Abstract
We conducted a double-blind, randomized controlled trial to compare a long-acting neuraminidase inhibitor, laninamivir octanoate, with oseltamivir. Eligible patients were children 9 years of age and under who had febrile influenza symptoms of no more than 36-h duration. Patients were randomized to 1 of 3 treatment groups: a group given 40 mg laninamivir (40-mg group), a group given 20 mg laninamivir (20-mg group), and an oseltamivir group. Laninamivir octanoate was administered as a single inhalation. Oseltamivir (2 mg/kg of body weight) was administered orally twice daily for 5 days. The primary end point was the time to alleviation of influenza illness. The primary analysis included 184 patients (61, 61, and 62 in the 40-mg group, 20-mg group, and oseltamivir group, respectively). Laninamivir octanoate markedly reduced the median time to illness alleviation in comparison with oseltamivir in patients infected with oseltamivir-resistant influenza A (H1N1) virus, and the reductions were 60.9 h for the 40-mg group and 66.2 h for the 20-mg group. On the other hand, there were no significant differences in the times to alleviation of illness between the laninamivir groups and oseltamivir group for patients with influenza A (H3N2) or B virus infection. Laninamivir octanoate was well tolerated. The most common adverse events were gastrointestinal events. Laninamivir octanoate was an effective and well-tolerated treatment for children with oseltamivir-resistant influenza A (H1N1) virus infection. Further study will be needed to confirm clinical efficacy against influenza A (H3N2) or B virus infection. Its ease of administration is noteworthy, because a single inhalation is required during the course of illness.
Swine origin influenza A (H1N1) virus (2009 pandemic H1N1 virus) was first detected in Mexico in the spring of 2009, and the World Health Organization declared a pandemic caused by 2009 pandemic H1N1 virus in June 2009 (21). Many otherwise healthy children and adults, as well as members of high-risk populations, who became infected with 2009 pandemic H1N1 virus developed severe illness and died. Neuraminidase inhibitors have recently been reported to be effective in preventing severe illness in patients with 2009 pandemic H1N1 virus infection (2, 8), and the importance of early treatment with neuraminidase inhibitors has been emphasized. However, appearance of oseltamivir-resistant 2009 pandemic H1N1 virus strains has been reported worldwide, up to 225 strains as of February 2010 (22), and the spread of oseltamivir-resistant 2009 pandemic H1N1 virus has become a concern. In fact, since almost 100% of seasonal influenza A (H1N1) viruses have become resistant to oseltamivir (20), there is an urgent need to develop anti-influenza agents that are effective not only against 2009 pandemic H1N1 virus but also against oseltamivir-resistant 2009 pandemic H1N1 virus.
Previous studies have reported the potential advantages of laninamivir octanoate (CS-8958; Daiichi Sankyo Co., Ltd., Tokyo, Japan), an octanoyl ester prodrug of laninamivir. Laninamivir has shown in vitro neuraminidase-inhibitory activity against various influenza A and B viruses, including subtypes N1 to N9 and oseltamivir-resistant viruses (23), and it has also been found to be effective against a swine origin H1N1 strain (7). Moreover, laninamivir octanoate has long-lasting antiviral activity. Preclinical studies of CS-8958 in mice showed that after intranasal administration it was rapidly converted to its active metabolite, laninamivir, that the laninamivir generated was retained in the lungs, where it had a long half-life of 41.4 h (10), and that a single intranasal dose of laninamivir octanoate exhibited efficacy similar to that of repeated doses of zanamivir or oseltamivir (12, 23). A study in healthy volunteers showed that laninamivir was slowly eliminated from the body over a period of up to 6 days after a single inhalation (6).
Influenza virus infection is one of the major causes of pediatric hospitalizations in the winter season (15, 17), and schoolchildren and children who attend day care centers are the principal transmitters of influenza in the community (13). The purpose of this trial was to compare the efficacy and safety of laninamivir octanoate to those of oseltamivir in children.
MATERIALS AND METHODS
Study design and criteria for enrollment.
This multicenter, double-blind, randomized controlled trial was conducted between December 2008 and March 2009 at 43 institutions in Japan. The trial was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice (4), and the final protocol was reviewed and approved by the institutional review board of each institution. All legally acceptable representatives provided written informed consent, and the patients were informed about the trial in a manner suitable for their ages.
Eligible patients were children 9 years of age and under who presented within 36 h of the onset of any influenza symptom, had an axillary temperature of 38.0°C or higher, and could inhale the test drug successfully. Influenza virus infection was diagnosed by the investigator based on the results obtained with a rapid diagnostic kit (mainly Capilia FluA+B [Tauns Co., Ltd., Shizuoka, Japan] [11] and QuickVue Rapid-SP influ [Quidel Corp., CA] [24]) and the clinical findings. Patients were excluded from the trial if they were suspected of having an infection by bacteria or a noninfluenza virus within 1 week before enrollment, reported any influenza-like symptoms within 1 week before the onset of influenza, had any chronic respiratory disease, cardiovascular disease, central nervous disorder, renal dysfunction, metabolic disorder, immune dysfunction, or other severe disorder, had a history of abnormal behavior while infected with influenza virus, or had been treated with amantadine, zanamivir, or oseltamivir within the previous 4 weeks.
Method of randomization and drug administration.
