Abstract
Drug resistance against dihydrofolate reductase (DHFR) inhibitors—such as pyrimethamine (PM)—has now spread to almost all regions where malaria is endemic, rendering antifolate-based malaria treatments highly ineffective. We have previously shown that the di-amino quinazoline QN254 [5-chloro-N′6′-(2,5-dimethoxy-benzyl)-quinazoline-2,4,6-triamine] is active against the highly PM-resistant Plasmodium falciparum V1S strain, suggesting that QN254 could be used to treat malaria in regions with a high prevalence of antifolate resistance. Here, we further demonstrate that QN254 is highly active against Plasmodium falciparum clinical isolates, displaying various levels of antifolate drug resistance, and we provide biochemical and structural evidence that QN254 binds and inhibits the function of both the wild-type and the quadruple-mutant (V1S) forms of the DHFR enzyme. In addition, we have assessed QN254 oral bioavailability, efficacy, and safety in vivo. The compound displays favorable pharmacokinetic properties after oral administration in rodents. The drug was remarkably efficacious against Plasmodium berghei and could fully cure infected mice with three daily oral doses of 30 mg/kg. In the course of these efficacy studies, we have uncovered some dose limiting toxicity at higher doses that was confirmed in rats. Thus, despite its relative in vitro selectivity toward the Plasmodium DHFR enzyme, QN254 does not show the adequate therapeutic index to justify its further development as a single agent.
Malaria control is a global public health priority that has been hampered by the rapid development and spread of resistance against antimalarials. As a consequence, the World Health Organization (WHO) recommends the use of artemisinin-containing combination therapies (ACTs) as a first-line treatment for malaria. Although ACTs are designed to reduce the chance of artemisinin drug resistance development, there are considerable concerns that this may already have occurred. For instance, there is now mounting evidence that the efficacy of artemisinin derivatives is reduced in Southeast Asia, where artemisinin derivatives have been used for a long time as monotherapies (7, 28, 53). This is a cause of concern since the spread of artemisinin resistance will compromise the usefulness of ACTs globally. Thus, there is an urgent need to discover and develop new alternative drugs.
For several decades, dihydrofolate reductase (DHFR) has been targeted with different classes of chemical entities for the development of new therapies for a broad range of therapeutic indications, including several parasitic diseases (13). DHFR catalyzes the reduction of dihydrofolate (DHF) to tetrahydrofolate (THF), which is an essential cofactor in the biosynthesis of deoxythymidylate monophosphate (dTMP), a metabolite essential to DNA synthesis and cell replication.
Pyrimethamine (PM) is a potent inhibitor of the Plasmodium DHFR enzyme, and this compound has been widely used in combination with the dihydropteroate synthetase (DHPS) inhibitor sulfadoxine. Unfortunately, PM resistance is now common, rendering this drug ineffective (30). One of the documented mechanisms of antifolate resistance is through the mutation of the target itself—the Plasmodium falciparum dhfr-ts (Pfdhfr-ts) gene, encoding for a bifunctional enzyme which possesses both DHFR and thymidylate synthase (TS) activities carried by two distinct subdomains (29). PM resistance is associated with the mutation of the amino acid Ser to Asn at codon 108 of DHFR (S108N). Ancillary mutations of N51I and C59R are associated with an increase in resistance, and the presence of the mutation I164L results in an even higher level of PM resistance (14, 47). The presence of these mutations significantly decreases the sensitivity of the PfDHFR enzyme to PM inhibition in a biochemical assay (48).
The antifolate triazine WR99210 (3, 22) is potent against P. falciparum bearing quadruple mutations of DHFR at S108N, N51I, C59R, and I164L (QM PfDHFR) (20). However, WR99210 has shown limited efficacy in vivo due to poor oral bioavailability and displayed some gastrointestinal toxicity. Attempts have been made to circumvent these issues and a prodrug form of WR99210 known as PS-15 has been shown to be orally active (2). The resolution of the three-dimensional structure of wild-type (WT) and QM PfDHFR—with either PM or WR99210 bound to its active site—provided structural insights into DHFR PM resistance mechanisms, as well as some understanding of the structural features of WR99210 that allow this compound to retain affinity for QM PfDHFR (55, 56).
The quinazoline pharmacophore has been successfully used to design drugs for the treatment of cancer and other human diseases. For example, the DHFR inhibitor, trimetrexate (5-methyl-6-[(3,4,5-trimethoxy-phenylamino)-methyl]-quinazoline-2,4-diamine) has been developed to treat various cancers in human patients (15, 32, 33). In a previous study, a series of quinazoline derivatives was tested against the highly PM-resistant P. falciparum strain (V1S) and the DHFR inhibitor 2,4-diamino-5-chloro-6-[N-(2,5-dimethoxybenzyl)-amino]quinazoline (or QN254 here and compound 1 in reference 34) was found to have potent activity—with an IC50 (i.e., the inhibitory concentration that reduces parasite growth by 50% in vitro) of 9 nM (34). Considering the increasingly widespread PM drug resistance, we set out to perform the experiments described here with the goal of further assessing the potential of QN254 as a candidate antimalarial to replace the failing PM. Collectively, our data demonstrate that QN254 (i) binds and inhibits the QM PfDHFR enzyme, (ii) is active on drug-resistant clinical isolates, and (iii) displays pharmacological properties compatible with an oral antimalarial drug candidate. However, preliminary toxicological findings indicate that QN254 does not show a therapeutic window sufficiently large to warrant its progression to the next development stage.
MATERIALS AND METHODS
Drugs and reagents.
PM and chloroquine used as standard drugs for evaluation on clinical isolates were purchased from Sigma (Cole, United Kingdom). WR99210 was a gift from Steve Ward (Liverpool Tropical School, Liverpool, United Kingdom). In the Ki determination, cycloguanil and WR99210 were gifts from Tirayut Vilaivan (Chulalongkorn University, Bangkok, Thailand). Standard drugs for in vivo efficacy testing were obtained from Mepha, Ltd., Switzerland (artesunate); Sigma (United States) (chloroquine diphosphate); and F. Hoffmann-LaRoche, Ltd., Switzerland (mefloquine hydrochloride). QN254 was prepared as described elsewhere (39).
DHFR biochemical assay and X-ray structure determination. (i) Ki determination.
Recombinant PfDHFR enzymes of P. falciparum and human were prepared from Escherichia coli BL21(DE3) bearing pET17b expression plasmids of P. falciparum WT and quadruple mutant (S108N, N51I, C59R, and I164L) [QM Pfdhfr] (17), and human dhfr (hdhfr) (18), using a methotrexate column as described previously (51). Binding affinities (Ki values) of QN254 and standard antifolate antimalarials, cycloguanil, PM, and WR99210 were determined as described elsewhere (51).
(ii) X-ray structure determination.
