Abstract
Arylimidamides (AIAs) represent a new class of molecules that exhibit potent antileishmanial activity (50% inhibitory concentration [IC50], <1 μM) against both Leishmania donovani axenic amastigotes and intracellular Leishmania, the causative agent for human visceral leishmaniasis (VL). A systematic lead discovery program was employed to characterize in vitro and in vivo antileishmanial activities, pharmacokinetics, mutagenicities, and toxicities of two novel AIAs, DB745 and DB766. They were exceptionally active (IC50 ≤ 0.12 μM) against intracellular L. donovani, Leishmania amazonensis, and Leishmania major and did not exhibit mutagenicity in an Ames screen. DB745 and DB766, given orally, produced a dose-dependent inhibition of liver parasitemia in two efficacy models, L. donovani-infected mice and hamsters. Most notably, DB766 (100 mg/kg of body weight/day for 5 days) reduced liver parasitemia in mice and hamsters by 71% and 89%, respectively. Marked reduction of parasitemia in the spleen (79%) and bone marrow (92%) of hamsters was also observed. Furthermore, these compounds distributed to target tissues (liver and spleen) and had a moderate oral bioavailability (up to 25%), a large volume of distribution, and an elimination half-life ranging from 1 to 2 days in mice. In a repeat-dose toxicity study of mice, there was no indication of liver or kidney toxicity for DB766 from serum chemistries, although mild hepatic cell eosinophilia, hypertrophy, and fatty changes were noted. These results demonstrated that arylimidamides are a promising class of molecules that possess good antileishmanial activity and desirable pharmacokinetics and should be considered for further preclinical development as an oral treatment for VL.
Leishmaniasis, a neglected tropical disease, is caused by parasitic protozoa of the genus Leishmania, including 20 species that are pathogenic for humans (21). Clinical manifestations of leishmaniasis mainly consist of cutaneous, mucocutaneous, visceral, and post-kala-azar dermal leishmaniasis, with symptoms ranging from skin and mucosal ulceration to systemic infection that is fatal if left untreated (6). An estimated 12 million people are currently infected with Leishmania, and up to 350 million people in 88 countries are at risk of infection (35). Approximately 2 million new cases of leishmaniasis are believed to occur annually, with 1.5 million for cutaneous leishmaniasis and 0.5 million for visceral leishmaniasis (VL). In macrophages, Leishmania amastigotes adapt to thrive in an acidic subcellular compartment, the parasitophorous vacuole (PV; pH ∼5) (2), where they maintain a neutral intracellular pH within the parasite by an energy-dependent process (9). Multiple layers of membrane barriers (i.e., host macrophage plasma membrane, phagolysosomal membrane, and Leishmania amastigote plasma membrane) presumably present a formidable challenge for chemotherapeutic agents to target Leishmania parasites in mammalian hosts.
Current chemotherapies for leishmaniasis have many limitations, including resistance, cost, parenteral administration, long treatment regimens, gastrointestinal intolerance, nephrotoxicity, and/or teratogenicity (10, 18, 29, 30, 36). Since effective vaccines do not exist, there is an urgent need to develop new chemotherapeutic candidates with improved pharmacokinetic and toxicologic properties for the treatment of leishmaniasis.
In our efforts to identify promising antiprotozoal agents from a library of aromatic diamidines and their analogues, we have identified a new class of molecules, arylimidamides (AIAs; previously referred to as “reversed” amidines [26, 27]), that exhibited submicromolar 50% inhibitory concentrations (IC50s) in the axenic Leishmania donovani amastigote assay and the L. donovani-infected macrophage assay (26) and nanomolar IC50s in Leishmania promastigote assays (22, 23). In addition, AIAs demonstrated submicromolar IC50s against Trypanosoma cruzi parasites (19, 24, 26), the causative agent for the devastating Chagas' disease (or human American trypanosomiasis, also a neglected tropical disease). Here, we describe a systematic lead discovery program for AIAs to characterize in vitro and in vivo antileishmanial activity, metabolism and pharmacokinetics, mutagenicity, and toxicity. The objective of this program was to provide the scientific foundation for future development of AIAs against leishmaniasis by determining the efficacies, pharmacokinetics, and toxicities of two promising members (Fig. 1) of this class.
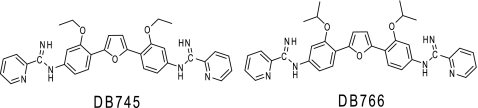
FIG. 1.
Chemical structures of two novel AIAs, DB745 and DB766.
MATERIALS AND METHODS
Chemicals.
DB745 {2,5-bis[2-ethoxy-4-(2-pyridylimino)aminophenyl]furan} and DB766 {2,5-bis[2-(2-propoxy)-4-(2-pyridylimino)aminophenyl]furan} were prepared according to a general, three-step route previously used to prepare other 2,5-diarylfuran AIA compounds (27). Chemical data for DB745, DB766, and synthetic intermediates are given in the supplemental material. Sodium stibogluconate was from IP Albert David Ltd., Kolkata, India. All other chemicals were purchased from Fisher Scientific (Pittsburgh, PA) or Sigma-Aldrich (St. Louis, MO), if not indicated otherwise.
In vitro susceptibility assays.
The activities of DB745, DB766, and control compounds against L. donovani axenic amastigotes and intracellular Leishmania amazonensis or Leishmania major β-lactamase-transfected parasites were determined as described previously (8). The activity of test compounds against intracellular L. donovani LV82 was assessed microscopically as follows. Peritoneal macrophages obtained from CD-1 mice were added to Lab-Tek chamber slides (Nunc) at a density of 5 × 104 cells/well in a volume of 100 μl in a macrophage medium (RPMI 1640 with Glutamax [Gibco] and 10% heat-inactivated fetal bovine serum [FBS], 50 units/ml penicillin, 50 μg/ml streptomycin [pH 7.4]) and were allowed to adhere overnight. Host cells were then infected overnight with L. donovani LV82 stationary-phase promastigotes at a parasite/macrophage ratio of 5:1 at 37°C in a 5% CO2 atmosphere. The following day, the used medium was replaced with fresh medium containing either test compound or vehicle. After incubation at 37°C for 72 h, slides were washed once with phosphate-buffered saline, fixed with methanol, and stained with Giemsa (977 μl distilled water, 23 μl Giemsa diluent [3.1 mM dibasic potassium phosphate, 8.3 mM monobasic sodium phosphate], 50 μl Giemsa stain) for 25 min. The percentage of infected macrophages in each chamber was determined microscopically at ×1,000 magnification. IC50s were determined by nonlinear regression analysis (four-parameter logistic curve) using SigmaPlot software. Compound activities against intracellular L. donovani clinical isolates AG83-S, MC4-R, and MC8-R were determined as described previously (11, 12). The toxicity of test compounds to mouse peritoneal macrophages was measured as follows. Starch-elicited macrophages were obtained from CD-1 mice by peritoneal lavage and were seeded in 96-well microtiter plates in RPMI 1640 with 10% FBS, 50 units/ml penicillin and 50 μg/ml streptomycin at a density of 1 × 106 cells/ml in 100 μl. The plate was incubated at 37°C under a 5% CO2 atmosphere. After 24 h, the medium was replaced with 100 μl fresh medium containing serial drug dilutions, and the plate was incubated for another 72 h. CellTiter reagent (20 μl; Promega, Madison, WI) was then added to each well, and incubation was continued for an additional 2 to 4 h. The plates were read at 490 nm on a SpectraMax Plus 384 spectrophotometer (Molecular Devices, Sunnyvale, CA), and IC50s were determined using the four-parameter dose response equation described previously (8).
Antileishmanial efficacy against L. donovani in mice.
Efficacy in the murine model of VL was measured as previously described (8), except that all compounds were dissolved in water prior to administration by the intraperitoneal (i.p.) or oral (p.o.) route.
Antileishmanial efficacy against L. donovani in hamsters.
