Abstract
Bacterial capsular polysaccharides and lipopolysaccharides are well-established ligands of innate and adaptive immune effectors and often exhibit structural and antigenic variability. Although many surface-localized glycoproteins have been identified in bacterial pathogens and symbionts, it not clear if and how selection impacts associated glycoform structure. Here, a systematic approach was devised to correlate gene repertoire with protein-associated glycoform structure in Neisseria species important to human health and disease. By manipulating the protein glycosylation (pgl) gene content and assessing the glycan structure by mass spectrometry and reactivity with monoclonal antibodies, it was established that protein-associated glycans are antigenically variable and that at least nine distinct glycoforms can be expressed in vitro. These studies also revealed that in addition to Neisseria gonorrhoeae strain N400, one other gonococcal strain and isolates of Neisseria meningitidis and Neisseria lactamica exhibit broad-spectrum O-linked protein glycosylation. Although a strong correlation between pgl gene content, glycoform expression, and serological profile was observed, there were significant exceptions, particularly with regard to levels of microheterogeneity. This work provides a technological platform for molecular serotyping of neisserial protein glycans and for elucidating pgl gene evolution.
It is now well established that protein glycosylation based on both N- and O-linked modifications occurs in bacterial species. In N-linked systems exemplified by the system in Campylobacter jejuni, large numbers of proteins that are translocated to the periplasm are glycosylated based on the presence of sequon elements and asparagine-targeting oligosaccharyltransferases related to those that operate in eukaryotes (21, 36, 69, 73). Two O-linked systems associated with covalent modification of type IV pilin subunits in pathogenic Neisseria species and in selected strains of Pseudomonas aeruginosa have been particularly well characterized (2, 16, 46-48, 54). The latter systems are remarkably similar to the N-linked system characterized in C. jejuni in that oligosaccharides are synthesized cytoplasmically as lipid-linked precursors that are then flipped into the periplasm. Protein-targeting oligosaccharyltransferases structurally related to the WaaL family of O-antigen ligases then transfer the oligosaccharides to protein substrates (2, 18, 49). The similarities between these N- and O-linked systems are perhaps best illustrated by genetic and functional interactions between components of the C. jejuni oligosaccharide biosynthetic machinery and elements of the neisserial pilin glycosylation pathway (2, 18). In contrast, the mechanisms operating in other bacterial O-linked systems are not completely understood yet, and there appears to be considerable diversity in the mechanisms of oligosaccharide synthesis, transfer of the glycan to the protein, and the cellular compartment in which glycan addition takes place. Prime examples of this diversity include the glycosylation of major subunits of S-layers (53), flagella (40), and type IV pili, as well as nonpilus adhesins, such as autotransporters (7, 51) and a family of serine-rich proteins identified in Gram-positive species (72). Recently, the pilin glycosylation system in the Gram-negative species Neisseria gonorrhoeae (the etiological agent of gonorrhea) was shown to be a general O-linked system in which a large set of structurally distinct periplasmic proteins undergo glycosylation (64). Likewise, a general O-linked glycosylation system targeting periplasmic and surface-exposed proteins has been documented in Bacteroides fragilis (19). In addition, an increasing number of lipoproteins in Mycobacterium tuberculosis have been found to be O glycosylated, and current evidence suggests that a single glycosylation pathway operates with these proteins (50).
The large number of bacterial protein glycosylation systems strongly suggests that these systems are advantageous and affect fitness. In fact, mutants with mutations in the general glycosylation systems of C. jejuni and B. fragilis are defective in mucosal colonization, although the fundamental basis for the observations is unclear (19, 23). In some cases, defects in protein stability and trafficking have been documented. Examples of the latter have been reported for the Aida and Ag43 autotransporter adhesins of Escherichia coli and the serine-rich Fap1 streptococcal adhesin (11, 35, 72). In these cases, the glycosylation status appears to influence protein integrity along with intracellular or membrane trafficking events.
Glycosylation may also influence protein structure and function or activity at the extracellular level. In the context of host-symbiont and host-pathogen interactions, bacterial cell surface polysaccharides and glycolipid glycans are well-established targets of both innate and adaptive immune responses (13, 61). However, the potential influence of protein-linked carbohydrate on immune recognition and signaling is only beginning to be investigated. Given the well-established effect of conjugating protein to carbohydrate on glycan-related immunogenicity, glycoproteins could be predicted to promote a robust T-cell-dependent antibody response directed toward glycan epitopes. In line with this, immunization of mice with O-glycosylated type IV pilin from P. aeruginosa strain 1244 (which bears a single repeat unit of the O antigen, the dominant component of its lipopolysaccharide) resulted in protection against challenge with immunological specificity for the O-polysaccharide (27). In addition, structural heterogeneity of carbohydrate modifications has been shown to affect the serospecificity of Campylobacter flagellins (41). With regard to innate immunity, the N-linked protein glycans of C. jejuni have been shown to influence interleukin-6 production by human dendritic cells via interaction with the macrophage galactose-type lectin (MGL) (62). Also, flagellin glycosylation of the phytopathogenic bacteria Pseudomonas syringae pv. glycinea and P. syringae pv. tomato appears to play an important role in hypersensitive cell death in nonhost plants and in host cell recognition (56, 57). Similarly, the flagellin glycosylation status in P. aeruginosa influences proinflammatory responses in human cell cultures (63).
