Abstract
ST-246, a novel compound that inhibits egress of orthopoxvirus from infected cells, is being evaluated as a treatment for pathogenic orthopoxvirus infections in humans. This phase I, double-blind, randomized, placebo-controlled, escalating multiple-dose study was conducted to determine the safety, tolerability, and pharmacokinetics of ST-246 administered as a single daily oral dose of 250, 400, or 800 mg for 21 days to nonfasting healthy human volunteers. ST-246 appeared to be well tolerated, with no serious adverse events (AEs). Headache, for which one subject in the 800-mg group discontinued the study, was the most commonly reported AE in all treatment groups. The multiple-dose pharmacokinetics of ST-246 was well characterized. The day 21 mean elimination half-lives were calculated at 18.8, 19.8, and 20.7 h for each of the 250-, 400-, and 800-mg/day dose groups, respectively. Steady state was reached by day 6 (within 3 to 5 half-lives), saturable absorption was observed at the 800-mg dose level, and the fraction of parent drug excreted in the urine was very low. Based on these results, administration of 400 mg/day ST-246 can be expected to provide plasma concentrations above the efficacious concentration demonstrated in nonhuman primate models in earlier studies.
Human orthopoxviruses cause a spectrum of diseases ranging from severe disseminated lesional disease characteristic of the most common type of variola virus infection (variola major) to localized lesional infection caused by vaccinia virus. Of the several species of orthopoxvirus known to infect humans, variola virus, the etiological agent of smallpox, causes far more serious infections than the other species of poxviruses (3). While variola virus no longer exists in the environment, other orthopoxviruses continue to circulate and cause disease. Monkeypox virus, which is endemic in some areas of the Democratic Republic of the Congo, causes a zoonotic disease that is characterized by a generalized infection resembling a milder version of smallpox (7). Vaccinia-like viruses have been isolated from patients in Brazil presenting with localized lesions of the hands and arms (10), and cowpox virus infections are increasing in certain parts of Europe (11). These viruses are believed to be maintained in the population through rodent reservoirs, and zoonotic disease is thought to arise from contact with infected animals or through an intermediate species such as cattle or domestic pets (7, 11). Disease severity in all cases is influenced by the status of the host immune system, with individuals who suffer from certain skin disorders or who are immunocompromised developing the most severe infections (2, 3).
There are currently no U.S. Food and Drug Administration-approved therapies other than early vaccination that can alter the outcome of disease or potentially prevent disease in a population that has been exposed to pathogenic orthopoxviruses (4, 6). Because vaccination has a lag period for antibody formation and carries the risk of certain severe side effects, and because it is not universally available to all who might potentially need it, there is clearly a need for a safe, small-molecule, oral medication that is highly active against variola virus and possibly other zoonotic poxviruses, such as monkeypox, cowpox, and vaccinia-like viruses.
ST-246 is a low-molecular-weight compound {Tecovirimat; 4-trifluoromethyl-N-(3,3a,4,4a,5,5a,6,6a-octahydro-1,3-dioxo-4,6-ethenocycloprop[f]isoindol-2(1H)-yl)-benzamide} that was discovered through a deliberate effort to develop orally available antiviral drugs for use in biodefense (1, 12). ST-246 is chemically unrelated to any substance currently approved for human use for human or veterinary applications. In a number of animal studies, oral administration of ST-246 not only protected nonhuman primates from variola and monkeypox viruses but also protected mice from lethal infection with vaccinia virus, cowpox virus, and ectromelia virus (8, 12) and squirrels from severe monkeypox disease (9), implying that ST-246 could also be used to control vaccination complications and to prevent or treat zoonotic poxvirus disease.
Safety pharmacology studies of mice and nonhuman primates demonstrated that ST-246 was well tolerated after a 28-day multiple-dose administration with a no-observable-effect level (NOEL) of 2,000 mg/kg and 300 mg/kg for mice and nonhuman primates, respectively. A phase I clinical study was conducted to determine the safety, tolerability, and clinical pharmacokinetics (PK) of ST-246 administered orally as a single dose of 500, 1,000, or 2,000 mg (fasting) or 1,000 mg (nonfasting) to healthy human volunteers (5). The study concluded that ST-246 at these dose levels was safe and well tolerated. The pharmacokinetics in plasma showed dose proportionality over 500- and 1,000-mg dose levels but not over 1,000- and 2,000-mg dose levels. At the 1,000-mg dose level, nonfasting subjects had greater apparent maximum concentration of drug in serum (Cmax), time to maximum concentration of drug in serum (Tmax), and area under the curve from zero hour to infinity (AUC0-∞) than fasting subjects (5).
