Abstract
The present study determined the pharmacokinetic profile of vancomycin in premature Malaysian infants. A one-compartment infusion model with first-order elimination was fitted to serum vancomycin concentration data (n = 835 points) obtained retrospectively from the drug monitoring records of 116 premature newborn infants. Vancomycin concentrations were estimated by a fluorescence polarization immunoassay. Population and individual estimates of clearance and distribution volume and the factors which affected the variability observed for the values of these parameters were obtained using a population pharmacokinetic modeling approach. The predictive performance of the population model was evaluated by visual inspections of diagnostic plots and nonparametric bootstrapping with replacement. Dosing guidelines targeting a value of ≥400 for the area under the concentration-time curve over 24 h in the steady state divided by the MIC (AUC24/MIC ratio) were explored using Monte Carlo simulation. Body size (weight), postmenstrual age, and small-for-gestational-age status are important factors explaining the between-subject variability of vancomycin pharmacokinetic parameter values for premature neonates. The typical population parameter estimates of clearance and distribution volume for a 1-kg premature appropriate-for-gestational-age neonate with a postmenstrual age of 30 weeks were 0.0426 liters/h and 0.523 liters, respectively. There was a 20% reduction in clearance for small-for-gestational-age infants compared to the level for the appropriate-for-gestational-age control. Dosage regimens based on a priori target response values were formulated. In conclusion, the pharmacokinetic parameter values for vancomycin in premature Malaysian neonates were estimated. Improved dosage regimens based on a priori target response values were formulated by incorporating body size, postmenstrual age, and small-for-gestational-age status, using Monte Carlo simulations with the model-estimated pharmacokinetic parameter values.
Bacterial sepsis is a major cause of neonatal complications, prolonged hospital stay, and death in premature newborns, especially those in developing countries. Sepsis-related mortality and morbidity rates were even higher in extremely preterm neonates and intrauterine-growth-restricted infants because of their innate immunological immaturity (36). Because coagulase-negative staphylococcus (CoNS) is a common pathogen for late-onset (72-h-postbirth) septicemia, and because methicillin-resistant Staphylococcus aureus (MRSA) is an important pathogen (10), vancomycin continues to be widely prescribed in neonatal intensive care units.
Kidney and vestibular/cochlear damage is a concern associated with vancomycin use. Very recently, it was shown that vancomycin trough concentrations were correlated with nephrotoxicity in hospitalized adult patients (27). Nonetheless, vancomycin-associated ototoxicity and nephrotoxicity among neonates are thought to be less frequent than those in adults (11), although more evidence-based investigations using appropriately designed and powered studies are needed. Until such data are forthcoming, it is prudent to avoid exposure to unnecessarily high peak or trough concentrations of vancomycin in the premature newborn.
There remain conflicting recommendations regarding the therapeutic range and monitoring practices that apply to vancomycin pharmacotherapy (20, 34, 37). Traditionally, for intermittent administration, various permutations of dose and dose frequency are often faithfully applied in order to obtain trough and peak plasma concentrations of 5 to 10 mg/liter and 20 to 50 mg/liter, respectively (37). Others have suggested that plasma trough concentrations of 10 to 15 mg/liter (23) or 15 to 20 mg/liter (18) may be suitable with respect to toxicity and efficacy against MRSA. One recent study (24) failed to demonstrate enhanced vancomycin efficacy in patients with MRSA infections when there were elevated plasma trough concentrations of 15 to 20 mg/liter. Other studies found vancomycin to be more efficacious against invasive MRSA when the ratio of the area under the concentration-time curve over 24 h (AUC24) to the MIC was at least 400 h (AUC24/MIC ratio of ≥400) (16, 28, 34).
There are no reports correlating vancomycin bactericidal effect and systemic exposure to vancomycin in neonatal patients. All information regarding the drug concentrations and efficacy response is, therefore, based on data derived from adult populations and extrapolated to the pediatric/neonatal population.
Malaysian infants are generally born smaller than their Caucasian counterparts. So far, there have been no data on the pharmacokinetics of vancomycin in very premature Malaysian neonates, despite the widespread use of this antibiotic in that country. Dosing practices have relied solely on literature data from Caucasian infants. Besides elucidating any alteration in pharmacokinetic parameter values, it was important to obtain estimates of the variability in this population since high interindividual pharmacokinetic variability is a feature of antimicrobial pharmacotherapeutics in premature infants (33). Accordingly, the aim of present study was to conduct a population pharmacokinetic analysis in order to obtain pharmacokinetic data for vancomycin in premature Malaysian infants. These findings were then applied using simulation to explore possibilities for improved vancomycin dosage regimens.
(Part of this work was presented at the PAGANZ [Population Approach Group of Australia and New Zealand] Scientific Meeting in February 2010.)
MATERIALS AND METHODS
Ethics and patient characteristics.
The study protocol was approved by the Medical Ethics Committee of the University of Malaya Medical Centre. Data were collected retrospectively from the records of the Special Care Nursery, (SCN), University of Malaya Medical Centre, Kuala Lumpur, Malaysia, from 1999 to 2005. Data on gestational age (GA), postmenstrual age (PMA), postnatal age (PNA), birth weight (BW), current weight (WT), Apgar scores (APG), and other concurrent treatment (CT) as well as laboratory clinical data were extracted from medical records. Infants having GAs of less than 32 weeks and PMAs of less than 36 weeks and having each had at least 2 vancomycin serum determinations performed were considered for inclusion in the study. An infant was considered to be small for gestational age (SGA) if the BW was below the 10th percentile of a specific weight threshold for a specific GA according to the fetal growth weight standard (29) for Singapore infants, since no Malaysian data on body weight for neonates born before the 28-week gestation were available.