Patients were randomly assigned to 1 of the following 3 treatment groups in a 1:1:1 ratio: a group given 40 mg laninamivir octanoate (CS-8958; Daiichi Sankyo Co., Ltd., Tokyo, Japan) (40-mg group), a group given 20 mg laninamivir octanoate (20-mg group), and an oseltamivir group. Laninamivir octanoate was administered as a single inhalation on day 1 of the trial calendar. Oseltamivir (2 mg/kg of body weight) was administered orally twice daily for 5 days (days 1 to 5) to patients whose body weight was under 37.5 kg. If the patient's weight was 37.5 kg or more, 75 mg oseltamivir was administered twice daily. The allocation sequence was generated by a computer, and it was stratified according to the institution and type of influenza virus based on the results of testing with a rapid diagnostic kit capable of detecting influenza A and B viruses separately. The patients, their legally acceptable representatives, the investigators, and the trial personnel were blinded to the allocation sequence throughout the trial by using a double-dummy method. Patients were allowed to use acetaminophen as a rescue medication for symptom relief.
Criteria for assessment.
A medical history of all patients was obtained, their axillary temperature was measured, and a physical examination was performed before the trial treatment. Routine laboratory tests (a hematologic examination [total white blood cell count, differential, hemoglobin concentration, red blood cell count, and platelet count], blood biochemistry examination [total protein, albumin, A/G ratio, total bilirubin, aspartate aminotransferase, alanine transaminase, alkaline phosphatase, γ-glutamyltransferase, lactate dehydrogenase, blood urea nitrogen, creatinine, sodium, potassium, and C-reactive protein], and urinalysis [protein, glucose, urobilinogen, and occult blood]) were performed at day 1 (baseline) and day 6. Caregivers recorded axillary temperature and the severity of influenza symptoms (i.e., nasal symptoms and cough) in diaries 4 times daily (morning, afternoon, evening, and bedtime) from day 1 to day 3 and twice daily from day 4 to day 15 (morning and bedtime). Severity of nasal symptoms and cough was rated on a scale of 0 to 3: 0 = absent, 1 = mild, 2 = moderate, and 3 = severe.
The primary end point was the time to alleviation of influenza illness, which was defined as the interval between the start of the trial treatment and the start of the first 21.5-hour period in which the nasal symptoms and cough had improved to “absent” or “mild” and axillary temperature had returned to 37.4°C or below. Patients whose influenza symptoms had not been alleviated at the time of their withdrawal from the study or at the end of the observation period were censored. Secondary end points were the median time to return to normal axillary temperature and the proportion of patients shedding virus at each time point.
Virological tests.
An anterior nasal and/or posterior pharyngeal throat swab was performed on days 1, 3, and 6 with the following acceptable time window: ±1 day for days 3 and 6. The swabs of the patients were placed in viral transport medium in a refrigerator, and within a maximum of 5 days they were divided into four portions (called specimens) and transported to a virological test facility, where they were stored at −80°C. After thawing, the specimens were used to infect Madin-Darby canine kidney (MDCK) cells. The cells were cultured for 7 days at 33°C, and the culture supernatants were used to test the susceptibility of the viral neuraminidase to inhibition by laninamivir and oseltamivir carboxylate. A fluorometric substrate, 4-methylumbelliferyl-N-acetyl-α-d-neuraminic acid, was used to measure the 50% inhibitory concentrations (IC50) of laninamivir and oseltamivir carboxylate (24). All specimens of the H1N1 subtype obtained at the patient's first visit were tested for the presence of the oseltamivir-resistant H274Y mutation (N2 numbering, the same as the H275Y mutation by N1 numbering) by a reverse transcription-PCR (RT-PCR)/restriction fragment length polymorphism assay according to a procedure reported elsewhere (3). The serially diluted specimens infected MDCK cells, which were cultured at 33°C for 7 days or until observation of a cytopathic effect in more than 75% of cells, and the culture supernatants were recovered. The culture supernatants obtained were tested for the presence of viruses by a hemagglutination assay with guinea pig red blood cells. The Behrens-Kärber equation was used to calculate viral titers as log10 50% tissue culture infective doses (TCID50)/ml of the viral transport medium (1). The lower detection limit of the viral titer was 1.5 log10 TCID50/ml in the assay. The type and subtype of the influenza virus (H1N1, H3N2, or B) were determined by performing RT-PCR with subtype-specific primers and viral RNA extracted from the specimens or by a serological test in which serum samples were collected at day 1 and day 22 and a hemagglutination inhibition assay was performed. All virological tests were performed by Mitsubishi Chemical Medience Corporation (Tokyo, Japan).
Statistical analysis.
The sample size of 60 patients in each group was determined on the basis of the previous randomized controlled trial (19). Since the difference between the median time to alleviation of influenza illness in the oseltamivir group and placebo group was 36 h in the previous trial, the sample size was determined to ensure that the upper limit of the 95% confidence interval (CI) of the difference between each laninamivir dose level group and the oseltamivir groups would be less than 36 h with a probability of 80% based on the Monte Carlo simulation.
In the efficacy analysis, we calculated the differences between the median times to alleviation of influenza illness in each laninamivir dose level group and the oseltamivir group and their two-sided nonparametric 95% CIs based on the generalized Wilcoxon test. Generalized Wilcoxon tests were also performed for each comparison between the treatment groups. The proportion of patients in each group who were shedding virus at each time point was calculated. Viral titers of <1.5 log10 TCID50/ml (the limit of detection by the method used) were recorded as 1.5 log10 TCID50/ml, and 1.5 log10 TCID50/ml was used to calculate the median values. All analyses were performed using SAS System Release 8.2 software (SAS Institute, Cary, NC). All reported P values are two sided, with no adjustments for multiple testing.
