Abstract
Objective
To evaluate the effect of atomoxetine hydrochloride versus placebo on attention-deficit/hyperactivity disorder (ADHD) and substance use disorder (SUD) in adolescents receiving motivational interviewing / cognitive behavioral therapy (MI/CBT) for SUD.
Method
This single-site, randomized, controlled trial was conducted between December 2005 and February 2008. Seventy adolescents (13-19 years) with Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) (DSM-IV) ADHD, a DSM-IV ADHD checklist score greater than or equal to 22, and at least one non-tobacco SUD were recruited from the community. All subjects received 12 weeks of atomoxetine hydrochloride + MI/CBT versus placebo + MI/CBT. The main outcome measure for ADHD was self-report DSM-IV ADHD checklist score. For SUD, the main outcome was self-report number of days used non-tobacco substances in the past 28 days using the Timeline Followback interview.
Results
Change in ADHD scores did not differ between atomoxetine + MI/CBT and placebo + MI/CBT (F4,191 = 1.23, p = 0.2975). Change in days used non-nicotine substances in the last 28 days did not differ between groups (F3,100 = 2.06, p = 0.1103).
Conclusions
There was no significant difference between the atomoxetine + MI/CBT and placebo + MI/CBT groups in ADHD or substance use change. The MI/CBT and/or a placebo effect may have contributed to a large treatment response in the placebo group.
Keywords: attention-deficit/hyperactivity disorder, adolescent, atomoxetine, substance use disorder
Background
Adolescents presenting for substance use disorder (SUD) treatment have rates of attention-deficit/hyperactivity disorder (ADHD) ranging from 30 to 50%.1-3 Co-occurring ADHD is associated with more severe substance use and worse SUD outcomes 3-4, but it is not known if treating ADHD during SUD treatment improves substance use.
There are two published, controlled medication trials for ADHD in adolescents with SUD.5,6 Both found medication (pemoline5 and methylphenidate-SODAS6) to be more efficacious than placebo for ADHD in adolescents not in SUD treatment. Neither study observed an impact on substance use.
At least four controlled pharmacotherapy trials for ADHD have been conducted in adults with co-occurring SUD. These include trials of atomoxetine, methylphenidate, and bupropion.7-10 The three trials that included patients concurrently enrolled in outpatient cognitive behavioral therapy (CBT) for SUD showed no differences between medication (methylphenidate or bupropion) and placebo for ADHD or SUD. 7-9 A trial that did not allow concurrent substance treatment showed a greater decline in ADHD symptoms and days of heavy drinking in the atomoxetine, compared to placebo, group.10
In summary, there are two main findings from the above studies. First, ADHD medications seem to be safe in patients with SUD and do not make SUD worse. Second, no study has shown a clear difference between active medication and placebo in patients with ADHD who are in SUD treatment. Interestingly, all three studies that included patients in outpatient SUD treatment attributed the reduction in ADHD symptoms in the placebo group to the CBT psychosocial intervention, 7-9 and the only studies to find a difference between medication and placebo did not allow concurrent SUD treatment.5,6,10
However, it is not known if these findings apply to adolescents enrolled in substance treatment, and the impact of ADHD treatment on both ADHD and SUD in this population is not known. These are the research gaps that this 12-week, double-blind, randomized, controlled trial of atomoxetine seeks to address. Atomoxetine was chosen as the study medication because of its limited abuse potential11. The primary study hypothesis was that atomoxetine would be more efficacious than placebo for ADHD in teens enrolled in substance treatment. The secondary hypothesis was that atomoxetine, compared to placebo, would lead to a greater decline in substance use in this population.
Method
Subjects
Subjects were 70 adolescents with the following inclusion/exclusion criteria: 1) age 13-19 years; 2) ability to understand and provide written, informed parental consent and minor assent, if under 18 years old, or individual consent if 18 years or older; 3) a diagnosis of ADHD using the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV)12 criteria and an adolescent, self-report DSM-IV ADHD checklist score greater than or equal to 22; 4) DSM-IV diagnosis of at least one non-nicotine SUD, 5) plans to live locally for at least four months; and 6) willingness to participate in motivational interviewing/cognitive behavioral therapy (MI/CBT) for SUD during the medication trial.
Exclusion criteria were: 1) mental illness that could not be managed as an outpatient (e.g. serious suicidal ideation), or without concurrent psychotropic medication; 2) history of bipolar disorder or psychosis; 3) medical contraindication to taking atomoxetine; 4) pregnancy, breast feeding, or unwillingness to use an effective form of birth control while in the study; and 5) SUD that could not be managed as an outpatient or without concurrent psychotropic medications (e.g. alcohol withdrawal, opioid withdrawal).
Study enrollment began in December 2005 and the last study visit occurred in February 2008.
Procedures
Prior approval was obtained from the Colorado Multiple Institutional Review Board. Written, informed consent was obtained from all subjects who were 18 years old or older. For minor subjects, informed consent from a guardian as well as written, minor assent were obtained. A Federal Certificate of Confidentiality strengthened the confidentiality of research data.
Active medication and placebo were supplied by the manufacturer (Eli Lilly & Company). A non-blinded research pharmacist bottled the medication and prepared the medication kits. The research pharmacist randomly assigned eligible participants to receive atomoxetine or placebo using a block size of 10 to balance the treatment assignment. All other research staff were blind to the medication assignment but not to the block size.
Participants weighing less than 70 kg were started at 0.5 mg/kg to 0.75 mg/kg per day and increased by 25 mg per week until their total dose was between 1.1 mg/kg and 1.5 mg/kg. Participants weighing more than 70 kg started at 50 mg per day and increased to 75 mg per day in the second week and 100 mg in the third week. Those who had significant side effects remained at their current dose, and an attempt was made to titrate the medication the following week. If side effects continued, the participant was maintained at the highest tolerable dose of medication. Subjects were instructed to take the study medication once daily in the morning.
