Abstract
We evaluated the prevalence of fks1 hot spot (HS) 1 mutations among 133 Candida strains from six species displaying various caspofungin MIC values (from ≤0.008 to >8 μg/ml). Only 4 (2.9%) strains displayed FKS1 HS1 amino acid substitutions: 1 C. albicans (F641Y) among 32 isolates tested (3.1%), 1 C. glabrata (S645P) among 34 isolates tested (2.9%), and 2 C. tropicalis (F641S) among 12 isolates tested (16.7%). The 4 isolates displaying FKS1 HS1 alterations showed elevated caspofungin MIC results (1 to >8 μg/ml) but lower anidulafungin and micafungin MIC values (0.12 to 4 μg/ml and 0.25 to 4 μg/ml, respectively) in some instances within the wild-type MIC population, as determined using the epidemiologic cutoff values (ECV). Candida krusei, C. parapsilosis, and C. guilliermondii isolates tested showed no FKS1 HS1 alterations regardless of echinocandin MIC result. We additionally analyzed 8 C. albicans and 7 C. glabrata strains for mutations on other HS regions of fks1 and fks2. Three C. glabrata strains showed alterations on FKS2 HS1 (two S645P and one L644W). In general, strains displaying S645P alteration showed higher echinocandin MIC values than strains harboring other mutations. Overall, Candida spp. strains showing caspofungin MIC values within the ECV did not display fks HS mutations. In contrast, strains showing alterations in this region displayed anidulafungin and/or micafungin MIC values within the wild-type population, suggesting that caspofungin could be the most sensitive agent for detection of these resistance mutations. Furthermore, results from this large, geographically diverse Candida spp. collection demonstrated that fks1 HS1 mutations remain uncommon among isolates with various echinocandin MIC levels.
Candida species are the most common cause of invasive fungal infections among hospitalized patients, accounting for 8 to 10% of all nosocomial bloodstream infections (5). Invasive candidiasis is associated with very high crude and attributable mortality rates, and treatment of these infections can be challenging, with several antifungal options available differing in cost and toxicity (9). The introduction of echinocandin compounds was an important advance in the treatment of invasive fungal infections, providing a well-tolerated and effective alternative to azoles and polyenes (5, 17). Echinocandins (caspofungin, anidulafungin, and micafungin) are often used for primary therapy for invasive candidiasis (9), based upon a favorable drug interaction profile, low toxicity, and good activity against species that may demonstrate resistance or reduced susceptibility to azoles and polyenes (e.g., C. glabrata, C. krusei) (8).
Echinocandins are lipopeptides that inhibit cell wall synthesis by targeting the 1,3-β-d-glucan synthase (GS) complex (1). Resistance to these compounds has been associated with mutations within two highly conserved regions of fks1 and fks2, and amino acid substitutions in the proteins encoded by these genes can occur within two hot spots (HSs) on each gene (4, 15). Strains carrying mutations on HSs of FKS-encoding genes were proven to have significantly reduced susceptibility of glucan synthase against echinocandins (6, 7). Additionally, in candidiasis mouse models, infection caused by strains harboring fks mutations required a 100- to 1,000-fold larger amount of antifungal agent to reduce kidney fungal burdens by 99% (99% effective dose [ED99]), the relative measure for in vivo susceptibility (12).
The majority of Candida spp. clinical strains displaying reduced echinocandin susceptibility possessed mutations in the HS1 region of fks1 (4), with amino acid substitutions in the serine residue of position 645. These clinical isolates were occasionally obtained from therapeutic failures or patients showing poor response to treatment with echinocandin compounds (12). However, the correlation of clinical failure and increased in vitro MIC values is not clear and studies suggested that the presence of an fks mutation or elevated MIC values was not a reliable predictor of treatment outcome (12).
In this study, we evaluated 133 Candida spp. strains for the presence of alterations within HS1 of fks1, and the presence of these mutations was correlated to the susceptibility testing results for the echinocandins according to the Clinical and Laboratory Standards Institute (CLSI) methodology. Subsets of C. albicans (eight strains) and C. glabrata (seven strains) were evaluated for mutations in other HSs of fks1 and fks2.