Preparation of recombinant PfDHFR-TS bifunctional enzymes was carried out as previously reported (4). Crystals were flash-frozen in liquid nitrogen by dipping for 10 s in a corresponding crystallization solution containing 20% glycerol as a cryoprotectant. X-ray diffraction data were collected under a cold nitrogen stream (100 K) at a wavelength of 1.54 Å on an FR591 rotating anode X-ray generator equipped with a nonius KappaCCD detector. The data were processed by using Denzo and Scalepack in the HKL2000 suite (35). Refinement was performed using a 2.1-Å 1J3K crystal structure as a template in both CNS (1) and REFMAC5 (26), along with model building in program O (16) and model validation in Procheck (24, 25) and Moleman2 (21). Figures were prepared with PyMOL (6).
Plasmodium strains and culturing methods. (i) Parasite strains and isolates.
We analyzed the in vitro activity of QN254 against clinical isolates from Kenya with different dhfr genotypes. These clinical isolates were collected in Kilifi between 2006 and 2008 as part of the malaria studies and were in vitro adapted for long-term culture as described elsewhere (40).
(ii) In vitro culture and chemosensitivity test.
P. falciparum cultures were carried out in RPMI 1640 (Gibco-BRL, United Kingdom) medium supplemented with 10% (vol/vol) normal human serum, 25 mM bicarbonate, 2 mM glutamine, 25 mM HEPES buffer, physiological concentrations of para-aminobenzoic acid (5 nM), and folic acid (23 nM). Antimalarial activity was measured in the presence of various concentrations of each compound by using radioisotopic incorporation (49). The results were expressed as the drug concentration required for 50% inhibition (IC50) of [3H]hypoxanthine incorporation into parasite nucleic acid, using nonlinear regression analysis of the dose-response curve.
(iii) Parasite genotyping of dhfr.
After in vitro adaptation of parasites, infected blood samples were spotted onto filter paper and stored. Parasite genomic material from these filter papers was prepared using the methanol procedure and point mutations at codons 108, 51, 59, and 164 of dhfr were analyzed by PCR and restriction fragment length polymorphism (PCR-RFLP) enzyme digestion as described elsewhere (31). Statistical analyses were carried out using Stata, version 9 (StataCorp, College Station, TX), using the Kruskal-Wallis nonparametric test for comparison of medians. The level of significance was set at P < 0.05.
In vivo pharmacokinetic (PK) studies.
The Institutional Animal Care and Use Committee of Novartis Institute for Tropical Diseases, registered with the Agri-Food and Veterinary Authority, Government of Singapore, approved all animal experimental protocols. QN254 was formulated at a concentration of 2.5 mg/ml for a dose of 25 mg/kg given orally (p.o.) and at 1-mg/ml concentration for a dose of 5 mg/kg given intravenously (i.v.). The solution formulation for i.v. dosing contained 10% NMP (n-methyl pyrrolidone), 30% PEG 400, and 60% of 10% vitamin E-TPGS. The suspension formulation for p.o. dosing was 0.5% carboxymethyl cellulose. For PK studies, blood samples were collected at several time points after p.o. and i.v. dosing in mice and rats.
(iv) Extraction and LC-MS/MS analysis of QN254.
Plasma samples were extracted with acidified acetonitrile for QN254 using an extractant/plasma ratio of 8:1. Analyte quantification was performed by liquid chromatography-tandem mass spectrometry (LC-MS/MS). LC was performed by using an Agilent 1100 HPLC system (Santa Clara, CA), with an Agilent Zorbax XDB Phenyl (3.5 μm, 4.6 by 75 mm) column at an oven temperature of 35°C, coupled with an API3200 triple quadruple mass spectrometer (Applied Biosystems, Foster City, CA). Instrument control and data acquisition were performed using Analyst 1.4.2 software (Applied Biosystems). The mobile phases used were phase A (water-acetic acid [99.8:0.2, vol/vol]) and phase B (acetonitrile-acetic acid [99.8:0.2, vol/vol]), using a gradient, with flow rate of 1.0 ml/min and a run time of 5 min. Under these conditions, the analyte retention time was 2.9 min. Compound detection on the mass spectrometer was performed in electrospray positive ionization mode and using multiple reaction monitoring for specificity, together with their optimized MS parameters. Analysis of study samples was performed on different days using an identical method with a ten-point calibration curve spanning 3 orders of magnitude in compound concentration. The lower and upper limits of quantification were 12 and 7,500 ng/ml, respectively. Intraday variability was established with triplicate quality control samples at three concentration levels. The relative standard deviation was under 15%. Interday variability is not reported as calibration curves were reprepared and reanalyzed with every set of study samples and results accepted if interday variability was found to be under 15%.
(v) PK data analysis.
The mean value from three animals at each time point was plotted against time to give the plasma concentration-time profile. PK parameters were determined by using WinNonlin Professional, version 5.0.1 (Pharsight, California), by noncompartmental modeling using software model 200 for oral dosing and model 201 for i.v. dosing. The oral bioavailability (F) was calculated as the ratio between the area under the curve (AUC) after p.o. administration and the AUC after i.v. administration corrected for dose (F = AUC p.o. × dose i.v./AUC i.v. × dose p.o.).
In vivo antimalarial efficacy studies.
All in vivo efficacy studies were carried out at the Swiss Tropical Institute, adhering to the regulations of the veterinary authorities of Basel-Stadt. The murine P. berghei ANKA was used as previously described (37). Experimental and control groups of five female NMRI mice (20 to 22 g) were infected i.v. with 108 parasitized erythrocytes/ml, in a volume of 0.2 ml (from a fresh mouse donor). In untreated control mice, parasitemia rises regularly to ∼30% by day 3 postinfection and causes death of the animals between day 5 and day 7 postinfection.
Experimental compounds were prepared in 0.5% carboxymethyl cellulose and administered orally. Comparators (artesunate, chloroquine, and mefloquine) were prepared in ETPGS vehicle (10% ethanol, 30% PEG 400, and 60% of a 10% vitamin ETPGS solution) and administered orally. Parasitemia was determined microscopically. The activity was calculated as the difference between parasitemia for the control and treated groups expressed as a percentage relative to the control group. The survival time was also recorded up to 30 days after infection. A compound was considered curative if the animal survived to day 30 after infection with no detectable parasites. The 50% effective dose (ED50) and ED90 values were assessed at day 3 by plotting the dose as a function of parasitemia, using nonlinear fitting with the Microcal Origin Statistical Program (OriginLab, Northampton, MA). All data reported are based on ≥10 mice, except for the three-times treatment with QN254 which, because of the observed toxicity, were performed only once with a cohort of five mice.
Rat toxicological study.
QN254 was suspended in 10% of a 1% (vol/vol) aqueous Solutol HS15 solution and 90% of a 0.5% aqueous methylcellulose M0555 solution and administered to groups of five male rats at daily oral (by gavage) doses of 50, 150, or 500 mg/kg/day. Animals (Wistar rats; Harlan Laboratories, Ltd., Füllinsdorf, Switzerland) were approximately 10 weeks of age (254 to 292 g) at the start of dosing. Clinical observations, body weight and food consumption determinations, clinical pathology (hematology and clinical chemistry) evaluations, and gross pathology examinations without organ weight determinations (due to the premature sacrifice of all treated groups) were performed on all groups. Microscopic examinations were conducted on all gross lesions and on a limited standard list of organs and tissues from animals assigned to the control and low-dose groups. At day 1 and day 8 blood samples were collected for toxicokinetic analyses.
