Abstract
Pseudomonas aeruginosa and other Gram-negative bacteria release membrane vesicles (MVs) from their surfaces, and MVs have an ability to interact with bacterial cells. Although it has been known that many bacteria have mechanisms that control their phenotypes with the transition from exponential phase to stationary phase, changes of properties in released MVs have been poorly understood. Here, we demonstrate that MVs released by P. aeruginosa during the exponential and stationary phases possess different physiochemical properties. MVs purified from the stationary phase had higher buoyant densities than did those purified from the exponential phase. Surface charge, characterized by zeta potential, of MVs tended to be more negative as the growth shifted to the stationary phase, although the charges of PAO1 cells were not altered. Pseudomonas quinolone signal (PQS), one of the regulators related to MV production in P. aeruginosa, was lower in MVs purified from the exponential phase than in those from the stationary phase. MVs from the stationary phase more strongly associated with P. aeruginosa cells than did those from the exponential phase. Our findings suggest that properties of MVs are altered to readily interact with bacterial cells along with the growth transition in P. aeruginosa.
Pseudomonas aeruginosa and many other Gram-negative bacteria naturally produce membrane vesicles (MVs), which are released from their outer surface. These bilayered spheres are made up of outer membrane proteins (OMPs) and lipopolysaccharide (LPS) and encapsulate periplasmic components. P. aeruginosa MVs also contain some virulence factors for killing host cells or other bacteria, including proteases, phospholipase C, alkaline phosphatase, and antibacterial factors, such as murein hydrolases (13, 15). MVs are also a component of the matrices of biofilm (27, 28). Moreover, P. aeruginosa MVs contain a quorum-sensing (QS) signaling molecule, 2-heptyl-3-hydroxyl-4-quinolone (Pseudomonas quinolone signal [PQS]) (18), which serves as a mediator of cell-cell communication (24, 34). PQS not only is packaged in MVs but also enhances MV production (18, 19).
P. aeruginosa MVs have an ability to interact not only with bacterial cells (12, 14, 16) but also with eukaryotic cells to deliver the pseudomonal toxins (20). P. aeruginosa MVs readily fuse with Gram-negative bacteria and attach with Gram-positive bacteria (12). It has been proposed that charge-charge interaction is important in adherence of MVs to bacterial cells (12). The outer surfaces of both Gram-negative and -positive bacteria are usually negatively charged and rich in divalent cations, such as Mg2+ or Ca2+, that stabilize surface charges. In the model of Kadurugamuwa and Beveridge (12), MVs have negatively charged LPSs, and the O side chains of LPSs are loosely packed and separated from one another because of high curvatures, resulting in little salt bridging by divalent cations between adjacent LPSs. Because of Mg2+ or Ca2+ depletion on the MV surface, it is likely that the anionic MV surface would become salt bridged to the bacterial cells. For that reason, MV surface charge would be an important factor that affects the interaction with bacterial cells through physiochemical forces. Surface charge is usually characterized by zeta potential, which is the electrical potential of the interfacial region between the bacterial surface and the aqueous environment (36, 38).
Many bacteria have exquisite regulatory mechanisms that control gene expression during the transition from the exponential phase to the stationary phase (11, 37). An example is QS, the mechanism of which enables it to coordinately tune multiple gene expression in a cell density-dependent manner. Although changes in gene transcription and cell behavior between the exponential phase and the stationary phase have been well studied, changes in the properties of the released MVs have not. To date, P. aeruginosa MVs have been well characterized in several studies, which have revealed their potential properties, virulence, and production mechanisms (3, 13, 25). However, each study focused on MVs isolated from a single growth phase. There is a likelihood that MV properties and production mechanisms are, to some degree, altered according to the growth transition.
In this study, we investigated the properties of MVs released during the transition from the exponential phase to the stationary phase. Since P. aeruginosa MVs play the role of a PQS carrier, it is thought that MVs take on some functions to easily interact with cells after PQS synthesis is initiated. We hypothesized that MV properties are altered with the growth transition to interact with bacterial cells. We showed that MVs developed more negative surface charges, contained more PQS, and interacted with PAO1 cells more readily as the growth shifted to the stationary phase. Our results contribute to understanding the mechanism of how MVs interact with bacterial cells.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The sequenced P. aeruginosa PAO1 Holloway strain (10) was used as a standard strain in this study. ΔpqsH, pqsH-xylE, pqsE-xylE, and ΔpqsH pqsE-xylE mutants of PAO1 were used (31, 32, 33). Bacterial cells were routinely grown at 37°C in Luria-Bertani (LB) medium at 150 rpm or LB containing 1.5% (wt/vol) agar.
MV isolation, purification, and quantification.
MV isolation, purification, and quantification were carried out as described previously (31). Crude MVs were isolated from an LB culture of P. aeruginosa. Cells were removed by centrifugation (6,000 × g, 15 min), and the supernatant was sequentially filtered through 0.45- and 0.22-μm cellulose acetate membranes, followed by ultracentrifugation at 150,000 × g for 3 h at 4°C. The pellets were washed with 10 mM HEPES (pH 6.8) containing 0.85% (wt/vol) NaCl (HEPES-NaCl). MV protein was quantified by the Bradford method (4), and MV phospholipid was quantified by the Stewart method (29, 31). In MV purification, pellets of ultracentrifuged MVs were resuspended in 500 μl of 45% (wt/vol) Optiprep in HEPES-NaCl, transferred to the bottom of an ultracentrifuge tube, and layered with Optiprep-HEPES-NaCl (1 ml of 40%, 35%, 30%, 25%, 20%, and 15% and 0.5 ml of 10%). The gradients were ultracentrifuged (100,000 × g, 3 h), and 500-μl fractions were collected from each gradient. Protein and phospholipid concentrations were measured in each fraction. Fractions containing MVs were resuspended in HEPES-NaCl buffer and ultracentrifuged again, and purified MVs were obtained. In the protein band pattern analysis, EDTA-free protease inhibitor (Roche, Mannheim, Germany) was added in all processes of MV purification per the manufacturer's instructions. All subsequent steps were carried out at 0 to 4°C to prevent the effect of proteolytic activities in the supernatant. We confirmed that proteolytic activities in the supernatants were substantially inhibited at 4°C (data not shown).
