Abstract
Purpose of Review
Circadian variation is commonly seen in healthy people; aberration in these biological rhythms is an early sign of disease. Impaired circadian variation of BP has been shown to be associated with greater target organ damage and to be associated with an elevated risk of cardiovascular events independent of the BP load. The purpose of this review is to examine the physiology of circadian BP variation and propose a tripartite model that explains the regulation of circadian BP
Recent findings
The time-keeper of the mammals resides centrally in the suprachiasmatic nucleus. Besides this central clock, molecular clocks exist in most peripheral tissues including vascular tissue and the kidney. These molecular clocks regulate sodium balance, sympathetic function and vascular tone. A physiological model is proposed that integrates our understanding of molecular clocks in mice to the circadian BP variation among humans. The master regulator in this proposed model is the sleep-activity cycle. The equivalents of peripheral clocks are endothelial and adrenergic functions. Thus, in the proposed model, the variation in circadian BP is dependent upon 3 major factors: physical activity, autonomic function, and sodium sensitivity.
Summary
The integrated consideration of physical activity, autonomic function, and sodium sensitivity appear to explain the physiology of circadian BP variation and the pathophysiology of disrupted BP rhythms in various conditions and disease states. Our understanding of molecular clocks in mice may help to explain the provenance of blunted circadian BP variation even among astronauts.
Keywords: blood pressure, circadian rhythms, molecular clocks, cosinor modeling, chronic kidney disease, pathophysiology
Introduction
Circadian variation is commonly seen in healthy people; aberration in these biological rhythms is an early sign of disease. Perhaps the best studied of these biological rhythms are circadian variations in temperature, heart rate, blood pressure (BP) [1]. In the general population impaired circadian variation of BP has been shown to be associated with greater target organ damage [2]. In addition it has been found to be associated with an elevated risk of cardiovascular events independent of the BP load [3;4].
The purpose of this review is to examine the physiology of circadian BP variation and propose a tripartite model that explains the regulation of circadian BP; the proposed model will allow us to better understand the provenance of deranged BP rhythms in disease. To prepare the framework for this tripartite model, the biology of circadian variation is first reviewed.
Circadian Biology of BP in Mice
The time-keeper of the mammals resides centrally in the suprachiasmatic nucleus. Besides this central clock, molecular clocks exist in most peripheral tissues including vascular tissue and the kidney [5;6]. Identified as transcription factors, these molecular clocks CLOCK and BMAL1, can heterodimerize and drive expression of Period (per) and Cryptochrome (Cry) genes [5]. The gene products, Per and Cry proteins, themselves then dimerize and complete the negative limb of the feedback loop by repressing their own transcription.
Circadian rhythmicity depends on BMAL1 and CLOCK genes. Genes relevant to catecholamine synthesis and disposition are under the control of the molecular clock [5]. Deletion in these genes results in significant loss of circadian mean arterial rhythms in mice [5]. Conversely, salt feeding in the Dahl salt-sensitive rat significantly blunts the expression of these clock genes in the liver, heart and the kidney [7]. Furthermore, deletion in these genes results in endothelial dysfunction, prothrombotic state and vascular injury [8]. Deletion in BMAL1 also results in significant blunting in pressor response to stress [5]. BMAL1 gene regulates sodium-proton exchange via NHE3 in the kidney [9]. More recently, Period 1 protein in the kidney has been shown to regulate the epithelial sodium channel which may be important in the control of sodium balance [6].
Before formulating a model that translates the circadian biology in mice to understand BP rhythms to men, a brief review of circadian BP variation is offered.
Description of Circadian BP Variation
BP varies over a 24 hour period with a peak during the day and nadir during the night. The decline in BP during sleep requires major physiological adjustments. Intuitively, assuming the supine posture increases preload which would logically increase cardiac output and BP. However, BP falls during sleep due to a coordinated and active switching off of autonomic activity in park evoked by baroreflex adjustments.
The abnormal pattern of BP detected by ambulatory BP monitoring is often described using the dichotomous definition based on dipper status. The lack of fall in BP during sleep is called non-dipping. Non-dipping has been defined in many ways and most investigators focus the definition on the systolic BP change. One of these definitions of non-dipping is night/day systolic BP ratio of >0.9 [4]. An inverted ratio (night/day ratio of >1.0) is called reverse dipping; night/day ratio of <0.8 is called extreme dipping; normal dipping is defined as night/day ratio of >0.8 to 0.9.
The dichotomous definition based on dipper status does not fully describe BP rhythms. A sinusoidal rhythm is observed in systolic blood pressure (BP) that varies about the mean (median estimating statistic or rhythm or MESOR) and generally peaks (acrophase) during the waking hours and nadirs (bathyphase) during sleep. The variation of BP about the MESOR is largely governed by activity [9]. This pattern of BP has acrophase during the waking hours and thus said to be “in-phase”. Patients with deranged rhythms often have the acrophase during sleeping hours, thus their rhythm is said to be “out-of-phase” (Figure 1). However, to be in-phase or out-of-phase the amplitude of circadian variation must be statistically greater than zero. If the circadian variation is so blunted that the amplitude of variation is no different from zero then acrophase may not be definable. This rhythm is called “phase-less”.
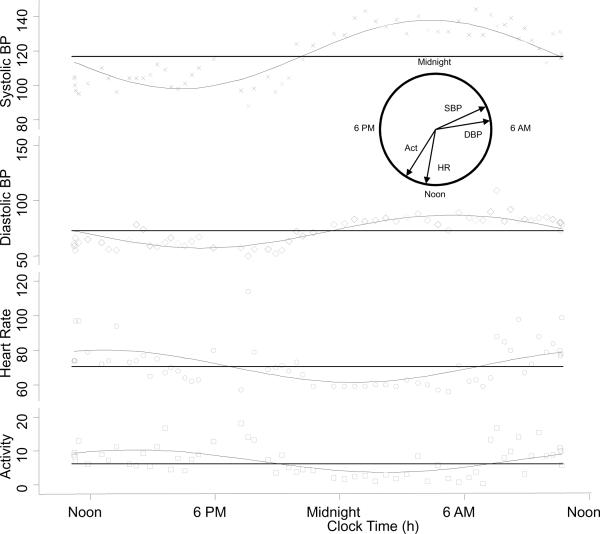
Figure 1.