RESULTS
Inhibitory activity (Ki): QN254 is a potent inhibitor of both the WT and quadruple mutant plasmodium DHFR enzymes.
QN254 was compared to the antimalarials PM and cycloguanil (inactive on QM PfDHFR), as well as the triazine WR99210, a potent inhibitor of all mutant PfDHFRs, including QM PfDHFR (20). The results of these experiments are summarized in Table 1. Consistent with previously published results showing that QN254 is active against the highly PM-resistant strain V1S, our results show that QN254 displays binding affinities comparable to WR99210 against both WT PfDHFR (Ki = 0.39 ± 0.05 nM) and QM PfDHFR (Ki = 0.58 ± 0.06 nM). It is also worth noting that QN254 appears to show a slightly better selectivity than WR99210, as shown by the higher human/plasmodium Ki ratio (26 for QN254 versus 15 for WR99210).
TABLE 1.
Results of competitive binding kinetics experiments on DHFR enzymes of P. falciparum WT (WT-PfDHFR) and quadruple mutant (QM-PfDHFR [S108N, N51I, C59R, and I164L]) and human (hDHFR) against QN254, WR99210, PM, and cycloguanila
| Agent | Mean Ki (nM) ± SD |
Ki ratio |
|||
|---|---|---|---|---|---|
| WT-PfDHFR | QM-PfDHFR | hDHFR | hDHFR/WT- pfDHFR | QM-PfDHFR/ WT-PfDHFR | |
| CLG | 1.51 ± 0.1 | 454 ± 38 | 55.6 ± 7.8 | 37 | 300 |
| PM | 0.59 ± 0.05 | 385 ± 163 | 30.8 ± 1.4 | 52 | 652 |
| WR99210 | 0.5 ± 0.1 | 1.9 ± 0.8 | 7.7 ± 0.4 | 15 | 1.8 |
| QN254 | 0.39 ± 0.05 | 0.58 ± 0.06 | 10.2 ± 0.6 | 26 | 1.5 |
PM, pyrimethamine; CLG, cycloguanil. Ki, binding constant.
WR99210 and QN254 have similar binding modes on QM PfDHFR.
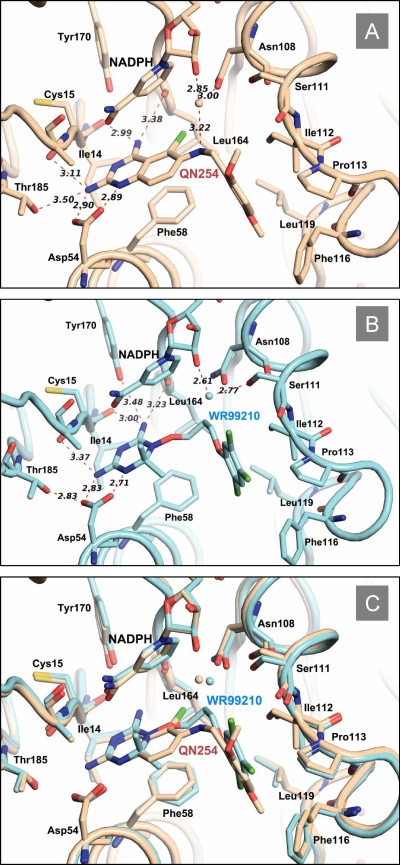
To determine the structural features important for QN254 binding to PfDHFR, we determined the crystal structure of a QN254/QM PfDHFR-TS (V1S) complex at 2.7-Å resolution (see Table S1 in the supplemental material). Figure 1 shows the structure of this complex and shows the key interactions required for QN254 binding. QN254 makes direct interactions with several key residues in the DHFR active site, as well as interactions via a water-mediated hydrogen bond. Compared to WR99210 binding (56), the diamino-quinazoline scaffold of QN254 hydrogen bonds with the side chain of Asp54 and gains an additional H-bond with Thr185 to replace the loss of an H-bond with Tyr170 presumably due to the larger nature of the quinazoline ring (Fig. 1A and B). Extensive hydrogen bonds between the enzyme carbonyl backbones at Ile14, Cys15, Leu164, and the diamino-quinazoline ring were established as observed in the WR99210 structure. Interactions that increase the binding of QN254 over WR99210 include a water-mediated hydrogen bond between the N-6 of the quinazoline ring with both the side chain of Asn108 and the C-2 hydroxyl of the nicotinamide adenosine dinucleotide phosphate (NADPH) ribose (Fig. 1A). In the WR99210 structure, a water molecule also mediates a hydrogen bond between Ser111 and the ribose of NADPH (Fig. 1B), but in this case, only enhances the interaction with NADPH (56). QN254 makes additional van der Waals contacts and π-π interactions with Leu46, Met55, and Phe58 side chains. The phenyl substituent of both inhibitors makes similar van der Waals interactions with Ile112, Pro113, Phe116, and Leu119. Consistent with the biochemical data reported above, comparison of the QN254/QM PfDHFR and WR99210/QM PfDHFR complexes (Fig. 1C) shows that both compounds overlap extensively within the active site of the QM PfDHFR enzyme. It appears that, in both cases, the flexibility of the linker region between the diamino-quinazoline or diamino-dihydrotriazine moieties, as well as the lipophilicity of the phenyl ring are critically important for binding to the QM PfDHFR. Moreover, QN254 takes advantage of the mutation at Asn108 by making an additional interaction via a water molecule (Fig. 1A, 6-N…O, 3.22 Å)—absent in the WR99210/V1S structure—that presumably contributes to its better affinity for the QM enzyme compared to WR99210.
FIG. 1.
Cocrystal structure of dihydrofolate reductase quadruple mutant (mutations at codons 108, 51, 59, and 164) showing H-bond interactions with QN254 (A) and WR99210 (B). Both complexes are superposed in panel C.
QN254 shows potent antimalarial activity against P. falciparum PM drug-resistant clinical isolates with various PfDHFR genotypes.
To further demonstrate that QN254 is active against P. falciparum even in areas of widespread PM drug resistance, we determined the in vitro activity of QN254 against 27 clinical isolates collected in Kilifi (Kenya) between 2006 and 2008. These isolates were part of a larger study in which we measured the activity of standard antifolate drugs (including PM) (19). For comparison purposes, in Table 2, we report again the data pertaining to PM along with the previously published results obtained with QN254 (19). As expected, PM was found to be largely inactive on these clinical isolates, with a median IC50 of 733.26 nM. In agreement with these data, and as discussed in our previous report, no WT isolates were found, and more than 72% of the tested isolates carried triple mutations in Pfdhfr (S108N, N51I, and C59R). Interestingly, one isolate carried the four mutations (S108N, N51I, C59R, and I164L), mutations present in the V1S form (19).
TABLE 2.