An 11-day procedure for screening compounds against VL in golden hamsters was adapted from the 8-day method reported by Stauber et al. (25). L. donovani (WR378) metacyclic promastigotes were obtained from cultured stationary-phase promastigotes harvested from liver homogenates of infected hamsters as previously described by Melby et al. (15). Five- to 6-week-old male Syrian hamsters (Mesocricetus auratus) were obtained from Harlan (Indianapolis, IN), and each hamster was infected intracardially (i.c.) with 2 × 108 metacyclic promastigotes suspended in 200 μl of Schneider's insect medium. Compounds were administered p.o. to L. donovani-infected hamsters, using a rubber gavage tube from day 4 postinfection (p.i.) to day 8 p.i., once daily. Controls consisted of two groups of infected hamsters (n = 4 per group). One group did not receive any treatment, and the other group received p.o. miltefosine (30 mg/kg of body weight/day) that served as the reference drug. Animals were euthanized on day 11 p.i. by CO2 asphyxiation. Liver, spleen, and femur were harvested in Schneider's insect medium supplemented with antibiotics.
Part of each liver and spleen tissue sample was used to prepare multiple touch smears on glass slides. Caution was taken that the same lobe of the liver or the same part of the spleen was used to prepare the smears from each tissue sample. To harvest bone marrow from the femur, the femur was cleaned, trimming all fat and muscles around the bone. A 3-ml syringe with 500 μl of Schneider's insect medium was inserted into one end of the bone, and the marrow was flushed into a microcentrifuge tube. The cells were centrifuged at 1,500 rpm for 2 min at room temperature. Supernatant was removed, and the cell pellet was gently broken and resuspended in the remaining medium. Smears were made on slides by the use of 10 to 12 μl of cell suspension. The smears were air dried, fixed in methanol, and Giemsa (15%) stained. The parasite burden in the liver and spleen of each animal was reported in Leishman-Donovan units (LDU), defined as the organ mass (g) multiplied by the number of amastigotes per 1,000 macrophage nuclei. The final numbers were converted to percentages of inhibition, considering the numbers of vehicle-treated control tissue to be 100%.
pKa and logD7.4 determination and DNA melting temperature.
The pKa and logD7.4 values were measured by pION Inc. (Woburn, MA), using a spectrophotometric method, with methanol as a cosolvent (3). DNA melting studies (melting temperature [Tm]) were conducted with the reference model system, poly(dA-dT), that has been extensively used with diamidines (13, 26, 27).
Metabolic stability, permeability, pharmacokinetics, and tissue distribution studies.
Metabolic stability of AIAs was evaluated using liver microsomes (XenoTech LLC, Lenexa, KS) derived from four relevant species, i.e., human, rat (potential species for toxicity studies), mouse, and hamster, supplemented with NADPH. Microsomal incubations were carried out according to a protocol described previously (33) with modifications. Briefly, DB745 and DB766 were dissolved in dimethyl sulfoxide and added to incubation buffer to achieve final concentrations of 0.1 μM, 1.0 μM, and 10 μM, containing 0.5% (vol/vol) organic solvent. Reactions were allowed to proceed for up to 60 min at 37°C and were stopped with 2 volumes of 7:1 methanol-water containing 0.1% trifluoroacetic acid (vol/vol) and 30 nM internal standard. DB745 was used as internal standard for DB766, and vice versa. After centrifugation (3,000 × g), the supernatant fractions were analyzed by high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS; described below) to quantify the amount of substrate remaining.
Permeability was measured using the Madin-Darby canine kidney (MDCK) cell monolayer as previously described (31). Briefly, the compound (3 μM for DB766 or 25 μM for furamidine) was added to the donor well (pH 7.4 in Hanks' balanced salt solution supplemented with 25 mM d-glucose and 25 mM HEPES), which was separated from the receiving well by the polarized MDCK cell monolayer grown on a polycarbonate membrane support. Transport (from apical to basolateral) was carried out for 60 min at 37°C in an incubator. Compound concentrations in donor and receiver wells were measured by HPLC-MS/MS to calculate apparent permeability (Papp) (31).
Single-dose pharmacokinetics and tissue distribution of DB745 and DB766 were evaluated in mice. All animal experiments adhered to protocols approved by the Institutional Animal Care and Use Committee at the University of North Carolina at Chapel Hill. Male Swiss Webster mice (n = 3 per time point) were given DB745 or DB766 via p.o. gavage or tail vein bolus injection (intravenous [i.v.]) as described previously (28) with modifications. Briefly, for i.v. administration, DB745 or DB766 was initially dissolved in sterile water at 2× concentrations and then diluted 1:1 (vol/vol) with 2× sterile saline (1.8% [wt/vol]). This dose solution was injected via a tail vein with administration over ∼10 to 20 s. For p.o. administration, DB745 or DB766 was dissolved in sterile water to form a clear solution at 5 mM or a gelatinous liquid at 20 mM and 40 mM. The dose volume was 5 ml/kg for both i.v. and p.o. administration. Terminal blood and tissue samples (liver, spleen, kidney, heart, and brain) were collected at selected time points from 5 min up to 72 h postdose. Plasma was obtained by centrifugation and processed for quantification of AIAs by HPLC-MS/MS (described below).
Ames screening assay.
The Ames screening assay was performed by SRI International (Menlo Park, CA) as previously described (1, 17). DB745 and DB766 were examined for their ability to induce genetic damage using the plate incorporation method with Salmonella enterica serovar Typhimurium tester strains TA98 and TA100, in the presence and absence of an Aroclor 1254-induced rat liver S9 metabolic activation system (10% [vol/vol]). DB745 and DB766 were dissolved in sterile water at 10 and 5 mg/ml, respectively, and then serially diluted to achieve doses ranging from 1 to 1,000 μg/plate (100 μl) for DB745 and 1 to 500 μg/plate (100 μl) for DB766. 2-Nitrofluorene and sodium azide were used as positive controls when metabolic activation was absent. 2-Aminoanthracene was used as positive control when metabolic activation was present. Sterile water and dimethyl sulfoxide (DMSO) were used as vehicle controls. Test plates were compared with the control plates for their revertant count and for the condition of the background bacterial lawn. A test compound was considered a mutagen when the mean number of revertant colonies on the test plates exceeded the mean solvent control counts by at least 2-fold.
Toxicity evaluation in female BALB/c mice.
Single-dose escalation and 5-day repeat-dose toxicity experiments were performed by SRI International. The no-observable-adverse-effect levels (NOAELs) and maximum tolerated doses (MTDs) were determined for DB745 and DB766 in female BALB/c mice following five daily p.o. or i.v. administrations. Dose formulations were prepared fresh daily under yellow light by dissolving appropriate amounts of compound in sterile water to achieve the target concentrations. Dosing volumes were 20 and 10 ml/kg for p.o. and i.v. administrations, respectively. For the single-dose escalation experiment, mice (n = 1 for each dose level per compound) were observed for 2 days after dose administration, and the dose levels were 5, 10, and 30 mg/kg i.v. and 25, 100, and 200 mg/kg p.o. for DB745 and 5, 20, and 50 mg/kg i.v. and 50 and 100 mg/kg p.o. for DB766. Doses larger than 100 mg/kg were not tested for DB766 due to limited aqueous solubility. For the 5-day repeat-dose toxicity experiment, mice (n = 5 for each dose level per compound) were administered a test compound once daily for five consecutive days and scheduled for necropsy on day 6 (1 day after the last dose). Dose levels were selected based on results from the single-dose escalation study described above and consisted of three dose levels (low, mid, and high) for each compound per route of administration. These dose levels were as follows: 5, 10, and 20 mg/kg/day i.v. and 25, 50, and 150 mg/kg/day p.o. for DB745; 5, 20, and 30 mg/kg/day i.v. and 25, 50, and 100 mg/kg/day p.o. for DB766. Control mice (n = 5 for each route of administration) were administered vehicle (sterile water) only. Body weights were recorded prior to day 1 and prior to each necropsy. Clinical hematology and serum chemistry were measured for blood samples collected 1 day after each last dose on day 6 prior to necropsy. Histopathologic examination of tissues retained in 10% neutral buffered formalin was performed by a board-certified veterinary pathologist.
Sample preparation for analytical assays.
A portion of the liver, one kidney, and the whole spleen, heart, and brain were weighed and diluted with 2 volumes of water (assuming 1 g of wet tissue is equal to 1 ml in volume), followed by homogenization using a sonic dismembrator (model 100; Fisher Scientific, Waltham, MA). Tissue homogenates or plasma samples (25 μl) were mixed with 200 μl of 7:1 (vol/vol) methanol-water containing 0.1% trifluoroacetic acid and internal standard (1 μM for tissue homogenates or 30 nM for plasma samples), followed by centrifugation (3,000 × g) to pellet proteins. The supernatants were dried using a 96-well microplate evaporator (model EVX-96; Apricot Designs Inc., Covina, CA) under N2 at 50°C and reconstituted with 100 μl 15% methanol containing 0.1% trifluoroacetic acid before HPLC/UV analysis for tissue homogenates or HPLC-MS/MS analysis for plasma samples (described below).
