Abstract
Males typically have greater variance in reproductive success than females, so mothers should benefit by producing sons under favorable conditions. Being paired with a better-than-average mate is one such favorable circumstance. High-quality fathers can improve conditions for their offspring by providing good genes, good resources, or both, so females paired to such males should invest preferentially in sons. Ornamentation has been linked to male quality in many birds and, in support of differential allocation theory, females of several avian species invest more in entire broods when paired to attractive mates. Additionally, the females of some bird species apparently manipulate the primary sex-ratio of their broods in relation to the attractiveness of their mates. However, empirical support for a link between mate ornamentation and preferential feeding of sons (another form of biased investment) is lacking. We tested for correlations between sex-biased parental investment and mate plumage colour in the eastern bluebird (Sialia sialis), a species in which juveniles have sexually dichromatic UV-blue plumage. We found that the proportion of maternal feeding attempts to fledgling sons (versus fledgling daughters) was positively correlated with structurally coloured plumage ornamentation of fathers. Additionally, paternal feeding attempts to sons were correlated with plumage ornamentation of mothers and increased in fathers exhibiting breast plumage characteristics typical of older males. These results provide further support for the idea that parental strategies are influenced by mate attractiveness and provide the first evidence that mate ornamentation can influence parental behavior even after offspring have left the nest.
Keywords: sex-ratio, parental behavior, resource allocation, ornaments, eastern bluebird, Sialia sialis, honest signal, plumage, colour
When the benefits of producing male and female offspring vary depending on context, parents are expected to maximize reproductive success by investing differentially in sons and daughters depending on their circumstances (Trivers and Willard 1973; Charnov 1982). Differential investment in sons and daughters can occur by varying the ratio of sons and daughters produced or through differential investment of energy in sons and daughters after birth or hatching. Animals have been shown to adjust investment in sons versus daughters in relation to season (Dijkstra et al. 1990; Sakisaka et al. 2000; Schultz 2008), diet (Bradbury and Blakey 1998; Opit & Throne 2008), maternal age (Blank and Nolan 1983; Isaac et al. 2005), mate quality (Svensson and Nilsson 1996; Roed et al. 2007), and mate attractiveness (Sheldon et al. 1999). In birds, differential investment in sons and daughters can take the form of primary sex-ratio manipulation via changes in the proportion of male eggs in a given brood. Alternatively, sex-biased investment strategies can manifest as differential resource allocation, which may occur if parents invest time and effort differently in sons and daughters after hatching.
The disparity in the value of males versus females stems from different reproductive opportunities for males and females of different qualities. In many species of animals, males have greater variance in reproductive output than females because the investment in offspring by females is larger than investment by males (Bateman 1948; Clutton-Brock 1988). Greater variance in male reproductive success arises because poor quality males are likely to produce fewer offspring than poor quality females, whereas high quality males can produce more offspring than high quality females. Therefore, parental investment in sons should be higher if 1) mothers are in good condition (Trivers and Willard 1973), or 2) if sons are fathered by high-quality males (Charnov 1982). Because high-quality males can provide a suite of direct (e.g., increased levels of food provisioning and nest-defense, Hoelzer 1989) and indirect (e.g., heritable genetic quality) benefits to their offspring (Andersson 1994), and because these benefits increase the likelihood that superior offspring will be produced, relative levels of parental investment in sons should also be influenced by the quality of their father.
Total parental investment in birds, typically measured in terms of investment to an entire brood, has been shown to vary with mate ornamentation in several species including blue tits Cyanistes caeruleus (Limbourg et al. 2004), barn swallows Hirundo rustica (de Lope and Moller 1993), and zebra finches Taeniopygia guttata (Burley 1988). Female blue tits, for example, feed broods at higher rates when mated to males with bright UV colouration (Limbourg et al. 2004) and defend their nests more vigorously than females paired to males with dull UV plumage (Johnsen et al. 2005). In contrast to these examples, female eastern bluebirds (Sialia sialis) do not feed nestlings at higher rates when mated to highly ornamented males (Siefferman and Hill 2003). Although no evidence has yet been published that supports the relationship between mate attractiveness and sex-biased provisioning for any avian species, bluebird parents may assess the ornamentation of their mates when making feeding decisions within a brood, altering relative investment in sons versus daughters.
We examined the potential for sex-biased parental investment via differential provisioning to sons and daughters in the eastern bluebird, a strongly sexually dichromatic species with sexually dichromatic offspring (Gowaty and Plissner 1998, Siefferman and Hill 2008). Because conspicuous sexual dichromatism is rare in juvenile birds (Kilner 2006) and presents a clear mechanism by which parents can distinguish sons from daughters, bluebirds present an ideal system in which to study sex-biased provisioning (sensu Stamps 1990). Additionally, extra-pair paternity is high in some populations of eastern bluebirds (Gowaty and Plissner 1998), potentially providing the reproductive variance between males and females required by the Trivers and Williard (1973) investment theory.
The bright UV-blue structural plumage colouration of adult male eastern bluebirds is positively correlated with male provisioning rates to incubating females (Siefferman and Hill 2005a), male provisioning rates to nestlings (Siefferman and Hill 2003), body condition, and age (Siefferman et al. 2005). Melanin-based, orange-coloured, breast plumage of males also varies with age, with older males possessing brighter breast feathers with less red chroma (Siefferman et al. 2005). Because males with more colourful structural plumage feed incubating females and nestlings at higher rates and are generally older and more experienced, we predicted that females would adjust their resource allocation to sons and daughters according to the ornamentation (perceived quality) of their mates. Specifically, females mated to colourful males should increase their investment in sons because having a bright male as a mate should increase the quality of those sons. Because the structural plumage of adult female eastern bluebirds is a condition-dependent trait that varies with food intake and predicts reproductive success in the wild (Siefferman and Hill 2005b), males also stand to benefit by modifying allocation to sons and daughters relative to the colouration of their mates. Thus, we predicted that adult male bluebirds should increase their investment in sons when mated to more colourful females.