All participants received weekly, individual, MI/CBT for SUD. The manual that was used was chosen because of its feasibility and empirical support as a psychosocial treatment for SUD in adolescents with co-occurring psychiatric and substance use disorders.13 Other studies also demonstrate the efficacy of MI/CBT as a treatment for adolescent SUD.1,14 The MI/CBT consisted of hour-long, weekly individual sessions and could include up to three family sessions. Cognitive, behavioral, and motivational techniques were used to help adolescents reduce their drug use and improve coping. Core modules included goal setting, a functional analysis of drug use, and coping with cravings. Subsequent modules included anger management, communication skills, mood management, drug refusal skills, and problem solving. The principal investigator and one of the research therapists were trained by the manual's developers. The principal investigator then trained the other five research therapists. Each therapist was audiotaped at least once during the study and chose a convenient session for the taping. A total of 12 sessions from a variety of different modules in the MI/CBT manual were reviewed by the principal investigator for fidelity and adherence, and no session fell below standards developed before the study began. The clinical program offered the MI/CBT sessions at no cost to research participants. There was also no cost to participants for the study procedures and medication.
Diagnostic and Outcome Measures
Baseline DSM-IV diagnoses of ADHD and SUD, were determined with the Kiddie Schedule for Affective Disorders and Schizophrenia - Present and Lifetime version (KSADS-PL), a semi-structured diagnostic interview with good reliability and validity in children and adolescents.15 This interview was administered at baseline by a board certified addiction and child/adolescent psychiatrist (C.T.).
The primary outcome measure for ADHD was the adolescent report DSM-IV ADHD symptom checklist 12 administered by study staff. This instrument measures all 18 ADHD criteria on a scale of 0 (“not present”) to 3 (“severe”). The scores are added to yield a result ranging from 0 (minimum) to 54 (maximum). Eliciting self-report of ADHD symptoms has been shown to be sensitive to medication effects in randomized, controlled trials of adults.16,17 A secondary ADHD outcome measure was the DSM-IV ADHD checklist administered monthly to the parents or guardians of minor children. This has been shown to be a reliable and valid method of assessing ADHD outcomes in pediatric samples.18 Adolescent self-report was used as the primary outcome measure of ADHD to enhance feasibility of data collection. At baseline, the principal investigator interviewed adolescents and guardians together to complete the DSM-IV ADHD checklist. Subsequent interviews with the adolescent were always face-to-face but the guardian interviews were either in person or by telephone. The principal investigator trained six research assistants (1 master's level, 4 bachelor's level, 1 with 3 years of college) in how to administer this DSM-IV ADHD symptom checklist. The training consisted of watching the principal investigator administer the instrument once and then being observed once by the principal investigator, who provided verbal feedback.
The Clinician Global Impression-Improvement (CGI-I) rating,19 as assessed by the study physician (C.T.) was also used as a secondary outcome measure of ADHD. A priori, subjects with a score of 1 (very much improved) or 2 (much improved) were considered ADHD responders.
The primary outcome measure for substance use was confidential, self-report number of days used substances (not including nicotine) in the past 28 days. This outcome was assessed using Timeline Followback (TLFB) procedures.20 This clinician-administered instrument records and sums the number of days used substances on a 28 day calendar. Urine drug screens (UDS) were collected at baseline and every other week (total possible UDS's = 7) and were sent to a laboratory (Redwood Toxicology Laboratory, Inc.) for evaluation of adulterants, alcohol, amphetamines, barbituates, benzodiazepines, cocaine, opiates, phencyclidine, and tetradyrdocannabinol. The sum of of drug-free UDS's was used as a secondary outcome measure for substance use.
Adverse events were assessed weekly with the Side Effects Form for Children and Adolescents (SEFCA).21 This form systematically assesses 52 possible side effects and has been widely used in pediatric samples. Safety was also assessed with weekly measurement of blood pressure and pulse, monthly measurement of weight, and pre/post serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST). Suicidal ideation, intent, and behavior were assessed weekly with the Treatment of Resistant Depression in Adolescents (TORDIA) suicide instrument.22 This instrument consists of two scales, one measuring suicidal ideation and intent and another assessing suicidal behavior. These scales measure suicidality on a scale of 0 (none) to 5 (e.g. “active suicidal ideation with plan and intent”). Medication adherence was assessed with a combination of weekly self-report and pill counts and was defined as the sum of days in which any of the prescribed study medication was taken divided by the total number of days a participant was prescribed medication. If a participant was prescribed no study medication (e.g. the study medication needed to be discontinued for clinical or safety reasons), then s/he was considered compliant for the days in which no medication was taken.
Statistical Methods
Data collection, entry, verification, transfer, confidentiality, storage, and analyses were conducted under the direction of the principal investigator (C.T.) and biostatistician (S.M.G.). Analyses were conducted using SPSS versions 15 23 and 17 24 and longitudinal analyses were completed using SAS 9.2.25 Baseline comparisons between atomoxetine + MI/CBT and placebo + MI/CBT groups on demographic and key variables were assessed with independent t-tests and chi-square tests, or non-parametric Mann-Whitney U and Fisher's exact tests, respectively, when the parametric assumptions were not upheld. Independent t-tests (or Mann-Whitney U tests, when necessary) were utilized to compare groups on: pre-post change in blood pressure, pulse, and weight; adolescent self-report ADHD scores; number of days used non-tobacco substances in the past 28 days; change in serum ALT and AST; medication adherence; and the number of negative UDS's. Each item of the SEFCA was dichotomized (the symptom occurred one or more times versus no times) and Pearson chi-square analyses or Fisher's exact tests compared groups on each item.