Based on these results, and given the variability in exposure levels in both monkeys and humans in the nonfasting and fasting states, it has been predicted that doses of 400 mg and 800 mg for humans who are nonfasting will encompass plasma drug exposure levels comparable to those that provide protective efficacy in the nonhuman primate model of orthopoxvirus disease. The purpose of this study was to determine the safety, tolerability, and clinical pharmacokinetics of ST-246 when administered orally as a single dose of 250, 400, or 800 mg every day for 21 days to nonfasting healthy human volunteers.
MATERIALS AND METHODS
Study design and population.
This was a randomized, double-blind, placebo-controlled, escalating multiple-dose study to assess the safety, tolerability, and PK of ST-246 when administered as a single daily oral dose (250 mg, 400 mg, 800 mg, or placebo) given for 21 days to healthy human volunteers in the nonfasted state. A total of 30 volunteers were expected to participate. The study protocol and amendment(s) as well as informed consent forms were reviewed and approved by an Institutional Review Board. This study was conducted in compliance with the protocol, International Conference on Harmonization Good Clinical Practice E6 (ICH-GCP), and applicable regulatory requirements, including the U.S. Code of Federal Regulations applicable to clinical studies. At the screening visit prior to performing any study-related procedures, subjects or their legally acceptable representatives provided their written consent to participate in the study after having been informed about the nature and purpose of the study, participation/termination conditions, and risks and benefits of treatment. A safety monitoring committee (SMC), an independent expert advisory group, evaluated accumulating safety data. The primary purpose of this SMC was to ensure the safety of the enrolled subjects. Before each cohort was dosed, the SMC reviewed the available safety data and made a recommendation as to whether the trial should proceed as planned.
The chosen sample size of 8 ST-246 subjects per dose level (24 total) is in the range generally considered appropriate for a first multiple-dose study of humans. The inclusion of two placebo subjects per dose level (six total) was designed to provide masking and thereby increase the objectivity of study assessments. It also provided a very small reference group for crude comparisons with respect to any anomalies among ST-246 subjects. In order to achieve this sample size, 67 subjects (males and nonpregnant females, 18 to 50 years old) were screened within a 28-day screening period prior to a 1-day enrollment/baseline period preceding study drug administration on day 1. During the screening period, a medical history, physical examination, vital signs, routine laboratory tests, pregnancy test, drug screen, and electrocardiogram (ECG) were conducted. Subjects were counseled on avoidance of pregnancy throughout the study. Pregnancy tests were performed at screening, on day 1 prior to drug administration, at the week 4 visit (day 28, 29, or 30), and at the week 7 visit (day 51, 52, or 53) prior to study completion. No concomitant medications were permitted during the study. An ECG was performed at screening, on day 1 (predose and 4 to 6 h postdose), during week 1 at 4 to 6 h postdose, and during week 3 at 4 to 6 h postdose. An amendment to the protocol added an ECG evaluation at week 4 and at week 7 or early discontinuation (if discontinuation was prior to week 4).
Twenty-four healthy volunteers were randomized, based on the SAS procedure PROC PLAN, to active drug in one of the three dosing groups (eight per dose group), while the six remaining volunteers (two per dose group) received placebo. Each dose group of 10 was divided into two cohorts of 5 subjects (4 active and 1 placebo). The first cohort was dosed approximately 4 to 8 weeks before the second cohort. Each dose group completed the study treatment approximately 5 weeks prior to the start of the following dose group.
On day 1, an intravenous catheter was inserted into the subject's forearm vein and a baseline (0-h) blood sample was obtained, followed by serial blood draws after medication administration. All volunteers received either a dose of ST-246 or placebo within 30 min after a standard light meal consisting of 400 to 450 cal and approximately 25% fat. Subsequently, subjects followed a single-dose/day administration of ST-246 for 21 days, with clinic visits at week 1 (day 6), week 2 (days 14 to 16), and week 3 (days 21, 22, 23, and 24). Subjects were followed for an additional 30 to 32 days, returning for follow-up visits at week 4 (days 28 to 31) and week 7 (days 51 to 53).
Subjects were instructed to eat their standard light meal and then take their study medication at the same time each day. These times were recorded on a diary card given to each subject on day 2. On days 1, 2, 6, 7, and 21, when the subjects were in the clinic for PK samples, the subjects were instructed to bring their study medication in with them so that the site staff could observe the subjects take their medication. Medication was given in the form of capsules, batch numbers 5J053 for 25 mg, 5J054 for 200 mg, and 5J052 for placebo.