Studies of O-linked flagellar glycosylation in P. aeruginosa, C. jejuni, and a number of Gram-positive species have revealed considerable variability in genomic glycosylation islands (40). In addition to differences in gene content, some genes localized in these loci are subject to phase (on-off) variation involving slipped-strand mispairing events. Similar findings have been obtained for the O-linked glycosylation system in N. gonorrhoeae and a related system in Neisseria meningitidis (2, 4, 29, 48). These observations strongly suggest that protein-associated glycans are positively selected. However, attempts to elucidate the evolutionary processes impacting these systems are complicated by difficulties in connecting genotype with phenotype. For example, predicting enzymatic activities of components involved in glycan biosynthesis based on the sequence alone is notoriously difficult. Therefore, glycosylation-related functions are characterized best by using purified components in in vitro assays. Moreover, despite recent advances in mass spectrometric (MS) and nuclear magnetic resonance (NMR) technologies, glycoprotein structural analysis is still arduous, particularly when proteins are expressed at low levels. Thus, current methodologies are not optimized for studies of large numbers of strains and mutants.
The broad-spectrum O-linked protein glycosylation system of N. gonorrhoeae is particularly well characterized with regard to the genetics of oligosaccharide biosynthesis, modification, and transfer to protein via the PglO/PglL oligosaccharyltransferase. As shown using strain N400, combined genetic and MS analyses, including interspecies complementation, have revealed that this system (designated the pgl [protein glycosylation] system) is remarkably similar to the N-linked system of C. jejuni with respect to the use of a peptide-proximal 2,4-diacetamido-2,4,6-trideoxyhexose (DATDH) sugar and related biosynthetic pathways for generating lipid-linked glycan substrates (2, 18, 39). The lipid-linked DATDH sugar can be further converted successively into hexose (Hex)-DATDH disaccharide and Hex-Hex-DATDH trisaccharide forms by the PglA and PglE glycosyltransferases, respectively (2). The hexoses in both the di- and trisaccharide forms can also undergo O acetylation by the PglI enzyme (2, 70). As pglA, pglE, and pglI are each predicted to be subject to phase variation in some backgrounds, strains have the potential to express five distinct glycoforms (2, 4, 29, 48, 70). A similar system operates in N. meningitidis strain c311, although to date only pilin and the AniA nitrite reductase proteins have been shown to be glycosylated (37). Pioneering analyses of pilin from this strain identified a trisaccharide with a terminal alpha-1-4-linked digalactose moiety attached to DATDH (54). Interestingly, nearly one-half of N. meningitidis isolates are reported to have a unique allele of pglB designated pglB2 associated with synthesis of a proximal glyceramido-acetamido trideoxyhexose (GATDH) rather than DATDH (10). In addition, some strains of both N. gonorrhoeae and N. meningitidis have been reported to contain additional genes predicted to encode glycosyltransferases linked to the core locus that includes the pglF, pglB, pglC, and pglD genes (32, 48). Thus, it appears that the number of protein-associated glycans may be far greater than currently perceived. The genus Neisseria also includes a number of related species that colonize humans, including Neisseria lactamica, which is closely related to N. gonorrhoeae and N. meningitidis but is rarely associated with disease (24), as well as other, more divergent commensal species. An examination of recently available genome sequences of these nonpathogenic species revealed that they contain open reading frames (ORFs) whose products share high levels of amino acid identity with many of the protein glycosylation components found in N. gonorrhoeae and N. meningitidis and with many of the N. gonorrhoeae proteins targeted for glycosylation. However, protein glycosylation has not been documented in any of these species yet.
Here, we developed a systematic approach for elucidating intra- and interstrain glycan diversity and its genetic basis in neisserial O-linked glycans by employing serotyping, mass spectrometric analyses, and genetically defined recombinant backgrounds. We then used these tools to demonstrate that protein-associated glycans are antigenically variable and that isolates of N. meningitidis and N. lactamica also exhibit broad-spectrum O-linked protein glycosylation.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The bacterial strains used in this study are described in Table 1 and were grown on conventional GC medium as described previously (20). Protein glycosylation mutations (pglA, pglC, pglEon, pglI) were introduced into various strain backgrounds using transformation as previously described (1, 2). Antibiotics were used for selection of transformants at the following concentrations: streptomycin, 750 μg/ml; erythromycin, 8 μg/ml; and kanamycin, 50 μg/ml. In the case of meningococcal transformants, 100 μg/ml kanamycin was used. In the high-pH treatment experiments, cells were incubated in GC media containing 50 mM NaOH at 37°C for 45 min.
TABLE 1.
