Abstract
Pseudomonas aeruginosa infection in ventilator-associated pneumonia is a serious and often life-threatening complication in intensive care unit patients, and new treatment options are needed. We used B-cell-enriched peripheral blood lymphocytes from a volunteer immunized with a P. aeruginosa O-polysaccharide-toxin A conjugate vaccine to generate human hybridoma cell lines producing monoclonal antibodies specific for individual P. aeruginosa lipopolysaccharide serotypes. The fully human monoclonal antibody secreted by one of these lines, KBPA101, is an IgM/κ antibody that binds P. aeruginosa of International Antigenic Typing System (IATS) serotype O11 with high avidity (5.81 × 107 M−1 ± 2.8 × 107 M−1) without cross-reacting with other serotypes. KBPA101 specifically opsonized the P. aeruginosa of IATS O11 serotype and mediated complement-dependent phagocytosis in vitro by the human monocyte-like cell line HL-60 at a very low concentration (half-maximal phagocytosis at 0.16 ng/ml). In vivo evaluation of KBPA101 demonstrated a dose-response relationship for protection against systemic infections in a murine burn wound sepsis model, where 70 to 100% of animals were protected against lethal challenges with P. aeruginosa at doses as low as 5 μg/animal. Furthermore, a high efficacy of KBPA101 in protection from local respiratory infections in an acute lung infection model in mice was demonstrated. Preclinical toxicology evaluation on human tissue, in rabbits, and in mice did not indicate any toxicity of KBPA101. Based on these preclinical findings, the first human clinical trials have been initiated.
Pseudomonas aeruginosa is one of the leading causes of hospital-acquired (nosocomial) infections, along with coagulase-negative staphylococci, Staphylococcus aureus, and Enterococcus spp. (9, 10, 37). Bloodstream infection and pneumonia in mechanically ventilated patients are among the most frequently observed forms of nosocomial infection. Many of the commonly found bacterial strains in nosocomial infections are multidrug resistant or extensively drug resistant to most antibiotics.
P. aeruginosa is a ubiquitous Gram-negative bacillus. Based on the phenotypic diversity of the O-polysaccharide moieties of surface lipopolysaccharide (LPS), P. aeruginosa is grouped into 20 unique O serotypes according to the International Antigenic Typing System (IATS) (15). Among these serotypes O11 is one of the most frequently observed ones accounting for 18 to 21% of P. aeruginosa infections (unpublished data by the authors and see also reference 28) with a very high virulence among at-risk hospital patients (42). Immunocompromised individuals in general and mechanically ventilated patients in particular are at high risk for developing pneumonia (ventilator-associated pneumonia [VAP]), for which P. aeruginosa is the most frequently detected Gram-negative bacterium (9, 37). VAP caused by P. aeruginosa is associated with significantly higher fatality rates than VAP caused by other bacterial pathogens (3, 16).
The increase in antibiotic-resistant microorganisms and the paucity of new small molecule drugs for treatment of infectious diseases have renewed the interest in antibody-based anti-infective therapy (2, 33). Although high-titer antibody preparations produced by human plasma fractionation methods are well accepted for treatment of selected viral and bacterial infections, only one monoclonal antibody (MAb), namely, Palivizumab for respiratory syncytial virus, has been licensed for immunotherapy of an infectious disease thus far. However, recent advances in the generation and large-scale production of chimeric or humanized MAbs allowed the clinical development of several MAbs against viral, bacterial, and fungal diseases (29, 33, 45).
IgM is the preferred isotype for complement-mediated killing and complement-dependent phagocytosis of infectious bacteria due to its pentameric form and its ability for effective complement activation. Furthermore, polysaccharides (including LPS) are T-cell-independent antigens, and antibodies induced in response to them are mostly of the IgM isotype. However, recombinant expression of pentameric IgM has not been achieved routinely thus far. There have been many attempts to generate human MAbs of different isotypes against various antigens of P. aeruginosa, such as LPS, alginate, or PcrV (reviewed by Doring and Pier [8]). Most of these MAbs demonstrated protection in animal models (18, 25, 30, 39, 41, 47, 48), and some were subsequently tested in clinical settings (12, 27, 38), but none of these MAbs have been licensed for routine use in humans thus far.
We describe here the generation and preclinical characterization of a fully human IgM/κ MAb termed KBPA101, directed against the LPS O polysaccharide of serotype O11 of P. aeruginosa. KBPA101 was generated by immortalizing human B lymphocytes and selected for high effector function. KBPA101 demonstrated high specificity and efficacy in vitro and in vivo and was already tested in a clinical phase 1 study (23).
MATERIALS AND METHODS
Generation of the cell line producing the human MAb KBPA101.
Human MAb KBPA101 has been generated by immunizing a healthy volunteer with an octovalent O-polysaccharide-toxin A conjugate vaccine. The vaccine contains polysaccharide of the O11 reference strain FT-2 (ATCC 27131). The vaccine has been described in detail (5, 6, 20, 21, 40). One week after the second immunization, antigen-specific B cells were enriched from peripheral blood by panning on immobilized LPS as described previously (19), followed by Epstein-Barr virus (EBV) transformation with a cell culture supernatant of the B95-8 marmoset cell line (7), resulting in lymphoblastoid cell lines (LCL). LCL producing antibodies against P. aeruginosa LPS of serotype O11 were fused to the hypoxanthine-aminopterin-thymidine-sensitive heterohybridoma cell line LA55 by a standard PEG 4000-dimethyl sulfoxide method (22). Hybridomas were grown in Iscove modified Dulbecco medium (IMDM; Sigma-Aldrich, Buchs, Switzerland) supplemented with 5 × 10−5 M β2-mercaptoethanol and 10% fetal calf serum (FCS) containing 50 pM hypoxanthine, 2.5 pg of azaserine/ml, and 2.5 pM ouabain (Sigma-Aldrich). Hybridomas producing MAb against P. aeruginosa O11 were selected and ranked based on antigen specificity, stability (determined by limiting dilution cultures), and a high antibody production rate (determined as pg of IgM/cell/24 h). One clone, producing the MAb KBPA101, was superior with respect to all criteria and therefore purified IgM formulated in phosphate-buffered saline (PBS) (23) was used for all subsequent experiments.
Sequencing of KBPA101.