Proteolytic activities.
Proteolytic activity was determined by an azocasein assay as previously reported, with some modification (31). Briefly, 10 μl of cell-free supernatant was added to 0.5 ml of 0.3% azocasein (Sigma) in 50 mM Tris-HCl (pH 7.2) and 0.5 mM CaCl2 and incubated at 37°C or 4°C for 15 min. The reaction was stopped by adding 0.5 ml of 10% trichloroacetic acid. After centrifugation (15,000 × g, 20 min), the absorbance at 400 nm in the supernatant was measured.
Transmission electron microscopy (TEM) observation.
Purified MVs were placed on Formvar-coated copper grids (200 mesh), which were then stained with 2% aqueous uranyl acetate for 1 min, rinsed, and observed with a JEOL-1010 TEM (JEOL, Tokyo, Japan) at 80 kV.
SDS-PAGE and protein identification.
OMPs were purified as described previously (30). Protein patterns of MVs and OMPs were examined by SDS-PAGE as previously described (31). MV protein bands were excised from SDS-polyacrylamide gels and placed into microcentrifuge tubes. Gel pieces were washed twice with 40% 1-propanol for 10 min and twice with 100 mM ammonium bicarbonate-50% acetonitrile for 10 min and dried using a centrifugal dryer. In-gel digestion was performed using 5 μl of trypsin (20 μg/ml) in 50 μl of 100 mM ammonium bicarbonate at 37°C for 10 to 16 h. Peptides were extracted twice from the gel with 0.1% trifluoroacetic acid (TFA)-50% acetonitrile for 10 min and dried to 10 μl using a centrifugal dryer. Peptide samples were desalted using a C18 ZipTip microcolumn (Millipore) as described by the manufacturer. For matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) analysis, 2 μl of samples was spotted onto the MALDI matrix and analyzed with a Shimadzu Axima-CFR+ (Shimadzu, Kyoto, Japan) mass spectrometer. Proteins were identified by peptide mass fingerprint analysis according to the molecular mass of each tryptic fragment and using the MASCOT search engine of the entire NCBI protein database of P. aeruginosa.
PQS detection.
PQS was quantified using thin-layer chromatography (TLC) as previously described (24). PQS was extracted from sonicated MVs with acidified ethyl acetate, and the solvent was evaporated. Extracts were suspended in 20 μl 1:1 ethyl acetate-acetonitrile. A 5-μl sample was analyzed using TLC with a 17:2:1 mixture of dichloromethane-acetonitrile-dioxane.
Measurement of hydrodynamic diameter and surface charge.
Particle size distribution of MVs was analyzed at 25°C with a Zetasizer Nano ZS particle analyzer (Malvern Instruments, Malvern, United Kingdom). Purified MVs were diluted to a concentration of approximately 10 μg protein/ml in 10 mM HEPES buffer. MV hydrodynamic zeta average diameter was calculated by the light scattering method.
The relative surface charges were estimated using a Zetasizer Nano ZS particle analyzer. The electrophoresis cell was filled with approximately 10 μg MV/ml in 10 mM HEPES buffer or PAO1 cells diluted to an optical density at 600 nm (OD600) of 0.1. The Smoluchowski approximation was applied for determining the zeta potential.
MV association assay.
To monitor the fusion of MVs with bacterial cells, MVs were fluorescently labeled with fluorescein isothiocyanate (FITC) as previously described with some modification (2). Purified MVs (100 μg protein) were incubated with FITC (100 μg) in 1 ml of 100 mM NaCl-50 mM Na2CO3 (pH 9) for 2 h at 25°C. FITC-labeled MVs were washed three times in HEPES-NaCl buffer (150,000 × g, 30 min). PAO1 cells grown to the late exponential phase were washed three times in phosphate-buffered saline (PBS) and diluted to an OD600 of 1.0. FITC-labeled MVs (10 μg/ml) were incubated with cells for 15 min to 120 min at 37°C. Cells were washed twice in PBS. Association of MVs with cells was observed by a scanning confocal laser microscope (LSM 5 Pascal; Carl Zeiss, Oberkochen, Germany) with an FITC filter (485 nm for excitation and 535 nm for emission), and total cells were visualized by differential interference microscopy. In the fluorometric quantification assay, FITC-labeled cells were lysed with a homogenizer. Fluorescence (485 nm for excitation and 535 nm for emission) was quantitated using a fluorometer (Arvo MX/Light; PerkinElmer, Yokohama, Japan). A standard curve was generated with a dilution series of purified FITC-labeled MVs. The fluorescence intensity of lysed cells, which are not incubated with MVs, was used for a blank. Cell-associated MV protein level per cell protein was calculated from a standard curve.
C23O specific activity assay.
To examine the transcription level of pqsH, the pqsH-xylE strain was used (31). To examine the transcription level of the pqsABCDE operon under pqsH deletion, the ΔpqsH pqsE-xylE mutant was used (32). An assay for activity of catechol-2,3-dioxygenase (C23O) (the xylE product) was performed as described previously (17). Cells were homogenized, and lysed protein samples were used. The A375 was recorded at 30°C. Specific activity was defined as nanomoles of product formed per minute per milligram of protein (ɛ1cm = 4.4 × 104).
RESULTS
MV production in the exponential and stationary phases.
