Abstract
A strain of human cytomegalovirus, T2211, modified from standard laboratory strain AD169 to contain a secreted alkaline phosphatase reporter gene for rapid viral quantitation, was cloned as a bacterial artificial chromosome, BA1, and then mutagenized to create recombinant viruses containing viral UL97 kinase sequence variants found in clinical specimens after ganciclovir treatment, but with no phenotypic data to determine their role in drug resistance. Seven control strains and 14 other recombinant strains were phenotyped for ganciclovir resistance and compared with similar strains created using prior technology to show a good concordance of findings. Sequence changes V466M, H469Y, A478V, N510S, A588V, K599R, L600I, G623S, T659I, and V665I were found to confer no significant ganciclovir resistance, while mutations L405P, M460T, A594E, and C603R conferred 3- to 9-fold increases in ganciclovir 50% inhibitory concentrations. Different mutations at codons 594 (A594V, A594E) and 603 (C603W, C603S) conferred varied amounts of ganciclovir resistance. Advances in recombinant phenotyping make it easier to show that many uncharacterized UL97 sequence variants do not confer ganciclovir resistance, but some are newly confirmed as resistance associated, including one (L405P) which is outside the codon range where such mutations are usually found. This information should improve the interpretation of genotypic data generated by diagnostic laboratories.
Prolonged use of ganciclovir (GCV) or its prodrug valganciclovir, often for several months in transplant recipients undergoing primary cytomegalovirus (CMV) infection, may result in the development of drug resistance, which is suspected when circulating viral loads increase during ongoing therapy (17). In current clinical practice, GCV resistance is usually confirmed by analysis of CMV DNA sequences amplified from posttreatment clinical specimens, since viral culture isolates or pretreatment baseline CMV sequences are not commonly available. GCV resistance typically results from mutations in the viral UL97 kinase gene responsible for the initial phosphorylation of GCV, but sometimes mutations in the viral UL54 DNA polymerase (pol) gene emerge to confer added GCV resistance and cross-resistance to cidofovir and/or foscarnet. With both genes, an extensive and evolving database of mutations related to drug resistance has been documented and used for interpretation of genotypic test results.
In the UL97 gene, a number of well-defined (“canonical”) mutations at codons 460, 520, and 590 to 607 are found in GCV-resistant clinical strains, with mutations M460V/I, H520Q, C592G, A594V, L595S, and C603W occurring most frequently (6). Point and deletion mutations in the codon range 590 to 607 confer various degrees of GCV resistance, while other changes in this region confer no significant resistance (6). Noncanonical UL97 sequence changes have repeatedly been found in patients who have received GCV. Sequences of pretreatment and/or drug-susceptible CMV isolates are available to indicate that certain sequence variants are probably normal interstrain polymorphisms unrelated to drug resistance (26), but in many cases, insufficient data exist to determine if the sequence variants represent polymorphisms, resistance-related mutations, or reporting errors (6, 16, 26, 32). Speculation about the significance of uncharacterized UL97 sequence variants has resulted in some misleading attributions of GCV resistance (5, 31), which can be confusing to clinicians and diagnostic laboratories.
The role of viral gene mutations in drug resistance is determined by recombinant phenotyping (marker transfer), whereby a specific sequence change is transferred to a reference laboratory strain of CMV and the resulting effect on drug susceptibility is assessed by the drug concentration required to reduce viral growth by 50% (EC50). Confirmed UL97 GCV resistance mutations usually confer 3- to 15-fold increases in the GCV EC50 (6). Many UL97 sequence changes have not been phenotyped, mainly because the size of the CMV genome makes its site-specific mutagenesis technically nontrivial. In recent years, however, the work has benefited from use of standard laboratory CMV strains modified with reporter genes to facilitate viral quantitation (10) and mutagenesis of the CMV genome as a bacterial artificial chromosome (BAC) (4, 27). Here, we combine these advances to determine the significance of many UL97 sequence changes described in the existing literature or encountered more recently in diagnostic laboratories.
MATERIALS AND METHODS
Virus strains and cells.
Baseline laboratory CMV strains T2211 (Fig. 1) and T2233 were derived from standard strain AD169 (ATCC VR-538) and have been published and used extensively for recombinant phenotyping (9-11). Inserted between genes US6 and US3, the strains contain a secreted alkaline phosphatase (SEAP) reporter gene with a unique PacI restriction site past its end, expressed under the control of the CMV major immediate-early promoter. They also contain other restriction sites inserted into their UL97 kinase and UL54 DNA polymerase (pol) gene sequences to facilitate the construction of mutant viruses by homologous recombination (10). Strain T2211 was modified (10) to contain UL97 mutation M460V (strain T2259), C592G (strain T2258), or A594V (strain T2255); these strains were used as controls to calibrate the levels of GCV resistance conferred by various mutations. By using the same methods (10, 11), additional recombinant viruses were newly derived from T2211 to contain mutations L405P (T2789), H469Y (T2924), A478V (T3215), A588V (T3216), A594E (T3217), and L600I (T3041). CMV strains were propagated in human foreskin fibroblast (HFF) cultures under standard conditions.
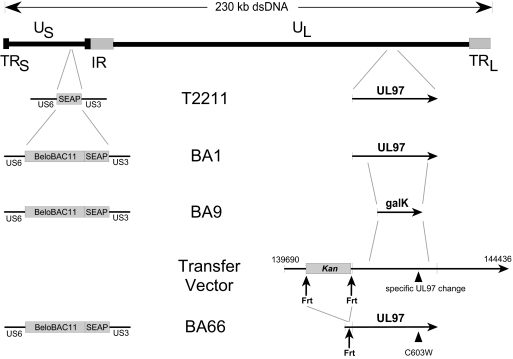
FIG. 1.
