Abstract
Klinefelter (47, XXY) syndrome (KS), the most common form of sex-chromosomal aneuploidy, is characterized by physical, endocrinologic, and reproductive abnormalities. Individuals with KS also exhibit a cognitive/behavioral phenotype characterized by language and language-based learning disabilities and executive and attentional dysfunction in the setting of normal general intelligence. The underlying neurobiologic mechanisms are just now beginning to be elucidated through structural and functional neuroimaging. Here, we review the literature of structural and functional neural findings in KS identified by neuroimaging and present preliminary results from a functional magnetic resonance imaging study examining brain activity during a verb generation task in KS.
Keywords: Klinefelter syndrome; XXY; MRI; neuroimage; Magnetic Resonance Imaging; Tomography, Emission-Computed; Tomography, Emission-Computed, Single-Photon
The 47, XXY karyotype is the most common form of sex-chromosomal aneuploidy, with an estimated prevalence in the general population between one in 500 and one in 1000 [Lanfranco et al., 2004]. The clinical manifestations associated with this genotype are referred to as Klinefelter syndrome (KS). Characteristic physical and medical attributes of infertility, small testes, gynecomastia, and tall body habitus with long legs are thought to result from testosterone deficiency [Wattendorf and Muenke, 2005]. Individuals with KS also exhibit a characteristic pattern of cognitive and behavioral dysfunction [Geschwind et al., 2000], which is reviewed in depth in another article in this issue [Boada et al., 2009]. In brief, the cognitive/behavioral phenotype includes language and language-based learning disabilities (similar to that observed in cytogenetically-normal children with dyslexia) as well as executive and attentional dysfunction [Lanfranco et al., 2004]. General intelligence appears to be within the typical range in men with KS, though global developmental delays in childhood include delayed language and (gross and fine) motor development [Wattendorf and Muenke, 2005].
Though a characteristic KS cognitive phenotype is now recognized, relatively little is known about the underlying neural mechanisms [Geschwind et al., 2000; Reiss et al., 2000; Geschwind and Dykens, 2004; Giedd et al., 2007; van Rijn et al., 2008]. In this article, we review the current literature of studies beyond case-report status that used structural and functional neuroimaging techniques to further understanding of the neurobiologic mechanisms underlying cognitive and behavioral differences in KS. We begin by presenting studies of volumetric differences in KS, organized by neuroanatomic level of the brain findings, including (1) widespread brain differences and differences in ventricular size; (2) cerebral lobar and cerebellar differences; (3) differences in intra-individual asymmetries; and (4) regional differences. This is followed by a section discussing findings from studies of non-volumetric structural differences. We then review the limited literature of functional neuroimaging studies and discuss preliminary results from an ongoing functional neuroimaging study focused on elucidating neural mechanisms underlying language in KS.
STRUCTURAL NEUROIMAGING
Traditional volumetric neuroimaging techniques quantify brain structure volumes on magnetic resonance imaging (MRI) using manually-traced neuroanatomical regions-of-interest (ROI). These techniques are subject to biases introduced by the variable subjective and objective criteria used by different individuals and different laboratories to delineate structural boundaries. Multiple automated, whole-brain MRI analysis technologies have been developed and become increasingly available over the last decade, including voxel-based morphometry (VBM) methods and the LowD technique. These techniques avoid the biases resulting from subjectivity and a priori determination of structures of interest mentioned above. VBM techniques [Ashburner and Friston, 2000] seek structural differences by comparing each voxel (each of the thousands of three-dimensional volume elements that comprise an image) of whole-brain images. The LowD technique [Rezaie et al., 2009] extracts gray matter (GM), white matter (WM), and total tissue (TT; gray plus white) volumes with a voxel-based approach, similar in its initial steps to VBM. In LowD, voxel-wise volumes are then integrated into a two-dimensional sagittal brain map of volume asymmetry for each subject. Finally, one-dimensional coronal slice profiles are created, representing degree of volumetric asymmetry for each coronal slice with a single value that can be presented as a graph along the anterior-posterior axis of the brain.
The neuroimaging analysis technique used for each KS imaging study is listed in Table 1, to enable consideration of the potential biases and assumptions underlying the results. We have chosen to include many study details in Table 1, opting to maximize critical information included about each study – at the expense of table brevity and optimized table format – with the goal of providing readers a single source for obtaining relevant information from the KS literature.
Table 1.
Structural Neuroimaging Studies
| Study | Subjects* | Method s |
Total Brain Volume (TBV) Measures terms used (definition provided) |
Regional Volume Correction |
Findings** | |
|---|---|---|---|---|---|---|
| TBV | Other | |||||
| Warwick 1999 | 10 KS (21.8±4.2) 26 CON (21.5±1.3) whole cohort (ages 16–28) |
T1 MRI, ROI |
“whole brain volume” (not defined; unclear if included Brstm, ventricles, or Crblm) |
Corrected as proportion of regional volume to TBV. For significantly abnormal brain regions, corrected with ANCOVA adjusting for TBV and height. |
KS<CON | Lateral ventricle (as proportion of TBV & ANCOVA-corr): KS>CON. AHC, “prefrontal” & Temp lobes, caudate, lentiform nuclei, thalami, 3rd & 4th ventricle: KS=CON. Regional volume asymmetries: none identified. |
| Patwardhan 2000 | 10 KS (27.3±3.0 [24–32]; 10 Nonmsc; 5 KS+T, 5 KS–T) 10 CON (26.8±3.3) |
T1 MRI, ROI |
“whole brain tissue volume” (TT; unclear if included Brstm or Crblm) “total cerebral gray volume” (GM; unclear if included Brstm or Crblm) |
Uncorrected & corrected with ANCOVA adjusting for GM TBV (Temp lobes) or for Temp lobe GM (STG). |
KS=CON (TT & GM) |
Ventricles: KS=CON. Tot Temp GM & L Temp GM: KS<CON. Tot Temp GM & L Temp GM (corr): KS=CON. Tot STG GM, R STG GM, L STG GM, R STG TT: KS<CON. Tot STG GM, R STG GM, L STG GM, R STG TT (corr): KS=CON. Testosterone Effects Tot Temp GM & L Temp GM, total STG GM, R STG GM, L STG GM, R STG TT: KS– T<CON, KS+T=CON. L Temp GM: KS–T<KS+T. Tot Temp GM (corr): KS–T=KS+T. L Temp GM (corr): KS–T<CON, KS–T<KS+T. |
| Patwardhan 2002 | 10 KS (27.3±3.0) 10 CON (27.3±3.5) |
T1 MRI, ROI |
“whole brain tissue volume” and “total brain volume” (TT; unclear if included Brstm or Crblm; used synonymously) |
Corrected with ANCOVA adjusting for TBV. |
KS=CON | HC (corr): KS=CON Amygdala (corr): KS<CON. |
| Rose 2004 | 20 KS (15.1±4.6) 40 CON (“age- matched”) |
T1 MRI, ROI |
No TBV. | No TBV correction. | None | L HC & R HC: KS<CON L amygdala: KS<CON R amygdala: KS=CON. |
|
Shen 2004; Giedd 2006 |
34 KS (12.6±4.3 [5.3–19.2]; 32 Nonmsc, 1 Msc, 1 Y-to-X translocation; age≥13: 10 KS+T & 8 KS–T, age≤12: 16 KS–T) 62 CON (12.9±4.3 [5.3–20.8]) |
T1 MRI, VBM |
“total brain tissue volumes of GM, WM, and ventricular CSF” (TBV for GM & for WM; unclear if included Brstm or Crblm) |
No TBV correction. | KS<CON (GM & WM) |
Ventricles: KS>CON Regional GM (insula (L), Temp gyri (B), amygdala (R), HC (L), cingulate, and Occ gyri (B)): KS<CON. Regional WM (R Prtl lobe): KS<CON. |
| DeLisi 2005 | 11 KS (34.6±12 [19– 54]; 6 Nonmsc, 5 Msc; 9 KS+T) 11 CON (36.5±13 [20–58]; 4 CON+T) |
T1 MRI, ROI & DTI voxel- wise analysis |
“total intracranial contents” (all voxels not removed during manual image-stripping of extracranial tissue) |
Corrected with ANCOVA adjusting for TBV & age. |
KS<CON | Ventricles (corr): KS=CON B Frnt & Temp lobe GM, STG GM, and posterior STG GM (corr): KS<CON. Anterior STG GM, AHC, & ventricles (corr): KS=CON Rightward STG asymmetry: KS<CON (non-significant trend; KS slightly leftward). Other asymmetries (lobes, ventricles, AHC): KS=CON. Correlations of volumes (in KS): All ROIs: no correlations with neuropsychologic measures scores in KS. Fractional anisotropy (DTI) L PLIC, L arcuate bundle, R & L AC: KS<CON. |
| Itti 2006 | 18 KS (35.8±11.8; 10 KS+T; 15 RH) 20 CON (32.3±11.3; 15 RH) |