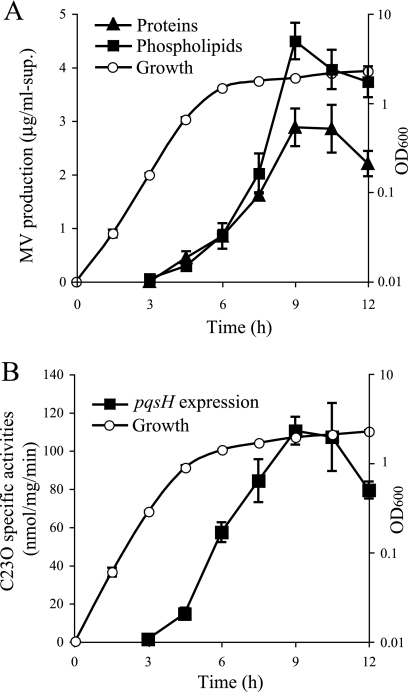
MVs from a P. aeruginosa LB culture were isolated by ultracentrifugation of the cell-free supernatant, and the MV quantities were assayed by measuring the protein and phospholipid concentrations of the pellets. We previously showed that over 90% of proteins and 99% of phospholipids of the ultracentrifuged pellets consist of MVs (31). Figure 1A shows that MV production was initiated at 4.5 h of growth and increased as the growth switched to the stationary phase.
FIG. 1.
Time courses of MV production and pqsH expression. (A) Growth curve and MV production in P. aeruginosa PAO1. The quantity of MVs in LB supernatants was determined by measuring the concentration of proteins and phospholipids in pellets from ultracentrifugation. Data are means ± standard deviations from triplicates. The results presented are representative of at least three independent experiments. (B) Growth curve of and pqsH transcription in the P. aeruginosa pqsH-xylE strain. The expression of the pqsH-xylE transcriptional fusion was monitored using a catechol-2,3-dioxygenase activity assay. Data are means ± standard deviations from triplicates. The results presented are representative of at least three independent experiments.
It has been reported elsewhere that a QS signal, PQS, which is released during the stationary phase, enhances MV production and that PQS is enclosed within MVs (18). To investigate the relationship between the initiation of MV production and that of PQS synthesis in P. aeruginosa, the reporter gene xylE was inserted downstream of the chromosomal pqsH gene, the product of which catalyzes the final reaction in PQS synthesis, and the expression was examined by assaying catechol-2,3-dioxygenase activity. The result showed that the transcription of pqsH was initiated from 4.5 h of growth and peaked in the early stationary phase (Fig. 1B).
We further examined how many MVs bacteria produce in the exponential (4.5-h growth) and stationary (12-h growth) phases. MVs were produced in both phases (Table 1), although the PQS synthesis might be very low in the exponential phase compared to the stationary phase. Interestingly, while MV protein concentration and MV phospholipid concentration were similar in the exponential-phase MVs (ex-MVs), phospholipid concentration was higher than protein concentration in the stationary-phase MVs (st-MVs). These results suggest that MVs are produced in both the exponential and stationary phases but that their properties are not identical in each growth phase.
TABLE 1.
MV concentrations in the supernatants and MV-producing activities in the exponential and stationary phasesa
| Cultivation time (h) | MV concn (μg/ml of supernatant) |
MV-producing activity (pg/CFU) |
||
|---|---|---|---|---|
| Protein | Phospholipid | Protein | Phospholipid | |
| 4.5 | 0.60 ± 0.04 | 0.62 ± 0.15 | 8.66 ± 0.16 | 8.91 ± 2.23 |
| 12 | 2.21 ± 0.13 | 3.28 ± 0.22 | 7.58 ± 0.63 | 11.32 ± 1.99 |
Data are the means ± standard deviations from triplicates.
Buoyant densities of MVs.
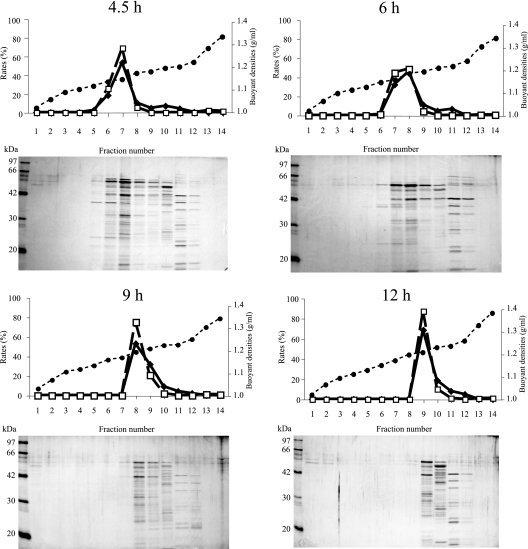
To investigate the properties of MVs with the growth transition, the differences in the buoyant density of MVs at each growth phase were examined. We fractionated crude MVs from 4.5, 6, 9, and 12 h of growth by an Optiprep density gradient method (31). For each sample, gradient fractions were collected and the concentrations of proteins and phospholipids were measured (Fig. 2). When MVs from the exponential phase (4.5 h of growth) were fractionated, both the protein and phospholipid concentrations were comparatively high in the light fractions (fractions 4 to 7), indicating that exponential-phase MVs fractioned to the light fractions. Indeed, the existence of MVs in the peak fractions could be confirmed by electron microscopy (data not shown). On the other hand, MVs from the stationary phase (12 h of growth) were found in the heavier fractions (fractions 8 and 9), suggesting that the buoyant density of st-MVs is higher than that of ex-MVs. As growth continued, the buoyant densities had a tendency to increase (Table 2). To further analyze the density of MVs, each fraction was subjected to SDS-PAGE and bands were detected by silver staining (Fig. 2). Probable MV bands were detected in the fractions with high protein and phospholipid concentrations. Some bands were detected in the heavier fractions (fractions 10 to 12) of each sample, and these fractions seemed to include flagellin (53 kDa), pilin (19 kDa), and other components of cells, as seen by Bauman and Kuehn (3). Thus, the buoyant density of MVs increases as the cells grow, suggesting that the MV component alters depending on the growth phase.