Neisseria strains used in this study
| Strain | Parent strain | Relevant genotype | Main glycan modification (identified by MS) | Reference |
|---|---|---|---|---|
| N. gonorrhoeae strains | ||||
| FA1090 (ST-1899) | ATCC 700825 | |||
| KS300 (FA1090) | FA1090 | recA6 | Ac-Hex-Hex-DATDH | 59 |
| KS301 | FA1090 | pglC::kan | No glycan | This study |
| KS302 | FA1090 | pglA::kan | DATDH | This study |
| KS303 | FA1090 | pglI::kan | Hex-Hex-DATDH | This study |
| KS100 (N400) | VD300 | recA6 | Ac-Hex-DATDH | 59 |
| KS104 (GGC) | N400 | pglC::kan | No glycan | 22 |
| KS141 | N400 | pglA::kan | DATDH | 2 |
| KS142 | N400 | pglEon | Ac-Hex-Hex-DATDH | 2 |
| KS144 | N400 | pglI::kan | Hex-DATDH | 2 |
| KS304 | N400 | pglEonpglI::kan | Hex-Hex-DATDH | This study |
| KS305 | N400 | pglBMC58 | Ac-Hex-DATDH | This study |
| KS306 | KS305 | pglBMC58pglA::kan | DATDH | This study |
| KS307 | KS142 | pglBMC58pglEon | Ac-Hex-Hex-DATDH | This study |
| KS308 | N400 | pglB28013 | Hex-GATDH | This study |
| KS309 | KS308 | pglB28013pglA::kan | GATDH | This study |
| KS310 | KS142 | pglB28013pglEon | Hex-Hex-GATDH | This study |
| KS101 (4/3/1) | VD300 | pilE ind | 71 | |
| KS105 | 4/3/1 | pglC::kan | 64 | |
| KS122 | 4/3/1 | pglA::kan | 64 | |
| KS127 | 4/3/1 | pglEon | 65 | |
| KS311 | 4/3/1 | pglB28013 | This study | |
| KS312 | 4/3/1 | pglB28013pglA::kan | This study | |
| KS313 | 4/3/1 | pglB28013pglEon | This study | |
| KS314 | 4/3/1 | pglI::kan | This study | |
| KS315 | 4/3/1 | pglEonpglI::kan | This study | |
| N. meningitidis strains | ||||
| KS316 (MC58, ST-74) | 58 | |||
| KS317 | MC58 | pglC::kan | This study | |
| KS318 | MC58 | pglA::kan | This study | |
| KS319 (H44/76, ST-32) | 26 | |||
| KS320 (Z2491, ST-4) | 44 | |||
| KS321 (8013, ST-177) | 33 | |||
| KS322 (FAM18, ST-11) | 6 | |||
| KS323 (BZ 10, ST-8) | 33 | |||
| KS324 (BZ 198, ST-41) | 33 | |||
| N. lactamica strains | ||||
| KS325 (ST-3787) | 5, ATCC 23970 | |||
| KS326 (ST-640) | 5 |
Allelic exchange of pglB/pglB2.
The MC58 and 8013 pglB alleles were introduced into N400 by using a two-step mutagenesis method that allowed gene replacement without introduction of a selectable marker into the final strain. This method uses a two-gene cassette containing both a selectable marker (ermC′) and a counterselectable marker (rpsL+) (31). First, the pCRII-pgl plasmid was constructed by PCR amplifying the whole N400 pgl locus with primers pgl-F (5′-AGCATATTGACGGGCTTGTCGC-3′) and pgl-R-(5′-AAGCGAAATCCTCGGACACG-3′) and inserting the PCR product into the pCRII-TOPO vector (Invitrogen). Then the ermC′/rpsL+ cassette from pFLOB4300 was amplified with primers pUC-F-MluI (5′-CCGACGCGTCCCAGTCACGACGTTGTAAAACG-3′) and pUC-R-MluI (5′-CCGACGCGTAGCGGATAACAATTTCACACAGG-3′) (MluI sites are underlined) and subsequently cloned into the MluI sites in pglF and pglB of the pCRII-pgl plasmid, which replaced some of the N400 pgl locus with the ermC′/rpsL+ cassette and resulted in the pCRII-pgl::ermC′/rpsL+ plasmid.
N400 was then transformed with pCRII-pgl::ermC′/rpsL+, and erythromycin-resistant gonococci were selected. N400 is naturally streptomycin resistant, but introduction of the rpsL+ allele made the intermediate strain, N400 pgl::ermC′/rpsL+, streptomycin sensitive. The next step was transformation with MC58 or 8013 genomic DNA, in which homologous recombination replaced the ermC′/rpsL+ cassette with the locus from the meningococcal genomic DNA, and the final strain was selected on streptomycin plates. For derived strains N400 pglB28013 and N400 pglBMC58 (in which the locus was exchanged without leaving any remaining antibiotic resistance cassette), DNA sequencing was used to confirm that no other mutations were present. Derivatives of strain 4/3/1 carrying pglB28013 were constructed by using the same two-step mutagenesis strategy.
SDS-PAGE and immunoblotting.
The procedures used for SDS-PAGE and immunoblotting have been described previously (20). Whole-cell lysates were prepared from equivalent numbers of cells by heating cell suspensions at 65°C for 10 min in SDS sample loading buffer. A rabbit polyclonal antibody against the nitrite reductase AniA was used at a 1:10,000 dilution. The mouse SM1 monoclonal antibody (MAb) (67), which specifically recognizes class I pilin types, was used at a 1:1,000 dilution. A rabbit polyclonal antiserum raised against the PilE-derived peptide 44KSAVTEYYLNHGKWPENNTSA64 (Research Genetics), which reacts with all pilin types, was used at a 1:10,000 dilution (3). Immunoreactive proteins were detected by immunoblotting using the glycan-specific rabbit antibodies described below and an alkaline phosphatase-coupled goat anti-rabbit secondary antibody (Sigma).
Development of glycan-specific rabbit polyclonal and monoclonal antibodies.