The longitudinal course of days of non-nicotine substance use, adolescent-report ADHD score, and parent report ADHD score were evaluated with mixed models using SAS 9.2.25 Models for each outcome variable included a fixed treatment effect (atomoxetine + MI/CBT vs. placebo + MI/CBT), a fixed time effect, and a treatment by time interaction, which estimated the average group-specific intercepts, rates of change over time, and group-specific differences in that rate, respectively.
The fixed time effect was measured as days since randomization. Although baseline assessments occurred up to 0 to 34 days before randomization, baseline dates for all three assessments were changed to the randomization date (i.e. day 0) to eliminate negative values for days. The rationale for this approach was based on the assumption that treatment (atomoxetine + MI/CBT or placebo + MI/CBT) did not affect the outcome variables before participants were randomized. In addition, the models included random effects which allowed the intercepts and curves (e.g., slope) of each fitted line to vary by subject. Polynomial models up to a cubic relationship with time in the fixed and random effects for all three outcome variables were assessed. The best fitting model for each variable was selected with likelihood ratio tests and minimum values for Akaike's Information Criterion,26 and we used variability estimates from those selected models to calculate the effect sizes that the completed study was powered to detect. For the parent report DSM-IV ADHD checklist, data from guardians who had minor children and who consented to participate were used (n = 56).
For the ADHD CGI-I assessment, a Pearson chi-square analysis was used to compare the number of completers (n = 65) in atomoxetine + MI/CBT versus placebo + MI/CBT who had a score < 3 (very much improved or much improved) at the end of the study.
All analyses used a two-tailed 0.05 significance level.
Results
Patient Disposition
A total of 91 adolescents were screened in person or by telephone (Figure 1). Of the 91 teens who were screened, 77 (85%) signed consent for full evaluation of inclusion and exclusion criteria. A total of 70 were randomized to atomoxetine + MI/CBT (N=35) or placebo + MI/CBT (N=35) and included in the analyses. Five (3 atomoxetine and 2 placebo) participants did not complete the study because they were lost to follow-up.
Figure 1.
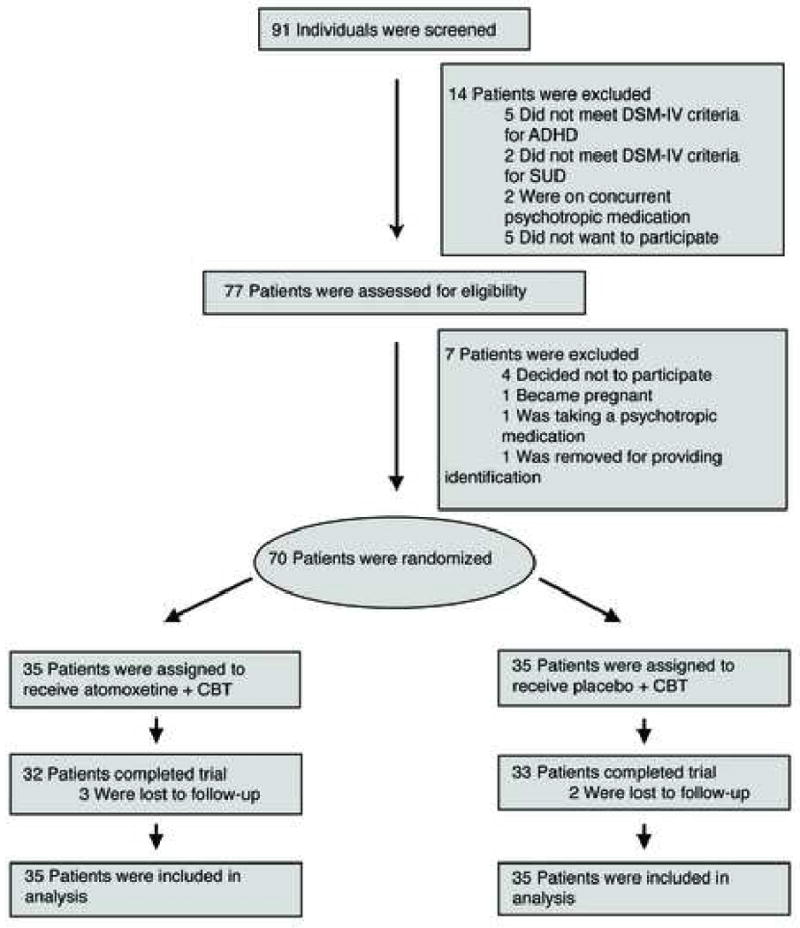
Patient Disposition. Note: ADHD = Attention-Deficit/Hyperactivity Disorder; CBT = Cognitive Behavioral Therapy; SUD = Substance Use Disorder
Table 1 describes the baseline variables for the sample. In general, the sample was predominantly male and Latino. Overall, 53 (75.7%) had ADHD, combined type, 12 (17.1%) had predominantly inattentive subtype, and 5 (7.1%) had predominantly hyperactive-impulsive subtype. Subjects in the atomoxetine group completed an average of 8.2 (SD = 2.6, range = 2-12) MI/CBT sessions, compared to 8.1 (SD = 2.9, range = 0 to 12) sessions for the placebo group (U = 606.5, p = 0.943).
Table 1.