T1 MRI, ROI; raw and CSF- corrected volumes† (p<0.01 for Multiple comparisons) |
“total-brain volume” (not defined, unclear if included Brstm or Crblm; ventricles included in raw volumes and excluded in CSF- corrected volumes†) |
No TBV correction. |
KS=CON (raw & atrophy- corrected) |
Lateral ventricles: KS>CON. B Crblm hemispheres: KS<CON. B Frnt, Temp, & Prtl lobes (GM & TT): KS=CON B HC: KS=CON. In RH subjects B Crblm hemisphere volumes (“atrophy-corrected”): KS<CON. L Temp lobe TT (“atrophy-corrected”): KS<CON. Correlations of volumes Lateral ventricles: inverse correlation (in entire cohort) with “verbal processing speed”, “nonverbal processing speed”, and “verbal executive function”. L Temp lobe TT: positive correlation (RH subjects only) with “language” scores and “verbal processing speed”. Testosterone Effects No significant effect on volumes (see text for details). |
| Giedd 2007 | 42 KS (12.8±5.0 [5.3-26]; 42 Nonmsc; age≥14: 14 KS+T, age≤12: 24 KS–T, age=13: 2 KS+T & 2 KS–T) 87 CON (12.7±5.0 [5.2–25.5]) |
T1 MRI, ROI & automated Cortical thickness |
“total cerebral volume” (GM, WM, & TT; unclear if included Brstm or Crblm; data indicates that ventricles not included) |
Uncorrected and corrected with ANCOVA adjusting for TBV (and SES & IQ) |
KS<CON (GM & TT) KS=CON (WM) |
Lateral ventricles (uncorr & corr): KS>CON. Frnt & Temp GM & WM, Par GM, Crblm: KS<CON. Prtl WM: KS=CON. Frnt GM & Temp GM (corr): KS<CON. Frnt WM, Temp WM, Prtl GM, Crblm (corr): KS=CON. Prtl WM (corr): KS>CON. Caudate (uncorr & corr): KS<CON. Cortical thickness (regional) B Temp lobes, L inferior Prtl lobe, L inferior Frnt area, and L motor strip: KS<CON. |
| Rezaie 2009 | 10 KS (35.4±12.7; 5 Nonmsc, 5 Msc; 8 KS+T) 10 CON (35.7±13.1; 4 CON+T) |
T1 MRI, LowD (automated) |
“hemispheric gray, white and total matter volumes” (extracted GM, WM, & TT of Cerebral hemispheres) |
No TBV correction. | KS<CON (B Hemispheric WM) KS=CON (B Hemispheric GM & TT) |
Hemispheric TBV Asymmetry R>L, KS=CON. 2D column map analysis Brain torque: KS=CON. Regional asymmetries (GM, WM, & TT): KS=CON. Lobar analysis of 2D column map analysis Torque index (TT): KS 13.8±6.1, CON 22.6±9.7, statistical analysis not reported. Frnt lobe volume (GM, WM, & TT) Rightward asymmetry: KS<CON (i.e. KS more symmetric), non- significant trend. 1D analysis WM cluster (mid-Frnt lobes), rightward asymmetry: KS<CON. |
Ages given as (mean age in years ± SD [range in years, if available]); # of nonmosaic and # and type of mosaic listed when available
Findings refer to volumes unless otherwise stated. Uncorrected for TBV unless specified.
TBV and all regional volumes (except ventricles) analyzed as “raw” volumes in the whole cohort and as “CSF-corrected” volumes amongst RH subjects only [Itti et al., 2006; Itti, personal communication, 2009]
AC=anterior cingulate; AHC=amygdala-hippocampal complex; ANCOVA=analysis of covariance; B=bilateral; Brstm=brainstem; Crblm=cerebellum; CON=control; CON+T=testosterone-treated control subject; corr=corrected for TBV; Frnt=Frontal; GM=gray matter; HC=hippocampus; KS=Klinefelter syndrome; KS+T= testosterone-treated KS subject; KS–T=testosterone-naïve KS subject; L=Left; Msc=mosaic; Nonmsc=nonmosaic; Occ=Occipital; PLIC = posterior limb of internal capsule; Prtl=Parietal; R=Right; RH=right-handed; ROI=region-of-interest analysis; SES=socioeconomic status; T1 MRI=T1-weighted magnetic resonance imaging; Temp=Temporal; TT=total tissue (gray plus white) matter; Tot=total (right and left combined); VBM=voxel based morphometry analysis; WM=white matter
Widespread Brain Differences and Differences in Ventricular Size
Widespread brain volume differences are commonly examined in neuroanatomical studies of neurodevelopmental disorders. Different investigators examine various overlapping combinations of structures considered together to constitute a measure of overall brain volume. These measures typically incorporate cerebral cortex and subcortical WM, but may or may not include subcortical/deep gray nuclei, cerebellum, brainstem, ventricles, or even other intracranial tissues. Overall brain measures are also referred to with a variety of terms, including “total brain volume,” “total cerebral volume,” “total tissue volume,” and “total intracranial contents.” Unfortunately, studies in KS vary not only in the terminology used, but also in whether a definition is provided for the measure examined. To enable us to speak about all the relevant studies, we will use the acronym TBV (“total brain volume”) throughout this paper to refer to measures of overall brain volume in all studies (whether a study used this term or not) and in speaking generally about changes involving the brain as a whole. When relevant, we will specify differences in the measure used. Further, Table 1 includes the actual term used by each study for their measure of overall brain volume and the definition, if provided.
Warwick et al. [1999] first published a study on TBV differences in KS comparing structural MRI of the brain in 10 men with KS to 26 control men. They found reduced TBV in KS compared to controls, as well as greater size of the lateral ventricles. Reduced TBV in KS has since been replicated in several studies [Shen et al., 2004; DeLisi et al., 2005; Giedd et al., 2007], though other investigators have found no difference [Patwardhan et al., 2000; Patwardhan et al., 2002] or only a non-significant trend for reduced TBV [Itti et al., 2006]. Conflicting results between these studies have multiple potential causes. First, the variable terminology and definitions for TBV measures prevent clear comparisons between studies. Demographic and methodological differences also likely contribute to conflicting results (and will be relevant for comparisons of brain volumes at other neuroanatomic levels as well). As in all neurodevelopmental studies of brain morphology, differences in subject age, study size, neuroimaging acquisition techniques, and neuroimaging analysis methodologies all influence results. (See Table 1 for the demographic and methodological characteristics of each structural MRI study.) Particular to research on KS, these studies also differ in the proportion of subjects with karyotypic mosaicism and the degree and type of mosaicism present (not always reported). Finally, most previous studies differ in their inclusion and reporting of subjects undergoing testosterone supplementation – a standard treatment, typically initiated in early adolescence in KS [Reiss et al., 2000; Lanfranco et al., 2004; Wattendorf and Muenke, 2005], that may also influence study results.
Giedd, Shen, and colleagues’ MRI VBM analysis [Shen et al., 2004; Giedd et al., 2006 (these studies included the same KS subjects, hence we cite only Shen et al., 2004, when referencing this data throughout the remainder of this review)] of a cohort of 34 KS boys and young men and 62 matched normal controls suggested that smaller TBV in KS is due to reductions in both the GM and WM portions of TBV. In a subsequent ROI-based study, Giedd et al. [2007] examined 42 KS boys and young men and 87 controls, including the subset of KS subjects included in the prior study [Shen et al., 2004]. Giedd and colleagues [2007] found the TT and GM components of TBV to be smaller in KS, but no significant difference in the WM component (though this discrepancy with their prior findings was not discussed by the authors). In contrast, a recent study by Rezaie et al. [2009] using a fully automated approach to TBV found relatively smaller bilateral hemispheric WM, but not GM, volumes in men with KS. Conflicting results regarding GM and WM contributions to smaller TBV may result from variability in study design, subject characteristics (e.g. inclusion of adult men only versus primarily boys with some young adults) and analysis techniques (see Table 1) as discussed above.
Hypothesizing that neuroanatomic differences could be related to the testosterone deficiency that occurs in KS, some investigators [Patwardhan et al., 2000; Itti et al., 2006] have examined the association between differences in whole brain morphology and use of testosterone supplementation in KS. Patwardhan et al. [2000] found no significant differences in TBV (TT or GM components) between five testosterone-treated and five testosterone-naïve XXY men, a finding later supported with a larger sample size (10 testosterone-treated, eight testosterone-naïve) by Itti et al. [2006] (though regional differences have been identified, as discussed below).