Relationship between PM and QN254 median inhibitory concentrations that kill 50% of parasitemia (IC50) and dihydrofolate reductase (dhfr) genotypes of clinical isolatesa
| DHFR genotypeb | IC50 (nM)c |
|
|---|---|---|
| PM | QN254 | |
| Double mutant* | 371 (8) | 4.48 (6) |
| Triple mutant* | 778.28 (24) | 11.66 (20) |
| Quadruple mutant | 3,690.79 (1) | 7.61 (1) |
No WT dhfr isolate was identified.
The double mutants are S108N and N51I or S108N and C59R; the triple mutants are S108, N51I and C59R; and the quadruple mutants are S108N, N51, C59R, and I164L. *, None of the tested differences between double and triple mutants were significant at P = 0.05 (Kruskal-Wallis test).
The number of isolates is given in parentheses. Data pertaining to PM were already published elsewhere (38).
In sharp contrast to the results obtained with PM, QN254 was potent against all isolates, with a median IC50 of 9.55 nM. The median IC50s of QN254 against double mutants (S108N/N51I or S108N/C59R) and triple mutants were 4.48 and 11.66 nM, respectively, and this difference was not statistically significant (Table 2). The quadruple mutant isolate had an IC50 of 7.61 nM. Collectively, these data clearly show that QN254 is active on clinically relevant P. falciparum isolates with a wide range of mutations in Pfdhfr (including quadruple mutations) associated with significant PM drug resistance.
QN254 is orally bioavailable and displays slow but complete absorption as well as a long half-life in rodents.
To determine the PK properties of QN254 in vivo, we measured the plasma concentration-time profile upon p.o. and i.v. administration in both mice and rats (Fig. 2). PK parameters are reported in Table S2 in the supplemental material. In both rodent species, QN254 displayed a high volume of distribution, greater than the total body water (volume of distribution at steady state [Vss] = 2.85 and 4.7 liters/kg in mice and rats, respectively), and the total systemic clearance was moderate to low at 57 and 30% of hepatic blood flow in mice and rats, respectively. Consistently, QN254 displayed an i.v. half-life of 0.8 h in mice and a relatively longer half-life of 3.5 h rats. After oral administration, the elimination half-lives were around 6 and 9 h in mice and rats, respectively. QN254 oral absorption was slow but complete with a time to maximum concentration (Tmax) of 4 h and apparent oral bioavailability of 100% in mice. These data indicate that the low aqueous solubility of the compound (∼2 mg/liter at pH 6.8) does not limit its absorption in rodents. In rats, QN254 absorption is similarly slow, and the relative oral bioavailability is 63%. Notably, the maximum plasma concentrations (Cmax) values were about 5.1 and 2.8 μM in mice and rats, respectively, which are concentrations several hundredfold above the P. falciparum IC50.
FIG. 2.
PK profile of QN254 upon i.v. and p.o. administration. QN254 was administered p.o. at 25 mg/kg (⧫) and i.v. at 5 mg/kg (□) to mice (A) and rats (B). Plasma concentrations were measured over time and are indicated in ng/ml ± the standard deviations (n = 3). Note that the timescale is different in panels A and B. Drug levels for the last time points in profile A were detectable but below the lower limit of quantification (12 ng/ml).
QN254 is orally efficacious in the P. berghei-infected malaria mouse model.
We tested the efficacy of QN254 in the P. berghei malaria mouse model, a reference animal model (12). Upon oral administration of single doses at 10, 15, 30, 60, and 100 mg/kg, QN254 showed a dose-dependent parasitemia reduction, 59 and 73% for 10- and 15-mg/kg doses, respectively; >99.99% for all other doses (Table 3). The efficacious doses of QN254 required to inhibit the growth of 50% (ED50) and 90% parasitemia (ED90) were 7.2 and 12.4 mg/kg, respectively, values that are comparable to those of artesunate (5.9 and 20.5 mg/kg) but slightly higher than those of mefloquine (3.8 and 5.2 mg/kg) and chloroquine (1.9 and 4.2 mg/kg) (52).
TABLE 3.
QN254 antimalarial activity in the P. berghei murine malaria modela
| Dosing regimen (agent and dose [mg/kg])b | Parasitemia reduction (%) | Avg mouse survival (days) | Cure rate (%) | Toxicity rate (%)c |
|---|---|---|---|---|
| QN254 | ||||
| 1 × 10 | 59 | 6.0 | 0 | 0 |
| 1 × 15 | 73 | 6.0 | 0 | 0 |
| 1 × 30 | >99.99 | 8.1 | 0 | 0 |
| 1 × 60 | >99.99 | 12.0 | 0 | 0 |
| 1 × 100 | >99.99 | 23.4 | 50 | 0 |
| 3 × 30 | >99.99 | 28.4 | 80 | 0 |
| 3 × 60 | >99.99 | NAd | 60 | 40* |
| 3 × 100 | >99.99 | NA | 20 | 80* |
| Artesunate | ||||
| 1 × 100 | 98 | 7.0 | 0 | 0 |
| 3 × 30 | 98.7 | 12.2 | 0 | 0 |
| Chloroquine | ||||
| 1 × 100 | 99.6 | 12.5 | 0 | 0 |
| 3 × 30 | 99.8 | 14.3 | 0 | 0 |
| Mefloquine | ||||
| 1 × 100 | 89 | 25.5 | 0 | 0 |
| 3 × 30 | 98.8 | 22.1 | 0 | 0 |
Results represent the percent inhibition of parasitemia compared to untreated controls. QN254 was prepared in 0.5% carboxymethyl cellulose. The mean survival of control animals was 5.6 to 6.2 days.
The solution formulation for artesunate, chloroquine, and mefloquine consisted of 10% ethanol, 30% PEG 400 (polyethylene glycol 400), and 60% of a 10% vitamin-ETPGS solution.
*, Mice died around day 10 and were parasite-free. There were 10 mice per test group except for the “3 ×” treatment with QN254 because of ethical reasons due to the observed toxicity.
NA, not available.
These results are consistent with the good PK data by the oral route reported above; however, one should bear in mind that the PK analysis was done in naive mice, and one cannot rule out that the PK parameters in infected mice might be different. Mouse survival was significantly increased to 8.1 days with a single dose treatment at 30 mg/kg and increased to 12 days at 60 mg/kg. At 100 mg/kg, QN254 displayed activity superior to the currently marketed antimalarial drugs artesunate and chloroquine, with parasitemia reduction >99.99% and an average mouse survival prolongation of 23.4 days. In addition, 50% of mice were cured with a single dose of 100 mg/kg QN254, whereas no mice were cured with a similar drug treatment regimen for all three standard antimalarial drugs.
At three daily oral doses of 30 mg/kg, the cure rate was improved to 80% with a mouse survival prolongation of about 28.4 days. We found some dose-limiting toxicity at three doses of 60 mg/kg and three doses of 100 mg/kg since six of the ten treated mice died at around day 10, despite being free of parasites at the time. The four surviving mice were cured and free of parasites at day 30.
Collectively, our data show that QN254 displays potent in vivo oral activity in the P. berghei mouse model and cured infected mice upon administration of three daily oral doses as low as 30 mg/kg. However, three-day dosing at higher doses (≥60 mg/kg) led to toxicity and death in some animals. These results indicate that the therapeutic window of QN254 might be too narrow.
