dev. 2002;16:1466–1471. doi: 10.1101/gad.991402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lando D, Peet DJ, Whelan DA, Gorman JJ, Whitelaw ML. Asparagine hydroxylation of the HIF transactivation domain a hypoxic switch. Science. 2002;295:858–861. doi: 10.1126/science.1068592. [DOI] [PubMed] [Google Scholar]
- 134.Mahon PC, Hirota K, Semenza GL. FIH-1: a novel protein that interacts with HIF-1alpha and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev. 2001;15:2675–2686. doi: 10.1101/gad.924501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hewitson KS, McNeill LA, Riordan MV, Tian YM, Bullock AN, Welford RW, Elkins JM, Oldham NJ, Bhattacharya S, Gleadle JM, Ratcliffe PJ, Pugh CW, Schofield CJ. Hypoxia-inducible Factor (HIF) Asparagine Hydroxylase Is Identical to Factor Inhibiting HIF (FIH) and Is Related to the Cupin Structural Family. J Biol Chem. 2002;277:26351–26355. doi: 10.1074/jbc.C200273200. [DOI] [PubMed] [Google Scholar]
- 136.Semenza GL. HIF-1 and human disease: one highly involved factor. Genes Dev. 2000;14:1983–1991. [PubMed] [Google Scholar]
- 137.Kaelin WG. Proline hydroxylation and gene expression. Annu Rev Biochem. 2005;74:115–128. doi: 10.1146/annurev.biochem.74.082803.133142. [DOI] [PubMed] [Google Scholar]
- 138.Pear WS, Simon MC. Lasting longer without oxygen: The influence of hypoxia on Notch signaling. Cancer Cell. 2005;8:435–437. doi: 10.1016/j.ccr.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 139.Yoshida H. ER stress and diseases. Febs J. 2007;274:630–658. doi: 10.1111/j.1742-4658.2007.05639.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.