To test these predictions we examined the provisioning rate of bluebird parents to fledgling sons and daughters. When offspring were of fledging-age, we placed one daughter and one son in a divided cage and allowed the parents to forage freely. Parents quickly adapted to the trial set-up and provisioned offspring through the wire cage. We analyzed feeding rates of parents as a proportion of feeding attempts to sons vs. daughters and examined the relationship between this proportion and the plumage ornamentation of each individual's mate.
MATERIALS AND METHODS
Study Population
We studied a banded population of eastern bluebirds (hereafter, bluebirds) in Lee County, Alabama, United States (32°35′N, 82°28′W) between March and August, 2008. Bluebirds are a sexually dimorphic passerine species (Family Turdidae) that breeds throughout eastern North America (Gowaty & Plissner 1998). Adult male bluebirds have rich blue colouration on their heads, backs, rumps, tails, and wings. The upper breasts of adult males are orange, and the bellies are white. Adult females have blue-gray upper parts with dull blue wings and tails and pale orange breasts.
We monitored nestboxes throughout the breeding season to determine when nests were in use by bluebirds, as well as to monitor the age and development of bluebird offspring. Bluebirds typically begin laying during the month of March in central Alabama and can produce up to three broods, averaging approximately four eggs per clutch (x̄ = 3.75 ± 1.1, Siefferman and Hill 2007), over the course of the breeding season. Nestlings typically hatch after 14 days of incubation and fledge 15-20 days after that (Gowaty and Plissner 1998).
Trial Procedures
Because fledglings spend much of their time hidden, it is typically difficult to observe patterns of parent-offspring interactions after young have fledged. In our study, we constrained the movements of fledgling bluebirds to an observation arena while simultaneously allowing their parents to forage naturally. Between 16 and 18 days of age, we selected one male and one female nestling from nests that contained one male nestling and at least one female nestling. We determined the sex of fledglings using plumage characteristics in the field, but later performed molecular techniques that allowed us to double-check our field assignments. Eastern bluebird nestlings typically fledge near this stage of development (Gowaty and Plissner 1998) and we chose specific trial dates on a brood-by-brood basis depending on the development and size of nestlings in each nestbox. When broods contained multiple female nestlings, we selected the individual with the mass closest to that of the male nestling.
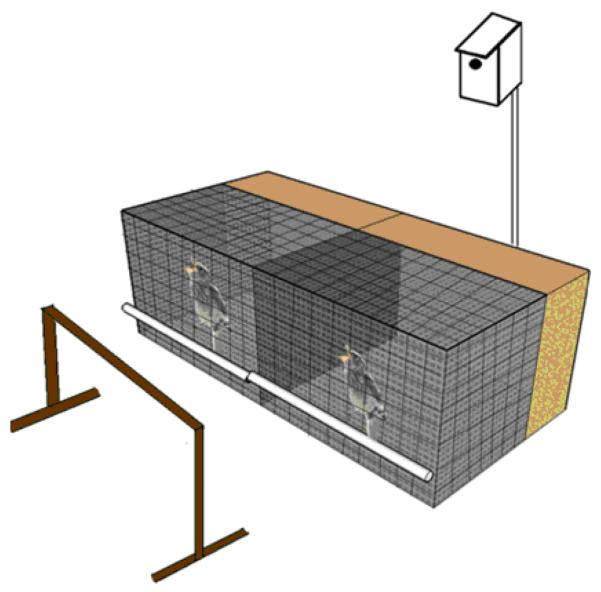
On the day of the trial, we gathered all nestlings from each box to measure their mass. In order to minimize the effects that different hunger levels might have on fledgling begging rate and intensity, as well as the effects that such differences might have on parental provisioning patterns, we fed each nestling one mealworm before all members of the brood were returned to the natal nestbox. At this point, we sealed the entrance to the nestbox to prevent any feeding by parents, as well as any premature fledging attempts. We then left the immediate area for 30 min to allow the nestlings to digest the recently consumed mealworm. After the 30 min pre-trial period, we returned to the nestbox, selected the pre-determined male and female, and placed them separately in a divided wire cage near the natal nestbox (Fig 1). A solid partition prevented any physical or visual contact between siblings in the wire cage. To create a location from which bluebird parents could assess their offspring, we placed a 50-cm tall perch one meter away from the front of the cage. We kept all remaining nestlings in a cloth box and fed them mealworms throughout the duration of the trial.
Fig 1.
The experimental setup used to constrain the movements of fledging bluebirds and facilitate quantification of provisioning by parents.
Parent bluebirds quickly adjusted to the trial setup and began to feed their offspring through the wire mesh of the cage. We used a tripod mounted video camera (Sony Hi-8) to record parent and chick interactions for one hour, at which point we reversed the position of the fledglings in the cage (to control for any possible effects that cage location might have) and resumed recording for one additional hour. After each trial, we removed the fledglings from the cage and returned them to their nestbox along with their siblings.
We quantified parental investment from video-tapes without knowledge of the sex of the fledglings or the identity of the parents in each trial. Although we were unable to reliably assess the size of food items brought to fledglings during the trial, previous research indicates that prey size does not vary with feeding rate in this population (Siefferman & Hill 2007). Food handling and transfer difficulties between parents and offspring, exacerbated by the wire mesh separating them, often caused parents to temporarily abandon feeding one fledgling and begin attempting to feed the other fledgling. Due to the inconsistency of food transfer, we used long-distance parental approaches to juveniles as a proximate measure of investment. Every time an adult directly approached one of the juveniles from outside the frame of the video screen, or from the perch one meter in front of the cage, we scored the event as a feeding attempt. This scoring method best captures the choices that parents make when delivering food and minimizes the effects that delivery complications and fledgling behavior had on parental feeding decisions. In another study using similar methodology (Ligon & Hill in review), begging by juveniles prior to parental approach was determined to be rare (twice in 366 feeding attempts), limiting the impact such behavior could have on feeding decisions made by parents.