Warwick and colleagues’ [1999] early finding of enlarged lateral ventricular volumes (both as a proportion of TBV and after adjusting for TBV using analysis of covariance) has also been of continued interest. Like Warwick et al. [1999], Giedd and colleagues have identified larger ventricular volumes in the setting of reduced TBV (both unadjusted [Shen et al., 2004; Giedd et al., 2007] and after adjustment for TBV [Giedd et al., 2007]). Larger ventricles (uncorrected for TBV) were also found by one group that did not find significantly smaller TBV [Itti et al., 2006], while no difference in ventricular volume was found in one study that did find smaller TBV (and adjusted for TBV) [DeLisi et al., 2005] and one study that did not find smaller TBV (and did not adjust for TBV) [Patwardhan et al., 2000]. Again, conflicting findings between these studies likely result from the variability of study design and subject characteristics (see Table 1), with added variability introduced by whether ventricular volume measurements were adjusted for TBV.
Lobar and Cerebellar Differences
Examination of cerebral lobar and cerebellar volumes has yielded both further insights and further discrepancies regarding neuroanatomical differences in males with KS. Though Warwick et al. [1999] identified significantly smaller TBV in KS, they found no differences in the TT volumes of bilateral temporal or “prefrontal” lobes (as the proportion of each lobar volume to TBV; parietal and occipital lobe volumes were not included in the study). Similarly, Itti et al. [2006] found no significant differences between bilateral temporal, frontal, or parietal lobe TT or GM volumes (uncorrected for TBV) in KS and controls.
Several other studies, however, have identified decreases in various lobar volumes in KS [Patwardhan et al., 2000; DeLisi et al., 2005; Giedd et al., 2007]. Patwardhan et al. [2000] found smaller temporal lobe GM volume (but not TT volume) in KS limited to the left side. However, this size difference did not persist after adjusting for TBV, suggesting that KS subjects do not have a disproportionate amount of reduced GM in the left temporal lobe compared to the rest of their brains. In contrast, DeLisi et al. [2005] found significantly smaller GM volumes in both left and right frontal and temporal lobes in eleven KS subjects compared to eleven controls. Additionally, these diminished lobar volumes remained significantly decreased when controlling for TBV (and age).
In a larger study focusing on a younger KS population, Giedd and colleagues [2007] found frontal and temporal lobe GM and WM volumes and parietal lobe GM volumes to be smaller in KS males than controls (using p≤0.05; only GM volumes were significant after Bonferroni correction) while parietal WM volumes were comparable. Of these differences in lobar tissue volumes, however, only decreased temporal and frontal GM volumes remained after adjusting for decreased TBV (as well as the additional covariates of socioeconomic status and IQ). In addition, after these adjustments, parietal lobe WM was noted to be disproportionately larger in KS subjects. These authors [Giedd et al., 2007] interpreted the findings of relatively smaller temporal and frontal GM volumes as consistent with the cognitive phenotype of KS, specifically impairments in language function, known be mediated by neural systems of the temporal and frontal lobes. In addition, they noted the similarity between their findings of smaller frontal lobe GM (and caudate, as seen below) volumes and MRI findings in attention-deficit hyperactivity disorder [Seidman et al., 2005], with which KS shares features of attentional and executive dysfunction. The authors also highlighted the possible relationship between “relative sparing of GM and larger WM volume in the parietal region” and the normal or above average performance on certain tasks (non-motor perceptual tasks and nonverbal visual memory) that are thought to rely on intact parietal functioning.
Both structural neuroimaging studies [Itti et al., 2006; Giedd et al., 2007] that examined the cerebellum as an ROI found reductions of bilateral cerebellar hemisphere volumes in men with KS. For Giedd et al. [2007], this difference did not persist after adjusting for TBV. No TBV correction was performed by Itti et al. [2006].
Taken together, these studies suggest that frontal and temporal lobe GM volumes, as well as cerebellar hemispheres, may be smaller in KS than in cytogenetically-typical males. Whether this is out of proportion to a possible overall decrease in TBV is less clear. Further, whether the lobar differences are more specific to the left side (specifically in the temporal lobe) as suggested by Patwardhan et al. [2000] remains uncertain. Differences in the temporal and frontal lobes of individuals with KS are certainly in keeping with the observed cognitive phenotype of KS, which includes language and executive dysfunction. Additionally, left-lateralized structural differences might be expected because language function is left-lateralized in a great majority of individuals [Toga and Thompson, 2003] and is known to be a dysfunctional domain of cognition in KS. Cerebellar differences might also be expected given the tendency to delayed motor development, as well as the increasingly recognized role of the cerebellum in cognition and language [Schmahmann and Caplan, 2006; De Smet et al., 2007].
Morphologic brain asymmetries, typically seen in all lobes of the human brain, are most evident and most prevalent amongst right-handers [Toga and Thompson, 2003]. Therefore, different distributions of subject handedness may also lead to discrepancies between study findings, especially in those studies that examine right- and left-sided volumes individually. Though Itti et al. [2006] found no significant differences in temporal lobe GM volume in their entire cohort (see above), when right-handed subjects were examined separately (KS, n=15; controls, n=15), a significant reduction of left temporal lobe TT (but not GM) volume was found; no significant differences were found between left-handed KS subjects (n=3) and typical controls (n=5). However, the right-handed subgroup analysis also differed from the whole-cohort analysis in that the former included only “cerebrospinal fluid (CSF)-corrected” volumes (automated subtraction of residual CSF, including ventricles, from the manually-traced ROIs [Itti et al., 2006; Itti, personal communication, 2009]), while the latter included only “raw” volumes (incorporating some CSF, including ventricles). Thus, caution must be used in drawing conclusions from this study about the effect of handedness.
The potential role of testosterone supplementation on structural neuroanatomy in KS at the lobar and cerebellar levels was examined in two of the above retrospective-design studies. Patwardhan et al. [2000] found that the smaller total (i.e., combined right and left) and left temporal GM volumes in KS subjects could be explained specifically by testosterone-naïve KS subjects who had significantly smaller total (left and right combined) and left temporal GM volumes (uncorrected) than controls, while testosterone-treated KS subjects were not significantly different from controls. Testosterone-treated subjects did, however, have significantly larger volumes of the left temporal lobe GM than testosterone-naïve subjects. After correction for TBV, left (but not total) temporal lobe GM volume in testosterone-naïve subjects remained significantly smaller than controls. In contrast, Itti et al. [2006] found no significant effect of testosterone therapy on MRI lobar volumes, though they did report a non-significant trend (no statistics provided) for smaller left cerebellar hemisphere volume in testosterone-treated right-handed KS subjects.
Volumetric Asymmetries
Another topic of interest in the study of KS is that of intra-individual brain asymmetries. An important concept in the study of brain asymmetries is brain (or Yakovlevian) torque [Toga and Thompson, 2003]. Brain torque describes the typical overall asymmetry of the human brain, with an anteriorly-protruded and wider right frontal lobe and a posteriorly-protruded and wider left occipital lobe. Another typical asymmetry of the human brain is the leftward volume asymmetry of perisylvian, language-related structures, most apparent and common in right-handed individuals [Toga and Thompson, 2003]. Cytogenetically-typical individuals with developmental language disorder or with dyslexia have been shown to exhibit alterations in perisylvian brain asymmetries compared to typical individuals [Semrud-Clikeman, 1997; Toga and Thompson, 2003; Herbert and Kenet, 2007; Leonard and Eckert, 2008]. Because of the language and language-based learning (e.g. reading) deficits seen in KS, and in light of findings from the few existing functional neuroimaging studies available (see below), atypicalities of structural brain asymmetries in KS may be anticipated.
The seminal study by Warwick et al. [1999] found no significant volume asymmetries of the lateral ventricles, “prefrontal” or temporal lobes, or amygdala-hippocampal complexes (AHC) in control men or those with KS. DeLisi et al. [2005] examined volume asymmetries of the same structures using a laterality index (left – right / 0.5*[left+right]). Both KS and controls exhibited asymmetries, but no differences in laterality index (i.e., degree of asymmetry) were found between KS and controls for frontal or temporal lobes, ventricles, or AHC. In the superior temporal gyrus (STG), a non-significant trend for less rightward asymmetry (specifically, slight leftward asymmetry) was seen in KS subjects. Whether their controls, who exhibited a rightward asymmetry, can be considered representative of the general population (where posterior STG, specifically the planum temporale, has a leftward asymmetry in most individuals [Toga and Thompson, 2003]) depends on whether this analysis was performed on anterior, posterior, or total (anterior plus posterior) STG, which was not specified.
Using the same cohort, Rezaie et al. [2009] examined the issue of intra-individual asymmetries with a variety of measures from the LowD technique. No differences in hemispheric brain asymmetry, brain torque (i.e. anterior rightward and posterior leftward asymmetry), or another measure of regional asymmetry using column-map analysis were identified between the groups in GM, WM, and TT. Analysis of lobar volumes (approximated from the column-map data) did, however, reveal a non-significant trend (p=0.056) for decreased rightward frontal lobe asymmetry in GM, WM, and TT volumes in KS (i.e., more symmetric than controls). Further analysis of coronal slice asymmetry profiles identified relatively reduced rightward asymmetry (resulting from reduced right hemisphere WM) in KS subjects for a (one-dimensional) region of frontal lobe WM in coronal slices anterior to the genu of the corpus callosum (Talairach y coordinate range 35–41). In sum, Rezaie et al. [2009] identified no differences in asymmetry at the hemispheric level, but found a trend suggesting less rightward frontal lobe asymmetry, and evidence for a specific decrease in rightward WM asymmetry in the mid-frontal lobe (along the anterior-posterior axis of the brain).