Map of CMV strain T2211 and derived BACs. Strain T2211 was derived from standard strain AD169 by insertion of a SEAP expression cassette between US3 and US6 and restriction sites in the UL54 and UL97 genes. BAC BA1 resulted from the insertion of vector BeloBAC11 adjacent to the SEAP cassette in T2211. BA9 was derived from BA1 by replacing a large part of UL97 with a galK selection marker. Desired UL97 mutants were constructed by recombination of a transfer vector containing an Frt-flanked kanamycin resistance marker, and its subsequent removal with Flp recombinase, resulting in UL97-modified BACs such as BA66, which contain a residual upstream 34-nucleotide Frt motif that does not affect the drug resistance phenotype. dsDNA, double-stranded DNA.
UL97 sequence variants.
Canonical GCV resistance mutations (C592G, A594V, and C603W) were selected as controls to compare their known phenotypes with those found after introduction into BAC clones. Common UL97 baseline sequence polymorphisms (N68D, L126Q, and I244V) not expected to confer resistance (7, 26) were also transferred together as a control. UL97 sequence variants without confirmed phenotypes include those published in connection with GCV resistance (M460T [1], V466M [3], H469Y [22], N510S [31], C603R [24], C603S [24], G623S [23], T659I [16], and V665I [7]) and unpublished ones encountered in diagnostic specimens from GCV-treated subjects (L405P, A478V, A588V, A594E, K599R, and L600I). In several cases, two recombinant viruses were constructed to represent the same sequence variant, using old and new technical approaches, in order to compare the resulting phenotypes.
Cloning vectors and E. coli host strains.
Reagents and protocols were obtained from the Biological Resources Branch of the National Cancer Institute (http://recombineering.ncifcrf.gov) and include a 7.5-kb BeloBAC11 vector, a galK expression vector, and Escherichia coli strains SW102 and SW105, which contain the temperature-inducible genetic elements exo, bet, and gam, which enable transient high-frequency homologous recombination into resident BACs, and a deletion of the galK gene, thus permitting its use as a selectable marker (34). SW105 also contains an arabinose-inducible Flpe gene for the transient expression of Flp recombinase (34). The standard Bluescript cloning vector (pBS2KS+; Stratagene) and the E. coli DH10B-derived strain GeneHogs (Invitrogen) were obtained commercially. The UL97 sequence context of strain AD169 (nucleotides 139690 to 144436 of the GenBank reference sequence X17403) was cloned into pBS2KS+ using the NotI and KpnI sites, as clone SC102 (10). A kanamycin resistance cassette (Kan), flanked by Frt motifs and a Bsu36I restriction site, was amplified by PCR using primers 5′-AGTGCAGCCTTAGGTGAAGTTCCTATACTTTCTAGAGAATAGGAACTTCAATAG-CCAATCTGATGCGGTA-3′ and 5′-TTACTGTTCCTAAGGAAGTTCCTATTCTCTAGAAAGTATAGGAACTTCAAGTCAGCGTAATGCTCTGC-3′ from plasmid gWizSEAP (Genlantis) and cloned into the native Bsu36I restriction site immediately upstream of the UL97 coding sequence in SC102, creating plasmid SC408.
BAC cloning of CMV strain T2211 as BA1.
The BeloBAC11 cloning vector was digested with enzymes BamHI and HindIII and ligated with a corresponding oligonucleotide linker containing a PacI restriction site between BamHI and HindIII. The modified vector was digested with PacI, and 0.4 μg was ligated to 3.5 μg of PacI-digested CMV strain T2211 genomic DNA extracted from infected cell cultures, using T4 DNA ligase (Invitrogen). The postligation reaction mixture was transfected into subconfluent HFF cell cultures (T3045) using Fugene 6 reagent (Roche), with the resulting viral cytopathic effect being visible 2 weeks later. A confluent 75-cm2 HFF culture flask was inoculated with 4 × 107 PFU of cell-free T3045 virus stock and incubated for 42 h. At this time, infected cell DNA was extracted by the Hirt method (19), and the entire DNA yield was electroporated into the GeneHogs strain of E. coli (Invitrogen). The resulting chloramphenicol-resistant colonies were evaluated by restriction digestion of plasmid DNA with HindIII and/or XbaI, and results were compared with those for the parental T2211 DNA. A colony which had restriction digestion patterns compatible with that of a BAC clone of the complete genome was propagated in E. coli, and its DNA was tested for production of infectious SEAP-producing CMV when transfected into HFF cultures. It was further retransformed into E. coli SW102 and SW105 by electroporation and rechecked for preservation of a complete HindIII digestion pattern and production of infectious CMV upon transfection into HFF. Transfections were performed using Fugene 6 reagent (Roche) according to the manufacturer's instructions, using ∼2 μg of BAC DNA, which was extracted from E. coli using standard alkaline lysis procedures (Tip-20; Qiagen). Viral genomic DNA was from infected cell cultures as previously described (12). After restriction digestion of 2 to 3 μg of DNA, fragments were resolved by field inversion gel electrophoresis through 0.8% agarose in 0.5× Tris-borate-EDTA buffer using a programmable power inverter (MJ Research PPI-200, program 2) and visualized by ethidium bromide staining.
Replacement of UL97 sequences of BA1 with galK gene cassettes.
The UL97 sequence of BA1 was replaced by PCR amplification of the bacterial galK expression cassette and recombineering into BA1 in E. coli SW102 as described previously (34). The PCR primers included 50-bp segments of CMV sequence delimiting the portion to be replaced by galK. Recombination of the PCR product into BA1, followed by plating on galactose minimal medium, enabled the selection of bacterial clones containing the recombinant BACs that incorporated the galK cassette, as confirmed by PCR showing the absence of the replaced UL97 segment. In one resulting BAC (BA9), codons 134 to 654 of the UL97 coding sequence were replaced by galK, and in another BAC (BA12), the replaced CMV sequence extended from 160 bases upstream of UL97 to codon 25 of UL98. These galK-modified BACs were used for further recombination to introduce the desired UL97 mutations without a preexisting background of wild-type UL97 sequence.