Safety assessments.
General safety parameters that were evaluated included adverse events (AEs), vital signs, physical exam, clinical laboratory safety tests (including hematology, blood chemistry for electrolytes and liver function, and urinalysis), and ECGs. The safety results of each dose group were examined by an independent safety monitoring committee prior to the initiation of study drug administration in the next-higher-dose group.
Blood and urine sample collection.
Venous blood (before and after study drug administration on day 1 [pre- and postdose] and on days 2, 6, 7, 21, 22, 23, and 24) and urine (before and after study drug administration on day 1 and day 21) were collected to determine the PK of ST-246 in humans. Plasma samples were collected and stored at −70°C until analyzed for maximum drug concentration (Cmax), time to maximum drug concentration (Tmax), terminal half-life (t1/2), area under the concentration-time curve (AUC), and renal clearance (CLR). Urine samples were immediately centrifuged at 4°C for 10 min at 2,000 × g and evaluated for urinary excretion.
Bioanalysis.
ST-246 was quantified from human plasma specimens by a validated liquid chromatography and tandem mass spectrometry method using an analog of ST-246 as an internal standard. Quality control (QC) samples were used to assess the performance of the assay method. Analytical run sequences were conducted with QC samples injected before and after a set of ≤10 study samples. The acceptance criteria for the analytical run were ≥66.6% of QC samples within ±15% relative error (RE) of the nominal value and ≥50% of the QC samples at each level within ±15% RE of the nominal value. The interday relative standard deviation for this assay (%RSD) for back-calculated concentration calibration standards ranged from 2.2% to 7.3%. The intrarun %RSD ranged from 0.5% to 20.0%. Human plasma (10 μl) was extracted with 90 μl of a solution containing 0.05% ammonium hydroxide and 0.05% acetic acid in methanol. The mixture was centrifuged at 10,000 × g for 4 min at 18°C, and the supernatant was injected onto a Phenomenex LUNA C18 column (30 by 2 mm; 5-μm particle size). The mobile phase consisted of water containing 0.05% acetic acid and 0.05% NH4OH. ST-246 was eluted using a 20% to 90% gradient of methanol containing 0.05% acetic acid and 0.05% NH4OH from 0.2 to 0.5 min, with a flow rate of 300 μl/min. Fractions were analyzed on a Sciex API 4000 triple quadrupole mass spectrometer, using turbospray ionization in the negative ion mode. Analytes were detected by multiple reactions monitoring the 375.1 m/z to 282.9 m/z transition for ST-246 and the 340.9 m/z to 248.9 m/z transition for the internal standard. The plasma and urine methods were validated for ranges of 50 to 4,000 ng/ml and 20 to 6,000 ng/ml, respectively.
Statistical analysis.
PK parameter values were summarized for each dose level of ST-246 (250 mg, 400 mg, and 800 mg), placebo (pooled over 3 dose levels), and total active treatment. All summaries and listings were based on the actual administration each subject received. Demographic and baseline data, including vital signs, laboratory tests, and ECGs, were summarized with descriptive statistics (number of subjects, mean, standard deviation [SD], median, minimum, maximum, and number of missing values). The number and percentage of subjects with AEs, including serious adverse events (SAEs) and discontinuations due to AEs, were tabulated. Summaries for each laboratory test based on Division of AIDS (DAIDS) AE grading criteria were generated. For data collected at multiple time points (e.g., vital signs, ECG), the scheduled visit day/time was used to summarize the data.
The PK parameter values were analyzed by noncompartmental methods using WinNonlin version 5.0.1 (Pharsight Corporation, Mountain View, CA) and were determined directly from the plasma and urine concentration-time data, including the area under the plasma concentration-time curve for each dosing interval (AUCtau), maximum concentration of drug in serum (Cmax) (ng/ml), time to Cmax (Tmax) (h), time from dose to the first measurable (nonzero) plasma concentration (Tlag), minimum drug concentration in plasma (Cmin), time to minimum drug concentration (Tmin), AUCtau/24 (Cavg), percent fluctuation, observed elimination rate constant (λz), terminal elimination half-life determined after the last dose on day 21 (t1/2), accumulation factor [AUCtau(Day21)/AUCtau(Day1)], total systemic clearance (CLss/F), volume of distribution (Vz/F), cumulative amount of ST-246 excreted unchanged in urine over 24 h (Ae0-24), fraction of dose excreted in urine (Fe), and CLR. Dose proportionality of ST-246 was assessed using log-transformed Cmax and AUCtau values. A two-sample t test was used to test for differences between males and females for those PK parameter values considered dose independent: AUCtau (dose normalized), λz, CLss/F, CLR, and Fe.