FIG. 2.
Protein concentrations, phospholipid concentrations, buoyant densities, and SDS-PAGE profiles of density gradient fractions. MVs were extracted from LB medium at 4.5, 6, 9, and 12 h of growth. Crude MV protein (100 μg) was separated on a 10% to 45% Optiprep gradient, and 14 fractions were collected (fraction 1 is the lightest and fraction 14 is the heaviest). The upper panels show the protein concentration (diamonds; left longitudinal axis), phospholipid concentration (squares; left longitudinal axis), and buoyant density (circles; right longitudinal axis) of each fraction. In the bottom panels, equal volumes of each fraction were subjected to SDS-PAGE (15% acrylamide) and silver stained.
TABLE 2.
Temporal changes in properties of MVs and cells
| Cultivation time (h) | MVs |
Cell zeta potentialc (mV) | ||
|---|---|---|---|---|
| Buoyant densitya (g/ml) | Hydrodynamic diamb (Z avg [nm]) | Zeta potentialc (mV) | ||
| 4.5 | 1.08, 1.11 | 105.2 ± 7.2 | −8.4 ± 1.3 | −35.0 ± 1.2 |
| 6 | 1.10, 1.14 | 107.3 ± 12.1 | −14.4 ± 1.8 | −34.3 ± 0.8 |
| 9 | 1.13, 1.14 | 111.8 ± 16.2 | −18.2 ± 1.9 | −34.2 ± 0.6 |
| 12 | 1.15, 1.17 | 118.8 ± 8.3 | −24.8 ± 1.4 | −34.7 ± 0.3 |
Data are minimum and maximum densities of fractions including MVs.
Data are the means ± standard deviations of the average intensity from three independent assays.
Data are the means ± standard deviations from three independent experiments carried out in triplicate.
Comparison of the exponential- and stationary-phase MV proteomes.
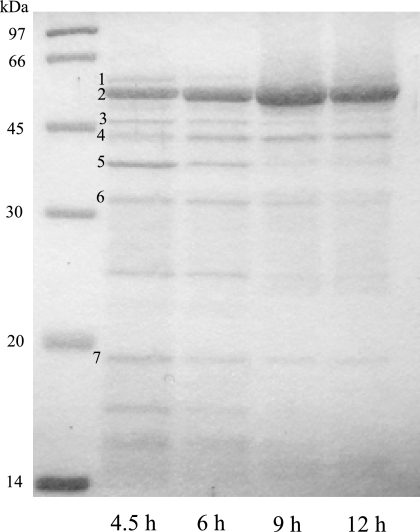
To investigate whether the protein profiles of MVs are altered according to the growth transition, MVs purified from 4.5-, 6-, 9-, and 12-h growth were compared using SDS-PAGE. Each band pattern was very similar but not identical to the others (Fig. 3). Some major bands of the exponential-phase MVs were excised and identified using MALDI-TOF MS after in-gel tryptic digestions. Major outer membrane proteins (OMPs), including OprD, OprF, and OprH, and extracellular proteins, including putative aminopeptidase PaAP and phospholipase C PlcB, and the bacteriophage protein PA0622 were identified (Table 3). The most abundant MV protein is aminopeptidase PaAP, and its identification is similar to the results from the work of Bauman and Kuehn (3). Interestingly, OMPs, including OprD, OprF, and OprH, were enriched in the exponential MVs compared to those in the stationary MVs (Fig. 3). On the other hand, the expression level of OprD, OprF, or OprH localized in PAO1 outer membrane remained constant irrespective of the growth phase based on SDS-PAGE analysis (data not shown). The proteolytic activity of the supernatant in the stationary phase was higher than that in the exponential phase (data not shown), suggesting that OMPs localized in MV may be degraded by extracellular proteases in the supernatant.
FIG. 3.
Protein profiles of MVs purified from 4.5, 6, 9, and 12 h of growth. Purified MV proteins (10 μg) were separated by SDS-PAGE (15% acrylamide) and stained with Coomassie brilliant blue. The number of each band corresponds to the numbers in Table 3.
TABLE 3.
Identified proteins in PAO1 MVs
| No. | Annotation name | PA no. | Score | tMWa,b | MPb | Cov.c |
|---|---|---|---|---|---|---|
| 1 | Putative aminopeptidase PaAP | PA2939 | 143 | 57.5 | 13/15 | 29 |
| 2 | Putative aminopeptidase PaAP | PA2939 | 143 | 57.5 | 13/15 | 29 |
| 3 | OprD | PA0958 | 67 | 48.4 | 7/11 | 26 |
| 4 | Bacteriophage protein | PA0622 | 65 | 41.2 | 8/15 | 30 |
| 5 | OprF | PA1777 | 76 | 37.6 | 5/11 | 24 |
| 6 | Phospholipase C, PlcB | PA0026 | 84 | 36.7 | 9/22 | 42 |
| 7 | OprH | PA1178 | 105 | 21.6 | 9/17 | 44 |
Theoretical mass.
Number of peptide masses matching the top hit from MASCOT peptide mass fingerprinting/total number of peptide masses.
Sequence coverage (%) in peptide mass fingerprinting.
Size and appearance of MVs.
To investigate whether the sizes of MVs vary according to the growth transition, the hydrodynamic diameter of MVs was examined in 10 mM HEPES (pH 6.8) by the light scattering method (Table 2). The hydrodynamic diameter of MVs did not vary among MVs at 4.5, 6, 9, and 12 h of growth (Table 2). Moreover, the distributions of MV diameters were also similar among these samples (data not shown). These data indicate that the MV's average size is invariable, regardless of the growth phase.
