Abstract
Background
Ionizing radiation (IR) initiates intracellular oxidative stress through enhanced formation of reactive oxygen species (ROS) that attack DNA leading to cell death. Because of the diversity of IR applied in medicine, agriculture, industry, and the growing threats of global terrorism, the acquisition of radioprotectors is an urgent need for the nation. However, the applicability of radioprotectors currently under investigation is limited due to their inherent toxicity.
Objective
This study investigated the effect of a standardized North American ginseng extract (NAGE, total ginsenoside content: 11.7%) on DNA damage in human lymphocytes at 90 minutes postirradiation.
Design
With the application of NAGE (250–1000 μg mL−1) at 90 minutes postirradiation (1 and 2 Gy), DNA damage in lymphocytes obtained from 40 healthy individuals was evaluated by cytokinesis-block micronucleus assay. Similar experiments were also performed in lymphocytes treated with WR-1065 (1 mmol/L or 3 mmol/L). In addition, before and after irradiation, lymphocytes obtained from 10 individuals were measured for their total antioxidant capacity (TAC) and the reactive oxygen species (ROS).
Results
The significant effect of NAGE against 137Cs-induced micronuclei (MN) in lymphocytes is concentration dependent. NAGE (750 μg mL−1) reduced MN yield by 50.7% after 1 Gy and 35.9% after 2 Gy exposures, respectively; these results were comparable to that of WR-1065. Furthermore, we also found that NAGE reduces MN yield and ROS but increases TAC in lymphocytes.
Conclusions
Our results suggest that NAGE is a relatively nontoxic natural compound that holds radioprotective potential in human lymphocytes even when applied at 90 minutes postirradiation. One of the radioprotective mechanisms may be mediated through the scavenging of free radicals and enhancement of the intracellular TAC.
Introduction
It is well known that exposure of normal tissue cells to ionizing radiation (IR) activates genetic cascades of signaling events, generating free radicals collectively known as reactive oxygen species (ROS), which attack DNA, ultimately leading to cell death. Due to the increased utilization of IR in human life and the growing threats of global terrorism, IR-induced normal tissue morbidities are of further importance to both civilians and military populations, since they are potentially subject to accidental or intentional nuclear mishaps. Hence, the development of an efficacious radioprotector would be a contribution to radiation oncology, public health, national defense, and environmental remediation.1–3
The term “radioprotector” primarily refers to free radical scavengers that avert the initial radiochemical events in cells following IR exposure. Currently, the majority of potential radioprotective chemical compounds under investigation are designed to scavenge IR-induced free radicals. Nevertheless, their efficacy is linked to high-drug dosages that will evoke unacceptable side-effects, and none of these agents is available for human use outside the clinic.2 Thus, the search for less- or nontoxic agents to counter the effects of IR remains an area of intense focus.1,2 Natural products such as herbal medicines with an abundance of antioxidant resources have received attention as possible radiation modifiers.4
Herbal medicine, or phytomedicine, is generally considered a well-established form of complementary medicine. Ginseng is one of the most frequently purchased herbs in the U.S. marketplace and is frequently taken orally as a traditional herbal medicine.5 The term ginseng refers to the dried root of several species in the plant genus Panax, which belongs to the Araliaceae family; it comprises two commonly used ginseng species (i.e., Panax ginseng C.A. Meyer [Asian ginseng] and Panax quinquefolius L. [North American ginseng]). These two forms of ginseng have drawn worldwide attention for their broad medicinal potential, such as antiaging, antidiabetic, anticarcinogenic, antihypertension, antipyretic, antistress, analgesic, and antifatigue effects, as well as their enhancement of immune response to polyclonal stimulation and promotion of DNA, RNA, and protein synthesis.5–9 Recently, in a 18.8 years cohort study based on 6282 human subjects, Yi et al. found that ginseng intake significantly decreased all-cause mortality in older Korean males.5 The predominant bioactive components of ginseng are a diverse group of triterpenoid saponins with steroidal structures, labeled ginsenosides. Although the mechanisms are still largely unknown, the medicinal properties of ginseng have been closely related to the effects of ginsenosides against free radical attack.6–8,10
After the exposure of mammalian cells to ionizing radiation (IR), an unregulated production of ROS, associated with a shift in the intracellular oxidant-antioxidant balance toward a pro-oxidant state, triggers damage to cellular membranes and DNA, leading to a state of oxidative stress. However, because effective antioxidants are free-radical scavengers that interfere with radical chain reactions, it is possible to protect cellular DNA from oxidative stress by supplementation with antioxidants.11–14
