Abstract
The growing number of infections caused by multidrug-resistant pathogens has prompted a more rational use of available antibiotics given the paucity of new, effective agents. Monte Carlo simulations were utilized to determine the appropriateness of several doripenem dosing regimens based on the probability of attaining the critical drug exposure metric of time that drug concentrations remain above the drug MIC (T>MIC) for 35% (and lower thresholds) of the dosing interval in >80 to 90% of the population (T>MIC 35% target). This exposure level generally correlates with in vivo efficacy for carbapenems. In patients with creatinine clearance of >50 ml/min, a 500-mg dose of doripenem infused over 1 h every 8 h is expected to be effective against bacilli with doripenem MICs of ≤1 μg/ml based on a T>MIC 35% target and MICs of ≤2 μg/ml based on lower targets. A longer, 4-hour infusion time improved target attainment in most cases, such that the T>MIC was adequate for pathogens with doripenem MICs as high as 4 μg/ml. Efficacy is expected for infections caused by pathogens with doripenem MICs of ≤2 μg/ml in patients with moderate renal impairment (creatinine clearance, 30 to 50 ml/min) who receive doripenem at 250 mg infused over 1 h every 8 h and in patients with severe impairment (creatinine clearance between 10 and 29 ml/min) who receive doripenem at 250 mg, infused over 1 h or 4 h, every 12 h. Results of pharmacokinetics/pharmacodynamics (PK/PD) modeling can guide dose optimization, thereby potentially increasing the clinical efficacy of doripenem against serious Gram-negative bacterial infections.
In a time of increasing antibiotic resistance (11, 14, 15, 17, 19, 37) and when few new antibiotics are being developed to treat serious Gram-negative bacterial infections (39), it is prudent to revisit how currently available antibiotics are being used and to determine whether they are being used to “best effect,” that is, whether their dosage and administration schedule meet the combined objectives of curing infection, minimizing safety risks, and curbing the emergence of antibiotic resistance. Standard parenteral antibiotic administration generally follows the “one size fits all” rule: most patients receive the same dose during a short infusion at the same interval, without regard to the severity and location of the infection being treated. In widely varying clinical scenarios, however, such as those involving critically ill patients in whom drug distribution and elimination are altered (35), a common (i.e., nonindividualized) approach is recognized as inadequate (42). In addition to having altered pharmacokinetics (PK), these patients may be at risk for infection with more antibiotic-resistant pathogens (higher MICs) and may therefore require greater antibiotic exposure, as accomplished through increased doses, an altered dosing interval or infusion duration, or a combination of these.
In this context, a critical step in dose selection is an understanding of the pharmacokinetics/pharmacodynamics (PK/PD) goal for treatment, derived from PK/PD modeling, to maximize the likelihood of a favorable clinical/microbiological response as well as to minimize the probability for exposure-related toxicities (26, 31). For carbapenem antibiotics, the fraction of time during the dosing interval that drug concentration remains above its MIC for the infecting pathogen(s) (T>MIC) is the target that best relates (directly) to patient outcomes (9). Bacteriostatic and bactericidal effects are expected when T>MIC exceeds 20 and 40, respectively (9, 12). By way of comparison, the PK/PD target for carbapenems is substantially shorter than that for penicillins (30 and 50, respectively) and cephalosporins (35 to 40 and 60 to 70, respectively) (12).
Doripenem, the newest carbapenem (β-lactam) antibiotic approved for use in the United States, Canada, and Europe (and currently approved in ∼60 countries worldwide), possesses greater antimicrobial activity against difficult-to-treat pathogens than do older members of the class—it is two to four times more potent than imipenem against Pseudomonas aeruginosa (38), for example—and is more stable in infusion solutions (5, 25, 32). Doripenem is the only antibiotic for which different infusion times (1 hour and 4 hours) have been formally evaluated in registrational studies and have received marketing approval (13). The pharmacokinetic profile of doripenem is similar to that of imipenem and meropenem (5, 25, 32). In animal models, doripenem has the lowest potential among the carbapenems to cause seizure (43). Furthermore, no drug-related seizures were reported in phase 3 clinical trials of doripenem (32, 34).
The objective of the work reported in this paper was to improve our understanding of doripenem dosing based on PK/PD modeling utilizing an updated population PK model, which was constructed from phase 1, 2, and 3 data from 303 subjects/patients (28), and Monte Carlo simulation techniques. Here we report the probability of attaining effective drug exposure with clinically relevant dosing regimens of doripenem over a range of MICs for pathogens commonly isolated in the hospital setting.