Rabbits were immunized with purified pili from strains N400 pglA, N400, and N400 pglEon, which express the DATDH-based monosaccharide, disaccharide, and trisaccharide glycoforms, respectively. Following a regimen with at least three immunizations (100 μg antigen/immunization), immune sera were harvested. These sera were designated pAb1, pAb2, and pAb3, respectively. To generate rabbit hybridomas, splenocytes from immunized rabbits were isolated and fused with a rabbit hybridoma fusion partner (34, 52). The antibodies were produced by Epitomics, Inc. (Burlingame, CA). Clones secreting glycan epitope-recognizing antibodies were selected by enzyme-linked immunosorbent assay (ELISA) screening of hybridoma supernatants using purified pili. Further screening of the ELISA-positive clones using immunoblotting identified hybridoma clones suitable for differentiating between the specific glycans. These clones were designated MAbs npg1 (neisseria protein glycan 1), npg2, and npg3, respectively. The rabbit monoclonal antibodies (10 mg/ml) were used at a 1:20,000 dilution for immunoblotting.
Development of GATDH-specific rabbit polyclonal antibodies.
Polyclonal antibodies (designated pGAb) were generated by rabbit immunization as described above using pili purified from strain N400 pglB28013 pglA, which expressed the protein-linked monosaccharide GATDH (Agrisera, Sweden). Solid-phase affinity purification was performed by immunoblotting a whole-cell lysate from the strain expressing the GATDH glycan (N400 pglB28013 pglA) and incubating it with pGAb (1:2,000 dilution). Specifically reactive glycoprotein bands were localized by developing strips cut from the edge of a polyvinylidene difluoride (PVDF) membrane, realigning them, and then excising the relevant area of the membrane. Antibodies were eluted from the membrane by two washes with 1 ml glycine-HCl buffer (5 mM, pH 2.3), which were collected and neutralized to physiologic pH by addition of 50 μl Tris-HCl (1 M, pH 7.8). The solution was then finally diluted in Tris-buffered saline (TBS) (1:3) before it was used for immunoblotting.
Sample preparation and ESI-MS analysis of intact PilE.
Type IV pili were isolated and treated using a methanol-chloroform washing and precipitation procedure, as described previously (2). Samples were subjected immediately to MS analysis or frozen at −80°C. Data were acquired with a quadrupole time of flight mass spectrometer (Q-Tof micro; Waters Corporation, Milford, MA) equipped with the standard Z-spray electrospray ionization (ESI) source as previously described (2).
Membrane protein fraction preparation and in-gel digestion.
N. meningitidis strain MC58 cells were harvested and washed with phosphate-buffered saline (PBS), and cell lysis was performed using a French press. Cellular debris was removed by centrifugation at 5,000 × g for 20 min, and the crude membranes were recovered from the supernatant by centrifugation at 100,000 × g for 60 min. The recovered membranes were washed two times in PBS and stored at −80°C.
Coomassie blue-stained protein bands were serially rehydrated and washed with 150 μl of high-performance liquid chromatography (HPLC)-grade water, 150 μl of acetonitrile-water (1:1, vol/vol), and 100% acetonitrile at room temperature. Protein reduction was carried out by addition of 50 μl of 10 mM dithiothreitol-0.05 M NH4HCO3 (60 min, 56°C) to the dehydrated gel pieces. Then the excess reduction buffer was removed, and thiol groups were alkylated by addition of 50 mM iodoacetamide and 0.05 M NH4HCO3 and incubation for 45 min at room temperature in the dark. The gel pieces were washed twice with 150 μl of acetonitrile-water (1:1, vol/vol) and 100% acetonitrile at room temperature. Digestion buffer (5 to 10 μl) containing 16 ng/μl trypsin (pig; modified; sequencing grade; Sigma-Aldrich) in 0.05 M NH4HCO3 was added, and samples were kept on ice for 30 min to allow rehydration of the gel pieces. To limit autoproteolysis of trypsin, the remaining buffer was removed and replaced with 35 to 50 μl of 0.05 M NH4HCO3, and digestion was carried out overnight at 37°C. Peptides were extracted successively with 5% formic acid, 5% formic acid-acetonitrile (1:1, vol/vol), and acetonitrile. The combined supernatants were dried with a SpeedVac and then redissolved in 0.1% formic acid. Samples were subjected immediately to mass spectrometric analysis or frozen at −80°C.
Nanoflow online liquid chromatographic MS analysis of proteolytic peptides.
Reverse-phase (C18) nano online liquid chromatographic MS/MS analyses of proteolytic peptides were performed using an HPLC system consisting of two Agilent 1200 HPLC binary pumps (one nano pump and one capillary pump) with a corresponding autosampler, column heater, and integrated switching valve. This liquid chromatography system was coupled via a nanoelectrospray ion source to an LTQ-Orbitrap mass spectrometer (Thermo Fisher Scientific, Bremen, Germany). For analyses, 4 μl of a peptide solution was injected onto the extraction column (5 by 0.3 mm) filled with Zorbax 300 SB-C18 (particle size, 5 μm; Agilent). Samples were washed with a mobile phase consisting of 0.1% formic acid, and 3% acetonitrile. The flow rate, provided by the capillary pump, was 4 μl/min. After 7 min, the integrated switching valve was activated, and the peptides were eluted in the back-flush mode from the extraction column onto a C18 3-μm resin column (150 by 0.075 mm; GlycproSIL C18-80Å; Glycpromass, Stove, Germany). The mobile phase consisted of acetonitrile and MS-grade water, both containing 0.1% formic acid. Chromatographic separation was obtained using a binary 5 to 55% acetonitrile gradient for 60 or 210 min. The flow rate, provided by the nanoflow pump, was 0.2 μl min−1.