Regional Differences in Volume
Amygdala-Hippocampal Complex (AHC)
Difficulties with social/emotional processing and abnormalities with auditory and verbal memory in KS [Geschwind et al., 2000; Geschwind and Dykens, 2004; Aleman et al., 2008] suggest possible dysfunction of the AHC. AHC structural differences have been identified in KS to varying degrees in various studies. Neither Warwick et al. [1999] nor DeLisi et al. [2005] found differences in the volumes of the entire AHC (adjusted for TBV) between individuals with KS and those without. Rather than measuring the AHC volume as a whole as in the previous two studies, Patwardhan et al. [2002] examined the volumes of the amygdalae and hippocampi (corrected for TBV) separately in men with KS compared to healthy adult male controls. They found reduced amygdalar volumes in KS, more comparable to those of women, while hippocampal volumes did not differ between the KS and control groups. Itti et al. [2006] also found no significant difference in the volumes of the hippocampal complex (unadjusted for TBV), though they reported a non-significant trend (no statistics provided) for smaller right-sided hippocampus in testosterone-treated right-handed KS subjects only. Also measuring amygdala and hippocampal volumes individually, Rose et al. [2004] found that KS men had smaller (uncorrected) volumes of the left amygdala, left hippocampus, and right hippocampus than controls. Though Patwardhan et al. [2002] suggested that more precise measurements of individual structures (amygdala and hippocampus) may explain the difference between their results and those that found no AHC differences, these last two studies [Rose et al., 2004; Itti et al., 2006] demonstrate that other factors (including neuroimaging methodologies and cohort factors) are likely also at play. It warrants consideration that the only ROI-based study [Rose et al., 2004] to find diminished volumes of the hippocampi (measured independent of the amygdalae) did not present TBV or adjust for the potential effects of this. This leaves open the question of whether the identified relative decrease in volumes is specific to those structures or is in proportion to an overall decrease in brain size in their KS subjects.
Instead of the ROI-based approaches used in the above studies, Giedd, Shen, and colleagues [Shen et al., 2004] utilized VBM to look for structural differences in KS. Among the various regions of decreased GM volume (uncorrected) found in individuals with KS (see below for others), they identified regions of decreased GM within the right amygdala and left hippocampus. Smaller regions that involve only a portion of a brain structure (such as the hippocampus or amygdala) are identifiable with VBM techniques since regions of difference need not be defined a priori. These smaller regions of difference can contribute to the conflicting findings of ROI studies depending upon how these studies define and measure the regions in question.
Superior Temporal Gyrus (STG)
The STG, a component of the neural networks underlying language as well as social processes [Bigler et al., 2007], has also been found to be altered in KS. In their whole-brain VBM analysis, Giedd, Shen, and coworkers [Shen et al., 2004] identified regions of reduced GM volume (uncorrected) in the left and the right STG compared to controls. Patwardhan et al. [2000] had previously found that individuals with KS had diminished bilateral STG GM volume and right STG TT volume compared to controls, though these differences did not persist after adjusting for the respective (left or right) temporal lobe GM volume. They also demonstrated an influence of treatment with testosterone in this region. Specifically, the same regional volumes (right STG GM and TT and left STG GM volumes, uncorrected) were significantly smaller in testosterone-naïve KS subjects than in controls, while these regions were comparable between testosterone-treated KS individuals and controls.
In a study that further examined the anterior and posterior subdivisions of the STG (using the anterior border of the first transverse temporal gyrus as the division), DeLisi et al. [2005] found reduced volume of bilateral posterior STG GM in KS compared to controls, while the anterior STG had comparable volumes to controls on both sides. This finding is of particular interest since the posterior, and not the anterior, portion of the STG is considered a component of Wernicke’s language area.
Caudate
Abnormalities in caudate morphology might be expected in KS given overlap in attentional and executive deficits with attention-deficit/hyperactivity disorder, a disorder in which caudate abnormalities have been identified repeatedly [Seidman et al., 2005]. Abnormalities of caudate morphology might also be postulated because of developmental motor delays present in KS, though other neurodevelopmental or neuropsychiatric disorders in which caudate abnormalities have been identified (e.g. obsessive-compulsive disorder [Santosh, 2000; Friedlander and Desrocher, 2006], fragile X syndrome [Gothelf et al., 2008]) typically involve repetitive motor behaviors not characteristic of KS. Using traditional ROI volumetric techniques, Giedd and colleagues [2007] found smaller caudate volumes (by 10%) in KS men, which were also present after adjusting for TBV, socioeconomic status, and IQ. This finding contrasts with a prior volumetric study by Warwick et al. [1999] that found no differences in caudate volume (corrected) as well as prior work by Giedd and colleagues using a VBM approach (uncorrected volumes) [Shen et al., 2004].
Other Regional Differences
As noted above, the work by Giedd, Shen, and colleagues [Shen et al., 2004] represents the only published whole-brain voxel-based approach to regional morphologic brain differences in KS. With this whole-brain approach, they identified multiple regions with decreased GM volume in KS. In addition to the amygdalar, hippocampal, and STG differences discussed above, they found regions of decreased GM volume in the left insular cortex, the cingulate region, the left middle temporal gyrus (MTG) and STG, and bilateral occipital gyri. The only regional WM difference identified was a region of relatively diminished WM volume in the right parietal lobe. Interestingly, this finding is on a subset of the subjects described in an ROI-based study by this group [Giedd et al., 2007] that identified relatively larger total (right and left combined) parietal lobe WM volume KS via an ROI analysis. Reasons for this discrepancy are unclear, but the authors [Giedd et al., 2007] note that it may be attributable either to hemispheric differences (not examined separately in the ROI analysis) or to differences in neuroimaging methods.
Non-Volumetric Structural Differences
Only two neuroimaging studies [DeLisi et al., 2005; Giedd et al., 2007] have examined structural neuroanatomical features other than volume in KS. Specifically, Giedd et al. [2007] evaluated cortical thickness in KS and control subjects. Implementing a false discovery rate of q=0.05, they found thinner cortex in KS in various parts of bilateral temporal lobes, as well as in portions of the left inferior parietal lobe, left inferior frontal lobe, and left precentral gyrus (PreCG). They proposed that thinning in temporal and frontal lobes corresponds to cognitive/behavior deficits in KS (language, attention, and executive dysfunction), and that thinner motor strip cortex, most prominently on the left, may correspond to motor difficulties (of the shoulders and upper trunk and of the musculature of the mouth and oropharynx) seen in KS.
DeLisi et al. [2005] published the only diffusion tensor imaging (DTI) study thus far in KS. They found decreased fractional anisotropy (a measure of WM directionality) in the left internal capsule, left arcuate bundle, and bilateral anterior cingulate regions of KS men compared with controls. This group noted that the more recent finding of decreased frontal WM asymmetry in the same cohort [Rezaie et al., 2009] is consistent with their DTI findings, both implicating abnormal WM morphology of the anterior frontal lobes. Most interesting, the first study identified WM asymmetry differences in coronal slices in the anterior frontal lobe that include the anterior cingulate and the more recent study found WM abnormalities localized to bilateral anterior cingulate regions, suggesting that the anterior cingulate region – implicated in both cognition and affect modulation [Devinsky et al., 1995; Drevets et al., 2008] – may warrant specific consideration in future studies of KS.
Structural-Cognitive Correlations
Four structural neuroimaging studies [Warwick et al., 1999; Patwardhan et al., 2000; DeLisi et al., 2005; Itti et al., 2006] have directly explored an association between altered brain morphology and cognitive differences in KS. DeLisi et al. [2005] found that reading scores on the Wide Range Achievement Test and scores (controlling for reading differences) on Stroop color-interference test (considered a measure of processing speed and inhibition) were significantly lower in KS subjects than in controls. Though correlations were present in the control group between reading scores and right hippocampal volume (uncorrected) and between Stroop scores and various uncorrected neuroanatomic volumes (TBV, left and right frontal lobe, and right hippocampus), no such correlations were present in the KS group. Warwick and colleagues [1999] also found no association between TBV in KS (which was smaller than in controls) and measures of IQ or behavioral characteristics (from the Structured Interview for Schizotypy [Kendler et al., 1989]).
