Abstract
Virulence gene profiles of atypical enteropathogenic Escherichia coli (aEPEC) and Shiga toxin-producing E. coli (STEC) from cattle, sheep, and humans were examined to determine the relationship between pathotypes. Shared virulence factors (intimin, EHEC hemolysin, serine protease, and a type II secretion system) were identified, suggesting a dynamic evolutionary relationship between aEPEC and STEC.
Diarrheagenic Escherichia coli represents a variety of different pathotypes with diverse virulence factors which often are conveniently used to subdivide E. coli associated with diarrheal disease (18, 22). For example, the primary virulence determinant of the enteropathogenic E. coli (EPEC) pathotype is the formation of attaching and effacing (A/E) lesions on cultured epithelial cells, a phenotype mediated by the locus for enterocyte effacement and the outer membrane protein intimin (eae) (15, 30). Similarly, the expression of Shiga toxins associated with lambdoid phage embedded within the chromosome defines the Shiga toxin-producing E. coli (STEC) pathotype (18, 22). A/E lesion formation has also been detected in certain STEC serogroups, such as O157, O26, O103, O111, and O145, which are commonly associated with more severe human disease (hemolytic-uremic syndrome [HUS] and hemorrhagic colitis [HC]) and are described as enterohemorrhagic E. coli (EHEC) (22). In addition to A/E lesion formation, many human EPEC strains possess the EPEC adherence factor (EAF) virulence plasmid containing an operon associated with the expression of bundle-forming pili (bfp) (30). Recent studies have suggested that EAF plasmid-negative EPEC may be associated with human diarrheal disease (1, 2, 16, 17, 23, 26, 31) and have also been isolated from ruminants (3, 9, 10, 14, 19, 20, 24). Consequently, these EAF plasmid-negative EPEC strains lacking the bfp locus have been described as atypical EPEC (aEPEC) strains to distinguish them from typical EAF plasmid-positive EPEC strains such as the progenitor strain O127:H6 E2348/69 (30). Loss of virulence determinants is not confined to the EPEC pathotype, however. Consecutive stool sampling of patients with HUS or HC has also revealed that certain EHEC serogroups (O157, O26, and O145) may lose stx genes during infection (4, 21). To distinguish these particular stx-negative, eae-positive serogroups associated with STEC/EHEC infection from aEPEC, it has been suggested that they be described as EHEC-LST (EHEC that lost stx) (4). It is uncertain whether ruminants are a reservoir of aEPEC which may cause human diarrheal disease or whether human aEPEC strains are distinct from ruminant aEPEC strains. Previous studies have indicated that plasmid-associated virulence factors more commonly associated with STEC, such as EHEC hemolysin (ehxA) (27), serine protease (espP) (6, 13), a catalase/peroxidise system (katP) (5), and a type II secretion pathway (etpD) (28), are occasionally associated with human aEPEC strains (1, 3, 16, 17) and that these same virulence factors may also be associated with ruminant aEPEC (8, 10-12). In addition, subtyping of ehxA and espP PCR products has indicated that subtypes of these virulence factors are shared between ruminant aEPEC and STEC (10, 12). This study, therefore, was done to investigate the virulence profiles of ruminant aEPEC and to compare them to thevirulence profiles of eae-positive STEC to provide insights into the relationship between the two pathotypes. A small number (n = 15) of STEC/aEPEC strains from humans were also included in the analyses where isolation was associated with symptoms associated with HUS/HC or diarrhea or there were no clinical details provided.