Mass spectra were acquired in the positive-ion mode by applying a data-dependent automatic switch between the survey scan and tandem mass spectrum (MS/MS) acquisition. Peptide samples were analyzed by a high-energy collision dissociation (HCD) fragmentation method by acquiring one Orbitrap survey scan in the mass range from m/z 300 to 2000, followed by MS/MS of the three most intense ions in the Orbitrap. The target value in the LTQ-Orbitrap was 1,000,000 for a survey scan at a resolution of 30,000 at m/z 400 using lock masses for recalibration to improve the mass accuracy of precursor ions. Fragmentation in the C-trap was performed by collision-induced dissociation with a target value of 5,000 ions. The ion selection threshold was 500 counts. Selected sequenced ions were dynamically excluded for 180 s.
Data analysis.
Mass spectrometric data were analyzed with an in-house neisserial protein sequence database using SEQUEST. The mass tolerances of a fragment ion and a parent ion were defined as 0.05 Da and 5 ppm, respectively. Methionine oxidation and cysteine carbamidomethylation were selected as a variable or fixed modification. A false discovery rate of 0.01 was required for proteins and peptides with a minimum length of 6 amino acids. Neisserial glycopeptide MS/MS spectra were manually searched by using Qual Browser, version 2.0.7.
Immunogold and transmission electron microscopy.
Sample grids were prepared by touching carbon-coated Formvar copper grids to individual bacterial colonies grown on GC agar (18 h, 37°C, 5% CO2) and were fixed with 1% glutaraldehyde in phosphate-buffered saline (PBS) (pH 7.4) for 4 min and four washes on drops of PBS containing 0.15% glycine. For immunogold labeling, the grids with fixed bacteria were first blocked with 0.8% bovine serum albumin (BSA) and then incubated with the rabbit MAbs (dilution, 1:100) for 30 min at room temperature. After four washes on drops of PBS containing 0.15% glycine, the grids were incubated with gold-conjugated protein A (10 nm) for 30 min. After six rinses on drops of water, the grids were stained for 3 min with uranyl acetate (2% aqueous solution) before they were viewed with a Philips CM100 transmission electron microscope.
RESULTS
DATDH-based glycoforms are immunogenic and antigenic.
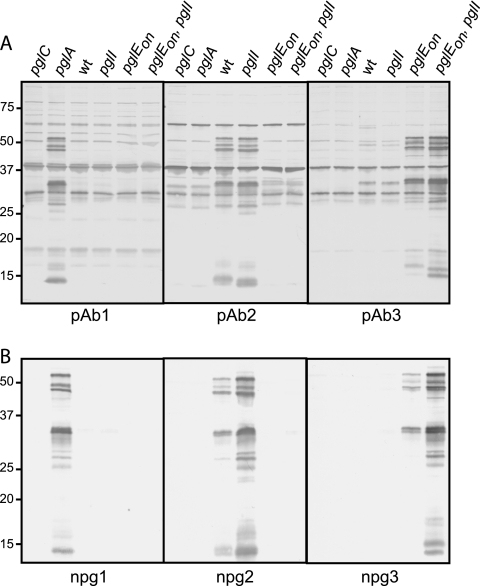
We observed previously that immunization of rabbits with glycosylated PilE protein (in purified type IV pili) resulted in antibodies that reacted in a glycan-specific manner with several other glycoproteins in N. gonorrhoeae (64). To examine glycan-related immunogenicity and antigenicity in more detail, rabbits were immunized with glycosylated PilE proteins derived from bacteria with different pgl backgrounds synthesizing either DATDH monosaccharide (pglA), acetyl (Ac)-Hex-DATDH disaccharide (wild type, pglEoff), or Ac-Hex-Hex-DATDH trisaccharide (pglEon). Immunoblotting was then used to examine the patterns of reactivity using whole-cell lysates from strains expressing the different glycoforms (Fig. 1A). To eliminate the potential confounding influence of antibodies directed to PilE, polypeptide backgrounds in which pilE was conditionally repressed were used. As a negative control, a pglC null mutant was used as glycosylation is disrupted in this background due to the obligatory role of PglC in synthesis of the basal DATDH sugar (2). Antibodies raised against PilE from the pglA strain displayed a specific pattern, reacting only with glycoproteins from the strain expressing DATDH monosaccharide, whereas antibodies raised against PilE from wild-type N400 (pglEoff) reacted specifically with glycoproteins from the strain synthesizing the Ac-Hex-DATDH disaccharide. Likewise, antibodies raised against trisaccharide PilE detected glycoproteins in the pglEon background but also showed weak reactivity with some glycoproteins in the disaccharide-expressing strain. The glycoprotein patterns seen by the antibodies were strikingly similar except for minor perturbations in relative mobility that coincided with glycan size. Similar effects were seen for the strains that differed in O-acetylation status, and they were most dramatic for glycoproteins in the lower-molecular-weight range. Taken together, the results indicate that there was a specific polyclonal antibody response in rabbits to epitopes associated with each glycoform. In addition, changes in glycoform expression were not associated with discernible changes at the macroheterogeneity level (protein substrate targeting).
FIG. 1.
DATDH-based glycans are immunogenic and antigenic: immunoblotting of whole-cell lysates of N. gonorrhoeae pgl mutant strains and variants with (A) the rabbit polyclonal antibodies pAb1, pAb2, and pAb3 raised against purified PilE bearing the DATDH, Ac-Hex-DATDH and Ac-Hex-Hex-DATDH glycans, respectively, and (B) the rabbit monoclonal antibodies npg1, npg2 and npg3 raised against the corresponding glycans. The strains used all had a 4/3/1 background in which pilE was conditionally repressed and were KS105 (pglC), KS122 (pglA), KS101 (wt), KS314 (pglI), KS127 (pglEon), and KS315 (pglEon pglI). wt, wild type.