Baseline Values by Treatment Group
| Atomoxetine + MI/CBT n = 35 n (%) or mean (SD) | Placebo + MI/CBT n = 35 n (%) or mean (SD) | Statistic | P-value | |
|---|---|---|---|---|
| Gender | ||||
| Female | 10 (28.6%) | 5 (14.3%) | χ21= 2.12 | 0.145 |
| Male | 25 (71.4%) | 30 (85.7%) | ||
| Ethnicity | ||||
| Hispanic/Latino | 20 (57.1%) | 28 (80%) | χ21= 4.24 | 0.039 |
| Not Hispanic Latino | 15 (42.9%) | 7 (20%) | ||
| Racea | ||||
| White | 11 (31.4%) | 2 (5.7%) | χ21 = 7.65 | 0.006 |
| Non-white | 24 (68.6%) | 33 (94.3%) | ||
| American Indian/Alaska Native | 0 | 2 (5.7%) | ||
| Asian | 0 | 1 (2.9%) | ||
| Black or African American | 3 (8.6%) | 3 (8.6%) | ||
| More than one race | 4 (11.4%) | 3 (8.6%) | ||
| Other | 17 (48.6%) | 24 (68.6%) | ||
| Age, in years | 16.06 (1.35) | 16.11 (1.78) | t63.4 = 0.15 | 0.88 |
| Baseline adolescent report ADHD score | 41.6 (7.24) | 38.46 (8.73) | t68 = 1.64 | 0.106 |
| Baseline parent report ADHD score | 42.63 (9.1) n = 30 | 41.77 (9.72) n = 26 | U = 375 | 0.805 |
| Baseline days of non-nicotine substance use | 18.37 (9.73) | 17.14 (10.4) | U = 590.5 | 0.794 |
| # Non-nicotine SUD diagnoses b | ||||
| 1 | 29 (82.9%) | 21 (60%) | χ21 = 4.48 | 0.0344 |
| 2 | 6 (17.1%) | 13 (37.1%) | ||
| 3 | 0 | 1 (2.9%) | ||
| SUD diagnoses | ||||
| Alcohol use disorder | 7 (20%) | 13 (37.1%) | χ21 = 2.52 | 0.112 |
| Cannabis use disorder | 34 (97.1%) | 33 (94.3%) | Fisher's Exact Test | 1 |
| Nicotine dependence | 21 (60%) | 19 (54.3%) | χ21= 0.23 | 0.629 |
| Cocaine use disorder | 0 | 2 (5.7%) | Fisher's Exact Test | 0.49 |
| Amphetamine use disorder | 0 | 1 (2.9%) | Fisher's Exact Test | 1 |
| Hallucinogen use disorder | 0 | 1 (2.9%) | Fisher's Exact Test | 1 |
| Psychiatric diagnoses | ||||
| Conduct disorder | 18 (51.4%) | 19 (54.3) | χ21 = 0.06 | 0.811 |
| Major depressive disorder | 9 (25.7%) | 11 (31.4%) | χ21= 2.52 | 0.597 |
Notes: ADHD = attention/deficit-hyperactivity disorder, MI/CBT = motivational interviewing/cognitive behavioral therapy, SUD = substance use disorder
A chi-square analysis was completed with two categories (white and non-white). The non-white category was composed of American Indian/Alaska native, Asian, black or African American, more than one race, and other.
A chi-square analysis was completed with two categories (1 diagnosis and 2-3 diagnoses).
Overall, the percentage of days during which at least a portion of the prescribed medication was taken was 84.47%. Medication adherence did not differ between the atomoxetine + MI/CBT (81.75%) and placebo + MI/CBT (87.18%) groups (U = 470.5, p = 0.095). For study completers weighing less than 70 kg, the mean dose prescribed for the last study week was 1.29 mg/kg (SD = 0.16, range = 1.05 to 1.59 mg/kg) in the placebo group, compared to 1.19 mg/kg (SD = 0.19, range = 0 to 1.81 mg/kg) in the atomoxetine group (U = 190.5, p = 0.810). For study completers weighing 70 or more kg, the mean dose was 86.7 mg (SD = 16.0, range = 50 to 100 mg) for the placebo group, compared to 88.8 mg (SD = 15.0, range = 62.5 to 100 mg) for the atomoxetine group (U = 71.0, p = 0.849). In the placebo group 25 of 33 (75.8%) completers were prescribed the maximum dose for the last study week, compared to 23 of 32 (71.9%) in the atomoxetine group (χ21= 0.13, p = 0.722).
Figure 2 shows the longitudinal change in DSM-IV ADHD checklist scores over time. Likelihood based criteria determined that the longitudinal course of adolescent self-report DSM-IV ADHD checklist scores followed a cubic curve with random subject and linear time effects. Groups did not differ in terms of change in adolescent self-report ADHD score over time (F4,191 = 1.23, p = 0.2975). From this model, there was an overall significant baseline to week 12 decrease in adolescent self-report ADHD score during treatment (mean pre-post decrease = 18.61, 95% CI: 22.08 to 15.13; t154 = -10.57, p = 0.00005). Separately, for atomoxetine + MI/CBT, the mean pre-post decrease was 18.19 (95% CI: 13.41 to 22.97, t137 = 7.53, p = 0.00005) and for placebo + MI/CBT the mean pre-post decrease was 19.02 (95% CI: 13.97 to 24.07, t171 = 7.43, p = 0.00005).
Figure 2.