In total, the virulence profiles of 154 eae-positive (91 cattle, 48 sheep, and 15 human) strains were examined using PCR amplification alone or in combination with PCR restriction fragment length polymorphism (RFLP). PCR-RFLP analysis of eae, ehxA, and espP amplicons was performed as described previously (10-12), and amplification of stx1, stx2, etpD, and katP (catalase/peroxidase) was performed using primers and amplification as outlined in other studies (5, 8, 9). Only bacterial strains that were eae positive were included in this study. Ruminant strains were originally isolated between November 2002 and January 2003 by using rectoanal mucosal swabs and selected on sorbitol MacConkey agar supplemented with cefixime and tellurite (CT-SMAC) or tryptone X-glucuronide agar (9). The human strains were from the Enteric Reference Laboratory, National Centre for Biosecurity & Infectious Diseases, Wallaceville, New Zealand. Serological analysis was carried out at the Enteric Reference Laboratory according to standard World Health Organization methods using antisera (Statens Serum Institute, Copenhagen, Denmark) raised for all known O and H groups.
Hierarchical clustering (complete linkage) was used to group samples based on the data generated from the seven PCR(-RFLP) analyses: stx1, stx2, eae, ehxA, espP, etpD, and katP. The similarity between samples was calculated by using s(si, sj) = N(matches)/[N(matches) + w·N(mismatches)] (32), where N(matches) is the number of matches between sample i and sample j and w is a weight applied to the number of mismatches. A Dice coefficient weighting factor (w) of 0.5 was used in this study. The distance metric was therefore calculated as d = 1 − s(si, sj). For example, two samples having the same genotype would have a similarity score of 1. If sample 1 had genotyping scores of (−, −, beta, C, −, +, −) and sample 2 had scores of (−, −, beta, −, −, −, −), N(matches) = 5, N(mismatch) = 2, the similarity score would be 0.83 and the distance score 0.17. The cluster analysis was performed using R (25) with the distance matrix computed by a custom R script.
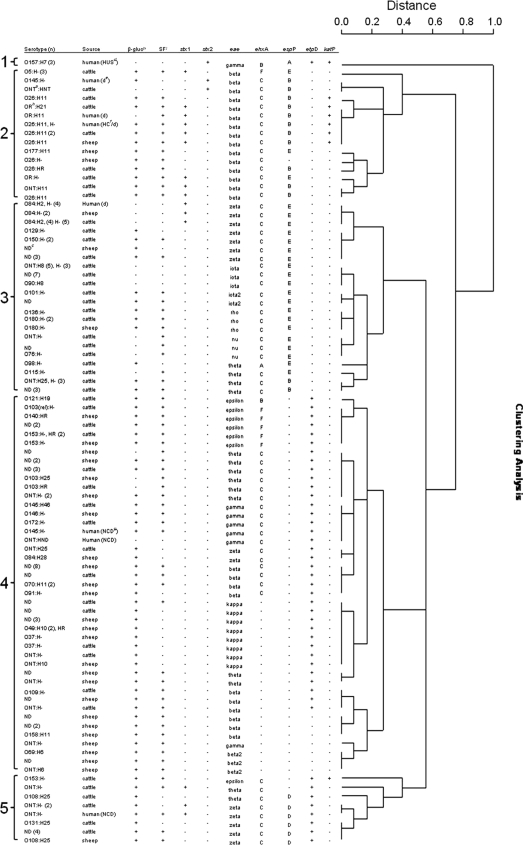
Using the data generated from the seven PCRs and differentiating by source, the E. coli strains clustered into 36 groups comprising five main clusters varying in heterogeneity (Fig. 1): 1, STEC O157; 2, eae β, ehxA, espP positive; 3, eae, ehxA, espP positive; 4, eae, ehxA, etpD positive or negative; 5, mainly (six of eight strains) eae, ehxA, espP positive, etpD positive. The espP and ehxA alleles were more common in cattle than in sheep (chi-square test, P < 0.001), and the etpD allele was more common in sheep than in cattle (P < 0.001). The katP allele was associated more commonly with STEC isolates than with aEPEC isolates (P < 0.001), whether the human isolates were included or not.
FIG. 1.
Hierarchical clustering (complete linkage) analysis of eae-positive E. coli isolates from cattle, sheep, and humans based on the data generated from seven PCR(-RFLP) analyses: stx1, stx2, eae, ehxA, espP, etpD, and katP. Superscripts: a, not typable; b, O rough (autoagglutination); c, not done; d, HUS; e, diarrhea; f, HC; g, no clinical details; h, β-glucuronidase activity; i, sorbitol fermentation.