Cotransfection of mutant UL97 sequences with BA12.
Because BAC BA12 is missing all of UL97 and the start of UL98, an essential gene, it was expected that cotransfection into HFF of BA12 DNA and an overlapping CMV DNA segment (nucleotides 139690 to 144436) containing the UL97 mutation of interest would result in recovery of viable recombinant CMV containing the mutation. This was performed using the same procedures as for traditional cotransfection (10), followed by plaque purification to obtain virus stock free of PCR-detectable galK sequences.
UL97 mutagenesis using Frt-flanked Kan selectable marker.
BAC mutagenesis using a Frt-flanked Kan selectable marker for the construction of other UL97 mutants has been described (27), although the exact positioning of the upstream Frt motif in that study differs from that in ours. The plasmid SC408 described above was modified to contain the desired UL97 mutation by replacing segments delimited by restriction site BamHI, PstI, or KpnI with products of PCR mutagenesis (12). The resulting clone was digested with NotI and KpnI to release the CMV sequence containing the UL97 mutation and the upstream Frt-flanked Kan cassette, and the DNA was purified by preparative agarose gel electrophoresis and recombineered into BAC BA9 in E. coli SW105 as described previously (34). DNA from a kanamycin-resistant colony was checked for the expected HindIII digestion pattern. The Kan cassette between the Frt motifs was removed by adding arabinose to a fresh culture to induce the resident Flp recombinase and plating for isolation of single colonies retaining chloramphenicol resistance but exhibiting kanamycin sensitivity. BAC DNA was then transfected into HFF cultures to reconstitute live CMV.
For all recombinant viruses, the entire UL97 sequence, including the upstream Frt motif, when present, was analyzed by standard automated fluorescent dideoxy sequencing to check for the presence of the desired mutation and the absence of unintended ones. PCR was also performed to confirm the absence of parental BAC galK sequences in the recombinant virus.
Phenotypic assays for GCV resistance.
Phenotypic assays for GCV resistance were done by SEAP yield reduction as described previously (10). Briefly, a row of 6 wells of a 24-well culture plate containing 2 × 105 confluent HFF cells was inoculated with cell-free virus stock at a multiplicity of infection (MOI) of 0.02. After 1.5 h, the inoculum was removed and replaced with culture medium containing serial 2-fold dilutions of GCV (Roche) in five of the wells, to a maximum concentration of 4 to 32 μM depending on its GCV susceptibility. The GCV concentration required to reduce the culture supernatant SEAP activity (as measured by a chemiluminescent substrate) by 50% at 6 days postinfection was determined (EC50) and reported as the mean and standard deviation of at least seven determinations done over at least four different setup dates to allow for variations in culture conditions. Assays were validated by criteria including supernatant SEAP values on day 1 compatible with an MOI of ∼0.02 (10), values on day 6 compatible with the expected level of viral growth, and a good exponential curve fit of the SEAP values under 2-fold serial drug dilutions (r2 > 0.89). Required controls for each setup date included a known sensitive and low-grade-resistant control (e.g., UL97 mutant C592G) to show the expected EC50 values and allow adequate discrimination of sensitive versus resistant strains. Although there are no universally accepted EC50 cutoffs to define GCV resistance, our current working definition of resistance is a 2-fold increase in EC50 based on observed standard deviations of ∼30% in mean EC50 values. This definition has also been used in previous work by others (13).
RESULTS
Cloning of strain T2211 as BAC BA1.
Ligation of the BeloBAC11 vector into T2211 DNA and transfection into HFF yielded infectious virus T3045, a Hirt DNA extract of which was transformed into E. coli, resulting in >100 chloramphenicol-resistant colonies. At least 3 of 114 colonies screened contained BAC DNA showing restriction digestion patterns with several common enzymes which closely resembled its parental strain. Of these, one was selected for further use and named BA1. Figure 2 shows the HindIII digestion pattern of BA1 (lane 2) to be consistent with the parental strain T2211 (lane 1), allowing for differences resulting from insertion of the BAC vector (marked in lane 2) and the multiple relative orientations of the long and short unique segments of the genome that are present in cell culture-grown viral DNA (upper bands in lane 1) but not in BACs (4). After transfection of BA1 into HFF, infectious CMV was recovered as T3099. A DNA extract of a T3099-infected HFF culture had a HindIII digestion pattern (Fig. 2, lane 6) which replicated that of the original strain, T2211 (lane 1), except for the bands resulting from insertion of the BAC vector, as marked in lane 6. The anticipated location of the BAC vector, immediately downstream of the SEAP expression cassette between US6 and US3 (Fig. 1), was validated by amplification of an appropriately sized PCR product using primers located within the SEAP and vector sequences and spanning the junction. Viral growth of strains T2211 and BA1-derived T3099 in cell culture appeared to be the same as that when monitored by cytopathic effect, infectivity of virus stocks generated, and culture supernatant SEAP activities on days 5 through 7 without added drug and after low-multiplicity infection. During this 2-day period, the SEAP signal increased about 5-fold to about 1.2 million relative light units from a baseline of less than 100 units before viral inoculation and 400 to 1,500 units 1 day after inoculation (10, 11).
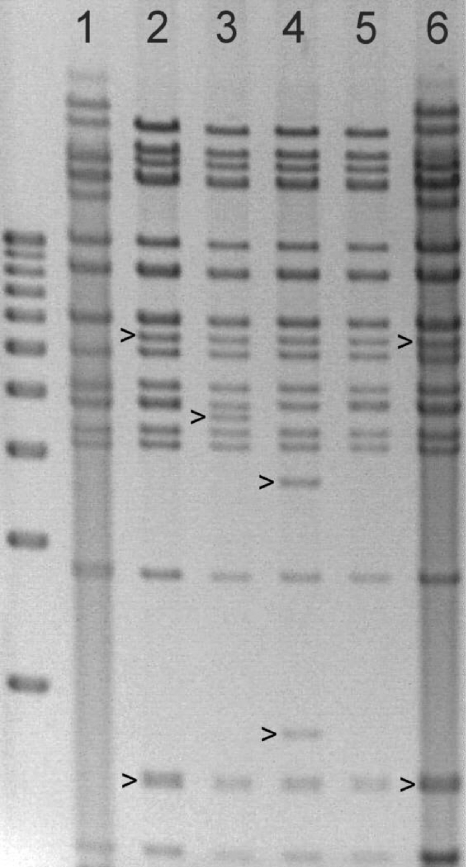
FIG. 2.