MATERIALS AND METHODS
A 5,000-patient Monte Carlo simulation implemented in S-Plus software (Insightful Corporation, Seattle, WA), using the mean PK parameter estimates and variance-covariance matrix from a population PK model (constructed using data from phase 1 studies with healthy volunteer and phase 2 and 3 studies [28]), was conducted to generate concentration-time profiles for several dosing regimens of doripenem. Since interoccasion variability was a significant component of the population PK model, the interindividual variability was inflated to include the interoccasion variability, as only one occasion per subject was simulated. Residual variability was not introduced into the calculations of simulated concentrations since it was found to be insignificant. Protein binding of 8.5% (6) was applied to correct for plasma protein binding in the simulated data.
The following dose regimens, each infused over either 1 hour (24, 27, 33) or 4 hours (7), were simulated: 500 mg administered every 8 h (normal renal function or mild renal impairment [creatinine clearance of >50 ml/min]), 250 mg administered every 8 h (moderate renal impairment [creatinine clearance of 50 ml/min or 30 ml/min]), and 250 mg administered every 12 h (severe renal impairment [creatinine clearance of 29 ml/min or 10 ml/min]).
Simulations were performed using creatinine clearances of 30 ml/min and 50 ml/min for the moderate level of renal impairment and 10 ml/min and 29 ml/min for severe renal impairment to bracket the ranges in these renal function categories. For each scenario, the results were calculated based on maximum likelihood parameter estimates with creatinine clearance at the above stated fixed values for all 5,000 subjects.
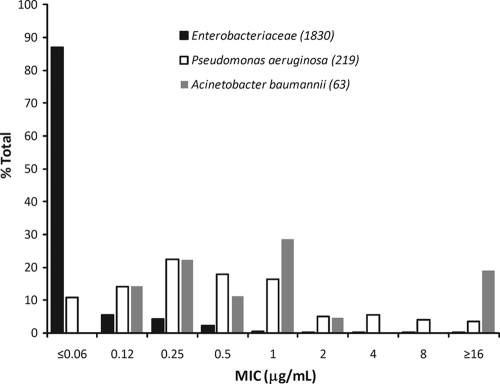
PK/PD target attainment probabilities for a free-drug T>MIC (fT>MIC) of 25%, 30%, or 35% were evaluated for each dosing regimen across a range of pathogens from large phase 3 clinical trials and from surveillance studies. The susceptibilities of pathogens from the clinical trials and from surveillance studies of doripenem are shown in Tables 1 and 2, respectively. The doripenem MIC distribution for selected Gram-negative pathogens from the clinical trials is shown in Fig. 1.
TABLE 1.
In vitro activities of doripenem against clinical isolates from all clinical studies
| Organism | n | MIC (μg/ml) |
||
|---|---|---|---|---|
| Range | 50% | 90% | ||
| Gram-negative aerobes | ||||
| Enterobacteriaceae | 1,830 | ≤0.03-32 | ≤0.03 | 0.12 |
| Escherichia coli | 1,183 | ≤0.03-2 | ≤0.03 | 0.03 |
| Klebsiella pneumoniae | 207 | ≤0.03-32 | 0.06 | 0.12 |
| Klebsiella oxytoca | 53 | ≤0.03-0.12 | 0.06 | 0.06 |
| Citrobacter freundii | 26 | ≤0.03-0.06 | ≤0.03 | 0.06 |
| Citrobacter koseri | 15 | ≤0.03-0.06 | ≤0.03 | 0.06 |