Overall, there was significant heterogeneity within the beta eae-positive STEC/aEPEC isolates (from humans, cattle, and sheep) with 12 groups and less variation with zeta isolates (from humans, cattle, and sheep) and theta isolates (from cattle and sheep) eae-positive STEC/aEPEC (5 and 7 groups, respectively, of each eae type). However, there was also heterogeneity within clusters with respect to the β-glucuronidase and non-sorbitol fermentation characteristics. Intimin subtype epsilon-, iota-, and kappa-positive aEPEC isolates were more homogeneous. Eight of 10 epsilon intimin-positive aEPEC isolates (ehxA subtype F) from cattle and sheep were clustered together with only single strains of ehxA subtypes B and C from cattle clustering separately. Iota-positive aEPEC isolates (n = 16) were associated with cattle exclusively and formed a single cluster. Kappa intimin-positive aEPEC isolates (n = 12) from cattle and sheep formed a single cluster, with only a single strain from cattle displaying a differing fermentation characteristic on CT-SMAC. Interestingly, multilocus sequence typing (MLST) of seven housekeeping genes from human aEPEC strains previously demonstrated the clustering of iota and kappa intimin types into specific clades where the majority of the strains were of the H8 (iota) or H10 (kappa) flagellum subtype, respectively (29). However, zeta intimin-positive and some theta intimin-positive aEPEC isolates could not be readily clustered using the MLST methodology (29). Furthermore, aEPEC serotypes ONT:H8 (intimin iota positive) and O49:H10 (intimin kappa positive) have been previously associated with human diarrheal disease (23, 29).
Six zeta intimin-positive aEPEC isolates, including an O131:H25 strain from cattle and a single O108:H25 isolates from sheep, had virulence profiles similar to those of two ONT:H− zeta intimin-positive STEC isolates, except for the presence of the stx1 gene in the STEC strains. Similarly, seven zeta intimin-positive aEPEC strains (six from cattle, one from a sheep), including two O150:H− strains and a single O129:H− strain, had virulence profiles similar to those of the 16 zeta intimin-positive O84:H2/H− STEC strains, except for the presence of the stx1 gene in the STEC strains. Generally, similarities between the virulence profiles of STEC and aEPEC isolates from sheep were less evident than those of isolates from cattle. Only a single O26:H11 aEPEC strain may have been an EHEC-LST strain, as other stx-positive O26:H11 strains with the same virulence profile were identified.
aEPEC strains are rarely isolated from human diarrheal disease in New Zealand, and only 2 of the 15 human strains in this study were stx negative (Fig. 1). More than 95% of the STEC isolates from human clinical cases in New Zealand are of serotype O157:H7. Non-O157 STEC strains (including eae-negative STEC strains) are isolated less frequently, and there are no uniform methods available for provincial health laboratories to screen for and identify aEPEC from stool specimens that are negative for STEC. Therefore, it is likely that non-O157 STEC strains may be somewhat underreported and aEPEC strains significantly overlooked, as they may be associated with nonbloody diarrheal disease or asymptomatic carriage. Despite the limited number of human isolates, the virulence profiles (ehxA subtype C and etpD positive) of the two human aEPEC strains (O145:H− and ONT:HND) that were intimin gamma positive matched those of two cattle strains (O172:H− and O145:H46) (Fig. 1) and a single sheep strain (O146:H−) (Fig. 1). As expected, the three STEC O157 strains clustered separately with their contrasting virulence profile containing O157:H7-specific ehxA and espP subtypes (Fig. 1). In addition, this work suggests that transmission of serogroups O26 and O84 between ruminants and humans may occur.