Restriction digestion of DNA from strain T2211 and derived BACs. HindIII restriction digestion of viral DNA extracted from infected fibroblast cultures (lane 1, strain T2211; lane 6, strain T3099) or BACs grown in E. coli (lane 2, BA1; lane 3, BA9; lane 4, BA9 after recombination with an Frt-Kan-Frt transfer vector during construction of BA66; lane 5, BAC from lane 4 after removal of the Kan marker to restore the BA1 pattern). Strain T3099 resulted from transfection of BAC BA1 into fibroblast cultures. Distinctive restriction digestion fragments in each lane are marked. The far left lane contains a sizing ladder of DNAs at 1-kb intervals from 3 kb at the bottom to 12 kb at the top (Invitrogen 15615-016).
Removal of wild-type UL97 sequences from BACs.
BAC BA1 was further mutagenized to BAC clones that had a part of the UL97 region (BA9) or the entire region (BA12) replaced by a galK selectable marker, to avoid a background of wild-type UL97 sequences when mutagenizing this gene region. This genetic alteration resulted in a distinctive change in the HindIII restriction digestion pattern of the BAC (Fig. 2, lane 3). Although recombineering as described previously (34) reliably resulted in isolation of bacterial colonies containing BACs with galK in place of UL97, PCR amplification using UL97 primers within the replaced segment showed that replating for isolated colonies or retransformation of BAC DNA was required to remove traces of the deleted UL97 segments. Subsequently, the desired mutant UL97 sequences were introduced by recombination, replacing the galK marker.
Recombination in cell culture.
Cotransfection into HFF of mutant UL97 DNA and BA12 DNA resulted in recovery of normally growing recombinant viruses with restored UL97 and UL98 sequences that are absent in BA12. This approach had the advantage of not requiring any additional BAC mutagenesis steps, and it added no new genetic elements, such as Frt. The live CMV that routinely grew following such cotransfection contained the intended UL97 mutation but was plaque purified to remove PCR-detectable galK sequence of BA12 that was present in viral DNA extracts, thus delaying the phenotype determination by a few weeks. Recombinant viruses produced using this method were T3135, in which the original UL97 sequence of T2211 was restored as a control, T3136 (UL97 variant H469Y), T3146 (K599R), and T3130 (V665I).
BAC mutagenesis using kanamycin selection.
BAC mutagenesis using kanamycin selection was used for the majority of the UL97 recombinants in this study and is based on recombineering (34) of BAC BA9 in E. coli SW105 with a DNA segment containing an Frt-flanked Kan cassette and then selecting for kanamycin-resistant colonies, similar to a strategy previously used (27). This resulted in a reliable yield of BACs containing mutant UL97 DNA with an upstream Frt-Kan-Frt marker, and showing a distinct HindIII restriction pattern (Fig. 2, lane 4). The Kan segment was then easily removed by arabinose induction of Flp recombinase in SW105, restoring the original HindIII restriction digestion pattern of BAC BA1 (Fig. 2, lane 5). After transfection of the BACs into HFF, this approach was successful in creating multiple recombinant CMVs, including the 18 strains labeled with a precursor BAC name in Table 1, all of which contained a residual 34-nucleotide Frt motif at the Bsu36I restriction site immediately upstream of UL97. Insertion of the Frt motif had no visible effect on the cell culture cytopathic effect, and the attained viral growth at 7 days after low-multiplicity infection was the same, as judged by the supernatant SEAP activity, which was in the range of 1.2 million relative light units. The strains consisted of controls with baseline UL97 sequences restored (T3185, T3261), or with known GCV resistance mutations C592G, A594V, and C603W (6), and various UL97 sequence changes not previously characterized by recombinant phenotyping (Table 1). In many cases, the same mutations had been introduced into recombinant CMV strains using other methods as well, in order to compare their drug resistance phenotypes for concordance.
TABLE 1.