| Enterobacter cloacae | 108 | ≤0.03-4 | 0.06 | 0.5 |
| Enterobacter aerogenes | 29 | ≤0.03-0.25 | 0.06 | 0.12 |
| Proteus mirabilis | 94 | ≤0.03-2 | 0.25 | 0.5 |
| Morganella morganii | 23 | ≤0.03-1 | 0.25 | 1 |
| Serratia marcescens | 35 | ≤0.03-8 | 0.12 | 0.25 |
| Non-Enterobacteriaceae | 245 | ≤0.03-32 | 0.25 | 4 |
| Pseudomonas aeruginosa | 219 | ≤0.03-32 | 0.5 | 4 |
| Acinetobacter spp. | 67 | ≤0.03->128 | 0.5 | 32 |
| Acinetobacter baumannii | 63 | ≤0.03->128 | 1 | 32 |
| Burkholderia cepacia | 2 | 2-8 | NAa | NA |
| Stenotrophomonas maltophilia | 16 | 4->128 | 64 | >128 |
| Haemophilus spp. | 101 | ≤0.03-1 | 0.06 | 0.25 |
| Gram-positive aerobes | ||||
| Enterococcus spp. | 172 | ≤0.03->128 | 4 | 32 |
| Enterococcus faecalis | 92 | ≤0.03-16 | 4 | 4 |
| Staphylococcus spp. | 333 | ≤0.03->128 | ≤0.03 | 16 |
| Staphylococcus aureus | 276 | ≤0.03->128 | ≤0.03 | 16 |
| Staphylococcus aureus (oxacillin susceptible) | 196 | ≤0.03-0.5 | ≤0.03 | 0.06 |
| Coagulase-negative staphylococci | 57 | ≤0.03-64 | 0.06 | 16 |
| Coagulase-negative staphylococci (oxacillin susceptible) | 44 | ≤0.03-1 | ≤0.03 | 0.25 |
| Streptococcus pneumoniae | 41 | ≤0.03-1 | ≤0.03 | 0.5 |
| Streptococcus spp. other than S. pneumoniae | 311 | ≤0.03-4 | ≤0.03 | 0.06 |
| All anaerobes | 660 | ≤0.03-32 | 0.25 | 0.5 |
NA, not applicable.
TABLE 2.
In vitro activities of doripenem against pathogens from TRUST 12 (2008)
| Organism | All isolates |
ICU isolates |
||||||
|---|---|---|---|---|---|---|---|---|
| No. of isolates | MIC (μg/ml) |
No. of isolates | MIC (μg/ml) |
|||||
| 50% | 90% | Range | 50% | 90% | Range | |||
| Gram-positive aerobes | ||||||||
| Enterococcus faecalis | 198 | 4 | 8 | 0.12-8 | 26 | 4 | 4 | 0.12-8 |
| Streptococcus pneumoniae | 2,858 | ≤0.015 | 1 | ≤0.015-2 | 436 | ≤0.015 | 0.5 | ≤0.015-1 |
| Staphylococcus aureus | ||||||||
| Methicillin susceptible | 555 | 0.03 | 0.06 | ≤0.015-1 | 48 | 0.03 | 0.06 | ≤0.015-0.06 |
| Methicillin resistant | 1,086 | 0.5 | 4 | ≤0.015->32 | 86 | 1 | 16 | 0.12->32 |
| Gram-negative aerobes | ||||||||
| Acinetobacter species | 349 | 1 | >32 | ≤0.015->32 | 92 | 16 | >32 | 0.12->32 |
| Enterobacter cloacae | 455 | 0.06 | 0.25 | ≤0.015-16 | 61 | 0.06 | 0.12 | 0.03-4 |
| Escherichia coli | 1,723 | 0.03 | 0.06 | ≤0.015-8 | 103 | 0.03 | 0.06 | ≤0.015-0.5 |
| Klebsiella pneumoniae | 1,540 | 0.06 | 0.12 | ≤0.015->32 | 133 | 0.06 | 0.25 | ≤0.015->32 |
| Haemophilus influenzae | 716 | 0.12 | 0.5 | ≤0.03-1 | 125 | 0.12 | 0.5 | ≤0.03-0.5 |
| Proteus mirabilis | 814 | 0.25 | 0.5 | ≤0.015-1 | 37 | 0.25 | 1 | 0.06-1 |
| Pseudomonas aeruginosa | 1,533 | 0.5 | 4 | ≤0.015->32 | 210 | 0.5 | 8 | 0.03->32 |
FIG. 1.
Doripenem MIC distribution for selected Gram-negative pathogens from phase 3 clinical studies.
