Abstract
Most approved drugs with activity against hepatitis B virus (HBV) have activity against human immunodeficiency virus type 1 (HIV-1), which precludes their use in patients who are coinfected with HBV and HIV-1 and who are not receiving antiretroviral therapy due to the risk of inducing resistance. The activity of telbivudine, a highly selective HBV inhibitor, against temporally and geographically distinct wild-type and multidrug-resistant HIV-1 clinical isolates was evaluated in vitro. No inhibition was observed with up to 600 μM drug, which supports further exploration of telbivudine as a therapeutic option for the treatment of HBV infections in patients coinfected with HIV-1.
Coinfection with hepatitis B virus (HBV) and human immunodeficiency virus type 1 (HIV-1) is common because both viruses are transmitted through the sexual and percutaneous routes. An estimated 10% of the 40 million HIV-infected individuals have developed chronic hepatitis B (1). The treatment of HBV infection in patients coinfected with HIV-1has become an important health issue due to the increased morbidity and mortality resulting from HBV-related chronic liver disease (15). Several nucleoside/nucleotide inhibitors with dual activities against both HIV-1 and HBV have been used as part of combination antiviral regimens in coinfected patients, including lamivudine, emtricitabine, adefovir, and tenofovir (2, 4-6, 16). However, few options exist for patients who are infected with HBV but who do not meet the current criteria for treatment with highly active antiretroviral therapy (HAART), as treatment with these anti-HBV agents alone has a proved (lamivudine and tenofovir) or theoretical (adefovir) risk of inducing resistance in HIV-1. Recently, it was reported that treatment with entecavir (ETC) also led to a consistent 1-log10 (copies/ml) reduction in the HIV-1 load in patients coinfected with HBV (9). Moreover, entecavir can select for the M184V mutation in the reverse transcriptase (RT) region of HIV-1. The M184V mutation confers phenotypic resistance to the drug both in vitro and in patients. Biochemical studies have confirmed that entecavir 5′-triphosphate is a substrate of HIV RT (3). Thus, treatment of HBV infection in patients coinfected with HIV-1 should be carefully selected and monitored to minimize the risk of developing HIV-1 resistance.
Telbivudine (LdT), a synthetic l-analogue of thymidine, was approved for the treatment of chronic HBV infection in 2006. The compound is a highly specific inhibitor of HBV polymerase. It was previously shown that telbivudine had no significant in vitro activity against 15 other DNA and RNA viruses, including two laboratory strains of wild-type HIV-1 (14). The lack of activity against HIV-1 presents an opportunity to use telbivudine for the treatment of HBV infection in patients coinfected with HIV-1 without introducing the risk of developing HIV-1 resistance in the absence of HAART, as recommended by an international panel on HIV-HBV coinfection (13). However, Low et al. recently reported a single case in which an patient who was coinfected with HIV and HBV and who was receiving adefovir-telbivudine combination therapy had a surprising reduction in the HIV-1 load that rebounded after telbivudine was withdrawn (8). The authors speculated that telbivudine was responsible for the inhibition of HIV-1; however, there were no data confirming the susceptibility of the HIV-1 isolate from that patient to either telbivudine or adefovir or the combination of both drugs in vitro, and no resistance-conferring mutation was identified. Nevertheless, an important question was raised, that is, whether telbivudine has any activity against HIV-1, particularly clinical isolates, since previous testing was performed only with a minimum number of laboratory strains. Therefore, prior to the further clinical investigation of the use of telbivudine in patients coinfected with HIV and HBV, a comprehensive in vitro study was initiated to examine the activity of the drug against HIV-1 clinical isolates.