Itti et al. [2006] explored the presence of correlations between their findings of abnormal volumes and 14 neuropsychological “domains” in KS subjects. Inverse correlations were identified between ventricular volume and both verbal processing speed and verbal executive function (r = −0.63, p=0.0001 and r = −0.51, p=0.003), two domains that were significantly impaired in KS compared to controls. Ventricular volume was also inversely correlated with nonverbal executive function (r = −0.51, p=0.003), though this domain was not significantly different (p=0.05) between groups. Further, these investigators identified correlations between (CSF-corrected) left temporal lobe TT (but not GM) volume and both language (r = +0.51, p=0.01) and verbal processing speed scores (r = +0.50, p=0.01) in right-handed subjects only. No correlations were identified between bilateral cerebellar hemisphere volumes (CSF-corrected) and any neuropsychological measure in right-handed subjects. It is important to note that these analyses were carried out on both groups (i.e., KS and controls) combined, so correlations identified may simply reflect known between-group, and not within-group, differences. Further, the exploratory nature of this analysis (including four neuroanatomic volumes and 14 neuropsychological tests) raises the question whether the imposed statistical threshold of p=0.01 is adequate to rule out type I errors.
Having found smaller left temporal lobe GM volumes in KS compared to controls, Patwardhan et al. [2000] investigated the association between left temporal GM volume (uncorrected) and verbal fluency, as measured by the Controlled Oral Word Association Test (COWAT). Within the KS group, a non-significant trend was identified of increased (i.e., better) COWAT score associated with increased left temporal GM volume. Examining the relationship to testosterone treatment, the authors found significantly better COWAT scores in the testosterone-treated KS subjects than the testosterone-naïve KS subjects. The distribution of left temporal lobe GM volumes versus COWAT scores amongst KS subjects was relatively segregated, revealing both increased left temporal GM volumes and COWAT scores in testosterone-treated subjects compared to their testosterone-naïve counterparts. Though it could not be demonstrated statistically given the small sample size, this was interpreted as suggesting a likely influence of testosterone supplementation on both verbal fluency and left temporal lobe GM volume in KS.
Summary of Structural Neuroimaging
Over the past decade, structural neuroimaging research has shown preliminary evidence for smaller brains in KS. Evidence for disproportionately-reduced regional volumes implicates the frontal and temporal lobes and the cerebellum as sites of specific abnormalities. Various investigators have provided some evidence for more specific regional changes, finding abnormally small amygdalae, hippocampi, STG, and caudate nuclei. Alterations in typical perisylvian asymmetries and brain torque have been anticipated because of language and language-based learning deficits in KS, though structural studies have, thus far, identified only limited evidence for aberrant asymmetry.
FUNCTIONAL NEUROIMAGING
Functional neuroimaging methods provide information about the neural mechanisms underlying cognitive and other brain processes that cannot be achieved by examination of brain structure alone. Two functional neuroimaging techniques have been used in the few published functional neuroimaging studies of KS in the literature: single photon emission computed tomography (SPECT) and functional magnetic resonance imaging (fMRI). SPECT is a functional neuroimaging technique that relies on quantifying emissions from an injected radiotracer to measure regional cerebral blood flow (rCBF) [Ors et al., 2005; Metting et al., 2007], considered an indirect measure of brain metabolism. Functional MRI (fMRI) is a non-invasive functional neuroimaging technique that measures blood-oxygen-level-dependent (BOLD) signal derived from local changes in the ratio of deoxygenated to oxygenated hemoglobin that occur in the setting of neuronal events. Both techniques are thought to demonstrate, indirectly, regions of the brain that are more active, either during the resting state or during a specific cognitive task.
Single photon emission computed tomography (SPECT)
Itti et al. [2003] used SPECT to characterize differences in resting-state cerebral perfusion. Their results indicated more symmetrical resting-state blood flow in nine KS men compared to nine healthy controls due to relative reduction in the typical leftward cerebral perfusion asymmetry, specifically in upper temporal and inferior parietal regions. Negative correlations were found between verbal neuropsychological test scores and increased resting right temporal blood flow. Positive correlations were identified between both verbal and non-verbal neuropsychological test scores and blood flow in regions of diminished flow in KS (including bilateral hippocampi, left cerebellar hemisphere, and cerebellar vermis). Another SPECT study [Junik and Kosowicz, 2005] reported greater number of “hypoperfusion foci” in subjects with KS, but significant methodologic and descriptive issues prevent clear interpretations from this study.
Functional MRI (fMRI)
To our knowledge, there has been only one published fMRI study in KS [van Rijn et al., 2008]. In this study, van Rijn and colleagues assessed hemispheric specialization for language in KS men using three different language tasks (verb generation, antonym generation, and a semantic decision task). Calculating a mean lateralization index across all three tasks, they found a significantly lower index (indicating less leftward lateralization) in KS than in controls. This difference in lateralization remained significant after correcting for performance on the language tasks. The relative decrease of leftward lateralization – seen in a combination of language-associated regions (STG, MTG, angular gyrus, supramarginal gyrus, and Broca’s area) and their homologous right-sided regions – resulted from increased language-related activity in the right hemisphere 455 (as opposed to decreased left hemisphere activity). When each homologous region pair was examined individually, the lateralization index was significantly lower in KS men than controls for both the STG and the supramarginal gyrus.
Thus, functional neuroimaging studies to date implicate a relative increase in right-sided cerebral activity, with a resulting decrease in typical leftward asymmetry, particularly in temporo-parietal regions. Interestingly, functional neuroimaging findings have demonstrated more evidence for diminished leftward asymmetry in posterior language-related regions, while structural imaging research has provided the most evidence of decreased rightward asymmetry in frontal regions – different but compatible findings in a theory of overall reduced asymmetry.
As the SPECT findings [Itti et al., 2003] represent resting-state blood flow, whether altered perfusion asymmetries reflect only baseline cerebral metabolic state in KS or differing patterns of perfusion and cerebral activity during language tasks (e.g., if internal language tasks were spontaneously carried out during SPECT imaging) is uncertain. The one published fMRI study [van Rijn et al., 2008] provides more specific information during cognitive activation tasks, but by combining data from three different language-oriented tasks, limits conclusions about altered neural systems used in KS for a specific language task.
We are currently undertaking a study aimed at extending present knowledge of structural and functional neuroanatomical differences in boys with KS. This study includes structural and functional MRI to examine the neural systems involved in verbal and non-verbal cognitive tasks. Boys age 7–14 years with KS participating in this study at Thomas Jefferson University were recruited from the Pediatric Endocrine clinic, self-referred, and referred from other physicians. Control subject boys, age 7–14 years, were recruited from internet notices of studies and from families participating in various research studies. The diagnosis of KS was established based on a 47,XXY karyotype. The study was approved by the Human Studies Committee of Thomas Jefferson University; informed consent was obtained from all parents and informed assent from all subjects.
Here we present the preliminary analysis of an fMRI study aimed at understanding differences in the neural systems underlying an expressive language task in this cohort. This preliminary analysis included 29 subjects (8 KS, 21 controls). Mean (±SD) age was 9.6 years (±1.8) for the KS group and 10.8 years (±1.9) for the control group. Right-handedness was present in 75% of the KS group (six right, one left, and one unknown) and 71% of the control group (15 right, five left, and one ambidextrous). All the KS subjects had nonmosaic 47,XXY karyotypes and only one received prior treatment with androgen/testosterone (for 3 months as a neonate).
This fMRI study used a verb generation task known to activate most commonly areas of language comprehension and execution (Broca’s and Wernicke’s areas) in the language dominant hemisphere [Drobyshevsky et al., 2006; Plante et al., 2006]. The task required silent generation of an action verb related to a noun (i.e., an object name) presented on the screen (“Verb Generation Task” condition) contrasted with a control condition in which subjects were asked to look at non-letter symbols (“####”) shown at the center of the screen. The five-minute scan consisted of five cycles of “30-second Control/30-second Task” conditions. The subject was instructed to think about a verb related to each noun, while not to think about anything during the “Control” epochs. Subjects responded by pressing one key each with the left and right index fingers once they were successful at generating a verb, allowing us to monitor the alertness of the subject and response time.
