Abstract
The in vitro activity of ceftaroline against 891 pneumococci collected in 2008 from 22 centers in the United States was investigated. Ceftaroline was the most potent agent tested, with the MICs being <0.008 to 0.5 μg/ml and the MIC90s being <0.008 to 0.25 μg/ml against 11 prevailing serotypes. The overall rates of susceptibility were as follows: penicillin G, 86.2%; ceftriaxone, 90.7%; cefuroxime, 70.1%; erythromycin, 61.6%; clindamycin, 79.2%; levofloxacin, 99.4%; and vancomycin, 100%. Serotype 19A isolates were the least susceptible. These results support the use of ceftaroline for the treatment of pneumococcal infections, including those caused by pneumococci resistant to other agents.
Streptococcus pneumoniae remains a significant respiratory pathogen. Although there was a decrease in the incidence of invasive and noninvasive disease in all age groups in the United States following the introduction in 2000 of the pediatric seven-valent pneumococcal protein conjugate vaccine (PCV7; Wyeth, Philadelphia, PA) (21, 22), it remains to be seen whether this will be permanent, whether replacement serotypes will fill the niche vacated by vaccine serotypes, or what the effect of the introduction of conjugate vaccines containing additional serotypes will be (10, 13, 15, 17, 25). A few replacement serotypes, predominantly 19A and 6C, are now among the most commonly isolated serotypes (12, 18, 19). Thus far, these serotypes have been less invasive than the types covered by PCV7, but antibiotic resistance is common (19).
Few new antimicrobial agents are on the horizon; therefore, structural modifications which improve the activity and stability of existing classes remain welcome. Ceftaroline is a novel cephalosporin for parenteral use with good in vitro activity against methicillin-resistant Staphylococcus aureus (7, 23). It has shown promising activity against S. pneumoniae and viridans group streptococci, as well as many common Gram-negative respiratory pathogens (24). Animal studies have been performed to determine its pharmacokinetic and pharmacodynamic parameters (1, 8, 9, 14), and phase III studies have been completed with both patients with complicated skin and skin structure infections (cSSSIs) and patients with community-acquired pneumonia (CAP) (5, 6, 26).
In the study described here, the activity of ceftaroline against clinical strains of S. pneumoniae isolated from various institutions across the United States in 2008 was investigated. Isolates were also characterized for their serotypes and their relationship to the serotypes covered by PCV7. Twenty-two medical centers from representative regions around the United States participated in the collection of S. pneumoniae strains for this study. Isolates (limited to one per patient) were collected from all specimen sources, and their identities were confirmed by standard procedures. Susceptibility testing was performed by broth microdilution in commercially prepared trays (TREK, Cleveland, OH), according to CLSI methods, and the susceptibilities were interpreted according to the CLSI breakpoints (3, 4). The comparator agents included penicillin, amoxicillin-clavulanate, cefuroxime, ceftriaxone, imipenem, erythromycin, clindamycin, levofloxacin, moxifloxacin, linezolid, vancomycin, and trimethoprim-sulfamethoxazole. Isolates were serotyped by use of the capsular swelling reaction with commercial group- and type-specific antisera (Statens Serum Institute, Copenhagen, Denmark). Serogroup 6 isolates were identified by PCR to differentiate between serotypes 6A, 6B, and 6C (11, 16). Data were analyzed by serotype and by the serotypes present in PCV7.
For this study, a total of 891 U.S. pneumococcal isolates were collected from 22 cities in 19 states. The isolates came from all body sites but were predominantly from respiratory tract sources, with lower respiratory tract isolates accounting for nearly 60% of the strains tested (Table 1). Blood isolates accounted for 15.6% of the strains, while 7.4% were from the middle ear; only 0.2% of the isolates were recovered from cerebrospinal fluid. The patients ranged from newborns to nonagenarians, with the isolates from individuals older than age 18 years accounting for 66.8% of the isolates. The majority of the isolates (58.4%) came from male patients; 39.2% of the strains came from adult males.
TABLE 1.