The full analysis set (FAS) (5) based on the intention-to-treat principle was defined as the primary analysis set in the efficacy analysis, and the per protocol set (PPS) (5) was used in the sensitivity analysis. The FAS included all randomized patients who met the major eligibility criteria, had received at least 1 dose of the trial treatment, and had undergone at least 1 assessment for influenza symptoms and axillary temperature. The PPS included all patients who met the criteria for FAS and were sufficiently compliant with the protocol.
RESULTS
Patient disposition and characteristics.
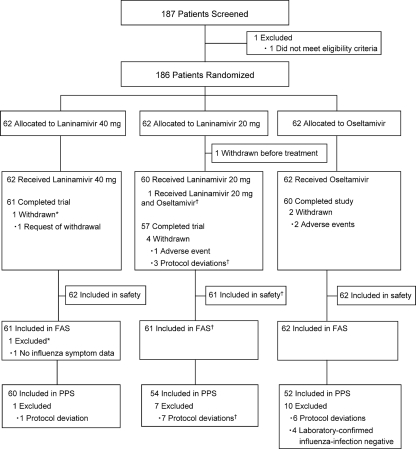
A total of 186 patients were enrolled in the trial (Fig. 1). Of these, 2 patients discontinued the trial before completing the first dose of the trial treatment and were excluded from the FAS: one discontinued the trial before receiving treatment, and the other did not complete inhalation. As a result, 184 patients (61, 61, and 62 in the laninamivir octanoate 40-mg group, laninamivir octanoate 20-mg group, and oseltamivir group, respectively) were included in the FAS. Of these, 1 patient in the 20-mg group received both laninamivir octanoate and oseltamivir and was analyzed as a member of the group to which the patient was originally allocated. The 1 patient who discontinued the trial before treatment (mentioned above) was excluded from the safety analysis.
FIG. 1.
Patient flow chart. *, did not complete inhalation of the test drug, and there were no postrandomization data; †, received both active drugs; this patient was analyzed as a member of the original treatment group in the full analysis set (FAS) and safety analysis set, but was excluded from the per protocol set (PPS).
The demographic and baseline characteristics of the 3 groups in the FAS were well balanced (Table 1). Nearly one-half of the patients had been vaccinated against influenza in the 2008 to 2009 season. Approximately 80% of the patients were infected with influenza virus A. Of these, 40, 40, and 32 patients in the 40-mg group, 20-mg group, and oseltamivir group, respectively, were infected with H1N1 strain. Viral culture or serology did not confirm influenza virus infection in four of the patients.
TABLE 1.
Demographic and baseline characteristicsa of the 184 patients included in the full analysis set
| Characteristic | Value for group receiving: |
||
|---|---|---|---|
| Laninamivir octanoate, 40 mg (n = 61) | Laninamivir octanoate, 20 mg (n = 61) | Oseltamivir (n = 62) | |
| Age (yr) | |||
| Mean ± SD | 6.8 ± 1.4 | 6.9 ± 1.5 | 6.7 ± 1.5 |
| Range | 3-9 | 4-9 | 3-9 |
| No. (%) female | 29 (47.5) | 25 (41.0) | 28 (45.2) |
| No. (%) male | 32 (52.5) | 36 (59.0) | 34 (54.8) |
| Mean ht (cm) ± SD | 120.72 ± 9.39 | 120.83 ± 9.43 | 121.60 ± 10.44 |
| Mean wt (kg) ± SD | 23.09 ± 5.40 | 23.12 ± 4.93 | 23.68 ± 5.23 |
| Vaccination against influenza | |||
| No. (%) vaccinated | 34 (55.7) | 30 (49.2) | 22 (35.5) |
| No. (%) not vaccinated | 27 (44.3) | 31 (50.8) | 40 (64.5) |
| No. (%) positive by rapid diagnostic test | 61 (100.0) | 61 (100.0) | 62 (100.0) |
| Laboratory-confirmed influenza virus infection | |||
| No. (%) positive | 61 (100.0) | 61 (100.0) | 58 (93.5) |
| No. (%) negative | 0 (0.0) | 0 (0.0) | 4 (6.5) |
| No. (%) infected withb: | |||
| A/H1N1 | 40 (65.6) | 40 (65.6) | 32 (51.6) |
| A/H3N2 | 11 (18.0) | 12 (19.7) | 16 (25.8) |
| B | 10 (16.4) | 9 (14.8) | 10 (16.1) |
| Mean axillary temp at enrollment (°C) ± SD | 38.86 ± 0.54 | 38.84 ± 0.65 | 38.63 ± 0.53 |
| Mean symptom score at enrollmentc ± SD | 2.2 ± 1.3 | 2.7 ± 1.3 | 2.6 ± 1.3 |
| Mean duration of illness before treatment (h) ± SD | 18.19 ± 7.74 | 18.19 ± 8.13 | 19.09 ± 8.50 |
There were no significant differences in demographic or baseline characteristics among the 3 groups.
The type of influenza virus was determined by RT-PCR with type-specific primers or by serological testing involving a hemagglutination inhibition assay.
See Materials and Methods for a description of symptom scores.
IC50 of laninamivir and oseltamivir.
We tested influenza viruses isolated from the patients prior to the start of the trial treatment for sensitivity to oseltamivir carboxylate and laninamivir. The mean IC50 of oseltamivir carboxylate for seasonal influenza A (H1N1) virus was 641 nmol/liter (range, 210 to 1,200 nmol/liter), whereas the mean IC50 of laninamivir was 1.79 nmol/liter (range, 0.81 to 3.60 nmol/liter). All H1N1 strains except those obtained from 4 patients had the H274Y mutation. The mean IC50 of oseltamivir carboxylate and laninamivir for influenza A (H3N2) virus were 0.65 nmol/liter (range, 0.29 to 0.92 nmol/liter) and 2.13 nmol/liter (range, 1.20 to 3.00 nmol/liter), respectively, and their mean IC50 for influenza B virus were comparable (ranges: 12 to 53 nmol/liter and 11 to 26 nmol/liter, respectively).