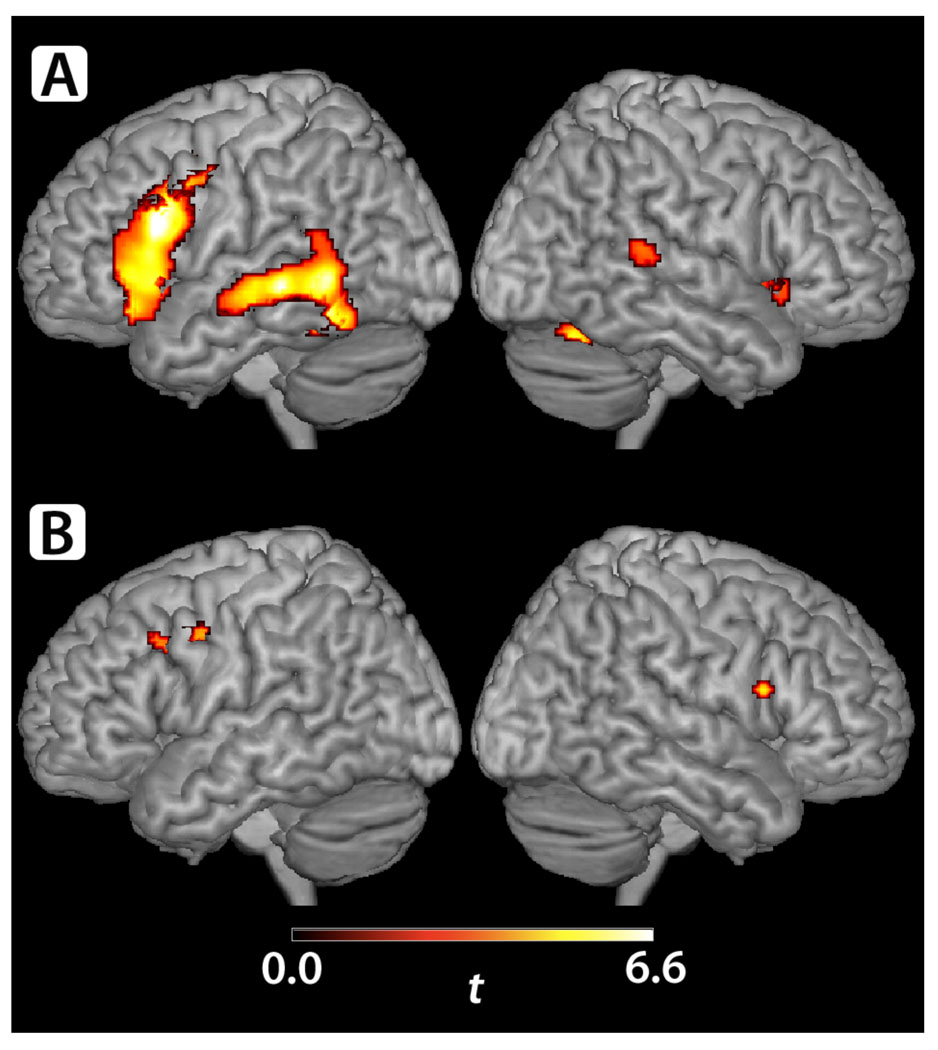
Functional MRI BOLD activity was examined for the whole cohort, within each group, and for the differences between the two groups. Given the preliminary nature of this study, we used a voxel-wise significance threshold of p<0.005 (uncorrected) and a cluster-wise extent threshold of >10 voxels. For the control subjects (n=21; Figure 1A), regions of task-dependent BOLD activity were seen in the left posterior lateral temporal lobe (including MTG, inferior temporal gyrus (ITG), and fusiform gyrus (FG)), inferomedial temporal lobe, and superior cerebellum and anteriorly on the left in Broca’s area as well as more superiorly and anteriorly in primary motor, premotor, and prefrontal cortex. Smaller regions of BOLD signal were also identified in the right-sided homologue of Broca’s area (IFG), the right posterior STG and MTG, inferomedial temporal lobe, and the cerebellum. KS subjects (n=8; Figure 1B) showed no significant BOLD activity in either Broca’s area or the posterior temporal regions identified in controls. A small region of BOLD activity was present in the KS group more dorsally in the left IFG (pars opercularis), PreCG, and middle frontal gyrus (MFG), consistent with the non-Broca’s area component of the frontal BOLD activity seen in control subjects. A small region of BOLD activity was also seen in the right PreCG and dorsal portion of the IFG, pars opercularis.
Figure 1.
A & B. (A) Control subjects (n=21) exhibited regions of BOLD activity during the verb generation task involving the left posterior MTG, ITG, STG, FG, lingual gyrus, PHG, hippocampus, deep gray nuclei, superior cerebellum, and vermis and involving the left IFG (including Broca’s area), PreCG, insula, and MFG. Smaller regions of right-sided BOLD activity were identified in the IFG (including the homologue to Broca’s area), posterior STG and MTG, cerebellum, lingual and calcarine gyri, FG, PHG, and hippocampus. (B) KS subjects (n=8) exhibited a small region BOLD activity during the verb generation task in the left PreCG, MFG, and IFG (pars opercularis, dorsal portion). Another region of activity was seen in the left deep grey nuclei. On the right, a region of BOLD activity was seen in the PreCG and in the dorsal portion of the IFG, pars opercularis.
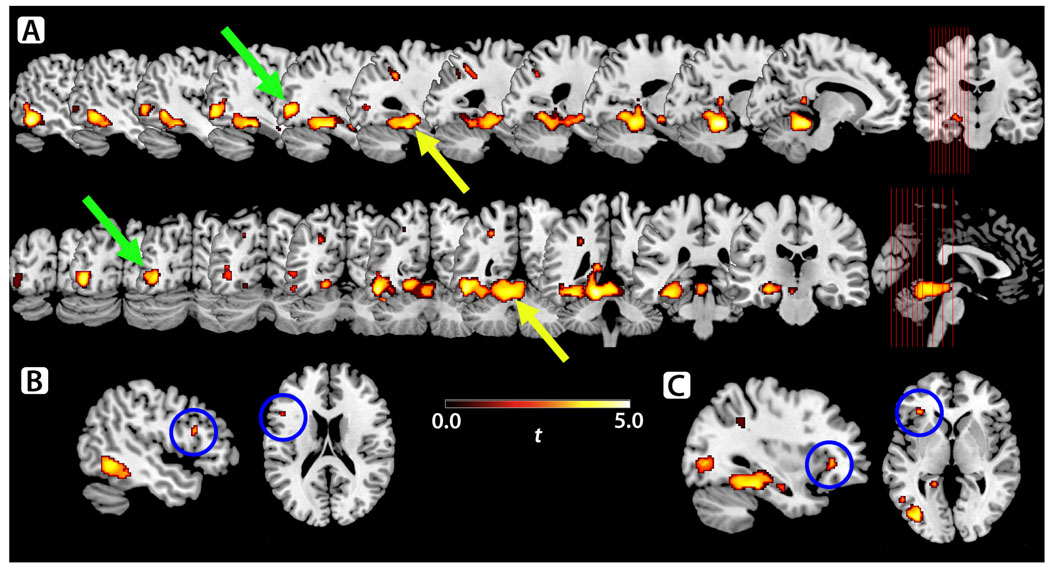
Between-group comparisons (Figure 2A–C) revealed significantly less activation in KS subjects than controls, mainly in left hemisphere regions. Notably, relatively less activation was seen in language-associated areas, including the left MTG (Figure 2A) and left IFG (Broca’s area; Figure 2B). The largest clusters of decreased activity involved the left MTG, ITG, parahippocampal gyrus (PHG), FG, and cerebellar vermis and the left middle occipital gyrus (MOG), inferior occipital gyrus (IOG), and ITG. No regions showed significantly greater BOLD activity in KS subjects compared to controls.
Figure 2.
A–C. KS showed significantly less BOLD signal activity than controls during the verb generation task in multiple left-sided and two right-sided regions. Clusters of decreased activity involved (A; yellow arrow) the left MTG, ITG, PHG, FG, superior cerebellum, and cerebellar vermis, and (green arrow) the left MOG, IOG, and ITG. Other regions of decreased activity were seen in parts of the left IFG: both Broca’s area (pars opercularis/triangularis, B; blue circle) and a more anteroinferior portion (pars triangularis/orbitalis, C; blue circle).
Preliminary findings of relatively decreased BOLD activity in language-related areas support the limited previous functional neuroimaging findings of reduced leftward functional asymmetry in the KS brain. Abnormalities of posterior language and reading-related areas (left MTG and FG) are consistent with known difficulties with reading and language-related tasks experienced by individuals with KS. Though the fMRI task (verb generation) was aimed at evoking expressive language cognitive processes, diminished activity in these posterior regions may relate to differences in the neural system used to read and comprehend the “object name” (noun) stimulus before generating a related verb.
Because of known difficulties with expressive language and word retrieval in KS [Geschwind et al., 2000], we hypothesized that abnormal BOLD activity would also be found in Broca’s area during an expressive language task (verb generation). This was confirmed by our preliminary analysis, representing the first neuroimaging evidence for decreased functional activity in anterior language-associated areas in KS.
Our findings of relatively reduced activation in the left superior cerebellum and cerebellar vermis corresponds to prior functional neuroimaging evidence of decreased cerebellar vermis and left cerebellar hemisphere blood flow in KS [Itti et al., 2003] as well as structural evidence for reduced cerebellar size [Itti et al., 2006] in KS. Evidence of cerebellar abnormalities in KS is in keeping with the increasingly recognized role of the cerebellum in language function [Schmahmann and Caplan, 2006; De Smet et al., 2007].