HPLC/UV analyses of DB745 and DB766 were performed using a previously described method (33) with modifications. All samples were monitored at 368 nm for the purpose of quantification with a flow rate of 0.5 ml/min. HPLC mobile phases consisted of 35 mM formic acid and 15 mM ammonium formate in HPLC-grade water (A) and 35 mM formic acid and 15 ammonium formate in 80:20 (vol/vol) acetonitrile-water (B). Mobile-phase composition began with 15% B and was increased to 35% B over 0.5 min and further increased to 55% B over the next 4.5 min. The mobile phase was then maintained at 95% B for 1 min to wash the column, followed by reequilibrating with 15% B for 4 min before injection of the next sample. Separation was carried out at 25°C. Typically, 20 μl of sample was injected. DB745 and DB766 eluted at 3.1 min and 3.5 min, respectively, with baseline separation. The calibration curve for DB766 neat standards ranged from 0.1 to 100 μM with a detection limit of 0.05 μM.
HPLC-MS/MS quantification of DB745 and DB766 was performed on an Applied Biosystems (Foster City, CA) API 3000 or 4000 triple quadrupole mass spectrometer equipped with a Turbo IonSpray interface (for electrospray ionization) (MDS Sciex, San Francisco, CA). Typically, 4 to 5 μl of sample was injected. DB745 and DB766 were eluted from an Aquasil C18 analytical column (2.1 by 50 mm, 5 μm; Thermo Electron, Waltham, MA) and analyzed in positive ion mode. HPLC mobile phases consisted of HPLC-grade water containing 0.3% formic acid (C) and methanol containing 0.3% formic acid (D). After a 0.4-min initial hold at 15% D, mobile-phase composition began with 15% D and was increased to 80% D over 1.6 min, followed by a 1.0-min hold, at a flow rate of 0.35 ml/min. The column was then washed with 95% D for 1.3 min at a flow rate of 0.5 ml/min and was reequilibrated with 15% D at a flow rate of 0.35 ml/min for 0.5 min before injection of the next sample. The characteristic selected reaction monitoring (SRM) transitions for DB745 and DB766 were m/z 547.4 → 426.1 and 575.3 → 454.1, respectively. The calibration curve for DB745 and DB766 ranged from 2.5 to 10,000 nM, using a quadratic equation with 1/x weighting.
Data analysis.
Statistical analysis of in vivo antileishmanial activity was performed by comparing the average liver parasitemia of treatment groups with that of the untreated control group using Student's unpaired t test (SigmaPlot 10.0); a P value of ≤0.05 was considered significant. Statistical analysis of serum clinical chemistry values was performed by the one-way analysis of variance (ANOVA), followed by the Dunnett's posttest if the ANOVA analysis was significant; a P value of ≤0.05 was considered significant. Microsomal half-life (t1/2) values were obtained by fitting the percentage of substrate remaining versus time curves to the 1-phase exponential decay equation (C = C0 × e−kt; t1/2 = 0.693/k). Noncompartmental pharmacokinetic analysis of plasma concentration versus time curves was performed to obtain the area under the concentration-time curve from 0 h to infinity (AUC0-∞), percentage of AUC that was extrapolated (AUC_%Extrap), maximum plasma concentration (Cmax), time to reach Cmax (Tmax), terminal elimination half-life (t1/2), clearance (CL), and steady-state volume of distribution (Vss). Oral bioavailability (F) was calculated using the equation:
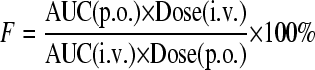 |
RESULTS
In vitro antileishmanial activities.
In vitro antileishmanial activities of two novel AIAs, DB745 and DB766, were evaluated using the axenic L. donovani amastigote assay and the Leishmania-infected macrophage assays (Table 1). Both AIAs exhibited submicromolar IC50s in all assays tested. Notably, DB766 had IC50s less than 0.1 μM in the intracellular Leishmania assays, substantially more potent than miltefosine, paromomycin, and pentamidine and similar in potency to amphotericin B. Both AIAs were selective against intracellular Leishmania parasites compared to murine peritoneal macrophages, with selectivity indexes of 250 and 78 (based on the L. donovani intracellular assay) for DB745 and DB766, respectively.
TABLE 1.
In vitro antileishmanial activities of AIAs and their physicochemical properties
| Compound | IC50 (μM)a vs: |
pKa | Log P | LogD7.4 | ΔTm(AT)e | ||||
|---|---|---|---|---|---|---|---|---|---|
| L. donovani axenic amastigotes | L. donovani LV82 intracellular amastigotes | L. amazonensis intracellular amastigotes | L. major intracellular amastigotes | Murine peritoneal macrophages | |||||
| DB745 | 0.50 ± 0.15 | 0.018 ± 0.007 | 0.12 ± 0.02 | 0.026 ± 0.011 | 4.5 ± 1.4 | 7.12 | 3.82 | 3.63 | 7.1 |
| DB766 | 0.50 ± 0.10 | 0.036 ± 0.005 | 0.087 ± 0.015 | 0.014 ± 0.004 | 2.8 ± 0.8 | 7.36 | 4.41 | 4.12 | 6.0 |
| Amphotericin B | 0.098 ± 0.013 | 0.066 ± 0.012 | 0.14 ± 0.01 | 0.21 ± 0.05 | 8.2 ± 1.1 | ND | ND | ND | ND |
| Miltefosine | 8.5 ± 1.2 | 2.7 ± 0.3 | 15 ± 3 | 25 ± 3 | 24 ± 15b | ND | ND | ND | ND |
| Paromomycin | >50 | >50 | 19 ± 3 | 25 ± 2 | >100 | ND | ND | ND | ND |
| Pentamidine | 1.8 ± 0.4 | >50d | 0.83 ± 0.17 | NDc | 14 ± 5 | 11.6 | 1.7 | <−2.0 | 12.8f |
Mean ± standard error of at least three independent determinations unless noted otherwise.
Mean ± range of two independent determinations.
ND, not determined.
Pentamidine is known to reduce the number of parasites per macrophage in in vitro experiments but not the number of infected macrophages as assessed here.
Increase in DNA thermal melting of poly(dA-dT).
See work by Stephens et al. (27).
Activity against antimony-resistant Leishmania isolates.
The potential of AIAs against drug-resistant Leishmania parasites was assessed using J774 macrophages infected with clinical isolates of antimony-resistant L. donovani (Table 2). DB745 and DB766 were active against these antimony-resistant Leishmania parasites, with IC50s ranging from 0.064 to 0.16 μM, similar to the IC50s against wild-type intracellular Leishmania parasites shown in Table 1.
TABLE 2.
In vitro antileishmanial activities of AIAs against intracellular L. donovani clinical isolates
| Compoundb | IC50 (μM)a vs L. donovani: |
||
|---|---|---|---|
| AG83-S | MC4-R | MC8-R | |
| DB745 | 0.13 ± 0.01 | 0.15 ± 0.02 | 0.16 ± 0.03 |
| DB766 | 0.090 ± 0.013 | 0.076 ± 0.005 | 0.064 ± 0.010 |
| Sb(V) | 9.0 ± 0.5 | 44 ± 3 | 40 ± 5 |
| Sb(III) | 6.2 ± 1.8 | 32 ± 5 | 26 ± 5 |
Mean ± standard deviation of at least three independent determinations.
Sb(V), sodium stibogluconate; Sb(III), sodium antimony tartrate.
In vivo efficacy in the murine VL model.
Prior to an in vivo efficacy study, the acute overt toxicity of DB745 and DB766 was initially evaluated in uninfected female BALB/c mice via repeat i.p. or p.o. administration (once daily for 5 days). When DB766 was given p.o. at doses of 25 and 50 mg/kg/day for 5 days, mice showed no signs of any overt adverse effect. However, when given i.p. at a dose of 50 mg/kg/day, DB766 caused some mild adverse effects (e.g., ruffled fur and hypoactivity), although all mice survived the 5 days of treatment. In contrast, when given i.p. at a dose of 50 mg/kg/day, DB745 caused moderate adverse effects, with tremors, ruffled fur, and a tendency to startle, but all mice survived the 5 days of treatment. At a dose of 100 mg/kg/day i.p., DB745 was lethal to mice after two doses. These mice displayed severe hypoactivity, ataxia, and tremors and had to be euthanized. Accordingly, appropriate dose regimens (shown in Fig. 2) were selected for further efficacy studies of mice. The highest DB766 (as a hydrochloride salt) dose given was 100 mg/kg/day due to its limited aqueous solubility.