Clinical outcomes.
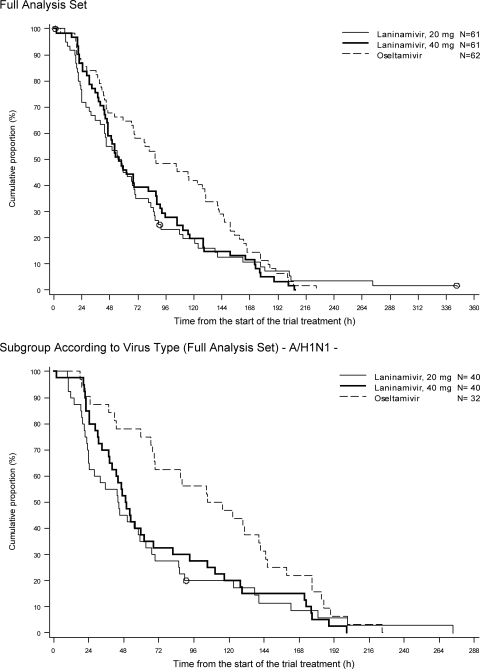
Both dosages of laninamivir octanoate (20 mg and 40 mg) alleviated influenza illness more rapidly than oseltamivir did (Fig. 2). The differences between the proportions of patients whose illness was alleviated were apparent within 24 h after the start of treatment and persisted until 168 h. In the FAS, the median time to alleviation of influenza illness was significantly shorter for the laninamivir octanoate groups than for the oseltamivir group, and the reductions were 31.9 h for the 40-mg group and 31.0 h for the 20-mg group (Table 2). Similar results were also obtained in the PPS.
FIG. 2.
Times to alleviation of influenza illness in patients included in the full analysis set.
TABLE 2.
Effects of laninamivir octanoate and oseltamivir on clinical outcomea
| Parameter | Value for: |
||
|---|---|---|---|
| LOG 40 | LOG 20 | OG | |
| All eligible patients | |||
| n | 61 | 61 | 62 |
| Median time (h) to alleviation of influenza illness (95% CI) | 55.4 (46.3-81.3) | 56.4 (43.7-69.2) | 87.3 (67.9-129.7) |
| LOG 40 and LOG 20 vs OG | |||
| Median differenceb (h) (95% CI) | −31.9 (−43.4 to 0.5) | −31.0 (−50.3 to −5.5) | |
| P | 0.059 | 0.009 | |
| LOG 40 vs LOG 20 | |||
| Median differencec (h) (95% CI) | −1.0 (−9.0 to 22.4) | ||
| P | 0.372 | ||
| A/H1N1-infected patients | |||
| n | 40 | 40 | 32 |
| Median time (h) to alleviation of influenza illness (95% CI) | 49.6 (39.7-62.1) | 44.3 (24.3-58.9) | 110.5 (68.8-141.9) |
| Median differenceb (h) (95% CI) | −60.9 (−71.0 to −10.2) | −66.2 (−81.2 to −18.5) | |
| P | 0.007 | 0.001 | |
| A/H3N2-infected patients | |||
| n | 11 | 12 | 16 |
| Median time (h) to alleviation of influenza illness (95% CI) | 88.6 (43.5-114.9) | 70.4 (30.3-110.9) | 44.3 (22.9-82.1) |
| Median differenceb (h) (95% CI) | 44.4 (−14.8 to 68.5) | 26.2 (−24.8 to 51.2) | |
| P | 0.168 | 0.591 | |
| B-infected patients | |||
| n | 10 | 9 | 10 |
| Median time (h) to alleviation of influenza illness (95% CI) | 77.6 (51.8-95.8) | 83.5 (66.6-107.8) | 127.8 (77.1-165.3) |
| Median differenceb (h) (95% CI) | −50.2 (−104.4 to 10.4) | −44.3 (−93.8 to 36.1) | |
| P | 0.147 | 0.413 | |
Data are for the FAS. LOG 20 and LOG 40, groups given 20 and 40 mg of laninamivir octanoate, respectively; OG, oseltamivir group. Median times to alleviation of influenza illness were estimated by the Kaplan-Meier method. P values were determined by the generalized Wilcoxon test.
Median time to alleviation of influenza illness for the LOG 40 or LOG 20 − median time for the OG.
Median time to alleviation of influenza illness for the LOG 40 − median time for the LOG 20.
The subgroup analyses showed that both dosages of laninamivir octanoate markedly reduced the median time to illness alleviation in the H1N1-infected subpopulation in comparison with oseltamivir, and the reductions were 60.9 h in the 40-mg group and 66.2 h in the 20-mg group (Table 2). The duration of clinical illness in the patients with influenza A (H3N2) virus infection appeared to be shorter in the oseltamivir group than in the laninamivir octanoate groups, whereas among the patients with influenza B virus infection, it appeared to be shorter in the laninamivir octanoate groups than in the oseltamivir group, but these differences were not statistically significant.
Among the patients infected with influenza A (H1N1) virus, the duration of fever was significantly shorter in the laninamivir octanoate groups than in the oseltamivir group (Table 3). The duration of cough and the duration of rhinorrhea were also shorter in the laninamivir octanoate groups, but the differences were statistically not significant (data not shown).