Vancomycin dosing, blood sampling, and measurement.
A vancomycin hydrochloride concentration (10 mg/ml) in normal saline for intravenous administration was reconstituted in the hospital pharmacy and infused at a constant rate over 60 min via a programmed syringe driver. Blood samples were collected by capillary heel pricking into clotting tubes and the sera isolated by centrifugation. Serum vancomycin concentrations were determined by a fluorescence polarization immunoassay method using the Cobas Integra 800 system from Roche Diagnostics, Switzerland. The lower detection limit of this assay was 1.39 mg/liter, and the between-day imprecision values (coefficients of variation [CV%]) were 3.0% at 8.70 mg/liter and 3.3% at 54.6 mg/liter.
Population pharmacokinetic analysis.
The pharmacokinetics from the concentration-time data were modeled by first-order conditional estimation with interaction (FOCE-I), using NONMEM version 6.10 (5). An overview of population modeling as implemented in NONMEM is described elsewhere (14). An initial analysis was performed to estimate the parameter values for a base model (i.e., no added covariates), and the influence of covariates was assessed by adding these values to the base model in turn. The likelihood ratio test was used to statistically assess the influence of covariates in improving the fit of a given model to the data; a change in the objective function value (OFV), which is generated in the NONMEM output, was noted for comparison of goodness-of-fit values among competing nested covariate models. The difference between a pair of OFV values observed when a covariate was included and then excluded was tested for statistical significance (P = 0.01). The difference between OFV values approximates the chi-square statistic with 1 degree of freedom (χ21, 0.01 = 6.6).
The between-subject variability (BSV) was modeled assuming a log-normal distribution, using the equation Pjk = PPOP·exp(ηj + ηk), where Pjk represents the true but unknown value of a parameter, e.g., clearance (CL) or volume of distribution (V), for the jth subject on the kth occasion, where PPOP is the population mean value for a parameter, where ηj is a normally distributed random variable having a variance of ω12 and mean of 0, representing the difference between a parameter value in the jth subject and PPOP, and where ηk is a random variable used to represent the variability of a given pharmacokinetic parameter value on different occasions (BOV), an occasion being defined a priori as a dose or sequence of doses followed by at least 1 observation. The BOV was assumed to be sampled from a normal distribution having a mean of 0 and a variance of ω22. In modeling the BOV, it was assumed that the variances of each parameter were sampled from the same distribution. In this study, any sample taken after an interval of 3 days was considered representative of a different occasion, as concentration monitoring of vancomycin typically occurred 3 days after initiation or change of dose.
The residual unexplained variability (RUV) among observed vancomycin concentrations and those predicted by the final population model were estimated by a combined proportional-additive error model, using the equation Cij = Cpred,ij·(1 + ɛ1,ij) + ɛ2,ij, where Cij is the ith observed concentration in the jth subject, Cpred,ij is the serum concentration predicted by the pharmacokinetic model, and ɛ1,ij and ɛ2,ij are randomly distributed variables having mean values of 0 and variances of σ12 and σ22, respectively.
Model evaluation.
The performance of the final covariate model was evaluated by visual inspection of diagnostic scatter plots. The robustness of the model was assessed using a nonparametric bootstrap, with replacement, of 1,000 NONMEM runs of the final model; the bootstrap median parameter values and the percentile bootstrap 95% intervals were compared with the respective values estimated from the final model.
Dosing regimen simulations.
Serum vancomycin concentration-time data sets for different dosing regimens were generated for 100 virtual infants with characteristics similar to those observed for the study patients by use of Monte Carlo simulation in conjunction with MASS (39) in R (version 2.8.1) (31). The final model was then used to produce 1,000 simulations of the serum concentration-time data for each patient's data set. In view of the efficacies and toxicities in relation to concentrations of vancomycin in serum, the criteria for recommended dosing regimens were as follows: >80% of the predicted AUC24/MIC ratios at ≥400, predicted trough concentrations from 5 to 20 mg/liter, and peak concentrations of ≤50 mg/liter. AUC24 was calculated by dividing a 24-h dose by CL estimated by NONMEM from the simulation. The MIC for MRSA was set at 1 mg/liter, according to the Malaysian hospital's institutional bacteriograms.
RESULTS
Patient characteristics.
Data comprising 835 serum vancomycin concentrations that were available for pharmacokinetic analysis were obtained from 116 premature neonates, comprising 105 single births, 4 twin births, and 1 triplet birth. More than half (58%) of these neonates had gestational ages of 28 weeks or less. Among these neonates, 56% were Malays, 25% Chinese, and 18% Indian and 2% had other racial backgrounds, but all patients were of Asian heritage. The baseline characteristics of the study subjects are summarized in Table 1.
TABLE 1.