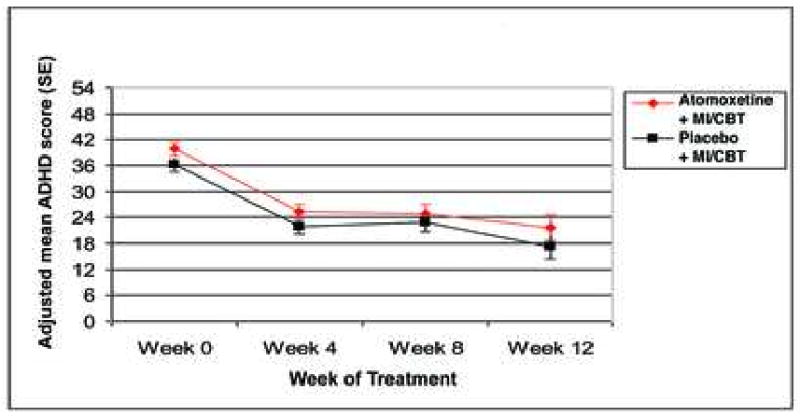
Change in adolescent report DSM-IV ADHD score. Note: ADHD = Attention-Deficit/Hyperactivity Disorder; MI/CBT = motivational interviewing / cognitive behavioral therapy.
The power calculations for designing the study were based on research in children and adolescents that suggested atomoxetine has an effect size of 0.7 for ADHD 27 and indicated that 35 subjects per group would provide 80% power to detect that effect size at a significance level of 0.05. At trial completion, the variability estimates from the final model indicated that the obtained sample size (35 per group) provided 80% power to detect a difference between groups in DSM-IV ADHD score of at least 9.9 as significant. This corresponds to an effect size of 0.67. Therefore, the study was adequately powered in terms of the primary hypothesis.
Likelihood-based criteria determined that the longitudinal course of ADHD scores reported by parents followed a quadratic curve with a random subject effect. Groups did not differ in terms of change in parent-report ADHD score over time (F3,113 = 1.34, p = 0.2654). From this model, there was an overall significant baseline to week 12 decrease during treatment in parent-report ADHD score (mean pre-post decrease = 11.32, 95% CI: 14.89 to 7.75, t106 = -6.28, p = 0.00005). Separately, for atomoxetine + MI/CBT, the mean pre-post decrease was 13.82 (95% CI: 9.21 to 18.43, t105 = 5.94, p = 0.00005), and for placebo + MI/CBT the mean pre-post decrease was 8.82 (95% CI: 3.37 to 14.28, t107 = 3.21, p = 0.0018).
There was no difference between the groups with respect to ADHD CGI-I scores at study end: the atomoxetine + MI/CBT group had 17 (53.1%) participants with a score < 3 and the placebo + MI/CBT had 20 (60.6%) participants (χ21 = 0.37, p = 0.543).
Figure 3 shows the change in the adolescent self-report number of days used non-tobacco substances in the past 28 days. Likelihood-based criteria determined that the longitudinal course of number of days used non-nicotine substances followed a quadratic curve with random subject and linear time effects. Again, the groups did not differ in terms of change in days used non-nicotine substances in the past 28 (F3,100 = 2.06, p = 0.1103). From this model, there was an overall significant baseline to week 12 decrease in using non-nicotine substances in the past 28 days (mean pre-post decrease = 4.01 days or 22.6% relative to baseline, 95% CI: 6.43 to 1.59 days, t69.4 = -3.3, p = 0.0015). Separately, for atomoxetine + MI/CBT, the mean pre-post decrease was 5.78 days (95% CI: 2.35 to 9.21, t67.3 = 3.36, p = 0.0013) and for placebo + MI/CBT the mean pre-post decrease was 2.24 days (95% CI: -1.18 to 5.67, t71.5 = 1.31, p = 0.1956). Groups did not differ on the mean number of negative UDSs (atomoxetine + MI/CBT = 1.03 versus placebo + MI/CBT = 1.11; U = 610, p = 0.972). Six subjects (3 in each treatment group) had zero days of non-nicotine substance use in the past 28 days that was confirmed by UDS at the end of treatment. Variability estimates from this model indicated that the obtained sample provided 80% power to detect a difference between groups of at least 6.8 days as significant. This corresponds to an effect size of 0.67.
Figure 3.
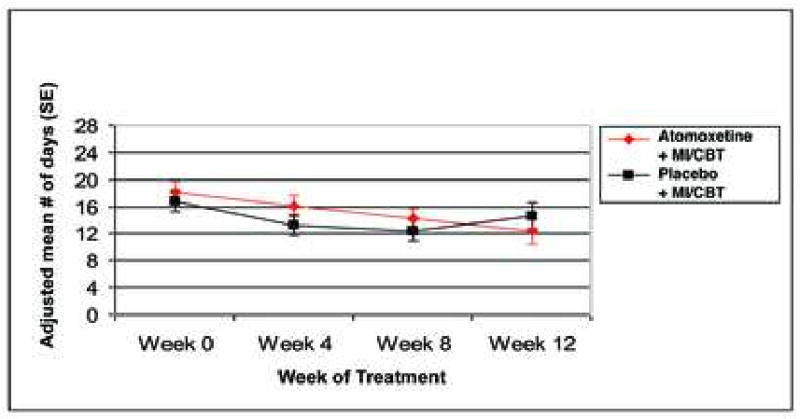
Change in number of days of non-nicotine substance use in the past 28 days. Note: MI/CBT = motivational interviewing / cognitive behavioral therapy.
Rates of adverse events were generally mild and short-lived. Overall, there were two serious adverse events. One person on placebo had a suicide attempt, and one on atomoxetine had a seizure after taking an overdose of bupropion in order to hallucinate. Eleven subjects expressed transient suicidal ideation or behavior after randomization. None required hospitalization. Of these, four were in the atomoxetine + MI/CBT group, and seven were in the placebo + MI/CBT group. One person in the atomoxetine + MI/CBT group had to discontinue the medication due to depression. Groups did not differ on pre-post change in ALT, AST, blood pressure (both systolic and diastolic), pulse, and weight. No subject reported an interaction between the study medication and substances of abuse. Table 2 compares in descending order the prevalence of adverse events between groups for the adverse events that occurred in at least 5% of subjects in either treatment arm.
