Abstract
Metabolic engineering of cyanobacteria has the advantage that sunlight and CO2 are the sole source of energy and carbon for these organisms. However, as photoautotrophs, cyanobacteria generally lack transporters to move hydrophilic primary metabolites across membranes. To address whether cyanobacteria could be engineered to produce and secrete organic primary metabolites, Synechococcus elongatus PCC7942 was engineered to express genes encoding an invertase and a glucose facilitator, which mediated secretion of glucose and fructose. Similarly, expression of lactate dehydrogenase- and lactate transporter-encoding genes allowed lactate accumulation in the extracellular medium. Expression of the relevant transporter was essential for secretion. Production of these molecules was further improved by expression of additional heterologous enzymes. Sugars secreted by the engineered cyanobacteria could be used to support Escherichia coli growth in the absence of additional nutrient sources. These results indicate that cyanobacteria can be engineered to produce and secrete high-value hydrophilic products.
Metabolic engineering of photosynthetic microbes is attractive because of the efficient use of light energy by these organisms and the potential for CO2 mitigation during production (21). Conventional terrestrial plants capture solar energy at low efficiencies (about 0.1 to 0.25% for corn and up to 1% for switchgrass), while fast-growing prokaryotic and eukaryotic microalgal species are about 1 order of magnitude more productive and their photosynthetic efficiencies can be >10% (12, 13). Genetic tools for engineering cyanobacterial species, including Synechococcus elongatus PCC7942 (Synechococcus), can be applied to metabolic engineering (7). For example, Deng and Coleman (8) expressed pyruvate decarboxylase and alcohol dehydrogenase in cyanobacteria to produce small amounts of ethanol, and Atsumi et al. recently described efficient synthesis of isobutanol using a four-step pathway established in Escherichia coli (2).
Much attention has been focused on metabolic engineering to produce fuels. However, fuel molecules are generally toxic to microbes even at moderate concentrations. In addition, on a per-photon basis, the actual market value of fuels is at best comparable to, and generally lower than, the market value of other commodity organic compounds, such as sugars, lactic acid, and amino acids. Engineering cyanobacteria to produce and secrete hydrophilic or charged molecules would thus be economically desirable.
Commonly used metabolic engineering organisms, such as E. coli and yeast (e.g., Saccharomyces cerevisiae), express a variety of transport systems for exporting waste products as well as importing nutrients. As photoautotrophs, cyanobacteria lack many of the transporters found in these organisms. In addition, while most microbes store energy by pumping protons across the plasma membrane, cyanobacteria store energy by transporting protons across the thylakoid membrane. In fact, cyanobacteria tend to alkalinize their growth medium in both laboratory and natural conditions (4), and thus how effective heterologous transporters can be in metabolic engineering of cyanobacteria is an open question. Here, we investigated whether heterologous transporters belonging to the major facilitator superfamily, in combination with relevant enzymes, could be introduced into cyanobacteria for production and secretion of useful products.
MATERIALS AND METHODS
Plasmid and strain construction.
Foreign genes were introduced into the Synechococcus genome by homologous recombination using neutral sites (7). Neutral site 1 (NS1) and NS2 are present in plasmids DS1321 and DS21, which confer spectinomycin and kanamycin resistance, respectively, and contain E. coli lacI and an isopropyl-β-d-thiogalactopyranoside (IPTG)-regulated trp-lac strong promoter (D. F. Savage, B. Afonso, and P. A. Silver, unpublished results). The invA and glf genes from Zymomonas mobilis were codon optimized for expression in Synechococcus and were synthesized so that they contained a C-terminal His6 tag, and ldhA and lldP were obtained by PCR amplification from E. coli DH5α using oligonucleotides that resulted in molecules with an N-terminal His6 tag. These genes were inserted into DS21 and DS1321 by using standard procedures. Transformation of Synechococcus was performed as described previously (7). Integration of vectors into neutral sites was verified by PCR to demonstrate the presence of appropriate novel chromosome-transgene junctions and the absence of uninserted sites.
Neutral site 3 (NS3) targeting vector.
A novel neutral site 3 plasmid was constructed to allow insertion of a transgene into Synechococcus independent of the vectors described above (see Fig. S2 in the supplemental material). This vector expresses E. coli lacI, confers chloramphenicol resistance, mediates integration into the remnant of a cryptic prophage in the Synechococcus genome, and contains a lac operon promoter followed by a multiple-cloning site. The galU and udhA genes were inserted into this vector.