The variable regions of the heavy and light chains of KBPA101 were amplified from the total cDNA by using the SMART (Becton Dickenson) universal primer, and the Cκ-specific primer AGCAGGCACACAACAGAGGCAGTTCC or the Cμ-specific primer GCCACGCTGCTCGTATCCGAC. The purified PCR fragments were used as templates for sequencing with the primers CACAACAGAGGCAGTTCC for light-chain and GCTGCTCGTATCCGACGG for heavy-chain sequences, performed at Microsynth AG (Balgach Switzerland). The sequences were compared to the V-base index (http://vbase.mrc-cpe.cam.ac.uk). The sequences of the IgVH variable region and the Vκ variable region are available under accession numbers GQ241317 and GQ241318, respectively.
Determination of LPS specificity by ELISA.
For screening and analysis of antibodies in cell culture supernatants, an enzyme-linked immunosorbent assay (ELISA) was used as described elsewhere (46), with some alterations. Briefly, P. aeruginosa LPS antigen (5 μg/ml) was bound to polystyrene microtiter plates (Nunc Maxisorp, Roskilde, Denmark) by methylated human serum albumin. All washing steps were performed with PBS containing 0.05% Tween 20 (Fluka Chemie AG, Buchs, Switzerland) (PBS-T). Cell culture supernatants were incubated for 2 h at 37°C. Bound antibodies were detected with horseradish peroxidase-conjugated goat anti-human IgM antibody (KPL, Inc., Gaithersburg, MD) diluted 1:2,000 in PBS containing 5% FCS (1 h at 37°C). Antibody binding was visualized with OPD (Orthophenyldiamin)-H2O2 substrate solution. Color reaction was stopped with 1 M HCl. Optical density was read on a Spectromax ELISA-plate reader (Paul Bucher AG, Basel, Switzerland) using SoftMax Pro software version 3.1.1 (Molecular Devices, Sunnyvale, CA).
Avidity determination by inhibition ELISA.
The avidity constant of KBPA101 for P. aeruginosa LPS serotype O11 was measured by inhibition ELISA as described by Bruderer et al. (1) and was defined as the reciprocal antigen concentration (in moles per liter) resulting in 50% inhibition of antibody binding. The cell lines RK-52 and RS-1H7 were ordered from the American Type Culture Collection (ATCC; ATCC HB-9149 and ATCC HB-9151), and HI-223 was ordered from IPOD (the International Patent Organism Depository) under the number FERM BP 3213.
Bacterial strains and clinical isolates.
P. aeruginosa strains were from various clinical isolates collected at hospitals in Bern and Zurich, Switzerland, and Jena Germany, and were serotyped by agglutination using a mouse MAb kit (Erfa Biotech, Westmount, Quebec, Canada) and by PCR using serotype-specific primers (32). The O-serotype designation used in the present study is according to the International Antigen Typing System (IATS). One of these strains, termed 2310.55, was used in the animal challenge studies due to its high virulence in the murine burn wound model. Reference strain FT-2 (O11, ATCC 27313) was used in the opsonophagocytosis assay.
Recognition of clinical isolates of P. aeruginosa by whole-cell ELISA.
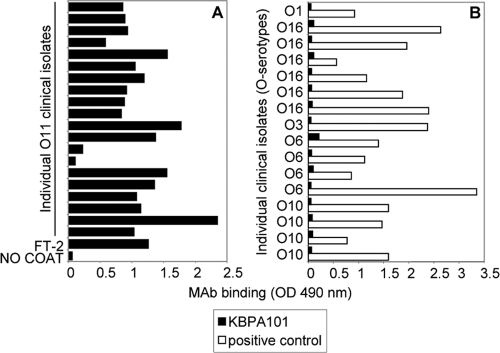
Bacteria from various clinical isolates were grown in Luria broth medium at 37°C to an optical density at 600 nm of 1.0 and fixed with 0.5% formalin overnight at 37°C. The fixed bacteria were diluted 1:50 in PBS and immobilized on ELISA plates. After the plates were blocked with PBS containing 5% FCS, KBPA101 was incubated with the fixed bacteria for 2 h at 37°C. Alternatively, isolates of other serotypes were grown as described above and incubated with KBPA101 or, as positive controls, with MAb specific for the respective serotypes (Fig. 1B, serotype-specific positive control MAb collectively called “positive control”). Bound antibodies were detected as described above.
FIG. 1.
KBPA101 specifically and exclusively reacts with clinical isolates of P. aeruginosa serotype O11. (A) The binding of KBPA101 to 20 clinical P. aeruginosa isolates of serotype O11 (serotype confirmed by agglutination and PCR) was tested by whole-cell ELISA. Reference strain FT-2 was used as a positive control. (B) The specificity of KBPA101 for O11 was confirmed by the absence of binding to clinical P. aeruginosa isolates of serotypes other than O11. Various murine MAbs with specificity for serotypes O1, O16, O3, O6, or O10 were used as a positive control.
Opsonophagocytosis assay.
HL60 cells were purchased from the ATCC (CCL-240) and cultured in IMDM containing 10% FCS at 37°C and 6.5% CO2. For use in the opsonophagocytosis assay, HL-60 cells were differentiated with 100 mM dimethyl formamide (Sigma-Aldrich) for 3 days (24). P. aeruginosa strain FT-2 was stained with fluorescein isothiocyanate (FITC) as described by Jansen et al. (13), and bacteria were incubated with serially diluted cell culture supernatants with or without complement for 30 min, followed by incubation for 90 min at 37°C with differentiated HL-60 cells (bacteria-HL-60, 7:1). Opsonophagocytosis was determined by analyzing the green fluorescence of the HL-60 cells in comparison with background staining by flow cytometry using a FACSCalibur (BD Biosciences, Allschwil, Switzerland). Background staining was determined by incubating FITC-conjugated bacteria in the presence of complement but in the absence of antibody with HL-60 cells. Internalization of FITC-labeled bacteria was confirmed by confocal microscopy.
Complement-dependent killing assay.
Bacterial cells were grown in Luria broth medium at 37°C to an optical density at 600 nm of 0.4. Cells were diluted 1:10 in Hanks balanced salt solution plus 0.1% bovine serum albumin (Sigma-Aldrich) and incubated with serially diluted antibody with or without complement for 90 min. Subsequently, the mixture was diluted in 0.9% NaCl and plated on brain heart infusion agar plates for enumeration of CFU after overnight incubation.