QN254 displays severe toxicity in rats and has a small therapeutic window.
In an exploratory 2-week rat toxicology study we found that QN254 was not tolerated upon repeated oral administration of daily dose of ≥50 mg/kg. In the present study, all animals had to be euthanized by day eight, because of serious adverse effects, body weight loss, decreased food consumption, and other clinical signs (soft feces, piloerection, and reduced motor activity).
In all rats sacrificed preterm, histopathological analysis revealed marked gastrointestinal tract and bone marrow toxicity. The major findings included degenerative/regenerative, atrophic and inflammatory changes in the gastric tract (mostly in the small intestine and cecal mucosa) and massive bone marrow atrophy. The affected tissues being very proliferative, this type of toxicity is consistent with on-target effects through sustained inhibition of DHFR and inhibition of cell proliferation, as has been previously reported for other DHFR inhibitors (2, 54).
Toxicokinetics data generated in the course of the present study showed that in rats, upon administration of a 50-mg/kg dose of QN254, daily plasma exposure at day 1 (AUC0-24 = 27,600 ng·h/ml) is only about twice the plasma exposure level reached at the efficacious dose in mice (AUC0-24 ≈ 16,990 ng·h/ml at an ED99 of 26.5 mg/kg). Thus, the QN254 therapeutic window is smaller than 2.
DISCUSSION
We have previously shown that QN254 is active in vitro against the P. falciparum strain (V1S) carrying a copy of the QM-Pfdhfr gene. Here, we provide data that the diamino-quinazoline DHFR inhibitor QN254 (i) binds to and inhibits the QM PfDHFR enzyme; (ii) displays potent antimalarial activity against antifolate drug-resistant P. falciparum clinical isolates with various DHFR genotypes, including QM-Pfdhfr; (iii) shows PK properties compatible with oral dosing; and (iv) displays excellent in vivo therapeutic efficacy in the P. berghei malaria mouse model.
Because antifolate drugs such as PM and proguanil have lost their efficacy due to resistance, it is crucial for any new molecule targeting this enzyme to show activity against all PM-resistant DHFR mutant forms. In an earlier study, we assessed the in vitro activity of WR99210 against the same clinical isolates tested in this publication (mean IC50 of about 0.72 nM [19]). Thus, compared to QN254, WR99210 is about 10 times more potent against parasite despite having a slightly lower binding affinity to PfDHFR than QN254. It remains to be determined whether better diffusion across membranes of parasitized red blood cells and/or an additional off-target effect could explain the higher WR99210 cellular potency.
Using a functional/biochemical assay and crystallographic structural data, we provide further evidence that QN254 binds to and inhibits Plasmodium WT and QM DHFR enzymes efficiently. The resolution of the three-dimensional structure of WT and QM PfDHFR—with either PM or WR99210 bound to its active site—provided structural insights into DHFR PM resistance mechanisms, as well as some understanding of the structural features allowing WR99210 to retain affinity for QM PfDHFR (55, 56). Our crystallographic data, describing the structure of a ternary complex of QN254 bound to QM PfDHFR, show that QN254 binds to its target in a manner similar to WR99210. Previous X-ray crystallographic studies (5) with a compound closely related to QN254 and the Pneumocystis carinii DHFR enzyme suggested that Phe69 (P. carinii numbering) or Phe116 (P. falciparum numbering) was engaged in close hydrophobic contact with the 5′ methoxy group on the phenyl ring of the lipophilic tail. The authors of that study also speculated that this interaction may contribute to the relative selectivity toward P. carinii (and possibly P. falciparum) since the conserved phenylalanine was not present in the mammalian DHFR enzymes. Careful analysis of our cocrystal QN254/PfDHFR structure failed to reveal such an interaction and in fact we could only identify weak van der Waals interactions between the lipophilic tail and the hydrophobic cavity present in the active site of QM PfDHFR. The structure-activity relationship (SAR) data for the class of quinazoline compounds are not very clear and are currently limited to what is described in previous publications (8-11, 38, 39).
Consistent with our structural data demonstrating the absence of specific interactions between the phenyl ring tail and PfDHFR, the available SAR data show that a large number of modifications of the lipophilic tails are tolerated. With respect to selectivity, our data do not provide any obvious structural or molecular explanation to rationalize the relative selectivity of QN254 for P. falciparum versus the human enzyme.
For more than 30 years, it has been known that QN derivatives inhibit the growth and proliferation of eukaryotic parasites such as Toxoplasma, Pneumocystis, Trypanosoma, and Plasmodium (8, 10, 11, 23, 36, 38, 45, 46). In fact, an extensive series of quinazolines have previously proved to be efficacious in rodent and simian malaria models (41-44). The most advanced candidate of this series, WR158122, entered clinical development as a candidate antimalarial drug but was later abandoned because of its relatively poor oral efficacy in humans (43).
After oral administration QN254 is well absorbed, displays a long half-life and an excellent oral bioavailability in rodents, as well as in larger species such as dog (data not shown). Although PAMPA and Caco-2 in vitro permeability data were predictive of the good absorption and bioavailability, our in vitro metabolic stability data predicted that QN254 would be subject to rapid hepatic clearance in all preclinical species, as well as in humans (data not shown). Nonetheless, in vivo QN254 showed a low (rats and dogs) to moderate (mouse) systemic clearance in all preclinical species. We cannot explain the discrepancy between in vitro and in vivo hepatic clearance, and further studies would be required to determine the main clearance mechanism in vivo.
Consistent with our PK results, QN254 displays good efficacy in the P. berghei mouse model through the oral route. However, in P. berghei-infected mice, we have found evidence of toxicity upon repeated daily dosing for 3 days at doses as low as 60 mg/kg and, indeed, further rat toxicology studies showed that QN254 does not possess an adequate therapeutic window. At this juncture we cannot unambiguously establish whether the observed toxicity is due to on- or off-target effects; as an example, we have evidence that QN254 presents some inherent risk of cardiotoxicity because of its ability to bind and inhibit the hERG channel in vitro (hERG IC50 = 1.4 μM). Nonetheless, the rat histopathological data showing that a highly proliferative tissue such as the bone marrow is massively affected is consistent with on-target toxicity and sustained inhibition of folates metabolism through DHFR inhibition (50, 54). Thus, despite its relative selectivity toward plasmodium DHFR enzyme in vitro, QN254 appears to potently inhibit mammalian (rat) DHFR in vivo. Based on the exposure reached at the efficacious dose and the exposure reached in rats upon dosing at 50 mg/kg in the toxicology study, we estimated that the therapeutic window is less than two. A narrow therapeutic window is indeed a hallmark of almost all antifolates and has historically been a significant hurdle to the development of new therapies based on DHFR inhibition. PM, for example, displays an acute LD50 of 130 mg/kg in mice (50). Mitigating this toxicity risk is the fact that antifolates such as PM are commonly used in combination at low doses (<5 mg/kg in humans). Because of the severe toxicity observed in rats, the Novartis Institute for Tropical Diseases is no longer pursuing the development of QN254. Nevertheless, further research into the feasibility of strategies—e.g., a prodrug approach, supplementation with folate derivatives such as 5-methyl-THF (27), or a combination with antimalarials showing synergistic effects with DHFR inhibitors—aiming to increase the therapeutic window of QN254 may be warranted.