In total, we recorded 49 feeding trials throughout the course of the breeding season. However, 5 of these trials were conducted with bluebird parents that had already been tested earlier in the year. We excluded these duplicate trials and used each set of bluebird parents only once in our analyses. Additionally, we were forced to exclude several trials from analysis because we failed to capture either the mother or father bluebird. These failures precluded analysis of plumage colour for these individuals. One additional trial was excluded from analysis because the sex assigned to fledglings in the field was later determined to be incorrect using molecular sexing techniques.
Colourimetric Data
We plucked feathers from adult bluebirds in March and April, 2008, and measured plumage colour from these feathers using an S-2000 spectroradiometer with a deuterium-halogen lamp (Ocean Optics, Dunedin, Florida, USA) following the procedures described in Siefferman and Hill (2003). When adults were captured, we collected 9 breast, 9 rump, and two outer tail feathers from each bird. Feather samples were plucked from the same location on all birds and were later placed on black paper for spectrophotometric analysis. Breast and rump feathers (both are types of contour feathers) attain colour by superposition of several feathers (sensu Quesada and Senar 2006), so we arranged them in a manner that mimicked the way these feathers lay naturally on bluebird bodies. One researcher (RL) then recorded spectral data using a micron fiber-optic probe at a 90° angle to the feather surface.
We used the spectral processing program ColouR (v1.7, Queens, Ontario) to calculate three standard descriptors of reflectance spectra: UV-chroma, hue, and brightness. Brightness (total amount to light reflected by the feather) is the summed reflectance from 300 to 700 nm. Chroma was calculated differently for UV-blue and chestnut colouration because of the inherent reflective properties of the two colours. For the rump and tail feathers, UVchroma, a measure of spectral purity, was calculated as the proportion of reflectance within the UV range to the total reflectance (R300-400/R300-700). For the chestnut breast feathers, red-chroma was calculated as the ratio of the total reflectance in the red range to the total reflectance of the entire spectrum (R575-700/R300-700). Hue is the principal colour reflected by the feather. For structural colouration (rump and tail), hue was defined as the wavelength of maximum reflectance (λ(Rmax)). Because hue (calculated as maximum slope) of the chestnut breast feathers expressed very little variation among males, we do not report hue for breast colouration.
Although structural plumage colour varies seasonally in blue tits (Örnborg et al. 2002), no such relationship has been found in eastern bluebirds (Seifferman and Hill 2005c). This fact, coupled with the relatively short time period (< 2 months) over which adults were captured, suggests that any effects of seasonality on our analyses in the present study were minimal.
Using Principal Components Analysis to Describe Plumage Colour
We performed separate principal components analyses (PCA) on measures of breast, rump, and tail colouration. We used PCA analysis because the plumage characteristics (brightness, chroma, hue) of each body region were correlated and because this analysis enabled us to reduce the number of colour variables to a more manageable number (from eight plumage variables to three). The results of the principal components analyses are summarized in Table 1.
Table 1.
Summary of principal components analyses of adult eastern bluebird plumage color and associated correlates of specific color variables. Positive and negative symbols indicate direction of associations, the zero symbol indicates no relationship.
| Brightness | Chroma | Hue | Agec | Conditiond,e | Description | |
|---|---|---|---|---|---|---|
| Male | ||||||
| aRump (PC1) | + | + | − | + | + | Bright, more UV chroma, left-shifted hues |
| aTail (PC1) | + | − | + | − | Bright, less UV chroma, right-shifted hues | |
| bBreast (PC1) | + | − | + | Bright, less red chroma | ||
| Female | ||||||
| aRump (PC1) | + | + | − | + | Bright, more UV chroma, left-shifted hues | |
| aTail (PC1) | + | − | More UV chroma, left-shifted hues | |||
| aTail (PC2) | + | Bright | ||||
| bBreast (PC1) | + | − | ⊘ | Bright, less red chroma |
Structural plumage colouration
Melanin plumage colouration
Siefferman et al. 2005
Siefferman and Hill 2005d
Siefferman and Hill 2005b
Male Plumage
The melanin breast colouration of males (brightness and chroma) reduced to one principal component that explained 64% of the variation among these variables (brightness loading, 0.80; red chroma loading, −0.80). A male with a high positive PC1 score for breast plumage had brighter feathers and less red chroma and was, therefore, less ornamented. The first principal component for structural rump colouration explained 64% of the variation among these variables and received strong loadings from brightness, hue, and UV chroma (0.45, −0.93, and 0.92, respectively). An individual with a high positive PC1 score for rump colour was more ornamented with brighter feathers, left-shifted hues, and greater UV chroma. The first principal component for tail colouration, which explained 60% of the variation among these variables, also received strong loadings from brightness, hue, and UV chroma (0.73, 0.73, −0.86). An individual with a high positive PC1 score for tail colour displayed bright feathers with right-shifted hues and decreased UV chroma (less ornamented).
Female Plumage
Melanin breast colouration (brightness and chroma) reduced to one principal component that explained 85% of the variation among these variables (brightness loading, 0.92; red chroma loading, −0.92). A female with a high positive PC1 score had brighter plumage and less red chroma and was, therefore, less ornamented. The first principal component for structural rump colouration explained 73% of the variation among these variables and received strong loadings from brightness, hue, and UV chroma (0.80, −0.80, and 0.95, respectively). An individual with a high positive PC1 score for rump colour was more ornamented with greater brightness and UV chroma and with a more left-shifted hue. The first principal component for tail colouration, which explained 52% of the variation among these variables, received strong loadings from hue and UV chroma (loadings; −0.91, 0.82). The second principal component for tail colouration, which explained 36% of the variation among these variables, received strong loading from brightness (0.94). Individuals with high positive PC1 scores for tail colour displayed feathers with increased UV chroma and a more left-shifted hue (more ornamented) and individuals with high positive PC2 scores for tail colour displayed brighter feathers.