Baseline characteristics of all neonatesa
| Demographic | Mean ± SD (range) | Total no. of neonates (%) |
|---|---|---|
| Gestational age (wk) | ||
| <24 | 23.0 ± 0 | 2 (1.7) |
| 24-28 | 26.4 ± 1.3 | 66 (56.9) |
| 29-31 | 29.7 ± 0.85 | 48 (41.4) |
| Postmenstrual age (wk) | ||
| <24 | 23.6 ± 0.4 | 2 (1.7) |
| 24-28 | 26.3 ± 1.1 | 39 (33.6) |
| >28-32 | 29.8 ± 1.1 | 70 (60.3) |
| >32-34 | 32.7 ± 0.7 | 5 (4.3) |
| Postnatal age (days) | ||
| 1-7 | 4.3 ± 1.6 | 86 (74.1) |
| 8-14 | 10.3 ± 1.8 | 21 (18.1) |
| 15-21 | 16.5 ± 1.8 | 6 (5.2) |
| 22-28 | 25.0 ± 2.8 | 2 (1.7) |
| 29-32 | 32 | 1 (0.9) |
| Birth wt (kg) | ||
| <0.5 | 0.42 | 1 (0.9) |
| 0.5-0.75 | 0.67 ± 0.06 | 19 (16.4) |
| >0.75-1.0 | 0.87 ± 0.07 | 51 (44.0) |
| >1.0-1.5 | 1.19 ± 0.13 | 42 (36.2) |
| >1.5-2 | 1.81 ± 0.17 | 3 (2.6) |
| Study wt (kg) | ||
| 0.5-0.75 | 0.67 ± 0.09 | 25 (21.6) |
| >0.75-1.0 | 0.91 ± 0.1 | 55 (47.4) |
| >1.0-1.5 | 1.20 ± 0.13 | 33 (28.4) |
| >1.5-2 | 1.77 ± 0.23 | 3 (2.6) |
| Serum creatinine concn (mmol/liter) | 0.076 ± 0.02 (0.031-0.143) | |
| Apgar score at 1 min | 5.0 ± 2.0 (1-9) | |
| Apgar score at 5 min | 7.4 ± 1.9 (2-10) |
n = 116 (66 males; 40% small-for-gestational-age neonates).
Population pharmacokinetics.
Both one- and two-compartment models were fitted to the data. The two-compartment model showed minimum advantages over the one-compartment model. Furthermore, large standard errors of the parameter estimates for the peripheral distribution volume and intercompartmental clearance revealed overparameterization (results not shown). Therefore, the pharmacokinetics of vancomycin was analyzed using a one-compartment open model with zero-order dosing (1 h intravenous infusion) and first-order elimination. Vancomycin concentrations versus sampling time after the last dose are presented in Fig. 1. A high correlation was found between PMA and WT (r = 0.64), and there was a negative correlation between PMA and SCR (r = −0.51), while separate correlations between SGA status and both WT and PMA were less pronounced (r = 0.34). In all covariate models, both CL and V were allometrically scaled to the size standard of a 70-kg adult to enable direct comparison with published adult values. Allometric exponents of 0.75 and 1 were assumed for CL and V, respectively (22), to uncouple correlations between size and values for developmental parameters, such as PMA, WT, and GA. The structural population model which best described the data comprised the equations CL = 1.0·(WT/70)0.75·(PMA/30)3.16·[0.83·SGA + 1.03·(1 − SGA)] and V = 36.6·WT/70, where CL is clearance (liters/h), V is the apparent volume of distribution (liters), PMA is the postmenstrual age (weeks), and SGA is 1 for small-for-gestational-age infants and 0 for appropriate-for-gestational-age infants.
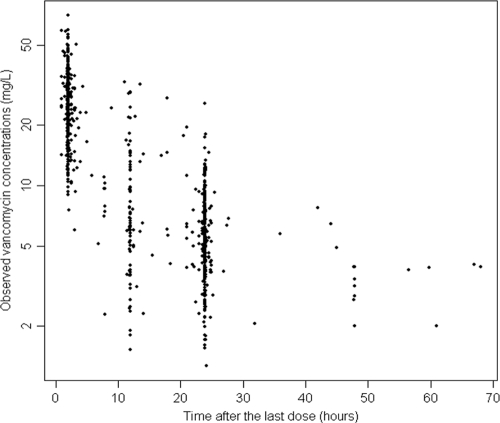
FIG. 1.
Scatter plot of vancomycin concentration versus time after the last dose for all study subjects.
The BSV values for both CL and V in the final model were less than half those estimated using the base model, while the proportional component of the RUV was reduced by more than a third, indicating that the final model accounted for much of the variability in the data (Table 2).
TABLE 2.
Variance changes related to the model-building processa
| Model | Covariate(s) | ΔOFV | BSVCL | BSVV | RUVσprop | RUVσadd |
|---|---|---|---|---|---|---|
| Base | None | 0 | 43.6 | 30.5 | 36.3 | 1.41 |
| 1 | Size on CL | −157 | 30.3 | 28.9 | 32.6 | 1.57 |
| 2 | PMA on CL | −276 | 33.3 | 28.8 | 27.5 | 1.82 |
| 3 | SCR on CL | −227 | 36.9 | 29.4 | 27.4 | 2.09 |
| 4 | PNA on CL | −204 | 38.5 | 31.8 | 29.3 | 1.74 |
| 5 | Ventilation on CL | −56 | 40.9 | 31.2 | 35.9 | 1.67 |
| 6 | SGA on CL | 0 | 43.4 | 30.5 | 36.3 | 1.41 |
| 7 | NSAID on CL | 0 | 43.5 | 31.2 | 36.3 | 1.99 |
| 8 | Race on CL | −5 | 44.4 | 30.3 | 36.2 | 1.45 |
| 9 | Size on V | −12 | 45.3 | 6.4 | 31.8 | 2.32 |
| 10 | Size and PMA on CL; size on V | −449 | 23.3 | 12.0 | 23.0 | 1.52 |
| 12 | Size, PMA, and race on CL; size on V | −458 | 23.1 | 12.5 | 23.0 | 1.52 |
| 13 | Size, PMA, and SCR on CL; size on V | −426 | 23.1 | 10.8 | 22.9 | 2.18 |
| Final | Size, PMA, and SGA on CL; size on V | −519 | 20.5 | 12.6 | 22.9 | 1.51 |
ΔOFV, change in objective function values; BSV, between-subject variability; RUVσprop, unexplained residual variability (proportional); RUVσadd, unexplained residual variability (additive); SCR, serum creatinine concentration.