Table 2.
Summary of adverse events
| Adverse event | Atomoxetine + MI/CBT (n = 35) |
Placebo + MI/CBT (n = 35) |
Statistic | P-Value |
|---|---|---|---|---|
| Difficulty concentrating | 23 (66%) | 16 (46%) | χ21= 2.84 | 0.092 |
| Appetite decrease | 21 (60%) | 13 (37%) | χ21= 3.66 | 0.056 |
| Difficulty falling asleep | 21 (60%) | 25 (71%) | χ21= 101 | 0.314 |
| Nasal congestion | 21 (60%) | 18 (51%) | χ21= 0.52 | 0.470 |
| Abdominal pain | 20 (57%) | 16 (46%) | χ21= 0.92 | 0.339 |
| Difficulty staying asleep | 18 (51%) | 21 (60%) | χ21= 0.52 | 0.470 |
| Drowsiness | 18 (51%) | 15 (43%) | χ21= 0.52 | 0.473 |
| Vomiting | 18 (51%) | 7 (20%) | χ21= 7.53 | 0.006* |
| Difficulty arousing in the A.M. | 17 (49%) | 18 (51%) | χ21= 0.06 | 0.811 |
| Irritability | 17 (49%) | 17 (49%) | χ21= 0 | 1.00 |
| Dizziness when standing up | 16 (46%) | 14 (40%) | χ21= 0.23 | 0.629 |
| Appetite increase | 15 (43%) | 10 (29%) | χ21= 1.56 | 0.212 |
| Nausea | 15 (43%) | 11 (31%) | χ21= 0.98 | 0.322 |
| Dizziness | 10 (29%) | 10 (29%) | χ21= 0 | 1.00 |
| Nausea | 12 (34%) | 14 (40%) | χ21= 0.25 | 0.621 |
| Dry mouth | 9 (26%) | 10 (29%) | χ21= 0.07 | 0.788 |
| Sweating | 8 (23%) | 10 (29%) | χ21= 0.30 | 0.585 |
| Depression | 7 (20%) | 13 (37%) | χ21= 2.52 | 0.112 |
| Blurry vision | 6 (17%) | 5 (14%) | Fisher's Exact Test | 1.00 |
| Heartburn | 6 (17%) | 3 (9%) | Fisher's Exact Test | 0.477 |
| Joint aches | 6 (17%) | 5 (14%) | Fisher's Exact Test | 1 |
| Motor tics | 6 (17%) | 5 (14%) | Fisher's Exact Test | 1 |
| Muscle cramps | 6 (17%) | 14 (40%) | χ21= 4.48 | 0.034* |
| Sadness | 6 (17%) | 14 (40%) | χ21= 4.48 | 0.034* |
| Slurred speech | 6 (17%) | 3 (9%) | Fisher's Exact Test | 0.477 |
| Tachycardia | 6 (17%) | 4 (11%) | Fisher's Exact Test | 0.734 |
| Excitement | 5 (14%) | 5 (14%) | Fisher's Exact Test | 1 |
| Tremor | 5 (14%) | 3 (9%) | Fisher's Exact Test | 0.710 |
| Frequent urination | 4 (11%) | 3 (9%) | Fisher's Exact Test | 1 |
| Fever | 3 (9%) | 5 (14%) | Fisher's Exact Test | 0.710 |
| Itching | 3 (9%) | 4 (11%) | Fisher's Exact Test | 1 |
| Pallor | 2 (6%) | 0 (0%) | Fisher's Exact Test | 0.493 |
| Monotonous speech | 2 (6%) | 1 (3%) | Fisher's Exact Test | 1 |
Notes: MI/CBT = motivational interviewing/cognitive behavioral therapy
denotes a p-value less than 0.05
Discussion
In a sample with psychiatric and substance use severity typical of teens referred to outpatient substance treatment,1,13 this placebo-controlled trial did not find a significant difference in ADHD change or substance use change between the atomoxetine + MI/CBT and placebo + MI/CBT groups. However, while average ADHD scores remained above the inclusion cut-off criterion, both groups had a significant pre-post decrease in ADHD symptoms. In addition, the side effect profile of atomoxetine in this sample is similar to that seen in subjects without SUD, despite the fact that most subjects in this sample continued to use substances while taking their medications.27
There are several limitations to the current study. First, this study was not adequately powered to fully assess atomoxetine's safety in this population. Second, because this study used a manual-standardized individual MI/CBT for SUD, the findings may not generalize to patients who are not in treatment or receive another type of other treatment.
It is interesting to compare these results to a meta-analysis of six randomized, placebo-controlled atomoxetine trials which reports the results for a sub-sample, ages 12-17 years.28 The meta-analysis used a very similar outcome measure, the ADHD RS, which assesses the 18 DSM-IV ADHD symptoms on a severity scale of 0 (none) to 3 (severe). Findings from the meta-analysis showed that, by parent-report, the atomoxetine group decreased 13.99 points (from 36.32 to 22.33), compared to a parent-report decrease of 13.82 points (from 42.63 to 28.81) in this sample. Thus, the decreases in ADHD symptoms for the two studies are similar.
Our trial also observed a significant decrease in ADHD symptoms among the placebo + MI/CBT group. It is interesting to compare our results to the ADHD trials that have been conducted in adults with SUD. Schubiner et al.7 reported a CGI-I response rate in their placebo + CBT group of 56% in adults with cocaine dependence, compared to 60.6% in our sample.8 Therefore, these placebo + CBT response rates also appear similar.