Test of Assortative Mating
Preferential investment in sons is predicted when mothers are themselves in good condition (Trivers and Williard 1973). If high-quality/highly ornamented mothers feed sons more than daughters, and if these same females are mated to high-quality/highly ornamented males, then a correlation between paternal ornamentation and maternal investment in sons could exist without mothers adjusting allocation to offspring in relation to the ornamentation of their mates. An absence of correlation between bluebird mothers and fathers with respect to quality/ornamentation, however, would allow a more straightforward interpretation of data with respect to the original hypothesis we set out to test. Therefore, we examined the potential for assortative mating by exploring correlations between the ornamentation of mated pairs.
Statistical Analyses
To determine which factors influenced the proportion of feeding attempts parents directed to their sons, we used generalized linear models (PROC GENMOD in SAS). Our experimental design forced the bluebird parents in our study to repeatedly choose between one of two offspring when delivering food, resulting in binary data. Therefore, we used binomial error distribution and logit link functions in our models, as is typical in biological investigations of binary data using generalized linear models (Donazar et al. 1993; Bustamante 1997; Martinez et al. 2003). Differences in sample sizes (e.g. number of feeding attempts per trial) are also accounted for in generalized linear models, an additional benefit of using this approach (Wilson and Hardy 2002). Using these models we were able to examine how the plumage colouration of the parents (their mates and themselves), the number of feeding attempts to sons by each parent's mate, the number of offspring in each brood, the difference in mass between sons and daughters, and the age of the nestlings influenced the proportion of feeding attempts to sons. Our final model selection was based on Akaike information criterion (AIC) values wherein models with the lowest AIC values were considered the most parsimonious. For the sake of simplicity, we plotted only the relationships between parental feeding behavior and the most significant variable in each model.
Ethical Note
We began each trial early in the morning to minimize the time that fledglings were exposed to high temperatures and returned all juveniles to their nestboxes within 2.5 hrs of the start of each trial. Field observations of fledglings following preliminary trials indicate that our caging protocol did not influence survival to the fledgling stage. This study was approved by the Auburn University Internal Animal Care and Use Committee (IACUC project registration no. 2008-1341) and conducted under Alabama State and U.S. Fish and Wildlife permits.
RESULTS
Eastern bluebird mothers fed their offspring significantly more than fathers during our study trials (Paired t-test: t43 = 2.82, p < 0.01). On average, mothers fed their offspring 25.47 times per trial (± SD 22.48, n = 43), while fathers averaged only 14.60 ±12.69 feeds per trial.
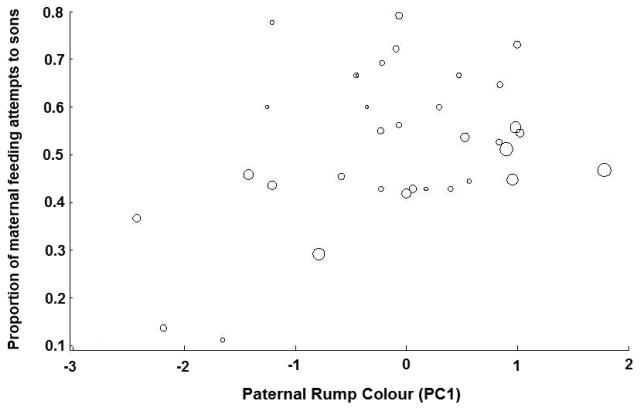
The generalized linear model with the lowest AIC value, built using data from 34 independent trials, included five predictive variables that explained a significant amount of variation in the proportion of feeding attempts that mother bluebirds directed towards their sons (Table 2). Specifically, we found significant relationships between the proportion of mothers' feeding attempts directed towards sons and the first principal component (PC1) of father's rump colouration, PC1 of father's tail colouration, PC1 of father's breast colour, the mother's own PC2 for tail colouration, and the number of offspring in the brood (Table 2). Females mated to males with more highly ornamented rumps (brighter, left-shifted hues, increased UV-chroma) increased their investment in sons (Fig. 2), as did females mated to males with greater tail ornamentation (left-shifted hues, increased UV-chroma, Fig. 3). Females also increased investment in sons when mated to males with brighter, less-red breast plumage (Fig. 4),when they themselves (mothers) had darker tails (Fig. 5), and when brood size was smaller (Table 2).
Table 2.
Optimized generalized linear model (lowest AIC value) for the proportion of feeding attempts that eastern bluebird mothers directed towards sons (out of their total feeding attempts).
| Parameter | Estimate | Standard Error |
Wald 95% Confidence Limits |
Chi- Square |
P value |
|
|---|---|---|---|---|---|---|
| Intercept | 0.55 | 0.25 | 0.05 | 1.04 | 4.74 | 0.03 |
| aFather rump PC1 | 0.19 | 0.07 | 0.06 | 0.32 | 8.66 | < 0.01 |
| aFather tail PC1 | −0.16 | 0.06 | −0.28 | −0.04 | 7.25 | < 0.01 |
| aFather breast PC1 | 0.21 | 0.08 | 0.05 | 0.38 | 6.51 | 0.01 |
| aMother tail PC2 | −0.16 | 0.07 | −0.29 | −0.03 | 5.85 | 0.02 |
| bBrood size | −0.16 | 0.07 | −0.29 | −0.03 | 5.74 | 0.02 |
Principal component plumage colour scores for the respective body regions
Number of offspring
Fig 2.
The relationship between paternal rump colour (PC1) and the proportion of maternal feeding attempts to sons. Higher PC1 scores indicate increased ornamentation. To facilitate a more accurate interpretation of the relative weights of each trial to the final model, symbol sizes are proportional to the number of maternal feeding attempts in each trial.