Murine burn wound model.
The in vivo protective capacity of KBPA101 was determined in the murine burn wound sepsis model in four independent experiments. All animal experiments were performed with approval of the Tierschutzkommission of the Canton of Bern. Temgesic (buprenorphine; Essex Pharma GmbH, Munich, Germany) was added to the drinking water from 3 days before challenge until the end of the experiment. Female NMRI mice (Charles River Laboratories, L'Arbresle, France) with an average body weight of 25 g were injected with a single dose of 0.005 to 0.4 mg/kg of KBPA101 in a volume of 0.1 ml at 4 h prior to challenge. For challenge, groups of 10 female mice were anesthetized by intraperitoneal injection with ketamine-xylasol. In addition, immediately before inflicting the burn wound, the mice were placed in an atmosphere of 3-chloro-1,1,2-trifluoroethyl-difluoromethyl ether (Ethrane; Abbott Laboratories, Chicago, IL) for ca. 30 s. Thereafter, mice were subjected to a 10-s ethanol burn of a 2-cm2 area of the back. A total of 2 × 107 CFU of P. aeruginosa clinical isolate 2310.55 suspended in 0.5 ml of PBS/mouse was injected immediately subcutaneously under the burned area. Mice were checked three times per day for 7 days after challenge, and moribund animals were euthanized. Survival rates of animals at 72 h postchallenge are reported.
For therapeutic evaluation of KBPA101, groups of 10 female mice were challenged as described above. At 2 h postchallenge the animals received a single dose of KBPA101 or PBS at the indicated dosing by the intravenous (i.v.) route. As a positive control, one group of animals was treated at the highest dose (4 mg/kg) immediately prior to challenge. Mice were checked three times per day for 4 days after challenge, and moribund animals were euthanized.
Acute lung infection model.
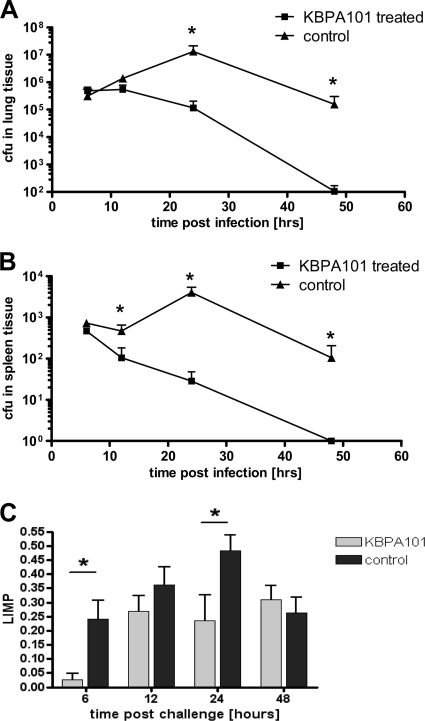
To evaluate the protective capacity of KBPA101 against lung infection with P. aeruginosa, an acute lung infection model was used. A total of 10 μg of KBPA101 (corresponding to ∼0.4 mg/kg) was injected i.v. into 40 BALB/c mice (M&B Laboratory Animals, Ry, Denmark), 40 control mice received i.v. injections of PBS. Thereafter, 40 μl of a 4.0 × 107/ml solution of strain 2310.55 (corresponding to 1.6 × 106 per mouse) was applied intratracheally into the lower left bronchus using a curved bead-tipped needle under deep anesthesia. This dose was chosen since it led to limited mortality only. After 6, 12, 24, and 48 h, mice (10 per group and time point) were sacrificed, and the lungs and spleens were removed aseptically. For quantification of bacteria in challenged mice, organs were suspended in PBS and homogenized with a blender on ice. Serially diluted organ homogenates were plated on modified Conradi Drigalski agar to determine the CFU/lung or the CFU/spleen. For the evaluation of macroscopic lung pathology the lung index of macroscopic pathology (LIMP) was used as described previously by Song et al. (43). LIMP equals the ratio of lung area with pathological changes divided by the area of the whole lung. The lung surface areas were calculated by measuring the widths and lengths of the areas using a ruler.
Toxicological evaluation of KBPA101.
All toxicity studies were performed with KBPA101 produced and purified according to good manufacturing practice (GMP) standards. These studies included the acute dose toxicity and repeat dose toxicity in mice, as well as a local tolerance study in New Zealand rabbits. For the repeat-dose toxicity study, KBPA101 was administered at the dose of 1.2, 4.0, or 12 mg/kg/dose, twice weekly for two consecutive weeks, by i.v. injection of BALB/c mice. All studies were performed by CIT, Evreux, France, according to good laboratory practice (GLP) standards (details are supplied in the supplemental material).
Statistical analysis.
All statistical analyses, including linear regression, were performed by using GraphPad Prism version 4 for Windows (GraphPad Software, San Diego, CA).
RESULTS
Generation and in vitro characterization of KBPA101.
KBPA101 is a vaccine-induced human MAb of IgM/κ isotype reactive with O polysaccharide of IATS serotype O11 of P. aeruginosa. It is secreted by a stable mouse-human heterohybridoma cell line generated by fusion of the mouse-human heterohybridoma cell line LA55 with EBV-transformed lymphocytes from a volunteer vaccinated with an octovalent O-polysaccharide-toxin A conjugate vaccine. Sequence analysis showed that the variable region of the KBPA101 heavy chain was encoded by VH3/DP-53 and JH3b, and the light chain variable region was encoded by Vκ/DPK18 and Jκ4. The heavy chain displayed 17 replacement and 3 silent mutations compared to DP-53, whereas the light chain displayed only 2 silent point mutations compared to DPK18. Testing of KBPA101 by ELISA against purified LPS of eight different serotypes (IATS-O1, -O3, -O4, -O5, -O6, -O10, -O11, and -O16 [Fisher immunotype 3]) showed exclusive reactivity with LPS of serotype O11 (data not shown). Functional avidity of KBPA101 to O11 LPS determined by inhibition ELISA was 5.81 × 107 M−1 ± 2.8 × 107 M−1. KBPA101 strongly bound to 18 of 20 clinical O11 isolates (Fig. 1A), confirming reactivity with clinically relevant P. aeruginosa strains. Further analysis revealed that the two nonrecognized isolates had low surface expression of LPS. No binding with various isolates of serotypes IATS-O1, -O16, -O3, -O6, or -O10 was observed (Fig. 1B).