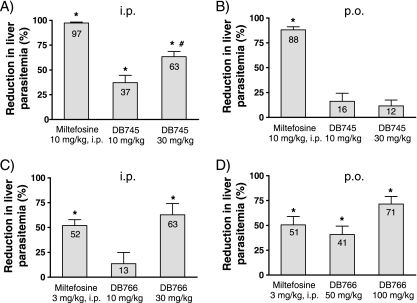
FIG. 2.
In vivo efficacy levels of DB745 and DB766 in the murine VL model. Compounds were given i.p. (A and C) or p.o. (B and D) once daily for 5 days to mice infected with L. donovani promastigotes. Results are presented as the percentage of reduction in liver parasitemia (LDU) versus untreated controls. Miltefosine given i.p. served as a positive control in all groups. Bars and error bars denote the means and standard deviations, respectively, of experimental groups containing four animals. *, P < 0.05, compared to untreated control; #, mice in this group received only four doses due to toxicity.
The reductions in liver parasitemia of mice treated with DB745 or DB766 compared to that of untreated controls are shown in Fig. 2. When given i.p., both DB745 and DB766 showed dose-dependent inhibition of liver parasitemia. A maximum reduction of 63% was observed for each compound administered at a dose of 30 mg/kg/day (DB766 was given for 5 days, but DB745 could be given for only 4 days due to adverse events in the mice). Furthermore, DB766 exhibited a promising dose-dependent p.o. activity in this murine VL model, with 71% inhibition of liver parasitemia at a dose of 100 mg/kg/day for 5 days. No signs of overt toxicity were observed for any of the mice treated with DB766.
In vivo efficacy in the hamster VL model.
Since the hamster VL model is considered to be clinicopathologically and immunopathologically more similar to human VL than to the murine model (14), DB766 was further evaluated in our 11-day hamster VL efficacy model. DB745 was not evaluated in this model primarily due to its toxicity being greater than that of DB766. The reductions in the parasitemia of three key tissues (i.e., liver, spleen, and bone marrow) in hamsters treated with DB766 are shown in Table 3. When given p.o. at a dose of 100 mg/kg/day for 5 days, DB766 reduced parasitemia (LDU) in liver, spleen, and bone marrow by 89%, 79%, and 92%, respectively. Moreover, DB766 inhibited the bone marrow parasitemia in a dose-dependent manner (24%, 70%, and 92% inhibition in LDU at doses of 30, 50, and 100 mg/kg/day for 5 days, respectively). However, using the same dose regimen, DB766 exhibited a relatively flat dose response in liver and spleen, ranging from 75% to 89% and 67% to 79% inhibition, respectively. Miltefosine, given p.o. at a dose of 30 mg/kg/day for 5 days as a positive control, afforded 88%, 79%, and 91% inhibition of parasitemia (LDU) in liver, spleen, and bone marrow, respectively.
TABLE 3.
DB766 in vivo efficacy in the hamster VL model
| Drug | Dose (mg/kg/day for 5 days) | % Inhibitiona in: |
|||||
|---|---|---|---|---|---|---|---|
| Liver |
Spleen |
Bone marrow |
|||||
| Mean | SD | Mean | SD | Mean | SD | ||
| DB766, p.o. | 10 | 10 | 10 | NDb | ND | ND | ND |
| 30 | 75 | 17 | 67 | 24 | 24 | 50 | |
| 50 | 76 | 21 | 74 | 29 | 70 | 22 | |
| 100 | 89 | 6 | 79 | 8 | 92 | 6 | |
| Miltefosine, p.o. | 30 | 88 | 8 | 79 | 6 | 91 | 9 |
All percent inhibition data are presented as the means and standard deviations of four replicate determinations in LDU.
ND, not determined.
Physicochemical properties and permeability.
The pKa and log P values for DB745 and DB766 were experimentally determined using a UV spectrophotometric method (Table 1). Unlike typical dicationic diamidines (e.g., pentamidine and furamidine), which often have pKa values above 10 (13, 20), DB745 and DB766 had near neutral pKa values (7.12 and 7.36, respectively). Furthermore, both AIAs were moderately lipophilic at physiologic pH with logD7.4 values of 3.63 and 4.12, respectively. DB766 was ∼20-fold more permeable across the MDCK cell monolayer than furamidine (65 versus 3.3 nm/s for Papp). DB745 and DB766 gave relatively small increases (6 to 7°C) in the DNA melting temperature (ΔTm), compared to the diamidines, furamidine (25°C) (26) and pentamidine (12.8°C) (Table 1).
In vitro metabolism in liver microsomes.
Metabolism of DB745 and DB766 was initially evaluated using pooled liver microsomes supplemented with NADPH, and microsomal t1/2 values were measured by monitoring substrate disappearance over time at three substrate concentrations (Table 4). In pooled mouse liver microsomes, DB745 and DB766 microsomal t1/2 values appeared to be concentration dependent (longer half-lives at higher substrate concentrations), indicating a saturable metabolism observed in the concentration range tested (0.1 to 10 μM). Similar results were observed for both compounds by using rat and hamster liver microsomes. In contrast, marginal substrate depletion was detected for DB745 and DB766 by using human liver microsomes with t1/2 values greater than 120 min at all concentrations tested.
TABLE 4.
Liver microsomal half-lives (t1/2) for AIAs
| Compound | Substrate concn (μM) | Liver microsomal t1/2 (min)a |
|||
|---|---|---|---|---|---|
| Human | Rat | Mouse | Hamster | ||
| DB745 | 0.1 | >120 | 80 | 15 | 75 |
| 1 | >120 | >120 | 47 | >120 | |
| 10 | >120 | >120 | >120 | >120 | |
| DB766 | 0.1 | >120 | 96 | 26 | 39 |
| 1 | >120 | >120 | >120 | >120 | |
| 10 | >120 | >120 | >120 | >120 | |
Pharmacokinetics in mice.
Single-dose i.v. and p.o. pharmacokinetics of DB745 and DB766 were evaluated in mice to examine the potential of these compounds as p.o. active drug candidates. After a single p.o. dose of 25, 100, and 200 μmol/kg in mice (or 16.5, 66, and 131 mg/kg), DB745 maximum plasma concentrations were 0.02, 0.40, and 1.68 μM, respectively, with terminal elimination t1/2 values ranging from 24 to 32 h (Table 5). DB745 p.o. bioavailability was dose dependent, improving from 3.3% to 25% as the dose increased from 25 to 200 μmol/kg. After a single p.o. dose of 100 μmol/kg (or 70 mg/kg) in mice, DB766 Cmax and AUC0-∞ were 4.4- and 2.4-fold higher than those of DB745, respectively (Table 6). Furthermore, the p.o. bioavailability of DB766 remained approximately the same as that of DB745 (10.4% versus 9.3%). In addition, a large volume of distribution was observed for both compounds, indicating extensive tissue binding.
TABLE 5.
Pharmacokinetic measurements of DB745 after single i.v. or p.o. administration in mice
| Parametera | Pharmacokinetic measurement of DB745 after indicated dose (μmol/kg) administration |
||||
|---|---|---|---|---|---|
| i.v. |
p.o. |
||||
| 1.5 (1.0 mg salt/kg) | 7.5 (4.9 mg salt/kg) | 25 (16.5 mg salt/kg) | 100 (66 mg salt/kg) | 200 (131 mg salt/kg) | |
| AUC0-∞ (h · μM) | 0.56 | 4.5 | 0.5 | 5.6 | 30.0 |
| AUC_%Extrap (%) | 9 | 3 | 42 | 19 | 13 |
| Cmax (μM) | 0.37 | 4.24 | 0.02 | 0.40 | 1.68 |
| Tmax (h) | 0.0833 | 0.0833 | 2 | 2 | 2 |
| t1/2 (h) | 17 | 18 | 24 | 30 | 32 |
| CL (ml/min/kg)b | 45 | 28 | 28 | 28 | 28 |
| Vss (liter/kg)c | 36 | 22 | 57 | 71 | 76 |
| F (%)d | 3.3 | 9.3 | 25 | ||
Noncompartmental analysis by WinNonlin 5.0.1, 1/y weighting, linear up/log down.