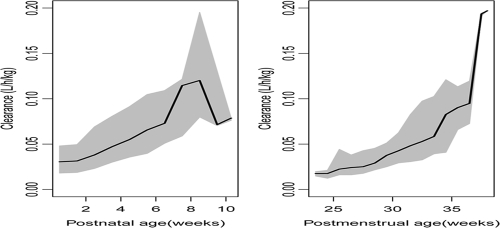
The typical population parameter estimates of CL and V for a 1-kg premature neonate with a PMA of 30 weeks were 0.0426 liters/h and 0.523 liters, respectively. The estimated population vancomycin elimination half-life was 8.5 h. There was a 20% reduction in CL for SGA infants, compared to the level for AGA infants. The BOV of CL was 16.7%; however, an estimate of the BOV of V could not be supported by the data. The correlation coefficient (r = 0.6) between CL and V was estimated from the values of the off-diagonal elements of the covariance-variance matrix, thereby justifying the simultaneous estimation of covariance and variances during the development of the variability model. The estimated clearance values in relation to the postmenstrual ages and postnatal ages of the study subjects are displayed in Fig. 2.
FIG. 2.
Estimated clearance in relation to the PMA and PNA stratified into weeks for study subjects. The solid lines are the median clearance values, while the shaded areas represent the 95th-percentile intervals.
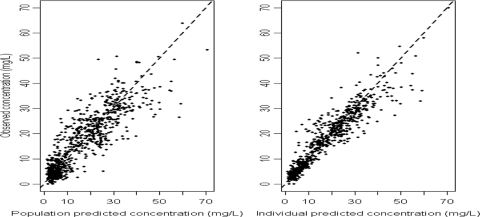
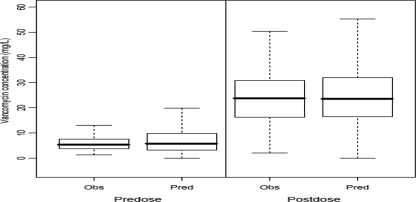
The plots of both the observed-versus-pre-Bayesian-estimation (population-predicted) values and the observed-versus-post-Bayesian-estimation (individual-predicted) values showed that the data points were distributed closely and symmetrically to the line of identity for the final model (Fig. 3). Box plots depicting the distributions of the predose and postdose concentrations of observation and predicted concentrations from 1,000 simulations derived from the final model are presented in Fig. 4. The median values for both the predose and the postdose concentrations from the observed data and the prediction as well as the interquartile range were similar, indicating the acceptable predictive capability of the final model.
FIG. 3.
Diagnostic scatter plots of observations versus pre-Bayesian estimations and observations versus post-Bayesian estimations for the final model. The broken lines represent the line of identity. Regression y = 0.8242x + 2.816, R2 = 0.77, imprecision = 5.97 (95% confidence interval [CI], 5.51 to 6.42) mg/liter, and bias = 0.03 (95% CI, −0.17 to 0.24) mg/liter for pre-Bayesian estimation, while regression y = 1.0915x − 0.0042, R2 = 0.89, imprecision = 4.13 (95% CI, 3.75 to 4.51) mg/liter, and bias = −0.30 (95% CI, −0.16 to −0.44) mg/liter for post-Bayesian estimation.
FIG. 4.
Box plots of the distributions of observed (Obs) and predicted (Pred) predose and postdose concentrations from 1,000 simulations from the final population model. The bold horizontal bars in the middle show the median values of vancomycin concentrations, while the outer boundaries of the boxes represent the ranges of the 25th and the 75th percentiles (interquartile ranges). The whiskers indicate the maximum and the minimum values of the concentrations. Outliers are not shown in these plots.
Pharmacokinetic parameter estimates for the final model and for the 1,000 bootstrap runs are presented in Table 3. For each parameter used, the median value from the bootstrapping runs was very close to the population median obtained using all the data and lay within the percentile bootstrap 95% intervals, indicating that the final model was stable and robust when the model was fitted to various combinations of concentration-time data sets.
TABLE 3.
Estimated population pharmacokinetic parameter values for the final model and for 1,000 bootstrap runs
| Parametera | Median value for final model | Value (2.5th-97.5th percentile) for 1,000 bootstrap runs |
|---|---|---|
| Structural model parameters | ||
| CLpop (liters h−1) | 1.00b | 0.982 (0.474-1.130)b |
| Vpop (liters) | 36.6b | 36.6 (34.9-38.3)b |
| Scaling factor of PMA on CL | 3.16 | 3.14 (2.52-3.77) |
| Scaling factor of SGA on CL | 0.83 | 0.84 (0.74-1.85) |
| Scaling factor of AGA on CL | 1.03 | 1.04 (0.91-2.23) |
| Variance model parameters | ||
| ωCL | 20.5 | 20.2 (16.4-23.9) |
| ωV | 12.6 | 12.3 (6.0-17.2) |
| ωBOV | 16.7 | 16.3 (11.2-20.8) |
| σproportional | 22.9 | 23.0 (19.7-26.3) |
| σadditive (mg/liter) | 1.51 | 1.50 (0.89-1.78) |
CLpop, population value for clearance; Vpop, population estimate for volume of distribution; PMA, postmenstrual age; SGA, small for gestational age; AGA, appropriate for gestational age; BOV, between-occasion variability of CL; ω, coefficient of variation (CV%); σ, unexplained residual variability (CV%).