(This work was presented in part at the 49th Interscience Conference on Antimicrobial Agents and Chemotherapy [ICAAC 2009], San Francisco, CA, 2009.)
MATERIALS AND METHODS
Strains.
A total of 133 Candida spp. clinical strains showing caspofungin MIC values ranging from ≤0.008 to 16 μg/ml were evaluated. These randomly selected Candida spp. strains belonged to 6 species, including C. glabrata (34 strains), C. albicans (32), C. parapsilosis (2 C. metapsilosis strains and 1 C. orthopsilosis strain) (25), C. guilliermondii (19), C. tropicalis (12), and C. krusei (11). These strains were collected during 2006-2007 as part of significant worldwide surveillance initiatives: the SENTRY Antimicrobial Surveillance Program and ARTEMIS (11, 14). Strains were identified at the participating medical centers by the established methods in use at each institution. Confirmation of species identification was performed at the central reference laboratory using Vitek (bioMérieux, Missouri), conventional reference methods, and/or 28S and internal transcribed spacer (ITS) sequencing as described elsewhere (10).
Antifungal susceptibility testing.
Broth microdilution MIC testing was performed according to Clinical and Laboratory Standards Institute (CLSI) methods (2). Panels were produced using RPMI 1640 broth supplemented with MOPS (morpholinepropanesulfonic acid) buffer. All results were recorded at 24 and 48 h, and the interpretive criteria used were those published in CLSI document M27-S2 (2006) (3). Quality control (QC) was performed as recommended in M27-A2 (2002) using the following strains: C. parapsilosis ATCC 22019 and C. krusei ATCC 6258.
DNA sequence analysis of fks.
DNA extraction was performed using the QIAamp DNA minikit (Qiagen, Hilden, Germany). Singleplex PCRs were set up with generic fks1 HS1 primers that were able to amplify most Candida species (6). C. glabrata and C. krusei required species-specific primers (4, 15) (Table 1).
TABLE 1.
Oligonucleotides used in this study
| Region | Oligonucleotide name | Sequence (5′ to 3′) | Reference |
|---|---|---|---|
| fks1 HS1 | fks-CSP-F | AAT GGG CTG GTG CTC AAC AT | 6 |
| fks-CSP-R | CCT TCA ATT TCA GAT GGA ACT TGA TG | 6 | |
| CKRUfksF | ACT GCA TCG TTT GCT CCT CT | 4 | |
| CKRUfksR | GAA CAT GAT CAA TTG CCA AC | 4 | |
| CGLAfks-F | CCA TTG GGT GGT CTG TTC ACG | 16 | |
| CGLAfks-R | GAT TGG GCA AAG AAA GAA ATA CGA C | 16 | |
| fks1 HS2 | CSP-fks1HS2-F | AAG ATT GGT GCT GGT ATG GG | 6 |
| CSP-fks1HS2-R | TAA TGG TGC TTG CCA ATG AG | 6 | |
| CGLA-fks1HS2-R | ATG GAG AGA ACA GCA GGG CG | 16 | |
| fks2 HS1 | CGLA-fks2HS1-F | GCT TCT CAG ACT TTC ACC G | 16 |
| CGLA-fks2HS1-R | CAG AAT AGT GTG GAG TCA AGA CG | 16 | |
| fks2 HS2 | CGLA-fks2HS2-F | TCT TGA CTT TCT ACT ATG CG | 16 |
| CGLA-fks2HS2-R | CTT GCC AAT GTG CCA CTG | 16 |
C. albicans (8 strains) and C. glabrata (7 strains) were randomly selected for evaluation of fks1 HS2 and fks2 HS1 and HS2 (C. glabrata only for fks2).
PCR amplicons were sequenced on both strands, and the nucleotide sequences and deduced amino acid sequences were analyzed using the Lasergene software package (DNASTAR, Madison, WI). Sequences were compared to others available via internet sources (http://www.ncbi.nlm.nih.gov/blast/).