TABLE 3.
Effects of laninamivir octanoate and oseltamivir on body temperaturea
| Parameter | Value for: |
||
|---|---|---|---|
| LOG 40 | LOG 20 | OG | |
| All eligible patients | |||
| n | 61 | 61 | 62 |
| Median time (h) to return to normal axillary temp (95% CI) | 38.1 (24.4-43.5) | 33.5 (22.5-43.8) | 40.9 (33.0-46.4) |
| Median differenceb (h) (95% CI) | −2.8 (−13.2 to 3.2) | −7.4 (−16.0 to 1.7) | |
| P | 0.423 | 0.128 | |
| A/H1N1-infected patients | |||
| n | 40 | 40 | 32 |
| Median time (h) to return to normal axillary temp (95% CI) | 30.5 (21.4-41.6) | 23.8 (20.1-38.3) | 49.3 (33.5-62.8) |
| Median differenceb (h) (95% CI) | −18.8 (−27.7 to −0.5) | −25.5 (−30.4 to −4.4) | |
| P | 0.034 | 0.006 | |
| A/H3N2-infected patients | |||
| n | 11 | 12 | 16 |
| Median time (h) to return to normal axillary temp (95% CI) | 42.9 (24.3-46.3) | 34.5 (9.6-70.4) | 21.3 (19.3-31.6) |
| Median differenceb (h) (95% CI) | 21.6 (1.3 to 25.8) | 13.2 (−10.2 to 35.0) | |
| P | 0.018 | 0.551 | |
| B-infected patients | |||
| n | 10 | 9 | 10 |
| Median time (h) to return to normal axillary temp (95% CI) | 48.6 (38.1-67.7) | 59.0 (43.6-83.5) | 45.6 (40.8-86.5) |
| Median differenceb (h) (95% CI) | 3.0 (−31.0 to 26.3) | 13.4 (−27.6 to 37.5) | |
| P | 0.911 | 0.872 | |
Data are for the FAS. LOG 20 and LOG 40, groups given 20 and 40 mg of laninamivir octanoate, respectively; OG, oseltamivir group. Median times to return to normal axillary temperature were estimated by the Kaplan-Meier method. P values were analyzed by the generalized Wilcoxon test.
Median time to return to normal axillary temperature for the LOG 40 or LOG 20 − median time for the OG.
Viral shedding.
In the H1N1-infected subpopulation, the proportion of patients shedding virus at day 6 was significantly lower in the laninamivir octanoate 20-mg group than in the oseltamivir group (0% [0/40] and 25.0% [8/32], respectively, P < 0.001) (Table 4). However, there were no significant differences in median virus titers on days 3 and 6 between the laninamivir octanoate groups and the oseltamivir group. There were no statistically significant differences between the laninamivir octanoate groups and the oseltamivir group in the H3N2-infected subpopulation or influenza B virus-infected subpopulation (Table 4).
TABLE 4.
Effects of laninamivir octanoate and oseltamivir on virus titera
| Parameter | Value for: |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| A/H1N1 infection of: |
A/H3N2 infection of: |
B infection of: |
|||||||
| LOG 40 | LOG 20 | OG | LOG 40 | LOG 20 | OG | LOG 40 | LOG 20 | OG | |
| Day 1 (baseline) | |||||||||
| n | 40 | 40 | 32 | 11 | 12 | 16 | 10 | 9 | 10 |
| Median virus titerb (log TCID50/ml) (range) | 2.20 (1.5-7.5) | 2.00 (1.5-4.5) | 2.35 (1.5-7.0) | 2.50 (1.5-7.5) | 2.60 (1.5-4.5) | 2.20 (1.5-5.2) | 6.65 (1.5-7.5) | 7.00 (2.0-7.5) | 6.80 (3.3-7.5) |
| No. (%) of patients shedding virusc | 37 (92.5) | 32 (80.0) | 28 (87.5) | 8 (72.7) | 10 (83.3) | 15 (93.8) | 10 (100.0) | 9 (100.0) | 10 (100.0) |
| Difference in %d (95% CI) | 5.0 (−9.1 to 19.1) | −7.5 (−24.4 to 9.4) | −21.0 (−49.9 to 7.8) | −10.4 (−34.6 to 13.8) | 0.0 | 0.0 | |||
| P | 0.692 | 0.529 | 0.272 | 0.560 | |||||
| Day 3 (days 2-4)e | |||||||||
| n | 40 | 39 | 32 | 11 | 12 | 16 | 10 | 9 | 10 |
| Median virus titerb (log TCID50/ml) (range) | 1.50 (1.5-3.5) | 1.50 (1.5-4.0) | 1.50 (1.5-4.3) | 1.50 (1.5-3.7) | 1.50 (1.5-4.0) | 1.50 (1.5-3.0) | 2.60 (1.5-5.7) | 2.00 (1.5-7.5) | 5.50 (1.5-7.0) |
| No. (%) of patients shedding virusc | 15 (37.5) | 17 (43.6) | 14 (43.8) | 4 (36.4) | 7 (58.3) | 4 (25.0) | 7 (70.0) | 7 (77.8) | 9 (90.0) |
| Difference in %d (95% CI) | −6.3 (−29.1 to 16.6) | −0.2 (−23.3 to 23.0) | 11.4 (−24.1 to 46.8) | 33.3 (−1.7 to 68.4) | −20.0 (−53.9 to 13.9) | −12.2 (−45.1 to 20.7) | |||
| P | 0.634 | 1.000 | 0.675 | 0.121 | 0.582 | 0.582 | |||
| Day 6 (days 5-7)e | |||||||||
| n | 40 | 40 | 32 | 11 | 11 | 16 | 10 | 9 | 10 |
| Median virus titer (log TCID50/ml)b (range) | 1.50 (1.5-2.0) | 1.50 (1.5-1.5) | 1.50 (1.5-3.5) | 1.50 (1.5-2.5) | 1.50 (1.5-1.7) | 1.50 (1.5-1.5) | 1.50 (1.5-2.0) | 1.50 (1.5-3.5) | 1.50 (1.5-5.0) |
| No. (%) of patients shedding virusc | 4 (10.0) | 0 (0.0) | 8 (25.0) | 1 (9.1) | 1 (9.1) | 0 (0.0) | 3 (30.0) | 3 (33.3) | 4 (40.0) |
| Difference in %d (95% CI) | −15.0 (−32.6 to 2.6) | −25.0 (−40.0 to −10.0) | 9.1 (−7.9 to 26.1) | 9.1 (−7.9 to 26.1) | −10.0 (−51.6 to 31.6) | −6.7 (−49.9 to 36.6) | |||
| P | 0.116 | <0.001 | 0.407 | 0.407 | 1.000 | 1.000 | |||
Data are for the FAS. LOG 20 and LOG 40, groups given 20 and 40 mg of laninamivir octanoate, respectively; OG, oseltamivir group. P values were determined by Fisher's exact test (comparison with OG).