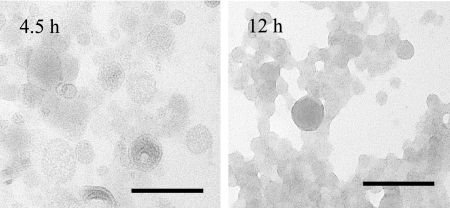
Moreover, the appearance of MVs was observed by TEM (Fig. 4). While changes in both the size and shape of MVs could not be observed, the translucence was different between two growth phases: ex-MVs were more translucent than st-MVs, suggesting that stationary-phase MVs contain a higher concentration of some substances, such as PQS or metals. This result reflects the earlier finding that MV density is enhanced as growth develops (Table 2).
FIG. 4.
Electron micrographs of exponential (4.5-h)- and stationary (12-h)-phase MVs. MVs were visualized by negative staining. Results are representative images. Bars, 100 nm.
PQS content in MVs.
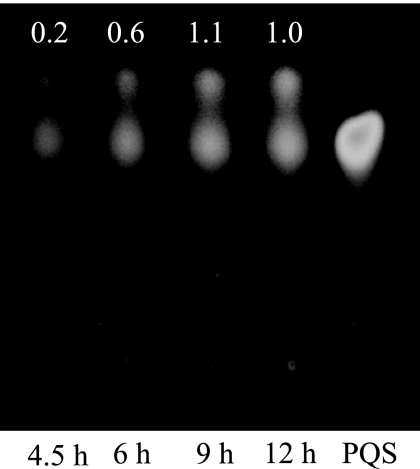
It has been known that MV production is regulated by PQS in P. aeruginosa (18). However, while PQS synthesis occurred at the onset of the stationary phase (9), MV production occurred detectably from the exponential phase. Hence, it is presumed that MV production is independent of PQS during the exponential phase. To corroborate the presumption, the existence of PQS in MVs was examined using TLC. The result showed that the amount of PQS in ex-MVs was smaller than that in st-MVs (Fig. 5). To further investigate the effect of PQS on MV production during the exponential phase, MV production of the ΔpqsH mutant, which does not synthesize PQS, was examined. While MV production of the ΔpqsH mutant was 1.94 (±0.19)-fold decreased in the exponential phase (4.5-h culture) over that of PAO1 wild type (WT), it was 4.15 (±1.35)-fold decreased in the stationary phase (12-h culture) over that of PAO1 WT, suggesting that the effect of PQS on MV production in the exponential phase is smaller than that in the stationary phase. Thus, it can be considered that dependence of MV production on PQS is low in the exponential phase.
FIG. 5.
Contents of PQS in MVs at 4.5, 6, 9, and 12 h of growth. PQS extracted from purified MVs (2.5 μg protein) was subjected to TLC. Chemically synthesized PQS (25 nmol) was used as a reference (right). Inset numbers are spot intensities relative to that of 12-h growth.
Zeta potential of MVs.
To consider the behavior of MVs, it is important to understand the zeta potential of MVs because it has been suggested that salt bridging by divalent cations between MVs and bacterial cells is important (12). Zeta potentials varied widely among MVs from different growth phases: MVs tended to be more negative as the growth switched to the stationary phase (Table 2). It was reported previously that the zeta potential of P. aeruginosa cells is as negative as −25 mV at pH 7 (6). In our experiment, the zeta potential of P. aeruginosa cells was around −35 mV and constant regardless of the growth phase (Table 2). These data indicate that MVs, which have different zeta potentials, were released according to growth phase without altering cellular zeta potentials.
Fusion of MVs to bacterial cells.
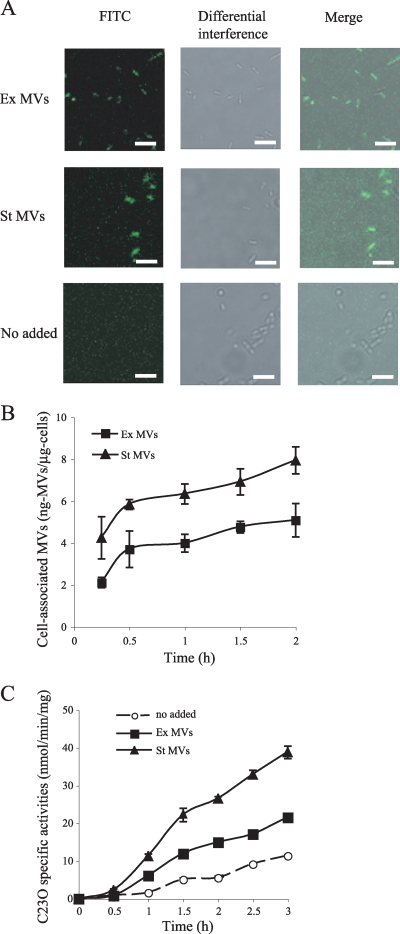
It has been reported elsewhere that P. aeruginosa MVs are fused to the Gram-negative bacterium Escherichia coli and attached to the Gram-positive bacterium Staphylococcus aureus (12). Since P. aeruginosa MVs are used as a carrier of interspecies communication (18), it can be considered that P. aeruginosa MVs fuse to their own cells. Purified ex-MVs and st-MVs were fluorescently labeled with FITC and incubated with P. aeruginosa PAO1 cells grown to the late exponential phase. When we incubated PAO1 cells with ex- or st-MVs at 37°C for 1 h, clear fluorescence was observed in both samples by microscopic observation (Fig. 6A). In contrast, no fluorescence was detected in cells when cells were not incubated with MVs. These results indicate that the FITC-labeled MVs were associated with PAO1 cells.
FIG. 6.
Association of MVs with PAO1 cells. (A) Visualization of MV association with PAO1 cells. FITC-labeled ex-MVs and st-MVs were incubated with PAO1 cells for 1 h at 37°C. Cells were visualized by confocal microscopy. Experiments were repeated three times, and representative images are shown. Scale bars, 5 μm. (B) Cell association activity of MVs. FITC-labeled ex-MVs and st-MVs were incubated with PAO1 cells for 15 min to 2 h at 37°C. Data are means ± standard deviations from triplicates. Representative data from three independent experiments are shown. (C) Catechol-2,3-dioxygenase activity assays of PpqsA transcriptional level in a ΔpqsH pqsE-xylE mutant in the presence and absence of MVs. The cells were grown to an OD600 of 1.0 in LB medium, MVs were added to cultures (final concentration, 5 μg protein/ml), and C23O was examined for 30 min to 3 h. Data are means ± standard deviations from triplicates. Representative data from three independent experiments are shown.
