Abstract
The activity of telavancin and comparators was assessed against a contemporary (2007 and 2008) global collection of 10,000 isolates of Staphylococcus aureus. Telavancin was very active against methicillin-susceptible and -resistant S. aureus (MSSA and MRSA, respectively; MIC50/90 for both, 0.12/0.25 μg/ml; 100.0% susceptible). This agent was 2-, 4-, and 8-fold more potent than daptomycin (MIC90, 0.5 μg/ml), vancomycin or quinupristin-dalfopristin (MIC90, 1 μg/ml), and linezolid (MIC90, 2 μg/ml) against MRSA, respectively. These data show a potent activity of telavancin tested against a current global collection of S. aureus.
Antimicrobial drug resistance among Gram-positive pathogens represents an ongoing worldwide therapeutic challenge. Since the 1990s, increasing rates of methicillin-resistant Staphylococcus aureus (MRSA) have been documented, and results from the National Healthcare Safety Network have recently shown that 56.2% of S. aureus isolates related to cases of device- and health care-associated infections in the United States were MRSA (10). This frequent phenotypic resistance feature found among hospital-acquired (HA) S. aureus strains is usually associated with resistance to other antimicrobial classes, such as macrolides, lincosamides, aminoglycosides, and tetracyclines (8).
In addition to HA MRSA, the emergence and rapid dissemination of community-associated MRSA have been commonly reported (4). Although other antimicrobial agents active against Gram-positive organisms (daptomycin, linezolid, and quinupristin-dalfopristin) demonstrate in vitro activity against S. aureus and are clinically available, they have shown limitations when treating serious infections caused by some Gram-positive pathogens. S. aureus nonsusceptibility during prolonged treatment with daptomycin has been reported (9), and linezolid and quinupristin-dalfopristin are considered bacteriostatic against some key Gram-positive organisms. Thus, vancomycin has remained the treatment of choice for many MRSA infections (18). However, ongoing reports of unfavorable clinical responses to vancomycin when treating infections caused by S. aureus displaying vancomycin MIC values at the limit of the susceptibility range (≥2 μg/ml) have led to considerable concern about the management of serious infections caused by this pathogen (5, 13).
This clinical scenario has prompted the pharmaceutical industry to develop new drugs with enhanced antimicrobial properties against Gram-positive cocci. Among the new agents, telavancin was recently approved in the United States and Canada as a once-daily treatment for adults with complicated skin and skin structure infections (cSSSI) caused by Gram-positive bacteria, including MRSA (1). Telavancin is a concentration-dependent, bactericidal lipoglycopeptide with a distinct dual mode of action, which includes inhibition of cell wall synthesis and disruption of essential bacterial membrane barrier functions (11, 16). This report summarizes the in vitro activity of telavancin versus currently marketed glycopeptides and other antimicrobial agents against MRSA and methicillin-susceptible S. aureus (MSSA) isolates collected from hospitalized patients during a comprehensive global surveillance program.
During 2007 and 2008, medical centers located in North America (27 centers in the United States), Europe (28 centers in 13 countries), Latin America (10 centers in 4 countries), and the Asia-Pacific region (APAC; 45 centers in 11 countries) were requested to forward to a monitoring central laboratory (JMI Laboratories, North Liberty, IA) consecutive, nonduplicate, clinically relevant pathogens recovered from prescribed specimen types. A total of 10,000 S. aureus isolates were selected for this investigation. These isolates were distributed among four regions, North America (5, 000), Europe (2, 000), APAC (2, 000), and Latin America (1, 000), with equal distribution of MRSA and MSSA. Bacterial identification was confirmed by the central monitoring site using standard algorithms. The isolates were tested for susceptibility by the reference Clinical and Laboratory Standards Institute (CLSI) broth microdilution method (2) using commercially prepared and validated panels (TREK Diagnostic Systems, Cleveland, OH) in cation-adjusted Mueller-Hinton broth. Antimicrobial agents representing the most common therapeutic classes and examples of drugs used for empirical or directed treatment of S. aureus were tested. Interpretation of MIC results was in accordance with published CLSI (M100-S19) (3) and European Committee on Antimicrobial Susceptibility Testing (EUCAST) criteria for clinical MIC breakpoints (http://www.srga.org/eucastwt/MICTAB/index.html, January 2010). The telavancin susceptibility breakpoint for S. aureus (≤1 μg/ml) was that recently approved by the U.S. FDA (1). The quality control strains utilized were S. aureus ATCC 29213 and Enterococcus faecalis ATCC 29212; all MIC results were within CLSI-listed ranges.