Studies of the radioprotective effect of ginseng have been performed primarily with the application of Asian ginseng in rodent models.8,15 Micronuclei (MN) in interphase mammalian cells are reliable biomarkers for evaluating IR-induced chromosome damage.16,17 We recently found that incubation with Asian ginseng dried root crude water extract (100 μg–2000 μg mL−1) 24 hours before 137Cs exposure significantly reduced radiation-induced (MN) yield in peripheral blood lymphocytes (PBL) obtained from 4 human subjects.18 However, although North American ginseng (NAG) is one of the best-selling herbs on the market, relatively few studies have involved NAG.8 The purpose of this study was to investigate whether a radioprotective effect of a standardized North American ginseng extract (NAGE) could also be achieved in human PBL when applied postradiation. The hypotheses behind this study are (1) that IR-induced oxidative injury in PBL is preventable by the administration of exogenous antioxidants; and (2) that the radioprotective effect of NAGE on human PBL is a result of modulation of the activity of the intracellular antioxidant defense systems. To test these hypotheses, we investigated the impact of NAGE when applied 90 minutes after 137Cs exposure on MN yield in PBL obtained from 40 healthy individuals. The MN results in PBL obtained from NAGE application were compared with similar experiments using WR-1065, the biologically active aminothiol form of amifostine (WR-2721), which is currently the only “gold standard” of radioprotectors approved by the U.S. Food and Drug Administration.19,20 In addition, in 10 of these individuals, we also evaluated the correlation between the effect of NAGE on intracellular total antioxidant capacity (TAC), levels of ROS production, and MN yield in PBL before and after 137Cs exposure. Although results are preliminary, we believe that the information generated from these in vitro studies will provide the foundation for in vivo trials to assess the potential of NAGE as a natural dietary radiation countermeasure.
Materials and Methods
Subjects
Our University Medical Center Institutional Review Board approved this study. A total of 40 healthy individuals (23 M/17 F) 43.3 ± 2.2 (mean ± standard error of mean [SEM]) years of age, without known history of exposure to mutagens, were recruited in this study. No individuals were currently taking any other pharmacologic agents, including medications, vitamins, or dietary supplements. All participants signed informed consent before enrollment.
NAGE preparation and ginsenosides content
The standardized NAGE powder (Lot-TKGS-010406) was purchased from Canadian Phytopharmaceuticals Corporation (Richmond, BC, Canada). Using high-performance liquid chromatography, the major ginsenosides in this NAGE powder were characterized by the vendor as follows: Rb1 (5.1%), Rb2 (0.99%), Rc (1.88%), Rd (1.23%), Re (2.14%), and Rg1 (0.36%) with total ginsenoside content (wt/wt) of 11.7%. To ensure stability, the NAGE was stored in a cool, dry, dark location over the course of the study. Before experimentation, 50 mg of the lyophilized NAGE powder was dissolved in 5 mL 1× RPMI culture medium (Sigma-Aldrich, MO), filtered through a 0.2-μm disc (Millipore, MA) under sterile conditions, and was used as the stock solution.
Cytokinesis-block (CB) MN assay
Fresh peripheral blood samples were drawn from each individual into Vacutainer Cell Preparation Tubes (Becton-Dickinson, NJ). Mononuclear cells were isolated by density gradient centrifugation at 1800g for 20 minutes, washed, and counted on a hemacytometer. Trypan blue exclusion showed the viability to be greater than 95%. The purity of mononuclear cells was >95% as determined by Hema-3 staining (Fisher Scientific, NC). For each culture, 2–3 × 105 cells mL−1 were incubated in polystyrene culture tubes containing 1 × RPMI 1640 culture medium (Sigma Chemical, MO), supplemented with 10% fetal calf serum, l-glutamine (0.03%), and penicillin (100 IU mL−1) and streptomycin (100 μg mL−1). The final volume of each culture was 1 mL. Duplicate cultures were set up for each experimental point within 60 minutes after venipuncture. Phytohemagglutinin (PHA, M Form, Invitrogen Corp., CA) was added to each culture (15 μL mL−1) immediately after ex vivo radiation exposure. Cytochalasin B (Sigma Chemical) was applied at 44 hours after the PHA stimulation, with a final concentration of 4 μg mL−1. All cultures were maintained in a humidified atmosphere of 5% CO2 at 37°C following another 24 hours, and cells were collected by centrifugation at 300g for 10 min. The slides, prepared according to the method of Fenech et al.,16,17 were stained with Hema-3 (Fisher Scientific).
Application of NAGE
To ascertain the optimum radioprotective dose of NAGE, a series of preliminary studies were carried out (data not shown). Treatment of PBL with NAGE at 500–750 μg mL−1 at 0 hours was found to cause a significant reduction in 137Cs-induced MN yield. Therefore, for the determination of a dose–response radioprotective effect of NAGE, in each experiment, four different concentrations (250, 500, 750, and 1000 μg mL−1) of NAGE were applied to mononuclear cell cultures (2 × 105 cells mL−1) in RPMI 1640 90 minutes after exposure to 137Cs- irradiation for CBMN assay.