RESULTS
Demographics.
A total of 30 subjects were enrolled and randomized to active drug or placebo in one of the three dosing groups. These subjects were all included in the safety analysis. A summary of the demographic characteristics of the subjects is presented in Table 1. The mean age of subjects in the study was approximately 34 years. There were more males than females in the active-treatment and placebo groups (total of 66.7% males); however, the distribution by gender varied for each dose group. Distribution by race also varied by dose group, with the majority of subjects in active-treatment groups (70.8%) and placebo groups (66.7%) being white. Of the remaining subjects, 25% of the active-treatment group and 33.3% of the placebo group were black and 4.2% of the active-treatment group were of another race. Hispanic or Latino ethnicity accounted for 25% of active-treatment and 33.3% of placebo groups. The mean weights were similar for all groups (71 to 75 kg), except for the 800-mg dose group, where the average weight was ∼87 kg.
TABLE 1.
Demographic and baseline characteristic (safety population)
| Characteristicc | Value for ST-246 treatment |
||||
|---|---|---|---|---|---|
| Placebo (n = 6) | 250 mg (n = 8) | 400 mg (n = 8) | 800 mg (n = 8) | Total active treatment (250-800 mg) (n = 24) | |
| Gender [no. (%)] | |||||
| Male | 4 (66.7) | 5 (62.5) | 4 (50.0) | 7 (87.5) | 16 (66.7) |
| Female | 2 (33.3) | 3 (37.5) | 4 (50.0) | 1 (12.5) | 8 (33.3) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Race [no. (%)] | |||||
| White | 4 (66.7) | 7 (87.5) | 6 (75.0) | 4 (50.0) | 17 (70.8) |
| Black | 2 (33.3) | 0 (0.0) | 2 (25.0) | 4 (50.0) | 6 (25.0) |
| Asian | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| American Indian or Alaska native | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Native Hawaiian or other Pacific islander | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Other | 0 (0.0) | 1 (12.5) | 0 (0.0) | 0 (0.0) | 1 (4.2) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Ethnicity [no. (%)] | |||||
| Hispanic or Latino | 2 (33.3) | 3 (37.5) | 3 (37.5) | 0 (0.0) | 6 (25.0) |
| Not Hispanic or Latino | 4 (66.7) | 5 (62.5) | 5 (62.5) | 8 (100.0) | 18 (75.0) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Agea (yr) | |||||
| n | 6 | 8 | 8 | 8 | 24 |
| Mean | 34.8 | 36.5 | 27.6 | 37.5 | 33.9 |
| SD | 8.9 | 8.2 | 7.8 | 7.6 | 8.8 |
| Median | 36.5 | 37.5 | 25.5 | 35.5 | 34.5 |
| Min-Max | 24-47 | 23-47 | 18-39 | 29-49 | 18-49 |
| Missing | 0 | 0 | 0 | 0 | 0 |
| Wtb (kg) | |||||
| n | 6 | 8 | 8 | 8 | 24 |
| Mean | 74.72 | 75.09 | 70.99 | 86.76 | 77.61 |
| SD | 14.63 | 15.28 | 15.34 | 11.95 | 15.26 |
| Median | 72.42 | 76.80 | 70.20 | 92.50 | 74.50 |
| Min-Max | 55.9-95.5 | 53.6-102.1 | 54.1-104.0 | 66.4-100.0 | 53.6-104.0 |
| Missing | 0 | 0 | 0 | 0 | 0 |
| Htb (cm) | |||||
| n | 6 | 8 | 8 | 8 | 24 |
| Mean | 174.5 | 170.1 | 168.0 | 178.3 | 172.1 |
| SD | 5.8 | 11.2 | 11.2 | 6.1 | 10.4 |
| Median | 174.5 | 174.0 | 166.0 | 178.5 | 174.0 |
| Min-Max | 167-182 | 151-181 | 155-191 | 168-186 | 151-191 |
| Missing | 0 | 0 | 0 | 0 | 0 |
| Body mass indexb (kg/m2) | |||||
| n | 6 | 8 | 8 | 8 | 24 |
| Mean | 24.4 | 25.9 | 24.9 | 27.2 | 26.0 |
| SD | 4.0 | 4.3 | 2.4 | 2.6 | 3.2 |
| Median | 24.6 | 26.2 | 25.1 | 27.5 | 26.2 |
| Min-Max | 19-29 | 18-31 | 20-29 | 22-30 | 18-31 |
| Missing | 0 | 0 | 0 | 0 | 0 |
Age = integer ([screening visit date − birth date]/365.25).