To test whether different potentials between ex- and st-MVs contribute to fusion activity with PAO1 cells, we quantified MVs associated with PAO1 cells. Cell-associated MV proteins were calculated from a standard curve relating fluorescence to ng of FITC MVs for each of the MV preparations. The results showed that st-MVs were 1.5- to 2-fold more highly associated with cells than were ex-MVs (Fig. 6B). When we tested temperature dependence of the MV-cell association, the amounts of st-MVs, which interact with cells, were not different between 37°C and 4°C (data not shown), suggesting that an enzymatic mechanism was not related to MV-bacterial cell association.
To further confirm the difference of MV-cell association activity between the ex- and st-MVs, the response of cells to PQS in MVs was examined. PQS acts as a ligand for PqsR, the transcriptional activator that controls the pqsABCDE operon (39). The gene products of pqsABCD catalyze the synthesis of 2-heptyl-4-quinolone (HHQ) from anthranilic acid, and the gene product of pqsH converts HHQ to PQS. The expression of pqsABCDE is autoregulated positively (7). To monitor the response of PQS, we used a ΔpqsH pqsE-xylE strain, and C23O (xylE product) specific activities were measured. Figure 6C shows that st-MVs promoted the expression of the pqsABCDE operon more than did ex-MVs. This result reflects the data that st-MVs possess higher levels of PQS and interact more easily with P. aeruginosa cells than do ex-MVs.
DISCUSSION
Although MVs are produced in both the exponential and stationary phases (Table 1), little attention has been given to changes in the properties of MVs within the growth phase. Under standard laboratory growth conditions, our data demonstrate that MVs released from the exponential phase and the stationary phase have different properties in terms of buoyant densities, surface charges, electron microscopic appearances, and PQS contents, whereas MVs retain the same hydrodynamic diameter. As far as we know, this is the first report to demonstrate a potential difference in MVs released during different growth phases.
Electron charge is an important factor in the interaction between MVs and bacterial cells. P. aeruginosa MVs have been reported to interact with both Gram-negative and Gram-positive bacteria (13). MVs are negatively charged because of LPS and small amounts of salt bridging by Ca2+ or Mg2+ between adjacent O side chains. For that reason, it has been proposed that MVs can be readily salt bridged to the Ca2+- or Mg2+-rich surface of bacterial cells. We showed that st-MVs were highly negatively charged (Table 2) and that they interact with bacterial cells more easily than do ex-MVs. Moreover, the st-MVs possessed high levels of PQS (Fig. 5) and induced the transcription of the pqsABCDE operon (Fig. 6C). Hence, such highly negatively charged MVs liberated during the stationary phase could be useful in cell-cell communication in P. aeruginosa.
The difference in the electron charges of MV surfaces and the PQS contents also provides a possible explanation for the difference in MV production mechanisms between the exponential phase and the stationary phase. Several studies have proposed that the electronegative charge of LPS causes charge-to-charge repulsion between adjacent molecules of LPS, which results in outer membrane blebbing in P. aeruginosa (13, 22, 26). Moreover, recent studies show that PQS interacts strongly with LPS, and ionic interactions between PQS and Mg2+ in the outer membrane enhance the anionic repulsion between neighboring LPS molecules, which enhances membrane blebbing (19, 20). In our study, st-MVs certainly contained PQS (Fig. 5), and their surfaces were highly negatively charged (Table 2), while PQS levels were low and the surface was more neutral in ex-MVs. It is possible that MVs are liberated by anionic repulsions that are dependent on PQS during the stationary phase but that another mechanism independent of PQS affects MV production during the exponential phase in P. aeruginosa. Our results also showed that st-MVs had higher densities and were less translucent than ex-MVs (Table 2 and Fig. 4). One possible explanation is that these differences would be derived from the increase of PQS concentration in MVs. We recently reported that misfolded OMPs would be an important factor to accelerate MV production and increase protein concentration in MVs (31). Since MV protein concentration was the same as that of phospholipid in the supernatant at the exponential phase, unlike in the case of the stationary phase (Table 1), it is possible that these proteins may be related to MV production during the exponential phase.
P. aeruginosa is a notorious pathogen that secretes many virulence exoproducts, most of which are produced when growth switches to the stationary phase. Cell density-dependent gene expression occurs in response to environmental signals, a process known as QS. Quinolones and phenazines are excreted from cells at specific times during growth, and their production is controlled by QS in P. aeruginosa (8, 9). The production of several secreted proteins such as azurin and proteases (e.g., elastase, PrlP, PasP, and PaAP) is regulated by QS (5, 23, 35). It has been suggested that secreted elastase is capable of degrading MV proteins (3). In this study, OMPs, including OprD, OprF, and OprH, were contained at lower levels in st-MVs than in ex-MVs (Fig. 3). Since many proteases are secreted during the stationary phase, these OMPs may be more labile than other MV proteins to proteases. PaAP has been characterized as a zinc-dependent leucine aminopeptidase and is secreted via Xcp-mediated type II secretion and regulated by the Las QS system (21, 23). A recent study demonstrated that PaAP is abundant in MVs from clinical strains compared to those from PAO1, and it promotes the association of MVs with lung cells (2). In our experiment, PaAP was consistently a most abundant protein in PAO1 MVs (Fig. 5). Since PaAP expression depends upon QS expression and oxygen concentration (1, 23), this discrepancy may be related to different culture conditions. In any event, the MVs that include large amounts of PaAP seem to be capable of attacking host cells. However, it has been unknown if PaAP is related to association with bacterial cells. Since cell association activity was not altered between 37°C and 4°C in our results, the effect of physiochemical factors, but not enzymatic factors, may be mostly related to MV-cell association.