Observed total body clearance; for p.o. administration, corrected with F.
Predicted steady-state volume of distribution for i.v. administration; predicted volume of distribution based on terminal phase.
Calculated based on the AUC at 7.5 μmol/kg i.v.
TABLE 6.
Pharmacokinetic measurements of DB766 after single i.v. or p.o. administration in mice
| Parametera | Pharmacokinetic measurement of DB766 after indicated dose (μmol/kg) administration |
||
|---|---|---|---|
| 1.5 (1.0 mg salt/kg) i.v. | 7.5 (5.0 mg salt/kg) i.v. | 100 (70 mg salt/kg) p.o. | |
| AUC0-∞ (h · μM) | 2.53 | 9.53 | 13.2 |
| AUC_%Extrap (%) | 8 | 14 | 13 |
| Cmax (μM) | 1.09 | 10.41 | 1.76 |
| Tmax (h) | 0.0833 | 0.0833 | 1 |
| t1/2 (h) | 22 | 24 | 48 |
| CL (ml/min/kg)b | 9.9 | 13.1 | 13.2 |
| Vss (liter/kg)c | 14 | 15 | 54 |
| F (%)d | 10.4 | ||
Noncompartmental analysis by WinNonlin 5.0.1, 1/y weighting, linear up/log down.
Observed total body clearance; for p.o. administration, corrected with F.
Predicted steady-state volume of distribution for i.v. administration; predicted volume of distribution based on terminal phase.
Calculated based on the AUC at 7.5 μmol/kg i.v.
Tissue distribution in mice.
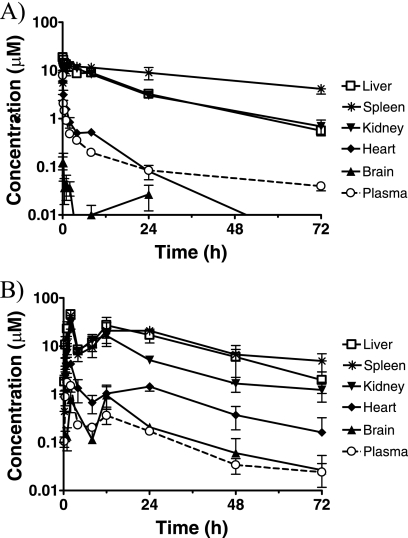
Tissue concentrations of DB766 after i.v. and p.o. administration in mice were determined using tissue homogenates from liver, spleen, kidney, heart, and brain (Fig. 3). After a single i.v. dose of 7.5 μmol/kg (or 5 mg/kg), DB766 maximum tissue concentrations were 10.7, 7.8, and 7.3 μg/g (or 18.7, 13.5, and 12.7 μM) in liver, spleen, and kidney, respectively, observed between 5 min and 2 h post-dose. DB766 brain concentrations were much lower than the corresponding plasma concentrations, indicating limited brain uptake. DB766 concentrations in the heart were higher than the corresponding plasma concentrations, albeit to a much lower extent compared to liver, spleen, and kidney. After a single p.o. dose of 100 μmol/kg (or 70 mg/kg), DB766 maximum tissue concentrations were 26.3, 24.8, and 17.4 μg/g (or 45.8, 43.1, and 30.3 μM) in liver, spleen, and kidney, respectively, all observed at 2 h postdose. Similar to i.v. administration, DB766 brain concentrations were lower than or close to the corresponding plasma concentrations, whereas DB766 heart concentrations were higher, albeit to a much lower extent than were those of the liver, spleen, and kidney. Furthermore, DB766 concentrations in tissues that had the highest levels of the compound (liver and spleen) paralleled plasma concentrations, indicating tissue t1/2 values similar to those of plasma.
FIG. 3.
DB766 tissue concentrations in mice after a single i.v. (A) or p.o. (B) administration of DB766. Plasma concentrations were plotted for the purpose of comparison. Tissue concentrations were calculated assuming that 1 g of wet tissue equals 1 ml in volume. Symbols and error bars denote the means and standard errors, respectively, of triplicate determinations.
Mutagenicity evaluation using the Ames screen.
Potential mutagenicity of DB745 and DB766 was assessed in a single experiment with two different Salmonella serovar Typhimurium tester strains (TA98 and TA100) with and without metabolic activation by using induced rat liver S9. DB745 was cytotoxic to TA98 at doses of ≥100 μg/plate and to TA100 at doses of ≥50 μg/plate in the absence of metabolic activation and to TA98 at doses of ≥500 μg/plate and to TA100 at doses of ≥100 μg/plate in the presence of metabolic activation. DB766 was cytotoxic only to TA100 at doses of ≥100 μg/plate in the absence of metabolic activation and at doses of ≥500 μg/plate in the presence of metabolic activation. No significant increase in revertant colonies was seen with or without metabolic activation for either DB745 (data not shown) or DB766 (Table 7), suggesting that these AIAs are not mutagenic.
TABLE 7.
DB766 mutagenicity evaluation using the Ames screen
| Strain | Compound | Dose/plate (μg) | Mean no. of revertant colonies/platea: |
|
|---|---|---|---|---|
| With metabolic activation | Without metabolic activation | |||
| TA98 | DB766 | 1 | 43 ± 4.2 | 38 ± 2.1 |
| 5 | 48 ± 0.0 | 29 ± 0.0 | ||
| 10 | 38 ± 9.2 | 29 ± 2.8 | ||
| 50 | 39 ± 7.1 | 28 ± 8.5 | ||
| 100 | 38 ± 0.0 | 34 ± 6.4 | ||
| 500 | 47 ± 1.4 | 28 ± 8.5 | ||
| Sterile water | 30 ± 4.2 | 36 ± 2.1 | ||
| DMSO | 42 ± 0.0 | 32 ± 3.5 | ||
| 2-Aminoanthracene | 2 | 2,744 ± 120 | ||
| 2-Nitrofluorene | 5 | 1,573 ± 140 | ||
| TA100 | DB766 | 1 | 159 ± 8 | 152 ± 1 |
| 5 | 154 ± 9 | 145 ± 0 | ||
| 10 | 148 ± 1 | 148 ± 3 | ||
| 50 | 135 ± 21 | 79 ± 27 | ||
| 100 | 98 ± 16 | Toxic | ||
| 500 | Toxic | Toxic | ||
| Sterile water | 153 ± 1 | 148 ± 13 | ||
| DMSO | 171 ± 18 | 130 ± 13 | ||
| 2-Aminoanthracene | 2 | 2,563 ± 59 | ||
| Sodium azide | 5 | 1,882 ± 110 | ||
Mean ± standard deviation of duplicate determinations. Toxic, test compound is cytotoxic to the Salmonella test strain.
Repeat-dose toxicity study of mice.
A single-dose escalation experiment was initially performed using adult female BALB/c mice to determine appropriate dose levels for the 5-day repeat-dose toxicity study. During the escalation experiment, all animals in the i.v. and p.o. treatment groups survived to their scheduled necropsy. The only test article-related clinical observation was tremors, which affected one animal in the 30 mg/kg DB745 i.v. group and one animal in the 50 mg/kg DB766 i.v. group. Accordingly, appropriate dose levels were chosen for the 5-day repeat-dose toxicity study and summarized in Materials and Methods.
Mortality/morbidity and clinical observations.
In the 5-day repeat-dose toxicity study, all mice in the i.v. groups and the p.o. DB766 groups survived until their scheduled necropsy (i.e., 1 day after each last dose). All mice in the p.o. DB766 groups (25, 50, and 100 mg/kg/day) appeared to be normal without significant clinical observations. However, mice that received a high p.o. dose of DB745 at 150 mg/kg/day either died or were euthanized in moribund condition on day 5. The most prominent adverse effect was tremors that occurred in all animals in the mid and high (10 and 20 mg/kg/day, respectively) i.v. dose DB745 groups and the high (30 mg/kg/day) i.v. dose DB766 group. These tremors generally started on day 2 or 3 and continued through day 6. For the high-p.o.-dose DB745 group, all mice were observed to have mild to severe ruffled fur and tremors on day 4, and two mice became hyperactive on day 5. These mice were either found dead (one animal) or euthanized in moribund condition (four animals) on day 5. For surviving animals, no changes in body weight or organ weight could be attributed to the treatment with either DB745 or DB766. For moribund animals (mice treated with the high p.o. dose of DB745 at 150 mg/kg/day), body weights and liver weights were decreased by 24% and 30%, respectively, compared to the final body weights and liver weights of the control animals treated with the vehicle.