The isolates included in this investigation were recovered from blood (39.2%), skin and skin structure (34.5%), respiratory (18.0%), urinary tract (1.2%), bone/joint (0.5%), catheter (0.3%), and other (6.3%) clinical specimens. The in vitro activity of telavancin and comparator agents tested against MRSA and MSSA is summarized in Table 1. Telavancin showed potent activity against MRSA (MIC90, 0.25 μg/ml; 100.0% susceptible), and it was 2-, 4-, and 8-fold more potent than daptomycin (MIC90, 0.5 μg/ml), vancomycin or quinupristin-dalfopristin (MIC90, 1 μg/ml), and linezolid (MIC90, 2 μg/ml), respectively. These comparators still showed high susceptibility rates (≥98.7% by CLSI and EUCAST criteria) against MRSA. Among the other comparators, only trimethoprim-sulfamethoxazole (TMP/SMX) demonstrated significant coverage against S. aureus (MIC50/90, ≤0.5/≤0.5 μg/ml; 92.4% susceptible).
TABLE 1.
In vitro activity of telavancin and other anti- Gram-positive comparator agents tested by reference methods against a worldwide collection of clinical S. aureus isolates
| Organism group (no. tested)/ antimicrobial agent | MIC50b | MIC90b | % Susceptible/resistanta |
|
|---|---|---|---|---|
| CLSI | EUCAST | |||
| MRSA (5,000) | ||||
| Telavancin | 0.12 | 0.25 | 100.0/—c | 100.0/ |
| Vancomycin | 1 | 1 | 99.9/0.0 | 99.9/0.1 |
| Teicoplanin | ≤2 | ≤2 | >99.9/<0.1 | 98.7/1.3 |
| Daptomycin | 0.25 | 0.5 | 99.9/— | 99.9/0.1 |
| Linezolid | 1 | 2 | >99.9/— | >99.9/<0.1 |
| Quinupristin-dalfopristin | 0.5 | 1 | 99.5/0.2 | 99.5/0.2 |
| Levofloxacin | >4 | >4 | 21.6/77.9 | 21.6/77.9 |
| Erythromycin | >4 | >4 | 13.9/85.6 | 14.2/85.6 |
| Clindamycin | ≤0.25 | >2 | 54.7/45.0 | 54.3/45.3 |
| Gentamicin | ≤1 | >8 | 77.9/21.1 | 77.1/22.9 |
| Tetracycline | ≤1 | >8 | 80.7/19.1 | 80.1/19.9 |
| TMP/SMX | ≤0.5 | ≤0.5 | 92.4/7.6 | 92.4/7.6 |
| MSSA (5,000) | ||||
| Telavancin | 0.12 | 0.25 | 100.0/— | 100.0/— |
| Vancomycin | 1 | 1 | 100.0/0.0 | 100.0/0.0 |
| Teicoplanin | ≤2 | ≤2 | 100.0/0.0 | 99.9/0.1 |
| Daptomycin | 0.25 | 0.5 | 100.0/— | 100.0/0.0 |
| Linezolid | 2 | 2 | 100.0/— | 100.0/0.0 |
| Quinupristin-dalfopristin | ≤0.25 | 0.5 | 99.9/0.0 | 99.9/0.0 |
| Levofloxacin | ≤0.5 | ≤0.5 | 92.9/6.8 | 92.9/6.8 |
| Erythromycin | ≤0.25 | >4 | 74.3/25.2 | 74.5/25.2 |
| Clindamycin | ≤0.25 | ≤0.25 | 95.0/4.9 | 94.6/5.0 |
| Gentamicin | ≤1 | ≤1 | 96.6/3.1 | 96.4/3.6 |
| Tetracycline | ≤1 | ≤1 | 94.6/5.0 | 94.2/5.8 |
| TMP/SMX | ≤0.5 | ≤0.5 | 98.7/1.3 | 98.7/1.3 |
The MIC interpretive criteria used were published by the CLSI (M100-S19) (3) and EUCAST (http://www.srga.org/eucastwt/MICTAB/index.html). The telavancin susceptibility breakpoint for S. aureus (≤1 μg/ml) was that recently approved by the U.S. FDA.