Sources of isolates included in this study
| Source | No. | % |
|---|---|---|
| Blood | 139 | 15.6 |
| Cerebrospinal fluid | 2 | 0.2 |
| Eye | 25 | 2.8 |
| Lower respiratory tract | 525 | 58.9 |
| Middle ear fluid | 66 | 7.4 |
| Sinus | 30 | 3.4 |
| Nasopharyngeal | 75 | 8.4 |
| Other | 29 | 3.2 |
| Total | 891 | 100 |
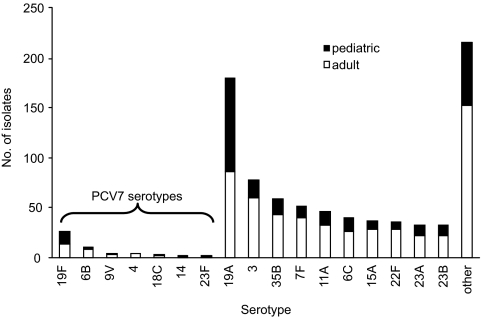
Forty-two serotypes were identified, with 11 serotypes accounting for 72.1% of the total. These 11 serotypes included serotypes 19A, 3, 35B, 7F, 11A, 6C, 15A, 22F, 23A, 23B, and 19F, which was the only serotype covered by PCV7 in this group. Serotype 19A was the most common serotype, accounting for 21.2% of the isolates tested. The serotypes covered by PCV7 accounted for only 6.3% of the isolates, with serotype 19F accounting for more than half of all serotypes covered by PCV7. A histogram showing the frequency distribution of the serotypes is presented in Fig. 1.
FIG. 1.
Distribution of pneumococcal serotypes isolated from adults and children (n = 891).
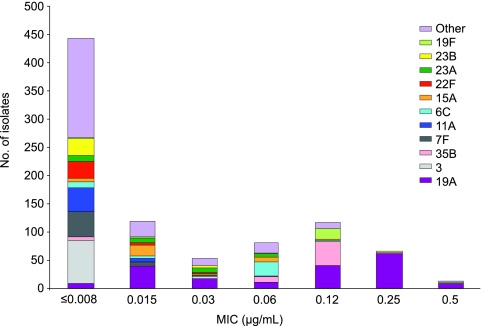
The susceptibilities of all isolates, as well as those of each of the 11 most common serotypes, are shown in Tables 2 and 3, which include the MIC ranges, the MIC50 and MIC90 values, and the percentage of strains susceptible to agents for which breakpoints are available. All isolates were susceptible to vancomycin and linezolid, whereas various degrees of susceptibility to the other agents tested were found. Only three isolates were resistant to quinolones (single strains of serotypes 19F and 23B and one untypeable strain). The ceftaroline MIC50s and MIC90s were low for all strains, ranging from ≤0.008 to 0.25 μg/ml, whereas these values for penicillin ranged from ≤0.03 to 4 μg/ml. A histogram of the MIC distribution of ceftaroline by serotype is shown in Fig. 2. Serotypes 19A and 19F accounted for most of the isolates for which the ceftaroline MICs were 0.12 to 0.5 μg/ml.
TABLE 2.