Eight temporally and geographically distinct HIV-1 clinical isolates were selected from the library of Monogram Biosciences (South San Francisco, CA) to cover isolates from a broad range of geographic locations and obtained at a variety of collection times, as well as to include various HIV-1 subtypes (subtypes A, B, BF, C, and D). In addition, two known multidrug-resistant HIV-1 isolates from the collection were also selected for evaluation. The first isolate has five mutations (D67N, K70R, T215F, K219E, and M184V) and represents an HIV-1 isolate with mid- to high-level resistance to both nucleoside RT inhibitors (NRTIs; zidovudine, lamivudine, and abacavir) and nonnucleoside RT inhibitors (NNRTIs; nevirapine, delavirdine, and efavirenz). It contains the signature M184V mutation that is responsible for resistance to both lamivudine and entecavir. The second isolate has three mutations (K103N, Y181C, and G190A) and represents an HIV-1 isolate with high-level resistance to NNRTIs (nevirapine, delavirdine, and efavirenz). Entecavir (synthesized by Moravek Biochemicals Inc.) was included in the study for comparison because it has previously been reported to have activity against HIV-1 clinical isolates (7). Lamivudine was also included as a positive control.
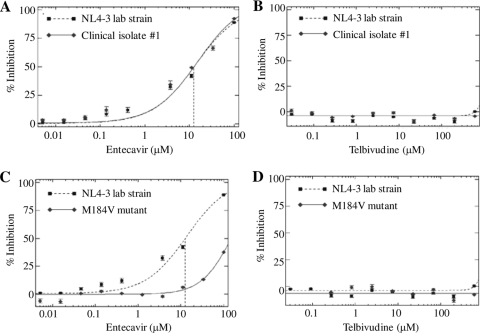
The activities of the drugs were assessed by the PhenoSense HIV drug susceptibility assay developed by Monogram Biosciences, the detailed methodology of which has been described previously (11). Briefly, the RT- and protease-coding regions of the HIV-1 clinical isolates were amplified from viral RNA by RT-PCR. The amplified product and a luciferase indicator gene were inserted into a resistance test vector plasmid. To produce a virus stock for the drug susceptibility assay, human embryonic kidney cells (HEK293 cells) were cotransfected with the resistance test vector plasmid DNA and an expression vector encoding the envelop proteins of amphotropic murine leukemia virus. Following transfection, pseudotype virus particles were harvested and used to infect fresh target cells. For susceptibility testing, the drugs were serially diluted in culture medium and added to naïve HEK293 cells for overnight incubation. By determination of the cell density, no significant cytotoxicity was observed either in cells treated with up to 600 μM telbivudine or 100 μM entecavir or in untreated cells. Drug-treated cells were infected with pseudotype viruses containing the RT and protease regions derived from the clinical isolates or a control laboratory strain, NL4-3. The ability of the viruses to replicate was assessed 72 h postinfection by measuring the luciferase activity in the target cells. Testing of the activities of telbivudine and entecavir against all HIV-1 isolates and the control laboratory strain was performed in the same experiment for direct comparison. The data were analyzed by plotting the percent inhibition (y axis) versus the log10 drug concentration (x axis) for each virus. Drug susceptibility curves for a representative HIV-1 wild-type clinical isolate (isolate 1), the M184V-containing multidrug-resistant strain, and the control laboratory strain are shown in Fig. 1. The 50% effective concentrations (EC50s) of telbivudine and entecavir against all the isolates were derived from the respective susceptibility curves and are summarized in Table 1. Entecavir exhibited inhibitory activity against all the wild-type HIV-1 clinical isolates and the NNRTI-resistant strain, with the EC50s ranging from 7.62 to 15.1 μM. These results are consistent with the anti-HIV activity of entecavir in vitro reported previously. The M184V-containing isolate was resistant to entecavir treatment and had an EC50 of >100 μM, or >8-fold higher than that of the control laboratory strain, suggesting that the effect of entecavir was specific and may have been due to the inhibition of HIV-1 RT. In contrast, telbivudine at up to 600 μM, the highest concentration tested, showed no inhibitory activity against any of the HIV-1 isolates. Higher concentrations were not tested, as cytotoxicity was observed in cells that were treated with 2,000 μM telbivudine. Telbivudine also had no effect on the resistant strains.
FIG. 1.