These parameter values were standardized to the size of a 70-kg adult with allometric exponents of 0.75 and 1 for clearance and volume of distribution, respectively.
Dosage regimen simulation.
Data sets were simulated for 100 virtual infants with the following values (means ± standard deviations [SD]): a weight of 1 ± 0.84 kg, a PMA of 30 ± 2.45 weeks, a correlation of WT and PMA of 0.64, and a probability of SGA status of 0.4. A loading dose of 15 mg/kg of body weight was administered if the maintenance doses were less than 15 mg per dose. As illustrated in Table 4, when the recommended dosage regimen for AGA infants was applied to SGA infants, more than 30% of these infants exhibited trough concentrations of >20 mg/liter. Likewise, when AGA infants were to receive the recommended dosage for SGA infants, about 20% of them failed to achieve a trough level of ≥5 mg/liter, and fewer than 75% of them attained the target AUC24/MIC ratio. The final dosing recommendations for intermittent vancomycin intravenous administration are presented in Table 5.
TABLE 4.
Exploration of vancomycin dosing recommendation from 1,000 simulations for each patient data set and corresponding concentrations and AUC24/MIC profilea
| Recommended dosage (mg/kg) | % of simulated concnb |
Median AUCc (2.5th-97.5th percentile) | % with AUC24/MIC ratio of >400 | |||
|---|---|---|---|---|---|---|
| Trough |
Peak |
|||||
| <5 | >20 | <10 | >50 | |||
| 15 q24h | 38.9 | 2.3 | 0.02 | 7.0 | 377 (213-704) | 47.0 |
| 20 q24h | 27.2 | 7.4 | 0.005 | 26.4 | 505 (272-9700) | 77.1 |
| Dosage for AGA neonates | 5.2 | 9.3 | 0.02 | 7.7 | 565 (314-1224) | 91.1 |
| Dosage for AGA neonates applied to SGA infants | 0.8 | 32 | 0.01 | 14.8 | 673 (412-1355) | 98.1 |
| Dosage for SGA neonates | 7.0 | 8.3 | 0.01 | 5.8 | 676 (360-1355) | 94.1 |
| Dosage for SGA neonates applied to AGA infants | 18.6 | 2.1 | 0.1 | 2.0 | 505 (282-1079) | 73.9 |
A loading dose of 15 mg/kg was given for maintenance doses of less than 15 mg per dose. q24h, every 24 h.
Unit of concentration in mg/liter.
Unit of AUC in mg·h/liter.
TABLE 5.
Recommended dose regimens for intermittent intravenous infusion of vancomycin for premature neonates
| Postmenstrual age (wk) | Recommended dose (mg/kg) for neonatesa |
|
|---|---|---|
| AGA | SGA | |
| <26 | 12.5 q24h | 10 q24h |
| 26 to <27 | 15 q24h | 12.5 q24h |
| 27 to <28 | 15 q24h | 12.5 q24h |
| 28 to <29 | 10 q12h | 15 q24h |
| 29 to <30 | 10 q12h | 15 q24h |
| 30 to <31 | 12.5 q12h | 10 q12h |
| 31 to <32 | 12.5 q12h | 10 q12h |
| 32 to <33 | 15 q12h | 10 q12h |
| 33 to <34 | 15 q12h | 12.5 q12h |
| 34 to <37 | 20 q12h | 15 q12h |
q24h, every 24 h; q12h, every 12 h.
DISCUSSION
This study presented some new findings with respect to the pharmacokinetic disposition of vancomycin in premature Malaysian infants within the first month of life, based on retrospective drug monitoring data. Both SGA status and PMA were shown to influence the CL of vancomycin, independent of size, by scaling the infants' weight to that of a 70-kg standard adult subject during model development. Allometric scaling, based on fractal geometry theory, has received increased acceptance as a convenient size standard in pharmacokinetics (22). Exponents of 0.75 and 1 for the allometric scaling of weight on clearance and volume of distribution, respectively, were assumed instead of being estimated, because one cannot reliably estimate exponents of the allometric model unless there is a very large weight range (4).
Both one- and two-compartment models have previously been used to describe vancomycin pharmacokinetics in neonates (3, 6, 12). The data in the current study did not support precise estimation of parameter values in a two-compartment model. The volume of distribution of vancomycin estimated in this study is comparable to that previously reported, while the clearance was lower than that estimated to occur in the Caucasian patients (3, 6, 12). Nonetheless, a degree of caution must be exercised when the parameter values obtained from various study groups are compared, as different models were used in parameter value estimation. A more reliable way of comparing these estimated parameter values might be to pool the data and analyze them simultaneously.
Decreased vancomycin clearance levels in small-for-gestational-age Caucasian infants were reported in two recent studies (2, 15). Compared with the proportions used in these two earlier studies, there was a markedly increased proportion of SGA infants enrolled in this study, which may partly explain the differences in the findings. Frattarelli et al. (15) ignored the influence of other potential covariates, such as weight and PMA, in assessing the impact of SGA status on vancomycin clearance. Notwithstanding the slightly different standards used for defining SGA status (13, 29, 38), genetic differences, cultural factors, maternal nutritional statuses, and perinatal infection rates in Malaysian facilities may account for the larger proportion of SGA infants enrolled in the present study.