MIC ranges, MIC50 and MIC90 values, and percentage of isolates susceptible to study agents at CLSI breakpointsa
| Serotype and agent (no. of isolates) | MIC (μg/ml) |
% susceptible | ||
|---|---|---|---|---|
| Range | 50% | 90% | ||
| All isolates (891) | ||||
| Ceftaroline | ≤0.08-0.5 | ≤0.008 | 0.12 | NA |
| Penicillin G | ≤0.03->4 | ≤0.03 | 4 | 86.2 |
| Ceftriaxone | ≤0.25-8 | ≤0.25 | 1 | 90.7 |
| Imipenem | ≤0.12-2 | ≤0.12 | 0.5 | 76.2 |
| Penicillin V | ≤0.03->4 | ≤0.03 | 4 | 58.6 |
| Amoxicillin-clavulanate | ≤1-16 | ≤1 | 8 | 83.1 |
| Cefuroxime | ≤1->8 | ≤1 | 8 | 70.1 |
| Erythromycin | ≤0.25->2 | ≤0.25 | >2 | 61.6 |
| Clindamycin | ≤0.25->2 | 0.25 | >2 | 79.2 |
| Levofloxacin | ≤0.5->4 | 1 | 1 | 99.4 |
| Moxifloxacin | ≤0.5-4 | ≤0.5 | ≤0.5 | 99.6 |
| Vancomycin | ≤1 | ≤1 | ≤1 | 100 |
| SXT | ≤0.5->2 | 0.5 | >2 | 66.2 |
| Linezolid | ≤0.12-2 | 1 | 1 | 100 |
| Serotype 19A (189) | ||||
| Ceftaroline | ≤0.008-0.5 | 0.12 | 0.25 | NA |
| Penicillin G | ≤0.03->4 | 4 | 4 | 46.6 |
| Ceftriaxone | ≤0.25-8 | 1 | 2 | 65.6 |
| Imipenem | ≤0.12-2 | 0.5 | 1 | 40.2 |
| Penicillin V | ≤0.03->4 | 4 | 4 | 13.8 |
| Amoxicillin-clavulanate | ≤1-16 | 8 | 8 | 45.0 |
| Cefuroxime | ≤1->8 | 8 | >8 | 36.1 |
| Erythromycin | ≤0.25->2 | >2 | >2 | 26.5 |
| Clindamycin | ≤0.25->2 | >2 | >2 | 43.4 |
| Levofloxacin | ≤0.25-2 | 1 | 1 | 100 |
| Moxifloxacin | ≤0.5 | ≤0.5 | ≤0.5 | 100 |
| Vancomycin | ≤1 | ≤1 | ≤1 | 100 |
| SXT | ≤0.5->2 | >8 | >2 | 16.9 |
| Linezolid | 0.25-1 | 0.5 | 1 | 100 |
| Serotype 3 (82) | ||||
| Ceftaroline | ≤0.008-0.03 | ≤0.008 | ≤0.008 | NA |
| Penicillin G | ≤0.03-0.12 | ≤0.03 | ≤0.03 | 100 |
| Ceftriaxone | ≤0.25 | ≤0.25 | ≤0.25 | 100 |
| Imipenem | ≤0.12 | ≤0.12 | ≤0.12 | 100 |
| Penicillin V | ≤0.03-0.12 | ≤0.03 | ≤0.03 | 98.8 |
| Amoxicillin-clavulanate | ≤1 | ≤1 | ≤1 | 100 |
| Cefuroxime | ≤1 | ≤1 | ≤1 | 100 |
| Erythromycin | ≤0.25->2 | ≤0.25 | ≤0.25 | 95.1 |
| Clindamycin | ≤0.25->2 | ≤0.25 | ≤0.25 | 96.3 |
| Levofloxacin | ≤0.5-2 | 1 | 1 | 100 |
| Moxifloxacin | ≤0.5 | ≤0.5 | ≤0.5 | 100 |
| Vancomycin | ≤1 | ≤1 | ≤1 | 100 |
| SXT | ≤0.5->1 | 0.03 | ≤0.25 | 97.6 |
| Linezolid | ≤0.12-1 | 0.5 | 1 | 100 |
| Serotype 35B (59) | ||||
| Ceftaroline | ≤0.008-0.25 | 0.12 | 0.12 | NA |
| Penicillin G | ≤0.03-4 | 2 | 2 | 98.3 |
| Ceftriaxone | ≤0.25-4 | 1 | 1 | 98.3 |
| Imipenem | ≤0.12-0.5 | 0.25 | 0.5 | 15.3 |
| Penicillin V | ≤0.03-4 | 2 | 2 | 10.2 |
| Amoxicillin-clavulanate | ≤1-8 | 2 | 4 | 62.7 |
| Cefuroxime | ≤1->8 | 4 | 4 | 9.6 |
| Erythromycin | ≤0.25->2 | ≤0.25 | >2 | 64.4 |