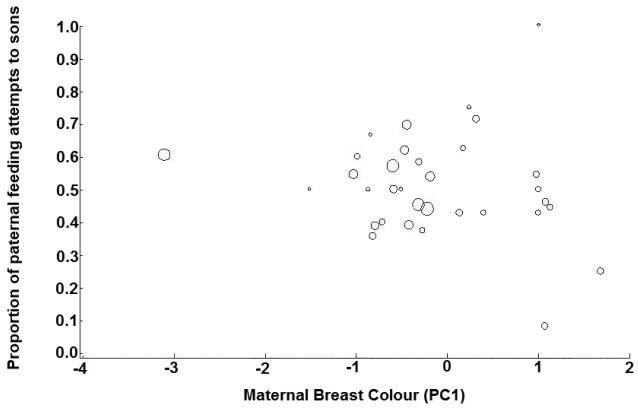
Fig 3.
The relationship between maternal breast colour (PC1) and the proportion of paternal feeding attempts to sons. Higher PC1 scores indicate increased ornamentation. Symbol sizes are proportional to the number of paternal feeding attempts in each trial.
The generalized linear model with the lowest AIC value for paternal feeding behavior, constructed using data from 34 feeding trials, contained only two predictive variables that explained a significant amount of variation in the proportion of feeding attempts that father bluebirds directed towards their sons (Table 3). Fathers fed sons relative to maternal breast ornamentation and in relation to their own breast plumage. Males mated to females with redder, darker breast plumage fed their sons more than their daughters, as did males with brighter, less-red breast plumage (Fig. 6).
Table 3.
Optimized generalized linear model (lowest AIC value) for the proportion of feeding attempts that eastern bluebird fathers directed towards sons (out of their total feeding attempts).
| Parameter | Estimate | Standard Error |
Wald 95% Confidence Limits |
Chi- Square |
P value |
|
|---|---|---|---|---|---|---|
| Intercept | − 0.12 | 0.10 | −0.31 | 0.07 | 1.52 | 0.22 |
| aMother breast PC1 | − 0.24 | 0.09 | −0.42 | −0.06 | 7.04 | 0.01 |
| aFather breast PC1 | 0.22 | 0.10 | 0.03 | 0.40 | 5.20 | 0.02 |
Principal component plumage colour scores for the respective body regions
Assortative Mating
None of the correlation coefficients between colour patches of mated fathers and mothers was statistically significant after we corrected the probability values for simultaneous tests by using the Bonferroni procedure (Table 4). The lack of assortative mating with respect to plumage ornamentation agrees with the results previously obtained examining this possibility in the same study population at an earlier time (Siefferman and Hill 2005b).
Table 4.
Uncorrected p-values and Pearson's correlation coefficients between principal component color scores of mated male and female bluebird parents (N = 37 pairs). None of the correlations was statistically significant after correction by the Bonferroni adjustment (corrected α = 0.004).
| Female Rump (PC1) |
Female Tail (PC1) |
Female Tail (PC2) |
Female Breast (PC1) |
|||||
|---|---|---|---|---|---|---|---|---|
| CCa | P | CCa | P | CCa | P | CCa | P | |
| Male Rump (PC1) | 0.09 | 0.59 | 0.02 | 0.92 | −0.16 | 0.33 | −0.39 | 0.02 |
| Male Tail (PC1) | −0.10 | 0.55 | −0.15 | 0.37 | −0.28 | 0.09 | −0.21 | 0.21 |
| Male Breast (PC1) | 0.02 | 0.93 | −0.14 | 0.42 | 0.07 | 0.68 | 0.14 | 0.42 |
Correlation Coefficient
DISCUSSION
Although the potential benefits associated with sex ratio manipulation in birds are numerous (Hasselquist and Kempenaers 2002), support for this possibility has not been universal (e.g. Dreiss et al. 2006; Saino et al. 1999). Nonetheless, we found a significant relationship between the plumage ornamentation of adult bluebirds and the parental feeding decisions of their mates. Specifically, females mated to males with more ornamented rump and tail feathers increased their feeding attempts to fledgling sons relative to daughters. Maternal investment in sons also increased when females were mated to males with brighter, less-red breast plumage, when mothers had darker tail feathers, and when broods were smaller. Additionally, paternal feeding decisions appear to be influenced by the breast colouration of their mates such that males mated to females with redder, darker breast plumage exhibited increased provisioning effort to sons. Taken together, these results support our predictions regarding the influence of mate ornamentation on parental feeding decisions.
Female eastern bluebirds should benefit from choosing brighter, generally older, males as mates because of the increased provisioning investment provided by such males (Siefferman and Hill 2003) and the inherent quality of males that have survived to an advanced age (Kokko and Lindström 1996). In this study, female bluebirds behaved as predicted if they are assessing the plumage colour of their mates when making reproductive decisions. Mothers fed sons at higher rates when mated to males with highly ornamented structural plumage (increased UV-chroma, left-shifted hues) and increased feeding attempts to sons when mated to males with melanin-based breast plumage qualities typical of older males (brighter plumage, less red-chroma; Seifferman et al. 2005). However, experimental mate-choice studies have shown that female bluebirds do not consistently choose males with brighter plumage (Liu et al. 2007, Liu et al. 2009). The lack of preference for traits that apparently signal multiple aspects of male quality (Siefferman and Hill 2003; Siefferman and Hill 2005a; Siefferman and Hill 2005c) presents something of a conundrum. One possible explanation is that females assess territory quality rather than male ornamentation when choosing mates. If highly ornamented males hold higher quality territories (Siefferman & Hill 2005c), then the observed relationship between paternal ornamentation and maternal investment in sons could arise if females made parenting decisions based solely on habitat quality. Only by experimentally manipulating adult plumage can we be entirely sure of the direct impact of mate plumage on parental feeding decisions, and such manipulations are an obvious next step in examining the factors influencing sex-biased provisioning.