Vancomycin is eliminated chiefly by glomerular filtration. Tubular damage arising from chronic fetal hypoxia in intrauterine-growth-retardation-complicated pregnancies (30), reduced glomerular filtration rate, and impaired sodium conservation in early postnatal life in SGA premature neonates (32) would markedly reduce the clearance. Moreover, a significant reduction in the number of functional nephrons in intrauterine-growth-retardation-affected neonates (21, 35) led us initially to predict that elevated serum creatinine concentration could be correlated with reduced vancomycin CL. Indeed, several previous studies reported that serum creatinine concentration was a significant predictor of the elimination of this antibiotic in the newborn (3, 6, 25). Although serum creatinine concentration was presently a predictor of CL when screened alone, its addition to the final model resulted in a markedly inferior fit suggesting overparameterization of the structural model. Clearly, the relationship among these variables with respect to vancomycin CL in the developmental physiology of premature infants is complex. For example, it is known that from several days to weeks after birth, there are elevated creatinine concentrations inappropriate to age and muscle mass in very preterm neonates. While placental transfer of maternal creatinine may contribute over the first day or two of life, a complex pattern of reabsorption and secretion of creatinine along the leaky immature renal tubules and vasculature during this period offers a more likely explanation (19), especially since 80% of the observations were provided by infants during the first month of life. A marked nonlinear increase in CL as a function of increasing PMA was clearly demonstrated (Fig. 2), reflecting the rapid functional development of eliminating organs observed when the infants were approaching full-term gestation.
Protein binding may hinder distribution of antimicrobial agents in tissues and hamper delivery of drug molecules to the site of action. Vancomycin is 25 to 35% protein bound, mainly to albumin and IgA. An in vitro study indicated that high albumin concentrations inhibit vancomycin bactericidal activity (17), but the clinical impact of changing plasma protein binding has not been demonstrated for adult patients (1). In the present study, total (bound-plus-unbound) concentrations were measured. The free fraction is constant from 2 to 80 mg/liter, which encompasses all concentrations in our data (9). Thus, dosage adjustment for attainment of targets on the basis of monitored total levels is valid and independent of protein binding.
In linking the pharmacodynamics of vancomycin killing and antibiotic systemic exposure, a target AUC24/MIC index of at least 400 was proposed for the treatment of invasive MRSA on the basis of the work of Moise-Broder et al. (28) and Frymoyer et al. (16) and was reinforced in a very recent consensus paper on vancomycin practice guidelines (34). Therefore, it was prudent to consider an AUC24/MIC target in addition to the traditional targeted peak (postinfusion) and trough (preinfusion) concentrations for any vancomycin dosage recommendation in order to minimize treatment failure, even though this dosing approach has been validated neither for adults nor for neonates. The ratio will probably need to be greater and would differ for babies with various degrees of prematurity, but this was beyond the scope of the present study.
The most prevalent pathogen in our SCN is methicillin-resistant Staphylococcus epidermidis (MRSE). Meropenem is often coadministered with vancomycin, and synergism between carbapenems and vancomycin has been documented (26). As such, the regimens developed from the population pharmacokinetic data in this study were predicated on an MIC of 1 mg/liter. The emergence of Staphylococcus warneri (7), which has reduced glycopeptide susceptibility (MIC > 2 mg/liter), may warrant proportionately higher vancomycin doses for treating nosocomial infections caused by this organism in neonatal intensive care units. The results also indicated that a lower maintenance dose is required for infants who are small for their gestational ages than for appropriate-for-gestational-age infants in order to maintain serum trough concentrations between 5 mg/liter and 20 mg/liter and an AUC24/MIC target greater than 400.
From a pharmacokinetic viewpoint, vancomycin serum concentration monitoring is still recommended for these infants, especially since we established from the variability model that the day-to-day variability (i.e., BOV) in CL was less than the variability between infants (i.e., BSV), which facilitates the adjustment of maintenance dosages on the basis of monitoring data; however, this would not be the case if the BOV were markedly greater than the BSV (8).
Conclusion.
The population pharmacokinetics of vancomycin have been described for premature Malaysian infants. Weight and postmenstrual age were important contributing factors for explaining variability in clearance, while weight alone was the most significant predictor for volume of distribution. Infants who were small for their gestational ages exhibited a 20% reduction in vancomycin clearance, compared to the level for appropriate-for-gestational-age infants. Dosing regimens incorporating weight, PMA, and SGA status to target an AUC24/MIC ratio of >400 are likely to improve clinical outcome, supported by routine serum concentration monitoring.
Acknowledgments
This study was supported by IRPA grant 06-02-03-0246-EA246 from the Ministry of Science, Technology and Innovation of Malaysia.
We thank the medical and nursing staffs of the Special Care Nursery, the Pharmacy staff, and the Clinical Diagnostics Laboratory staff for their kind cooperation.
We declare that we have no competing interests.
Footnotes
Published ahead of print on 12 April 2010.