An important difference must be noted between our preliminary findings and those of the few prior functional neuroimaging studies in KS [Itti et al., 2003; van Rijn et al., 2008]. Though both show evidence for altered left lateralized functional cerebral activity, our preliminary findings identified less left-sided cerebral activity than controls in language-associated regions, rather than increased right-sided activity with a resulting reduction in leftward functional asymmetry, as seen in the other two studies [Itti et al., 2003; van Rijn et al., 2008]. This discrepancy has multiple potential contributing factors. First, this may be due to the different types of activity being measured – activity during a single expressive language task in the current study as opposed to combined activity during multiple (receptive and expressive) language tasks [van Rijn et al., 2008] or resting state activity [Itti et al., 2003]. Our examination of boys, rather than adult men as in the other two studies, is another possible cause for the discrepancy. Developmental shifts in the pattern of brain activation associated with the verb generation task have been demonstrated in typical individuals [Holland et al., 2001]. Since the degree of leftward lateralization has been showed to increase with age from 7- to 18-years-old, age must be taken into account when drawing conclusions about altered asymmetry. Other recognized limitations of this preliminary analysis include the mixed handedness of the cohort and the potential influences of KS subjects’ prior treatment with testosterone (one subject), general cognitive ability, language abilities, and their performance on the fMRI language task. Finally, KS sample size is also very small and the groups are unbalanced, thus the data should be interpreted with caution. Once more data are available in this cohort, we intend to explore many of the above factors with further fMRI data analyses. The overall goal of this ongoing MRI study of KS – to examine the effects of testosterone treatment on the brain of individuals with KS – will be presented in a more complete form in the future, including analyses of structural neuroimaging and other fMRI tasks.
Summary of Functional Neuroimaging
Functional neuroimaging studies during rest and during language tasks have suggested that less leftward functional brain asymmetry, particularly in temporo-parietal brain regions, may indeed be present in KS. Our preliminary fMRI data further support this notion, demonstrating that during a specific expressive language (i.e. verb generation) task requiring both generation of language and reading, diminished brain activity was present in KS subjects in multiple left-sided regions, including prominent language- and reading-associated areas (Broca’s area, left MTG, and left FG). The influence of general cognitive ability, language ability, task performance, and age may contribute to variability in relative activity differences between hemispheres, and will be explored further in future analyses of the cohort currently being studied.
CONCLUSION
Though Klinefelter Syndrome is a relatively common disorder with a recognized cognitive and behavioral phenotype including deficits in language and language-based learning disabilities and executive and attentional dysfunction, surprisingly little work has been done to examine the neurobiologic differences that underlie these abnormalities.
As we have shown, the current literature provides only a preliminary understanding of the neural differences in the disorder. Interpretation of the discrepant findings from currently available studies is complicated by the differences in study design, cohort characteristics, and neuroimaging analysis methods. Such study variability is unavoidable as research is conducted internationally in many different laboratories and setting. However, we make several recommendations to improve cross-study interpretation. First, we recommend that cohort characteristics be clearly identified. In studies of KS, it is important that this includes the proportion of mosaic and nonmosaic subjects and, when possible, the degree and type of mosaicism. Also, reporting the proportion of subjects receiving (or previously receiving) testosterone supplementation is important. With respect to structural image analysis, the measure of TBV should be specified and explicitly defined to make clear what structures beyond the cerebral cortex and subcortical WM are incorporated (including subcortical/deep gray nuclei, cerebellum, brainstem, and ventricles). This will allow for more accurate comparisons between results of TBV differences. Also, it will allow for appropriate interpretation of lobar, ventricular, or regional volumes that have been corrected for TBV. Whether correction has or has not been implemented should also be explicitly stated for all methodologies (and analyses with and without correction are suggested).
Much work remains to obtain a clearer understanding of the neurobiologic underpinnings of cognitive and behavioral abnormalities in KS. More research, using sophisticated neuroimaging techniques and, hopefully, incorporating the above recommendations, will provide scientists and clinicians with a greater understanding of the poorly understood neurologic abnormalities that form part of the constellation of this relatively common genetic disorder. Additionally, this population’s cognitive phenotype exhibits strong similarities to those constituting developmental dyslexia and attention deficit/hyperactivity disorder. The relatively homogenous genetic basis for the cognitive phenotype in KS provides a population full of potential for furthering knowledge about these even more common disorders through ongoing structural and functional neuroimaging research.
CallOuts
Though a characteristic KS cognitive phenotype is now recognized, relatively little is known about the underlying neural mechanisms.
…[S]tructural neuroimaging research has shown preliminary evidence for smaller brains in KS. Evidence for disproportionately-reduced regional volumes implicates the frontal and temporal lobes and the cerebellum as sites of specific abnormalities.
…[F]unctional neuroimaging findings have demonstrated more evidence for diminished leftward asymmetry in posterior language-related regions, while structural imaging research has provided the most evidence of decreased rightward asymmetry in frontal regions – different but compatible findings in a theory of overall reduced asymmetry.
Interpretation of the discrepant findings from currently available studies is complicated by the differences in study design, cohort characteristics, and neuroimaging analysis methods.
Table 2.
Functional Neuroimaging Studies
| Study | Groups* | Methods | Findings |
|---|---|---|---|
| Itti 2003 | 9 KS men (27.8±6.6 [18–37]; 9 KS–T) 9 CON men ([18–35]) |
SPECT, VOI |
VOIs with rCBF in KS>CON R SFG, middle frontal gyrus, PoCG, IPL, SMG, AG, STG, MTG, insula. L SFG, medial frontal gyrus. VOIs with rCBF in KS<CON R HC, cingulate gyrus. L HC, cerebellar hemisphere. Cerebellar vermis, fornix. rCBF Asymmetry in CN, leftward asymmetry in 11 pairs of homologous VOIs (3 perirolandic gyri, insula, STG, MTG, TTG, cuneus, superior & inferior parietal gyri, & cerebellum) in KS, leftward asymmetry in 3 pairs: PreCG, TTG, & cerebellum. Others pairs symmetric. Correlations of rCBF Inverse correlation with verbal skills test scores: R STG, MTG, insula. Positive correlation with verbal & nonverbal skills test scores: B HC, L cerebellar hemisphere, R oCG, R IPL, R AG |
| Junik 2005 | 27 KS men ([17–47]) 26 CON men |
SPECT, VOI | More “hypoperfusion foci” in KS. (see text regarding interpretation) |
| Van Rijn 2008 | 15 KS men (36.9±11.8; 14 KS+T, 1 KS–T) 14 CON men (35.5±9.5) |
fMRI, VOI analysis of 3 conjoint tasks (paced verb generation, antonym generation, semantic decision) |
Overall MLI (5 VOIs combined): KS<CON; BOLD activity in right hemisphere VOIs KS>CON. STG MLI & SMG MLI: KS<CON. Correlations of MLI Overall MLI: inverse correlation with higher total PANSS scores. STG MLI: inverse correlation with SPQ disorganization dimension. |
ages given as (mean age in years ± SD [range in years, if available])
AG=angular gyrus; B=bilateral; CON=control; fMRI=functional magnetic resonance imaging; HC=hippocampus; IPL=inferior parietal lobule; KS=Klinefelter syndrome; KS+T= testosterone-treated KS subject; KS–T= testosterone-naïve KS subject; L=left; MLI=mean lateralization index ([left active voxels – right active voxels]/total active voxels); MTG=middle temporal gyrus; PANSS=Positive and Negative Syndrome Scale (schizophrenia symptomatology); PoCG= post-central gyrus; PreCG=pre-central gyrus; R=right; rCBF= regional cerebral blood flow; SFG=superior frontal gyrus; SMG=supramarginal gyrus; SPECT=Single photon emission computed tomography; SPQ=Schizotypal Personality Questionnaire; STG=superior temporal gyrus; TTG=transverse temporal gyrus; VOI=volumes-of-interest
Table 3.
Regions of less BOLD activity in KS than Controls during verb generation task
| Side | Region | Brodmann Areas |
Talairach Coordinates |
Extent (k) |
T value | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Left | Culmen (anterior cerebellar vermis), Middle Temporal Gyrus, Inferior Temporal Gyrus, Parahippocampal Gyrus, Fusiform Gyrus |
20, 36, 37 | −16 | −45 | −11 | 2686 | 5.09 |
| Left | Middle Occipital Gyrus, Inferior Occipital Gyrus, Inferior Temporal Gyrus | 19 | −38 | −74 | 2 | 264 | 4.55 |
| Left | Temporal Lobe, Hippocampus | 20 | −34 | −12 | −16 | 12 | 3.41 |
| Left | Superior Parietal Lobule, Inferior Parietal Lobule, Precuneus | 7, 40 | −30 | −43 | 35 | 53 | 3.41 |
| Left | Inferior Frontal Gyrus | 47 | −32 | 31 | 0 | 29 | 3.31 |
| Left | Inferior Frontal Gyrus | 45 | −48 | 18 | 18 | 12 | 3.16 |
| Right | Declive (posterior cerebellar vermis) | –– | 36 | −63 | −15 | 12 | 2.95 |
| Left | Precuneus, Superior Parietal Lobule | 7 | −24 | −60 | 38 | 14 | 2.92 |
| Right | Lingual Gyrus | 18 | 22 | −74 | −8 | 11 | 2.85 |
ACKNOWLEDGEMENTS
The authors would like to thank Masanori Nagamine for assistance with fMRI data processing.