Circadian variation in blood pressure, heart rate, and concomitant activity is shown for a patient with kidney disease. The horizontal lines are the median estimating statistic of rhythm (MESOR). The circular inset shows the time of peak effect (acrophase) on a 24 hour clock. Acrophases for both systolic and diastolic BP are seen during the hours of sleep. Acrophases for heart rate and activity are seen between noon and 6 PM. Activity units are arbitrary and taken as the square root of the average activity 5 minutes prior to each blood pressure measurement.
Physiology of Circadian BP Variation
A physiological model is proposed that integrates our understanding of molecular clocks in mice to the circadian BP variation in humans. This model is proposed to provide explanations for the causes of non-dipping in various conditions and disease states.
The master regulator in this model is the sleep-activity cycle. The surrogate of the sleep-activity cycle is physical activity in the proposed model. The equivalents of peripheral clocks are endothelial and adrenergic functions. Thus, in the proposed model, the variation in circadian BP is dependent upon 3 major factors: physical activity, autonomic function, and sodium sensitivity (Figure 2). These factors are further discussed below:
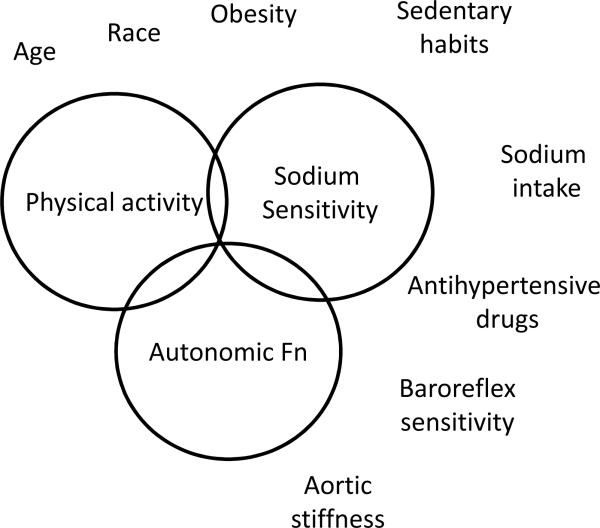
Figure 2.
Factors that mediate dipping and conditions that impair dipping. The 3 factors that are primarily responsible for dipping are physical activity, sodium sensitivity and autonomic function. Impairments of any of these 3 functions can lead to non-dipping. Some conditions associated with non-dipping are shown outside these circles and discussed in the text. Diseases such as CKD are often associated with non-dipping the pathophysiology of which is complex and discussed in the text.
Physical activity
During the day when people are most active, BP is higher. Among individuals who are normally active during the day, if physical activity is increased during the night, the nocturnal BP decline is blunted [10].
There is a direct relationship between physical activity and systolic BP; increase in physical activity raises the systolic BP [10–12]. Clark et al were among the first to report that physical activity explains much of the circadian variation in BP [13]. Subsequent investigators utilizing actigraphy extended these observations to demonstrate that between 20–60% of variation in daytime blood pressure could be explained by physical activity [14–18]. The first study to evaluate the association of directly measured activity with circadian BP variation was that of Kario et al who found that the sleep/awake ratio of systolic BP was directly related to the night/day physical activity. In another study, the relationship between physical activity and circadian BP variation was revealed by directly measuring physical activity with an actimeter and BP with an ambulatory monitor [19]. A novel feature of this study was the use of cosinor analysis to model circadian rhythms. A higher level of directly measured physical activity was associated with important changes in systolic BP profiles which include the following: 1) lower overall BP; 2) higher amplitude of variation (and thus greater dipping) and; 3) restoration of acrophase to the early hours of the morning.
In contrast to systolic BP, circadian variation for diastolic BP was not entirely explained by activity; even among participants in the lowest 25 percent of activity a circadian variation in diastolic BP was apparent [19]. The disparate relationship of circadian variation in systolic BP with activity and diastolic BP with activity may be because systolic BP is influenced to a greater extent by changes in cardiac output, which in turn is heavily influenced by physical activity. It follows that lack of physical activity is associated with lack of circadian variation in systolic BP. Astronauts in zero gravity also show blunting in systolic BP circadian variation [20]. This may be because the zero gravity environment leads to relative physical inactivity. In addition, the sympathetic nervous system is activated due to hypovolemia induced by the space flight (the effects of sympathetic activation are discussed below) [21].
Although physical activity magnifies the amplitude of variation in diastolic BP and heart rate, activity does not perturb the MESOR or acrophase [19]. Diastolic BP is influenced by systemic vascular resistance which is regulated by the autonomic nervous system. Heart rate is also directly regulated by the autonomic nervous system. Given that both hemodynamic variables are under direct autonomic control it is little surprise that both are influenced in similar ways by increased physical activity. Even when physical activity is very low a circadian variation was seen in the case of diastolic BP and heart rate. This observation suggests an important influence of variation in endogenous autonomic tone in regulation of these hemodynamic variables.
Autonomic Function
The sympathetic nervous system regulates the circadian variation in blood vessel tone and is closely linked to dipping [22]. A distinct circadian rhythm is observed for epinephrine and norepinephrine in healthy volunteers; awakening evokes epinephrine release and orthostasis both epinephrine and norepinephrine release [23]. Among healthy volunteers, higher resting measurements of sympathetic traffic are associated with greater daytime BP variability and a more marked nocturnal decline in BP [24]. Conversely, among patients with tetraplegia, dipping is abolished; patients with tetraplegia, unlike those with paraplegia, have complete interruption of the sympathetic pathway [25]. Patients with paraplegia have less impairment in circadian variation of BP. Increased sodium intake can impair baroreflex sensitivity which can then cause sympathetic activation [26]. Compared to dippers, there are several lines of evidence for sympathetic activation among non-dippers: non-dippers have 1) impaired renal sodium excretion in the upright posture [27];. 2) inappropriate release of norepinephrine during supine rest [28]; 3) a blunted nocturnal fall in urinary epinephrine and norepinephrine excretion rates [29]; and 4) an increased responsiveness of alpha1 adrenergic receptors [29].