RESULTS
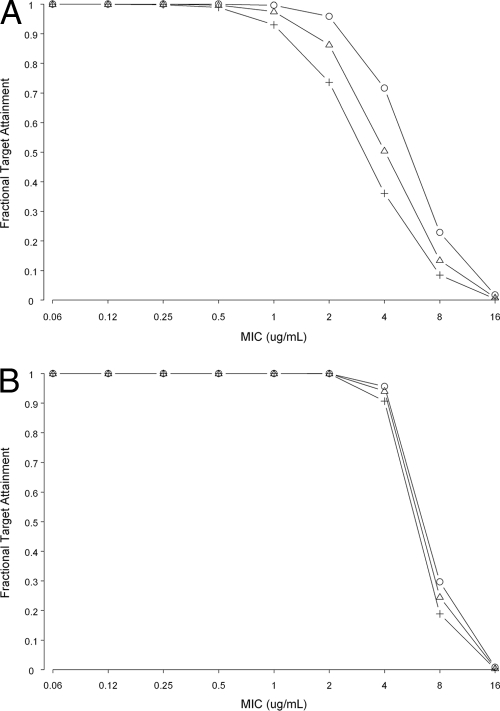
Table 3 shows the probability of PK/PD target attainment by renal function, duration of doripenem infusion, and MIC. Figure 2 shows PK/PD target attainment for selected dosing regimens of doripenem infused over 1 or 4 h over a wide range of creatinine clearances observed in phase 1, 2, and 3 studies. The MIC90 and ranges utilized in this analysis are consistent with those observed in a large database of 9,551 pathogens isolated from patients—including a substantial number in an intensive care unit (ICU) at the time of specimen collection—at 56 geographically distributed centers in the United States (Table 2; TRUST 2008).
TABLE 3.
Probability of PK/PD target attainment by renal function, duration of doripenem infusion, and MIC of pathogen
| Renal function and dose regimena | MIC (μg/ml)b | % Probability of attaining PK/PD target (% fT>MIC)c |
||
|---|---|---|---|---|
| 25 | 30 | 35 | ||
| Normal function to mild impairment (CLCR > 50 ml/min), 500 mg q8h | ||||
| 1-h infusion | 1 | 99.58 | 97.24 | 91.84 |
| 2 | 95.34 | 82.96 | 68.4 | |
| 4 | 66.46 | 43.1 | 25.3 | |
| 4-h infusion | 1 | 100 | 100 | 100 |
| 2 | 100 | 100 | 100 | |
| 4 | 94.54 | 93.06 | 90.0 | |
| Moderate impairment, 250 mg q8h | ||||
| Upper bound (CLCR = 50 ml/min) | ||||
| 1-h infusion | 1 | 100 | 100 | 99.78 |
| 2 | 99.8 | 98.06 | 91 | |
| 4 | 70.4 | 38.5 | 16.18 | |
| 4-h infusion | 1 | 100 | 100 | 100 |
| 2 | 100 | 100 | 99.94 | |
| 4 | 73.2 | 63.18 | 49.48 | |
| Lower bound (CLCR = 30 ml/min) | ||||
| 1-h infusion | 1 | 100 | 100 | 100 |
| 2 | 100 | 99.96 | 99.84 | |
| 4 | 97.42 | 89.06 | 71.4 | |
| 4-h infusion | 1 | 100 | 100 | 100 |
| 2 | 100 | 100 | 100 | |
| 4 | 94.46 | 91.28 | 85.54 | |
| Severe impairment, 250 mg q12h | ||||
| Upper bound (CLCR = 29 ml/min) | ||||
| 1-h infusion | 1 | 100 | 99.96 | 99.44 |
| 2 | 99.7 | 96.5 | 86.98 | |
| 4 | 63.96 | 33.64 | 14.78 | |
| 4-h infusion | 1 | 100 | 100 | 100 |
| 2 | 100 | 99.98 | 99.86 | |
| 4 | 82.7 | 64.92 | 40.84 | |
| Lower bound (CLCR = 10 ml/min) | ||||
| 1-h infusion | 1 | 100 | 100 | 100 |
| 2 | 100 | 100 | 100 | |
| 4 | 99.9 | 99.38 | 97.46 | |
| 4-h infusion | 1 | 100 | 100 | 100 |
| 2 | 100 | 100 | 100 | |
| 4 | 99.78 | 99.54 | 98.66 | |
Abbreviations: CLCR, creatinine clearance; q8h, every 8 h; q12h, every 12 h.
Note that results are presented for MICs of 1 to 4 μg/ml because it is in this range of pathogen susceptibilities that the greatest amount of variation was seen in the target attainment rates.
Proportion of patients with higher than PK/PD target, % fT>MIC.
FIG. 2.
Target attainment results for doripenem at 500 mg every 8 h infused over 1 h and 4 h over a wide range of creatinine clearances observed in phase 1, 2, and 3 studies (○, 25% T>MIC; ▵, 30% T>MIC; +, 35% T>MIC). (A) One-hour infusion; (B) 4-hour infusion.