Synechococcus culture conditions.
Synechococcus strains were cultured in BG-11 medium (1) at 30°C. Unless indicated otherwise, cultures were grown with a light cycle consisting of 18 h of light and 6 h of darkness. To select for homologous recombination and integration of heterologous DNA into the genome and for culture maintenance of engineered strains, kanamycin (25 μg/ml or 2.5 μg/ml if in combination with other drugs), spectinomycin (25 μg/ml or 2 μg/ml if in combination with other drugs), and/or chloramphenicol (12.5 μg/ml or 2.5 μg/ml if in combination with other drugs) was added to the medium. For all experiments, Synechococcus cultures were tested for possible contamination by spotting cultures onto LB medium plates; only data obtained with uncontaminated cultures are reported here.
Sugar and lactic acid production and assay.
For sugar and lactate production exponentially growing cultures at an optical density at 750 nm (OD750) of 0.05 were used unless indicated otherwise. Sugar production was induced with 100 to 300 mM NaCl (see Table S1 in the supplemental material) and 100 μM IPTG. Growth was monitored by measuring the OD750. Sugar production was determined using a sucrose-d-fructose-d-glucose assay kit (catalogue number K-SUFRG; Megazyme, Ltd., Bray, Ireland). Assays were performed using culture supernatants prepared by centrifuging samples for 5 min at 21,130 × g. To determine combined intracellular and extracellular sucrose concentrations, the samples were sonicated prior to removal of cell debris by centrifugation. Lactic acid assays were performed with an ld-lactic acid determination kit from R-Biopharm (catalogue number 11112821035) adapted for a 96-well plate reader (Victor 3V; Perkin-Elmer) by using 1/10 of the amounts of the reagents recommended by the manufacturer in each assay mixture. Assays were performed in triplicate, and standard deviations were determined.
Synechococcus-E. coli coculture.
To test whether the sugar-secreting Synechococcus strain could support growth of E. coli, cultures of the Synechococcus glf invA strain and a non-sugar-secreting control strain were grown to an OD750 of 0.1. Sugar secretion was induced with 200 mM NaCl and 100 μM IPTG. E. coli cells were washed in phosphate-buffered saline (PBS) three times and incubated with shaking in PBS containing 100 μM IPTG and antibiotics for 1 h at 37°C, after which 106 cells/ml were added. The medium used was BG-11 medium with 100 μM IPTG, 2.5 μg/ml kanamycin, 2 μg/ml spectinomycin, and 1 mg/ml NH4Cl. Growth of E. coli was monitored by plating serial dilutions on LB agar and measuring the yellow fluorescent protein (YFP) fluorescence of culture samples with a Victor 3V plate reader. For microscopic quantitation of Synechococcus and E. coli, bacteria in samples of the liquid coculture were visualized by red chlorophyll autofluorescence and YFP fluorescence, respectively. To observe microcolony formation, 100 μl of the initial coculture was plated on BG-11 agar containing the compounds listed above. After 4 days pieces of agar were cut out and placed onto a MatTek glass bottom dish for microscopy.
Invertase activity assay.
Crude cell extracts of Synechococcus strains expressing invA and glf were prepared from a culture that had been induced with 100 μM IPTG for 3 days. Cell pellets were resuspended in invertase assay buffer and disrupted by sonication on ice for 2.5 s followed by a 5-s break, using a total sonication time of 5 min. Cell debris was removed by centrifugation. Invertase activity was measured as described previously (22) using an assay mixture containing crude cell extracts in 100 mM sodium acetate buffer (pH 5) at 30°C. The reaction was started by addition of 150 mM sucrose. After 10 min of incubation, the reaction was stopped by incubation at 100°C for 2 min. The heat-denatured proteins were pelleted, and the glucose concentration in the supernatant was determined using a sucrose-glucose-fructose assay kit (Megazyme). To determine the invertase specific activity in the crude cell extracts, the protein concentration was measured by performing a Bradford protein assay (Bio-Rad).
Nucleotide sequence accession numbers.
The sequences of the integrated transgenes have been deposited in the GenBank database under accession numbers HM026754 (ldhA in NS2), HM026755 (lldP in NS1), HM026756 (glf in NS1), HM026757 (invA in NS2), and HM026758 (novel transgene in NS3).
RESULTS AND DISCUSSION
Engineering strategy.