| Clindamycin | ≤0.25 | ≤0.25 | ≤0.25 | 100 |
| Levofloxacin | ≤0.5-4 | 1 | 1 | 98.3 |
| Moxifloxacin | ≤0.5 | ≤0.5 | ≤0.5 | 100 |
| Vancomycin | ≤1 | ≤1 | ≤1 | 100 |
| SXT | ≤0.5->2 | ≤0.06 | ≤0.25 | 100 |
| Linezolid | 0.5-1 | 1 | 1 | 100 |
| Serotype 7F (52) | ||||
| Ceftaroline | ≤0.008-0.03 | ≤0.008 | 0.015 | NA |
| Penicillin G | ≤0.03-0.06 | ≤0.03 | ≤0.03 | 100 |
| Ceftriaxone | ≤0.25 | ≤0.25 | ≤0.25 | 100 |
| Imipenem | ≤0.12 | ≤0.12 | ≤0.12 | 100 |
| Penicillin V | ≤0.03-0.06 | ≤0.03 | ≤0.03 | 100 |
| Amoxicillin-clavulanate | ≤1-2 | ≤1 | ≤1 | 100 |
| Cefuroxime | ≤1 | ≤1 | ≤1 | 100 |
| Erythromycin | ≤0.25 | ≤0.25 | ≤0.25 | 100 |
| Clindamycin | ≤0.25 | ≤0.25 | ≤0.25 | 100 |
| Levofloxacin | ≤0.5-2 | 1 | 1 | 100 |
| Moxifloxacin | ≤0.5 | ≤0.5 | ≤0.5 | 100 |
| Vancomycin | ≤1 | ≤1 | ≤1 | 100 |
| SXT | ≤0.5 | ≤0.06 | ≤0.25 | 100 |
| Linezolid | 0.25-1 | 0.5 | 1 | 100 |
| Serotype 11A (49) | ||||
| Ceftaroline | ≤0.008-0.12 | 0.06 | 0.06 | NA |
| Penicillin G | ≤0.03-2 | ≤0.03 | ≤0.03 | 100 |
| Ceftriaxone | ≤0.25-2 | ≤0.25 | ≤0.25 | 98.0 |
| Imipenem | ≤0.12-0.25 | ≤0.12 | ≤0.12 | 95.9 |
| Penicillin V | ≤0.03-2 | ≤0.03 | ≤0.03 | 95.9 |
| Amoxicillin-clavulanate | ≤1-4 | ≤1 | ≤1 | 98.0 |
| Cefuroxime | ≤1-8 | ≤1 | ≤1 | 95.2 |
| Erythromycin | ≤0.25->2 | ≤0.25 | >2 | 75.5 |
| Clindamycin | ≤0.25->2 | ≤0.25 | ≤0.25 | 95.9 |
| Levofloxacin | ≤0.5-2 | 1 | 2 | 100 |
| Moxifloxacin | ≤0.5 | ≤0.5 | ≤0.5 | 100 |
| Vancomycin | ≤1 | ≤1 | ≤1 | 100 |
| SXT | ≤0.5->2 | 0.12 | ≤0.25 | 87.8 |
| Linezolid | 0.5-2 | 1 | 1 | 100 |
| Serotype 6C (43) | ||||
| Ceftaroline | ≤0.008-0.12 | 0.015 | 0.06 | NA |
| Penicillin G | ≤0.03-2 | 0.5 | 1 | 100 |
| Ceftriaxone | ≤0.25-1 | 0.25 | 0.25 | 100 |
| Imipenem | ≤0.12-0.5 | ≤0.12 | ≤0.12 | 90.7 |
| Penicillin V | ≤0.03-2 | 0.5 | 1 | 37.2 |
| Amoxicillin-clavulanate | ≤1-2 | ≤1 | ≤1 | 100 |
| Cefuroxime | ≤1-8 | 2 | 4 | 47.5 |
| Erythromycin | ≤0.25->2 | >2 | >2 | 41.9 |
| Clindamycin | ≤0.25->2 | ≤0.25 | ≤0.25 | 97.7 |
| Levofloxacin | ≤0.5-1 | 1 | 1 | 100 |
| Moxifloxacin | ≤0.5 | ≤0.5 | ≤0.5 | 100 |
| Vancomycin | ≤1 | ≤1 | ≤1 | 100 |
| SXT | ≤0.5->2 | >8 | >2 | 34.9 |
| Linezolid | 0.5-2 | 1 | 1 | 100 |
| MDR isolates (123)b | ||||
| Ceftaroline | 0.06-0.5 | 0.25 | 0.25 | NA |
| Penicillin G | 1->4 | 4 | 4 | 11.4 |
| Ceftriaxone | 0.5-8 | 2 | 4 | 43.9 |
| Imipenem | ≤0.12-2 | 1 | 1 | 0.8 |
| Penicillin V | 1->4 | 4 | 4 | 0 |
| Amoxicillin-clavulanate | ≤1-16 | 8 | 16 | 8.1 |
| Cefuroxime | 2->8 | 8 | >8 | 0 |
| Erythromycin | 8->8 | >8 | >8 | 0 |