In patients with creatinine clearances greater than 50 ml/min, a 500-mg dose of doripenem infused over 1 h every 8 h would be expected to be effective for bacilli with MICs to doripenem of 1 μg/ml or less based on a T>MIC 35% target and up to 2 μg/ml with lower targets (Fig. 2). Given its potent in vitro activity against nosocomial pathogens (Tables 1 and 2)—the majority of pathogens had a MIC90 of ≤1 μg/ml—efficacy would be expected. Enterococcus faecalis, Acinetobacter baumannii, and Pseudomonas aeruginosa would require a longer infusion time or a higher dose to enhance target attainment (41). The longer, 4-hour infusion time improved target attainment over that with the 1-hour infusion time such that the T>MIC was adequate for pathogens with a MIC as high as 4 μg/ml.
When the conservative target (T>MIC 35%) is used, efficacy would be expected for infections caused by pathogens with higher MICs to doripenem (≤2 μg/ml) in patients with moderate renal impairment (creatinine clearance between 30 and 50 ml/min) who receive doripenem at 250 mg infused over 1 h every 8 h and in patients with severe impairment (creatinine clearance between 10 and 29 ml/min) who receive doripenem at 250 mg, infused over 1 h or 4 h, every 12 h. These doses yield exposures in renally impaired subjects comparable to those achieved in subjects with normal renal function or with mild renal impairment (6).
DISCUSSION
It is becoming more common in intensive care units to alter dosing strategy to maximize carbapenem PK/PD target attainment, by either increasing the drug dose, prolonging the infusion time, or both. Since inadequate initial antimicrobial coverage increases medical costs and leads to higher mortality (1), which is twice as high among patients infected with P. aeruginosa as among those infected with other pathogens (16, 20), it makes sense to maximize coverage at the initiation of treatment and to de-escalate therapy, when feasible, based on definitive identification of the infecting pathogen and determination of antimicrobial susceptibilities.
The impact of altering β-lactam dosing strategies for critically ill patients has been evaluated by several groups of investigators. For instance, Lodise et al. compared a prolonged 4-hour infusion of piperacillin-tazobactam at 3.75 g every 8 h with the usual dose of 3.375 g infused over 30 min every 4 or 6 h in 194 patients with pseudomonal infection (22). Among patients with an acute physiological and chronic health evaluation II score of ≥17, mortality was lower (12.2% versus 31.6% for shorter infusion; P = 0.04) and length of stay was shorter (21 versus 38 days for shorter infusion; P = 0.02) in those who received the prolonged infusion. The 4-hour infusion provided a T>MIC of 50% or greater for a MIC up to 16 μg/ml, whereas the 30-min infusion provided confident coverage when the MIC was no higher than 4 μg/ml. In a number of modeling exercises, Monte Carlo simulations showed that T>MIC was increased by extending the meropenem infusion time. T>MIC of ≥40% for a MIC of up to 8 μg/ml was predicted with a 2-g meropenem dose infused over 3 h every 8 h (18, 21). Theoretically, high doses and long infusions of meropenem or other modeled β-lactams should provide improved bacterial eradication (23, 36). However, meropenem has limited stability in solution: 4 h in normal saline and 1 h in 5% dextrose (5). Depending on how quickly a patient can receive the infusion solution, the opportunity for extended infusion of meropenem is limited. Imipenem is less conducive to “designer dosing,” as the dose is limited by its potential to cause seizure as well as its short period of stability in solution. In addition, imipenem is the least potent of the antipseudomonal carbapenems (imipenem, meropenem, and doripenem) (10, 38).
Because doripenem demonstrates high relative potency against Gram-negative pathogens, particularly P. aeruginosa (e.g., 7.3% of isolates had a MIC of ≥4 μg/ml in the TRUST 2008 database), long stability in infusion solutions, and no measurable concentration-dependent toxic effects such as seizures, it can be administered over varied infusion times (1 and 4 h) and in a 500-mg or 1-g dose. In our model, a 500-mg dose of doripenem infused over 4 h every 8 h would be expected to be effective for bacilli with MICs to doripenem of up to 4 μg/ml based on a T>MIC 35% target. Even higher target attainment is likely achieved with 4-hour infusions of 1-g doses. According to PD modeling by Van Wart et al., a 1-g dose of doripenem infused over 4 h would provide adequate coverage for pathogens with a MIC as high as 8 μg/ml (41). It is worth mentioning that the choice of doses is not solely based upon probability of target attainment but rather takes the safety of the patients into consideration (6). The 1-g dose regimen has been evaluated in over 300 subjects participating in phase 1 and phase 2 studies without observed dose-related adverse reactions (8, 29, 30, 40). Of course, the 1-g dose will be used with more confidence once the safety and efficacy data from recently completed phase 3 clinical studies are published (2-4).