Supplementary Material
Acknowledgments
We thank the director of the Kenya Medical Research Institute for permission to publish these data.
S.K. is an international research scholar of Howard Hughes Medical Institute. The Kenya Medical Research Institute received support from the EU Commission under Framework 6 as part of the AntiMal Integrated Project 018834, the European and Developing Countries Clinical Trials Partnership, and the Wellcome Trust WT077092. BIOTEC was supported by grants from Thailand-TDR (T-2) and the Medicines for Malaria Venture. Novartis Institute for Tropical Diseases and the Swiss Tropical Institute receive funding from a joint grant of the Medicines for Malaria Venture and the Wellcome Trust (WT078285).
Footnotes
Published ahead of print on 29 March 2010.
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1.Brunger, A. T., P. D. Adams, G. M. Clore, W. L. DeLano, P. Gros, R. W. Grosse-Kunstleve, J. S. Jiang, J. Kuszewski, M. Nilges, N. S. Pannu, R. J. Read, L. M. Rice, T. Simonson, and G. L. Warren. 1998. Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. D 54(Pt. 5):905-921. [DOI] [PubMed] [Google Scholar]
- 2.Canfield, C. J., W. K. Milhous, A. L. Ager, R. N. Rossan, T. R. Sweeney, N. J. Lewis, and D. P. Jacobus. 1993. PS-15: a potent, orally active antimalarial from a new class of folic acid antagonists. Am. J. Trop. Med. Hyg. 49:121-126. [DOI] [PubMed] [Google Scholar]
- 3.Childs, G. E., and C. Lambros. 1986. Analogues of N-benzyloxydihydrotriazines: in vitro antimalarial activity against Plasmodium falciparum. Ann. Trop. Med. Parasitol. 80:177-181. [DOI] [PubMed] [Google Scholar]
- 4.Chitnumsub, P., J. Yuvaniyama, J. Vanichtanankul, S. Kamchonwongpaisan, M. D. Walkinshaw, and Y. Yuthavong. 2004. Characterization, crystallization and preliminary X-ray analysis of bifunctional dihydrofolate reductase-thymidylate synthase from Plasmodium falciparum. Acta Crystallogr. D Biol. Crystallogr. 60:780-783. [DOI] [PubMed] [Google Scholar]
- 5.Cody, V., N. Galitsky, J. R. Luft, W. Pangborn, S. F. Queener, and A. Gangjee. 2002. Analysis of quinazoline and pyrido[2,3-d]pyrimidine N9-C10 reversed-bridge antifolates in complex with NADP+ and Pneumocystis carinii dihydrofolate reductase. Acta Crystallogr. D Biol. Crystallogr. 58:1393-1399. [DOI] [PubMed] [Google Scholar]
- 6.Delano, W. L. 2002. The PyMOL molecular graphics system. Delano Scientific, Palo Alto, CA.
- 7.Dondorp, A. M., F. Nosten, P. Yi, D. Das, A. P. Phyo, J. Tarning, K. M. Lwin, F. Ariey, W. Hanpithakpong, S. J. Lee, P. Ringwald, K. Silamut, M. Imwong, K. Chotivanich, P. Lim, T. Herdman, S. S. An, S. Yeung, P. Singhasivanon, N. P. Day, N. Lindegardh, D. Socheat, and N. J. White. 2009. Artemisinin resistance in Plasmodium falciparum malaria. N. Engl. J. Med. 361:455-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elslager, E. F., O. D. Bird, J. Clarke, S. C. Perricone, and D. F. Worth. 1972. Folate antagonists. 9. 2,4-Diamino-6-((aralkyl)alkylamino)quinazolines, a potent class of antimetabolites with prodigious antimalarial effects. J. Med. Chem. 15:1138-1146. [DOI] [PubMed] [Google Scholar]
- 9.Elslager, E. F., J. Clarke, L. M. Werbel, D. F. Worth, and J. Davoll. 1972. Folate antagonists. 3. 2,4-Diamino-6-(heterocyclic)quinazolines, a novel class of antimetabolites with potent antimalarial and antibacterial activity. J. Med. Chem. 15:827-836. [DOI] [PubMed] [Google Scholar]
- 10.Elslager, E. F., M. P. Hutt, P. Jacob, J. Johnson, B. Temporelli, L. M. Werbel, D. F. Worth, and L. Rane. 1979. Folate antagonists. 15. 2,3-Diamino-6-(2-naphthylsulfonyl)quinazoline and related 2,4-diamino-6-[(phenyl and naphthyl)sulfinyl and sulfonyl]quinazolines, a potent new class of antimetabolites with phenomenal antimalarial activity. J. Med. Chem. 22:1247-1257. [DOI] [PubMed] [Google Scholar]
- 11.Elslager, E. F., P. Jacob, J. Johnson, L. M. Werbel, D. F. Worth, and L. Rane. 1978. Folate antagonists. 13. 2,4-Diamino-6-](alpha,alpha,alpha-trifluoro-m-tolyl)thio]quinazoline and related 2,4-diamino-6-[(phenyl- and naphthyl)thio]quinazolines, a unique class of antimetabolites with extraordinary antimalarial and antibacterial effects. J. Med. Chem. 21:1059-1070. [DOI] [PubMed] [Google Scholar]
- 12.Fidock, D. A., P. J. Rosenthal, S. L. Croft, R. Brun, and S. Nwaka. 2004. Antimalarial drug discovery: efficacy models for compound screening. Nat. Rev. Drug Discov. 3:509-520. [DOI] [PubMed] [Google Scholar]
- 13.Gangjee, A., S. Kurup, and O. Namjoshi. 2007. Dihydrofolate reductase as a target for chemotherapy in parasites. Curr. Pharm. Des. 13:609-639. [DOI] [PubMed] [Google Scholar]
- 14.Gregson, A., and C. V. Plowe. 2005. Mechanisms of resistance of malaria parasites to antifolates. Pharmacol. Rev. 57:117-145. [DOI] [PubMed] [Google Scholar]
- 15.Jackman, A. L., F. T. Boyle, and K. R. Harrap. 1996. Tomudex (ZD1694): from concept to care, a programme in rational drug discovery. Invest. New Drugs 14:305-316. [DOI] [PubMed] [Google Scholar]
- 16.Jones, T. A., J. Y. Zou, S. W. Cowan, and M. Kjeldgaard. 1991. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A 47:110-119. [DOI] [PubMed] [Google Scholar]
- 17.Kamchonwongpaisan, S., R. Quarrell, N. Charoensetakul, R. Ponsinet, T. Vilaivan, J. Vanichtanankul, B. Tarnchompoo, W. Sirawaraporn, G. Lowe, and Y. Yuthavong. 2004. Inhibitors of multiple mutants of Plasmodium falciparum dihydrofolate reductase and their antimalarial activities. J. Med. Chem. 47:673-680. [DOI] [PubMed] [Google Scholar]