| Clindamycin | 1->2 | >2 | >2 | 0 |
| Levofloxacin | ≤0.5->4 | 1 | 1 | 99.2 |
| Moxifloxacin | ≤0.5-4 | ≤0.5 | ≤0.5 | 99.2 |
| Vancomycin | ≤1 | ≤1 | ≤1 | 100 |
| SXT | 1->2 | >2 | >2 | 0 |
| Linezolid | 0.25-1 | 0.5 | 1 | 100 |
Data are shown for all isolates, for serotypes with >40 isolates, and for MDR strains. MDR, resistant to penicillin, macrolides, lincosamides, and trimethoprim-sulfamethoxazole; NA, no susceptibility breakpoints established for ceftaroline; SXT, trimethoprim-sulfamethoxazole.
MDR isolates included isolates of serotypes 19A (n = 101), 19F (n = 19), and 6B (n = 3).
TABLE 3.
Susceptibilities of isolates against all agents tested for all isolates and for the 11 predominating serotypes
| Agent (CLSI breakpoint for susceptibility [μg/ml]) | % susceptibility of isolates of the following serotype (no. of isolates): |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All (891) | 19A (189) | 3 (82) | 35B (59) | 7F (52) | 11A (49) | 6C (43) | 15A (38) | 22F (35) | 23A (33) | 23B (33) | 19F (29) | |
| Ceftaroline (NAa) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Penicillin G (≤2) | 86.2 | 46.6 | 100 | 98.3 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 37.9 |
| Ceftriaxone (≤1) | 90.7 | 65.6 | 100 | 98.3 | 100 | 98.0 | 100 | 100 | 100 | 100 | 97.0 | 65.5 |
| Imipenem (≤0.12) | 76.2 | 40.2 | 100 | 15.3 | 100 | 95.9 | 90.7 | 100 | 100 | 100 | 100 | 20.7 |
| Penicillin V (≤0.06) | 58.6 | 13.8 | 98.8 | 10.2 | 100 | 95.9 | 37.2 | 10.5 | 100 | 36.4 | 84.8 | 20.7 |
| Amoxicillin/clavulanate (≤2) | 83.1 | 45.0 | 100 | 62.7 | 100 | 98.0 | 100 | 100 | 100 | 100 | 100 | 37.9 |
| Cefuroxime (≤1) | 70.1 | 36.1 | 100 | 9.6 | 100 | 95.2 | 47.5 | 91.4 | 100 | 96.4 | 96.8 | 20.7 |
| Erythromycin (≤0.25) | 61.6 | 26.5 | 95.1 | 64.4 | 100 | 75.5 | 41.9 | 5.3 | 88.6 | 78.8 | 75.8 | 17.2 |
| Clindamycin (≤0.25) | 79.2 | 43.4 | 96.3 | 100 | 100 | 95.9 | 97.7 | 10.5 | 100 | 81.8 | 100 | 31.0 |
| Levofloxacin (≤2) | 99.4 | 100 | 100 | 98.3 | 100 | 100 | 100 | 100 | 100 | 100 | 97.0 | 96.6 |
| Moxifloxacin (≤1) | 99.6 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 97.0 | 96.6 |
| Vancomycin (≤1) | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| SXTb (≤0.5) | 66.2 | 16.9 | 97.6 | 100 | 100 | 87.8 | 34.9 | 55.3 | 97.1 | 90.9 | 84.8 | 17.2 |
| Linezolid (≤2) | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
NA, no susceptibility breakpoints established for ceftaroline
SXT, trimethoprim-sulfamethoxazole.
FIG. 2.
Distribution of ceftaroline MICs (in μg/ml), by serotype.