To engineer Synechococcus to produce the hydrophilic compounds glucose or fructose and lactic acid, we expressed enzymes that generated these molecules intracellularly and also relevant transporters that allowed export (Fig. 1; see Materials and Methods and Fig. S1 in the supplemental material). For glucose or fructose transport we used the GLF protein from Zymomonas mobilis (3), and for lactate transport we used the LldP protein from E. coli (17). Both proteins are in the major facilitator superfamily whose members have 12 transmembrane alpha-helical segments and generally lack cleaved signal sequences (9, 20). The GLF protein facilitates diffusion of glucose and fructose in either direction across the membrane, and the transported sugar is not phosphorylated (14). The LldP protein cotransports lactate with a proton (17); during Synechococcus growth in BG-11 medium, when the culture reaches an OD750 of 1, the pH of the medium is generally about 9, so lactate should be exported from the cell if it is produced intracellularly.
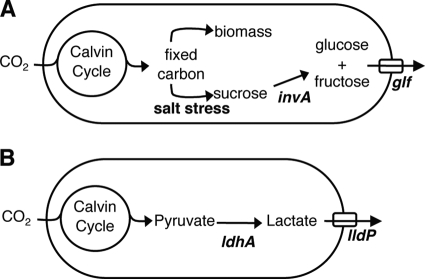
FIG. 1.
Engineering schemes for cyanobacterial production of hexose sugars and lactic acid. In both schemes, carbon is fixed by natural pathways, yielding precursors. (A) Sugar production scheme. Synechococcus naturally produces sucrose in response to salt stress. The bacteria are engineered to express invertase (encoded by invA), which cleaves sucrose into glucose and fructose, and the glf gene encoding a glucose- and fructose-facilitated diffusion transporter, which allows export of the sugars from the cell. (B) Lactic acid production scheme. Synechococcus naturally produces pyruvate as a metabolic intermediate. The bacteria are engineered to express lactate dehydrogenase (encoded by ldhA), which produces lactic acid, and a transporter encoded by lldP, which cotransports lactate and H+ from the cell.
To engineer intracellular production of glucose and fructose, we used the fact that the freshwater cyanobacteria accumulate intracellular sucrose to balance the osmolarity when they are grown in salt water (5, 16). We introduced the Z. mobilis invA gene encoding a soluble, cytoplasmic invertase (22) to cleave NaCl-induced sucrose. To produce lactate, we introduced the E. coli ldhA gene encoding lactate dehydrogenase, which catalyzes the reduction of pyruvate to lactate (18).
Heterologous genes were integrated into the Synechococcus genome by homologous recombination using vectors with so-called “neutral sites” (NS) that can tolerate insertion with no phenotypic effects (7) (see Materials and Methods and Fig. S2 in the supplemental material). All proteins were expressed as His-tagged proteins from an E. coli trp/lac promoter, and the E. coli lacI gene was also expressed in Synechococcus from its own promoter. To express additional genes for pathway optimization, we constructed a new Synechococcus integrating vector that uses chloramphenicol resistance and integrates into a distinct genomic site such that the vector can be used independent of the previously described vectors (see Materials and Methods and Fig. S2 in the supplemental material).
Activity of transgenes in vivo.
The expression and functions of heterologous genes were determined by performing direct assays, as well as by examining secretion of molecules of interest in engineered Synechococcus strains. The functionality of the cloned Z. mobilis invA and glf genes in E. coli was shown as follows: the glf expression plasmid rescued growth of an E. coli ptsI mutant (CHE30) (18) on glucose, and E. coli DH5α containing the invA expression plasmid was able to use sucrose as a carbon source (data not shown). Synechococcus was transformed with combinations of the enzyme and transporter expression plasmids and the corresponding empty vectors. Expression of InvA-His6 and LdhA-His6 in Synechococcus was observed by Western blotting, while GLF-His6 and LldP-His6 were not detected (see Fig. S3A and B in the supplemental material), perhaps due to low levels of expression combined with poor extraction from membranes. The invertase activity in Synechococcus extracts was increased about 18-fold by expression of invA (see Fig. S3C in the supplemental material). The functionality of glf in Synechococcus was shown by adding 500 μM glucose or fructose to the culture medium, which allowed slow, heterotrophic growth of the cyanobacteria in the dark, while the sugar disappeared from the medium (see Fig. S3D in the supplemental material). Expression of ldhA in Synechococcus resulted in increased levels of intracellular lactate (data not shown).