Opsonophagocytic activity.
The biological activity of KBPA101 in vitro was assessed by using a flow cytometry-based opsonophagocytosis assay. KBPA101 efficiently mediated phagocytosis of O11 reference strain FT-2 (Fig. 2A) and of the highly virulent clinical isolate 2310.55 (Fig. 2B). Phagocytosis was dependent of the dose of KBPA101 and of the presence of complement (Fig. 2 [▴], baby rabbit serum [BRS]). No phagocytosis was observed if heat-inactivated complement was used (Fig. 2 [▪], heated BRS [HBRS]). Half-maximal opsonophagocytic activity was observed at 0.16 ng/ml. In order to confirm internalization of FITC-conjugated bacteria, after opsonization, HL-60 cells were fixed on a glass slide and analyzed by confocal microscopy (Fig. 2C). In addition, KBPA101 showed direct complement-dependent killing of bacterial cells in a dose-dependent manner (Fig. 2D). These results are in line with the observation that KBPA101 complexed with O11 LPS strongly activated the complement system and immobilized C1q, whereas free antibody did not activate complement (data not shown).
FIG. 2.
KBPA101 specifically mediates complement-dependent opsonophagocytosis of P. aeruginosa serotype O11 in vitro. FITC-conjugated P. aeruginosa (reference strain FT2, ATCC 27131 [A]; clinical isolate 2310.55 [B]) were incubated with KBPA101, together with baby rabbit complement (BRS, ▴) and the phagocytic cell line (HL-60). The amount of phagocytosis was measured by flow cytometry. KBPA101 mediated phagocytosis in a dose-dependent manner, showing half-maximal phagocytosis at a concentration of 0.1 ng/ml for reference strain FT2, as well as the clinical isolate 2310.55. No opsonophagocytosis was observed in the absence of active complement (heat-inactivated complement, HBRS, ▪). (C) Cross-sectional confocal microscopy of HL-60 cells after phagocytosis of opsonized FITC-conjugated P. aeruginosa strain FT2. On the right side and at the bottom, overlays of 10 sections are shown. (D) KBPA101 mediated complement-dependent killing of P. aeruginosa serotype O11. Bacteria were incubated in the presence or absence of rabbit complement with increasing concentrations of KBPA101 for 90 min. Surviving bacteria were plated on agarose plates and incubated overnight at 37°C. Percent survival rates are plotted against MAb concentrations.
Prevention of systemic P. aeruginosa infection in a murine burn wound sepsis model.
The capacity of KBPA101 to prevent systemic P. aeruginosa infection was evaluated in a murine burn wound sepsis model (4). Decreasing doses of KBPA101 were administered to NMRI mice 4 h prior to burn wound challenge and P. aeruginosa infection. Doses greater than 0.2 mg/kg (body weight) resulted in 70 to 100% survival (Fig. 3), whereas the administration of lower doses resulted in reduced survival rates. Nontreated, challenged mice had a mortality rate of close to 100%, whereas mice with burn wounds but no Pseudomonas infection had a 100% survival rate (data not shown). In the treatment groups, most animals succumbing to the infection died within the first 72 h. Since the serum half-life of KBPA101 in normal mice was estimated to be ∼18 h, it can be assumed that by 72 h most of the antibody is eliminated from the system. Animals were routinely followed up several days beyond that time point, and yet no recurrences of the infections were observed.
FIG. 3.

KBPA101 protects mice from systemic infection with P. aeruginosa serotype O11 in a murine burn wound model. Various doses of KBPA101 were given to NMRI mice (n = 10/dose/experiment) prior to challenge with a lethal dose of live bacteria. Upon inflicting a burn wound to animals under deep anesthesia, P. aeruginosa serotype O11 (clinical isolate 2310.55) was injected subcutaneously (s.c.) under the burn wound. The experiment was repeated four times (experiments 1 through 4). The average survival rates 3 days after challenge are shown from the four independent experiments. A linear regression was calculated from the mean values. Control animals receiving no antibody had survival rates of <10%, whereas KBPA101-treated animals had increasing survival rates in a dose-dependent manner. No mortality was observed in mice treated equally but injected s.c. with PBS instead of live bacteria (data not shown).
Therapy of systemic P. aeruginosa infection in a murine burn wound sepsis model.
The therapeutic potential of KBPA101 was assessed in the same murine burn wound sepsis model (Fig. 4) when animals were challenged with a lethal dose of P. aeruginosa and treated 4 h postchallenge with a single injection of KBPA101 (1, 2, or 4 mg/kg [body weight]). In general, a therapeutic setting requires higher doses of antibody in order to demonstrate an effect, and we therefore chose 4 mg/kg as the highest dose, reflecting a 10-fold greater amount of antibody conferring protection in a prophylactic model. As a positive control a group of animals was treated at the time of challenge with the highest dose. KBPA101 administered either immediately or 4 h postchallenge significantly reduced mortality compared to untreated control animals (Fig. 4, P < 0.001 [log-rank test]). Surprisingly, no dose effect was observed in this experiment. However, the bacterial load in splenic tissue was lower in mice receiving 4 mg/kg than in animals treated with 1 mg/kg (2.9 × 102 CFU versus 1.8 × 105 CFU per g of splenic tissue), hinting at a higher efficacy with higher dosing.
FIG. 4.
Treatment with KBPA101 extends the survival of mice with systemic infection with P. aeruginosa serotype O11 in a murine burn wound model. Upon inflicting a burn wound to animals under deep anesthesia, a lethal dose of live P. aeruginosa serotype O11 (clinical isolate 2310.55) was injected s.c. under the burn wound. Control animals were treated with the highest dose of KBPA101 immediately after challenge, whereas experimental animals were treated 4 h postchallenge with a single i.v. injection of various doses of KBPA101. A significant survival benefit was observed for treated animals compared to untreated animals. No dose response was seen for treatment effect. Significant survival rates compared to control group are marked with an asterisk (*, P < 0.001 [log-rank test]).
Prevention of respiratory P. aeruginosa in an acute lung infection model.