Genotypes and phenotypes of recombinant viruses
| BACa | Virus | UL97 |
Ganciclovir |
EC50 ratiog | |||
|---|---|---|---|---|---|---|---|
| Mutationb | Baselinec | EC50 (μM)d | SDe | No. of assaysf | |||
| T2211 | None | H587Y | 1.10 | 0.22 | 95 | ||
| T2233 | None | 1.11 | 0.32 | 61 | |||
| BA1 | T3099 | None | H587Y | 1.02 | 0.30 | 12 | |
| T3135i | None | H587Y | 1.00 | 0.16 | 13 | ||
| BA14 | T3185 | None | Frt-H587Y | 0.96 | 0.19 | 14 | |
| BA29 | T3261 | None | Frt | 0.99 | 0.28 | 23 | |
| BA63 | T3326 | None | Frt N68D L126Q I244V | 1.09 | 0.27 | 14 | |
| T2789 | L405Ph | L126Q I244V | 3.02 | 0.67 | 14 | 2.7 | |
| BA13 | T3184 | L405P | Frt L126Q I244V | 2.76 | 0.98 | 12 | 2.5 |
| BA77 | T3346 | M460T | Frt | 9.24 | 1.76 | 10 | 9.3 |
| T2259 | M460V | 8.52 | 1.83 | 18 | 8.6 | ||
| BA25 | T3257 | V466M | Frt | 1.24 | 0.21 | 8 | 1.3 |
| T2924 | H469Y | I244V | 1.33 | 0.31 | 13 | 1.2 | |
| T3136i | H469Y | I244V | 1.05 | 0.39 | 8 | 1.1 | |
| BA19 | T3242 | A478V | Frt | 0.77 | 0.16 | 11 | 0.8 |
| T3215 | A478V | 0.95 | 0.20 | 7 | 0.9 | ||
| BA17 | T3240 | N510S | Frt | 1.14 | 0.25 | 10 | 1.2 |
| T3216 | A588V | 1.23 | 0.24 | 9 | 1.1 | ||
| BA21 | T3244 | A588V | Frt | 0.99 | 0.21 | 9 | 1.0 |
| T2258 | C592G | 3.36 | 1.19 | 76 | 3.0 | ||
| BA27 | T3259 | C592G | Frt | 3.00 | 0.84 | 39 | 3.0 |
| T3217 | A594E | 3.31 | 0.44 | 11 | 3.0 | ||
| BA26 | T3258 | A594E | Frt | 2.98 | 0.83 | 10 | 3.0 |
| T2255 | A594V | 9.24 | 2.37 | 14 | 8.3 | ||
| BA22 | T3252 | A594V | Frt | 8.48 | 2.52 | 12 | 8.6 |
| BA23 | T3253 | K599R | Frt | 1.23 | 0.29 | 11 | 1.2 |
| T3146i | K599R | 0.94 | 0.23 | 9 | 0.9 | ||
| T3041 | L600I | 1.50 | 0.32 | 24 | 1.4 | ||
| BA65 | T3331 | C603R | Frt | 8.18 | 1.77 | 14 | 8.3 |
| BA64 | T3327 | C603S | Frt | 1.85 | 0.38 | 11 | 1.9 |
| BA66 | T3329 | C603W | Frt | 7.81 | 1.68 | 12 | 7.9 |
| BA30 | T3264 | G623S | Frt | 1.13 | 0.35 | 11 | 1.1 |
| BA20 | T3243 | T659I | Frt | 1.26 | 0.31 | 11 | 1.3 |
| BA16 | T3239 | V665I | Frt | 1.06 | 0.28 | 9 | 1.1 |
| T3130i | V665I | 1.22 | 0.33 | 8 | 1.2 | ||
BAC, bacterial artificial chromosome CMV clone name.
UL97 sequence change targeted for phenotyping.
Frt, Flp recombinase recognition site upstream of the gene. Baseline changes from strain AD169 resulting from strain variation, introduced restriction sites, or Frt motif.
Mean drug concentration required to reduce SEAP growth by 50% at 6 days postinfection.
Standard deviation of the EC50 values.
Number of assays performed over at least four separate dates.
Ratio of ganciclovir EC50 to that of baseline strain (matched for origin and Frt).
Items associated with GCV resistance are shown in boldface type.
Derived by cotransfection of UL97 variant DNA and BAC BA12, which lacks the UL97 sequence.
Effect of UL97 sequence changes on GCV susceptibility.
All recombinant viruses were phenotyped for GCV susceptibility by SEAP yield reduction (10) on day 6. Results are shown in Table 1 and include more replicates per virus than is customary for traditional plaque reduction assays. Although standard deviations for all phenotypic assays are commonly about 30% of the mean EC50 values because of variation in the growth condition of cell cultures, the EC50s and ratios of different viruses with the same genotype tended to converge toward the same value with increasing numbers of replicates. Various BAC-derived strains with baseline UL97 sequences, with or without the Frt motif upstream (T3099, T3135, T3185, T3261 and T3326), had GCV EC50 values very similar to those of the antecedent non-BAC, SEAP-reporter baseline strains T2211 and T2233. UL97 mutations known to confer various levels of GCV resistance conferred the same levels of resistance after their introduction into BAC clones and reconstitution of live virus. This included mutations C592G (T3259 versus T2258) and A594V (T3252 versus T2255), where results using SEAP reporter strains correlate with traditional plaque reduction data (10), and mutation C603W (T3329; EC50 ratio, 7.9) (Table 1), where results based on standard strain AD169 and plaque reduction assays had given an GCV EC50 ratio of 8.0 (8). For the uncharacterized UL97 sequence variants that were constructed in replicate as recombinant viruses with and without BAC cloning, the resulting GCV resistance phenotypes were the same, including the susceptible and resistant strains containing mutations L405P, H469Y, A478V, A588V, A594E, K599R, and V665I.
All newly phenotyped UL97 sequence variants outside the canonical codon range, 460, 520, or 590 to 607, were shown to confer no significant GCV resistance, with the exception of L405P. This unpublished mutation was found in a PCR product clone of a highly GCV-resistant clinical isolate that also contained UL97 mutation C607F (12) and pol mutation P522S (13), both known GCV resistance markers. These and other drug resistance mutations evolved in an HIV-infected subject over many months of exposure to various anti-CMV therapies, not including maribavir, an experimental UL97 inhibitor (6). L405P was phenotyped because of its proximity to known maribavir resistance mutations at UL97 codons 409 and 411 (6, 11) and the possibility of cross-resistance. Neither L405P recombinant (T2789 nor T3184) showed evidence of maribavir resistance (EC50, <0.12 μM), but the two showed the same low-grade GCV resistance, comparable to levels conferred by C592G and A594E (Table 1). The L126Q and I244V baseline strain variation transferred with L405P was previously shown to have no effect on susceptibility to GCV or maribavir (11), consistent with results for strain T3326 in the present study (Table 1). The L405P mutant virus had normal-appearing cytopathic effect in cell culture, and supernatant SEAP activity measured at 6 days was in the same range as that of baseline strains, suggesting normal growth. Mutation M460T conferred a level of GCV resistance comparable to that of the canonical mutation M460V, whereas A594E conferred a level of GCV resistance that was less than that of the canonical mutation A594V but similar to that of the less-common mutation A594T (12). Mutations K599R, L600I, and C603S (24) conferred little or no GCV resistance despite their locations within the codon range 590 to 607; some other mutations at the same codons, such as K599T (15), C603R (Table 1), and C603W (Table 1 and reference 8), confer higher levels of GCV resistance.