Sodium sensitivity
Salt-sensitivity can be defined as reduction in BP upon restriction of dietary sodium intake. Patients with non-dipping are salt-sensitive and commonly have deranged endothelial function. Normal endothelial function appears to be important in the regulation of circadian BP variation. Compared to dippers, non-dippers have impairment in directly measured endothelial function [30]. The relationship of endothelial function and salt-sensitivity were demonstrated by administering L-arginine, a substrate for endothelial nitric oxide synthase, to Dahl salt-sensitive rats; L-arginine restored endothelial function which corrected the salt-sensitivity in these animals [31].
Measures that improve sodium sensitivity such as dietary sodium restriction, ACE inhibitors, and diuretics may also restore deranged circadian rhythms. Among hypertensive patients, dietary sodium restriction to between 1–2 g/d restored circadian BP derangements [32]. Interestingly, this improvement was observed only among those who were sodium-sensitive [33]. Thiazide diruetics also restored circadian BP profile, but only among non-dippers [34]. Likewise, administration of the ACE inhibitor restored the dipping profile [35].
There are likely other mechanisms that mediate dipping. For example, impaired melatonin patterns among non-dippers suggest a physiological response to darkness independent of the factors discussed above [36]. However, other studies have not found differences in melatonin between dippers and non-dippers [28]. Others suggest existence of a central mechanism to regulate the baroreflex which mediates dipping [37]. The latter study was limited to 12 subjects, so will require further confirmation.
Conditions associated with non-dipping
Epidemiological observations suggest multiple correlates of non-dipping. These correlates are not diseases but nonetheless conditions that are associated with non-dipping. These conditions are directly associated with a deranged physiology of dipping. The relationship between these conditions and physiological derangements may be multifactorial as discussed below.
The correlates of non-dipping are the following: age, aortic stiffness, race, impaired fasting glucose, lack of social support, reduced physical quality of life. Age is directly associated with non-dipping [38;39] which may be due to reduced physical activity and sympathetic activation due to impaired baroreflex sensitivity. Aortic stiffness is associated with endothelial dysfunction and impaired baroreflex sensitivity; the latter leads to autonomic dysfunction and non-dipping [40]. African Americans have a high prevalence of non-dipping likely from salt-sensitivity, a marker of endothelial dysfunction [41]. African American adolescent often have low potassium intake which can activate the sympathetic nervous system. Among salt-sensitive African American adolescents, potassium supplementation leads to dipping due to drop in sleep diastolic BP (from 69 to 57 mmHg) [42]. Impaired fasting glucose is associated with non-dipping even among normotensive individuals and is associated with endothelial dysfunction [43]. Lack of social support is associated with non-dipping; non-dipping in this situation may be mediated by activation of autonomic pathways [44].
Diseases associated with impaired circadian BP rhythms
Several disease states are associated with a high prevalence of non-dipping. The foremost among these is chronic kidney disease (CKD).
Chronic Kidney Disease
All the 3 factors that regulate circadian BP are deranged among patients with CKD. How these factors lead to deranged circadian rhythms is discussed further.
Patients with CKD often are sedentary and have low physical activity. Those with low physical activity have lower circadian BP variation. This aberration in circadian BP variation is particularly pronounced for systolic BP and less so for diastolic BP or pulse pressure. Non-dippers have a greater level of sleep activity compared to dippers, although the awake activity level is similar between dippers and non-dippers [10]. One reason for increased nocturnal activity among patients with CKD is nocturia, which is associated with non-dipping [45].
Patients with CKD have sympathetic activation which has been documented by direct microneurography [46]. Microneurography records the bursts of sympathetic nerve activity in the peroneal nerve and has demonstrated a heightened state of sympathetic activation among patients on hemodialysis and among those with CKD [46]. This activation is directly related to diseased kidneys; anephric patients have lower sympathetic activation [46]. Sympathetic activation can also occur in these patients due to arterial stiffness and consequently reduced baroreceptor sensitivity. Increased arterial stiffness is strongly related to proteinuria [47]. Sympathetic activation is associated with non-dipping among patients with type 2 diabetes and diabetic nephropathy [28].
Patients with CKD have endothelial dysfunction [48]. A manifestation of endothelial dysfunction sodium sensitivity, is seen even at the earliest states of CKD [49]. The sympathetic nervous system can directly modulate renal sodium excretion and this can further induce endothelial dysfunction [50].
Given the above pathophysiology, it is not surprising to note that non-dipping is seen early in the course of CKD. Some reports have indicated that non-dipping may precede sustained elevation in BP in patients with type 1 diabetes who subsequently develop microalbuminuria [51]. Others have reported that non-dipping may reveal the presence of diabetic glomerulopathy even in the absence of microalbuminuria [52].
Among patients with established CKD, further impairment of GFR is not associated with greater non-dipping. Rosansky et al reported that in 53 older veterans with hypertension increasing severity of renal dysfunction was not related to the extent of dipping [41]. Among 30 patients with CKD, Portaluppi et al found no significant relationship between creatinine clearance and day to night BP changes [53]. However, among 380 patients with essential hypertension, secondary hypertension, and on renal replacement therapy Farmer et al reported a direct relationship between plasma creatinine and non-dipping [54]. These differences may be due to heterogeneity among the populations and differences in the statistical methods. For example, a non-linear relationship between non-dipping and plasma creatinine may exist but if these nonlinear relationships are not formally tested one may wrongly conclude the presence of a linear relationship. In a large prospective study which included a large number of patients with CKD, Agarwal et al reported that among 336 older veterans not on renal replacement therapy, dipping was not further impaired with advancing stages of renal failure [19]. A graded relationship emerged between the mean level of BP and kidney function. However, the relationship of kidney function to circadian variation was non-graded. Whether the derangement in kidney function was assessed by proteinuria or eGFR, circadian BP variation was markedly impaired in patients even with the earliest stages of CKD [19;55]. Furthermore, the time it takes to dip is prolonged among patients with CKD [56].