The most common highly resistant serotype, 19A, had ceftaroline MIC50 and MIC90 values of 0.12 and 0.25 μg/ml, respectively, and these values were 4 and 4 μg/ml, respectively, for penicillin and 1 and 2 μg/ml, respectively, for ceftriaxone. The rates of susceptibility for this serotype were as follows: penicillin G, 46.6%; penicillin V, 13.8%; amoxicillin-clavulanate, 45.0%; ceftriaxone 65.6%; and cefuroxime, 36.1%. Isolates of serotype 19A showed lower levels of susceptibility to erythromycin, clindamycin, imipenem, and trimethoprim-sulfamethoxazole than isolates of the other serotypes. The other serotypes with decreased susceptibility included serotypes 19F and 35B and newly identified serotype 6C. One hundred twenty-three (14% of the total) isolates were multidrug resistant (MDR), which was defined as resistance to penicillin, macrolides, lincosamides, and trimethoprim-sulfamethoxazole, with the few quinolone-resistant strains detected in this study being found among the isolates in this group. Almost all (96%) of these MDR isolates were serogroup 19: 101 were serotype 19A and 19 were serotype 19F. The rates of MDR were 53.4% and 82.6% for serotype 19A and 19F isolates, respectively. The ceftaroline MICs for the MDR isolates ranged from 0.06 to 0.5 μg/ml, with the both the MIC50 and the MIC90 values being 0.25 μg/ml. The distribution of serotypes by patient age (adult versus pediatric) is shown in Fig. 1. The highest proportion of pediatric isolates for any serotype was for serotypes 19A and 19F, approximately 50% of which were from pediatric patients.
With the increasing prevalence of MDR serotypes of S. pneumoniae not present in PCV7 (replacement serotypes), such as serotype 19A, the development of new antimicrobial agents continues to be critical. Trends in the United States show an increasing prevalence of invasive disease caused by serotype 19A isolates: 9.3 cases per 100,000 population in 2005 compared with 2.6 cases per 100,000 population from 1998 to 1999 in children less than 5 years of age (2). In 2005, isolates of serotype 19A accounted for 40% of the cases of invasive pneumococcal disease in children less than 5 years of age. Improving coverage with existing conjugate and polysaccharide vaccines, the use of new higher-valency conjugate vaccines, and the use of conjugate vaccines among adults, especially those with underlying illnesses, have been recommended to further decrease the incidence of pneumococcal disease (20).
This study identifies significant in vitro limitations to the currently recommended parenteral β-lactam, macrolide, and lincosamide classes of agents, as well as several oral agents, including macrolides, lincosamides, and trimethoprim-sulfamethoxazole, particularly for serotypes 19A and 19F. Two randomized, double-blinded, multicenter phase III trials of the efficacy and safety of ceftaroline versus those of ceftriaxone in community-acquired pneumonia showed that the two agents have comparable efficacies, although few pneumococci with high MICs to either agent were found (6). On the basis of our results, it would appear that ceftaroline has the potential to advance the effectiveness of the antimicrobial arsenal available to clinicians for the treatment of drug-resistant pneumococcal infections, with the drug having the added advantage of having activity against methicillin-resistant S. aureus.
Acknowledgments
This study was funded by a grant from Forest Laboratories, Inc. (New York, NY). Scientific Therapeutics Information, Inc. (Springfield, NJ), provided editorial assistance with the manuscript. Funding for the editorial assistance was provided by Forest Laboratories, Inc.
Footnotes
Published ahead of print on 22 March 2010.
REFERENCES
- 1.Andes, D., and W. A. Craig. 2006. Pharmacodynamics of a new cephalosporin, PPI-0903 (TAK-599), active against methicillin-resistant Staphylococcus aureus in murine thigh and lung infection models: identification of an in vivo pharmacokinetic-pharmacodynamic target. Antimicrob. Agents Chemother. 50:1376-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. 2008. Invasive pneumococcal disease in children 5 years after conjugate vaccine introduction—eight states, 1998-2005. MMWR Morb. Mortal. Wkly. Rep. 57:144-148. [PubMed] [Google Scholar]
- 3.Clinical and Laboratory Standards Institute. 2009. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 8th ed. Approved standard M7-A8. Clinical and Laboratory Standards Institute, Wayne, PA.