DISCUSSION
Cloning of a SEAP-expressing derivative of laboratory CMV strain AD169 as BAC BA1 facilitated the construction of recombinant viruses containing UL97 mutations and the determination of their GCV resistance phenotypes. This helped to resolve longstanding questions about the diagnostic significance of certain UL97 amino acid changes, and it newly validated several UL97 mutations as conferring GCV resistance (Fig. 3).
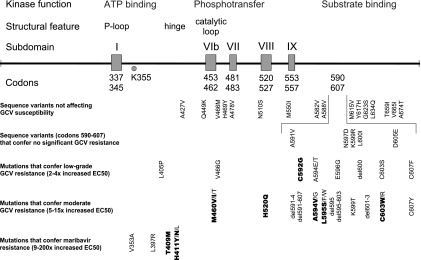
FIG. 3.
Map of UL97 sequence variants. The UL97 codon range 300 to 707 is displayed with boxes defining codon ranges representing conserved sequence subdomains (18) and associated with the structural features and kinase functions indicated. The lysine residue K355 is critical for kinase function. UL97 sequence variants are mapped by category, with the most-common drug resistance mutations found in clinical CMV isolates shown in bold. All of the sequence variants or mutations have been characterized by phenotyping of recombinant CMVs that contain the specific mutation. GCV resistance mutations are clustered at codons 460, 520, and 590 to 607; the latter segment is shown in expanded layout in the middle rows of data. Information was compiled from Table 1 and published literature (6, 12, 27, 28).
Several UL97 variants shown not to confer GCV resistance (Table 1) are noteworthy for divergent and confusing opinions expressed in the literature. Although amino acid changes H469Y and N510S were found in pretreatment or GCV-susceptible CMV isolates (14, 26) and should be considered unlikely to be resistance related, they have been speculatively linked to GCV resistance (5, 22, 31, 32). Some other sequence variants, such as V466M (3) and T659I (35), were reported as a change from a pretreatment sequence after therapy. T659I was classified as a GCV resistance marker without specific phenotypic data (16, 20, 33). The findings in Table 1 add to several other UL97 sequence variants that did not confer GCV resistance after BAC mutagenesis (27). It is unclear if these variants found in GCV-treated subjects represent unrecognized UL97 baseline sequence polymorphisms, an adaptive change to GCV that is too small to measure by current phenotypic drug resistance assays, or artifacts of PCR or data reporting, but they should be clearly distinguished from confirmed GCV resistance mutations for genotypic resistance testing purposes, since they did not confer drug resistance when transferred into a control CMV strain.
Mutations L405P, M460T, and A594E (Fig. 3) were newly confirmed to confer GCV resistance, and these include one mutation (L405P) located outside the usual range of codons for such mutations. L405P was found as a minor component discernible only in a PCR clone from a clinical isolate that also contained other known GCV resistance mutations in UL97 and UL54. It is near the locations of known maribavir resistance mutations (6), but the lack of maribavir cross-resistance, the normal cytopathic appearance, and the growth in cell culture of the L405P recombinant suggest that the mutation affects the phosphorylation of GCV and not the natural substrates of UL97, similar to the effect of common UL97 GCV resistance mutations, such as M460V (29). Confirmed GCV resistance mutations in clinical isolates outside the codon range of 460, 520, and 590 to 607 are rare. Another recent example is the mutation V466G (28) which, in contrast to V466M in Table 1, was reported to confer GCV resistance. It should be noted that laboratory-generated UL97 kinase-defective mutants (e.g., K355M or large deletions) that are unable to phosphorylate any substrate display an abnormal cytopathic effect, are severely growth impaired (30), and are inherently resistant to both GCV and MBV because UL97 kinase activity is missing, but such mutants have been considered unlikely to occur in clinical isolates because of their severe growth defect.
Mutation M460T was found to confer as much GCV resistance as the better-known mutations M460V (10) and M460I (25), and together, these mutations represent instances of change at each of the 3 nucleotides of this codon. Mutation A594E confers less GCV resistance than the common mutation A594V (10), and C603R (24) confers GCV resistance at a level similar to that of C603W (8). On the other hand, K599R and C603S (24) do not confer the GCV resistance associated with other known mutations at these codons (8, 15), illustrating the divergence of phenotypes arising out of different amino acid changes at the same codon in a resistance-related locus, as has also been reported for the UL54 pol gene (9). From these data and the phenotypes of variants N597D (21), L600I (Table 1), and D605E (10), it is becoming clear that not all point mutations in the codon range 590 to 607 confer significant GCV resistance, even though this range contains canonical resistance mutations (6). Variation in the levels of GCV resistance conferred by different UL97 mutations probably relates to their individual effects on GCV phosphorylation, as reported for a vaccinia virus expression system, in which the mutation C592G that confers low-level GCV resistance (Table 1) also conferred a lesser impairment of GCV phosphorylation than mutations such as M460V that confer greater GCV resistance (2).
Phenotypic data obtained after mutagenesis of a SEAP reporter CMV BAC clone were found to be closely comparable to those obtained by previous methods. BAC mutagenesis uses readily available materials and methods for the rapid selection and cloning of the mutants and eliminates the need for plaque purification of recombinant viruses prior to phenotyping. Use of an Frt-flanked kanamycin-positive selection marker speeds the construction of mutants, although it requires strategic placement of the Frt motif to avoid an effect on viral growth. Substantial comparative data were produced here to show that the 34-nucleotide Frt motif remaining after removal of the kanamycin resistance marker in BAC clones did not affect the GCV resistance phenotype. Although the genetic purity and stability of BAC clones remain a quality control concern, current experience suggests that recombineering (34) of SEAP reporter BACs is an efficient approach to the recombinant phenotyping of unknown sequence variants in clinical specimens from subjects receiving antiviral therapy.