Measurement at screening.
Percentages are based on the number of subjects in each group. Min-Max, range of minimum to maximum.
Safety.
Thirteen of active-treated subjects (54.2%) and four placebo subjects (66.7%) reported at least one treatment-emergent adverse event (TEAE). The most commonly reported AEs are summarized in Table 2. Eight subjects in the active-treatment groups (33.3%) and two placebo subjects (33.3%) reported at least one AE considered to be drug related (definitely, probably, or possibly) in the opinion of the investigator. Most AEs (30/37 AEs [81.1%] in the active-treated groups and 6/8 AEs [75%] in the placebo group) were mild in intensity. Headache was the most commonly reported AE in all treatment groups. One subject in the 800-mg dose group experienced an AE (severe headache) which led to discontinuation from the study; the investigator considered this AE to be related to the study drug. No deaths or SAEs occurred during the course of the study. There were no other trends in the data that suggested the presence of other drug-related toxicity.
TABLE 2.
Incidence of treatment-emergent adverse events (TEAEs) categorized as related to study drug summarized by system organ class and preferred terma
| Adverse events | No. (%) for ST-246 treatment |
||||
|---|---|---|---|---|---|
| Placebo (n = 6) | 250 mg (n = 8) | 400 mg (n = 8) | 800 mg (n = 8) | Total active treatment (250-800 mg) (n = 24) | |
| Total no. of TEAEs | 5 | 5 | 0 | 11 | 16 |
| Subjects reporting at least one TEAE | 2 (33.3) | 3 (37.5) | 0 (0.0) | 5 (62.5) | 8 (33.3) |
| Eye disorders: abnormal sensation in eye | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (12.5) | 1 (4.2) |
| Gastrointestinal disorders | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (37.5) | 3 (12.5) |
| Dry mouth | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (12.5) | 1 (4.2) |
| Flatulence | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (25.0) | 2 (8.3) |
| Nausea | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (12.5) | 1 (4.2) |
| General disorders and administration site conditions: fatigue | 0 (0.0) | 1 (12.5) | 0 (0.0) | 0 (0.0) | 1 (4.2) |
| Nervous system disorders | 1 (16.7) | 3 (37.5) | 0 (0.0) | 3 (37.5) | 6 (25.0) |
| Headache | 1 (16.7) | 3 (37.5) | 0 (0.0) | 2 (25.0) | 5 (20.8) |
| Somnolence | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (12.5) | 1 (4.2) |
| Skin and subcutaneous tissue disorders: urticaria | 1 (16.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
TEAEs are nonserious adverse events on or after day 1 postdose through week 4 follow-up and serious adverse events starting on or after day 1 postdose through week 7 study completion. For the number of subjects with TEAEs, subjects were counted once for each system organ class and once for each preferred term. Adverse events are coded in accordance with the MedDRA (Medical Dictionary for Regulatory Activities, version 10.0; www.meddramsso.com). Percentages are based on the number of subjects in each treatment group.
Most baseline vital signs were similar between groups, and none of the ECG results obtained at baseline (pulse, systolic blood pressure, and QT interval, a measure of the time between the start of the Q wave and the end of the T wave in the heart's electrical cycle) were considered clinically significant by the investigator (data not shown).
Pharmacokinetics.