- 4.Clinical and Laboratory Standards Institute. 2009. Performance standards for antimicrobial susceptibility testing; 19th informational supplement. CLSI document M100-S19. Clinical and Laboratory Standards Institute, Wayne, PA.
- 5.Corey, R., M. Wilcox, G. Talbot, T. Baculik, and D. Thye. 2008. CANVAS-1: randomized, double-blinded, phase 3 study (P903-06) of the efficacy and safety of ceftaroline versus vancomycin plus aztreonam in complicated skin and skin structure infections (cSSSI), abstr. L-1515a. Abstr. 48th Annu. Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC)-Infect. Dis. Soc. Am. (IDSA) 46th Annu. Meet. American Society for Microbiology and Infectious Diseases Society of America, Washington, DC.
- 6.Eckburg, P., H. Friedland, J. Lee, L. Llorens, I. Critchley, and D. Thye. 2009. FOCUS 1 and 2: randomized, double-blinded, multicenter phase 3 trials of the efficacy and safety of ceftaroline (CPT) vs ceftriaxone (CRO) in community-acquired pneumonia (CAP), abstr. L1-345a. Abstr. 49th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 7.Ge, Y., D. Biek, G. H. Talbot, and D. F. Sahm. 2008. In vitro profiling of ceftaroline against a collection of recent bacterial clinical isolates from across the United States. Antimicrob. Agents Chemother. 52:3398-3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ge, Y., L. Floren, R. Redman, S. Liao, and M. Wilker. 2006. The pharmacokinetics and safety of ceftaroline (PPI-0903) in healthy subjects receiving multiple-dose intravenous infusions, abstr. A-1937. Abstr. 46th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 9.Ge, Y., and A. Hubbel. 2006. In vitro evaluation of plasma protein binding and metabolic stability of ceftaroline (PPI-0903), abstr. A-1935. Abstr. 46th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 10.Hicks, L. A., L. H. Harrison, B. Flannery, J. L. Hadler, W. Schaffner, A. S. Craig, D. Jackson, A. Thomas, B. Beall, R. Lynfield, A. Reingold, M. M. Farley, and C. G. Whitney. 2007. Incidence of pneumococcal disease due to non-pneumococcal conjugate vaccine (PCV7) serotypes in the United States during the era of widespread PCV7 vaccination, 1998-2004. J. Infect. Dis. 196:1346-1354. [DOI] [PubMed] [Google Scholar]
- 11.Jacobs, M. R., S. Bajaksouzian, R. A. Bonomo, C. E. Good, A. R. Windau, A. M. Hujer, C. Massire, R. Melton, L. B. Blyn, D. J. Ecker, and R. Sampath. 2009. Occurrence, distribution, and origins of Streptococcus pneumoniae serotype 6C, a recently recognized serotype. J. Clin. Microbiol. 47:64-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobs, M. R., C. E. Good, S. Bajaksouzian, and A. R. Windau. 2008. Emergence of Streptococcus pneumoniae serotypes 19A, 6C, and 22F and serogroup 15 in Cleveland, Ohio, in relation to introduction of the protein-conjugated pneumococcal vaccine. Clin. Infect. Dis. 47:1388-1395. [DOI] [PubMed] [Google Scholar]
- 13.Jacobs, M. R., C. E. Good, B. Beall, S. Bajaksouzian, A. R. Windau, and C. G. Whitney. 2008. Changes in serotypes and antimicrobial susceptibility of invasive Streptococcus pneumoniae strains in Cleveland: a quarter century of experience. J. Clin. Microbiol. 46:982-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacqueline, C., J. Caillon, V. Le Mabecque, A. F. Miegeville, A. Hamel, D. Bugnon, J. Y. Ge, and G. Potel. 2007. In vivo efficacy of ceftaroline (PPI-0903), a new broad-spectrum cephalosporin, compared with linezolid and vancomycin against methicillin-resistant and vancomycin-intermediate Staphylococcus aureus in a rabbit endocarditis model. Antimicrob. Agents Chemother. 51:3397-3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Messina, A. F., K. Katz-Gaynor, T. Barton, N. Ahmad, F. Ghaffar, D. Rasko, and G. H. McCracken, Jr. 2007. Impact of the pneumococcal conjugate vaccine on serotype distribution and antimicrobial resistance of invasive Streptococcus pneumoniae isolates in Dallas, TX, children from 1999 through 2005. Pediatr. Infect. Dis. J. 26:461-467. [DOI] [PubMed] [Google Scholar]