Given these findings and our goal of generating more specific glycan-recognizing reagents, monoclonal antibody (MAb)-producing cell lines were derived from immunized rabbits (52). Following screening for the cell lines with glycan specificity, three cell lines and corresponding MAbs were chosen for further study based on recognition of mono-, di-, and trisaccharide forms. When used for immunoblotting, these MAbs produced reactivity patterns that largely mimicked the reactivity patterns seen with the corresponding polyclonal antisera (Fig. 1B). However, the signals seen with the MAbs specific for the di- and trisaccharides were stronger for the pglI backgrounds lacking O acetylation. Thus, the epitopes recognized by these MAbs may be masked or otherwise compromised by acetylation of the hexose sugars.
Neisserial glycoprotein profiles and diversity in selected strains and species.
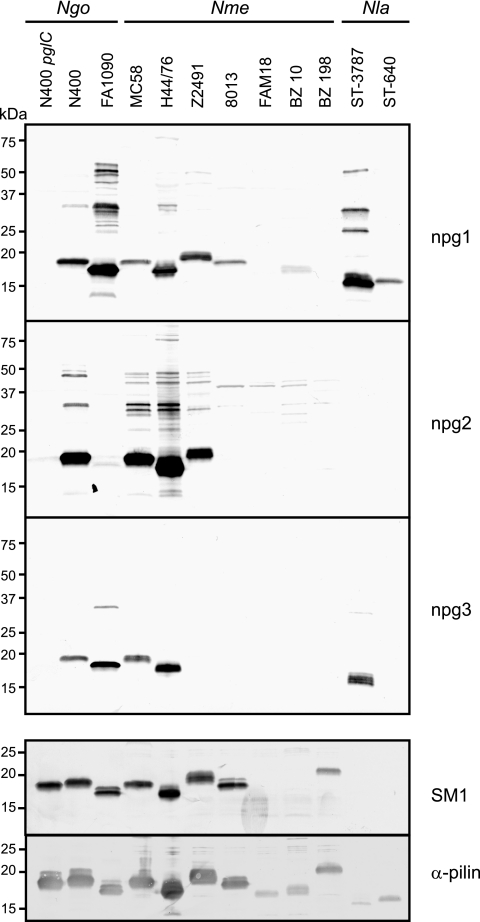
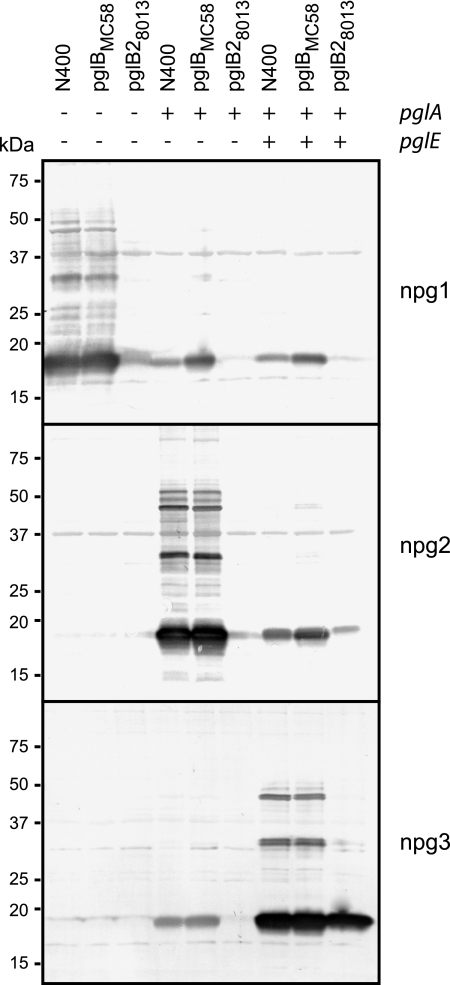
Although broad-spectrum O-linked protein glycosylation has been well characterized in N. gonorrhoeae N400, it is not clear if general protein glycosylation occurs in other neisserial strains and species. To begin to address this possibility, selected strains were subjected to immunoblotting using the three glycan-specific MAbs. The strains were chosen primarily on the basis of the availability of their genome sequences or previous pgl characterization and included one N. gonorrhoeae strain, seven N. meningitidis strains, and two strains of N. lactamica. For strains reactive with one of the MAbs, the major reactive species was PilE (as shown by immunoblotting using the SM1 pilin MAb and a pilin peptide-directed antiserum that reacts with all PilE forms). As shown in Fig. 2, diverse reactivity patterns were detected, which nonetheless could be placed into groups based on similarities and levels of microheterogeneity. For example, N. gonorrhoeae N400 showed the strongest reactivity with the npg2 MAb and weaker reactivity with the mono- and trisaccharide-directed MAbs. This pattern was consistent with the results of MS analyses of N400 glycoproteins, which revealed that the disaccharide glycoform was the predominant glycoform (2), and with the presumed presence of less abundant cells carrying phase-on variants of pglE. This pattern was also seen for N. meningitidis strains MC58, H44/76, and Z2491, although the latter strain was negative for reactivity with the trisaccharide MAb. Also, the disaccharide-directed MAb detected multiple proteins in all three of these strains, and the overall patterns were similar to one another but somewhat distinct from those for the N. gonorrhoeae strains. N. meningitidis strains 8013, FAM18, BZ 10, and BZ 198 were essentially nonreactive with all three MAbs, except for weak signals for the monosaccharide epitope associated with PilE in 8013 and BZ 10. The overall lack of reactivity observed with these strains appears to correlate with the presence of the pglB2 gene, which was implicated in the synthesis of the lipid-linked GATDH sugar intermediate. N. lactamica strain ST-640 reacted only with the monosaccharide MAb, as expected since the pglA allele is phase off. Finally, N. gonorrhoeae FA1090 and N. lactamica ST-3787 displayed similar patterns marked by predominant reactivity with the monosaccharide MAb, no reactivity with the disaccharide MAb, and weak reactivity with the trisaccharide MAb.