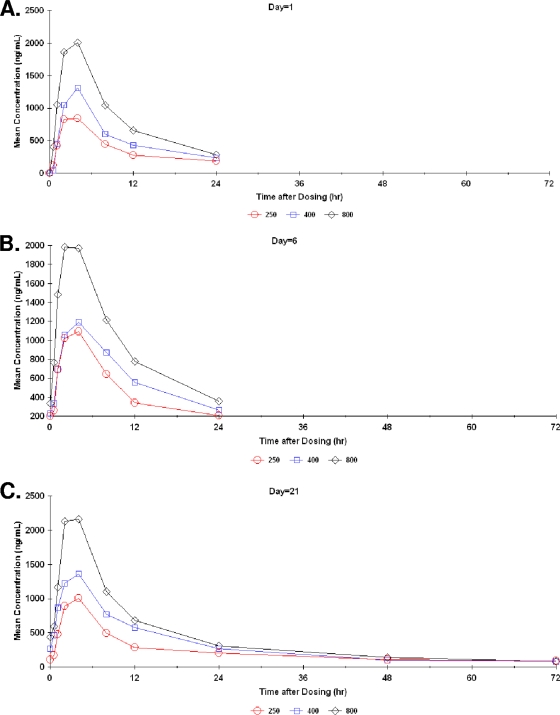
A total of 24, 22, and 19 subjects were used for day 1, 6, and 21 PK parameter value estimates, respectively. A total of 5 subjects failed to complete the study. One discontinued due to severe headache, one was lost to follow-up, two were unable to complete the study procedures, and one discontinued for unspecified reasons. The mean ST-246 concentration-versus-time profiles obtained from all human subjects at doses of 250, 400, and 800 mg/day declined in a biexponential manner postadministration (Fig. 1).
FIG. 1.
Mean ST-246 plasma concentrations over time for day 1 (A), day 6 (B), and day 21 (C) (PK population).
A summary of ST-246 exposure, i.e., mean Cmax (and Tmax) and mean AUCtau values for 250-, 400-, and 800-mg/day dose levels, is displayed in Table 3. At 21 days of dosing, the mean exposure values (using Cmax and AUCtau) of ST-246 were similar to those observed at 6 days of dosing. Steady-state ST-246 plasma concentration values were achieved by day 6, within three to five elimination half-lives. The mean (median) elimination half-lives (T) for the 250-, 400-, and 800-mg/day dose groups were 18.8 (22.6), 19.8 (18.1), and 20.7 (21.1) hours, respectively. Mean Tmax was independent of dose.
TABLE 3.
ST-246 Exposure summary
| ST-246 dose group/ parameter (mean) | Value for: |
||
|---|---|---|---|
| Day 1 | Day 6 | Day 21 | |
| 250 mg/day | |||
| Mean Cmax (ng/ml) | 985 | 1,212 | 1,101 |
| Median Cmax (ng/ml) | 984 | 1,065 | 954 |
| SD | 346 | 350 | 378 |
| Cmax range (ng/ml) | 518-1,430 | 907-1,970 | 835-1,910 |
| Mean AUCtau (ng·h/ml) | 9,101 | 11,892 | 10,083 |
| Median AUCtau (ng·h/ml) | 8,042 | 10,436 | 9,755 |
| SD | 3,492 | 4,389 | 2,843 |
| AUCtau range (ng·h/ml) | 5,136-14,443 | 6,932-20,846 | 6,257-14,511 |
| t1/2 (h) | NRa | NR | 18.8 |
| 400 mg/day | |||
| Mean Cmax (ng/ml) | 1,392 | 1,298 | 1,457 |
| Median Cmax (ng/ml) | 1,300 | 1,330 | 1,635 |
| SD | 411 | 277 | 451 |
| Cmax range (ng/ml) | 755-2,050 | 778-1,700 | 742-1,870 |
| Median AUCtau (ng·h/ml) | 14,785 | 14,834 | 17,118 |
| SD | 3,626 | 4,708 | 5,534 |
| Mean AUCtau (ng·h/ml) | 13,146 | 15,432 | 16,182 |
| AUCtau range (ng·h/ml) | 7,291-16,641 | 8,777-21,980 | 7,040-23,143 |
| t1/2 (h) | NR | NR | 19.8 |
| 800 mg/day | |||
| Mean Cmax (ng/ml) | 2,279 | 2,337 | 2,437 |
| Median Cmax (ng/ml) | 2,385 | 2,190 | 2,275 |
| SD | 395 | 437 | 496 |
| Cmax range (ng/ml) | 1,570-2,680 | 1,880-3,060 | 1,960-3,130 |
| Median AUCtau (ng·h/ml) | 20,269 | 21,387 | 22,526 |
| SD | 6,091 | 4,696 | 4,579 |
| Mean AUCtau (ng·h/ml) | 20,959 | 23,352 | 22,684 |
| AUCtau range (ng·h/ml) | 14,962-31,670 | 18,903-30,592 | 17,684-28,009 |
| t1/2 (h) | NR | NR | 20.7 |
| Result for all dose groups | |||
| Tmax (h) | 2.88-3.25 | 2.63-3.57 | 2.67-3.17 |
NR, not reported.