- 16.Park, I. H., S. Park, S. K. Hollingshead, and M. H. Nahm. 2007. Genetic basis for the new pneumococcal serotype, 6C. Infect. Immun. 75:4482-4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park, S. Y., C. A. Van Beneden, T. Pilishvili, M. Martin, R. R. Facklam, and C. G. Whitney. 2010. Invasive pneumococcal infections among vaccinated children in the United States. J. Pediatr. 156:478-483. [DOI] [PubMed] [Google Scholar]
- 18.Pelton, S. I., H. Huot, J. A. Finkelstein, C. J. Bishop, K. K. Hsu, J. Kellenberg, S. S. Huang, R. Goldstein, and W. P. Hanage. 2007. Emergence of 19A as virulent and multidrug resistant Pneumococcus in Massachusetts following universal immunization of infants with pneumococcal conjugate vaccine. Pediatr. Infect. Dis. J. 26:468-472. [DOI] [PubMed] [Google Scholar]
- 19.Pichichero, M. E., and J. R. Casey. 2007. Emergence of a multiresistant serotype 19A pneumococcal strain not included in the 7-valent conjugate vaccine as an otopathogen in children. JAMA 298:1772-1778. [DOI] [PubMed] [Google Scholar]
- 20.Pilishvili, T., C. Lexau, M. M. Farley, J. Hadler, L. H. Harrison, N. M. Bennett, A. Reingold, A. Thomas, W. Schaffner, A. S. Craig, P. J. Smith, B. W. Beall, C. G. Whitney, and M. R. Moore. 2010. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J. Infect. Dis. 201:32-41. [DOI] [PubMed] [Google Scholar]
- 21.Ray, G. T., S. I. Pelton, K. P. Klugman, D. R. Strutton, and M. R. Moore. 2009. Cost-effectiveness of pneumococcal conjugate vaccine: an update after 7 years of use in the United States. Vaccine 27:6483-6494. [DOI] [PubMed] [Google Scholar]
- 22.Ray, G. T., C. G. Whitney, B. H. Fireman, V. Ciuryla, and S. B. Black. 2006. Cost-effectiveness of pneumococcal conjugate vaccine: evidence from the first 5 years of use in the United States incorporating herd effects. Pediatr. Infect. Dis. J. 25:494-501. [DOI] [PubMed] [Google Scholar]
- 23.Sader, H. S., T. R. Fritsche, and R. N. Jones. 2008. Antimicrobial activities of ceftaroline and ME1036 tested against clinical strains of community-acquired methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 52:1153-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sader, H. S., T. R. Fritsche, K. Kaniga, Y. Ge, and R. N. Jones. 2005. Antimicrobial activity and spectrum of PPI-0903M (T-91825), a novel cephalosporin, tested against a worldwide collection of clinical strains. Antimicrob. Agents Chemother. 49:3501-3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singleton, R. J., T. W. Hennessy, L. R. Bulkow, L. L. Hammitt, T. Zulz, D. A. Hurlburt, J. C. Butler, K. Rudolph, and A. Parkinson. 2007. Invasive pneumococcal disease caused by nonvaccine serotypes among Alaska native children with high levels of 7-valent pneumococcal conjugate vaccine coverage. JAMA 297:1784-1792. [DOI] [PubMed] [Google Scholar]
- 26.Wilcox, M., R. Corey, G. Talbot, T. Baculik, and D. Thye. 2008. CANVAS-2: randomized, double-blinded, phase 3 study (P903-07) of the efficacy and safety of ceftaroline vs vancomycin plus aztreonam in complicated skin and skin structure infections, abstr. P1792. Abstr. 19th Annu. Meet. Eur. Congr. Clin. Microbiol. Infect. Dis.