Kidney Transplantation
Whether dipping can be restored by kidney transplantation has yielded conflicting reports. With transplantation, some studies suggest that dipping can be restored [57] whereas others have reported a high prevalence of non-dipping [54;58;59]. Whereas successful kidney transplantation may restore kidney function to normal, more often these patients continue to have impaired kidney function and hypertension [60]. If even the slightest impairment of GFR is associated with non-dipping this would be one reason why kidney transplantation may not restore dipping. In a study of 36 kidney transplant recipients, all patients had either proteinuria or impaired GFR and 95% were non-dippers [61]. In another study of 49 kidney transplant recipients where all patients had either proteinuria or impaired GFR and 82% were non-dippers [58]. In a longitudinal cohort of kidney transplant recipients, GFR and age were independent correlates of non-dipping [62]Other reasons could be the use of corticosteroids which can cause endothelial dysfunction and the use of calcineurin inhibitors that can cause both endothelial dysfunction and sympathetic activation. The dose and plasma concentration of the calcineurin inhibitor cyclosporine has been associated with non-dipping [63]. In fact, among kidney transplant patients dipping is restored over time [57]. This restoration is consistent with the hypothesis that over time these patients have less immunosuppression and consequently reduced sodium sensitivity and better endothelial function.
Hemodialysis
Diuretics and sodium restriction in salt-sensitive hypertension but without CKD can restore dipping. Therefore it stands to reason that volume challenge among hypertensive hemodialysis patients can restore dipping. In a randomized trial, although reducing dry-weight resulted in reduced systolic and diastolic BP within one month [64], there was no restoration of dipping [65]. Hypertensive hemodialysis patients have marked sympathetic activation and volume reduction is unlikely to mitigate sympathetic activation; in fact it may even aggravate it. Accordingly, it is not surprising that dipping was not restored with volume reduction therapy in these patients.
Sleep Apnea
A high prevalence of non-dipping is noted among patients with sleep apnea. For example, among those with obstructive sleep apnea non-dipping was found in 28 of 40 patients (70%) [66]. Non-dipping was found even in these patients even when they were normotensive. The effect of sleep disordered breathing on circadian BP rhythms is independent of physical activity and obesity [67]. Among children with moderate sleep disordered breathing, impaired nocturnal decline in systolic and diastolic BP has been reported [67]. All 3 circadian regulatory factors—physical activity, sodium sensitivity and autonomic function—are deranged in these patients. Physical activity is lower because these patients are often obese. Nocturnal activity may be increased due to increased nocturnal awakening and nocturia. Sodium sensitivity is deranged due to a variety of causes which together constitute the metabolic syndrome. Sympathetic activation has been reported in these patients especially in relationship to apneic spells. In fact, the apnea-hypopnea indices correlate closely with non-dipping [66]. Furthermore, sympathetic activation, as inferred from daytime urine norepinephrine is related to night-time BP and daytime BP variability [68].
Heart failure
In one study of 50 patients with symptomatic heart failure, 78% were found to be non-dippers [69]. Non-dipping was also found to be worse in those with more advanced stages of heart failure. Heart failure is associated with reduced physical activity, autonomic activation, and endothelial dysfunction. Accordingly, it is unsurprising to find such a high prevalence of non-dipping among these patients.
Metabolic syndrome
Metabolic syndrome and its components are associated with non-dipping [70;71]. Among patients with metabolic syndrome, Hermida et al have demonstrated reduced physical activity and factors associated with endothelial dysfunction such as obesity, hypertension, and hyperglycemia [70]. Interestingly, smoking was associated with dipping because smoking evoked an increase in BP during the day. During sleep when people did not smoke a decline in BP was noted.
Conclusion
The tripartite activity-autonomic-sodium sensitivity model appears to explain the physiology of circadian BP variation and the pathophysiology of disrupted BP rhythms in various conditions and disease states. Our understanding of molecular clocks in mice helps to explain the provenance of blunted circadian BP variation among astronauts.
Acknowledgement
The technical assistance of Shathabish S. Kariyanna is gratefully acknowledged.
Grant Support: NIH 5RO1-DK062030-06
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Verdecchia P, Schillaci G, Porcellati C. Dippers versus non-dippers. J.Hypertens.Suppl. 1991;9:S42–S44. [PubMed] [Google Scholar]
- 2.Verdecchia P, Schillaci G, Guerrieri M, Gatteschi C, Benemio G, Boldrini F, Porcellati C. Circadian blood pressure changes and left ventricular hypertrophy in essential hypertension. Circulation. 1990;81:528–536. doi: 10.1161/01.cir.81.2.528. [DOI] [PubMed] [Google Scholar]
- 3.Ohkubo T, Hozawa A, Yamaguchi J, Kikuya M, Ohmori K, Michimata M, Matsubara M, Hashimoto J, Hoshi H, Araki T, Tsuji I, Satoh H, Hisamichi S, Imai Y. Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24-h blood pressure: the Ohasama study. J.Hypertens. 2002;20:2183–2189. doi: 10.1097/00004872-200211000-00017. [DOI] [PubMed] [Google Scholar]
- 4.Muxfeldt ES, Cardoso CR, Salles GF. Prognostic value of nocturnal blood pressure reduction in resistant hypertension. Arch.Intern.Med. 2009;169:874–880. doi: 10.1001/archinternmed.2009.68. [DOI] [PubMed] [Google Scholar]; *A larger Brazilian study among patients with resistant hypertension that reports the prognostic value of dipping.