In this study, bluebird mothers varied investment in sons and daughters relative to the ornamentation of their mates, but females' provisioning decisions were also tied to their own tail colouration. Mothers with darker (less bright) tail feathers increased provisioning attempts to sons. Given that the brightness of structural plumage is condition-dependent in female eastern bluebirds (Siefferman and Hill 2005b), this relationship runs counter to predictions of the Trivers and Willard (1973) hypothesis. Females in poor condition (less ornamented plumage) should invest more in daughters (Trivers and Willard 1973), given the lower variance in reproductive success for daughters relative to sons. At this point, we have no simple explanation for why drab females feed sons more.
We predicted that bluebird fathers, like bluebird mothers, would adjust provisioning to sons and daughters relative to the ornamentation of their mates. Indeed, we found that fathers increased investment in sons when mated to females with darker, redder breast plumage, a finding supporting our general prediction. Melanin-based plumage has frequently been found to serve a signaling role in antagonistic intraspecific interactions (Senar 2006), though no such function has been shown in eastern bluebirds. If, in fact, melanin breast plumage of female bluebirds conveys information about how individuals fare in intrasexual competition (for mates or resources), then males that assess such signals when making offspring investment decisions might increase their own fitness. Contrary to our findings regarding melanin-based plumage, we did not discover any relationship between the structural plumage colour of mothers and the feeding decisions of fathers, despite the fact that such plumage appears to be condition-dependent in female bluebirds (Siefferman and Hill 2005b). However, fathers performed significantly fewer feeding attempts per trial than mothers, suggesting that males follow a different overall provisioning strategy compared to females. The contribution of eastern bluebird fathers to fledglings may come primarily in the form of nest and territory defense (e.g. Gibson and Moehrenshlager 2008; Hogstad 2005; Rytkönen et al. 1993). In support of this idea, there is an increased tendency for mountain bluebird Sialia currucoides fathers to physically attack model predators during the latter stages of the nesting cycles (Gibson and Moehrenshlager 2008). If paternal investment is primarily comprised of non-feeding behaviors at the fledgling stage, then the costs (Siefferman and Hill 2008) and benefits of selective feeding are low and a paternal strategy of assessing maternal ornaments to optimize feeding decisions might not have evolved. Alternatively, the low rate of food delivery by males could have obscured all but the strongest effects of male assessment of mate quality on allocation decisions.
In addition to assessing female breast ornamentation, fathers made feeding decisions with respect to their own breast plumage. Males with brighter, less red-chromatic breast plumage (typical of older males, Siefferman et al. 2005) fed their sons more than their daughters. In eastern bluebirds, age and breast colour are tightly linked (Siefferman et al. 2005) such that males with brighter breasts are older. If age is a predictor of individual quality (Kokko and Lindström 1996), then increased investment in sons by brighter males is simply a greater investment in sons by older, higher quality individuals.
Why should bluebird parents invest differentially in sons and daughters during the fledgling stage? We propose three explanations to account for the delayed nature of the sex-specific investment we have demonstrated here. First, flexible investment strategies that can be adjusted as environmental variables change should be favored over those strategies that only take effect at the beginning of parental investment (Trivers and Willard 1973), although parents should generally be favored to make such adjustments as early as possible. Second, the cues that bluebird parents use to discriminate between sons and daughters may not be available throughout the entire duration of parental investment–the structural blue plumage of juvenile eastern bluebirds does not appear until relatively late in development (Gowaty and Plissner 1998). Although nestling vocalizations may enable parents to discriminate sons from daughters in some bird species (e.g. barn swallows, Saino et al. 2003), there are no differences in vocalizations between male and female nestlings in the closely related western bluebird Sialia mexicana (Monk and Koenig 1997). Adults may, therefore, wait to invest differentially in sons and daughters until they can tell them apart, i.e. when they can evaluate plumage differences outside of the nest. Third, the demands placed on bluebird parents likely correspond to the increasing energetic demands of offspring as they grow (Ricklefs and Williams 1984). As the cost of provisioning nestlings increases throughout the nesting period, parents should benefit by becoming increasingly selective about which of their offspring receive food. If costliness of provisioning offspring increases choosiness by parents with respect to which offspring they feed, then the increased selectivity shown by provisioning bluebird mothers (i.e. the correlation between paternal ornamentation and maternal investment in sons) fits with previously discovered sex-specific costs of reproduction in bluebirds wherein mothers bear a larger costs of reproduction than fathers (Siefferman and Hill 2008).
While the potential mechanisms of primary sex-ratio manipulation in birds are poorly understood (reviewed in Pike and Petrie 2003), the mechanisms by which parental behaviors can influence sex allocation and sex ratio are relatively straightforward. To change secondary offspring sex-ratio, parents can 1) selectively destroy offspring of one sex at an early stage (Charnov 1982), 2) selectively incubate eggs of different sexes (Ligon and Ligon 1990; Pike and Petrie 2003), and 3) they can give more food to offspring of a particular sex (Charnov 1982). There are obvious costs to the first and second strategies, namely the loss of energy and time expended in producing a fertile egg and/or incubating it. However, the third strategy is plastic and allows parents to match allocation decisions with current environmental conditions. Such a benefit might be particularly important for species in which the period of parental care is extended and during which time the optimal investment strategy (sons vs. daughters) may change.
Ornamental traits have been a focus of interest among evolutionary biologists since the discussions of Darwin and Wallace (Cronin 1991), but most studies of such traits in birds have focused on ornaments in the context of female mate choice (Hill 2006) or male-male competition (Senar 2006). However, if ornamental traits are condition-dependent signals of quality they should be assessed in contexts other than mate choice and competition. Here we show evidence that female bluebirds assess male quality via expression of plumage colouration as a means to optimize resource allocation among offspring. It seems probable that assessment of condition-dependent ornaments occurs across a wide range of contexts, but additional experimental tests are required to confirm such possibilities.