Values are in micrograms per milliliter.
—, no breakpoints available.
The methicillin resistance phenotype did not adversely affect the telavancin MIC values, a finding also noted for vancomycin, teicoplanin, daptomycin, linezolid, and TMP/SMX when comparing the MRSA MIC90 results directly with the MSSA MIC90 results (Table 1). A slight increase in the quinupristin-dalfopristin MIC90 values was observed when it was tested against MRSA (MIC90, 1 μg/ml) compared to those obtained for MSSA isolates (MIC90, 0.5 μg/ml). Comparator agents such as levofloxacin (92.9% susceptible), clindamycin (≥94.6% susceptible), gentamicin (≥96.4% susceptible), and tetracycline (≥94.2% susceptible) were only active when tested against MSSA (Table 1).
Table 2 shows the telavancin MIC distribution for MRSA isolates collected from four geographic regions. A log-normal distribution and an overall modal MIC value of 0.12 μg/ml were observed, as well as differences in the telavancin MIC distribution among the four evaluated regions. A telavancin modal MIC value of 0.12 μg/ml was observed for MRSA isolates originating from North America and Europe (62.8 and 61.7% of the MRSA isolates were inhibited at 0.12 μg/ml, respectively), while a modal MIC of 0.25 μg/ml was noted for the isolates from Latin America and the APAC region (55.4 and 50.7% of the MRSA isolates were inhibited at 0.25 μg/ml, respectively). However, telavancin inhibited all staphylococci at ≤0.5 μg/ml.
TABLE 2.
Telavancin MIC distribution for 5,000 MRSA isolates collected in four geographic regions in 2007 and 2008
| Region (no. of isolates tested) | % Occurrence at telavancin MIC (μg/ml) of: |
|||||
|---|---|---|---|---|---|---|
| ≤0.015 | 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | |
| North America (2,500) | 0.1 | 0.1 | 3.8 | 62.8a | 31.4 | 1.8 |
| Europe (1,000) | 0.1 | 8.1 | 61.7 | 29.4 | 0.7 | |
| APAC (1,000) | 2.3 | 39.6 | 50.7 | 7.4 | ||
| Latin America (500) | 0.2 | 1.0 | 37.8 | 55.4 | 5.6 | |
| All (5,000) | <0.1 | <0.1 | 4.1 | 55.5 | 37.2 | 3.1 |
Modal MICs are in bold.
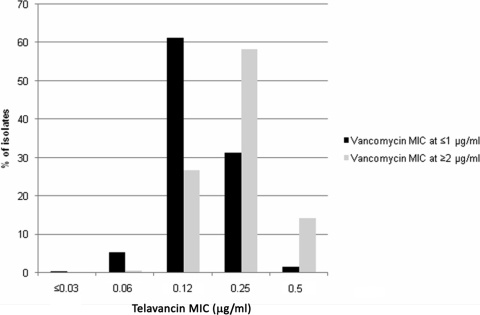
Several studies have documented increased clinical failure rates when treating infections caused by MRSA showing elevated vancomycin MIC values (>1 μg/ml), and some published clinical outcome studies suggest adjusting the vancomycin susceptibility breakpoint to ≤1 μg/ml (17). When telavancin activity was evaluated against S. aureus isolates with vancomycin MIC values of ≥2 μg/ml (3.8% of the S. aureus isolates), the telavancin modal MIC (58.2% at 0.25 μg/ml) and MIC90 values (0.5 μg/ml) shifted only 1 doubling dilution higher than those of isolates having lower (≤1 μg/ml) vancomycin MIC results (modal MIC, 61.3% at 0.12 μg/ml; MIC90, 0.25 μg/ml; Fig. 1). Furthermore, five and three S. aureus isolates displayed a phenotype of nonsusceptibility to daptomycin (MIC, 2 to 4 μg/ml) and intermediate susceptibility to vancomycin (VISA; MIC, 4 μg/ml), respectively. The telavancin MIC values obtained against daptomycin-nonsusceptible and VISA isolates were 0.25 to 0.5 and 0.12 to 0.25 μg/ml, respectively (data not shown).