In conclusion, we demonstrated that properties of MVs were altered as growth switches to the stationary phase in P. aeruginosa. We have shown that MVs released at the stationary phase possess negatively charged surfaces and high activity of association with cells. These findings would be useful for understanding the mechanism of interaction between bacterial cells and MVs.
Acknowledgments
We are very grateful to Fumiko Yukuhiro of the National Institute of Agrobiological Sciences (NIAS) for advising and helping with the TEM analysis. We also express thanks to Yutaka Yawata for technical advice on confocal microscopy analysis.
Y. Tashiro and M. Toyofuku were supported by a Scientific Research fellowship from the Japan Society for the Promotion of Sciences (JSPS). This study was supported in part by grants-in-aid for scientific research to N. Nomura from the Ministry of Education, Culture, Sports, and Technology of Japan.
Footnotes
Published ahead of print on 9 April 2010.
REFERENCES
- 1.Alvarez-Ortega, C., and C. Harwood. 2007. Responses of Pseudomonas aeruginosa to low oxygen indicate that growth in the cystic fibrosis lung is by aerobic respiration. Mol. Microbiol. 65:153-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bauman, S. J., and M. J. Kuehn. 2009. Pseudomonas aeruginosa vesicles associate with and are internalized by human lung epithelial cells. BMC Microbiol. 9:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bauman, S. J., and M. J. Kuehn. 2006. Purification of outer membrane vesicles from Pseudomonas aeruginosa and their activation of an IL-8 response. Microbes Infect. 8:2400-2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 5.Brint, J. M., and D. E. Ohman. 1995. Synthesis of multiple exoproducts in Pseudomonas aeruginosa is under the control of RhlR-RhlI, another set of regulators in strain PAO1 with homology to the autoinducer-responsive LuxR-LuxI family. J. Bacteriol. 177:7155-7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruinsma, G. M., M. Rustema-Abbing, H. C. van der Mei, C. Lakkis, and H. J. Busscher. 2006. Resistance to a polyquaternium-1 lens care solution and isoelectric points of Pseudomonas aeruginosa strains. J. Antimicrob. Chemother. 57:764-766. [DOI] [PubMed] [Google Scholar]
- 7.Déziel, E., F. Lépine, S. Milot, J. He, M. N. Mindrinos, R. G. Tompkins, and L. G. Rahme. 2004. Analysis of Pseudomonas aeruginosa 4-hydroxy-2-alkylquinolines (HAQs) reveals a role for 4-hydroxy-2-heptylquinoline in cell-to-cell communication. Proc. Natl. Acad. Sci. U. S. A. 101:1339-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dietrich, L. E., A. Price-Whelan, A. Petersen, M. Whiteley, and D. K. Newman. 2006. The phenazine pyocyanin is a terminal signalling factor in the quorum sensing network of Pseudomonas aeruginosa. Mol. Microbiol. 61:1308-1321. [DOI] [PubMed] [Google Scholar]
- 9.Diggle, S. P., K. Winzer, S. R. Chhabra, K. E. Worrall, M. Cámara, and P. Williams. 2003. The Pseudomonas aeruginosa quinolone signal molecule overcomes the cell density-dependency of the quorum sensing hierarchy, regulates rhl-dependent genes at the onset of stationary phase and can be produced in the absence of LasR. Mol. Microbiol. 50:29-43. [DOI] [PubMed] [Google Scholar]
- 10.Holloway, B. W., V. Krishnapillai, and A. E. Morgan. 1979. Chromosomal genetics of Pseudomonas. Microbiol. Rev. 43:73-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishihama, A. 1999. Modulation of the nucleoid, the transcription apparatus, and the translation machinery in bacteria for stationary phase survival. Genes Cells 4:135-143. [DOI] [PubMed] [Google Scholar]
- 12.Kadurugamuwa, J. L., and T. J. Beveridge. 1996. Bacteriolytic effect of membrane vesicles from Pseudomonas aeruginosa on other bacteria including pathogens: conceptually new antibiotics. J. Bacteriol. 178:2767-2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kadurugamuwa, J. L., and T. J. Beveridge. 1995. Virulence factors are released from Pseudomonas aeruginosa in association with membrane vesicles during normal growth and exposure to gentamicin: a novel mechanism of enzyme secretion. J. Bacteriol. 177:3998-4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kadurugamuwa, J. L., A. Mayer, P. Messner, M. Sára, U. B. Sleytr, and T. J. Beveridge. 1998. S-layered Aneurinibacillus and Bacillus spp. are susceptible to the lytic action of Pseudomonas aeruginosa membrane vesicles. J. Bacteriol. 180:2306-2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li, Z., A. J. Clarke, and T. J. Beveridge. 1996. A major autolysin of Pseudomonas aeruginosa: subcellular distribution, potential role in cell growth and division and secretion in surface membrane vesicles. J. Bacteriol. 178:2479-2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li, Z., A. J. Clarke, and T. J. Beveridge. 1998. Gram-negative bacteria produce membrane vesicles which are capable of killing other bacteria. J. Bacteriol. 180:5478-5483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maseda, H., I. Sawada, K. Saito, H. Uchiyama, T. Nakae, and N. Nomura. 2004. Enhancement of the mexAB-oprM efflux pump expression by a quorum-sensing autoinducer and its cancellation by a regulator, MexT, of the mexEF-oprN efflux pump operon in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 48:1320-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mashburn, L. M., and M. Whiteley. 2005. Membrane vesicles traffic signals and facilitate group activities in a prokaryote. Nature 437:422-425. [DOI] [PubMed] [Google Scholar]