These data indicate that, except for katP, ruminant aEPEC may harbor the same plasmid-encoded virulence factors as STEC and this may be host associated. STEC plasmid-associated virulence factors that may be acquired by horizontal gene transfer, such as EHEC hemolysin, serine protease, and a type II secretion system (7), are commonly found in ruminant aEPEC and the prevalence of these virulence factors in ruminant aEPEC is greater (ehxA, 57.8%; espP, 45.6%; etpD, 53.5%) than their reported presence in human aEPEC strains (≤36%) (1, 4, 16, 17). Bacterial isolates that are intimin types beta (human, cattle, and sheep isolates), zeta (human, cattle, and sheep isolates), and theta (cattle and sheep isolates) form heterogeneous groups which contain both STEC and aEPEC, indicating that horizontal gene transfer of stx-containing prophage may occur within these groups. To our knowledge, intimin types iota and kappa are not readily associated with STEC (10, 19, 20, 24, 33) and appear from this study to be more homogeneous and clonal and could therefore be more refractory to the insertion of stx-containing prophage. However, intimin types iota and kappa have been associated with human diarrheal disease (23, 26, 29) and certain serotypes such as O49:H10 and ONT:H8 that have been found in both ruminants and humans may be genuine zoonotic pathogens. We believe that the aEPEC pathotype is a heterogeneous group of bacteria that may encompass several different groups with various host specificities: (i) EHEC-LST, (ii) classical aEPEC strains that have lost the EAF plasmid, (iii) human aEPEC strains associated with diarrheal disease that contain STEC virulence factors, and (iv) commensal aEPEC strains that reside in cattle and sheep and contain STEC virulence factors. Further studies are required to elucidate any zoonotic relationship between humans and ruminant aEPEC and to characterize the integrity or availability of stx-containing phage insertion sites within STEC and aEPEC to determine whether a dynamic relationship between aEPEC and STEC occurs within the ruminant and/or human gastrointestinal tract with cycles of horizontal gene transfer and acquisition/loss of stx-containing phage by A/E lesion-producing bacteria.
Acknowledgments
This work was supported by AgResearch repositioning funds.
Footnotes
Published ahead of print on 16 April 2010.
REFERENCES
- 1.Afset, J. E., G. Bruant, R. Brousseau, J. Harel, E. Anderssen, L. Bevanger, and K. Bergh. 2006. Identification of virulence genes linked with diarrhea due to atypical enteropathogenic Escherichia coli by DNA microarray analysis and PCR. J. Clin. Microbiol. 44:3703-3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Afset, J. E., E. Anderssen, G. Bruant, J. Harel, L. Wieler, and K. Bergh. 2008. Phylogenetic background and virulence profile of atypical enteropathogenic Escherichia coli from a case control study using multilocus sequence typing and DNA microarray. J. Clin. Microbiol. 46:2280-2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aktan, I., B. Carter, H. Wilking, R. M. La Ragione, L. Wieler, M. J. Woodward, and M. F. Anjum. 2007. Influence of geographical origin, host animal and stx gene on the virulence characteristics of Escherichia coli O26 strains. J. Med. Microbiol. 56:1431-1439. [DOI] [PubMed] [Google Scholar]
- 4.Bielaszewska, M., B. Middendorf, R. Köck, A. W. Friedrich, A. Fruth, H. Karch, M. A. Schmidt, and A. Mellmann. 2008. Shiga toxin-negative attaching and effacing Escherichia coli: distinct clinical associations with bacterial phylogeny and virulence traits and inferred in-host pathogen evolution. Clin. Infect. Dis. 47:208-217. [DOI] [PubMed] [Google Scholar]