Acknowledgments
Gail Marousek, Laura Van Wechel, Heather Lichy, Daniel Mitchell, and Alwin Borgmann provided technical assistance. Reagents for recombineering were provided by Court and Copeland and the Biological Resources Branch of the National Cancer Institute. Adriana Weinberg (Denver), Lawrence Drew (San Francisco), Ajit Limaye (Seattle), and Pascal Meylan (Lausanne, Switzerland) referred unpublished UL97 sequence variants from clinical genotypic resistance assays.
This work was supported by NIH grant AI39938 and Department of Veterans Affairs research funds.
Footnotes
Published ahead of print on 2 April 2010.
REFERENCES
- 1.Abraham, B., S. Lastere, J. Reynes, F. Bibollet-Ruche, N. Vidal, and M. Segondy. 1999. Ganciclovir resistance and UL97 gene mutations in cytomegalovirus blood isolates from patients with AIDS treated with ganciclovir. J. Clin. Virol. 13:141-148. [DOI] [PubMed] [Google Scholar]
- 2.Baldanti, F., D. Michel, L. Simoncini, M. Heuschmid, A. Zimmermann, R. Minisini, P. Schaarschmidt, T. Schmid, G. Gerna, and T. Mertens. 2002. Mutations in the UL97 ORF of ganciclovir-resistant clinical cytomegalovirus isolates differentially affect GCV phosphorylation as determined in a recombinant vaccinia virus system. Antiviral Res. 54:59-67. [DOI] [PubMed] [Google Scholar]
- 3.Boivin, G., C. Gilbert, A. Gaudreau, I. Greenfield, R. Sudlow, and N. A. Roberts. 2001. Rate of emergence of cytomegalovirus (CMV) mutations in leukocytes of patients with acquired immunodeficiency syndrome who are receiving valganciclovir as induction and maintenance therapy for CMV retinitis. J. Infect. Dis. 184:1598-1602. [DOI] [PubMed] [Google Scholar]
- 4.Borst, E. M., G. Hahn, U. H. Koszinowski, and M. Messerle. 1999. Cloning of the human cytomegalovirus (HCMV) genome as an infectious bacterial artificial chromosome in Escherichia coli: a new approach for construction of HCMV mutants. J. Virol. 73:8320-8329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castor, J., L. Cook, L. Corey, and K. R. Jerome. 2007. Rapid detection directly from patient serum samples of human cytomegalovirus UL97 mutations conferring ganciclovir resistance. J. Clin. Microbiol. 45:2681-2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chou, S. 2008. Cytomegalovirus UL97 mutations in the era of ganciclovir and maribavir. Rev. Med. Virol. 18:233-246. [DOI] [PubMed] [Google Scholar]
- 7.Chou, S., A. Erice, M. C. Jordan, G. M. Vercellotti, K. R. Michels, C. L. Talarico, S. C. Stanat, and K. K. Biron. 1995. Analysis of the UL97 phosphotransferase coding sequence in clinical cytomegalovirus isolates and identification of mutations conferring ganciclovir resistance. J. Infect. Dis. 171:576-583. [DOI] [PubMed] [Google Scholar]
- 8.Chou, S., G. Marousek, S. Guentzel, S. E. Follansbee, M. E. Poscher, J. P. Lalezari, R. C. Miner, and W. L. Drew. 1997. Evolution of mutations conferring multidrug resistance during prophylaxis and therapy for cytomegalovirus disease. J. Infect. Dis. 176:786-789. [DOI] [PubMed] [Google Scholar]
- 9.Chou, S., G. Marousek, S. Li, and A. Weinberg. 2008. Contrasting drug resistance phenotypes resulting from cytomegalovirus DNA polymerase mutations at the same exonuclease locus. J. Clin. Virol. 43:107-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chou, S., L. C. Van Wechel, H. M. Lichy, and G. I. Marousek. 2005. Phenotyping of cytomegalovirus drug resistance mutations by using recombinant viruses incorporating a reporter gene. Antimicrob. Agents Chemother. 49:2710-2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chou, S., L. C. Van Wechel, and G. I. Marousek. 2007. Cytomegalovirus UL97 kinase mutations that confer maribavir resistance. J. Infect. Dis. 196:91-94. [DOI] [PubMed] [Google Scholar]
- 12.Chou, S., R. H. Waldemer, A. E. Senters, K. S. Michels, G. W. Kemble, R. C. Miner, and W. L. Drew. 2002. Cytomegalovirus UL97 phosphotransferase mutations that affect susceptibility to ganciclovir. J. Infect. Dis. 185:162-169. [DOI] [PubMed] [Google Scholar]
- 13.Cihlar, T., M. D. Fuller, and J. M. Cherrington. 1998. Characterization of drug resistance-associated mutations in the human cytomegalovirus DNA polymerase gene by using recombinant mutant viruses generated from overlapping DNA fragments. J. Virol. 72:5927-5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erice, A., N. Borrell, W. Li, W. J. Miller, and H. H. Balfour, Jr. 1998. Ganciclovir susceptibilities and analysis of UL97 region in cytomegalovirus (CMV) isolates from bone marrow recipients with CMV disease after antiviral prophylaxis. J. Infect. Dis. 178:531-534. [DOI] [PubMed] [Google Scholar]
- 15.Faizi Khan, R., S. Mori, Y. Eizuru, K. Kumura Ishii, and Y. Minamishima. 1998. Genetic analysis of a ganciclovir-resistant human cytomegalovirus mutant. Antiviral Res. 40:95-103. [DOI] [PubMed] [Google Scholar]
- 16.Gilbert, C., J. Bestman-Smith, and G. Boivin. 2002. Resistance of herpesviruses to antiviral drugs: clinical impacts and molecular mechanisms. Drug Resist. Updat. 5:88-114. [DOI] [PubMed] [Google Scholar]