Research Support: This research was supported by NIH; NS050597 (JR). KJS was supported by the NINDS Neurological Sciences Academic Development Award (K12 NS01692). FH is supported by NARSAD Young Investigator Award, the Child Health Research Program, and NICHD 5K23HD054720.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to report.
REFERENCES
- Aleman A, Swart M, van Rijn S. Brain imaging, genetics and emotion. Biol Psychol. 2008;79:58–69. doi: 10.1016/j.biopsycho.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-Based morphometry-the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Bigler ED, Mortensen S, Neeley ES, et al. Superior temporal gyrus, language function, and autism. Dev Neuropsychol. 2007;31:217–238. doi: 10.1080/87565640701190841. [DOI] [PubMed] [Google Scholar]
- Boada R, Janusz J, Hutaff-Lee C, et al. The cognitive phenotype in Klinefelter syndrome: A review of the literature including genetic and hormonal factors. Dev Disabil Res Rev. 2009 doi: 10.1002/ddrr.83. (this issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLisi LE, Maurizio AM, Svetina C, et al. Klinefelter’s syndrome (XXY) as a genetic model for psychotic disorders. Am J Med Genet B Neuropsychiatr Genet. 2005;135B:15–23. doi: 10.1002/ajmg.b.30163. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118(Pt 1):279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr. 2008;13:663–681. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobyshevsky A, Baumann SB, Schneider W. A rapid fMRI task battery for mapping of visual, motor, cognitive, and emotional function. Neuroimage. 2006;31:732–744. doi: 10.1016/j.neuroimage.2005.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlander L, Desrocher M. Neuroimaging studies of obsessive-compulsive disorder in adults and children. Clin Psychol Rev. 2006;26:32–49. doi: 10.1016/j.cpr.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Geschwind DH, Boone KB, Miller BL, et al. Neurobehavioral phenotype of Klinefelter syndrome. Ment Retard Dev Disabil Res Rev. 2000;6:107–116. doi: 10.1002/1098-2779(2000)6:2<107::AID-MRDD4>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Geschwind DH, Dykens E. Neurobehavioral and psychosocial issues in Klinefelter syndrome. Learn Disabil Res Pract. 2004;19:166–173. [Google Scholar]
- Giedd JN, Clasen LS, Lenroot R, et al. Puberty-Related influences on brain development. Mol Cell Endocrinol. 2006;254–255:154–162. doi: 10.1016/j.mce.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Clasen LS, Wallace GL, et al. XXY (Klinefelter syndrome): A pediatric quantitative brain magnetic resonance imaging case-control study. Pediatrics. 2007;119:e232–e240. doi: 10.1542/peds.2005-2969. [DOI] [PubMed] [Google Scholar]
- Gothelf D, Furfaro JA, Hoeft F, et al. Neuroanatomy of fragile X syndrome is associated with aberrant behavior and the fragile X mental retardation protein (FMRP) Ann Neurol. 2008;63:40–51. doi: 10.1002/ana.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert MR, Kenet T. Brain abnormalities in language disorders and in autism. Pediatr Clin North Am. 2007;54:563–583. doi: 10.1016/j.pcl.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Itti E, Gaw Gonzalo IT, Boone KB, et al. Functional neuroimaging provides evidence of anomalous cerebral laterality in adults with Klinefelter’s syndrome. Ann Neurol. 2003;54:669–673. doi: 10.1002/ana.10735. [DOI] [PubMed] [Google Scholar]
- Itti E, Gaw Gonzalo IT, Pawlikowska-Haddal A, et al. The structural brain correlates of cognitive deficits in adults with Klinefelter’s syndrome. J Clin Endocrinol Metab. 2006;91:1423–1427. doi: 10.1210/jc.2005-1596. [DOI] [PubMed] [Google Scholar]
- Junik R, Kosowicz J. Reduced brain perfusion and neurocranial shape abnormalities of the temporal regions in patients with Klinefelter syndrome. Neuro Endocrinol Lett. 2005;26:593–598. [PubMed] [Google Scholar]
- Kendler KS, Lieberman JA, Walsh D. The structured interview for schizotypy (SIS): A preliminary report. Schizophr Bull. 1989;15:559–571. doi: 10.1093/schbul/15.4.559. [DOI] [PubMed] [Google Scholar]
- Lanfranco F, Kamischke A, Zitzmann M, et al. Klinefelter’s syndrome. Lancet. 2004;364:273–283. doi: 10.1016/S0140-6736(04)16678-6. [DOI] [PubMed] [Google Scholar]
- Leonard CM, Eckert MA. Asymmetry and dyslexia. Dev Neuropsychol. 2008;33:663–681. doi: 10.1080/87565640802418597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metting Z, Rödiger LA, De Keyser J, et al. Structural and functional neuroimaging in mild-to-moderate head injury. Lancet Neurol. 2007;6:699–710. doi: 10.1016/S1474-4422(07)70191-6. [DOI] [PubMed] [Google Scholar]
- Ors M, Ryding E, Lindgren M, et al. SPECT findings in children with specific language impairment. Cortex. 2005;41:316–326. doi: 10.1016/s0010-9452(08)70269-7. [DOI] [PubMed] [Google Scholar]
- Patwardhan AJ, Brown WE, Bender BG, et al. Reduced size of the amygdala in individuals with 47,XXY and 47,XXX karyotypes. Am J Med Genet. 2002;114:93–98. doi: 10.1002/ajmg.10154. [DOI] [PubMed] [Google Scholar]
- Patwardhan AJ, Eliez S, Bender B, et al. Brain morphology in Klinefelter syndrome: Extra X chromosome and testosterone supplementation. Neurology. 2000;54:2218–2223. doi: 10.1212/wnl.54.12.2218. [DOI] [PubMed] [Google Scholar]
- Plante E, Schmithorst VJ, Holland SK, et al. Sex differences in the activation of language cortex during childhood. Neuropsychologia. 2006;44:1210–1221. doi: 10.1016/j.neuropsychologia.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Eliez S, Schmitt JE, et al. Brain imaging in neurogenetic conditions: Realizing the potential of behavioral neurogenetics research. Ment Retard Dev Disabil Res Rev. 2000;6:186–197. doi: 10.1002/1098-2779(2000)6:3<186::AID-MRDD6>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Rezaie R, Daly EM, Cutter WJ, et al. The influence of sex chromosome aneuploidy on brain asymmetry. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:74–85. doi: 10.1002/ajmg.b.30772. [DOI] [PubMed] [Google Scholar]
- Rose AB, Merke DP, Clasen LS, et al. Effects of hormones and sex chromosomes on stress-influenced regions of the developing pediatric brain. Ann N Y Acad Sci. 2004;1032:231–233. doi: 10.1196/annals.1314.027. [DOI] [PubMed] [Google Scholar]
- Santosh PJ. Neuroimaging in child and adolescent psychiatric disorders. Arch Dis Child. 2000;82:412–419. doi: 10.1136/adc.82.5.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD, Caplan D. Cognition, emotion and the cerebellum. Brain. 2006;129:290–292. doi: 10.1093/brain/awh729. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Valera EM, Makris N. Structural brain imaging of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1263–1272. doi: 10.1016/j.biopsych.2004.11.019. [DOI] [PubMed] [Google Scholar]
- Semrud-Clikeman M. Evidence from imaging on the relationship between brain structure and developmental language disorders. Semin Pediatr Neurol. 1997;4:117–124. doi: 10.1016/s1071-9091(97)80028-9. [DOI] [PubMed] [Google Scholar]
- Shen D, Liu D, Liu H, et al. Automated morphometric study of brain variation in XXY males. Neuroimage. 2004;23:648–653. doi: 10.1016/j.neuroimage.2004.08.018. [DOI] [PubMed] [Google Scholar]
- De Smet HJ, Baillieux H, De Deyn PP, et al. The cerebellum and language: The story so far. Folia Phoniatr Logop. 2007;59:165–170. doi: 10.1159/000102927. [DOI] [PubMed] [Google Scholar]
- Toga AW, Thompson PM. Mapping brain asymmetry. Nat Rev Neurosci. 2003;4:37–48. doi: 10.1038/nrn1009. [DOI] [PubMed] [Google Scholar]
- van Rijn S, Aleman A, Swaab H, et al. Effects of an extra X chromosome on language lateralization: An fMRI study with Klinefelter men (47,XXY) Schizophr Res. 2008;101:17–25. doi: 10.1016/j.schres.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Warwick MM, Doody GA, Lawrie SM, et al. Volumetric magnetic resonance imaging study of the brain in subjects with sex chromosome aneuploidies. J Neurol Neurosurg Psychiatry. 1999;66:628–632. doi: 10.1136/jnnp.66.5.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattendorf DJ, Muenke M. Klinefelter syndrome. Am Fam Physician. 2005;72:2259–2262. [PubMed] [Google Scholar]