RESULTS AND DISCUSSION
A total of 133 Candida spp. collected from medical centers located in North America (41.0% of the strains), Europe (23.9%), Asia-Pacific region (22.4%), and Latin America (12.7%) were analyzed. These strains belonged to six Candida species: C. glabrata, C. albicans, C. parapsilosis, C. guilliermondii, C. tropicalis, and C. krusei. The MIC distribution for echinocandin compounds (caspofungin, anidulafungin, and micafungin) is presented in Table 2.
TABLE 2.
MIC distributions of echinocandin compounds against 133 Candida spp. strains tested for the presence of fks mutations
| Organism (no. of strains) | No. of strains at MIC (μg/ml) (no. of strains demonstrating fks mutations)a |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤0.008 | 0.015 | 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | >8 | |
| C. glabrata (34) | ||||||||||||
| Anidulafungin | —b | — | 7 | 15 | 4 (1) | [2] | — | 3 | — | 3 (3) | — | — |
| Caspofungin | — | — | 1 | 19 | [2] | 1 (1) | 5 | 1 | 2 | 2 | 1 (1) | 2 (2) |
| Micafungin | 2 | 21 | [2] | 1 (1) | 2 | 2 | 1 | — | 1 (1) | 2 (2) | — | — |
| C. albicans (32) | ||||||||||||
| Anidulafungin | 3 | 10 | 13 | 4 | [2 (1)] | — | — | — | — | — | — | — |
| Caspofungin | 3 | 2 | 9 | 17 | [0] | — | — | 1 (1) | — | — | — | — |
| Micafungin | 3 | 21 | [7] | — | — | 1 (1) | — | — | — | — | — | — |
| C. parapsilosis (25) | ||||||||||||
| Anidulafungin | — | — | — | — | — | 2 | 2 | 5 | 13 | [3] | — | — |
| Caspofungin | — | — | — | — | — | 1 | 14 | [10] | — | — | — | — |
| Micafungin | — | — | — | — | — | 2 | 3 | 3 | 15 | [2] | — | — |
| C. guilliermondii (19) | ||||||||||||
| Anidulafungin | — | — | — | — | — | — | — | — | 10 | 9 | — | [0] |
| Caspofungin | — | — | — | — | — | 1 | 1 | 12 | 2 | [2] | 1 | — |
| Micafungin | — | — | — | — | — | — | 4 | 6 | 7 | [2] | — | — |
| C. tropicalis (12) | ||||||||||||
| Anidulafungin | 1 | 6 | 3 | — | [0] | — | — | 2 (2) | — | — | — | — |
| Caspofungin | — | 2 | 6 | 2 | [0] | — | — | — | — | 2 (2) | — | — |
| Micafungin | 1 | 2 | 6 | 1 | [0] | — | 2 (2) | — | — | — | — | — |
| C. krusei (11) | ||||||||||||
| Anidulafungin | — | 1 | 3 | 7 | [0] | — | — | — | — | — | — | — |
| Caspofungin | — | — | 1 | 4 | — | [1] | 1 | 3 | 1 | — | — | — |
| Micafungin | — | — | — | 8 | [3] | — | — | — | — | — | — | — |
Epidemiological cutoff values (ECV, a statistical method used to determine the wild-type MIC range) are shown in brackets (13).
—, data not available.
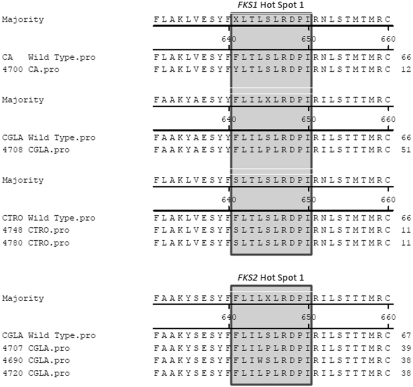
Among 133 Candida spp. tested, only four (2.9%) strains displayed fks1 HS1 mutations generating amino acid substitutions: 1 C. albicans (F641Y), 1 C. glabrata (S645P), and 2 C. tropicalis (F641S) strains (Table 3 and Fig. 1). Candida spp. strains harboring fks mutations demonstrated elevated caspofungin MIC results (1 to ≥8 μg/ml) (Table 2) and lower anidulafungin and micafungin MIC values (by at least 2-fold) (Table 3). Previous reports also observed that strains possessing altered FKS usually display higher caspofungin MIC values than those of the other echinocandins. Additionally, these strains appear to have lower anidulafungin MIC results (12), often within the susceptible range.