Virus titers <1.5 log TCID50/ml were set equal to 1.5 log TCID50/ml.
Number of patients with detectable virus (at least 1.5 log TCID50/ml).
Percentage of LOG 40 or LOG 20 patients shedding virus − percentage of OG patients.
Acceptable range of patient visit.
Tolerability and safety.
Both drugs were well tolerated. The most common adverse events were gastrointestinal events, including diarrhea, nausea, and vomiting. The event rates for diarrhea were 3.2% (2/62), 6.6% (4/61), and 1.6% (1/62) in the laninamivir octanoate 40-mg group, laninamivir octanoate 20-mg group, and oseltamivir group, respectively; for vomiting they were 3.2% (2/62), 4.9% (3/61), and 6.5% (4/62), respectively; and for nausea they were 1.6% (1/62), 1.6% (1/61), and 0.0% (0/62), respectively. In addition, gastroenteritis was observed in 1.6% (1/62), 6.6% (4/61), and 3.2% (2/62) of the patients, respectively. These events were generally mild to moderate and resolved within several days. Psychiatric disorders were observed in 3 patients treated with laninamivir octanoate; they consisted of abnormal behavior in the 40-mg group and crying and delirium in the 20-mg group, but they were mild and did not require any treatment. No clinically meaningful laboratory changes were observed in any of the treatment groups.
DISCUSSION
In this trial, a single inhalation of laninamivir octanoate reduced the duration of influenza illness by more than 30 h in comparison with 10 doses of oseltamivir administered orally over 5 days, mainly because most patients were infected with oseltamivir-resistant seasonal influenza A (H1N1) virus with an H274Y (by N2 numbering) mutation. Accordingly, the differences in duration of illness between the laninamivir octanoate groups and oseltamivir group were marked in patients infected with influenza A (H1N1) virus; that is, both dose levels of laninamivir octanoate reduced the duration of illness by more than 60 h in comparison with oseltamivir. Thus, the results of this study demonstrated that laninamivir octanoate is fully effective against oseltamivir-resistant seasonal influenza A (H1N1) virus strains clinically, even though the effectiveness of oseltamivir against the H1N1 strain in children in the 2008 to 2009 season has been reported to be reduced (9).
A comparison between the durations of viral shedding in the laninamivir octanoate and oseltamivir groups also demonstrated greater efficacy of laninamivir octanoate against oseltamivir-resistant influenza A (H1N1) virus virologically. The proportion of patients shedding virus at day 6 was significantly lower in the laninamivir octanoate 20-mg group than in the oseltamivir group (Table 4). However, there were no significant differences in median virus titers between laninamivir groups and the oseltamivir group, probably because the virus titers on day 1 were low (2.0 to 2.35 log10 TCID50/ml) for unknown reasons. In addition, we assumed the virus titer to be 1.5 log10 TCID50/ml if the virus titer was less than 1.5 log10 TCID50/ml. This approximation would affect the results.
There were no statistically significant differences in clinical efficacy between the laninamivir octanoate groups and oseltamivir group against influenza A (H3N2) or B virus infection, mainly because the number of patients was small in this study. However, the results of our previous study showed that oseltamivir is more effective clinically against influenza A (H3N2) virus infection than against influenza A (H1N1) or B virus (16, 18).
Both dosages of laninamivir octanoate (20 mg and 40 mg) were well tolerated. The gastrointestinal adverse events were generally mild to moderate, and their rates were similar in all 3 groups. A recent meta-analysis of randomized controlled trials showed that diarrhea, vomiting, and nausea occurred in 6.6%, 6.7%, and 3.4%, respectively, of children treated with a placebo (14), findings that are comparable to our own. As the clinical effects in the 40-mg group and 20-mg group were similar and there was no statistically significant difference in the virological effects between these groups, a higher dose is preferable for antiviral therapy to prevent the emergence of drug-resistant variants.