Clinical pathology evaluations.
Since DB766 appeared to accumulate in the mouse liver and kidney (Fig. 3), special attention was paid to clinical chemistries that could indicate liver or kidney toxicity (Table 8). No treatment-related increase in liver enzymes or blood urea nitrogen was detected in any groups except the moribund animals in the high-p.o.-dose DB745 group. For the p.o. treatment groups, statistically significant and dose-related increases in phosphorus levels (up to 74% compared to the control animals) were observed and the largest increase was associated with the moribund animals in the high-p.o.-dose DB745 group, whereas slight increases in phosphorus levels were noted in the i.v. treatment groups. Other statistically significant changes in clinical chemistries of surviving animals were noted, but are considered to be too small to be toxicologically relevant. In addition, no treatment-related changes in hematology were found in either the i.v. or the p.o. treatment groups (data not shown).
TABLE 8.
Serum clinical chemistry evaluations for the 5-day repeat-dose toxicity study in mice
| Compound | Dose (mg/kg/day) | Mean (SD)a |
||||
|---|---|---|---|---|---|---|
| AST (U/liter) | ALT (U/liter) | BUN (mg/dl) | ALP (U/liter) | PHO (mg/dl) | ||
| Vehicle (i.v.) | 0 | 65 (14) | 34 (10) | 20 (2) | 134 (10) | 6.1 (1.2) |
| DB745 (i.v.) | 5 | 80 (49) | 43 (15) | 18 (2) | 113 (14) | 7.1 (0.7) |
| 10 | 80 (12) | 40 (16) | 24 (6) | 118 (16) | 8.1 (0.9) | |
| 20 | 89 (24) | 57 (30) | 27 (3) | 107 (7) | 7.3 (1.1) | |
| DB766 (i.v.) | 5 | 76 (30) | 36 (9) | 19 (1) | 103 (12) | 7 (1) |
| 20 | 76 (18) | 50 (23) | 26 (3) | 104 (26) | 6.5 (0.4) | |
| 30 | 73 (27) | 35 (8) | 24 (4) | 118 (7) | 7.6 (0.6) | |
| Vehicle (p.o.) | 0 | 70 (16) | 36 (8) | 29 (5) | 129 (23) | 5.3 (0.3) |
| DB745 (p.o.) | 25 | 67 (10) | 31 (6) | 29 (6) | 136 (19) | 6.9 (0.4)c |
| 50 | 77 (14) | 34 (8) | 27 (4) | 130 (25) | 7.2 (0.8)d | |
| 150b | 770 (370) | 220 (100) | 52 (13) | 120 (7) | 9.2 (1.8) | |
| DB766 (p.o.) | 25 | 75 (26) | 44 (24) | 27 (2) | 126 (25) | 7.0 (1.2)c |
| 50 | 90 (16) | 45 (19) | 25 (4) | 123 (11) | 8.3 (1.0)d | |
| 100 | 68 (9) | 34 (11) | 29 (2) | 142 (18) | 7.5 (1.0)d | |
Mean (standard deviation [SD]) of five animals. AST, aspartate aminotransferase; ALT, alanine aminotransferase; BUN, blood urea nitrogen; ALP, alkaline phosphatase; PHO, phosphorus.
One animal in this group died before scheduled necropsy, and the other four were in moribund condition on day 5. Values in this group represent means and SD of four moribund animals and were not included in statistical significance analysis.
P ≤ 0.05.
P ≤ 0.01.
Gross necropsy and histopathology.
The only treatment-related gross necropsy finding was discolored pale liver, which was observed in moribund animals in the high-p.o.-dose DB745 group. Other findings, including discolored or roughened heart and mottled lung, were noted in most groups. However, these findings are considered to be attributable to spontaneous changes in BALB/c mice as indicated by microscopic examination of the heart tissue and results of sodium pentobarbital euthanasia, respectively. No organ weight changes could be attributed to treatment with either DB745 or DB766, except for moribund animals in the high-p.o.-dose DB745 group, where a reduction in liver weight (30%) compared to that of the control group was present. The key histopathology findings are minimal to mild hepatic cell eosinophilia, hypertrophy, and fatty changes that were directly related to the high i.v. and high p.o. doses of DB745 and DB766. One moribund animal in the high-p.o.-dose DB745 group showed a moderate fatty change in the liver. In contrast, liver necrosis, gastric inflammation, edema, and hemorrhage, as well as splenic lymphoid depletion were found only in moribund animals from the high-p.o.-dose DB745 group at 150 mg/kg/day and are considered to have been secondary findings related to stress.
Taken together, based on the morbidity, clinical observations, clinical chemistry, and histopathology, the NOAELs and MTDs for female BALB/c mice treated p.o. or i.v. once a day for five consecutive days are summarized in Table 9.
TABLE 9.
NOAELs and MTDs of AIAs in female BALB/c mice treated p.o. or i.v. once a day for five consecutive days
| Compound | NOAEL (mg/kg/day) |
MTD (mg/kg/day) |
||
|---|---|---|---|---|
| p.o. | i.v. | p.o. | i.v. | |
| DB745 | 50 | 5 | Between 50 and 150 | 10 |
| DB766a | 50 | 20 | 100 | 30 |
Doses higher than 100 mg/kg/day were not tested due to limited aqueous solubility.
DISCUSSION
In this work, we have evaluated two novel AIAs regarding their in vitro and in vivo antileishmanial activity, physicochemical and pharmacokinetic properties, mutagenicity, and toxicity. Our data demonstrate that AIAs are promising preclinical development candidates for the p.o. treatment of VL. First, AIAs are potent leishmanicidals with submicromolar IC50s against intracellular amastigotes. In particular, DB766 is among the most potent, with an IC50 of <0.1 μM (Table 1), which is similar to that of amphotericin B and substantially more active than miltefosine and paromomycin. Moreover, AIAs are equally active against wild-type and antimony-resistant Leishmania parasites, suggesting a potential role as a rescue treatment for antimony treatment failures or as a partner in combination therapy with currently used antileishmanials. Second, AIAs are active in both mouse and hamster VL models, markedly reducing liver parasitemia in these systems. In addition, DB766 caused a substantial decrease in spleen and bone marrow parasitemia in the hamster VL model, which was considered to be clinicopathologically and immunopathologically more similar to human VL than the murine model (14). Third, AIAs have desirable physicochemical and pharmacokinetic properties, including moderate lipophilicity, p.o. bioavailability, and metabolic stability, as well as preferred tissue distribution to target organs (liver and spleen). Considering that species-dependent rates of metabolism were observed (Table 4), a slower metabolism in humans may benefit efficacy and dosing frequency, although a potential increase in toxicity needs to be considered for dose selection. Fourth, DB745 and DB766 are not mutagenic in the Ames screening assay using two Salmonella serovar Typhimurium tester strains (Table 7). Finally, the 5-day repeat-dose toxicity study of mice showed that DB766 was better tolerated than was DB745, as indicated by the NOAELs and MTDs following i.v. administration (direct comparison following p.o. administration was not possible due to insufficient data). Furthermore, no treatment-related changes in hematology, liver function tests, or kidney function tests (Table 8) were found in DB766-treated mice. It should be noted that higher doses of DB766 (i.e., >100 mg/kg) were not examined for efficacy or toxicity due to the limited aqueous solubility of this hydrochloride salt. Studies are in progress evaluating efficacy and toxicity of the mesylate salt of DB766, as this mesylate salt afforded markedly improved aqueous solubility.
Although AIAs are structural analogues of cationic diamidines, e.g., pentamidine and furamidine, they differ significantly in physicochemical properties and bioavailability. AIAs are much more lipophilic than diamidines at neutral pHs based on logD7.4 values (4.12 and <−2.0 for DB766 and furamidine [13], respectively). In contrast to the extremely low p.o. bioavailability exhibited by cationic diamidines (e.g., ∼0.24% for furamidine in mice) (our unpublished data), AIAs have moderate p.o. bioavailability (up to 25% in mice). Furthermore, AIAs are generally more active against intracellular parasites, Leishmania and T. cruzi, and less active against Trypanosoma brucei subsp. brucei than their diamidine counterparts (34). This difference in the antiparasitic activity may indicate distinctions in mechanism of action and/or cellular uptake between AIAs and diamidines.