Secretion of sugars and lactic acid from engineered cyanobacteria.
Coexpression of catabolic enzymes and transporters led to secretion of hexose sugars and lactic acid. When Synechococcus containing the invA and glf transgenes was treated with 200 mM NaCl to induce sucrose synthesis and with 100 μM IPTG to induce invA and glf expression, glucose and fructose were secreted, and the maximum total concentration was about 200 μM (Fig. 2A and B). Both invA expression and glf expression were required for this effect. The glucose levels in the medium were significantly lower than the fructose levels, and the fructose levels declined as the cell density increased (Fig. 2A and B); these observations suggest that glucose and fructose were metabolized by the engineered cyanobacteria. Extracellular sucrose was not observed in strains expressing invA, and there were not significant differences between the wild-type and glf-expressing Synechococcus strains. The exponential growth rates for Synechococcus expressing neither transgene, invA, glf, and invA plus glf were 0.80 ± 0.002, 0.61 ± 0.01, 0.74 ± 0.01, and 0.70 ± 0.04 per day, respectively (Fig. 2D), suggesting that under the conditions employed, sugar production did not lead to a major diversion of the carbon flux.
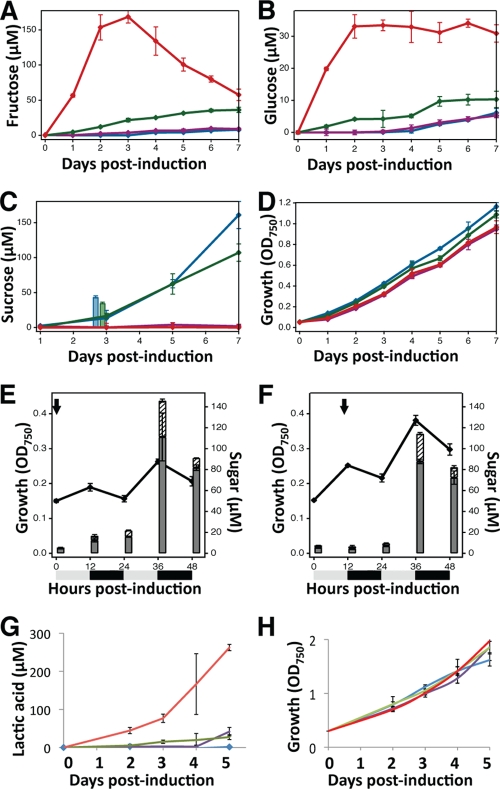
FIG. 2.
Sugar and lactate production and growth of Synechococcus strains expressing heterologous enzymes and transporters. (A to D) Production of sugars and growth of strains expressing Z. mobilis glf and invA (red), only glf (green), only invA (purple), or neither transgene (blue). The x axis shows the time after induction of sucrose synthesis with 200 mM NaCl and of transgenes with 100 μM IPTG. (A) Extracellular concentration of fructose. (B) Extracellular concentration of glucose. (C) Extracellular concentration of sucrose. The bars in panel C indicate the intracellular concentration of sucrose plus the extracellular concentration of sucrose; less than 3 μM sucrose was detected in the invA-expressing strains. (D) Growth after induction of transgenes with NaCl and IPTG. (E and F) Growth and sugar production or consumption as a function of a cycle consisting of 12-h days and 12-h nights. The lines indicate OD750, and the bars indicate the concentrations of extracellular fructose (gray bars) and glucose (striped bars). The arrows indicate the induction with NaCl and IPTG at dawn (E) and at dusk (F). (G and H) Concentration of secreted lactic acid (G) and growth (H) of Synechococcus expressing E. coli ldhA and lldA (red), only lldA (green), only ldhA (purple), or neither transgene (blue). The error bars indicate standard deviations.
Cyanobacterial cultures maintained with alternating dark and light periods accumulate polysaccharides when they are exposed to light and mobilize this intracellular reserve material in the dark (19). To address whether induced sugar synthesis occurs during the day or whether stored energy reserves are used during the night, we monitored the growth and fructose and glucose sugar secretion of Synechococcus expressing glf and invA as a function of the day-night cycle. Increases in the cell mass and net hexose sugar secretion occurred only in the presence of light; both parameters decreased in the dark (Fig. 2E and F). This observation suggests that sucrose is synthesized only during the day and illustrates how engineered secretion systems might be used to noninvasively assay metabolic states.