Drug susceptibility curves for telbivudine and entecavir against selected HIV-1 isolates. The activities of the compounds were measured by the PhenoSense HIV drug susceptibility assay. The results for a representative wild-type clinical isolate (isolate 1) and an M184V mutation-containing multidrug-resistant strain are shown in comparison to those for the control laboratory strain, NL4-3. Each dilution of the compound was tested singly. Error bars in the susceptibility curves were defined from the standard deviation of the bootstrapped values from the measured and the curve-fitted data points.
TABLE 1.
EC50 values of telbivudine, lamivudine, and entecavir against HIV-1 isolatesa
| HIV-1 strain | Description | Collection date | EC50 (μM) |
||
|---|---|---|---|---|---|
| Lamivudine | Entecavir | Telbivudine | |||
| Laboratory strain | NL4-3 | NA | 4.68 | 12.6 | >600 |
| Clinical isolates (wild type) | |||||
| 1 | Subtype A | Jun 2004 | 3.54 | 12.5 | >600 |
| 2 | Subtype A | Feb 2007 | 4.48 | 13.2 | >600 |
| 3 | Subtype B | Jul 2004 | 4.78 | 15.1 | >600 |
| 4 | Subtype B | Oct 2004 | 5.43 | 13.5 | >600 |
| 5 | Subtype BF | Dec 2006 | 4.24 | 13.6 | >600 |
| 6 | Subtype C | Apr 2004 | 3.90 | 9.51 | >600 |
| 7 | Subtype C | Jan 2007 | 4.06 | 9.95 | >600 |
| 8 | Subtype D | Jan 2007 | 3.25 | 7.62 | >600 |
| Resistant strains | |||||
| 1 | D67N/K70R/T215F/K219E/M184V | NA | >300 | >100 | >600 |
| 2 | K103N/Y181C/G190A | NA | 7.16 | 11.7 | >600 |
The isolates included eight temporally and geographically distinct wild-type clinical isolates, two multidrug-resistant isolates, and a control laboratory strain. Subtype A HIV-1 is predominantly found in West and Central Africa and also causes much of the Russian epidemic; subtype B is the most common subtype in Europe, the Americas, Japan, and Australia; subtype BF is predominantly found in South America; subtype C is mainly in found in southern and East Asia, India, and Nepal; and subtype D is generally limited to East and Central Africa. NA, not available.
In conclusion, telbivudine did not exhibit any direct activity against HIV-1 clinical isolates in vitro. The concentrations of telbivudine tested in vitro (600 μM) far exceeded the physiologically relevant concentrations of the drug in patients: the maximum concentration of telbivudine in plasma was 13.2 ± 4.5 μM when it was used at its therapeutic dose (600 mg). In the case reported by Low et al. (8), the patient received adefovir, in addition to telbivudine, and the patient underwent multiple interruptions to the combination treatment and received only a short-term follow-up. It was not clear what effect the dual therapy may have on the HIV-1 load. There was no confirmation testing of the susceptibility of the patient isolate to either adefovir or telbivudine, and no drug resistance-conferring mutation was reported. Thus, the results for this single case should be interpreted with caution. As Monogram's PhenoSense HIV drug susceptibility assay is not designed or intended for use for assessment of synergistic or antagonistic drug interactions, these types of experiments were not performed in this study. As such, the effect of the combination of telbivudine and adefovir remains to be determined. More recently, Milazzo et al. (10) reported on the clinical histories of three patients with HIV-HBV coinfection who were treated for HBV infection with telbivudine monotherapy and who were followed up without the interruption of treatment for 24 weeks. In all three patients, telbivudine produced a rapid and potent reduction in HBV DNA levels. The baseline HIV-1 load in the patients ranged from 103 to 104 copies/ml, and all patients had high baseline CD4 counts. In two of the three patients, the HIV load declined by 2 to 3 log10 copies/ml and became undetectable (<300 copies/ml) within 1 to 2 weeks of the start of telbivudine treatment but subsequently rebounded to the baseline levels within 4 weeks of treatment. One patient had no change in the HIV-1 load from the baseline level throughout the duration of treatment. Viral sequencing analysis showed that all three patients had wild-type HIV-1 (subtype B) in their plasma and peripheral blood mononuclear cells at the baseline and that no drug resistance-conferring mutations were found in either HIV-1 or HBV at up to 24 weeks of follow-up. It was suggested that the reduction in the HIV-1 load may be caused by lymphocyte activation, cytokine production, and liver inflammation, i.e., effects that resulted indirectly from the blocking of HBV replication by telbivudine. The observation of an early and transient reduction in the HIV-1 load was interesting and to our knowledge has not been reported previously. Sheldon et al. showed that 10 mg/day adefovir reduced the HBV load but not the HIV-1 load after 6 months in patients coinfected with HBV and HIV-1; however, no early viral kinetics data were reported (12). In summary, there appeared to be a clear difference between the cases associated with telbivudine and those associated with entecavir or other anti-HBV drugs, in which the direct inhibition of HIV-1 replication was detected and specific drug resistance-conferring mutations, such as M184V, were selected in HIV-1 RT.