- 19.Mashburn-Warren, L., J. Howe, P. Garidel, W. Richter, F. Steiniger, M. Roessle, K. Brandenburg, and M. Whiteley. 2008. Interaction of quorum signals with outer membrane lipids: insights into prokaryotic membrane vesicle formation. Mol. Microbiol. 69:491-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mashburn-Warren, L. M., and M. Whiteley. 2006. Special delivery: vesicle trafficking in prokaryotes. Mol. Microbiol. 61:839-846. [DOI] [PubMed] [Google Scholar]
- 21.Michel, G. P., E. Durand, and A. Filloux. 2007. XphA/XqhA, a novel GspCD subunit for type II secretion in Pseudomonas aeruginosa. J. Bacteriol. 189:3776-3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen, T. T., A. Saxena, and T. J. Beveridge. 2003. Effect of surface lipopolysaccharide on the nature of membrane vesicles liberated from the Gram-negative bacterium Pseudomonas aeruginosa. J. Electron Microsc. (Tokyo) 52:465-469. [DOI] [PubMed] [Google Scholar]
- 23.Nouwens, A. S., S. A. Beatson, C. B. Whitchurch, B. J. Walsh, H. P. Schweizer, J. S. Mattick, and S. J. Cordwell. 2003. Proteome analysis of extracellular proteins regulated by the las and rhl quorum sensing systems in Pseudomonas aeruginosa PAO1. Microbiology 149:1311-1322. [DOI] [PubMed] [Google Scholar]
- 24.Pesci, E. C., J. B. Milbank, J. P. Pearson, S. McKnight, A. S. Kende, E. P. Greenberg, and B. H. Iglewski. 1999. Quinolone signaling in the cell-to-cell communication system of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. U. S. A. 96:11229-11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Renelli, M., V. Matias, R. Y. Lo, and T. J. Beveridge. 2004. DNA-containing membrane vesicles of Pseudomonas aeruginosa PAO1 and their genetic transformation potential. Microbiology 150:2161-2169. [DOI] [PubMed] [Google Scholar]
- 26.Sabra, W., H. Lünsdorf, and A. P. Zeng. 2003. Alterations in the formation of lipopolysaccharide and membrane vesicles on the surface of Pseudomonas aeruginosa PAO1 under oxygen stress conditions. Microbiology 149:2789-2795. [DOI] [PubMed] [Google Scholar]
- 27.Schooling, S., and T. J. Beveridge. 2006. Membrane vesicles: an overlooked component of the matrices of biofilms. J. Bacteriol. 188:5945-5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schooling, S., A. Hubley, and T. J. Beveridge. 2009. Interactions of DNA with biofilm-derived membrane vesicles. J. Bacteriol. 191:4097-4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stewart, J. 1980. Colorimetric determination of phospholipids with ammonium ferrothiocyanate. Anal. Biochem. 104:10-14. [DOI] [PubMed] [Google Scholar]
- 30.Tashiro, Y., N. Nomura, R. Nakao, H. Senpuku, R. Kariyama, H. Kumon, S. Kosono, H. Watanabe, T. Nakajima, and H. Uchiyama. 2008. Opr86 is essential for viability and is a potential candidate for a protective antigen against biofilm formation by Pseudomonas aeruginosa. J. Bacteriol. 190:3969-3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tashiro, Y., R. Sakai, M. Toyofuku, I. Sawada, T. Nakajima-Kambe, H. Uchiyama, and N. Nomura. 2009. Outer membrane machinery and alginate synthesis regulators control membrane vesicle production in Pseudomonas aeruginosa. J. Bacteriol. 191:7509-7519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tashiro, Y., M. Toyofuku, T. Nakajima-Kambe, H. Uchiyama, and N. Nomura. 2010. Bicyclic compounds repress membrane vesicle production and Pseudomonas quinolone signal synthesis in Pseudomonas aeruginosa. FEMS Microbiol. Lett. 304:123-130. [DOI] [PubMed] [Google Scholar]
- 33.Toyofuku, M., T. Nakajima-Kambe, H. Uchiyama, and N. Nomura. 2010. The effect of a cell-to-cell communication molecule, Pseudomonas quinolone signal (PQS), produced by P. aeruginosa on other bacterial species. Microbes Environ. 25:1-7. [DOI] [PubMed] [Google Scholar]
- 34.Toyofuku, M., N. Nomura, E. Kuno, Y. Tashiro, T. Nakajima, and H. Uchiyama. 2008. Influence of the Pseudomonas quinolone signal on denitrification in Pseudomonas aeruginosa. J. Bacteriol. 190:7947-7956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Upritchard, H. G., S. J. Cordwell, and I. L. Lamont. 2008. Immunoproteomics to examine cystic fibrosis host interactions with extracellular Pseudomonas aeruginosa proteins. Infect. Immun. 76:4624-4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van der Mei, H. C., and H. J. Busscher. 2001. Electrophoretic mobility distributions of single-strain microbial populations. Appl. Environ. Microbiol. 67:491-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wagner, V. E., D. Bushnell, L. Passador, A. I. Brooks, and B. H. Iglewski. 2003. Microarray analysis of Pseudomonas aeruginosa quorum-sensing regulons: effects of growth phase and environment. J. Bacteriol. 185:2080-2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilson, W. W., M. M. Wade, S. C. Holman, and F. R. Champlin. 2001. Status of methods for assessing bacterial cell surface charge properties based on zeta potential measurements. J. Microbiol. Methods 43:153-164. [DOI] [PubMed] [Google Scholar]
- 39.Xiao, G., E. Déziel, J. He, F. Lépine, B. Lesic, M. H. Castonguay, S. Milot, A. P. Tampakaki, S. E. Stachel, and L. G. Rahme. 2006. MvfR, a key Pseudomonas aeruginosa pathogenicity LTTR-class regulatory protein, has dual ligands. Mol. Microbiol. 62:1689-1699. [DOI] [PubMed] [Google Scholar]