- 5.Curtis AM, Cheng Y, Kapoor S, Reilly D, Price TS, FitzGerald GA. Circadian variation of blood pressure and the vascular response to asynchronous stress. Proc.Natl.Acad.Sci.U.S.A. 2007;104:3450–3455. doi: 10.1073/pnas.0611680104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gumz ML, Stow LR, Lynch IJ, Greenlee MM, Rudin A, Cain BD, Weaver DR, Wingo CS. The circadian clock protein Period 1 regulates expression of the renal epithelial sodium channel in mice. J Clin Invest. 2009;119:2423–2434. doi: 10.1172/JCI36908. [DOI] [PMC free article] [PubMed] [Google Scholar]; **Novel report of regulation of ENAC in mice by the period 1 protein. This discovery has implications for the circadian variation of Na balance.
- 7.Mohri T, Emoto N, Nonaka H, Fukuya H, Yagita K, Okamura H, Yokoyama M. Alterations of circadian expressions of clock genes in Dahl salt-sensitive rats fed a high-salt diet. Hypertension. 2003;42:189–194. doi: 10.1161/01.HYP.0000082766.63952.49. [DOI] [PubMed] [Google Scholar]
- 8.Anea CB, Zhang M, Stepp DW, Simkins GB, Reed G, Fulton DJ, Rudic RD. Vascular disease in mice with a dysfunctional circadian clock. Circulation. 2009;119:1510–1517. doi: 10.1161/CIRCULATIONAHA.108.827477. [DOI] [PMC free article] [PubMed] [Google Scholar]; **Deletion of clock genes in mice leads to severe vascular disease and a prothrombotic state.
- 9.Saifur RM, Emoto N, Nonaka H, Okura R, Nishimura M, Yagita K, van der Horst GT, Matsuo M, Okamura H, Yokoyama M. Circadian clock genes directly regulate expression of the Na(+)/H(+) exchanger NHE3 in the kidney. Kidney Int. 2005;67:1410–1419. doi: 10.1111/j.1523-1755.2005.00218.x. [DOI] [PubMed] [Google Scholar]
- 10.Agarwal R, Light RP. Physical activity and hemodynamic reactivity in chronic kidney disease. Clin J Am Soc Nephrol. 2008;3:1660–1668. doi: 10.2215/CJN.02920608. [DOI] [PMC free article] [PubMed] [Google Scholar]; *A study that directly demonstrates the relationship between physical activity and BP.
- 11.Leary AC, Donnan PT, MacDonald TM, Murphy MB. Physical activity level is an independent predictor of the diurnal variation in blood pressure. J Hypertens. 2000;18:405–410. doi: 10.1097/00004872-200018040-00008. [DOI] [PubMed] [Google Scholar]
- 12.Miyai N, Arita M, Miyashita K, Morioka I, Shiraishi T, Nishio I. Blood pressure response to heart rate during exercise test and risk of future hypertension. Hypertension. 2002;39:761–766. doi: 10.1161/hy0302.105777. [DOI] [PubMed] [Google Scholar]
- 13.Clark LA, Denby L, Pregibon D, Harshfield GA, Pickering TG, Blank S, Laragh JH. A quantitative analysis of the effects of activity and time of day on the diurnal variations of blood pressure. J Chronic.Dis. 1987;40:671–681. doi: 10.1016/0021-9681(87)90103-2. [DOI] [PubMed] [Google Scholar]
- 14.Kuwajima I, Hamamatsu A, Suzuki Y, Kuramoto K. The relationship between ambulatory blood pressure and physical activity in young and older shiftworkers. A quantitative assessment of physical activity using a microcomputer with acceleration sensor. Jpn.Heart J. 1993;34:279–289. doi: 10.1536/ihj.34.279. [DOI] [PubMed] [Google Scholar]
- 15.Van Egeren LF. Monitoring activity and blood pressure. J Hypertens.Suppl. 1991;9:S25–S27. [PubMed] [Google Scholar]
- 16.Stewart MJ, Brown H, Padfield PL. Can simultaneous ambulatory blood pressure and activity monitoring improve the definition of blood pressure? Am.J Hypertens. 1993;6:174S–178S. doi: 10.1093/ajh/6.6.174s. [DOI] [PubMed] [Google Scholar]
- 17.Gretler DD, Carlson GF, Montano AV, Murphy MB. Diurnal blood pressure variability and physical activity measured electronically and by diary. Am.J Hypertens. 1993;6:127–133. doi: 10.1093/ajh/6.2.127. [DOI] [PubMed] [Google Scholar]
- 18.Siche JP, Larota C, Charbonnier S, Baguet JP, Diourte B, Bonnet JL, Mallion JM. [A quantitative analysis of a predictive model of ambulatory blood pressure monitoring integrating physical activity recording] Arch.Mal Coeur Vaiss. 1998;91:979–984. [PubMed] [Google Scholar]
- 19.Agarwal R, Light RP. GFR, proteinuria and circadian blood pressure. Nephrol.Dial.Transplant. 2009;24:2400–2406. doi: 10.1093/ndt/gfp074. [DOI] [PubMed] [Google Scholar]; **Whether kidney dysfunction is measured by proteinuria or impaired GFR, non-dipping is seen early in the course of kidney disease.
- 20.Karemaker JM, Berecki-Gisolf J. 24-h blood pressure in Space: The dark side of being an astronaut. Respir.Physiol Neurobiol. 2009 doi: 10.1016/j.resp.2009.05.006. [DOI] [PubMed] [Google Scholar]; **Astronauts do not have a change in overall BP in space, just a disruption in the circadian rhythm.