- 18.Kamchonwongpaisan, S., J. Vanichtanankul, B. Tarnchompoo, J. Yuvaniyama, S. Taweechai, and Y. Yuthavong. 2005. Stoichiometric selection of tight-binding inhibitors by wild-type and mutant forms of malarial (Plasmodium falciparum) dihydrofolate reductase. Anal. Chem. 77:1222-1227. [DOI] [PubMed] [Google Scholar]
- 19.Kiara, S. M., J. Okombo, V. Masseno, L. Mwai, I. Ochola, S. Borrmann, and A. Nzila. 2009. In vitro activity of antifolate and polymorphism in dihydrofolate reductase of Plasmodium falciparum isolates from Kenyan coast: emergence of parasites with Ile-164-Leu mutation. Antimicrob. Agents Chemother. [DOI] [PMC free article] [PubMed]
- 20.Kinyanjui, S. M., E. K. Mberu, P. A. Winstanley, D. P. Jacobus, and W. M. Watkins. 1999. The antimalarial triazine WR99210 and the prodrug PS-15: folate reversal of in vitro activity against Plasmodium falciparum and a non-antifolate mode of action of the prodrug. Am. J. Trop. Med. Hyg. 60:943-947. [DOI] [PubMed] [Google Scholar]
- 21.Kleywegt, G. J. 1997. Validation of protein models from CA coordinates alone. J. Mol. Biol. 273:371-376. [DOI] [PubMed] [Google Scholar]
- 22.Knight, D. J., P. Mamalis, and W. Peters. 1982. The antimalarial activity of N-benzyl-oxydihydrotriazines. III. The activity of 4,6-diamino-1,2-dihydro-2,2-dimethyl-1-(2,4,5,-trichloropropyloxy)-1,3,5-triazine hydrobromide (BRL 51084) and hydrochloride (BRL 6231). Ann. Trop. Med. Parasitol. 76:1-7. [PubMed] [Google Scholar]
- 23.Kovacs, J. A., C. J. Allegra, B. A. Chabner, J. C. Swan, J. Drake, M. Lunde, J. E. Parrillo, and H. Masur. 1987. Potent effect of trimetrexate, a lipid-soluble antifolate, on Toxoplasma gondii. J. Infect. Dis. 155:1027-1032. [DOI] [PubMed] [Google Scholar]
- 24.Laskowski, R. A., J. A. Rullmann, M. W. MacArthur, R. Kaptein, and J. M. Thornton. 1996. AQUA and PROCHECK-NMR: programs for checking the quality of protein structures solved by NMR. J. Biomol. NMR 8:477-486. [DOI] [PubMed] [Google Scholar]
- 25.Morris, A. L., M. W. MacArthur, E. G. Hutchinson, and J. M. Thronton. 1992. Stereochemical quality of protein structure coordinates. Proteins 12:345-364. [DOI] [PubMed] [Google Scholar]
- 26.Murshudov, G. N., A. A. Vagin, and E. J. Dodson. 1997. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D 53:240-255. [DOI] [PubMed] [Google Scholar]
- 27.Nduati, E., A. Diriye, S. Ommeh, L. Mwai, S. Kiara, V. Masseno, G. Kokwaro, and A. Nzila. 2008. Effect of folate derivatives on the activity of antifolate drugs used against malaria and cancer. Parasitol. Res. 102:1227-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noedl, H., Y. Se, K. Schaecher, B. L. Smith, D. Socheat, and M. M. Fukuda. 2008. Evidence of artemisinin-resistant malaria in western Cambodia. N. Engl. J. Med. 359:2619-2620. [DOI] [PubMed] [Google Scholar]
- 29.Nzila, A. 2006. Inhibitors of de novo folate enzymes in Plasmodium falciparum. Drug Discov. Today 11:939-944. [DOI] [PubMed] [Google Scholar]
- 30.Nzila, A. 2006. The past, present and future of antifolates in the treatment of Plasmodium falciparum infection. J. Antimicrob. Chemother. 57:1043-1054. [DOI] [PubMed] [Google Scholar]
- 31.Nzila, A. M., E. K. Mberu, J. Sulo, H. Dayo, P. A. Winstanley, C. H. Sibley, and W. M. Watkins. 2000. Towards an understanding of the mechanism of pyrimethamine-sulfadoxine resistance in Plasmodium falciparum: genotyping of dihydrofolate reductase and dihydropteroate synthase of Kenyan parasites. Antimicrob. Agents Chemother. 44:991-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Dwyer, P. J., R. J. DeLap, S. A. King, A. J. Grillo-Lopez, D. F. Hoth, and B. Leyland-Jones. 1987. Trimetrexate: clinical development of a nonclassical antifolate. NCI Monogr. 1987:105-109. [PubMed] [Google Scholar]
- 33.O'Dwyer, P. J., D. D. Shoemaker, J. Plowman, J. Cradock, A. Grillo-Lopez, and B. Leyland-Jones. 1985. Trimetrexate: a new antifol entering clinical trials. Invest. New Drugs 3:71-75. [DOI] [PubMed] [Google Scholar]
- 34.Ommeh, S., E. Nduati, E. Mberu, G. Kokwaro, K. Marsh, A. Rosowsky, and A. Nzila. 2004. In vitro activities of 2,4-diaminoquinazoline and 2,4-diaminopteridine derivatives against Plasmodium falciparum. Antimicrob. Agents Chemother. 48:3711-3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Otwinowski, Z. M., and W. Minor. 1979. Processing of X-ray diffraction data collected in oscillation mode. Method Enzymol. 276:307-326. [DOI] [PubMed] [Google Scholar]
- 36.Piper, J. R., C. A. Johnson, C. A. Krauth, R. L. Carter, C. A. Hosmer, S. F. Queener, S. E. Borotz, and E. R. Pfefferkorn. 1996. Lipophilic antifolates as agents against opportunistic infections. 1. Agents superior to trimetrexate and piritrexim against Toxoplasma gondii and Pneumocystis carinii in in vitro evaluations. J. Med. Chem. 39:1271-1280. [DOI] [PubMed] [Google Scholar]
- 37.Ridley, R. G., H. Matile, C. Jaquet, A. Dorn, W. Hofheinz, W. Leupin, R. Masciadri, F. P. Theil, W. F. Richter, M. A. Girometta, A. Guenzi, H. Urwyler, E. Gocke, J. M. Potthast, M. Csato, A. Thomas, and W. Peters. 1997. Antimalarial activity of the bisquinoline trans-N1,N2-bis(7-chloroquinolin-4-yl)cyclohexane-1,2-diamine: comparison of two stereoisomers and detailed evaluation of the S,S enantiomer, Ro 47-7737. Antimicrob. Agents Chemother. 41:677-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosowsky, A., C. E. Mota, J. E. Wright, J. H. Freisheim, J. J. Heusner, J. J. McCormack, and S. F. Queener. 1993. 2,4-Diaminothieno[2,3-d]pyrimidine analogues of trimetrexate and piritrexim as potential inhibitors of Pneumocystis carinii and Toxoplasma gondii dihydrofolate reductase. J. Med. Chem. 36:3103-3112. [DOI] [PubMed] [Google Scholar]