To evaluate whether KBPA101 might protect from respiratory Pseudomonas infection, a model of acute lung infection in BALB/c mice was used. For this purpose, KBPA101 (∼0.4 mg/kg [body weight]) or an equivalent volume of PBS was administered i.v. prior to intratracheal lung challenge P. aeruginosa. The challenge dose was chosen to induce significant lung infection but only minimal mortality (based on pilot experiments). Application of KBPA101 led to rapid clearance of P. aeruginosa from the lung (Fig. 5A). Pseudomonas infection was completely resolved in treated animals 48 h postchallenge, whereas at this time point infection was still ongoing in untreated animals. Similarly, KBPA101 completely cleared systemic P. aeruginosa from the spleen, whereas live bacteria were still present in untreated mice at 48 h postchallenge (Fig. 5B). In addition, KBPA101-treated mice, compared to PBS-treated mice, showed milder macroscopic lung pathology at 6 and 24 h after infection (Fig. 5C).
FIG. 5.
KBPA101 protects mice from local lung infection with P. aeruginosa serotype O11 in an acute lung infection model. KBPA101 or an equivalent volume of PBS was injected i.v. into BALB/c mice 2 h before challenge with 3.5 × 107 CFU of P. aeruginosa serotype O11 (clinical isolate 2310.55). Groups of 10 mice were sacrificed 6, 12, 24, and 48 h after intratracheal challenge, and the bacterial load was determined in homogenates of the lungs (A) and spleens (B). Time points with significant differences between treated and untreated controls are marked with an asterisk (*, P < 0.05 [Mann-Whitney test]). (C) Pathological evaluation, wherein the macroscopic lung pathology was expressed as the lung index of macroscopic pathology (LIMP) as described previously (the LIMP is the ratio of the lung area with pathological changes divided by the area of the whole lung). Statistical significant differences are marked with an asterisk (*, P < 0.05 [Mann-Whitney test]).
Toxicological evaluation of KBPA101.
Histological evaluation of clinical-grade KBPA101 binding to 36 different tissues from three different, unrelated donors did not indicate any nonspecific binding (as presented in the supplemental material). In addition, KBPA101 underwent toxicological evaluation, including an acute dose toxicity study and a repeat-dose toxicity study in rats, as well as a local tolerance study in rabbits. KBPA101 was well tolerated locally, as well as systemically at all dose levels with no adverse clinical signs observed. A slightly lower body weight gain was noted in females given 12 mg/kg/dose, but no laboratory changes were noted at the end of the treatment or the treatment-free follow-up period. No effects on the organ weights and no macroscopic or microscopic findings were observed at the end of the treatment and treatment-free follow-up periods. Consequently, under the experimental conditions of the study, the “no observed effect level” (NOEL) was considered to be 4.0 mg/kg/dose.
DISCUSSION
Here we describe the generation and characterization of a highly specific human monoclonal IgM antibody targeting the outer polysaccharide side chain of P. aeruginosa serotype IATS O11 as a potential treatment for infections with P. aeruginosa. We chose to target the surface O polysaccharide of P. aeruginosa because LPS is an immunologically highly relevant antigen and represents one of the major surface-associated virulence factor of this pathogen (31, 36). It might seem more straightforward to use LPS core-specific MAbs to avoid the need for serotype specificity. However, this approach is not appropriate since it has been shown that such MAbs confer only limited protection (26). Second, it is known that under anaerobic growth conditions Pseudomonas might change to a mucoid phenotype characterized by the loss of LPS surface expression and the production of alginate. However, the mucoid phenotype is usually observed in chronic infection, e.g., in cystic fibrosis patients, whereas in acute (nosocomial) infection the LPS-expressing phenotype predominates and, as such, an LPS-specific MAb is a valid treatment option for acute infections.
The scarcity in the development of new highly active antibiotics for Gram-negative bacteria demonstrates the need for new treatment options. The B cells used for the generation of the MAb-secreting hybridoma were induced by active vaccination with an O-polysaccharide-toxin A conjugate vaccine (37). This step was essential as naturally induced IgM antibodies against T-independent antigens (including polysaccharides) usually are of low affinity and have low effector potential. Indeed, we have shown previously that naturally (i.e., infection induced) anti-LPS antibodies mostly were of low affinity and specific for LPS-core determinants rather than O-polysaccharide epitopes. Consequently, they were ineffective in mediating clearance of P. aeruginosa infection (46). Thus, the approach of using immunized volunteers as the source of human lymphocytes for the generation of hybridomas was clearly useful. We chose to work with an MAb of IgM isotype rather than of IgG isotype, based on the facts that the major effector mechanism against bacterial infection is complement-dependent phagocytosis and that IgM is known to be the most effective isotype regarding complement activation (44). This notion is supported by the high opsonophagocytic activity of KBPA101 (Fig. 2).
Several cell lines secreting human monoclonal IgM antibodies specific for P. aeruginosa LPS serotype IATS O11 are available at the ATCC, e.g., the hybridomas RK-52 and RS-1H7 (ATCC HB-9149 and ATCC HB-9151), both originally derived from the EBV-transformed lymphoblastoid cell line RM5 (47) by fusion with KR-4 (a lymphoblastoid cell line described by Kozbor et al. [17]) to form the cell line RK-52 or by fusion with the immortalized cell line SHM-D33 to form RS-1H7. A side-by-side comparison of the two antibodies with KBPA101 for specificity and affinity to the O11 LPS antigen revealed a significantly higher avidity to the antigen for KBPA101 (1.3 × 106 M−1 for RK-52 and 5.5 × 106 M−1 for RS-1H7, as determined by three independent experiments versus 5.81 × 107 M−1 for KBPA101). In addition, Harrison et al. used the hybridoma cell line HI223 to produce an IgM antibody for the treatment of P. aeruginosa O11 infections in a clinical trial. Although the monoclonal IgM HI223 antibody has an affinity similar to that of KBPA101 (7.5 × 107 M−1), the reported opsonophagocytic effector function in vitro at 100 ng/ml is significantly lower than that of KBPA101, with an 50% effector function of 0.16 ng/ml (14). Nevertheless, none of these antibodies has been developed for clinical trials beyond a few patients, such as described by Harrison et al. Thus, it is certainly important to evaluate KBPA101 in a clinical setting to evaluate whether the combination of higher affinity and better effector function will translate to a better clinical outcome.