One possible explanation for this finding is that all of our participants received MI/CBT for SUD. Other medication trials for ADHD in adults undergoing SUD treatment also found large ADHD responses in their placebo + CBT groups.7-9 Interestingly, a recent study supporting the efficacy of CBT for ADHD in adults used a treatment that is remarkably similar to our CBT for SUD.29 Both manual-standardized treatments are individual, use motivational interviewing and psychoeducation, and include modules on communication skills, problem solving, and anger management. Therefore, there may be significant overlap between the CBT treatment for SUD and a proven CBT treatment for ADHD. Furthermore, the fact that the three positive ADHD medication trials in subjects with SUD did not include a psychosocial treatment 5,6,10 and that all the studies that did include a psychosocial treatment have been negative 7-9 supports the possibility that the CBT may have had an impact on ADHD.
Another possible explanation is the placebo effect. In the above meta-analysis28, adolescents received atomoxetine or placebo without a co-occurring psychosocial treatment. Subjects on placebo had a mean decrease in parent or teacher report ADHD symptoms of 6.95 points, compared to a parent-report decrease of 19.02 points in our sample. Therefore, our sample receiving placebo + MI/CBT had a larger decrease in ADHD symptoms compared to the sample receiving placebo alone.
Finally, it is possible that parent-report or other measures may be needed to detect differences between medication and placebo. For example, comparing our results to the meta-analysis, the difference in ADHD change between atomoxetine and placebo in the meta-analysis was 7.04 points, compared to 5.00 points for parent-report in our sample. These differences seem similar, and it is possible that with a larger sample, the difference that we observed in parent-report would have reached statistical significance.
The results of this study suggest several future research directions. First, further research is needed to determine the impact of MI/CBT on ADHD symptoms in this population. One way to assess this might be to continue the study medication after the MI/CBT treatment ends. Second, future studies should use various measures, such as self-report, parent-report, and an objective measure, if possible. Third, more research is needed to inform the statistical methods needed to describe the longitudinal relationship between change in SUD and change in ADHD symptoms. Finally, a placebo lead-in phase may decrease the placebo effect. However, a placebo lead-in may make subject recruitment less feasible.
Acknowledgments
This project was funded by the American Academy of Child and Adolescent Psychiatry Physician Scientist Program in Substance Abuse K12 Award (DA 000357-06AK12) and National Institute on Drug Abuse grants U10 DA013732, DA012845 and 5R01DA022284. Medication and matching placebo were supplied by Eli Lilly.
Footnotes
Disclosure: Drs. Thurstone, Riggs, and Mikulich-Gilbertson, and Ms. Salomonsen-Sautel report no biomedical financial interests or potential conflicts of interest.
Clinical trial registration information -- A Randomized, Placebo-Controlled Trial of Atomoxetine for Attention-Deficit/Hyperactivity Disorder in Adolescents with Substance Use Disorder. URL: http://www.clinicaltrials.gov. Unique identifier: NCT00399763
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Dr. Christian Thurstone, Denver Health and Hospital Authority and the University of Colorado Denver
Dr. Paula D. Riggs, University of Colorado Denver
Ms. Stacy Salomonsen-Sautel, University of Colorado Denver
Dr. Susan K. Mikulich-Gilbertson, University of Colorado Denver
References
- 1.Dennis M, Godley SH, Diamond G, et al. The Cannabis Youth Treatment (CYT) Study: main findings from two randomized trials. J Subst Abuse Treat. 2004;27:197–213. doi: 10.1016/j.jsat.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Greenbaum PE, Foster-Johnson L, Petrila A. Co-occurring addictive and mental disorders among adolescents: prevalence research and future directions. Am J Orthopsychiatry. 1996;66:52–60. doi: 10.1037/h0080154. [DOI] [PubMed] [Google Scholar]
- 3.Horner BR, Scheibe KE. Prevalence and implications of attention-deficit hyperactivity disorder among adolescents in treatment for substance abuse. J Am Acad Child Adolesc Psychiatry. 1997;36:30–36. doi: 10.1097/00004583-199701000-00014. [DOI] [PubMed] [Google Scholar]
- 4.Wise BK, Cuffe SP, Fischer T. Dual diagnosis and successful participation of adolescents in substance abuse treatment. J Subst Abuse Treat. 2001;21:161–165. doi: 10.1016/s0740-5472(01)00193-3. [DOI] [PubMed] [Google Scholar]
- 5.Riggs PD, Hall SK, Mikulich-Gilbertson SK, Lohman M, Kayser A. A randomized controlled trial of pemoline for attention-deficit/hyperactivity disorder in substance-abusing adolescents. J Am Acad Child Adolesc Psychiatry. 2004;43:420–429. doi: 10.1097/00004583-200404000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Szobot CM, Rohde LA, Katz B, et al. A randomized crossover clinical study showing that methylphenidate-SODAS improves attention-deficit/hyperactivity disorder symptoms in adolescents with substance use disorder. Braz J Med Biol Res. 2008;41:250–257. doi: 10.1590/s0100-879x2008005000011. [DOI] [PubMed] [Google Scholar]