WR-1065 preparation and application
WR-1065 was kindly provided by Dr. Robert J. Schultz (Drug Synthesis and Chemistry Branch, National Institutes of Health (NIH)-National Cancer Institutes, Bethesda, MD). A stock solution (10 mmol/L) of WR-1065 was made up with RPMI 1640 culture medium and was kept frozen. The stock solution was thawed on ice immediately before use and was filtered through a 0.2-μm disc (Millipore, MA). The remainder was quickly frozen again after use.
For each experimental condition, we serially diluted the stock solution of WR-1065 with the culture medium to the final concentrations (1 mmol/L or 3 mmol/L). We then applied WR-1065 (1 mmol/L or 3 mmol/L) to mononuclear cell cultures (6 × 105 cells mL−1) at 90 minutes postirradiation. After the 10-minute treatment with WR-1065, the cell cultures were centrifuged, washed with phosphate-buffered saline to remove the WR-1065, and were resuspended in the RPMI 1640 culture medium for the completion of the CBMN assay.
Ex vivo irradiation
The human G0 PBL were exposed ex vivo to 137Cs γ-rays (Gamma Cell 40, Radiation Machinery, Ontario, Canada) with 1 or 2 Gy (0.6 Gy/min) at room temperature (22°C), and NAGE was applied to the culture medium 90 minutes after irradiation.
Microscopy
Slides were coded and randomized to ensure anonymity upon scoring. For consistency, the microscopy was performed by one researcher (WW). Under 400 × magnification, in continuous fields from two slides prepared for each experimental checkpoint, he scored a minimum of 1000 binucleated (BN) cells where possible. The quantification of MN yield was restricted to BN cells with distinct intact cytoplasm and included those with nuclear bridges. MN with smooth edges touching the main nucleus and those with clearly defined overlap were also included in the count. The distribution of MN number in each BN cell was also recorded. The MN yield was determined as MN yield = (Total number of MN in BN cells/Total number of scored BN cells) × 1000. Percentage reduction of MN was determined as 137Cs-induced MN yield in varying concentrations of NAGE compared to that with radiation alone.
Measurement of the intracellular total antioxidant capacity (TAC) in PBL
In addition to their CBMN assay, blood samples obtained from 10 (6 males/4 females, 42.7 ± 4.6 years of age) of the 40 individuals were studied to determine the intracellular TAC level in PBL. Using the Antioxidant Assay Kit (Sigma, CS-0790), PBL (1 × 106 cells mL−1) before and at 90 minutes postirradiation were incubated with different concentrations of NAGE for 24 hours for the determination of intracellular TAC. The antioxidant assay is based on the formation of a ferryl myoglobin radical from myoglobin and hydrogen peroxide, which oxidizes ABTS [2,2'-azinobis-(3-ethyl-benzothialozine-6-sulfonic acid)] to produce a radical cation ABTS+, a soluble green chromogen that can be determined at 405 nm. In the presence of antioxidants, the radical cation is suppressed to an extent dependent on antioxidant activity, and the color intensity is decreased proportionally. Trolox, a water-soluble vitamin E analogue, served as a control antioxidant. In brief, at the end of the 24-hour incubation, PBL were sonicated on ice in 1 mL of cold 1 × assay buffer and centrifuged at 12,000g for 15 minutes (4°C). In a 96-well culture plate, the supernatant of PBL lysates (10 μL) in each well was mixed with 1 × myoglobin working solution (20 μL), ABTS substrate working solution (150 μL), and 3% hydrogen peroxide (25 μL) and allowed to incubate at 25°C for 30 minutes. For the Trolox standard curve, 10 μL of a Trolox standard and 20 μL of myoglobin working solution were added to each well. Kit stop solution (100 μL) was then added to each well. Samples were read immediately at 406 nm excitation/530 nm emission on a plate reader. Results were calculated using a reference curve based on Trolox as a standard, and intracellular TAC in PBL was expressed in mM equivalent/L.
Measurement of intracellular oxidative stress in PBL
In addition to their CBMN assay, blood samples obtained from 10 (6 male/4 female, 42.7 ± 4.6 years of age) of the 40 individuals were studied to determine the intracellular ROS level in PBL. In brief, using the Live Cell Fluorescent Reactive Oxygen Species Detection Kit (MGT-M1049, Marker Gene Technologies, Eugene, OR), before and at 90 minutes post 137Cs exposure, PBL (1 × 106 cells mL−1) were incubated with different concentrations of NAGE for 24 hours. At the end of the incubation, the culture medium was aspirated after centrifugation (1000g, 15 minutes) and pellets were suspended in ROS inducer (i.e., t-butyl hydroperoxide working solution [100 μmol/L]), then incubated for 60 minutes at 37°C. After centrifugation, PBL pellets were resuspended and incubated in darkness for 45 minutes in 2′,7′′-dichlorofluorescein diacetate (25 μmol/L), which is a cell-permeable substrate and a reliable fluorogenic marker for ROS detection. Upon enzyme activity, the highly fluorescent dye, 2′,7′′-dichlorofluorescein, was produced. The cellular fluorescence from each well was determined using 480-nm excitation/535-nm emission with a microplate spectrofluorometer. The values of fluorescence intensity, reflecting the intracellular concentration of ROS in PBL, were expressed as percentage of controls.