- 39.Rosowsky, A., C. E. Mota, J. E. Wright, and S. F. Queener. 1994. 2,4-Diamino-5-chloroquinazoline analogues of trimetrexate and piritrexim: synthesis and antifolate activity. J. Med. Chem. 37:4522-4528. [DOI] [PubMed] [Google Scholar]
- 40.Sasi, P., A. Abdulrahaman, L. Mwai, S. Muriithi, J. Straimer, E. Schieck, A. Rippert, M. Bashraheil, A. Salim, J. Peshu, K. Awuondo, B. Lowe, M. Pirmohamed, P. Winstanley, S. Ward, A. Nzila, and S. Borrmann. 2009. In vivo and in vitro efficacy of amodiaquine against Plasmodium falciparum in an area of continued use of 4-aminoquinolines in East Africa. J. Infect. Dis. 199:1575-1582. [DOI] [PubMed] [Google Scholar]
- 41.Schmidt, L. H. 1978. Plasmodium falciparum and Plasmodium vivax infections in the owl monkey (Aotus trivirgatus). III. Methods employed in the search for new blood schizonticidal drugs. Am. J. Trop. Med. Hyg. 27:718-737. [DOI] [PubMed] [Google Scholar]
- 42.Schmidt, L. H. 1979. Studies on the 2,4-diamino-6-substituted quinazolines. II. Activities of selected derivatives against infections with various drug-susceptible and drug-resistant strains of Plasmodium falciparum and Plasmodium vivax in owl monkeys. Am. J. Trop. Med. Hyg. 28:793-807. [PubMed] [Google Scholar]
- 43.Schmidt, L. H. 1979. Studies on the 2,4-diamino-6-substituted quinazolines. III. The capacity of sulfadiazine to enhance the activities of WR-158,122 and WR-159,412 against infections with various drug-susceptible and drug-resistant strains of Plasmodium falciparum and Plasmodium vivax in owl monkeys. Am. J. Trop. Med. Hyg. 28:808-818. [PubMed] [Google Scholar]
- 44.Schmidt, L. H., and R. N. Rossan. 1979. Studies on the 2,4-diamino-6 substituted quinazolines. I. Antimalarial activities of 2,4-diamino-6-[(3,4-dichlorobenzyl)-nitrosoamino]-quinazoline (CI-679) as exhibited in rhesus monkeys infected with the Ro or Ro/PM strains of Plasmodium cynomolgi. Am. J. Trop. Med. Hyg. 28:781-792. [PubMed] [Google Scholar]
- 45.Senkovich, O., V. Bhatia, N. Garg, and D. Chattopadhyay. 2005. Lipophilic antifolate trimetrexate is a potent inhibitor of Trypanosoma cruzi: prospect for chemotherapy of Chagas’ disease. Antimicrob. Agents Chemother. 49:3234-3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Senkovich, O., B. Pal, N. Schormann, and D. Chattopadhyay. 2003. Trypanosoma cruzi genome encodes a pteridine reductase 2 protein. Mol. Biochem. Parasitol. 127:89-92. [DOI] [PubMed] [Google Scholar]
- 47.Sibley, C. H., J. E. Hyde, P. F. Sims, C. V. Plowe, J. G. Kublin, E. K. Mberu, A. F. Cowman, P. A. Winstanley, W. M. Watkins, and A. M. Nzila. 2001. Pyrimethamine-sulfadoxine resistance in Plasmodium falciparum: what next? Trends Parasitol. 17:582-588. [DOI] [PubMed] [Google Scholar]
- 48.Sirawaraporn, W., R. Sirawaraporn, S. Yongkiettrakul, A. Anuwatwora, G. Rastelli, S. Kamchonwongpaisan, and Y. Yuthavong. 2002. Mutational analysis of Plasmodium falciparum dihydrofolate reductase: the role of aspartate 54 and phenylalanine 223 on catalytic activity and antifolate binding. Mol. Biochem. Parasitol. 121:185-193. [DOI] [PubMed] [Google Scholar]
- 49.Sixsmith, D. G., W. M. Watkins, J. D. Chulay, and H. C. Spencer. 1984. In vitro antimalarial activity of tetrahydrofolate dehydrogenase inhibitors. Am. J. Trop. Med. Hyg. 33:772-776. [DOI] [PubMed] [Google Scholar]
- 50.Stephan-Guldner, M. 1993. Preclinical toxicology and safety pharmacology of brodimoprim in comparison to trimethoprim and analogs. J. Chemother. 5:400-410. [PubMed] [Google Scholar]
- 51.Tarnchompoo, B., C. Sirichaiwat, W. Phupong, C. Intaraudom, W. Sirawaraporn, S. Kamchonwongpaisan, J. Vanichtanankul, Y. Thebtaranonth, and Y. Yuthavong. 2002. Development of 2,4-diaminopyrimidines as antimalarials based on inhibition of the S108N and C59R+S108N mutants of dihydrofolate reductase from pyrimethamine-resistant Plasmodium falciparum. J. Med. Chem. 45:1244-1252. [DOI] [PubMed] [Google Scholar]
- 52.Vennerstrom, J. L., S. Arbe-Barnes, R. Brun, S. A. Charman, F. C. Chiu, J. Chollet, Y. Dong, A. Dorn, D. Hunziker, H. Matile, K. McIntosh, M. Padmanilayam, J. Santo Tomas, C. Scheurer, B. Scorneaux, Y. Tang, H. Urwyler, S. Wittlin, and W. N. Charman. 2004. Identification of an antimalarial synthetic trioxolane drug development candidate. Nature 430:900-904. [DOI] [PubMed] [Google Scholar]
- 53.White, N. J. 2008. Qinghaosu (artemisinin): the price of success. Science 320:330-334. [DOI] [PubMed] [Google Scholar]
- 54.Winstanley, P. A., E. K. Mberu, I. S. Szwandt, A. M. Breckenridge, and W. M. Watkins. 1995. In vitro activities of novel antifolate drug combinations against Plasmodium falciparum and human granulocyte CFUs. Antimicrob. Agents Chemother. 39:948-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yuthavong, Y., J. Yuvaniyama, P. Chitnumsub, J. Vanichtanankul, S. Chusacultanachai, B. Tarnchompoo, T. Vilaivan, and S. Kamchonwongpaisan. 2005. Malarial (Plasmodium falciparum) dihydrofolate reductase-thymidylate synthase: structural basis for antifolate resistance and development of effective inhibitors. Parasitol. 130:249-259. [DOI] [PubMed] [Google Scholar]
- 56.Yuvaniyama, J., P. Chitnumsub, S. Kamchonwongpaisan, J. Vanichtanankul, W. Sirawaraporn, P. Taylor, M. D. Walkinshaw, and Y. Yuthavong. 2003. Insights into antifolate resistance from malarial DHFR-TS structures. Nat. Struct. Biol. 10:357-365. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.