The accumulation factors (day 21) ranged from 1.16 to 1.21, indicating that ST-246 concentrations accumulated by only 16% to 21% after chronic (once per day) dosing. The fraction of ST-246 excreted unchanged in urine (expressed as percentage of dose) was very low; mean values varied from 0.02% to 0.03% of the dose.
Based on a formal statistical assessment of ST-246 PK parameter values (Cmax and AUCtau), a conclusion of dose proportionality could not be confirmed, since the 90% confidence index (CI) of the slope did not include 1 (Table 4). However, as the dose increased from 250 to 400 mg/day (1.6-fold), the mean day 21 AUCtau also increased 1.6-fold (a dose-proportionate increase). When the dose was further increased to 800 mg (a 3.2-fold increase from 250 mg), the AUCtau increased 2.25-fold, a less-than-proportionate increase. These results are indicative of saturable absorption at the 800-mg dose level. Similar results were observed for Cmaxs.
TABLE 4.
Dose proportionality of ST-246 assessed using pharmacokinetic parameter results (PK population)
| Study day and parametera | Estimate of slope (β)b | SE of estimate of slope (β) | 90% CI of estimate of slope (β)c |
|---|---|---|---|
| 1 | |||
| Cmax (ng/ml) | 0.76 | 0.13 | 0.54-0.97 |
| AUCtau (ng·h/ml) | 0.74 | 0.14 | 0.50-0.98 |
| 6 | |||
| Cmax (ng/ml) | 0.59 | 0.11 | 0.41-0.78 |
| AUCtau (ng·h/ml) | 0.61 | 0.13 | 0.39-0.84 |
| 21 | |||
| Cmax (ng/ml) | 0.71 | 0.14 | 0.47-0.94 |
| AUCtau (ng·h/ml) | 0.70 | 0.15 | 0.45-0.96 |
Actual values used (not dose normalized).
General linear model was fitted to the data using the log-transformed model: log (parameter) = intercept + ß·log (dose) + error.
For a tentative conclusion of dose proportionality of the dose-dependent parameters, the 90% CI for the slope should include 1.
DISCUSSION
This was the first multiple-dose study that assessed the safety, tolerability, and pharmacokinetics of the antiorthopoxvirus compound ST-246 when administered as a single daily oral dose (250, 400, or 800 mg) for 21 days in 24 healthy male and female volunteers in the nonfasted (fed) state. ST-246 was well tolerated across all doses. There were no deaths or serious adverse events reported during this study. The 250-mg dose group had the largest percentage of subjects reporting at least one AE (6/8, 75%), followed by the placebo group (4/6, 66.7%), the 800-mg treatment dose group (5/8, 62.5%), and then the 400-mg dose group (2/8, 25%).
Ten of 30 (33.3%) active-treated subjects (eight active-treated subjects [33.3%] and two placebo-treated subjects [33.3%]) reported at least one AE considered by the investigator to be drug related. The 800-mg dose group had the largest percentage of subjects reporting at least one drug-related AE (5/8, 62.5%), followed by the 250-mg dose group (3/8, 37.5%) and the placebo group (2/6, 33.3%). There were no drug-related AEs reported in the 400-mg dose group. The most common of these was headache, reported by 11 (45.8%) active-treated subjects and 2 placebo subjects (33.3%). The second most common AEs in the active-treated groups were gastrointestinal disorders (dry mouth, flatulence, nausea, and vomiting), reported by six (25%) active-treated subjects and none of the placebo subjects. One subject in the 800-mg dose group experienced a drug-related AE (severe headache) which led to discontinuation from the study.
Laboratory results and vital sign results were unremarkable. ECG analysis did not reveal any clinically significant QTcB (Bazett's correction of the QT interval) parameters or changes from baseline.
Based on these results, we concluded that (i) headache appeared to be a possible AE of treatment with ST-246 and (ii) the incidence of AEs and drug-related AEs is not strictly dose dependent.
The multiple-dose pharmacokinetics of ST-246 was well characterized in these healthy volunteers. The collection of blood samples on days 1, 6, and 21, and urine samples on days 1 and 21, allowed determination of pharmacokinetic parameter values after a single dose on day 1 through 24 h, at an intervening time point (day 6), and for 72 h after the last dose on day 21. In particular, the day 21 elimination half-life was calculated, steady state was reached by day 6 (within 3 to 5 half-lives), saturable absorption was observed at the 800-mg dose level, and very little drug was excreted unchanged in urine.
Overall, ST-246 appears safe and well tolerated when administered orally as a single daily dose for 21 days to normal, healthy nonfasted volunteers. These results support further testing of ST-246 in multicenter pivotal clinical safety trials.