- 17.Gilbert, C., and G. Boivin. 2005. Human cytomegalovirus resistance to antiviral drugs. Antimicrob. Agents Chemother. 49:873-883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanks, S. K., A. M. Quinn, and T. Hunter. 1988. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science 241:42-52. [DOI] [PubMed] [Google Scholar]
- 19.Hirt, B. 1967. Selective extraction of polyoma DNA from infected mouse cell cultures. J. Mol. Biol. 26:365-369. [DOI] [PubMed] [Google Scholar]
- 20.Humar, A., D. Kumar, J. Preiksaitis, G. Boivin, D. Siegal, J. Fenton, K. Jackson, S. Nia, and D. Lien. 2005. A trial of valganciclovir prophylaxis for cytomegalovirus prevention in lung transplant recipients. Am. J. Transplant. 5:1462-1468. [DOI] [PubMed] [Google Scholar]
- 21.Iwasenko, J. M., G. M. Scott, W. D. Rawlinson, A. Keogh, D. Mitchell, and S. Chou. 2009. Successful valganciclovir treatment of post-transplant cytomegalovirus infection in the presence of UL97 mutation N597D. J. Med. Virol. 81:507-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jabs, D. A., B. K. Martin, M. S. Forman, J. P. Dunn, J. L. Davis, D. V. Weinberg, K. K. Biron, and F. Baldanti. 2001. Mutations conferring ganciclovir resistance in a cohort of patients with acquired immunodeficiency syndrome and cytomegalovirus retinitis. J. Infect. Dis. 183:333-337. [DOI] [PubMed] [Google Scholar]
- 23.Limaye, A. P., L. Corey, D. M. Koelle, C. L. Davis, and M. Boeckh. 2000. Emergence of ganciclovir-resistant cytomegalovirus disease among recipients of solid-organ transplants. Lancet 356:645-649. [DOI] [PubMed] [Google Scholar]
- 24.Lurain, N. S., S. M. Bhorade, K. J. Pursell, R. K. Avery, V. V. Yeldandi, C. M. Isada, E. S. Robert, D. J. Kohn, M. Q. Arens, E. R. Garrity, A. J. Taege, M. G. Mullen, K. M. Todd, J. W. Bremer, and B. Yen-Lieberman. 2002. Analysis and characterization of antiviral drug-resistant cytomegalovirus isolates from solid organ transplant recipients. J. Infect. Dis. 186:760-768. [DOI] [PubMed] [Google Scholar]
- 25.Lurain, N. S., L. E. Spafford, and K. D. Thompson. 1994. Mutation in the UL97 open reading frame of human cytomegalovirus strains resistant to ganciclovir. J. Virol. 68:4427-4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lurain, N. S., A. Weinberg, C. S. Crumpacker, and S. Chou. 2001. Sequencing of cytomegalovirus UL97 gene for genotypic antiviral resistance testing. Antimicrob. Agents Chemother. 45:2775-2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin, M., C. Gilbert, E. Covington, and G. Boivin. 2006. Characterization of human cytomegalovirus (HCMV) UL97 mutations found in a valganciclovir/oral ganciclovir prophylactic trial by use of a bacterial artificial chromosome containing the HCMV genome. J. Infect. Dis. 194:579-583. [DOI] [PubMed] [Google Scholar]
- 28.Martin, M., N. Goyette, J. Ives, and G. Boivin. 2010. Incidence and characterization of cytomegalovirus resistance mutations among pediatric solid organ transplant patients who received valganciclovir prophylaxis. J. Clin. Virol. 47:321-324. [DOI] [PubMed] [Google Scholar]
- 29.Michel, D., P. Schaarschmidt, K. Wunderlich, M. Heuschmid, L. Simoncini, D. Muhlberger, A. Zimmermann, I. Pavic, and T. Mertens. 1998. Functional regions of the human cytomegalovirus protein pUL97 involved in nuclear localization and phosphorylation of ganciclovir and pUL97 itself. J. Gen. Virol. 79:2105-2112. [DOI] [PubMed] [Google Scholar]
- 30.Prichard, M. N. 2009. Function of human cytomegalovirus UL97 kinase in viral infection and its inhibition by maribavir. Rev. Med. Virol. 19:215-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reddy, A. J., A. K. Zaas, K. E. Hanson, and S. M. Palmer. 2007. A single-center experience with ganciclovir-resistant cytomegalovirus in lung transplant recipients: treatment and outcome. J. Heart Lung Transplant. 26:1286-1292. [DOI] [PubMed] [Google Scholar]
- 32.Smith, I. L., J. M. Cherrington, R. E. Jiles, M. D. Fuller, W. R. Freeman, and S. A. Spector. 1997. High-level resistance of cytomegalovirus to ganciclovir is associated with alterations in both the UL97 and DNA polymerase genes. J. Infect. Dis. 176:69-77. [DOI] [PubMed] [Google Scholar]
- 33.Spector, S. A., K. Hsia, D. Wolf, M. Shinkai, and I. Smith. 1995. Molecular detection of human cytomegalovirus and determination of genotypic ganciclovir resistance in clinical specimens. Clin. Infect. Dis. 21(Suppl. 2):S170-S173. [DOI] [PubMed] [Google Scholar]
- 34.Warming, S., N. Costantino, D. L. Court, N. A. Jenkins, and N. G. Copeland. 2005. Simple and highly efficient BAC recombineering using galK selection. Nucleic Acids Res. 33:e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolf, D. G., I. L. Smith, D. J. Lee, W. R. Freeman, M. Flores-Aguilar, and S. A. Spector. 1995. Mutations in human cytomegalovirus UL97 gene confer clinical resistance to ganciclovir and can be detected directly in patient plasma. J. Clin. Invest. 95:257-263. [DOI] [PMC free article] [PubMed] [Google Scholar]