- 5.Brunder, W., H. Schmidt, and H. Karch. 1996. KatP, a novel catalase peroxidase encoded by the large plasmid of enterohemorrhagic Escherichia coli O157:H7. Microbiology 142:3305-3315. [DOI] [PubMed] [Google Scholar]
- 6.Brunder, W., H. Schmidt, and H. Karch. 1997. EspP, a novel extracellular serine protease of enterohemorrhagic Escherichia coli O157:H7 cleaves human coagulation factor V. Mol. Microbiol. 24:767-778. [DOI] [PubMed] [Google Scholar]
- 7.Brunder, B. W., H. Schmidt, M. Frosch, and H. Karch. 1999. The large plasmids of Shiga-toxin-producing Escherichia coli (STEC) are highly variable genetic elements. Microbiology 145:1005-1014. [DOI] [PubMed] [Google Scholar]
- 8.Cookson, A. L., C. M. Hayes, G. R. Pearson, J. M. Roe, A. D. Wales, and M. J. Woodward. 2002. Isolation from sheep of an attaching and effacing Escherichia coli O115:H− with a novel combination of virulence factors. J. Med. Microbiol. 51:1041-1049. [DOI] [PubMed] [Google Scholar]
- 9.Cookson, A. L., S. C. S. Taylor, and G. T. Attwood. 2006. The prevalence of Shiga toxin-producing Escherichia coli in cattle and sheep in the lower North Island, New Zealand. N. Z. Vet. J. 54:28-33. [DOI] [PubMed] [Google Scholar]
- 10.Cookson, A. L., J. Bennett, C. Nicol, F. Thomson-Carter, and G. T. Attwood. 2009. Molecular subtyping and distribution of the serine protease from Shiga toxin-producing Escherichia coli among atypical enteropathogenic E. coli strains. Appl. Environ. Microbiol. 75:2246-2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cookson, A. L., J. Bennett, F. Thomson-Carter, and G. T. Attwood. 2007. Intimin subtyping of Escherichia coli: concomitant carriage of multiple intimin subtypes from forage-fed cattle and sheep. FEMS Microbiol. Lett. 272:163-171. [DOI] [PubMed] [Google Scholar]
- 12.Cookson, A. L., J. Bennett, F. Thomson-Carter, and G. T. Attwood. 2007. Molecular subtyping and genetic analysis of the enterohemolysin gene (ehxA) from Shiga toxin-producing Escherichia coli and atypical enteropathogenic E. coli. Appl. Environ. Microbiol. 73:6360-6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Djafari, S., F. Ebel, C. Deibel, S. Krämer, M. Hudel, and T. Chakraborty. 1997. Characterization of an exported protease from Shiga toxin-producing Escherichia coli. Mol. Microbiol. 25:771-784. [DOI] [PubMed] [Google Scholar]
- 14.Ewers, C., C. Schüffner, R. Weiss, G. Baljer, and L. H. Wieler. 2004. Molecular characteristics of Escherichia coli serogroup O78 strains isolated from diarrheal cases in bovines urge further investigations on their zoonotic potential. Mol. Nutr. Food Res. 48:504-514. [DOI] [PubMed] [Google Scholar]
- 15.Garmendia, J., G. Frankel, and V. F. Crepin. 2005. Enteropathogenic and enterohemorrhagic Escherichia coli infections: translocation, translocation, translocation. Infect. Immun. 73:2573-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hernandes, R. T., W. P. Elias, M. A. M. Vieira, and T. A. T. Gomes. 2009. An overview of atypical enteropathogenic Escherichia coli. FEMS Microbiol. Lett. 297:137-149. [DOI] [PubMed] [Google Scholar]
- 17.Jensen, C., S. Ethelberg, B. Olesen, P. Scheillerup, K. E. P. Olsen, F. Scheutz, E. M. Nielsen, J. Neimann, B. Høgh, P. Gerner-Smidt, K. Mølbak, and K. A. Krogfelt. 2007. Attaching and effacing Escherichia coli isolates from Danish children: clinical significance and microbiological characteristics. Eur. Soc. Clin. Microbiol. Infect. Dis. 13:863-872. [DOI] [PubMed] [Google Scholar]