Our studies demonstrated the preclinical efficacy of KBPA101 against systemic P. aeruginosa infection after burn wound injury. Prophylactic treatment of mice with KBPA101 resulted in dose-dependent protection, as well as a significant survival benefit in therapeutic treatment. The protective effect of KBPA101 cannot be simply attributed to an anti-inflammatory effect of IgM or to a nonspecific stimulation of the immune response. Although not formally proven here, our own data indicated that the KBPA101 antibody, if applied in an infection model with P. aeruginosa strains of different serotypes, did not show any protective effects for the animals. In addition, IgM antibodies specific for a different serotype did not show any effect in a therapeutic lung infection model, whereas KBPA101 had a significant beneficial effect on treated animals (unpublished data). Thus, a specific interaction of KBPA101 with its target seems to be a prerequisite for efficacy in vivo. The notion that the antibody might solely promote the uptake and intracellular survival of the bacterial cells inside phagocytes seems rather unlikely. First, the MAb itself has in the presence of complement a bactericidal activity, thus having a direct effect on the bacterial load independent of phagocytes. Second, we have no indication in our animal experiments that surviving animals will later develop recurrent infections since we have never observed such events, even after long-term follow-up. Thus, it seems very unlikely that the uptake of bacterial cells by phagocytes promoted through KBPA101 will lead to persistent infections.
Surprisingly, therapy with KBPA101 in the murine burn wound model did not indicate any dose dependence. This may be due to technical reasons inherent to the model: the course of acute P. aeruginosa infection in currently available mouse models is very rapid, i.e., clearly more rapid than in humans. Similar observations were made recently in independent animal experiments using KBPA101 in a therapeutic lung challenge model in combination with antibiotic treatment, where increasing doses greater than 1 mg/kg did not improve the outcome with regard to faster clearance of bacterial loads in lungs or faster recovery from the challenge (unpublished). Thus, it could be speculated that doses between 0.4 and 1 mg/kg are optimal for efficacy and that increasing the amounts would not improve the effects. The underlying cause of the plateau effect of KBPA101 remains elusive at the moment.
Our data from an acute lung infection model indicate that KBPA101 might be a feasible candidate for treatment of lung infection with P. aeruginosa. This was surprising, since it is generally assumed and it has been demonstrated that IgM poorly penetrates into lung tissues (11, 34, 35). Since we see efficacy with KBPA101 in an acute pulmonary infection model, it could be speculated that exudation into the lung lumen of macromolecules, including antibodies, from the blood circulation may be facilitated by the inflammatory reaction at the site of an acute infection.
For an eventual use for treatment of pneumonia it will be critical to exclude exaggerated immunopathology in the lung that could be associated with effector mechanisms of IgM. Our findings from the acute lung infection model in mice did not indicate enhanced macroscopic lung pathology in KBPA101-treated mice. In the past tolerability and pharmacokinetics of other MAb preparations of IgM isotype in humans has been documented in patients with pneumonia, bacteremia and burn wounds associated with P. aeruginosa infection (12, 38). In both studies the preparations of antibodies were demonstrated to be safe and well tolerated with no evidence of acute complement consumption. These data indicate that infusion of monoclonal IgM preparations can be safe.
Overall, the preclinical evaluation of the fully human MAb KBPA101 indicated efficacy against systemic and/or respiratory P. aeruginosa infection. In comparison to the anti-P. aeruginosa IgM MAbs described in previous clinical studies (12), KBPA101 demonstrated a higher opsonophagocytic activity in vitro. Furthermore, KBPA101 demonstrated a significant biological activity in vivo in a burn wound sepsis model, as well as in a lung challenge model. Based on the favorable toxicological profile of KBPA101 (see the supplemental material), a clinical phase I safety/tolerability study in human volunteers with KBPA101 has been conducted (23) and a phase II study in patients with ventilator-associated pneumonia is in progress.
Supplementary Material
Acknowledgments
We thank Silvana Manolio, Marianne Wyss, and Sandra Jampen for their excellent technical work.
Footnotes
Published ahead of print on 22 March 2010.
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1.Bruderer, U., S. J. Cryz, Jr., U. B. Schaad, M. Deusinger, J. U. Que, and A. B. Lang. 1992. Affinity constants of naturally acquired and vaccine-induced anti-Pseudomonas aeruginosa antibodies in healthy adults and cystic fibrosis patients. J. Infect. Dis. 166:344-349. [DOI] [PubMed] [Google Scholar]
- 2.Casadevall, A., E. Dadachova, and L. A. Pirofski. 2004. Passive antibody therapy for infectious diseases. Nat. Rev. Microbiol. 2:695-703. [DOI] [PubMed] [Google Scholar]
- 3.Crouch Brewer, S., R. G. Wunderink, C. B. Jones, and K. V. Leeper, Jr. 1996. Ventilator-associated pneumonia due to Pseudomonas aeruginosa. Chest 109:1019-1029. [DOI] [PubMed] [Google Scholar]
- 4.Cryz, S. J., Jr., E. Furer, and R. Germanier. 1983. Protection against Pseudomonas aeruginosa infection in a murine burn wound sepsis model by passive transfer of antitoxin A, antielastase, and antilipopolysaccharide. Infect. Immun. 39:1072-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cryz, S. J., Jr., A. Lang, A. Rudeberg, J. Wedgwood, J. U. Que, E. Furer, and U. Schaad. 1997. Immunization of cystic fibrosis patients with a Pseudomonas aeruginosa O-polysaccharide-toxin A conjugate vaccine. Behring Inst. Mitt:345-349. [PubMed]
- 6.Cryz, S. J., Jr., A. B. Lang, J. C. Sadoff, R. Germanier, and E. Furer. 1987. Vaccine potential of Pseudomonas aeruginosa O-polysaccharide-toxin A conjugates. Infect. Immun. 55:1547-1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desgranges, C., M. F. Lavoue, J. Patet, and G. de-The. 1979. In vitro transforming activity of Epstein-Barr virus (EBV). II. Differences between M81 and B95-8 EBV strains. Biomedicine 30:102-108. [PubMed] [Google Scholar]
- 8.Doring, G., and G. B. Pier. 2008. Vaccines and immunotherapy against Pseudomonas aeruginosa. Vaccine 26:1011-1024. [DOI] [PubMed] [Google Scholar]
- 9.Eggimann, P., S. Hugonnet, H. Sax, S. Touveneau, J. C. Chevrolet, and D. Pittet. 2003. Ventilator-associated pneumonia: caveats for benchmarking. Intensive Care Med. 29:2086-2089. [DOI] [PubMed] [Google Scholar]
- 10.Eggimann, P., and D. Pittet. 2001. Infection control in the ICU. Chest 120:2059-2093. [DOI] [PubMed] [Google Scholar]
- 11.Falk, G. A., A. J. Okinaka, and G. W. Siskind. 1972. Immunoglobulins in the bronchial washings of patients with chronic obstructive pulmonary disease. Am. Rev. Respir. Dis. 105:14-21. [DOI] [PubMed] [Google Scholar]
- 12.Harrison, F. J., D. Rohm, T. Kohzuki, and H. Noguchi. 1997. Pharmacokinetics, tolerability, and preliminary efficacy of human anti-Pseudomonas aeruginosa monoclonal antibodies in pneumonia and burn infection patients. Hybridoma 16:413-420. [DOI] [PubMed] [Google Scholar]
- 13.Jansen, W. T., J. Gootjes, M. Zelle, D. V. Madore, J. Verhoef, H. Snippe, and A. F. Verheul. 1998. Use of highly encapsulated Streptococcus pneumoniae strains in a flow-cytometric assay for assessment of the phagocytic capacity of serotype-specific antibodies. Clin. Diagn. Lab. Immunol. 5:703-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kahzuki, T., I. Uezumi, K. Irie, and H. Ochi. 1991. Human monoclonal antibody and pharmaceutical composition containing the same for treatment of pseudomonas aeruginosa.