ACKNOWLEDGEMENTS
We thank T. Parker, K.J. Navara, J.D. Ligon, C. Okekpe, P. Dunn, J. Quesada and members of the Hill lab group for suggestions improving this manuscript. Additionally, we thank M. Liu for guidance with laboratory procedures, M. Buschow, and J. Hill for assistance with data collection, R. Montgomery for the use of his program Colour, S. H. Ligon for her assistance logging video data, and V. Rebal for her assistance taping thousands of feathers and her unfailing support to RAL throughout the duration of this project. This research was supported by a grant from the National Institute of Allergy and Infectious Diseases, Project # R01AI049724 to GEH and by grants from the Birmingham Audubon Society, Sigma Xi, and the American Ornithologists' Union to RAL.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE CITED
- Andersson M. Sexual Selection. Princeton University Press; Princeton: 1994. [Google Scholar]
- Bateman AJ. Intra-sexual selection in Drosophila. Heredity. 1948;2:349–368. doi: 10.1038/hdy.1948.21. [DOI] [PubMed] [Google Scholar]
- Blank JL, Nolan V. Offspring sex-ratio in red-winged blackbirds is dependent on maternal age. Proceedings of the National Academy of Sciences. 1983;80:6141–6145. doi: 10.1073/pnas.80.19.6141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury RR, Blakey JK. Diet, maternal condition, and offspring sex ratio in the zebra finch, Poephila guttata. Proceedings of the Royal Society B. 1998;265:895–899. [Google Scholar]
- Burley N. The differential-allocation hypothesis: an experimental test. American Naturalist. 1988;132:611–628. [Google Scholar]
- Bustamante J. Predictive models for Lesser Kestrel Falco naumanni distribution, abundance, and extinction in southern Spain. Biological Conservation. 1997;80:153–160. [Google Scholar]
- Charnov EL. The Theory of Sex Allocation. Princeton University Press; Princeton: 1982. [PubMed] [Google Scholar]
- Clutton-Brock TH. Reproductive Success. In: Clutton-Brock TH, editor. Reproductive Success. University of Chicago Press; Chicago: 1988. pp. 472–485. [Google Scholar]
- Cronin H. The Ant and the Peacock. Cambridge University Press; Princeton: 1991. [Google Scholar]
- Dijkstra C, Daan S, Buker JB. Adaptive seasonal variation in the sex ratio of kestrel broods. Functional Ecology. 1990;4:143–147. [Google Scholar]
- Donazar JA, Hiraldo F, Bustamante J. Factors influencing nest site selection, breeding density and breeding success in the bearded vulture (Gypaetus barbatus) Journal of Applied Ecology. 1993;30:504–514. [Google Scholar]
- Dreiss A, Richard M, Moyen F, White J, Møller AP, Danchin E. Sex ratio and male sexual characters in a population of blue tits, Parus caeruleus. Behavioral Ecology. 2006;17:13–19. [Google Scholar]
- Gibson KW, Moehrenschlager A. A sex difference in the behavioural response of nesting mountain bluebirds (Sialia currucoides) to a mounted predator. Ethology. 2008;26:185–189. [Google Scholar]
- Gowaty PA, Plissner JH. Eastern Bluebird (Sialia sialis) In: Poole A, editor. The Birds of North America Online. No. 381. Cornell Lab of Ornithology, Ithaca; New York: 1998. doi:10.2173/bna.381. http://bna.birds.cornell.edu/bna/species/381. [Google Scholar]
- Hill GE. Female Mate Choice for Ornamental Coloration. In: Hill GE GE, McGraw KJ, editors. Bird Coloration Volume II: Function and Evolution. Harvard University Press; Harvard: 2006. pp. 137–200. [Google Scholar]
- Hoelzer GA. The good parent process of sexual selection. Animal Behaviour. 1989;38:1067–1078. [Google Scholar]
- Hogstad O. Sex-differences in nest defence in Fieldfares Turdus pilaris in relation to their size and physical condition. Ibis. 2005;147:375–380. [Google Scholar]
- Isaac JL, Krockenberger AK, Johnson CN. Adaptive sex allocation in relation to life-history in the common brushtail possum, Trichosurus vulpecula. Journal of Animal Ecology. 2005;74:552–558. [Google Scholar]
- Johnsen A, Delhey K, Schlicht E, Peters A, Kempenaers B. Male sexual attractiveness and parental effort in blue tits: a test of the differential allocation hypothesis. Animimal Behaviour. 2005;70:877–888. [Google Scholar]
- Kilner RM. Function and Evolution of Color in Young Birds. In: Hill GE GE, McGraw KJ, editors. Bird Coloration Volume II: Function and Evolution. Harvard University Press; Harvard: 2006. pp. 201–232. [Google Scholar]
- Kokko H, Lindström J. Evolution of female preference for old mates. Proceedings of the Royal Society B. 1996;263:1533–1538. [Google Scholar]
- Ligon JD, Ligon SH. Female-biased sex ratio at hatching in the green woodhoopoe. Auk. 1990;107:765–771. [Google Scholar]
- Limbourg T, Mateman AC, Andersson S, Lessells CM. Female blue tits adjust parental effort to manipulated male UV attractiveness. Proceedings of the Royal Society B. 2004;271:1903–1908. doi: 10.1098/rspb.2004.2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Siefferman L, Hill GE. An experimental test of female choice relative to male structural coloration in eastern bluebirds. Behavioral Ecology & Sociobiology. 2007;61:623–630. doi: 10.1007/s00265-007-0416-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Siefferman L, Mays H, Steffen JE, Hill GE. A field test of female mate preference for male plumage coloration in eastern bluebirds. Animal Behaviour. 2009;78:879–885. doi: 10.1016/j.anbehav.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lope F, Moller AP. Female reproductive effort depends on the degree of ornamentation of their mates. Evolution. 1993;47:1152–1160. doi: 10.1111/j.1558-5646.1993.tb02142.x. [DOI] [PubMed] [Google Scholar]