FIG. 1.
Distribution of telavancin MICs when the drug was tested against S. aureus isolates displaying vancomycin MIC values of ≤1 (9,624 strains) and ≥2 μg/ml (376 strains).
The results obtained during this investigation corroborate previous reports describing the potent in vitro activity (MIC90) of telavancin against clinical S. aureus isolates (MIC90, 0.25 to 0.5 μg/ml) (6, 7, 12, 14). Differences in the modal MIC values of telavancin were noted when it was tested against MRSA isolates from distinct geographic regions. It is tempting to speculate that these results could be due to the presence of specific MRSA lineages within each geographic area, which may reflect different susceptibility profiles. When telavancin activity was evaluated against S. aureus isolates with higher vancomycin MIC values (≥2 μg/ml), including VISA isolates (MIC, 4 μg/ml), a slight decrease in potency (2-fold) was observed. These results are also in agreement with earlier publications that demonstrated telavancin MIC values between 0.25 and 1 μg/ml when it was tested against VISA isolates (6, 15).
In summary, based upon MIC90 values, telavancin had stable activity over time against S. aureus, including isolates exhibiting a methicillin resistance phenotype, regardless of the geographic origin of the strains (6, 7). The results of this surveillance report confirm the stable potency of telavancin and emphasize its potential role as an effective alternative to current approved agents for the therapy of cSSSI due to S. aureus organisms, especially MRSA (1).
Acknowledgments
Expert technical and informatic support was kindly provided by D. Biedenbach, P. Rhomberg, J. Ross, A. Small, and M. Stillwell.
The study and publication process were funded by Astellas Pharma Global Development, Inc., and Theravance, Inc. Circulation of the draft manuscript for scientific review by Astellas Pharma Global Development, Inc., and Theravance, Inc., and collation of comments were conducted by Emily Hutchinson, a medical writer at Envision Scientific Solutions funded by Astellas Pharma Global Development, Inc.
Footnotes
Published ahead of print on 12 April 2010.
REFERENCES
- 1.Astellas Pharma Global Development, Inc. Last accessed 8 January 2010. VIBATIV package insert. Astellas Pharma Global Development, Inc., Deerfield, IL. http://www.vibativ.com.
- 2.Clinical and Laboratory Standards Institute. 2006. M07-A7, Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard—seventh edition. Clinical and Laboratory Standards Institute, Wayne, PA.
- 3.Clinical and Laboratory Standards Institute. 2009. M100-S19. Performance standards for antimicrobial susceptibility testing. 19th informational supplement. Clinical and Laboratory Standards Institute, Wayne, PA.