Given the fact that there are few options for the treatment of HBV infection in patients coinfected with HIV-1 not receiving HAART, the confirmation that telbivudine lacks direct activity against HIV-1 clinical isolates in vitro is of great significance. Our results support the further clinical investigation of telbivudine as the therapeutic option for HBV infection in patients coinfected with HIV-1 who do not require treatment for their HIV-1 infection.
Acknowledgments
We thank Chingha Lai and Teresa Compton for their support and Christos Petropoulos and Jeannette Whitcomb for helpful discussions and interpretation of the data.
Footnotes
Published ahead of print on 22 March 2010.
REFERENCES
- 1.Alter, M. J. 2006. Epidemiology of viral hepatitis and HIV co-infection. J. Hepatol. 44:S6-S9. [DOI] [PubMed] [Google Scholar]
- 2.Benhamou, Y., V. Thibault, P. Vig, V. Calvez, A. G. Marcelin, M. H. Fievet, G. Currie, C. G. Chang, L. Biao, S. Xiong, C. Brosgart, and T. Poynard. 2006. Safety and efficacy of adefovir dipivoxil in patients infected with lamivudine-resistant hepatitis B and HIV-1. J. Hepatol. 44:62-67. [DOI] [PubMed] [Google Scholar]
- 3.Domaoal, R. A., M. McMahon, C. L. Thio, C. M. Bailey, J. Tirado-Rives, A. Obikhod, M. Detorio, K. L. Rapp, R. F. Siliciano, R. F. Schinazi, and K. S. Anderson. 2008. Pre-steady-state kinetic studies establish entecavir 5′-triphosphate as a substrate for HIV-1 reverse transcriptase. J. Biol. Chem. 283:5452-5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dore, G. J., D. A. Cooper, C. Barrett, L. E. Goh, B. Thakrar, and M. Atkins. 1999. Dual efficacy of lamivudine treatment in human immunodeficiency virus/hepatitis B virus-coinfected persons in a randomized, controlled study (CAESAR). The CAESAR Coordinating Committee. J. Infect. Dis. 180:607-613. [DOI] [PubMed] [Google Scholar]
- 5.Dore, G. J., D. A. Cooper, A. L. Pozniak, E. DeJesus, L. Zhong, M. D. Miller, B. Lu, and A. K. Cheng. 2004. Efficacy of tenofovir disoproxil fumarate in antiretroviral therapy-naive and -experienced patients coinfected with HIV-1 and hepatitis B virus. J. Infect. Dis. 189:1185-1192. [DOI] [PubMed] [Google Scholar]
- 6.Gish, R. G., H. Trinh, N. Leung, F. K. Chan, M. W. Fried, T. L. Wright, C. Wang, J. Anderson, E. Mondou, A. Snow, J. Sorbel, F. Rousseau, and L. Corey. 2005. Safety and antiviral activity of emtricitabine (FTC) for the treatment of chronic hepatitis B infection: a two-year study. J. Hepatol. 43:60-66. [DOI] [PubMed] [Google Scholar]