TABLE 3.
Summary of FKS alterations detected in Candida spp. strains
| Isolate | Species | Alterationsa |
MIC (μg/ml) |
Location | |||
|---|---|---|---|---|---|---|---|
| fks1 HS1 | fks2 HS1 | Caspofungin | Anidulafungin | Micafungin | |||
| 4700 | C. albicans | F641Y | NT | 1 | 0.12 | 0.25 | New York, NY |
| 4748 | C. tropicalis | F641S | NT | 4 | 1 | 0.5 | Akron, OH |
| 4780 | C. tropicalis | F641S | NT | 4 | 1 | 0.5 | Akron, OH |
| 4708 | C. glabrata | S645P | NT | ≥8 | 4 | 4 | Akron, OH |
| 4707 | C. glabrata | NM | S645P | 8 | 4 | 2 | Cleveland, OH |
| 4690 | C. glabrata | NM | L644W | 0.25 | 0.12 | 0.06 | Japan |
| 4720 | C. glabrata | NM | S645P | ≥8 | 4 | 4 | Detroit, MI |
NT, not tested. NM, no mutation.
FIG. 1.
Alignments of FKS hot spot regions for amino acid alterations observed in this study. Amino acid positions are defined as equivalent to those for C. albicans.
A recent study analyzing 8 C. albicans strains showing fks1 mutation F641S, caspofungin MIC values from 2 to 4 μg/ml, and micafungin values of 0.5 to 8 μg/ml had anidulafungin MIC results at 0.12 to 0.5 μg/ml, suggesting that anidulafungin or in some cases micafungin could maintain the potency against these strains (17). However, similar levels of in vivo efficacy are noted for all echinocandin agents, raising concerns that switching to another compound of the same class when resistance is observed could not increase efficacy of the treatment (4, 17). Further studies are necessary to confirm the clinical and in vivo significance of these phenotypic differences.
C. glabrata and C. tropicalis strains exhibiting fks1 HS1 mutations demonstrated elevated caspofungin, anidulafungin, and micafungin MIC values (0.5 to ≥ 8 μg/ml) (Table 3), and these results were at or greater than recently established epidemiologic cutoff values (ECV) (Table 2), the ECV being the statistical method used to determine the wild-type MIC range (13, 16); in most cases, the values were distinctly higher than those for the wild-type population. Conversely, the C. albicans strain displaying F641Y FKS1 alteration displayed modestly elevated caspofungin MIC values (MIC at 1 μg/ml) and lower anidulafungin and micafungin MIC results (0.12 and 0.25 μg/ml, respectively). It is noteworthy that the anidulafungin MIC result for this strain was at the established ECV (Table 2).
Diploid species (C. albicans and C. krusei) carrying multiple alleles of fks1 displayed silent mutations on fks1 HS1. These silent mutations were heterozygous and were observed in 15 (46.9%) C. albicans (A1929T or C1923T) and 5 (45.4%) C. krusei (A2034G) isolates. All but two C. krusei strains showing the silent mutations displayed very low echinocandin MIC values (≤0.06 μg/ml). Two C. krusei strains exhibited modestly elevated caspofungin MIC results (1 and 2 μg/ml) but significantly lower micafungin and anidulafungin MIC values (0.06 μg/ml). Silent mutations were previously described among C. albicans strains (4), but considerably lower rates were observed in comparison to those in this study (6 out of 28 strains; 21.4%).
C. parapsilosis and C. guilliermondii routinely display higher echinocandin MIC values than those of other Candida species, and regardless of the higher MIC values, successful treatment of infections caused by these organisms at indicated dosages has been achieved (12). According to a previous report (6), the mechanism of this intrinsic reduced susceptibility appears to be the presence of polymorphisms in the HS regions of fks1. C. guilliermondii and C. parapsilosis strains tested in this study showed no mutations or polymorphism on the fks1 HS1 region.