- 4.Cornaglia, G., and G. M. Rossolini. 2009. Forthcoming therapeutic perspectives for infections due to multidrug-resistant Gram-positive pathogens. Clin. Microbiol. Infect. 15:218-223. [DOI] [PubMed] [Google Scholar]
- 5.Cosgrove, S. E., and V. G. Fowler, Jr. 2008. Management of methicillin-resistant Staphylococcus aureus bacteremia. Clin. Infect. Dis. 46(Suppl. 5):S386-S393. [DOI] [PubMed] [Google Scholar]
- 6.Draghi, D. C., B. M. Benton, K. M. Krause, C. Thornsberry, C. Pillar, and D. F. Sahm. 2008. Comparative surveillance study of telavancin activity against recently collected Gram-positive clinical isolates from across the United States. Antimicrob. Agents Chemother. 52:2383-2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Draghi, D. C., B. M. Benton, K. M. Krause, C. Thornsberry, C. Pillar, and D. F. Sahm. 2008. In vitro activity of telavancin against recent Gram-positive clinical isolates: results of the 2004-05 Prospective European Surveillance Initiative. J. Antimicrob. Chemother. 62:116-121. [DOI] [PubMed] [Google Scholar]
- 8.Dunbar, L. M., D. M. Tang, and R. M. Manausa. 2008. A review of telavancin in the treatment of complicated skin and skin structure infections (cSSSI). Ther. Clin. Risk Manag. 4:235-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayden, M. K., K. Rezai, R. A. Hayes, K. Lolans, J. P. Quinn, and R. A. Weinstein. 2005. Development of daptomycin resistance in vivo in methicillin-resistant Staphylococcus aureus. J. Clin. Microbiol. 43:5285-5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hidron, A. I., J. R. Edwards, J. Patel, T. C. Horan, D. M. Sievert, D. A. Pollock, and S. K. Fridkin. 2008. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006-2007. Infect. Control Hosp. Epidemiol. 29:996-1011. [DOI] [PubMed] [Google Scholar]
- 11.Higgins, D. L., R. Chang, D. V. Debabov, J. Leung, T. Wu, K. M. Krause, E. Sandvik, J. M. Hubbard, K. Kaniga, D. E. Schmidt, Jr., Q. Gao, R. T. Cass, D. E. Karr, B. M. Benton, and P. P. Humphrey. 2005. Telavancin, a multifunctional lipoglycopeptide, disrupts both cell wall synthesis and cell membrane integrity in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 49:1127-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jansen, W. T., A. Verel, J. Verhoef, and D. Milatovic. 2007. In vitro activity of telavancin against gram-positive clinical isolates recently obtained in Europe. Antimicrob. Agents Chemother. 51:3420-3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones, R. N. 2008. Key considerations in the treatment of complicated staphylococcal infections. Clin. Microbiol. Infect. 14(Suppl. 2):3-9. [DOI] [PubMed] [Google Scholar]
- 14.Krause, K. M., M. Renelli, S. Difuntorum, T. X. Wu, D. V. Debabov, and B. M. Benton. 2008. In vitro activity of telavancin against resistant gram-positive bacteria. Antimicrob. Agents Chemother. 52:2647-2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leuthner, K. D., C. M. Cheung, and M. J. Rybak. 2006. Comparative activity of the new lipoglycopeptide telavancin in the presence and absence of serum against 50 glycopeptide non-susceptible staphylococci and three vancomycin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 58:338-343. [DOI] [PubMed] [Google Scholar]
- 16.Lunde, C. S., S. R. Hartouni, J. W. Janc, M. Mammen, P. P. Humphrey, and B. M. Benton. 2009. Telavancin disrupts the functional integrity of the bacterial membrane through targeted interaction with the cell wall precursor lipid II. Antimicrob. Agents Chemother. 53:3375-3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neoh, H. M., S. Hori, M. Komatsu, T. Oguri, F. Takeuchi, L. Cui, and K. Hiramatsu. 2007. Impact of reduced vancomycin susceptibility on the therapeutic outcome of MRSA bloodstream infections. Ann. Clin. Microbiol. Antimicrob. 6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakoulas, G., P. A. Moise-Broder, J. Schentag, A. Forrest, R. C. Moellering, Jr., and G. M. Eliopoulos. 2004. Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. J. Clin. Microbiol. 42:2398-2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stryjewski, M. E., D. R. Graham, S. E. Wilson, W. O'Riordan, D. Young, A. Lentnek, D. P. Ross, V. G. Fowler, A. Hopkins, H. D. Friedland, S. L. Barriere, M. M. Kitt, and G. R. Corey. 2008. Telavancin versus vancomycin for the treatment of complicated skin and skin-structure infections caused by Gram-positive organisms. Clin. Infect. Dis. 46:1683-1693. [DOI] [PubMed] [Google Scholar]