- Martinez JA, Serrano D, Zuberogoitia I. Predictive models of habitat preferences for the Eurasian eagle owl Bubo bubo: a multiscale approach. Ecography. 2003;26:21–28. [Google Scholar]
- Monk DS, Koenig WD. Individual, brood, and sex variation in begging calls of Western Bluebirds. Wilson Bulletin. 1997;109:328–332. [Google Scholar]
- Opit GP, Throne JE. Effects of diet on population growth of psocids Lepinotus reticulatus and Liposcelis entomophila. Journal of Economic Entomology. 2008;101:616–622. doi: 10.1603/0022-0493(2008)101[616:eodopg]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Örnborg J, Andersson S, Griffith SC, Sheldon BC. Seasonal changes in the ultraviolet structural colour signals in blue tits, Parus caeruleus. Biological Journal of the Linnean Society. 2002;76:237–245. [Google Scholar]
- Pike TW, Petrie M. Potential mechanisms of avian sex manipulation. Biological Reviews. 2003;78:553–574. doi: 10.1017/s1464793103006146. [DOI] [PubMed] [Google Scholar]
- Quesada J, Senar JC. Comparing plumage colour measurements obtained directly from live birds and from collected feathers: the case of the great tit Parus major. Journal of Avian Biology. 2006;37:609–616. [Google Scholar]
- Ricklefs RE, Williams JB. Daily energy expenditure and water-turnover rate of adult European Starlings (Sturnus vulgaris) during the nesting cycle. Auk. 1984;101:707–716. [Google Scholar]
- Roed KH, Holand O, Mysterud A, Tveradal A, Kumpula J, Nieminen M. Male phenotypic quality influences offspring sex ratio in a polygynous ungulate. Proceedings of the Royal Society B. 2007;274:727–733. doi: 10.1098/rspb.2006.0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rytkönen S, Orell M, Kolvula K. Sex-role reversal in Willow Tit nest defense. Behavioral Ecology & Sociobiology. 1993;33:275–282. [Google Scholar]
- Saino N, Ellegren H, Møller AP. No evidence for adjustment of sex allocation in relation to paternal ornamentation and paternity in barn swallows. Molecular Ecology. 1999;8:399–406. [Google Scholar]
- Saino N, Galeotti P, Sacchi R, Boncoraglio G, Martinelli R, Moller AP. Sex differences in begging vocalizations of nestling barn swallows, Hirundo rustica. Animal Behaviour. 2003;66:1003–1010. [Google Scholar]
- Sakisaka Y, Yahara T, Miura I, Kasuya E. Maternal control of sex ratio in Rana rugosa: evidence from DNA sexing. Molecular Ecology. 2000;9:1711–1715. doi: 10.1046/j.1365-294x.2000.01038.x. [DOI] [PubMed] [Google Scholar]
- Schultz ET. A sex difference in seasonal timing of birth in a livebearing fish. Copeia. 2008;3:673–679. [Google Scholar]
- Senar JC. Bird colors as intrasexual signals of aggression and dominance. In: Hill GE GE, McGraw KJ, editors. Bird Coloration Volume II: Function and Evolution. Harvard University Press; Harvard: 2006. pp. 87–136. [Google Scholar]
- Sheldon BC, Andersson S, Griffith SC, Örnborg J, Sendecka J. Ultraviolet colour variation influences blue tit sex ratios. Nature. 1999;402:874–877. [Google Scholar]
- Siefferman L, Hill GE. Structural and melanin coloration indicate parental effort and reproductive success in male eastern bluebirds. Behavioral Ecology. 2003;14:855–861. [Google Scholar]
- Siefferman L, Hill GE. Blue structural coloration of male eastern bluebirds Sialia sialis predicts incubation provisioning to females. Journal of Avian Biology. 2005a;36:488–493. [Google Scholar]
- Siefferman L, Hill GE. Evidence for sexual selection on structural plumage coloration in female eastern bluebirds (Sialia sialis) Evolution. 2005b;59:1819–1828. [PubMed] [Google Scholar]
- Siefferman L, Hill GE. UV-blue structural coloration and competition for nestboxes in male eastern bluebirds. Animal Behaviour. 2005c;69:67–72. [Google Scholar]
- Siefferman L, Hill GE. Male eastern bluebirds trade future ornamentation for current reproductive investment. Biology Letters. 2005d;1:208–211. doi: 10.1098/rsbl.2004.0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siefferman L, Hill GE. The effect of rearing environment on blue structural coloration of eastern bluebirds (Sialia sialis) Behavioral Ecology & Sociobiology. 2007;61:1839–1846. doi: 10.1007/s00265-007-0416-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siefferman L, Hill GE, Dobson FS. Ornamental plumage coloration and condition are dependent on age in eastern bluebirds Sialia sialis. Journal of Avian Biology. 2005;36:428–435. [Google Scholar]
- Siefferman L, Hill GE. Sex-specific costs of reproduction in Eastern Bluebirds Sialia sialis. Ibis. 2008;150:32–39. doi: 10.1111/j.1474-919X.2007.00723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamps J. When should avian parents differentially provision sons and daughters? American Naturalist. 1990;135:671–685. [Google Scholar]
- Svensson E, Nilsson JA. Mate quality affects offspring sex ratio in blue tits. Proceedings of the Royal Society B. 1996;263:357–361. [Google Scholar]
- Trivers RL, Willard DE. Natural Selection of Parental Ability to Vary the Sex Ratio of Offspring. Science. 1973;179:90–92. doi: 10.1126/science.179.4068.90. [DOI] [PubMed] [Google Scholar]
- Wilson K, Hardy ICW. Statistical analysis of sex ratios: an introduction. In: Hardy ICW, editor. Sex Ratios: Concepts and Research Methods. Cambridge University Press; Cambridge: 2002. pp. 48–92. [Google Scholar]