Synechococcus cells engineered to express lactate dehydrogenase and/or a lactate transporter behaved analogously to produce lactate (Fig. 2G and H). Cells expressing the E. coli ldhA and lldP genes from IPTG-inducible promoters secreted relatively high levels of lactate into the medium, while cells expressing only ldhA or lldP produced about 4-fold-lower levels. The rate of accumulation of extracellular lactate was much lower than the rate of accumulation of hexose sugars, but it increased steadily for at least 9 days. These observations indicate that the net intracellular rate of lactate production is lower than the net intracellular rate of glucose and fructose production but that after secretion lactate cannot be taken up and metabolized.
Further engineering to enhance metabolite production.
To demonstrate that secretion of products of interest from Synechococcus could be further enhanced by increasing the levels of intracellular precursors, we expressed heterologous enzymes to improve the production of intracellular sucrose and lactate. Sucrose is normally synthesized from fructose-1-phosphate and UDP-glucose, which are condensed to form sucrose phosphate that is then dephosphorylated to generate sucrose (Fig. 3A). UDP-glucose is synthesized by UDP-glucose phosphorylase, encoded by the E. coli galU gene (15). Integration of the galU gene expressed from an IPTG-regulated promoter at a distinct Synechococcus neutral site (see Materials and Methods and Fig. S1 and S2 in the supplemental material) further increased hexose sugar production by more than 30% in cells expressing invA and glf (Fig. 3B).
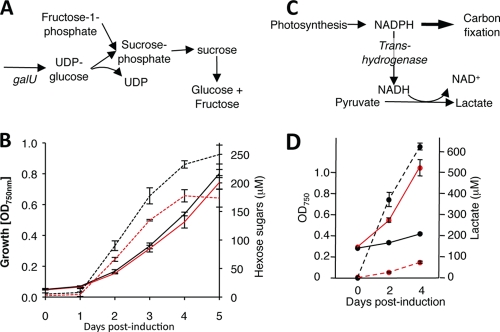
FIG. 3.
Enhancement of sugar and lactate production by rational metabolic engineering. (A) UDP-glucose and fructose-1-phosphate are the precursors of glucose and fructose in the artificial pathway. UDP-glucose is produced by UDP-glucose phosphorylase, which is encoded by the galU gene. (B) Total glucose concentration plus total fructose concentration in culture supernatants of Synechococcus expressing glf plus invA, either with a galU expression construct (black) or without galU (red). Dashed lines, glucose concentration plus fructose concentration; solid lines, bacterial density. (C) NADPH is the major carrier of reducing equivalents in photosynthetic cells. Exchange with NAD+ is catalyzed by NADP/NAD transhydrogenase, yielding NADH, the reducing agent substrate for lactate dehydrogenase. (D) Lactate concentrations in culture supernatants of induced Synechococcus with ldhA plus lldP with (black) or without (red) a udhA expression construct. Dashed lines, lactate concentration; solid lines, bacterial density. The error bars indicate standard deviations.
Photosynthetic cells produce NADPH as the major carrier of reducing equivalents, but lactate dehydrogenase uses NADH as its reducing substrate (Fig. 3C). Expression of the E. coli udhA gene, which encodes a soluble NADPH/NADH transhydrogenase (6), in Synechococcus also expressing ldhA and lldP markedly enhanced lactate secretion (Fig. 3D). Expression of the transhydrogenase also reduced the growth rate of Synechococcus. This could have been directly due to a flux of energy and carbon into lactate, to alterations in the intracellular pH, or to lower levels of NADPH impacting the regulatory protein OpcA and activating the oxidative pentose phosphate pathway (11).
Cyanobacterial sugar production supports E. coli growth.
The cost of the carbon source in commercial fermentation is significant and can be as much as 30 to 50% of the overall operating cost (10). When cocultured with sugar-secreting Synechococcus, E. coli was able to grow without addition of an external carbon source in liquid culture or on solid medium (Fig. 4). In principle, coculture of sugar-secreting cyanobacteria and a second engineered microbe could allow production of a desired product without a reduced-carbon feedstock in situations where synthesis of the product is incompatible with cyanobacterial metabolism.
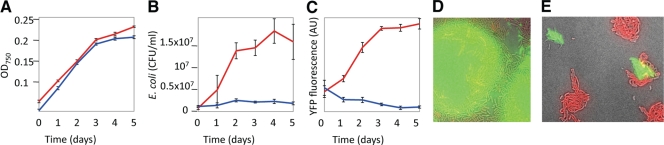
FIG. 4.