Statistical analyses
All measurements were represented as the mean and standard error of the mean (±SEM) and were blinded as to subject status. We used the software package SPSS for the data analysis.21 Statistical methods consisted of repeated measures of analysis of variance and linear regression using a mixed-model approach with random intercepts. Linear contrasts were used to examine the effect of NAGE (0–1000 μg mL−1) on radiation-induced MN yield in PBL and were completely cross-classified in a factorial fashion. The effect of radiation on MN yield of PBL in the presence and absence of NAGE, and the interactions between radiation doses and concentrations of NAGE were evaluated separately. Associations between MN yield and intracellular TAC and ROS levels in PBL were assessed by Pearson's correlation test.
Results
Effect of NAGE and WR-1065 on MN yield in PBL before 137Cs exposure (Table 1)
Table 1.
Comparison of the Effect of North American Ginseng Extract (NAGE) (μg mL−1) and WR-1065 (mmol/L) Applied 90 Minutes Postirradiation on 137Cs-Induced Micronuclei (MN) Yield (per 1000 Binucleated Cells) in Lymphocytes Obtained from 40 Healthy Individuals
| |
|
MN |
|
|
MN |
||||
|---|---|---|---|---|---|---|---|---|---|
| Gy | NAGE | Mean | SEM | Reduction (%) | Gy | WR-1065 | Mean | SEM | Reduction (%) |
| 0 | 0 | 16.7* | 0.9 | – | 0 | 0 | 16.7 | 0.9 | – |
| 250 | 14.4 | 1.3 | – | 1 mmol/L | 15.6 | 1.3 | – | ||
| 500 | 15.6 | 1.4 | – | 3 mmol/L | 15.9 | 1.8 | – | ||
| 750 | 10.5 | 1.2 | – | ||||||
| 1000 | 14.2 | 1.0 | |||||||
| 1 | 0 | 111.4*† | 4.4 | – | 1 | 0 | 111.4# | 4.4 | – |
| 250 | 89.7† | 7.5 | 19.5 | 1 mmol/L | 63.9# | 5.9 | 42.6 | ||
| 500 | 71.1† | 3.6 | 36.2 | 3 mmol/L | 53.5# | 5.6 | 52.0 | ||
| 750 | 54.9† | 8.1 | 50.7 | ||||||
| 1000 | 69.1† | 4.7 | 38.0 | ||||||
| 2 | 0 | 212.8*‡ | 6.9 | – | 2 | 0 | 212.8** | 6.9 | – |
| 250 | 172.2‡ | 12.5 | 19.1 | 1 mmol/L | 131.4** | 12.5 | 38.3 | ||
| 500 | 159.5‡ | 9.0 | 25.0 | 3 mmol/L | 141.8** | 16.7 | 33.4 | ||
| 750 | 136.4‡ | 12.8 | 35.9 | ||||||
| 1000 | 139.6‡ | 8.5 | 34.4 | ||||||
Percentage reduction and significance of difference were determined as 137Cs-induced MN yield in varying concentrations of NAGE and WR-1065 compared to that of radiation alone.
p-Value based on comparisons of MN from 0 Gy versus 1 Gy versus 2 Gy when NAGE was not applied.
p-Values of MN induced by 1 Gy with the presence of different concentrations of NAGE.
p-Values of MN induced by 2 Gyn with the presence of different concentrations of NAGE.
p-Value of MN induced by 1 Gy with different concentrations of WR-1065 (1 mmol and 2 mmol).
p-Value of MN induced by 2 Gy with different concentrations of WR-1065 (1 mmol and 2 mmol).
p < 0.001. †#p < 0.01.
SEM, standard error of mean.
Before irradiation and in the absence of both NAGE and WR-1065, mean (±SEM) baseline MN yield of PBL obtained from the 40 healthy individuals was 16.7 ± 0.9 per 1000 BN cells. The presence of NAGE (250–1000 μg mL−1) or WR-1065 (1 mmol/L or 3 mmol/L) in PBL culture medium did not affect the MN yield significantly.