The median time to illness alleviation did not differ significantly between the laninamivir octanoate groups and oseltamivir group in the parallel trial in adult patients, although most of the adult patients were infected with oseltamivir-resistant influenza A (H1N1) virus. The detailed analysis of the trial in adult patients will be reported elsewhere. Thus, there seems to be a difference between the efficacies of laninamivir octanoate in adults and children. If there actually is a difference in efficacy, we think that the difference in clinical response is related to the patients’ ages or to their immune status. Since the influenza illness in adults in the parallel trial was mild, most adults may have recovered rapidly irrespective of whether they were treated with laninamivir or oseltamivir or not treated at all. By contrast, the illness caused by the same oseltamivir-resistant virus in the children in our trial may have been severe enough to require treatment with a neuraminidase inhibitor. We previously reported finding that oseltamivir was less effective against influenza B virus than against influenza A virus in children (16), and in that study, we observed a phenomenon in which the effectiveness of oseltamivir against influenza B virus increased with patient age. Therefore, the adult patients treated with oseltamivir in the parallel trial may have recovered because they were immune and not because oseltamivir was effective.
In conclusion, laninamivir octanoate was an effective and well-tolerated drug for the treatment of children with oseltamivir-resistant influenza A (H1N1) virus infection. Further study will be needed to confirm the clinical efficacy of laninamivir against influenza A (H3N2) or B virus infection. Its ease of administration is especially noteworthy, because only a single inhalation is required during the course of illness.
Acknowledgments
This study was supported by Daiichi Sankyo Co., Ltd.
The persons listed below are members of the CS-8958 Influenza in Children Study Group. Project Steering Committee members were Akira Watanabe (Research Division for Development of Anti-Infective Agents, Institute of Development, Aging and Cancer, Tohoku University, Sendai, Japan), Norio Sugaya (Keiyu Hospital, Kanagawa, Japan), Takao Takahashi (Department of Pediatrics, Keio University School of Medicine, Tokyo, Japan), and Daisuke Hiro, Katsuyasu Ishida, Hiroshi Takahashi, Shinichiro Awamura, and Mitsutoshi Uemori (Daiichi Sankyo Co., Ltd.). Investigators were Takashi Abe (Abe Child Clinic), Masahiro Bamba (Yokosuka Kyosai Hospital), Shinya Enomoto (Children's Enomoto Clinic), Hirofumi Furuta (Furuta Children's Clinic), Tsunekazu Haruta (Kobe City Medical Center General Hospital), Terue Hayashi (Matsunami Clinic), Kazumi Hiraba (Mokubo Children's Clinic), Masataka Ichikawa (Ichikawa Children's Clinic), Keisaku Imamura (Suzuran Children's Clinic), Jun Ishihara (Yokohama Municipal Citizen's Hospital), Satoshi Iwata (National Hospital Organization Tokyo Medical Center), Takehito Kuroda (Kuroda Clinic), Haruo Kuroki (Sotobo Children's Clinic), Koji Maehara (Maehara Children's Clinic), Shigenori Matsubara (Matsubara Otorhinolaryngology Clinic), Yutaka Minohara (Bunny Children's Clinic), Keiko Mitamura (Eiju General Hospital), Masaru Miura (Tokyo Metropolitan Kiyose Children's Hospital), Akiko Miyata (Miyata Clinic), Hiroo Miyazawa (Miyazawa Clinic), Toshikazu Nagakura (Yoga Allergy Clinic), Mari Nirasawa (Saiseikai Central Hospital), Kiyoshi Nishikawa (Nishikawa Clinic), Hiromasa Noda (Sunrise Children's Clinic), Takashige Okada (Okada Children's Clinic), Kota Saito (Saito Pediatric Clinic), Seiji Sato (Saitama Municipal Hospital), Yoshitake Sato (General Ota Hospital, Society of Health Incurrence of Fuji Heavy Industries, Ltd.), Kyoko Shibao (Shibao Pediatric Clinic), Tomoyuki Shibuya (Shibuya Clinic), Shizuo Shindo (Shindo Pediatric Clinic), Hiroyuki Shiro (Yokohama Rosai Hospital), Mikiko Takano (Kose Children's Clinic), Hirotaka Takahashi (Tokyo Metropolitan Ohtsuka Hospital), Toshikazu Takahashi (Takahashi Clinic), Yoshio Takasaki (Takasaki Pediatric Clinic), Jiro Takei (Takei Clinic), Toru Tsubota (Tsubota Children's Clinic), Toshiaki Tsuchie (Otakanomori Hospital), Toru Watanabe (Watanabe Pediatric Allergy Clinic), Yuji Yamashita (Yamashita Pediatric Clinic), Takashi Yokoyama (Yokoyama Pediatric Clinic), and Ryota Yoshimura (Yoshimura Children's Clinic).
Footnotes
Published ahead of print on 5 April 2010.
REFERENCES
- 1.Behrens, B., and G. Kärber. 1935. Wie sind Rehenversuche für biologishe Auswertungen am zweckmäsigsten anzuordnen? Naunyn Schmiedebergs Arch. Pharmakol. Exp. Pathol. 177:377-388. [Google Scholar]
- 2.Dominguez-Cherit, G., S. E. Lapinsky, A. E. Macias, et al. 2009. Critically ill patients with 2009 influenza A (H1N1) in Mexico. JAMA 302:1880-1887. [DOI] [PubMed] [Google Scholar]
- 3.Guo, L., R. J. Garten, A. S. Foust, et al. 2009. Rapid identification of oseltamivir-resistant influenza A (H1N1) viruses with H274Y mutation by RT-PCR/restriction fragment length polymorphism assay. Antiviral Res. 82:29-33. [DOI] [PubMed] [Google Scholar]
- 4.International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use. 1996. ICH harmonized tripartite guideline: guideline for good clinical practice. International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use, Geneva, Switzerland. http://www.ich.org/cache/compo/276-254-1.html.