Sugar-secreting Synechococcus supports E. coli growth in coculture. (A to C) E. coli DH5α containing a YFP expression plasmid was diluted to obtain a concentration of 106 cells/ml in wild-type Synechococcus cultures (blue) or Synechococcus cultures expressing glf and invA (red) in BG-11 medium with 200 mM NaCl, 1 mg/ml NH4Cl, and appropriate antibiotics. (A) Growth of Synechococcus in the presence of E. coli. (B and C) Growth of E. coli, as determined by the number of CFU (B) or YFP fluorescence (C). The error bars indicate standard deviations. (D and E) Coculture of sugar-secreting Synechococcus and E. coli on agar plates. E. coli YFP fluorescence is yellow-green; Synechococcus chlorophyll autofluorescence is red. Microcolonies formed on BG-11 agar with 100 mM NaCl (D) and 200 mM NaCl (E) 4 days after plating.
Comparison with cyanobacterial production of fuel molecules.
Previous efforts to metabolically engineer cyanobacteria have focused on ethanol, isobutanol, and isobutyraldehyde, which are relatively lipophilic molecules that can directly cross cell membranes. Experiments described here demonstrated that cyanobacteria can be engineered to produce a much wider variety of compounds, using transporters to export the molecule of choice. Because cyanobacteria start with light and CO2 as feedstocks, the cost of production of any carbon-based molecule is a function of the number of photons needed to drive the synthesis of the molecule and the efficiency of the engineered pathway. For example, at present, the cost of lactic acid is higher than the cost of sugar because sugar is used as a feedstock to produce lactic acid. In principle, production by cyanobacteria could abolish this distinction, and in the experiments described here, the rates of production of sugars and lactic acid were comparable. Moreover, the initial rate of production of lactate in the strain expressing lactate dehydrogenase, the lactate transporter, and NADP/NAD transhydrogenase was about 54 mg/liter/day/OD750 unit (Fig. 3D), which is comparable to initial rate of production of isobutyraldehyde measured by Atsumi et al. (2), although many details of the production system were different.
Because of the urgent need to replace fossil fuels with carbon-neutral fuels, much discussion has focused on algae as a source of biofuels (12). However, as commodity chemicals, fossil fuels are still quite inexpensive. Production of fuel-type molecules from engineered cyanobacteria will require large-scale photobioreactors that operate very efficiently. Since such photobioreactors are still in the design phase, the initial product yields may not be high enough to economically justify fuel production but could allow production of molecules that are currently more expensive, such as lactic acid. As the design of such bioreactors is optimized, biofuel production using cyanobacteria may become economically feasible. The results presented here provide an attractive strategy for achieving these goals.
Supplementary Material
Acknowledgments
This work was supported by funds from Harvard University and the Wyss Institute for Biologically Inspired Engineering. H.N. and B.T.W. were supported by fellowships from the German National Academic Foundation and Deutscher Akademischer Austauschdienst, respectively. D.F.S. is a DOE Energy Biosciences Fellow of the Life Sciences Research Foundation.
We thank Susan Golden for plasmids and advice and Patrick McCroskey and Geoff Duyk for strategic suggestions.