REFERENCES
- 1.Albrecht, L., M. Rybak, L. Warbasse, and D. Edwards. 1991. Vancomycin protein binding in patients with infections caused by Staphylococcus aureus. Ann. Pharmacother. 25:713-715. [DOI] [PubMed] [Google Scholar]
- 2.Allegaert, K., B. J. Anderson, J. N. van den Anker, S. Vanhaesebrouck, and F. de Zegher. 2007. Renal drug clearance in preterm neonates: relation to prenatal growth. Ther. Drug Monit. 29:284-291. [DOI] [PubMed] [Google Scholar]
- 3.Anderson, B. J., K. Allegaert, J. N. Van den Anker, V. Cossey, and N. H. Holford. 2007. Vancomycin pharmacokinetics in preterm neonates and the prediction of adult clearance. Br. J. Clin. Pharmacol. 63:75-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson, B. J., and N. H. Holford. 2008. Mechanism-based concepts of size and maturity in pharmacokinetics. Annu. Rev. Pharmacol. Toxicol. 48:303-332. [DOI] [PubMed] [Google Scholar]
- 5.Beal, S. L., L. B. Sheiner, and A. Boeckmann (ed.). 2006. NONMEM user's guide (1989-2006). Icon Development Solutions, Ellicott City, MD.
- 6.Capparelli, E. V., J. R. Lane, G. L. Romanowski, E. J. McFeely, W. Murray, P. Sousa, C. Kildoo, and J. D. Connor. 2001. The influences of renal function and maturation on vancomycin elimination in newborns and infants. J. Clin. Pharmacol. 41:927-934. [DOI] [PubMed] [Google Scholar]
- 7.Center, K. J., A. C. Reboli, R. Hubler, G. L. Rodgers, and S. S. Long. 2003. Decreased vancomycin susceptibility of coagulase-negative staphylococci in a neonatal intensive care unit: evidence of spread of Staphylococcus warneri. J. Clin. Microbiol. 41:4660-4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charles, B. G. P., S. R. B. Townsend, P. A. F. Steer, V. J. R. M. Flenady, P. H. F. Gray, and A. M. Shearman. 2008. Caffeine citrate treatment for extremely premature infants with apnea: population pharmacokinetics, absolute bioavailability, and implications for therapeutic drug monitoring. Ther. Drug Monit. 30:709-716. [DOI] [PubMed] [Google Scholar]
- 9.Chen, Y., R. L. G. Norris, J. J. Schneider, and P. J. Ravenscroft. 1992. The influence of vancomycin concentration and the pH of plasma on vancomycin protein binding. J. Pharmacol. Toxicol. Methods 28:57-60. [DOI] [PubMed] [Google Scholar]
- 10.Couto, R. C., E. A. A. Carvalho, T. M. G. Pedrosa, E. R. Pedroso, M. C. Neto, and F. M. Biscione. 2007. A 10-year prospective surveillance of nosocomial infections in neonatal intensive care units. Am. J. Infect. Control 35:183-189. [DOI] [PubMed] [Google Scholar]
- 11.de Hoog, M., J. W. Mouton, and J. N. van den Anker. 2004. Vancomycin: pharmacokinetics and administration regimens in neonates. Clin. Pharmacokinet. 43:417-440. [DOI] [PubMed] [Google Scholar]
- 12.de Hoog, M., R. C. Schoemaker, J. W. Mouton, and J. N. van den Anker. 2000. Vancomycin population pharmacokinetics in neonates. Clin. Pharmacol. Ther. 67:360-367. [DOI] [PubMed] [Google Scholar]
- 13.Devlieger, H., G. Martens, A. Bekaert, R. Eeckels, and R. Vlietinck. 1996. Perinatal activities in Flanders, p. 94-116. The Flemish Centre for the Study of Perinatal Epidemiology, Brussels, Belgium.
- 14.Ette, E. I., and P. J. Williams. 2004. Population pharmacokinetics I: background, concepts, and models. Ann. Pharmacother. 38:1702-1706. [DOI] [PubMed] [Google Scholar]
- 15.Frattarelli, D. A., H. Ergun, M. Lulic-Botica, V. T. Lehr, and J. V. Aranda. 2005. Vancomycin elimination in human infants with intrauterine growth retardation. Pediatr. Infect. Dis. J. 24:979-983. [DOI] [PubMed] [Google Scholar]
- 16.Frymoyer, A., A. L. Hersh, L. Z. Benet, and B. J. Guglielmo. 2009. Current recommended dosing of vancomycin for children with invasive methicillin-resistant Staphylococcus aureus infections is inadequate. Pediatr. Infect. Dis. J. 28:398-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garrison, M. W., K. Vance-Bryan, T. A. Larson, J. P. Toscano, and J. C. Rotschafer. 1990. Assessment of effects of protein binding on daptomycin and vancomycin killing of Staphylococcus aureus by using an in vitro pharmacodynamic model. Antimicrob. Agents Chemother. 34:1925-1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gemmell, C. G., D. I. Edwards, A. P. Fraise, F. K. Gould, G. L. Ridgway, and R. E. Warren on behalf of the Joint Working Party of the British Society for Antimicrobial Chemotherapy, Hospital Infection Society and Infection Control Nurses Association. 2006. Guidelines for the prophylaxis and treatment of methicillin-resistant Staphylococcus aureus (MRSA) infections in the UK. J. Antimicrob. Chemother. 57:589-608. [DOI] [PubMed] [Google Scholar]