FIG. 2.
Neisserial glycoprotein profiles and glycan diversity. (Top panels) Immunoblotting of whole-cell lysates from strains of N. gonorrhoeae (Ngo) (N400 pglC, N400, and FA1090), N. meningitidis (Nme) (MC58, H44/76, Z2491, 8013, FAM18, BZ 10, and BZ 198), and N. lactamica (Nla) (ST-3787 and ST-640) with glycan-specific monoclonal antibodies. (Bottom panels) Immunoblotting using the SM1 MAb specifically recognizing class I pilin types and a polyclonal antiserum raised against the PilE-derived peptide 44KSAVTEYYLNHGKWPENNTSA64, which reacts with all pilin types. Thus, strains FAM18, BZ 10, ST-3787, and ST-640 all express class II pilins. Also, strains 8013, FAM18, BZ 10, and BZ 198 carry the pglB2 allele that is associated with synthesis of GATDH glycans, while all of the other strains have the pglB allele that is associated with synthesis of DATDH glycans.
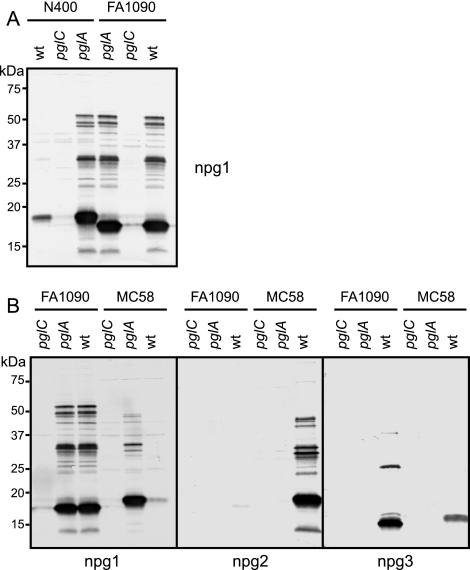
The unique pattern of microheterogeneity observed for strain FA1090 was not anticipated as this strain carries the pglB gene and phase-on alleles of pglA and pglE. Thus, one would predict that this strain should express the DATDH trisaccharide form and thus behave phenotypically like the N400 pglEon strain, but this was not the case. To examine this finding in more detail, we constructed mutants of FA1090 carrying null alleles of either pglA or pglC and compared them to equivalent mutants of N. gonorrhoeae N400 and N. meningitidis MC58. As shown Fig. 3A, introduction of the pglC mutation into FA1090 eliminated reactivity with the monosaccharide-specific MAb, as it did in N400. However, while introduction of the null allele of pglA into FA1090 had no effect on reactivity with the monosaccharide MAb, the same mutation in N400 resulted in a dramatic increase in reactivity. Also, comparison of the pglA null-allele mutants revealed remarkably similar glycoprotein profiles for the N400 and FA1090 mutants (Fig. 3A). Evaluation of the equivalent mutants of N. meningitidis MC58 revealed a pattern identical to that of N400 with respect to glycan antigenicity, although the relative mobilities of the predominant glycoproteins appeared to be distinct from those observed for the two N. gonorrhoeae strains (Fig. 3B).
FIG. 3.
Glycoprotein profiles and glycan antigenicity in strains N400, FA1090, and MC58. (A) Whole-cell lysates of N. gonorrhoeae N400 and FA1090 wild-type (wt) strains and pglA (monosaccharide-expressing) and pglC (glycosylation-null) mutants probed with MAb npg1. The strains used were KS100 (N400 wt), KS104 (N400 pglC), KS141 (N400 pglA), KS302 (FA1090 pglA), KS301 (FA1090 pglC), and KS300 (FA1090 wt). (B) Immunoblotting of whole-cell lysates of N. gonorrhoeae FA1090 and N. meningitidis MC58 wild-type strains and pglA (monosaccharide-expressing) and pglC (glycosylation-null) mutants probed with MAbs npg1, npg2, and npg3. The strains used were KS301 (FA1090 pglC), KS302 (FA1090 pglA), KS300 (FA1090 wt), KS317 (MC58 pglC), KS318 (MC58 pglA), and KS316 (MC58 wt).
Direct effect of O acetylation on protein-associated glycan antigenicity.