- 15.Knirel, Y. A. 1990. Polysaccharide antigens of Pseudomonas aeruginosa. Crit. Rev. Microbiol. 17:273-304. [DOI] [PubMed] [Google Scholar]
- 16.Kollef, M. H., P. Silver, D. M. Murphy, and E. Trovillion. 1995. The effect of late-onset ventilator-associated pneumonia in determining patient mortality. Chest 108:1655-1662. [DOI] [PubMed] [Google Scholar]
- 17.Kozbor, D., P. Tripputi, J. C. Roder, and C. M. Croce. 1984. A human hybrid myeloma for production of human monoclonal antibodies. J. Immunol. 133:3001-3005. [PubMed] [Google Scholar]
- 18.Landsperger, W. J., K. D. Kelly-Wintenberg, T. C. Montie, L. S. Knight, M. B. Hansen, C. C. Huntenburg, and M. J. Schneidkraut. 1994. Inhibition of bacterial motility with human antiflagellar monoclonal antibodies attenuates Pseudomonas aeruginosa-induced pneumonia in the immunocompetent rat. Infect. Immun. 62:4825-4830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lang, A. B., E. Furer, G. Senyk, J. W. Larrick, and S. J. Cryz, Jr. 1990. Systematic generation of antigen specific human monoclonal antibodies with therapeutical activities using active immunization. Hum. Antibodies Hybridomas 1:96-103. [PubMed] [Google Scholar]
- 20.Lang, A. B., A. Rudeberg, M. H. Schoni, J. U. Que, E. Furer, and U. B. Schaad. 2004. Vaccination of cystic fibrosis patients against Pseudomonas aeruginosa reduces the proportion of patients infected and delays time to infection. Pediatr. Infect. Dis. J. 23:504-510. [DOI] [PubMed] [Google Scholar]
- 21.Lang, A. B., U. B. Schaad, A. Rudeberg, J. Wedgwood, J. U. Que, E. Furer, and S. J. Cryz, Jr. 1995. Effect of high-affinity anti-Pseudomonas aeruginosa lipopolysaccharide antibodies induced by immunization on the rate of Pseudomonas aeruginosa infection in patients with cystic fibrosis. J. Pediatr. 127:711-717. [DOI] [PubMed] [Google Scholar]
- 22.Lang, A. B., U. Schurch, F. Zimmermann, and U. Bruderer. 1992. Selective generation of antigen-specific human hybridomas optimized for large scale growth in serum-free medium. J. Immunol. Methods 154:21-26. [DOI] [PubMed] [Google Scholar]
- 23.Lazar, H., M. P. Horn, A. W. Zuercher, M. A. Imboden, P. Durrer, M. Seiberling, R. Pokorny, C. Hammer, and A. B. Lang. 2009. Pharmacokinetics and safety profile of the human anti-Pseudomonas aeruginosa serotype O11 immunoglobulin M monoclonal antibody KBPA-101 in healthy volunteers. Antimicrob. Agents Chemother. 53:3442-3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martinez, J. E., S. Romero-Steiner, T. Pilishvili, S. Barnard, J. Schinsky, D. Goldblatt, and G. M. Carlone. 1999. A flow cytometric opsonophagocytic assay for measurement of functional antibodies elicited after vaccination with the 23-valent pneumococcal polysaccharide vaccine. Clin. Diagn. Lab. Immunol. 6:581-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsumoto, T., K. Tateda, S. Miyazaki, N. Furuya, A. Ohno, Y. Ishii, Y. Hirakata, and K. Yamaguchi. 1999. Effect of antiflagellar human monoclonal antibody on gut-derived Pseudomonas aeruginosa sepsis in mice. Clin. Diagn. Lab. Immunol. 6:537-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCloskey, R. V., R. C. Straube, C. Sanders, S. M. Smith, C. R. Smith, et al. 1994. Treatment of septic shock with human monoclonal antibody HA-1A. A randomized, double-blind, placebo-controlled trial. Ann. Intern. Med. 121:1-5. [DOI] [PubMed] [Google Scholar]
- 27.Meng, Y. G., T. Wong, L. D. Saravolatz, and J. E. Pennington. 1993. Pharmacokinetics of an IgM human monoclonal antibody against Pseudomonas aeruginosa in nonseptic patients. J. Infect. Dis. 167:784-785. [DOI] [PubMed] [Google Scholar]
- 28.Muller-Premru, M., and M. Gubina. 2000. Serotype, antimicrobial susceptibility and clone distribution of Pseudomonas aeruginosa in a university hospital. Zentralbl. Bakteriol. 289:857-867. [DOI] [PubMed] [Google Scholar]
- 29.Nagy, E., C. Giefing, and A. von Gabain. 2008. Anti-infective antibodies: a novel tool to prevent and treat nosocomial diseases. Expert Rev. Anti-Infect. Ther. 6:21-30. [DOI] [PubMed] [Google Scholar]
- 30.Ochi, H., H. Ohtsuka, S. Yokota, I. Uezumi, M. Terashima, K. Irie, and H. Noguchi. 1991. Inhibitory activity on bacterial motility and in vivo protective activity of human monoclonal antibodies against flagella of Pseudomonas aeruginosa. Infect. Immun. 59:550-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pier, G. B. 2007. Pseudomonas aeruginosa lipopolysaccharide: a major virulence factor, initiator of inflammation, and target for effective immunity. Int. J. Med. Microbiol. 297:277-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raymond, C. K., E. H. Sims, A. Kas, D. H. Spencer, T. V. Kutyavin, R. G. Ivey, Y. Zhou, R. Kaul, J. B. Clendenning, and M. V. Olson. 2002. Genetic variation at the O-antigen biosynthetic locus in Pseudomonas aeruginosa. J. Bacteriol. 184:3614-3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reichert, J. M., and M. C. Dewitz. 2006. Anti-infective monoclonal antibodies: perils and promise of development. Nat. Rev. Drug Discov. 5:191-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reynolds, H. Y., and H. H. Newball. 1974. Analysis of proteins and respiratory cells obtained from human lungs by bronchial lavage. J. Lab. Clin. Med. 84:559-573. [PubMed] [Google Scholar]