It is still unclear how AIAs exert their antileishmanial effect. Recent studies demonstrated that AIAs caused profound ultrastructural alterations in the nuclei and mitochondria, as well as in the microtubule organization of T. cruzi (24). Similarly, pentamidine induced prominent mitochondrial distention and disrupted the mitochondrial membrane potential in Leishmania parasites (7, 32) and in rat liver mitochondria (16). Pentamidine was also shown to inhibit the plasma membrane Ca2+-ATPase of T. brucei (5). Studies into pentamidine resistance in Leishmania mexicana also suggested a mitochondrial target in the mode of action for cationic diamidines (4). Considering that DB745 and DB766 bind to synthetic poly(dA-dT) DNA, albeit with much weaker affinity than to pentamidine (Table 1), the mitochondrion may still play a role in the mode of action for AIAs in Leishmania parasites. However, recent studies with new AIAs showed that these compounds retained submicromolar antileishmanial activity even when DNA binding is lacking [ΔTm(AT) ≤ 2°C, where ΔTm(AT) is the increase in DNA thermal melting of poly(dA-dT)] (our unpublished data). Thus, processes that do not require DNA binding could also play a role in parasite killing. Further study into potential mechanisms of action of AIAs in Leishmania is in progress.
Compared to cationic diamidines, AIAs displayed appreciably higher p.o. bioavailability, exhibiting promising p.o. activity in both mouse and hamster VL models. The enhanced p.o. bioavailability of AIAs is likely due to a combination of an improved gastrointestinal absorption as a result of increased lipophilicity at physiologic pHs (Table 1) and potentially saturated metabolism at higher doses (Table 4). The observed saturable metabolism of DB745 in mouse liver microsomes could partially contribute to its dose-dependent p.o. bioavailability in mice (Table 5). In addition, increased lipophilicity and near neutral pKa values provide a possible explanation for the potent activity of AIAs against intracellular Leishmania parasites, because lipophilic AIAs should traverse the host macrophage plasma membrane and phagolysosomal membrane via passive diffusion at physiologic pHs before reaching parasites. Once in the phagolysosomes or PV, where the pH value is ∼5 (2), AIAs could form cations before being taken up by the parasite via an unknown process in order to exert their antileishmanial activities. It would be interesting to confirm the above hypothesis and identify the uptake process, as it may have implications in determining potential drug resistance.
AIAs accumulated in the mouse liver and spleen at the highest concentrations among all tissues examined, a potentially positive attribute because the liver and spleen are target organs of chemotherapeutic agents for VL and Leishmania parasites predominantly reside in these organs, in addition to the bone marrow (6). Furthermore, appreciable concentrations of AIAs were detected in the kidney and heart (concentrations in the bone marrow were not determined in this study), whereas markedly lower concentrations were detected in the brain, suggesting limited central nervous system (CNS) penetration by AIAs. The extensive tissue distribution and accumulation described above are consistent with the large volume of distribution obtained from the pharmacokinetic analysis of the plasma concentration-time profiles (Tables 5 and 6) and have raised toxicity concerns, particularly for the spleen, liver, and kidney. Accordingly, biomarkers for liver or kidney toxicity and histopathology on spleen, liver, and kidney were specifically included in the 5-day repeat-dose toxicity study of mice. Results showed that DB766 did not cause significant increase in liver enzymes or blood urea nitrogen (Table 8), despite relatively high liver and kidney concentrations even after a single dose (Fig. 3). DB745 and DB766 caused minimal to mild hepatic cell eosinophilia, hypertrophy, and fatty changes at the high i.v. and p.o. doses tested, which were the primary determinants for their NOAEL and MTD levels (Table 9). Moreover, increases in phosphorus levels following i.v. and p.o. treatment with DB745 and DB766 are not likely due to kidney or bone marrow damage because microscopic examination of these two tissues did not reveal any treatment-related damages. Another possible cause of increased phosphorus levels is calcium deposition (mineralization) in tissues. Since cardiac mineralization is a commonly reported spontaneous change in BALB/c mice and was also present in this study of both i.v. and p.o. treatment groups, the changes in phosphorus levels may, in part, have been secondary to the process of cardiac mineralization in these mice.
The high tissue concentrations (relative to in vitro IC50s) of AIAs may explain the observed promising efficacy in mice (Fig. 3). However, it was anticipated that the levels of compound in the liver and spleen should have resulted in near complete reduction of parasitemia. Studies are in progress to understand how AIAs are distributed within the target organs (liver, spleen, and bone marrow) and subcellularly, as it may provide an explanation for the above-described disconnect between tissue concentrations and efficacy. Moreover, additional pharmacokinetic and efficacy studies are ongoing in the hamster VL model to investigate pharmacokinetic-pharmacodynamic relationships and the potential impact of Leishmania infection on pharmacokinetics.
In conclusion, studies presented here confirmed that AIAs possess potent antileishmanial activities in vitro and good activities in animal models of VL. Our data demonstrate that DB766 is a promising preclinical development candidate for the p.o. treatment of VL, and other AIA analogues should be further explored to obtain improved efficacy and safety profiles.
Supplementary Material
Acknowledgments
This study was supported by a grant from the Bill and Melinda Gates Foundation and by contract N01-AI-60011 with SRI International from the National Institute of Allergy and Infectious Diseases.
We are grateful to Stephen Wring (Scynexis Inc., NC) and Brian Vesely (College of Public Health, University of South Florida, FL) for providing help on the MDCK transport study and technical assistance, respectively.
Footnotes
Published ahead of print on 5 April 2010.
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1.Ames, B. N., J. McCann, and E. Yamasaki. 1975. Methods for detecting carcinogens and mutagens with the Salmonella/mammalian-microsome mutagenicity test. Mutat. Res. 31:347-364. [DOI] [PubMed] [Google Scholar]
- 2.Antoine, J. C., E. Prina, C. Jouanne, and P. Bongrand. 1990. Parasitophorous vacuoles of Leishmania amazonensis-infected macrophages maintain an acidic pH. Infect. Immun. 58:779-787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avdeef, A. 2001. Physicochemical profiling (solubility, permeability and charge state). Curr. Top. Med. Chem. 1:277-351. [DOI] [PubMed] [Google Scholar]
- 4.Basselin, M., H. Denise, G. H. Coombs, and M. P. Barrett. 2002. Resistance to pentamidine in Leishmania mexicana involves exclusion of the drug from the mitochondrion. Antimicrob. Agents Chemother. 46:3731-3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benaim, G., C. Lopez-Estrano, R. Docampo, and S. N. Moreno. 1993. A calmodulin-stimulated Ca2+ pump in plasma-membrane vesicles from Trypanosoma brucei; selective inhibition by pentamidine. Biochem. J. 296(Pt. 3):759-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chappuis, F., S. Sundar, A. Hailu, H. Ghalib, S. Rijal, R. W. Peeling, J. Alvar, and M. Boelaert. 2007. Visceral leishmaniasis: what are the needs for diagnosis, treatment and control? Nat. Rev. Microbiol. 5:873-882. [DOI] [PubMed] [Google Scholar]
- 7.Croft, S. L., and R. P. Brazil. 1982. Effect of pentamidine isethionate on the ultrastructure and morphology of Leishmania mexicana amazonensis in vitro. Ann. Trop. Med. Parasitol. 76:37-43. [DOI] [PubMed] [Google Scholar]