- 18.Kaper, J. B., J. P. Nataro, and H. L. T. Mobley. 2004. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2:123-140. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi, H., A. Miura, H. Hayashi, T. Ogawa, T. Endô, E. Hata, M. Eguchi, and K. Yamamoto. 2003. Prevalence and characteristics of eae-positive Escherichia coli from healthy cattle in Japan. Appl. Environ. Microbiol. 69:5690-5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krause, G., S. Zimmermann, and L. Beutin. 2005. Investigation of domestic animals and pets as a reservoir for intimin (eae) gene positive Escherichia coli types. Vet. Microbiol. 106:87-95. [DOI] [PubMed] [Google Scholar]
- 21.Mellmann, A., M. Bielaszewska, L. B. Zimmerhackl, R. Prager, D. Harmsen, H. Tschäpe, and H. Karch. 2005. Enterohemorrhagic Escherichia coli in human infection: in vivo evolution of a bacterial pathogen. Clin. Infect. Dis. 41:785-792. [DOI] [PubMed] [Google Scholar]
- 22.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nguyen, R. N., L. S. Taylor, M. Tauschek, and R. M. Robins-Browne. 2006. Atypical enteropathogenic Escherichia coli infection and prolonged diarrhea in children. Emerg. Infect. Dis. 12:597-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramachandran, V., K. Brett, M. A. Hornitzky, M. Dowton, K. A. Bettelheim, M. J. Walker, and S. P. Djordjevic. 2003. Distribution of intimin subtypes among Escherichia coli isolates from ruminant and human sources. J. Clin. Microbiol. 41:5022-5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.R Foundation for Statistical Computing. 2009. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
- 26.Robins-Browne, R. M., A. M. Bordun, M. Tauschek, V. R. Bennett-Wood, J. Russell, F. Oppedisano, N. A. Lister, K. A. Bettelheim, C. K. Fairley, M. I. Sinclair, and M. E. Hellard. 2004. Escherichia coli and community-acquired gastroenteritis, Melbourne, Australia. Emerg. Infect. Dis. 10:1797-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmidt, H., C. Kernbach, and H. Karch. 1996. Analysis of the EHEC hly operon and its location in the physical map of the large plasmid of enterohaemorrhagic Escherichia coli O157:H7. Microbiology 142:907-914. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt, H., B. Henkel, and H. Karch. 1997. A gene cluster closely related to type II secretion pathway operons of gram-negative bacteria is located on the large plasmid of enterohemorrhagic Escherichia coli O157 strains. FEMS Microbiol. Lett. 148:265-272. [DOI] [PubMed] [Google Scholar]
- 29.Tennant, S. M., M. Tauschek, K. Azzopardi, A. Bigham, V. Bennett-Wood, E. L. Hartland, W. Qi, T. S. Whittam, and R. M. Robins-Browne. 2009. Characterisation of atypical enteropathogenic E. coli strains of clinical origin. BMC Microbiol. 9:117-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trabulsi, L. R., R. Keller, and T. A. Tardelli Gomes. 2002. Typical and atypical enteropathogenic Escherichia coli. Emerg. Infect. Dis. 8:508-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vieira, M. A., J. R. C. Andrade, L. R. Trabulsi, A. C. Rosa, A. M. Dias, S. R. Ramos, G. Frankel, and T. A. Gomes. 2001. Phenotypic and genotypic characteristics of Escherichia coli strains of non-enteropathogenic E. coli (EPEC) serogroups that carry EAE and lack the EPEC adherence factor and Shiga toxin DNA probe sequences. J. Infect. Dis. 183:762-772. [DOI] [PubMed] [Google Scholar]
- 32.Xu, R., and D. C. Wunsch II. 2009. Proximity measures, p. 15-30. In R. Xu and D. C. Wunsch II (ed.), Clustering. Wiley-Interscience, Hoboken, NJ.
- 33.Zhang, W. L., B. Köhler, E. Oswald, L. Beutin, H. Karch, S. Morabito, A. Caprioli, S. Suerbaum, and H. Schmidt. 2002. Genetic diversity of intimin genes of attaching and effacing Escherichia coli strains. J. Clin. Microbiol. 40:4486-4492. [DOI] [PMC free article] [PubMed] [Google Scholar]