- 21.Eckberg DL. Bursting into space: alterations of sympathetic control by space travel. Acta Physiol Scand. 2003;177:299–311. doi: 10.1046/j.1365-201X.2003.01073.x. [DOI] [PubMed] [Google Scholar]
- 22.Panza JA, Epstein SE, Quyyumi AA. Circadian variation in vascular tone and its relation to alpha-sympathetic vasoconstrictor activity. N Engl J Med. 1991;325:986–990. doi: 10.1056/NEJM199110033251402. [DOI] [PubMed] [Google Scholar]
- 23.Dodt C, Breckling U, Derad I, Fehm HL, Born J. Plasma epinephrine and norepinephrine concentrations of healthy humans associated with nighttime sleep and morning arousal. Hypertension. 1997;30:71–76. doi: 10.1161/01.hyp.30.1.71. [DOI] [PubMed] [Google Scholar]
- 24.Narkiewicz K, Winnicki M, Schroeder K, Phillips BG, Kato M, Cwalina E, Somers VK. Relationship between muscle sympathetic nerve activity and diurnal blood pressure profile. Hypertension. 2002;39:168–172. doi: 10.1161/hy1201.097302. [DOI] [PubMed] [Google Scholar]
- 25.Munakata M, Kameyama J, Kanazawa M, Nunokawa T, Moriai N, Yoshinaga K. Circadian blood pressure rhythm in patients with higher and lower spinal cord injury: simultaneous evaluation of autonomic nervous activity and physical activity. J.Hypertens. 1997;15:1745–1749. doi: 10.1097/00004872-199715120-00083. [DOI] [PubMed] [Google Scholar]
- 26.Creager MA, Roddy MA, Holland KM, Hirsch AT, Dzau VJ. Sodium depresses arterial baroreceptor reflex function in normotensive humans. Hypertension. 1991;17:989–996. doi: 10.1161/01.hyp.17.6.989. [DOI] [PubMed] [Google Scholar]
- 27.Uzu T, Takeji M, Yamauchi A, Kimura G. Circadian rhythm and postural change in natriuresis in non-dipper type of essential hypertension. J Hum.Hypertens. 2001;15:323–327. doi: 10.1038/sj.jhh.1001185. [DOI] [PubMed] [Google Scholar]
- 28.Nielsen FS, Hansen HP, Jacobsen P, Rossing P, Smidt UM, Christensen NJ, Pevet P, Vivien-Roels B, Parving HH. Increased sympathetic activity during sleep and nocturnal hypertension in Type 2 diabetic patients with diabetic nephropathy. Diabet.Med. 1999;16:555–562. doi: 10.1046/j.1464-5491.1999.00127.x. [DOI] [PubMed] [Google Scholar]
- 29.Sherwood A, Steffen PR, Blumenthal JA, Kuhn C, Hinderliter AL. Nighttime blood pressure dipping: the role of the sympathetic nervous system. Am.J.Hypertens. 2002;15:111–118. doi: 10.1016/s0895-7061(01)02251-8. [DOI] [PubMed] [Google Scholar]
- 30.Higashi Y, Nakagawa K, Kimura M, Noma K, Hara K, Sasaki S, Goto C, Oshima T, Chayama K, Yoshizumi M. Circadian variation of blood pressure and endothelial function in patients with essential hypertension:a comparison of dippers and non-dippers. J.Am.Coll.Cardiol. 2002;40:2039–2043. doi: 10.1016/s0735-1097(02)02535-4. [DOI] [PubMed] [Google Scholar]
- 31.Chen PY, Sanders PW. L-arginine abrogates salt-sensitive hypertension in Dahl/Rapp rats. J Clin Invest. 1991;88:1559–1567. doi: 10.1172/JCI115467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fujii T, Uzu T, Nishimura M, Takeji M, Kuroda S, Nakamura S, Inenaga T, Kimura G. Circadian rhythm of natriuresis is disturbed in nondipper type of essential hypertension. Am J Kidney Dis. 1999;33:29–35. doi: 10.1016/s0272-6386(99)70254-4. [DOI] [PubMed] [Google Scholar]
- 33.Uzu T, Ishikawa K, Fujii T, Nakamura S, Inenaga T, Kimura G. Sodium restriction shifts circadian rhythm of blood pressure from nondipper to dipper in essential hypertension. Circulation. 1997;96:1859–1862. doi: 10.1161/01.cir.96.6.1859. [DOI] [PubMed] [Google Scholar]
- 34.Uzu T, Kimura G. Diuretics shift circadian rhythm of blood pressure from nondipper to dipper in essential hypertension. Circulation. 1999;100:1635–1638. doi: 10.1161/01.cir.100.15.1635. [DOI] [PubMed] [Google Scholar]
- 35.Svensson P, de Faire U, Sleight P, Yusuf S, Ostergren J. Comparative effects of ramipril on ambulatory and office blood pressures: a HOPE Substudy. Hypertension. 2001;38:E28–E32. doi: 10.1161/hy1101.099502. [DOI] [PubMed] [Google Scholar]
- 36.Jonas M, Garfinkel D, Zisapel N, Laudon M, Grossman E. Impaired nocturnal melatonin secretion in non-dipper hypertensive patients. Blood Press. 2003;12:19–24. [PubMed] [Google Scholar]
- 37.Sayk F, Becker C, Teckentrup C, Fehm HL, Struck J, Wellhoener JP, Dodt C. To dip or not to dip: on the physiology of blood pressure decrease during nocturnal sleep in healthy humans. Hypertension. 2007;49:1070–1076. doi: 10.1161/HYPERTENSIONAHA.106.084343. [DOI] [PubMed] [Google Scholar]
- 38.Agarwal R, Andersen MJ. Correlates of systolic hypertension in patients with chronic kidney disease. Hypertension. 2005;46:514–520. doi: 10.1161/01.HYP.0000178102.85718.66. [DOI] [PubMed] [Google Scholar]
- 39.Minutolo R, Borrelli S, Chiodini P, Scigliano R, Bellizzi V, Cianciaruso B, Nappi F, Zamboli P, Catapano F, Conte G, De NL. Effects of age on hypertensive status in patients with chronic kidney disease. J.Hypertens. 2007;25:2325–2333. doi: 10.1097/HJH.0b013e3282ef549e. [DOI] [PubMed] [Google Scholar]
- 40.Castelpoggi CH, Pereira VS, Fiszman R, Cardoso CR, Muxfeldt ES, Salles GF. A blunted decrease in nocturnal blood pressure is independently associated with increased aortic stiffness in patients with resistant hypertension. Hypertens.Res. 2009;32:591–596. doi: 10.1038/hr.2009.71. [DOI] [PubMed] [Google Scholar]; *A study that demonstrates that is consistent with the notion that baroreflex sensitivity which is impaired in those with stiff aortas is associated with sympathetic activation and non-dipping.