Acknowledgments
This project has been funded in whole or in part with Federal funds from the Biomedical Advanced Research and Development Authority, Department of Health and Human Services, in conjunction with the National Institute of Allergy and Infectious Disease, National Institutes of Health, Department of Health and Human Services, under contract no. HHSN266200600014C.
Robert Jordan, Jarasvech Chinsangaram, Tove' C. Bolken, Shanthakumar R. Tyavanagimatt, Deborah Tien, Kevin F. Jones, Annie Frimm, Jean Clarke, and Dennis E. Hruby are shareholders of SIGA Technologies, Inc.
Footnotes
Published ahead of print on 12 April 2010.
REFERENCES
- 1.Bailey, T. R., S. R. Rippin, E. Opsitnick, C. J. Burns, D. C. Pevear, M. S. Collett, G. Rhodes, S. Tohan, J. W. Huggins, R. O. Baker, E. R. Kern, K. A. Keith, D. Dai, G. Yang, D. Hruby, and R. Jordan. 2007. N-(3,3a,4,4a,5,5a,6,6a-octahydro-1,3-dioxo-4,6-ethenocycloprop[f]isoindol-2-(1H)-yl)carboxamides: identification of novel orthopoxvirus egress inhibitors. J. Med. Chem. 50:1442-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bray, M. 2003. Pathogenesis and potential antiviral therapy of complications of smallpox vaccination. Antiviral Res. 58:101-114. [DOI] [PubMed] [Google Scholar]
- 3.Fenner, F., D. A. Henderson, I. Arita, Z. Jazek, and I. D. Ladnyi. 1988. Smallpox and its eradication. World Health Organization, Geneva, Switzerland.
- 4.Institute of Medicine. 1999. Assessment of future scientific needs for live variola virus. National Academy Press, Washington, DC. [PubMed]
- 5.Jordan, R., D. Tien, T. C. Bolken, K. F. Jones, S. R. Tyavanagimatt, J. Strasser, A. Frimm, M. L. Corrado, P. G. Strome, and D. E. Hruby. 2008. Single-dose safety and pharmacokinetics of ST-246, a novel orthopoxvirus egress inhibitor. Antimicrob. Agents Chemother. 52:1721-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.LeDuc, J. W., and P. B. Jahrling. 2001. Strengthening national preparedness for smallpox: an update. Emerg. Infect. Dis. 7:155-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parker, S., A. Nuara, R. M. Buller, and D. A. Schultz. 2007. Human monkeypox: an emerging zoonotic disease. Future Microbiol. 2:17-34. [DOI] [PubMed] [Google Scholar]
- 8.Quenelle, D. C., R. M. Buller, S. Parker, K. A. Keith, D. E. Hruby, R. Jordan, and E. R. Kern. 2007. Efficacy of delayed treatment with ST-246 given orally against systemic orthopoxvirus infections in mice. Antimicrob. Agents Chemother. 51:689-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sbrana, E., R. Jordan, D. E. Hruby, R. I. Mateo, S. Y. Xiao, M. Siirin, P. C. Newman, A. P. A. Travassos da Rosa, and R. B. Tesh. 2007. Efficacy of the antipoxvirus compound ST-246 for treatment of severe orthopoxvirus infection. Am. J. Trop. Med. Hyg. 76:768-773. [PubMed] [Google Scholar]
- 10.Trindade, G. S., G. L. Emerson, D. S. Carroll, E. G. Kroon, and I. K. Damon. 2007. Brazilian vaccinia viruses and their origins. Emerg. Infect. Dis. 13:965-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vorou, R. M., V. G. Papavassiliou, and I. N. Pierroutsakos. 2008. Cowpox virus infection: an emerging health threat. Curr. Opin. Infect. Dis. 21:153-156. [DOI] [PubMed] [Google Scholar]
- 12.Yang, G., D. C. Pevear, M. H. Davies, M. S. Collett, T. Bailey, S. Rippen, L. Barone, C. Burns, G. Rhodes, S. Tohan, J. W. Huggins, R. O. Baker, R. L. Buller, E. Touchette, K. Waller, J. Schriewer, J. Neyts, E. DeClercq, K. Jones, D. Hruby, and R. Jordan. 2005. An orally bioavailable antipoxvirus compound (ST-246) inhibits extracellular virus formation and protects mice from lethal orthopoxvirus challenge. J. Virol. 79:13139-13149. [DOI] [PMC free article] [PubMed] [Google Scholar]