Effect of NAGE and WR-1065 applied at 90 minutes after 137Cs exposure on MN yield in PBL
Radiation alone (1 Gy and 2 Gy) sharply increased the MN yield in PBL in a dose-dependent manner (Table 2, p < 0.001). However, both NAGE (250–1000 μg mL−1) and WR-1065 (1 mmol/L or 3 mmol/L) significantly reduced the MN yields as their concentration increased (Table 1). The best-fitting line for this relationship was Y = C + αD + βD2 (Table 2, p < 0.001), where Y is the MN per 1000 BN cells and C is the intercept, D is the concentration of NAGE or WR-1065, and α and β are the linear and quadratic coefficients, respectively. Table 1 shows that, when compared with radiation alone, application of NAGE (750 μg mL−1) reduced MN yield by 50.7% after 1 Gy and 35.9% after 2 Gy exposure, respectively; the application of WR-1065 (3 mmol/L) reduced MN yield by 52.0% after 1 Gy and 33.4% after 2 Gy exposure, respectively.
Table 2.
Regression Coefficients of 137Cs Dose–Response Relationship of Micronuclei Yield in Binucleated Lymphocytes Obtained from Healthy Individuals (n = 40) When NAGE or WR-1065 Applied to the Culture Medium 90 Minutes After the Radiation Exposure (*p < 0.001)
| Gy | Intercept | Slope | Curvature | |
|---|---|---|---|---|
| NAGE | 0 | 14.0* | 1.53E−3 | −2.94E−6 |
| WR-1065 | 0 | 15.3* | 2.59E−1 | −3.00E−2 |
| NAGE | 1 | 123.0* | −1.65E−1* | 1.17E−4* |
| WR-1065 | 1 | 130.5* | −8.72E+1* | 2.05E+1* |
| NAGE | 2 | 194.6* | −1.47E−1* | 7.03E−5 |
| WR-1065 | 2 | 241.7* | −1.49E+2* | 3.85E+2* |
Effect of NAGE applied before and 90 minutes after 137Cs irradiation on intracellular TAC status (mmol/L Trolox equivalent/L) in PBL
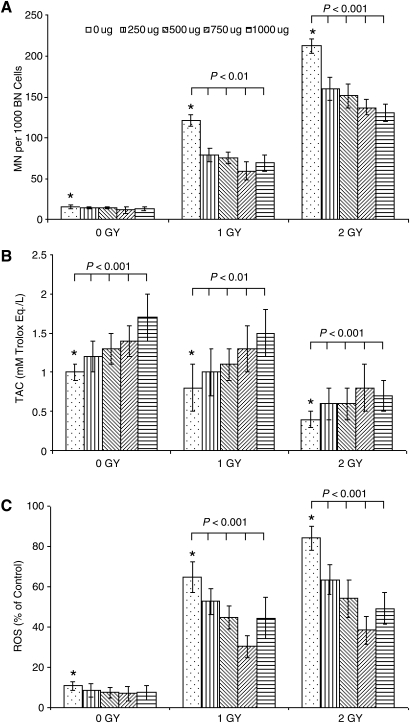
Figure 1B illustrates the variations of intracellular TAC levels in PBL obtained from 10 healthy individuals. Before 137Cs irradiation and in the absence of NAGE in the PBL culture medium, the baseline TAC level in PBL was 1.0 ± 0.1. However, it increased in PBL before irradiation with increments in NAGE concentration (250–1000 μg mL−1) in a concentration-dependent manner (p < 0.001). In contrast, IR exposure of PBL results in a decline in the intracellular TAC level in PBL. After 137Cs exposure (1 Gy and 2 Gy) of PBL, baseline TAC levels in PBL decreased with increasing radiation dose (p < 0.001). When NAGE was applied to the culture medium 90 minutes after radiation exposure, as compared with radiation alone (1 Gy and 2 Gy), TAC levels in irradiated PBL increased significantly (p < 0.01).
FIG. 1.
Effect of North American ginseng extract (μg/mL) applied at 90 minutes postirradiation of PBL obtained from 10 individuals on (A) 137Cs-induced micronuclei (MN) yields in peripheral blood lymphocytes (PBL); BN, binucleated; (B) intracellular total antioxidant capacity (TAC) levels (mmol/L Trolox Eq/L) in PBL before and after 137Cs irradiation; and (C) intracellular reactive oxygen species (ROS) levels (% of control) in PBL before and after 137Cs irradiation. Each bar represents the mean ± standard error of the mean of duplicate independent measurements pooled from each individual compared to their respective irradiated controls (*p < 0.001).
Effect of NAGE on intracellular ROS status (% of control) in PBL before and 90 minutes after 137Cs irradiation (Fig. 1C)
Before irradiation and in the absence of NAGE in the PBL culture medium, baseline fluorescent ROS level in PBL obtained from 10 healthy individuals was 11.1 ± 2.2. IR exposure of PBL results in an increase in the intracellular ROS level in PBL. The ROS level in irradiated PBL increased significantly with radiation dose to 62.5 ± 6.6 after 1 Gy and 85.3 ± 6.6 after 2 Gy exposure. However, when NAGE was applied to culture medium 90 minutes postexposure, the intracellular ROS level in irradiated PBL decreased significantly with the NAGE concentration (p < 0.001).