Footnotes
Published ahead of print on 2 April 2010.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Allen, M. M., and R. Y. Stanier. 1968. Growth and division of some unicellular blue-green algae. J. Gen. Microbiol. 51:199-202. [DOI] [PubMed] [Google Scholar]
- 2.Atsumi, S., W. Higashide, and J. C. Liao. 2009. Direct photosynthetic recycling of carbon dioxide to isobutyraldehyde. Nat. Biotechnol. 27:1177-1180. [DOI] [PubMed] [Google Scholar]
- 3.Barnell, W. O., K. C. Yi, and T. Conway. 1990. Sequence and genetic organization of a Zymomonas mobilis gene cluster that encodes several enzymes of glucose metabolism. J. Bacteriol. 172:7227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becking, L. G. M. B., I. R. Kaplan, and D. Moore. 1960. Limits of the natural environment in terms of pH and oxidation-reduction potentials. J. Geol. 68:243-284. [Google Scholar]
- 5.Blumwald, E., R. J. Mehlhorn, and L. Packer. 1983. Studies of osmoregulation in salt adaptation of cyanobacteria with ESR spin-probe techniques. Proc. Natl. Acad. Sci. U. S. A. 80:2599-2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boonstra, B., C. E. French, I. Wainwright, and N. C. Bruce. 1999. The udhA gene of Escherichia coli encodes a soluble pyridine nucleotide transhydrogenase. J. Bacteriol. 181:1030-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clerico, E. M., J. L. Ditty, and S. S. Golden. 2007. Specialized techniques for site-directed mutagenesis in cyanobacteria. Methods Mol. Biol. 362:155-171. [DOI] [PubMed] [Google Scholar]
- 8.Deng, M. D., and J. R. Coleman. 1999. Ethanol synthesis by genetic engineering in cyanobacteria. Appl. Environ. Microbiol. 65:523-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DiMarco, A. A., and A. H. Romano. 1985. d-Glucose transport system of Zymomonas mobilis. Appl. Environ. Microbiol. 49:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galbe, M., P. Sassner, A. Wingren, and G. Zacchi. 2007. Process engineering economics of bioethanol production. Adv. Biochem. Eng. Biotechnol. 108:303-327. [DOI] [PubMed] [Google Scholar]
- 11.Hagen, K. D., and J. C. Meeks. 2001. The unique cyanobacterial protein OpcA is an allosteric effector of glucose-6-phosphate dehydrogenase in Nostoc punctiforme ATCC 29133. J. Biol. Chem. 276:11477-11486. [DOI] [PubMed] [Google Scholar]
- 12.Huntley, M. E., and D. G. Redalje. 2007. CO2 mitigation and renewable oil from photosynthetic microbes: a new appraisal. Mitigat. Adapt. Strat. Global Change 12:573-608. [Google Scholar]
- 13.Li, Y., M. Horsman, N. Wu, C. Q. Lan, and N. Dubois-Calero. 2008. Biofuels from microalgae. Biotechnol. Prog. 24:815-820. [DOI] [PubMed] [Google Scholar]
- 14.Marger, M. D., and M. H. Saier, Jr. 1993. A major superfamily of transmembrane facilitators that catalyse uniport, symport and antiport. Trends Biochem. Sci. 18:13-20. [DOI] [PubMed] [Google Scholar]
- 15.Marolda, C. L., and M. A. Valvano. 1996. The GalF protein of Escherichia coli is not a UDP-glucose pyrophosphorylase but interacts with the GalU protein possibly to regulate cellular levels of UDP-glucose. Mol. Microbiol. 22:827-840. [DOI] [PubMed] [Google Scholar]
- 16.Miao, X., Q. Wu, G. Wu, and N. Zhao. 2003. Sucrose accumulation in salt-stressed cells of agp gene deletion-mutant in cyanobacterium Synechocystis sp. PCC 6803. FEMS Microbiol. Lett. 218:71-78. [DOI] [PubMed] [Google Scholar]
- 17.Núñez, M. F., O. Kwon, T. H. Wilson, J. Aguilar, L. Baldoma, and E. C. C. Lin. 2002. Transport of l-lactate, d-lactate, and glycolate by the LldP and GlcA membrane carriers of Escherichia coli. Biochem. Biophys. Res. Commun. 290:824-829. [DOI] [PubMed] [Google Scholar]
- 18.Plumbridge, J. 1999. Expression of the phosphotransferase system both mediates and is mediated by Mlc regulation in Escherichia coli. Mol. Microbiol. 33:260-273. [DOI] [PubMed] [Google Scholar]
- 19.Smith, A. J. 1983. Modes of cyanobacterial carbon metabolism. Ann. Microbiol. (Paris) 134B:93-113. [DOI] [PubMed] [Google Scholar]
- 20.Snoep, J. L., N. Arfman, L. P. Yomano, R. K. Fliege, T. Conway, and L. O. Ingram. 1994. Reconstruction of glucose uptake and phosphorylation in a glucose-negative mutant of Escherichia coli by using Zymomonas mobilis genes encoding the glucose facilitator protein and glucokinase. J. Bacteriol. 176:2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spolaore, P., C. Joannis-Cassan, E. Duran, and A. Isambert. 2006. Commercial applications of microalgae. J. Biosci. Bioeng. 101:87-96. [DOI] [PubMed] [Google Scholar]
- 22.Yanase, H., H. Fukushi, N. Ueda, Y. Maeda, A. Toyoda, and K. Tonomura. 1991. Cloning, sequencing, and characterization of the intracellular invertase gene from Zymomonas mobilis. Agric. Biol. Chem. 55:1383-1390. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.