Tigecycline is often one of the few therapeutic options for infections by multidrug-resistant Gram-negative bacteria. Therefore, accurate MIC testing is critical. Since in many laboratories tigecycline is not included in the routine panel of antibiotics for susceptibility testing, tigecycline MICs are frequently determined by Etest. However, previous studies have shown that tigecycline MICs determined by Etest may be influenced by the brand of Mueller-Hinton (MH) agar, hypothetically due to variations in the concentration of manganese (2). Furthermore, for Acinetobacter species with MICs ≥ 2 mg/liter by Etest, the MIC was at least 2 doubling dilutions lower by broth microdilution (BMD) (1). The aim of this study was to evaluate the Etest as a method to determine tigecycline MICs for Enterobacter species.
Tigecycline MICs were determined by BMD and Etest for 271 Enterobacter blood culture isolates (84% Enterobacter cloacae isolates and 16% Enterobacter aerogenes isolates) obtained in 2006 and 2007 from 12 laboratories in the Netherlands. BMD was performed using Becton Dickinson (BD) MH II medium for BMD (Merlin, Germany), according to International Organization for Standardization (ISO) guideline 20776-1 (3) (the reference method of the Clinical and Laboratory Standards Institute [CLSI]) and the European Committee on Antimicrobial Susceptibility Testing [EUCAST]). Etests (bioMérieux, France) were performed on agar with the same MH medium used in the BMD method and on Iso-Sensitest agar (Oxoid, United Kingdom). In each experiment, Escherichia coli ATCC 25922 was used as a control strain. FDA and EUCAST breakpoints were used (susceptible ≤ 2 mg/liter and ≤ 1 mg/liter, respectively). The manganese concentrations of the MH broth and both agars were determined using induction-coupled plasma atomic emission spectrometry (ICP-AES) after the medium was dissolved with 65% nitric acid.
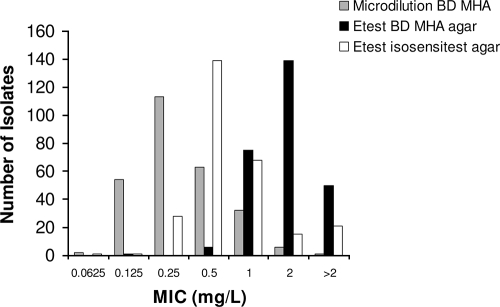
Compared to BMD, MICs determined by Etest were significantly higher using either MH agar or Iso-Sensitest agar (Fig. 1 and Table 1). The correlation between the results of the BMD and Etest on MH agar was high (Spearman's nonparametric correlation coefficient, 0.9; P < 0.001), and the geometric mean difference ± standard deviation (SD) was 2.4 ± 0.4 doubling dilutions, pointing toward a systematic difference between the methods. In 84% of the isolates, tigecycline MICs were 2 or more doubling dilutions higher by Etest on MH agar compared to the values found by BMD. The MICs determined by Etest on MH agar were also significantly higher than by Etest on Iso-Sensitest agar. No correlation between the manganese concentration in the test medium and the tigecycline MIC was observed (Table 1).
FIG. 1.
Distribution of MICs of 271 Enterobacter isolates by BMD, Etest on BD Mueller-Hinton agar (MHA), and Etest on Iso-Sensitest agar.
TABLE 1.
Susceptibility results for 271 Enterobacter isolates using BMD, Etest on MHA (BD), and Etest on Iso-Sensitest agar
| Method and medium | Manganese concn in medium (mg/kg) | MIC (mg/liter) |
Susceptibility rate (%)a: |
|||
|---|---|---|---|---|---|---|
| 50% | 90% | Geometric mean ± SDb | EUCAST (S ≤ 1) | FDA (S ≤ 2) | ||
| BMD on BD MH broth | 0.029c | 0.25 | 1 | 0.42 ± 0.41* | 97** | 100** |
| Etest | ||||||
| BD MH agar | 0.043c | 1.5 | 3 | 1.94 ± 1.6* | 30** | 82** |
| Iso-Sensitest agar | 0.48 | 0.5 | 1.5 | 0.88 ± 1.2* | 88** | 94** |
Susceptibility rates found by using EUCAST and FDA susceptibility breakpoints (S), which were ≤1 and ≤2 mg/liter, respectively. **, P < 0.001 for comparisons of susceptibility rates between each detection method using the Fisher exact test.
*, P < 0.001 for comparisons of MICs between each detection method using a t test.
The higher manganese concentration in the BD MH agar compared to BD MH broth could be explained by the manganese content of pure agar (0.002 mg/kg).
The susceptibility rates were significantly higher by the BMD method than by Etest (Table 1). When FDA breakpoints were used, false nonsusceptibility was observed in 18% of the isolates when performing Etest on MH agar. When EUCAST breakpoints were used, 97% of isolates were susceptible by the BMD method, whereas only 30% were susceptible by Etest on MH agar, corresponding to false nonsusceptibility in 67% of the isolates. No false-susceptible Etest results were observed using either MH agar or Iso-Sensitest agar.
The MICs of the E. coli control strain were within the specified range (0.03 mg/liter to 0.25 mg/liter) for the three methods, although the MICs were 0.0325 mg/liter using BMD and 0.25 mg/liter using Etest on MH agar.
In conclusion, this study shows that tigecycline MICs for Enterobacter species determined by Etest are significantly higher than MICs determined by BMD. Differences in the manganese concentration of the test media could not explain the observed differences, and further research is required to resolve this issue. We conclude that nonsusceptible tigecycline Etest results should be confirmed using a BMD method. The misclassification of isolates as tigecycline nonsusceptible may deny patients an important antibiotic option.
Footnotes
Published ahead of print on 29 March 2010.
REFERENCES
- 1.Casal, M., F. Rodriguez, B. Johnson, E. Garduno, F. Tubau, R. O. de Lejarazu, A. Tenorio, M. J. Gimenez, R. Bartolome, C. Garcia-Rey, L. Aguilar, and N. Garcia-Escribano. 2009. Influence of testing methodology on the tigecycline activity profile against presumably tigecycline-non-susceptible Acinetobacter spp. J. Antimicrob. Chemother. 64:69-72. [DOI] [PubMed] [Google Scholar]
- 2.Fernandez-Mazarrasa, C., O. Mazarrasa, J. Calvo, A. del Arco, and L. Martinez-Martinez. 2009. High concentrations of manganese in Mueller-Hinton agar increase MICs of tigecycline determined by Etest. J. Clin. Microbiol. 47:827-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.International Organization for Standardization. 2006. Clinical laboratory testing and in vitro diagnostic test systems. Susceptibility testing of infectious agents and evaluation of performance of antimicrobial susceptibility test devices. Part 1. Reference method for testing the in vitro activity of antimicrobial agents against rapidly growing aerobic bacteria involved in infectious diseases. International Standard 20776-1. International Organization for Standardization, Geneva, Switzerland.