Among 7 C. glabrata strains randomly selected for evaluation of fks1 HS2 and fks2 HS1 and HS2 mutations, 3 strains displayed amino acid alterations within FKS2 HS1 (S645P and L644W) (Fig. 1 and Table 3). Two C. glabrata strains carrying fks2 HS1 mutations encoding S645P substitution produced less-susceptible or resistant MIC values for all three echinocandin compounds (MIC results at 2 to ≥8 μg/ml). The MIC results for these two strains were comparable to the values observed for the C. glabrata strain carrying the same amino acid alteration (S645P) on FKS1 HS1 (Table 3). FKS1 HS1 amino acid alteration in position S645 appears to generate highly elevated MIC levels compared to other substitutions, with a decrease in the catalytic activity of the 1,3-β-d-GS by approximately one-half that of a wild-type strain (7). These observations seem to be in agreement with the elevated MIC results obtained in the present study for S645P alterations in FKS1 HS1 and FKS2 HS1.
The C. glabrata strain possessing FKS2 HS1 L644W substitution had lower MIC values for all three echinocandin agents, similar to or at the ECV (Table 2). The presence of an fks1 HS1 mutation leading to alteration L644W was previously detected in one C. tropicalis isolate and one C. krusei isolate, displaying very distinct caspofungin MIC results (1 and 8 μg/ml, respectively; tested using EUCAST methodologies). C. tropicalis possessed only the alteration L644W and low caspofungin MIC results, whereas the C. krusei strain showed an additional alteration that was not located within the HS but could be involved in the higher MIC values observed for this compound. The C. glabrata strain harboring the mutation leading to the alteration L644W from this study had only modest elevation of the caspofungin MIC result (0.25 μg/ml) but lower micafungin and anidulafungin MIC values (0.06 and 0.12 μg/ml, respectively). The role of this mutation in the resistance to different echinocandins should be further investigated.
None of the 8 selected C. albicans strains displayed fks1 HS2 mutations, including four strains showing silent mutations on fks1 HS1 (A1929T and C1923T). All C. albicans strains and four C. glabrata strains lacking fks1 HS2 and fks2 HS1 and HS2 mutations demonstrated very low MIC values for all echinocandin compounds (MIC values at ≤0.06 μg/ml for caspofungin and micafungin and ≤0.12 μg/ml for anidulafungin), below the ECVs for most of the strains tested (97 strains for caspofungin).
Echinocandins are valuable treatment options for invasive fungal infections due to low toxicity, infrequent side effects, and lack of cross-resistance with azoles. Reduced susceptibility to echinocandins in Candida spp. has been primarily associated with mutations in the HS1 fks1 subunit of 1,3-β-d-GS. In the present study, Candida spp. isolates showing distinct echinocandin MIC values were selected from worldwide collections and tested for the presence of fks1 HS1 mutations. Our results indicate that fks mutations appear to be uncommon despite the recent increase in the use of these antifungal agents.
Acknowledgments
The present study was funded by educational/research grants from Pfizer Inc. and Astellas Pharma Global Development, Inc.
Footnotes
Published ahead of print on 5 April 2010.
REFERENCES
- 1.Baixench, M. T., N. Aoun, M. Desnos-Ollivier, D. Garcia-Hermoso, S. Bretagne, S. Ramires, C. Piketty, and E. Dannaoui. 2007. Acquired resistance to echinocandins in Candida albicans: case report and review. J. Antimicrob. Chemother. 59:1076-1083. [DOI] [PubMed] [Google Scholar]
- 2.Clinical and Laboratory Standards Institute. 2008. M27-A3. Reference method for broth dilution antifungal susceptibility testing of yeasts. Third edition. Clinical and Laboratory Standards Institute, Wayne, PA.
- 3.Clinical and Laboratory Standards Institute. 2008. M27-S3. Reference method for broth dilution antifungal susceptibility testing of yeasts. 3rd informational supplement. Clinical and Laboratory Standards Institute, Wayne, PA.