- 41.Rosansky SJ, Menachery SJ, Wagner CM, Jackson K. Circadian blood pressure variation versus renal function. Am J Kidney Dis. 1995;26:716–721. doi: 10.1016/0272-6386(95)90433-6. [DOI] [PubMed] [Google Scholar]
- 42.Wilson DK, Sica DA, Miller SB. Effects of potassium on blood pressure in salt-sensitive and salt-resistant black adolescents. Hypertension. 1999;34:181–186. doi: 10.1161/01.hyp.34.2.181. [DOI] [PubMed] [Google Scholar]
- 43.Ishikawa J, Hoshide S, Eguchi K, Ishikawa S, Pickering TG, Shimada K, Kario K. Plasma tissue inhibitor of matrix metalloproteinase-1 level is increased in normotensive non-dippers in association with impaired glucose metabolism. Hypertens.Res. 2008;31:2045–2051. doi: 10.1291/hypres.31.2045. [DOI] [PubMed] [Google Scholar]
- 44.Rodriguez CJ, Burg MM, Meng J, Pickering TG, Jin Z, Sacco RL, Boden-Albala B, Homma S, Di Tullio MR. Effect of social support on nocturnal blood pressure dipping. Psychosom.Med. 2008;70:7–12. doi: 10.1097/PSY.0b013e31815aab4e. [DOI] [PubMed] [Google Scholar]
- 45.Agarwal R, Light RP, Bills JE, Hummel LA. Nocturia, Nocturnal Activity, and Nondipping. Hypertension. 2009;54:646–651. doi: 10.1161/HYPERTENSIONAHA.109.135822. [DOI] [PubMed] [Google Scholar]; *Nocturia is associated with increased nocturnal awakening and non-dipping.
- 46.Converse RL, Jr, Jacobsen TN, Toto RD, Jost CMT, Cosentino F, Fouad-Tarazi F, Victor RG. Sympathetic overactivity in patients with chronic renal failure. N Engl J Med. 1992;327:1912–1918. doi: 10.1056/NEJM199212313272704. [DOI] [PubMed] [Google Scholar]
- 47.Agarwal R. Antihypertensive agents and arterial stiffness: relevance to reducing cardiovascular risk in the chronic kidney disease patient. Curr.Opin.Nephrol Hypertens. 2007;16:409–415. doi: 10.1097/MNH.0b013e3282063b86. [DOI] [PubMed] [Google Scholar]
- 48.Clausen P, Jensen JS, Jensen G, Borch-Johnsen K, Feldt-Rasmussen B. Elevated urinary albumin excretion is associated with impaired arterial dilatory capacity in clinically healthy subjects. Circulation. 2001;103:1869–1874. doi: 10.1161/01.cir.103.14.1869. [DOI] [PubMed] [Google Scholar]
- 49.Johnson RJ, Herrera-Acosta J, Schreiner GF, Rodriguez-Iturbe B. Subtle acquired renal injury as a mechanism of salt-sensitive hypertension. N.Engl.J Med. 2002;346:913–923. doi: 10.1056/NEJMra011078. [DOI] [PubMed] [Google Scholar]
- 50.van Tilborg KA, Rabelink TJ, van Rijn HJ, Boomsma F, Koomans HA. Arterial baroreflex control of renal hemodynamics in humans. Circulation. 1994;90:1883–1890. doi: 10.1161/01.cir.90.4.1883. [DOI] [PubMed] [Google Scholar]
- 51.Lurbe E, Redon J, Kesani A, Pascual JM, Tacons J, Alvarez V, Batlle D. Increase in nocturnal blood pressure and progression to microalbuminuria in type 1 diabetes. N.Engl.J Med. 2002;347:797–805. doi: 10.1056/NEJMoa013410. [DOI] [PubMed] [Google Scholar]
- 52.Torbjornsdotter TB, Jaremko GA, Berg UB. Ambulatory blood pressure and heart rate in relation to kidney structure and metabolic control in adolescents with Type I diabetes. Diabetologia. 2001;44:865–873. doi: 10.1007/s001250100528. [DOI] [PubMed] [Google Scholar]
- 53.Portaluppi F, Montanari L, Massari M, Di Chiara V, Capanna M. Loss of nocturnal decline of blood pressure in hypertension due to chronic renal failure. Am J Hypertens. 1991;4:20–26. doi: 10.1093/ajh/4.1.20. [DOI] [PubMed] [Google Scholar]
- 54.Farmer CK, Goldsmith DJ, Cox J, Dallyn P, Kingswood JC, Sharpstone P. An investigation of the effect of advancing uraemia, renal replacement therapy and renal transplantation on blood pressure diurnal variability. Nephrol Dial.Transplant. 1997;12:2301–2307. doi: 10.1093/ndt/12.11.2301. [DOI] [PubMed] [Google Scholar]
- 55.Elung-Jensen T, Strandgaard S, Kamper AL. Longitudinal observations on circadian blood pressure variation in chronic kidney disease stages 3–5. Nephrol.Dial.Transplant. 2008 doi: 10.1093/ndt/gfn126. [DOI] [PubMed] [Google Scholar]; *Dipping is impaired early in the course of CKD.