- 35.Reynolds, H. Y., and R. E. Thompson. 1973. Pulmonary host defenses. I. Analysis of protein and lipids in bronchial secretions and antibody responses after vaccination with pseudomonas aeruginosa. J. Immunol. 111:358-368. [PubMed] [Google Scholar]
- 36.Rocchetta, H. L., L. L. Burrows, and J. S. Lam. 1999. Genetics of O-antigen biosynthesis in Pseudomonas aeruginosa. Microbiol. Mol. Biol. Rev. 63:523-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosenthal, V. D., D. G. Maki, A. Mehta, C. Alvarez-Moreno, H. Leblebicioglu, F. Higuera, L. E. Cuellar, N. Madani, Z. Mitrev, L. Duenas, J. A. Navoa-Ng, H. G. Garcell, L. Raka, R. F. Hidalgo, E. A. Medeiros, S. S. Kanj, S. Abubakar, P. Nercelles, and R. D. Pratesi. 2008. International Nosocomial Infection Control Consortium report, data summary for 2002-2007, issued January 2008. Am. J. Infect. Control 36:627-637. [DOI] [PubMed] [Google Scholar]
- 38.Saravolatz, L. D., N. Markowitz, M. S. Collins, D. Bogdanoff, and J. E. Pennington. 1991. Safety, pharmacokinetics, and functional activity of human anti-Pseudomonas aeruginosa monoclonal antibodies in septic and nonseptic patients. J. Infect. Dis. 164:803-806. [DOI] [PubMed] [Google Scholar]
- 39.Sawada, S., T. Kawamura, and Y. Masuho. 1987. Immunoprotective human monoclonal antibodies against five major serotypes of Pseudomonas aeruginosa. J. Gen. Microbiol. 133:3581-3590. [DOI] [PubMed] [Google Scholar]
- 40.Schaad, U. B., A. B. Lang, J. Wedgwood, A. Ruedeberg, J. U. Que, E. Furer, and S. J. Cryz, Jr. 1991. Safety and immunogenicity of Pseudomonas aeruginosa conjugate A vaccine in cystic fibrosis. Lancet 338:1236-1237. [DOI] [PubMed] [Google Scholar]
- 41.Seifert, M., G. Schoenherr, D. Roggenbuck, U. Marx, and R. von Baehr. 1996. Generation and characterization of a human monoclonal IgM antibody that recognizes a conserved epitope shared by lipopolysaccharides of different gram-negative bacteria. Hybridoma 15:191-198. [DOI] [PubMed] [Google Scholar]
- 42.Sekiguchi, J., T. Asagi, T. Miyoshi-Akiyama, A. Kasai, Y. Mizuguchi, M. Araake, T. Fujino, H. Kikuchi, S. Sasaki, H. Watari, T. Kojima, H. Miki, K. Kanemitsu, H. Kunishima, Y. Kikuchi, M. Kaku, H. Yoshikura, T. Kuratsuji, and T. Kirikae. 2007. Outbreaks of multidrug-resistant Pseudomonas aeruginosa in community hospitals in Japan. J. Clin. Microbiol. 45:979-989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Song, Z., A. Kharazmi, H. Wu, V. Faber, C. Moser, H. K. Krogh, J. Rygaard, and N. Hoiby. 1998. Effects of ginseng treatment on neutrophil chemiluminescence and immunoglobulin G subclasses in a rat model of chronic Pseudomonas aeruginosa pneumonia. Clin. Diagn. Lab. Immunol. 5:882-887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spiegelberg, H. L. 1989. Biological role of different antibody classes. Int. Arch. Allergy Appl. Immunol. 90(Suppl. 1):22-27. [DOI] [PubMed] [Google Scholar]
- 45.ter Meulen, J. 2007. Monoclonal antibodies for prophylaxis and therapy of infectious diseases. Expert Opin. Emerg. Drugs 12:525-540. [DOI] [PubMed] [Google Scholar]
- 46.Zuercher, A. W., M. P. Horn, J. U. Que, A. Ruedeberg, M. H. Schoeni, U. B. Schaad, P. Marcus, and A. B. Lang. 2006. Antibody responses induced by long-term vaccination with an octovalent conjugate Pseudomonas aeruginosa vaccine in children with cystic fibrosis. FEMS Immunol. Med. Microbiol. 47:302-308. [DOI] [PubMed] [Google Scholar]
- 47.Zweerink, H. J., M. C. Gammon, C. F. Hutchison, J. J. Jackson, D. Lombardo, K. M. Miner, J. M. Puckett, T. J. Sewell, and N. H. Sigal. 1988. Human monoclonal antibodies that protect mice against challenge with Pseudomonas aeruginosa. Infect. Immun. 56:1873-1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zweerink, H. J., M. C. Gammon, C. F. Hutchison, J. J. Jackson, G. B. Pier, J. M. Puckett, T. J. Sewell, and N. H. Sigal. 1988. X-linked immunodeficient mice as a model for testing the protective efficacy of monoclonal antibodies against Pseudomonas aeruginosa. Infect. Immun. 56:1209-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.