Discussion
In this ex vivo study of PBL obtained from 40 healthy human subjects, we have demonstrated the potential of a standardized NAGE and WR-1065 to modulate 137Cs-induced oxidative stress in PBL at 90 minutes after exposure, thereby indicating their postexposure radioprotective effect. The cell membrane of PBL has very high phospholipid content, rendering PBL vulnerable to oxidative damage.7 MN formation in PBL is a well-established biomarker for radiation-induced damage and free-radical impacts.22 We irradiated PBL with 1–2 Gy, because this dose range induces significant DNA damage, without the possibility of instant killing or causing selective interphase cell death.
The radioprotective effect and the antioxidative potentials of ginseng are no longer novel findings;15,18,23,24 nevertheless, to the best of our knowledge, no published data have ever been used to analyze the effect of NAGE on intracellular oxidant–antioxidant homeostasis in human PBL after irradiation. We discovered three interesting findings in this study. First, at 90 minutes postirradiation, both NAGE and WR-1065 significantly reduced the 137Cs-induced MN yields in a concentration-dependent manner (Tables 1 and 2). This relatively long postirradiation protective window of the two agents could be of potential clinical interest if it is upheld in in vivo studies.
Second, we also found that at 90 minutes postirradiation, the maximum reduction rates by NAGE (750 μg mL−1) and WR (3 mmol/L) of MN yields in PBL were 50.7% and 52% after 1 Gy and 35.9% and 33.4% after 2 Gy irradiation, respectively (Table 1). These results suggest that the potential of NAGE against radiation-induced MN production in PBL is comparable to that of WR-1065. In addition, at concentrations up to 1000 μg mL−1 (NAGE) or 3 mmol/L (WR-1065), respectively, no modulation of MN yield was found in PBL before irradiation (Table 1), indicating that this protection is accomplished without apparent genomic toxicity in PBL.
Third, we further observed in PBL obtained from 10 human subjects that the rise in 137Cs-induced MN yields in PBL is paralleled by the ROS level at the time of irradiation in a radiation dose-dependent manner (p = 0.03–0.005), but is inversely correlated with the reduction of TAC (p = 0.005–0.001) (Fig. 1A–C). This finding suggests that the existing endogenous antioxidants in PBL were not able to counteract the 137Cs-induced oxidative stress, and thus the increased MN production was a function of ROS accumulation and DNA damage. However, with the application of NAGE to the culture medium (250–1000 μg mL−1) at 90 minutes postirradiation, the observed significant increase in intracellular TAC level and concomitant decrease of both MN yield and ROS level strongly indicate a restoration of antioxidant capacity in PBL by NAGE treatment.
Both ginseng and WR-1065 confer radioprotection by scavenging IR-induced ROS. However, the exact mechanism underlying the 90 minutes postexposure radioprotective effect of NAGE is unclear. It may be related to the upregulation of antioxidant enzymes induced by NAGE at the level of gene expression 90 minutes postexposure.8 As demonstrated in vitro,19,25,26 the delayed radioprotection of WR-1065 is associated with the effect of manganese superoxide dismutase (SOD2). Since the molecular components of ginseng responsible for this scavenging action are ginsenosides,7,8 the radioprotective potential of ginseng is likely directly related to its ginsenoside content, which is quite high in our NAGE formulation (11.7%). Ginsenosides in NAGE are capable of intercalating in the plasma membrane, leading to changes in membrane fluidity and eliciting a cellular response to IR-induced cytotoxic stress.27 In this study, we found that the application of NAGE to the culture medium at 90 minutes post 137Cs exposure significantly increased intracellular TAC levels and was accompanied by a significant decrease in both ROS and MN yields in PBL (Fig. 1). Under our experimental design, the intracellular TAC in PBL represents the cumulative antioxidant capacity, including both NAGE-derived antioxidants and those of endogenous origin. Our findings suggest that (1) the lipid-soluble and water-soluble antioxidant of NAGE permeates into PBL and suppresses 137Cs-induced MN and ROS via OH radical scavenging; and (2) this action could be occurring directly through free-radical scavenging or indirectly through upregulation of antioxidant enzymes.10,28 Based on our findings concerning antioxidant activity at the cellular level, it appears that the postexposure protection of NAGE may be due either to its potential for modulating the redox homeostasis or for boosting the intracellular antioxidant defense system in human PBL.