In summary, the PK/PD findings as presented in this paper may facilitate customized dosing to optimize the PD behavior of doripenem.
Acknowledgments
We acknowledge Sandra Norris of Norris Communications Group for her writing and editorial assistance on the manuscript. We also thank Susan C. Nicholson and Behin Yektashenas of Ortho-McNeil-Janssen Scientific Affairs, LLC, for their assistance with the preparation of the manuscript.
Footnotes
Published ahead of print on 12 April 2010.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1.Alvarez-Lerma, F. 1996. Modification of empiric antibiotic treatment in patients with pneumonia acquired in the intensive care unit: ICU-Acquired Pneumonia Study Group. Intensive Care Med. 22:387-394. [DOI] [PubMed] [Google Scholar]
- 2.Anonymous. A study of the safety and effectiveness of doripenem compared with imipenem in the treatment of patients with ventilator-associated pneumonia. ClinicalTrials.gov identifier NCT00589693. http://www.clinicaltrials.gov/ct2/show/NCT00589693?term=doripenem&rank=1. Accessed 30 December 2009.
- 3.Anonymous. A safety and tolerability study of doripenem in patients with abdominal infections or pneumonia. ClinicalTrials.gov identifier NCT00515034. http://www.clinicaltrials.gov/ct2/show/NCT00515034?term=doripenem&rank=2. Accessed 30 December 2009.
- 4.Anonymous. An effectiveness, safety, and microbiology study of doripenem in patients with nosocomial (hospital-acquired) pneumonia. ClinicalTrials.gov identifier NCT00502801. http://www.clinicaltrials .gov/ct2/show/NCT00502801?term=doripenem&rank=8. Accessed 30 December 2009.
- 5.AstraZeneca Pharmaceuticals, L.P. 2008. Merrem (meropenem for injection). Prescribing information. AstraZeneca Pharmaceuticals, L.P., Wilmington, DE.
- 6.Bhavnani, S. M., J. P. Hammel, B. B. Cirincione, M. A. Wikler, and P. G. Ambrose. 2005. Use of pharmacokinetic-pharmacodynamic target attainment analyses to support phase 2 and 3 dosing strategies for doripenem. Antimicrob. Agents Chemother. 49:3944-3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chastre, J., R. Wunderink, P. Prokocimer, M. Lee, K. Kaniga, and I. Friedland. 2008. Efficacy and safety of intravenous infusion of doripenem versus imipenem in ventilator-associated pneumonia: a multicenter, randomized study. Crit. Care Med. 36:1089-1096. [DOI] [PubMed] [Google Scholar]
- 8.Cirillo, I., N. Vaccaro, R. Evans, R. Redman, and G. L. Kearns. 2009. Pharmacokinetics of doripenem in adult subjects with cystic fibrosis, abstr. A1-017. Abstr. 49th Intersci. Conf. Antimicrob. Agents Chemother.
- 9.Craig, W. A. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1-10. [DOI] [PubMed] [Google Scholar]
- 10.DeRyke, C. A., J. L. Kuti, and D. P. Nicolau. 2007. Pharmacodynamic target attainment of six beta-lactams and two fluoroquinolones against Pseudomonas aeruginosa, Acinetobacter baumannii, Escherichia coli, and Klebsiella species collected from United States intensive care units in 2004. Pharmacotherapy 27:333-342. [DOI] [PubMed] [Google Scholar]
- 11.Doern, G. V., S. S. Richter, A. Miller, N. Miller, C. Rice, K. Heilmann, and S. Beekmann. 2005. Antimicrobial resistance among Streptococcus pneumoniae in the United States: have we begun to turn the corner on resistance to certain antimicrobial classes? Clin. Infect. Dis. 41:139-148. [DOI] [PubMed] [Google Scholar]
- 12.Drusano, G. L. 2004. Antimicrobial pharmacodynamics: critical interactions of “bug and drug.” Nat. Rev. Microbiol. 2:289-300. [DOI] [PubMed] [Google Scholar]
- 13.European Medicines Agency. 2009. Summary of product characteristics: Doribax. European Medicines Agency, London, United Kingdom. http://www.emea.europa.eu/humandocs/Humans/EPAR/doribax/doribax.htm.