- 7.Lin, P. F., B. Nowicka-Sans, B. Terry, S. Zhang, C. Wang, L. Fan, I. Dicker, V. Gali, H. Higley, N. Parkin, D. Tenney, M. Krystal, and R. Colonno. 2008. Entecavir exhibits inhibitory activity against human immunodeficiency virus under conditions of reduced viral challenge. Antimicrob. Agents Chemother. 52:1759-1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Low, E., A. Cox, M. Atkins, and M. Nelson. 2009. Telbivudine has activity against HIV-1. AIDS 23:546-547. [DOI] [PubMed] [Google Scholar]
- 9.McMahon, M. A., B. L. Jilek, T. P. Brennan, L. Shen, Y. Zhou, M. Wind-Rotolo, S. Xing, S. Bhat, B. Hale, R. Hegarty, C. R. Chong, J. O. Liu, R. F. Siliciano, and C. L. Thio. 2007. The HBV drug entecavir—effects on HIV-1 replication and resistance. N. Engl. J. Med. 356:2614-2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Milazzo, L., I. Caramma, A. Lai, M. Violin, C. De Maddalena, M. Cesari, M. Galli, and C. Balotta. 2009. Telbivudine in the treatment of chronic hepatitis B: experience in HIV type-1-infected patients naive for antiretroviral therapy. Antivir. Ther. 14:869-872. [DOI] [PubMed] [Google Scholar]
- 11.Petropoulos, C. J., N. T. Parkin, K. L. Limoli, Y. S. Lie, T. Wrin, W. Huang, H. Tian, D. Smith, G. A. Winslow, D. J. Capon, and J. M. Whitcomb. 2000. A novel phenotypic drug susceptibility assay for human immunodeficiency virus type 1. Antimicrob. Agents Chemother. 44:920-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheldon, J. A., A. Corral, B. Rodes, S. Mauss, J. Rockstroh, F. Berger, C. Schwarze-Zander, and V. Soriano. 2005. Risk of selecting K65R in antiretroviral-naive HIV-infected individuals with chronic hepatitis B treated with adefovir. AIDS 19:2036-2038. [DOI] [PubMed] [Google Scholar]
- 13.Soriano, V., M. Puoti, M. Peters, Y. Benhamou, M. Sulkowski, F. Zoulim, S. Mauss, and J. Rockstroh. 2008. Care of HIV patients with chronic hepatitis B: updated recommendations from the HIV-Hepatitis B Virus International Panel. AIDS 22:1399-1410. [DOI] [PubMed] [Google Scholar]
- 14.Standring, D. N., E. G. Bridges, L. Placidi, A. Faraj, A. G. Loi, C. Pierra, D. Dukhan, G. Gosselin, J. L. Imbach, B. Hernandez, A. Juodawlkis, B. Tennant, B. Korba, P. Cote, E. Cretton-Scott, R. F. Schinazi, M. Myers, M. L. Bryant, and J. P. Sommadossi. 2001. Antiviral beta-l-nucleosides specific for hepatitis B virus infection. Antivir. Chem. Chemother. 12(Suppl 1):119-129. [PubMed] [Google Scholar]
- 15.Thio, C. L. 2009. Hepatitis B and human immunodeficiency virus coinfection. Hepatology 49:S138-S145. [DOI] [PubMed] [Google Scholar]
- 16.Wolters, L. M., H. G. Niesters, B. E. Hansen, M. E. van der Ende, F. P. Kroon, C. Richter, K. Brinkman, P. L. Meenhorst, and R. A. de Man. 2002. Development of hepatitis B virus resistance for lamivudine in chronic hepatitis B patients co-infected with the human immunodeficiency virus in a Dutch cohort. J. Clin. Virol. 24:173-181. [DOI] [PubMed] [Google Scholar]