Our findings are in agreement with the belief that supplementation of antioxidants could inhibit the ROS-induced DNA damage in human PBL.13 However, our results were generated from ex vivo experiments after up to 2 Gy irradiation of PBL. Also, the antioxidant capacities of NAGE measured ex vivo may not be consistent with their effects in vivo. For instance, after oral ingestion of ginseng, both gastric digestion and hydrolysis by intestinal microflora lead to the biotransformation of ginsenosides.29 Subsequently, ginsenoside metabolites can be absorbed into the blood, whence they can exert their active pharmacological effects. Since intestinal bacteria are sensitive to host conditions, the individual physiologic variations in bacteria-hydrolyzing potentials may affect the radioprotective efficiency of NAGE. Thus, the clinical relevance of our findings that the concentration of NAGE at 750 μg mL−1 reduced both MN yields and ROS levels in human PBL ex vivo (Table 1, Fig. 1) would be hard to predict. We are planning to answer these questions in a future research project.
Currently, amifostine (WR-2721) is the only radioprotective agent approved by the U.S. Food and Drug Administration for cancer patients undergoing radiotherapy. However, the limitations associated with amifostine include its inherent toxicity, high cost, intravenous administration route to be applied 15 minutes before radiotherapy, and possible tumor protection.1,2,30 In contrast, NAGE is a relatively nontoxic, inexpensive natural product with broad medicinal and pharmacological activities, including antitumor activity that can be orally administered under emergency conditions or as a dietary supplement. We believe, therefore, that NAGE is a candidate eminently suited for addition to the list of potential radioprotectors.
Acknowledgments
This research was supported by National Institutes of Health/National Center for Complementary and Alternative Medicine grant R21-AT002639-02. We are grateful to Miriam Wildeman, M.D. for her dedication in editing and proofreading of the manuscript; and to Mark Harrington for his technical support. Our sincere appreciation also extends to the healthy volunteers who gave their blood to make this study possible, and to the reviewers for their constructive suggestions.
Disclosure Statement
No competing financial interests exist.
References
- 1.Moulder JE. Report on an interagency workshop on the radiobiology of nuclear terrorism. Molecular and cellular biology of moderate dose (1–10 Sv) radiation and potential mechanisms of radiation protection (Bethesda, Maryland, December 17–18, 2001) Radiat Res. 2002;158:118–124. doi: 10.1667/0033-7587(2002)158[0118:roaiwo]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 2.Stone HB. Moulder JE. Coleman CN, et al. Models for evaluating agents intended for the prophylaxis, mitigation and treatment of radiation injuries report of an NCI workshop, December 3–4, 2003. Radiat Res. 2004;162:711–728. doi: 10.1667/rr3276. [DOI] [PubMed] [Google Scholar]
- 3.Pellmar TC. Rockwell S. Radiological/Nuclear Threat Countermeasures Working Group. Priority list of research areas for radiological nuclear threat countermeasures. Radiat Res. 2005;163:115–123. doi: 10.1667/rr3283. [DOI] [PubMed] [Google Scholar]
- 4.Weiss JF. Landauer MR. Protection against ionizing radiation by antioxidant nutrients and phytochemicals. Toxicol. 2003;189:1–20. doi: 10.1016/s0300-483x(03)00149-5. [DOI] [PubMed] [Google Scholar]
- 5.Yi SW. Sull JW. Hong JS, et al. Association between ginseng intake and mortality: Kangwha cohort study. TJ Altern Complement Med. 2009;15:921–928. doi: 10.1089/acm.2008.0296. [DOI] [PubMed] [Google Scholar]
- 6.Kitts DD. Hu C. Efficacy and safety of ginseng. Pub Health Nutri. 2000a;4A:473–485. doi: 10.1017/s1368980000000550. [DOI] [PubMed] [Google Scholar]
- 7.Block KI. Mead MN. Immune system effects of Echinacea, ginseng, and astragals: A review. Interg Cancer Therap. 2003;2:247–267. doi: 10.1177/1534735403256419. [DOI] [PubMed] [Google Scholar]
- 8.Lee TK. Johnke RM. Allison RR, et al. The radioprotective potential of ginseng. Mutagenesis. 2005;20:237–243. doi: 10.1093/mutage/gei041. [DOI] [PubMed] [Google Scholar]
- 9.Lee SH. Ahn YM. Ahn SY, et al. Interaction between Warfarin and Panax ginseng in ischemic stroke patients. TJ Altern Complement Med. 2008;14:715–721. doi: 10.1089/acm.2007.0799. [DOI] [PubMed] [Google Scholar]
- 10.Kitts DD. Wijewickreme AN. Hu C. Antioxidant properties of a North American ginseng extract. Molec Cell Biochem. 2000b;203:1–10. doi: 10.1023/a:1007078414639. [DOI] [PubMed] [Google Scholar]