- 4.Desnos-Ollivier, M., S. Bretagne, D. Raoux, D. Hoinard, F. Dromer, and E. Dannaoui. 2008. Mutations in the fks1 gene in Candida albicans, C. tropicalis, and C. krusei correlate with elevated caspofungin MICs uncovered in AM3 medium using the method of the European Committee on Antibiotic Susceptibility Testing. Antimicrob. Agents Chemother. 52:3092-3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gafter-Gvili, A., L. Vidal, E. Goldberg, L. Leibovici, and M. Paul. 2008. Treatment of invasive candidal infections: systematic review and meta-analysis. Mayo Clin. Proc. 83:1011-1021. [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Effron, G., S. K. Katiyar, S. Park, T. D. Edlind, and D. S. Perlin. 2008. A naturally occurring proline-to-alanine amino acid change in Fks1p in Candida parapsilosis, Candida orthopsilosis, and Candida metapsilosis accounts for reduced echinocandin susceptibility. Antimicrob. Agents Chemother. 52:2305-2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia-Effron, G., S. Park, and D. S. Perlin. 2009. Correlating echinocandin MIC and kinetic inhibition of fks1 mutant glucan synthases for Candida albicans: implications for interpretive breakpoints. Antimicrob. Agents Chemother. 53:112-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katiyar, S., M. Pfaller, and T. Edlind. 2006. Candida albicans and Candida glabrata clinical isolates exhibiting reduced echinocandin susceptibility. Antimicrob. Agents Chemother. 50:2892-2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kett, D. H., and G. F. Cubillos. 2008. Anidulafungin in the treatment of patients with invasive candidiasis. Int. J. Antimicrob. Agents 32(Suppl. 2):S99-S102. [DOI] [PubMed] [Google Scholar]
- 10.Leaw, S. N., H. C. Chang, H. F. Sun, R. Barton, J. P. Bouchara, and T. C. Chang. 2006. Identification of medically important yeast species by sequence analysis of the internal transcribed spacer regions. J. Clin. Microbiol. 44:693-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Messer, S. A., G. J. Moet, J. T. Kirby, and R. N. Jones. 2009. Activity of contemporary antifungal agents, including the novel echinocandin anidulafungin, tested against Candida spp., Cryptococcus spp., and Aspergillus spp.: report from the SENTRY Antimicrobial Surveillance Program (2006 to 2007). J. Clin. Microbiol. 47:1942-1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perlin, D. S. 2007. Resistance to echinocandin-class antifungal drugs. Drug Resist. Updat. 10:121-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pfaller, M. A., L. Boyken, R. J. Hollis, J. Kroeger, S. A. Messer, S. Tendolkar, R. N. Jones, and D. Diekema. 2010. Wild-type MIC distributions and epidemiological cutoff values (ECVs) for the echinocandins and Candida spp. J. Clin. Microbiol. 48:52-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pfaller, M. A., L. Boyken, R. J. Hollis, S. A. Messer, S. Tendolkar, and D. J. Diekema. 2005. In vitro activities of anidulafungin against more than 2,500 clinical isolates of Candida spp., including 315 isolates resistant to fluconazole. J. Clin. Microbiol. 43:5425-5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson, G. R., III, N. P. Wiederhold, A. C. Vallor, N. C. Villareal, J. S. Lewis, Jr., and T. F. Patterson. 2008. Development of caspofungin resistance following prolonged therapy for invasive candidiasis secondary to Candida glabrata infection. Antimicrob. Agents Chemother. 52:3783-3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turnidge, J., G. Kahlmeter, and G. Kronvall. 2006. Statistical characterisation of bacterial wild-type MIC value distributions and the determination of epidemiological cut-off values. Clin. Microbiol. Infect. 12:418-425. [DOI] [PubMed] [Google Scholar]
- 17.Wiederhold, N. P., J. L. Grabinski, G. Garcia-Effron, D. S. Perlin, and S. A. Lee. 2008. Pyrosequencing to detect mutations in FKS1 that confer reduced echinocandin susceptibility in Candida albicans. Antimicrob. Agents Chemother. 52:4145-4148. [DOI] [PMC free article] [PubMed] [Google Scholar]