- 8.Delfin, D. A., R. E. Morgan, X. Zhu, and K. A. Werbovetz. 2009. Redox-active dinitrodiphenylthioethers against Leishmania: synthesis, structure-activity relationships and mechanism of action studies. Bioorg. Med. Chem. 17:820-829. [DOI] [PubMed] [Google Scholar]
- 9.Glaser, T. A., J. E. Baatz, G. P. Kreishman, and A. J. Mukkada. 1988. pH homeostasis in Leishmania donovani amastigotes and promastigotes. Proc. Natl. Acad. Sci. U. S. A. 85:7602-7606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar, D., A. Kulshrestha, R. Singh, and P. Salotra. 2009. In vitro susceptibility of field Isolates of Leishmania donovani to miltefosine and amphotericin B: correlation with sodium antimony gluconate susceptibility and implications for treatment in areas of endemicity. Antimicrob. Agents Chemother. 53:835-838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mandal, S., M. Maharjan, S. Ganguly, M. Chatterjee, S. Singh, F. Buckner, and R. Madhubala. 2009. High-throughput screening of amastigotes of Leishmania donovani clinical isolates against drugs using a colorimetric β-lactamase assay. Indian J. Exp. Biol. 47:475-479. [PMC free article] [PubMed] [Google Scholar]
- 12.Mandal, S., M. Maharjan, S. Singh, M. Chatterjee, and R. Madhubala. 2010. Assessing aquaglyceroporin gene status and expression profile in antimony-susceptible and -resistant clinical isolates of Leishmania donovani from India. J. Antimicrob. Chemother. 65:496-507. [DOI] [PubMed]
- 13.Mathis, A. M., A. S. Bridges, M. A. Ismail, A. Kumar, I. Francesconi, M. Anbazhagan, Q. Hu, F. A. Tanious, T. Wenzler, J. Saulter, W. D. Wilson, R. Brun, D. W. Boykin, R. R. Tidwell, and J. E. Hall. 2007. Diphenyl furans and aza analogs: effects of structural modification on in vitro activity, DNA binding, and accumulation and distribution in trypanosomes. Antimicrob. Agents Chemother. 51:2801-2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Melby, P. C., B. Chandrasekar, W. Zhao, and J. E. Coe. 2001. The hamster as a model of human visceral leishmaniasis: progressive disease and impaired generation of nitric oxide in the face of a prominent Th1-like cytokine response. J. Immunol. 166:1912-1920. [DOI] [PubMed] [Google Scholar]
- 15.Melby, P. C., Y. Z. Yang, J. Cheng, and W. Zhao. 1998. Regional differences in the cellular immune response to experimental cutaneous or visceral infection with Leishmania donovani. Infect. Immun. 66:18-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moreno, S. N. 1996. Pentamidine is an uncoupler of oxidative phosphorylation in rat liver mitochondria. Arch. Biochem. Biophys. 326:15-20. [DOI] [PubMed] [Google Scholar]
- 17.Mortelmans, K., and E. Zeiger. 2000. The Ames Salmonella/microsome mutagenicity assay. Mutat. Res. 455:29-60. [DOI] [PubMed] [Google Scholar]
- 18.Olliaro, P. L., P. J. Guerin, S. Gerstl, A. A. Haaskjold, J. A. Rottingen, and S. Sundar. 2005. Treatment options for visceral leishmaniasis: a systematic review of clinical studies done in India, 1980-2004. Lancet Infect. Dis. 5:763-774. [DOI] [PubMed] [Google Scholar]
- 19.Pacheco, M. G., C. F. da Silva, E. M. de Souza, M. M. Batista, P. B. da Silva, A. Kumar, C. E. Stephens, D. W. Boykin, and N. Soeiro Mde. 2009. Trypanosoma cruzi: activity of heterocyclic cationic molecules in vitro. Exp. Parasitol. 123:73-80. [DOI] [PubMed] [Google Scholar]
- 20.Paul, M., R. Durand, Y. Boulard, T. Fusai, C. Fernandez, D. Rivollet, M. Deniau, and A. Astier. 1998. Physicochemical characteristics of pentamidine-loaded polymethacrylate nanoparticles: implication in the intracellular drug release in Leishmania major infected mice. J. Drug Target. 5:481-490. [DOI] [PubMed] [Google Scholar]
- 21.Pearson, R. D., and A. Q. Sousa. 1996. Clinical spectrum of leishmaniasis. Clin. Infect. Dis. 22:1-13. [DOI] [PubMed] [Google Scholar]
- 22.Rosypal, A. C., J. E. Hall, S. Bakunova, D. A. Patrick, S. Bakunov, C. E. Stephens, A. Kumar, D. W. Boykin, and R. R. Tidwell. 2007. In vitro activity of dicationic compounds against a North American foxhound isolate of Leishmania infantum. Vet. Parasitol. 145:207-216. [DOI] [PubMed] [Google Scholar]
- 23.Rosypal, A. C., K. A. Werbovetz, M. Salem, C. E. Stephens, A. Kumar, D. W. Boykin, J. E. Hall, and R. R. Tidwell. 2008. Inhibition by dications of in vitro growth of Leishmania major and Leishmania tropica: causative agents of old world cutaneous leishmaniasis. J. Parasitol. 94:743-749. [DOI] [PubMed] [Google Scholar]
- 24.Silva, C. F., M. B. Meuser, E. M. De Souza, M. N. Meirelles, C. E. Stephens, P. Som, D. W. Boykin, and M. N. Soeiro. 2007. Cellular effects of reversed amidines on Trypanosoma cruzi. Antimicrob. Agents Chemother. 51:3803-3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stauber, L. A., E. M. Franchino, and J. Grun. 1958. An eight-day method for screening compounds against Leishmania donovani in the golden hamster. J. Eukaryot. Microbiol. 5:269-273. [Google Scholar]
- 26.Stephens, C. E., R. Brun, M. M. Salem, K. A. Werbovetz, F. Tanious, W. D. Wilson, and D. W. Boykin. 2003. The activity of diguanidino and “reversed” diamidino 2,5-diarylfurans versus Trypanosoma cruzi and Leishmania donovani. Bioorg. Med. Chem. Lett. 13:2065-2069. [DOI] [PubMed] [Google Scholar]
- 27.Stephens, C. E., F. Tanious, S. Kim, W. D. Wilson, W. A. Schell, J. R. Perfect, S. G. Franzblau, and D. W. Boykin. 2001. Diguanidino and “reversed” diamidino 2,5-diarylfurans as antimicrobial agents. J. Med. Chem. 44:1741-1748. [DOI] [PubMed] [Google Scholar]
- 28.Sturk, L. M., J. L. Brock, C. R. Bagnell, J. E. Hall, and R. R. Tidwell. 2004. Distribution and quantitation of the anti-trypanosomal diamidine 2,5-bis(4-amidinophenyl)furan (DB75) and its N-methoxy prodrug DB289 in murine brain tissue. Acta Trop. 91:131-143. [DOI] [PubMed] [Google Scholar]
- 29.Sundar, S., T. K. Jha, C. P. Thakur, J. Engel, H. Sindermann, C. Fischer, K. Junge, A. Bryceson, and J. Berman. 2002. Oral miltefosine for Indian visceral leishmaniasis. N. Engl. J. Med. 347:1739-1746. [DOI] [PubMed] [Google Scholar]
- 30.Sundar, S., H. Mehta, A. V. Suresh, S. P. Singh, M. Rai, and H. W. Murray. 2004. Amphotericin B treatment for Indian visceral leishmaniasis: conventional versus lipid formulations. Clin. Infect. Dis. 38:377-383. [DOI] [PubMed] [Google Scholar]
- 31.Troutman, M. D., and D. R. Thakker. 2003. Novel experimental parameters to quantify the modulation of absorptive and secretory transport of compounds by P-glycoprotein in cell culture models of intestinal epithelium. Pharm. Res. 20:1210-1224. [DOI] [PubMed] [Google Scholar]
- 32.Vercesi, A. E., and R. Docampo. 1992. Ca2+ transport by digitonin-permeabilized Leishmania donovani. Effects of Ca2+, pentamidine and WR-6026 on mitochondrial membrane potential in situ. Biochem. J. 284(Pt. 2):463-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang, M. Z., J. Y. Saulter, E. Usuki, Y. L. Cheung, M. Hall, A. S. Bridges, G. Loewen, O. T. Parkinson, C. E. Stephens, J. L. Allen, D. C. Zeldin, D. W. Boykin, R. R. Tidwell, A. Parkinson, M. F. Paine, and J. E. Hall. 2006. CYP4F enzymes are the major enzymes in human liver microsomes that catalyze the O-demethylation of the antiparasitic prodrug DB289 [2,5-bis(4-amidinophenyl)furan-bis-O-methylamidoxime]. Drug Metab. Dispos. 34:1985-1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Werbovetz, K. 2006. Diamidines as antitrypanosomal, antileishmanial and antimalarial agents. Curr. Opin. Invest. Drugs 7:147-157. [PubMed] [Google Scholar]
- 35.WHO. Leishmaniasis: the global trend. WHO, Geneva, Switzerland. http://www.who.int/neglected_diseases/integrated_media_leishmaniasis/en/index.html.
- 36.Yeates, C. 2002. Sitamaquine (GlaxoSmithKline/Walter Reed Army Institute). Curr. Opin. Invest. Drugs 3:1446-1452. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.