- 7.Schubiner H, Saules KK, Arfken CL, et al. Double-blind placebo-controlled trial of methylphenidate in the treatment of adult ADHD patients with comorbid cocaine dependence. Exp Clin Psychopharmacol. 2002;10:286–294. doi: 10.1037//1064-1297.10.3.286. [DOI] [PubMed] [Google Scholar]
- 8.Levin FR, Evans SM, Brooks DJ, Kalbag AS, Garawi F, Nunes EV. Treatment of methadone-maintained patients with adult ADHD: double-blind comparison of methylphenidate, bupropion and placebo. Drug Alcohol Depend. 2006;81:137–148. doi: 10.1016/j.drugalcdep.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 9.Levin FR, Evans SM, Brooks DJ, Garawi F. Treatment of cocaine dependent treatment seekers with adult ADHD: double-blind comparison of methylphenidate and placebo. Drug Alcohol Depend. 2007;87:20–29. doi: 10.1016/j.drugalcdep.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Wilens TE, Adler LA, Weiss MD, et al. Atomoxetine treatment of adults with ADHD comorbid alcohol use disorders. Drug Alcohol Depend. 2008;96:145–154. doi: 10.1016/j.drugalcdep.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Heil SH, Holmes HW, Bickel WK, et al. Comparison of the subjective, physiological, and psychomotor effects of atomoxetine and methylphenidate in light drug users. Drug Alcohol Depend. 2002;67:149–156. doi: 10.1016/s0376-8716(02)00053-4. [DOI] [PubMed] [Google Scholar]
- 12.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. Washington, D.C.: American Psychiatric Association; 1994. [Google Scholar]
- 13.Riggs PD, Mikulich-Gilbertson SK, Davies RD, Lohman M, Klein C, Stover SK. A randomized controlled trial of fluoxetine and cognitive behavioral therapy in adolescents with major depression, behavior problems, and substance use disorders. Arch Pediatr Adolesc Med. 2007;161:1026–1034. doi: 10.1001/archpedi.161.11.1026. [DOI] [PubMed] [Google Scholar]
- 14.Waldron HB, Slesnick N, Brody JL, Turner CW, Peterson TR. Treatment outcomes for adolescent substance abuse at 4- and 7-month assessments. J Consult Clin Psychol. 2001;69:802–813. [PubMed] [Google Scholar]
- 15.Ambrosini PJ. Historical development and present status of the schedule for affective disorders and schizophrenia for school-age children (K-SADS) J Am Acad Child Adolesc Psychiatry. 2000;39:49–58. doi: 10.1097/00004583-200001000-00016. [DOI] [PubMed] [Google Scholar]
- 16.Adler LA, Goodman DW, Kollins SH, et al. Double-blind placebo-controlled study of the efficacy and safety of lisdexamfetamine dimesylate in adults with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2008;69:1364–1373. doi: 10.4088/jcp.v69n0903. [DOI] [PubMed] [Google Scholar]
- 17.Adler LA, Zimmerman B, Starr HL, et al. Efficacy and safety of OROS methylphenidate in adults with attention-deficit/hyperactivity disorder: a randomized, placebo-controlled, double-blind, parallel group, dose-escalation study. J Clin Psychopharmacol. 2009;29:239–247. doi: 10.1097/JCP.0b013e3181a390ce. [DOI] [PubMed] [Google Scholar]
- 18.Bostic JQ, Biederman J, Spencer TJ, et al. Pemoline treatment of adolescents with attention deficit hyperactivity disorder: a short-term controlled trial. J Child Adolesc Psychopharmacol. 2000;10:205–216. doi: 10.1089/10445460050167313. [DOI] [PubMed] [Google Scholar]
- 19.Conners CK, Barkley RA. Rating scales and checklists for child psychopharmacology. Psychopharmacol Bull. 1985;21:809–843. [PubMed] [Google Scholar]
- 20.Sobell LC, Sobell SM. Timeline follow-back: a technique for assessing self-reported alcohol consumption. In: Litten R, Allen J, editors. Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Totowa, NJ: Humana Press; 1992. pp. 41–72. [Google Scholar]
- 21.Klein RGAH, Barkley RA, Campbell M, Leckman JF, Ryan ND, Solanto MV, Whalen CK. Clinical Evaluation of Psychotropic Drugs: Principles and Guidelines. New York: Raven Press Ltd; 1994. [Google Scholar]
- 22.Brent D, Emslie G, Clarke G, et al. Switching to another SSRI or to venlafaxine with or without cognitive behavioral therapy for adolescents with SSRI-resistant depression: the TORDIA randomized controlled trial. JAMA. 2008;299:901–913. doi: 10.1001/jama.299.8.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.SPSS Inc. SPSS for Windows, Version 15.0. Chicago, IL: SPSS Inc; 2008. [Google Scholar]
- 24.SPSS Inc. SPSS for Windows, Version 17. Chicago, IL: SPSS Inc.; 2009. [Google Scholar]
- 25.SAS Institute Inc. SAS/STAT User's Guide, Version 9.2. Cary, NC: SAS Institute Inc.; 2008. [Google Scholar]
- 26.Jones R. Longitudinal Data with Serial Correlation: A State-Space Approach. New York, NY: Chapman & Hall; 1993. [Google Scholar]
- 27.Michelson D, Faries D, Wernicke J, et al. Atomoxetine in the treatment of children and adolescents with attention-deficit/hyperactivity disorder: a randomized, placebo-controlled study. Am J Psychiatry. 2002;159:1896–1901. doi: 10.1176/appi.ajp.159.11.1896. [DOI] [PubMed] [Google Scholar]
- 28.Wilens TE, Kratochvil C, Newcorn JH, Gao H. Do children and adolescents with ADHD respond differently to atomoxetine? J Am Acad Child Adolesc Psychiatry. 2006;45:149–157. doi: 10.1097/01.chi.0000190352.90946.0b. [DOI] [PubMed] [Google Scholar]
- 29.Safren SA, Otto MW, Sprich S, Winett CL, Wilens TE, Biederman J. Cognitive-behavioral therapy for ADHD in medication-treated adults with continued symptoms. Behav Res Ther. 2005;43:831–842. doi: 10.1016/j.brat.2004.07.001. [DOI] [PubMed] [Google Scholar]