- 5.International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use. 1998. ICH harmonized tripartite guideline: statistical principles for clinical trials. International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use, Geneva, Switzerland. http://www.ich.org/cache/compo/276-254-1.html.
- 6.Ishizuka, H., S. Yoshiba, H. Okabe, and K. Yoshihara. Clinical pharmacokinetics of laninamivir, a novel long-acting neuraminidase inhibitor, after single and multiple inhaled doses of its prodrug, CS-8958, in healthy male volunteers. J. Clin. Pharmacol., in press. [DOI] [PubMed]
- 7.Itoh, Y., K. Shinya, M. Kiso, et al. 2009. In vitro and in vivo characterization of new swine-origin H1N1 influenza viruses. Nature 460:1021-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jain, S., L. Kamimoto, A. M. Bramley, et al. 2009. Hospitalized patients with 2009 H1N1 influenza in the United States, April-June. N. Engl. J. Med. 361:1935-1944. [DOI] [PubMed] [Google Scholar]
- 9.Kawai, N., H. Ikematsu, N. Iwaki, et al. 2009. Clinical effectiveness of oseltamivir for influenza A(H1N1) virus with H274Y neuraminidase mutation. J. Infect. 59:207-212. [DOI] [PubMed] [Google Scholar]
- 10.Koyama, K., M. Takahashi, M. Oitate, et al. 2009. CS-8958, a prodrug of the novel neuraminidase inhibitor R-125489, demonstrates a favorable long retention profile in the mouse respiratory tract. Antimicrob. Agents Chemother. 53:4845-4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kubo, N., H. Ikematsu, S. Nabeshima, et al. 2003. Evaluation of an immunochromatography test kit for rapid diagnosis of influenza. Kansenshogaku Zasshi 77:1007-1014. [DOI] [PubMed] [Google Scholar]
- 12.Kubo, S., T. Tomozawa, M. Kakuta, A. Tokumitsu, and M. Yamashita. 2010. Laninamivir prodrug CS-8958, a long-acting neuraminidase inhibitor, shows superior anti-influenza virus activity after a single administration. Antimicrob. Agents Chemother. 54:1256-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reichert, T. A., N. Sugaya, D. S. Fedson, W. P. Glezen, L. Simonsen, and M. Tashiro. 2001. The Japanese experience with vaccinating schoolchildren against influenza. N. Engl. J. Med. 344:889-896. [DOI] [PubMed] [Google Scholar]
- 14.Shun-Shin, M., M. Thompson, C. Heneghan, R. Perera, A. Harnden, and D. Mant. 2009. Neuraminidase inhibitors for treatment and prophylaxis of influenza in children: systematic review and meta-analysis of randomised controlled trials. BMJ 339:b3172. doi: 10.1136/bmj.b3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sugaya, N., K. Mitamura, M. Nirasawa, and K. Takahashi. 2000. The impact of winter epidemics of influenza and respiratory syncytial virus on paediatric admissions to an urban general hospital. J. Med. Virol. 60:102-106. [PubMed] [Google Scholar]
- 16.Sugaya, N., K. Mitamura, M. Yamazaki, et al. 2007. Lower clinical effectiveness of oseltamivir against influenza B contrasted with influenza A infection in children. Clin. Infect. Dis. 44:197-202. [DOI] [PubMed] [Google Scholar]
- 17.Sugaya, N., K. Nerome, M. Ishida, et al. 1992. Impact of influenza virus infection as a cause of pediatric hospitalization. J. Infect. Dis. 165:373-375. [DOI] [PubMed] [Google Scholar]
- 18.Sugaya, N., D. Tamura, M. Yamazaki, et al. 2008. Comparison of the clinical effectiveness of oseltamivir and zanamivir against influenza virus infection in children. Clin. Infect. Dis. 47:339-345. [DOI] [PubMed] [Google Scholar]
- 19.Whitley, R. J., F. G. Hayden, K. S. Reisinger, et al. 2001. Oral oseltamivir treatment of influenza in children. Pediatr. Infect. Dis. J. 20:127-133. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization. 2009. Influenza A(H1N1) virus resistance to oseltamivir—2008/2009 influenza season, northern hemisphere. World Health Organization, Geneva, Switzerland. http://www.who.int/csr/disease/influenza/H1N1webupdate20090318%20ed_ns.pdf.
- 21.World Health Organization. 2009. World now at the start of 2009 influenza pandemic. June 2009. World Health Organization, Geneva, Switzerland. http://www.who.int/mediacentre/news/statements/2009/h1n1_pandemic_phase6_20090611/en/index.html.
- 22.World Health Organization. 2010. Pandemic (H1N1) 2009—update 86. World Health Organization, Geneva, Switzerland. http://www.who.int/csr/disease/swineflu/laboratory05_02_2010/en/index.html.
- 23.Yamashita, M., T. Tomozawa, M. Kakuta, A. Tokumitsu, H. Nasu, and S. Kubo. 2009. CS-8958, a prodrug of the new neuraminidase inhibitor R-125489, shows long-acting anti-influenza virus activity. Antimicrob. Agents Chemother. 53:186-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamazaki, M., K. Mitamura, K. Kimura, et al. 2001. Clinical evaluation of an immunochromatography test for rapid diagnosis of influenza. Kansenshogaku Zasshi 75:1047-1053. [DOI] [PubMed] [Google Scholar]