- 14.Felmingham, D., R. Cantón, and S. G. Jenkins. 2007. Regional trends in beta-lactam, macrolide, fluoroquinolone and telithromycin resistance among Streptococcus pneumoniae isolates 2001-2004. J. Infect. 55:111-118. [DOI] [PubMed] [Google Scholar]
- 15.Fritsche, T. R., H. S. Sader, and R. N. Jones. 2008. Antimicrobial activity of ceftobiprole, a novel anti-methicillin-resistant Staphylococcus aureus cephalosporin, tested against contemporary pathogens: results from the SENTRY Antimicrobial Surveillance Program (2005-2006). Diagn. Microbiol. Infect. Dis. 61:86-95. [DOI] [PubMed] [Google Scholar]
- 16.Ibrahim, E. H., G. Sherman, S. Ward, V. J. Fraser, and M. H. Kollef. 2000. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest 118:146-155. [DOI] [PubMed] [Google Scholar]
- 17.Jacobs, M. R., D. Felmingham, P. C. Appelbaum, R. N. Grüneberg, and The Alexander Project Group. 2003. The Alexander Project 1998-2000: susceptibility of pathogens isolated from community-acquired respiratory tract infection to commonly used antimicrobial agents. J. Antimicrob. Chemother. 52:229-246. [DOI] [PubMed] [Google Scholar]
- 18.Jaruratanasirikul, S., S. Sriwiriyajan, and J. Punyo. 2005. Comparison of the pharmacodynamics of meropenem in patients with ventilator-associated pneumonia following administration by 3-hour infusion or bolus injection. Antimicrob. Agents Chemother. 49:1337-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jenkins, S. G., S. D. Brown, and D. J. Farrell. 2008. Trends in antibacterial resistance among Streptococcus pneumoniae isolated in the U.S.A.: update from PROTEKT US Years 1-4. Ann. Clin. Microbiol. Antimicrob. 7:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kollef, M. H. 2000. Inadequate antimicrobial treatment: an important determinant of outcome for hospitalized patients. Clin. Infect. Dis. 31(Suppl. 4):S131-S138. [DOI] [PubMed] [Google Scholar]
- 21.Li, C., J. L. Kuti, C. H. Nightingale, and D. P. Nicolau. 2006. Population pharmacokinetic analysis and dosing regimen optimization of meropenem in adult patients. J. Clin. Pharmacol. 46:1171-1178. [DOI] [PubMed] [Google Scholar]
- 22.Lodise, T. P., Jr., B. Lomaestro, and G. L. Drusano. 2007. Piperacillin-tazobactam for Pseudomonas aeruginosa infection: clinical implications of an extended-infusion dosing strategy. Clin. Infect. Dis. 44:357-363. [DOI] [PubMed] [Google Scholar]
- 23.Lorente, L., L. Lorenzo, M. M. Martin, A. Jimenez, and M. L. Mora. 2006. Meropenem by continuous versus intermittent infusion in ventilator-associated pneumonia due to gram-negative bacilli. Ann. Pharmacother. 40:219-223. [DOI] [PubMed] [Google Scholar]
- 24.Lucasti, C., A. Jasovich, O. Umeh, J. Jiang, K. Kaniga, and I. Friedland. 2008. Efficacy and tolerability of IV doripenem versus meropenem in adults with complicated intra-abdominal infection: a phase III, prospective, multicenter, randomized, double-blind, noninferiority study. Clin. Ther. 30:868-883. [DOI] [PubMed] [Google Scholar]
- 25.Merck & Co., Inc. 2007. Primaxin IV (imipenem and cilastatin for injection). Prescribing information. Merck & Co., Inc., Whitehouse Station, NJ.
- 26.Mouton, J. W., and A. A. Vinks. 2007. Continuous infusion of beta-lactams. Curr. Opin. Crit. Care 13:598-606. [DOI] [PubMed] [Google Scholar]
- 27.Naber, K. G., L. Llorens, K. Kaniga, P. Kotey, D. Hedrich, and R. Redman. 2009. Intravenous doripenem 500 mg versus levofloxacin 250 mg with an option to switch to oral therapy for the treatment of complicated lower urinary tract infection and pyelonephritis. Antimicrob. Agents Chemother. 53:3782-3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nandy, P., M. Samtani, and R. Lin. 2010. Population pharmacokinetics of doripenem based on data from phase 1 studies with healthy volunteers and phase 2 and 3 studies with critically ill patients. Antimicrob. Agents Chemother. 54:2354-2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nicholson, S. C., J. Sambrowski, B. Yektashenas, and M. Ambruzs. 2008. Overcoming challenges associated with clinical trials of anti-infectives (AI) in subjects with ventilator-associated pneumonia (VAP): experience with doripenem 1gram q8h (abstract 473). Crit. Care Med. 36(Suppl.):A118. [Google Scholar]
- 30.Nicholson, S. C., J. Peterson, M. Ambruzs, B. Yektashenas, and J. Xiang. 2009. Doripenem 1 g infused for 4 hours (h) in the treatment of nosocomial pneumonia/ventilator associated pneumonia (NP/VAP) due to Acinetobacter baumannii (A. baumannii), abstr. 386. Abstr. 47th Annu. Meet. Infect. Dis. Soc. Am. (IDSA).