- 56.Fukuda M, Mizuno M, Yamanaka T, Motokawa M, Shirasawa Y, Nishio T, Miyagi S, Yoshida A, Kimura G. Patients with renal dysfunction require a longer duration until blood pressure dips during the night. Hypertension. 2008;52:1155–1160. doi: 10.1161/HYPERTENSIONAHA.108.115329. [DOI] [PubMed] [Google Scholar]
- 57.Gatzka CD, Schobel HP, Klingbeil AU, Neumayer HH, Schmieder RE. Normalization of circadian blood pressure profiles after renal transplantation. Transplantation. 1995;59:1270–1274. [PubMed] [Google Scholar]
- 58.Stenehjem AE, Gudmundsdottir H, Os I. Office blood pressure measurements overestimate blood pressure control in renal transplant patients. Blood Press Monit. 2006;11:125–133. doi: 10.1097/01.mbp.0000209080.24870.2d. [DOI] [PubMed] [Google Scholar]
- 59.Ferreira SR, Moises VA, Tavares A, Pacheco-Silva A. Cardiovascular effects of successful renal transplantation: a 1-year sequential study of left ventricular morphology and function, and 24-hour blood pressure profile. Transplantation. 2002;74:1580–1587. doi: 10.1097/00007890-200212150-00016. [DOI] [PubMed] [Google Scholar]
- 60.Jacobi J, Rockstroh J, John S, Schreiber M, Schlaich MP, Neumayer HH, Schmieder RE. Prospective analysis of the value of 24-hour ambulatory blood pressure on renal function after kidney transplantation. Transplantation. 2000;70:819–827. doi: 10.1097/00007890-200009150-00020. [DOI] [PubMed] [Google Scholar]
- 61.Kooman JP, Christiaans MH, Boots JM, Van der Sande FM, Leunissen KM, Van Hooff JP. A comparison between office and ambulatory blood pressure measurements in renal transplant patients with chronic transplant nephropathy. Am.J.Kidney Dis. 2001;37:1170–1176. doi: 10.1053/ajkd.2001.24518. [DOI] [PubMed] [Google Scholar]
- 62.Haydar AA, Covic A, Jayawardene S, Agharazii M, Smith E, Gordon I, O'Sullivan H, Goldsmith DJ. Insights from ambulatory blood pressure monitoring: diagnosis of hypertension and diurnal blood pressure in renal transplant recipients. Transplantation. 2004;77:849–853. doi: 10.1097/01.tp.0000115345.16853.51. [DOI] [PubMed] [Google Scholar]
- 63.Covic A, Gusbeth-Tatomir P, Mardare N, Buhaescu I, Goldsmith DJ. Dynamics of the circadian blood pressure profiles after renal transplantation. Transplantation. 2005;80:1168–1173. doi: 10.1097/01.tp.0000167003.97452.a8. [DOI] [PubMed] [Google Scholar]
- 64.Agarwal R, Alborzi P, Satyan S, Light RP. Dry-weight reduction in hypertensive hemodialysis patients (DRIP): a randomized, controlled trial. Hypertension. 2009;53:500–507. doi: 10.1161/HYPERTENSIONAHA.108.125674. [DOI] [PMC free article] [PubMed] [Google Scholar]; **A landmark trial that illustrates the value of dry-weight management among patients with hypertension on hemodialysis.
- 65.Agarwal R. Volume-Associated Ambulatory Blood Pressure Patterns in Hemodialysis Patients. Hypertension. 2009;54:241–247. doi: 10.1161/HYPERTENSIONAHA.109.136366. [DOI] [PMC free article] [PubMed] [Google Scholar]; **Although dry-weight reduction reduces BP, it does not restore dipping. A unique pattern of interdialytic ambulatory BP is described in patients who have euvolemia.
- 66.Suzuki M, Guilleminault C, Otsuka K, Shiomi T. Blood pressure “dipping” and “non-dipping” in obstructive sleep apnea syndrome patients. Sleep. 1996;19:382–387. doi: 10.1093/sleep/19.5.382. [DOI] [PubMed] [Google Scholar]
- 67.Amin R, Somers VK, McConnell K, Willging P, Myer C, Sherman M, McPhail G, Morgenthal A, Fenchel M, Bean J, Kimball T, Daniels S. Activity-adjusted 24-hour ambulatory blood pressure and cardiac remodeling in children with sleep disordered breathing. Hypertension. 2008;51:84–91. doi: 10.1161/HYPERTENSIONAHA.107.099762. [DOI] [PubMed] [Google Scholar]
- 68.Bao X, Nelesen RA, Loredo JS, Dimsdale JE, Ziegler MG. Blood pressure variability in obstructive sleep apnea: role of sympathetic nervous activity and effect of continuous positive airway pressure. Blood Press Monit. 2002;7:301–307. doi: 10.1097/00126097-200212000-00002. [DOI] [PubMed] [Google Scholar]
- 69.Mallion JM, Neuder Y, Ormezzano O, Rochette GB, Salvat M, Baguet JP. Study of nycthemeral variations in blood pressure in patients with heart failure. Blood Press Monit. 2008;13:163–165. doi: 10.1097/MBP.0b013e3282fd16f8. [DOI] [PubMed] [Google Scholar]
- 70.Hermida RC, Chayan L, Ayala DE, Mojon A, Dominguez MJ, Fontao MJ, Soler R, Alonso I, Fernandez JR. Association of metabolic syndrome and blood pressure nondipping profile in untreated hypertension. Am.J.Hypertens. 2009;22:307–313. doi: 10.1038/ajh.2008.358. [DOI] [PubMed] [Google Scholar]; **Metabolic syndrome is now added to the list of conditions associated with non-dipping.
- 71.Ayala DE, Hermida RC, Chayan L, Mojon A, Fontao MJ, Fernandez JR. Circadian pattern of ambulatory blood pressure in untreated hypertensive patients with and without metabolic syndrome. Chronobiol.Int. 2009;26:1189–1205. doi: 10.3109/07420520903206294. [DOI] [PubMed] [Google Scholar]; **Patients with metabolic syndrome are more often non-dippers.