- 19.Guignard, J. P., and A. Drukker. 1999. Why do newborn infants have a high plasma creatinine? Pediatrics 103:e49. [DOI] [PubMed] [Google Scholar]
- 20.Helgason, K. O., A. H. Thomson, and C. Ferguson. 2008. A review of vancomycin therapeutic drug monitoring recommendations in Scotland. J. Antimicrob. Chemother. 61:1398-1399. [DOI] [PubMed] [Google Scholar]
- 21.Hinchliffe, S. A., M. R. J. Lynch, P. H. Sargent, C. V. Howard, and D. Velzen. 1992. The effect of intrauterine growth retardation on the development of renal nephrons. Br. J. Obstet. Gynaecol. 99:296-301. [DOI] [PubMed] [Google Scholar]
- 22.Holford, N. H. G. 1996. A size standard for pharmacokinetics. Clin. Pharmacokinet. 30:329-332. [DOI] [PubMed] [Google Scholar]
- 23.Iwamoto, T., Y. Kagawa, M. Kojima, T. Iwamoto, Y. Kagawa, and M. Kojima. 2003. Clinical efficacy of therapeutic drug monitoring in patients receiving vancomycin. Biol. Pharm. Bull. 26:876-879. [DOI] [PubMed] [Google Scholar]
- 24.Jeffres, M. N., W. Isakow, J. A. Doherty, P. S. McKinnon, D. J. Ritchie, S. T. Micek, and M. H. Kollef. 2006. Predictors of mortality for methicillin-resistant Staphylococcus aureus health-care-associated pneumonia: specific evaluation of vancomycin pharmacokinetic indices. Chest 130:947-955. [DOI] [PubMed] [Google Scholar]
- 25.Kimura, T., K. Sunakawa, N. Matsuura, H. Kubo, S. Shimada, and K. Yago. 2004. Population pharmacokinetics of arbekacin, vancomycin, and panipenem in neonates. Antimicrob. Agents Chemother. 48:1159-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kobayashi, Y. 2005. Study of the synergism between carbapenems and vancomycin or teicoplanin against MRSA, focusing on S-4661, a carbapenem newly developed in Japan. J. Infect. Chemother. 11:259-261. [DOI] [PubMed] [Google Scholar]
- 27.Lodise, T. P., N. Patel, B. M. Lomaestro, K. A. Rodvold, and G. L. Drusano. 2009. Relationship between initial vancomycin concentration-time profile and nephrotoxicity among hospitalized patients. Clin. Infect. Dis. 49:507-514. [DOI] [PubMed] [Google Scholar]
- 28.Moise-Broder, P. A., A. Forrest, M. C. Birmingham, and J. J. Schentag. 2004. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin. Pharmacokinet. 43:925-942. [DOI] [PubMed] [Google Scholar]
- 29.Mongelli, M., and A. Biswas. 2001. A fetal growth standard derived from multiple modalities. Early Hum. Dev. 60:171-177. [DOI] [PubMed] [Google Scholar]
- 30.Pachi, A., R. Lubrano, E. Maggi, A. Giancotti, M. Elli, O. Mannarino, and M. A. Castello. 1993. Renal tubular damage in fetuses with intrauterine growth retardation. Fetal Diagn. Ther. 8:109-113. [DOI] [PubMed] [Google Scholar]
- 31.R Development Core Team. 2008. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
- 32.Robinson, D., C. P. Weiner, K. T. Nakamura, and J. E. Robillard. 1990. Effect of intrauterine growth retardation on renal function on day one of life. Am. J. Perinatol. 7:343-346. [DOI] [PubMed] [Google Scholar]
- 33.Rodvold, K. A., J. A. Everett, R. D. Pryka, and D. M. Kraus. 1997. Pharmacokinetics and administration regimens of vancomycin in neonates, infants and children. Clin. Pharmacokinet. 33:32-51. [DOI] [PubMed] [Google Scholar]
- 34.Rybak, M., B. Lomaestro, J. C. Rotschafer, R. Moellering, Jr., W. Craig, M. Billeter, J. R. Dalovisio, and D. P. Levine. 2009. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am. J. Health Syst. Pharm. 66:82-98. [DOI] [PubMed] [Google Scholar]
- 35.Silver, L. E., P. J. Decamps, L. M. Korst, L. D. Platt, and L. C. Castro. 2003. Intrauterine growth restriction is accompanied by decreased renal volume in the human fetus. Am. J. Obstet. Gynecol. 188:1320-1325. [DOI] [PubMed] [Google Scholar]
- 36.Tiskumara, R., S. H. Fakharee, C. Q. Liu, P. Nuntnarumit, K. M. Lui, M. Hammoud, J. K. Lee, C. B. Chow, A. Shenoi, R. Halliday, and D. Isaacs. 2009. Neonatal infections in Asia. Arch. Dis. Child. Fetal Neonatal Ed. 94:F144-F148. [DOI] [PubMed] [Google Scholar]
- 37.Tobin, C. M., J. M. Darville, A. H. Thomson, G. Sweeney, J. F. Wilson, A. P. MacGowan, and L. O. White. 2002. Vancomycin therapeutic drug monitoring: is there a consensus view? The results of a UK National External Quality Assessment Scheme (UK NEQAS) for Antibiotic Assays questionnaire. J. Antimicrob. Chemother. 50:713-718. [DOI] [PubMed] [Google Scholar]
- 38.Usher, R., and F. McLean. 1969. Intrauterine growth of live-born Caucasian infants at sea level: standards obtained from measurements in 7 dimensions of infants born between 25 and 44 weeks. J. Pediatr. 74:901-910. [DOI] [PubMed] [Google Scholar]
- 39.Venables, W. N., and B. D. Ripley. 2002. Modern applied statistics with S, 4th ed. Springer, New York, NY.