- 31.Nicolau, D. P. 2008. Pharmacodynamic optimization of beta-lactams in the patient care setting. Crit. Care 12(Suppl. 4):S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ortho-McNeil-Janssen Pharmaceutical, Inc. 2008. Doribax (doripenem for injection). Prescribing information. Ortho-McNeil-Janssen Pharmaceutical, Inc., Raritan, NJ.
- 33.Réa-Neto, A., M. Niederman, S. M. Lobo, E. Schroeder, M. Lee, K. Kaniga, N. Ketter, P. Prokocimer, and I. Friedland. 2008. Efficacy and safety of doripenem versus piperacillin/tazobactam in nosocomial pneumonia: a randomized, open-label, multicenter study. Curr. Med. Res. Opin. 24:2113-2126. [DOI] [PubMed] [Google Scholar]
- 34.Redman, R., and T. M. File. 2009. Safety of intravenous infusion of doripenem. Clin. Infect. Dis. 49(S1):S28-S35. [DOI] [PubMed] [Google Scholar]
- 35.Roberts, J. A., and J. Lipman. 2007. Optimizing use of beta-lactam antibiotics in the critically ill. Semin. Respir. Crit. Care Med. 28:579-585. [DOI] [PubMed] [Google Scholar]
- 36.Roberts, J. A., J. Lipman, S. Blot, and J. Rello. 2008. Better outcomes through continuous infusion of time-dependent antibiotics to critically ill patients? Curr. Opin. Crit. Care 14:390-396. [DOI] [PubMed] [Google Scholar]
- 37.Sahm, D. F., N. P. Brown, D. C. Draghi, A. T. Evangelista, Y. C. Yee, and C. Thornsberry. 2008. Tracking resistance among bacterial respiratory tract pathogens: summary of findings of the TRUST Surveillance Initiative, 2001-2005. Postgrad. Med. 120(Suppl. 1):8-15. [DOI] [PubMed] [Google Scholar]
- 38.Sahm, D. 2009. In vitro activity of doripenem. Clin. Infect. Dis. 49(S1):S11-S16. [DOI] [PubMed] [Google Scholar]
- 39.Talbot, G. H., J. Bradley, J. E. Edwards, Jr., D. Gilbert, M. Scheld, J. G. Bartlett, and Antimicrobial Availability Task Force of the Infectious Diseases Society of America. 2006. Bad bugs need drugs: an update on the development pipeline from the Antimicrobial Availability Task Force of the Infectious Diseases Society of America. Clin. Infect. Dis. 42:657-668. [DOI] [PubMed] [Google Scholar]
- 40.Vaccaro, N., O. Umeh, R. Redman, and I. Cirillo. 2009. Pharmacokinetics of doripenem 1 g administered over 4 hours in patients with ventilator-associated pneumonia, abstr. A1-018. Abstr. 49th Intersci. Conf. Antimicrob. Agents Chemother.
- 41.Van Wart, S. A., D. R. Andes, P. G. Ambrose, and S. M. Bhavnani. 2009. Pharmacokinetic-pharmacodynamic modeling to support doripenem dose regimen optimization for critically ill patients. Diagn. Microbiol. Infect. Dis. 63:409-414. [DOI] [PubMed] [Google Scholar]
- 42.Vinks, A. A. 2002. The application of population pharmacokinetic modeling to individualized antibiotic therapy. Int. J. Antimicrob. Agents 19:313-322. [DOI] [PubMed] [Google Scholar]
- 43.Zhanel, G. G., N. Ketter, E. Rubinstein, I. Friedland, and R. Redman. 2009. Overview of seizure-inducing potential of doripenem. Drug Saf. 32:709-716. [DOI] [PubMed] [Google Scholar]