- 11.Lemon JA. Rollo CD. Boreham DR. Elevated DNA damage in a mouse model of oxidative stress: Impacts of ionizing radiation and a protective dietary supplement. Mutagenesis. 2008;23:473–482. doi: 10.1093/mutage/gen036. [DOI] [PubMed] [Google Scholar]
- 12.Møller P. Loft S. Interventions with antioxidants and nutrients in relation to oxidative DNA damage and repair. Mutat Res. 2004;551:79–89. doi: 10.1016/j.mrfmmm.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 13.Møller P. Loft S. Dietary antioxidants and beneficial effect on oxidatively damaged DNA. Free Rad Biol Med. 2006;41:388–415. doi: 10.1016/j.freeradbiomed.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Dusinska M. Collins AR. The comet assay in human biomonitoring: Gene-environment interactions. Mutagenesis. 2008;23:191–205. doi: 10.1093/mutage/gen007. [DOI] [PubMed] [Google Scholar]
- 15.Lee HJ. Kim SR. Kim JC, et al. In vivo radioprotective effect of Panax ginseng C.A. Meyer and identification of active ginsenosides. Phytother Res. 2006;20:392–395. doi: 10.1002/ptr.1867. [DOI] [PubMed] [Google Scholar]
- 16.Fenech M. Morley AA. Measurement of micronuclei in lymphocytes. Mutat Res. 1985;147:29–36. doi: 10.1016/0165-1161(85)90015-9. [DOI] [PubMed] [Google Scholar]
- 17.Fenech M. Denham J. Francis W. Micronuclei in cytokinesis-blocked lymphocytes of cancer patients following fractionated partial-body radiotherapy. Int J Radiat Biol. 1990;57:373–384. doi: 10.1080/09553009014552471. [DOI] [PubMed] [Google Scholar]
- 18.Lee TK. Allison RR. O'Brien KF, et al. Ginseng reduces the micronuclei yield in lymphocytes after irradiation. Mutat Res. 2004;557:75–84. doi: 10.1016/j.mrgentox.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Dziegielewski J. Baulch JE. Goetz W, et al. WR-1065, the active metabolite of amifostine, mitigates radiation-induced delayed genomic instability. Free Rad Biol Med. 2008;45:1674–1681. doi: 10.1016/j.freeradbiomed.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walker DM. Kajon AE. Torres SM, et al. WR1065 mitigates AZT-ddl-induced mutagenesis and inhibits viral replication. Environ Mol Mutagen. 2009;50:460–472. doi: 10.1002/em.20482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.SPSS. Base 10.0 Application Guide. SPSS Inc. 2001 [Google Scholar]
- 22.Greenrod W. Fenech M. The principal phenolic and alcoholic components of wine protect human lymphocytes against hydrogen peroxide- and ionizing radiation-induced DNA damage in vitro. Mutagenesis. 2003;18:119–126. doi: 10.1093/mutage/18.2.119. [DOI] [PubMed] [Google Scholar]
- 23.Lee LS. Wise SD. Chan C, et al. Possible differential induction of phase 2 enzyme and antioxidant pathways by American ginseng, Panax quinquefolius. J Clin Pharmacol. 2008;48:599–609. doi: 10.1177/0091270008314252. [DOI] [PubMed] [Google Scholar]
- 24.Kim TK. Yoo KM. Lee JW, et al. Protective effect of steamed American ginseng (Panax quinquefolius L.) on V79-4 cells induced by oxidative stress. J Ethnopharmacol. 2007;111:443–450. doi: 10.1016/j.jep.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Murley JS. Nantajit D. Baker KL, et al. Maintenance of manganese superoxide dismutase (SOD2)-mediated delayed radioprotection induced by repeated administration of the free thiol form of amifostine. Radiat Res. 2008;169:495–505. doi: 10.1667/RR1194.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murley JS. Kataoka Y. Weydert CJ, et al. Delayed cytoprotection after enhancement of Sod2 (MnSOD) gene expression in SA-NH mouse sarcoma cells exposed to WR-1065, the active metabolite of amifostine. Radiat Res. 2002;158:101–109. doi: 10.1667/0033-7587(2002)158[0101:dcaeos]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 27.Attele AS. Wu JA. Yuan CS. Ginseng pharmacology. Biochem Pharmacol. 1999;58:1685–1693. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 28.Xie JT. Shao ZH. Vanden Hoek TL, et al. Antioxidant effects of ginsenoside Re in cardiomyocytes. Eur J Pharmacol. 2006;532:201–207. doi: 10.1016/j.ejphar.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 29.Hasegawa H. Proof of the mysterious efficacy of ginseng: Basic and clinical trials: Metabolic activation of ginsenoside. Deglycosylation by intestinal bacteria and esterification with fatty acid. J Pharmacol Sci. 2004;95:153–157. doi: 10.1254/jphs.fmj04001x4. [DOI] [PubMed] [Google Scholar]
- 30.Grdina DJ. Murley JS. Kataoka Y, et al. Radioprotectors: Current status and new directions. Radiat Res. 2005;163:704–705. [PubMed] [Google Scholar]