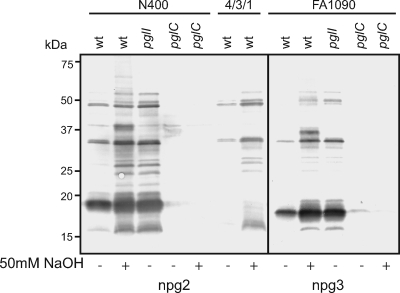
To distinguish whether the effect of O acetylation on the reactivity of the di- and trisaccharide-recognizing MAbs was due to its influence on macroheterogeneity or due to masking of the epitopes, we exploited the established lability of O-linked acetate bonds under high-pH conditions. As shown in Fig. 4, suspending cells of N400 in medium with 50 mM NaOH prior to sample preparation increased the immunoreactivity of the disaccharide-recognizing MAb to the level observed for the untreated pglI mutant. In addition, a novel band was detected, which corresponded to an aberrantly migrating form of PilE (as it was not present in treated samples of a strain in which pilE was conditionally repressed and it reacted with the pilin-specific antibody [data not shown]). Both increased immunoreactivity and the aberrantly migrating form of PilE were observed for the treated samples of FA1090 probed with the trisaccharide-recognizing MAb (Fig. 4) and of MC58 probed with the di- and trisaccharide-recognizing MAbs (data not shown). Similar to what has been observed previously for capsular and O antigens (9, 43), in this study O acetylation had a documented effect on glycan antigenicity, presumably by blocking epitope accessibility.
FIG. 4.
Increased npg2 and npg3 immunoreactivity at high pH and in pglI mutants. Immunoblotting of whole-cell lysates of wild-type strains, pglI mutants, and high-pH-treated wild-type strains revealed increased reactivity with the npg2 and npg3 monoclonal antibodies due to the lack of an O-acetyl group in pglI mutants or with the high-pH treatment. The extraneous band that appeared during high-pH treatment is PilE (as determined by immunoblotting with SM1 [data not shown]). This band was not present with the 4/3/1 background, in which pilE was conditionally repressed. The strains used were KS100 (N400 wt), KS144 (N400 pglI), KS104 (N400 pglC), KS101 (4/3/1 wt), KS300 (FA1090 wt), KS303 (FA1090 pglI), and KS301 (FA1090 pglC). wt, wild type.
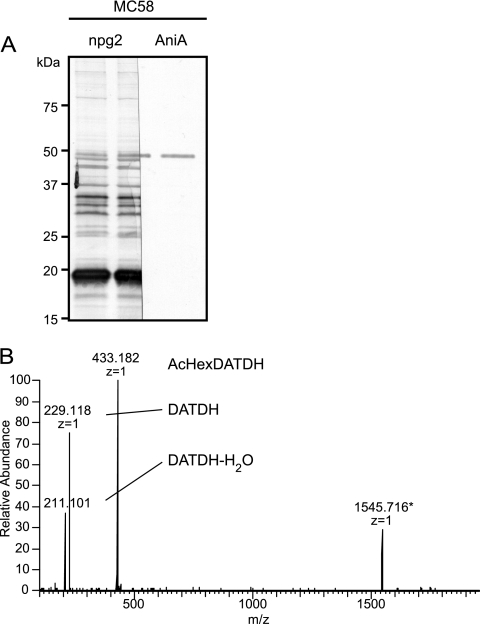
Although our data clearly document that there are multiple glycoproteins in N. meningitidis strains MC58, H44/76, and Z2491 and N. lactamica strain ST-3787, the repertoire of glycoproteins in each strain has not been characterized. There has been only one report of an N. meningitidis glycoprotein other than PilE, and this glycoprotein is the AniA nitrite reductase of strain C311, which was identified by its immunoreactivity with antibodies raised against glycosylated PilE and was characterized by MS (37). Concurrently, AniA was also shown to be glycosylated in N. gonorrhoeae N400 (64). To examine the AniA glycosylation status in MC58, immunoblotting analyses using the disaccharide-recognizing MAb and antibodies to AniA were carried out in parallel. As shown in Fig. 5A, the AniA band was readily identified as the slowest-migrating MAb-specific band, at approximately 48 kDa. Moreover, this protein reacts with the disaccharide-specific MAb, and equivalent signals were observed for the H44/76 and Z2491 strains using the disaccharide-specific MAb (Fig. 2). Using an alternative “shotgun” approach in which tryptic peptides in gel slices from SDS-PAGE of an MC58 membrane protein fraction were directly examined for glycan-related signals by using MS2, a glycopeptide from protein GNA1946 was identified (Fig. 5B). This glycopeptide was equivalent to that reported for the orthologous Ng1237 glycoprotein in N. gonorrhoeae N400 (64). A more detailed analysis of the meningococcal glycoproteomes will be presented elsewhere.
FIG. 5.
AniA and GNA1946 are glycosylated in MC58. (A) Equivalent whole-cell lysate samples of N. meningitidis MC58 were electrophoresed in adjacent lanes, and following transfer to a PVDF membrane, the membrane was divided in half lengthwise. The two pieces were then probed with MAb npg2 and antibodies to AniA. The results demonstrate that the same band corresponding to AniA was identified with both antibodies. (B) Identification of the glycopeptide 6DSAPAASASAAADNGAAK23 from lipoprotein GNA1946 derived by tryptic cleavage of a membrane protein-enriched fraction of MC58. High-energy collision dissociation (HCD) fragmentation of [M+2H]2+ at m/z 989.4505 revealed characteristic oxonium ions at m/z 433.182 and m/z 229.118 corresponding to O-Ac-Hex-DATDH and DATDH glycans, respectively. The signal at m/z 1545.716 is a signal from the peptide backbone after loss of the glycan moiety; the monoisotopic theoretical molecular mass is 1,545.714 Da.
Influence of the pglB2 allele on glycan structure and antigenicity.
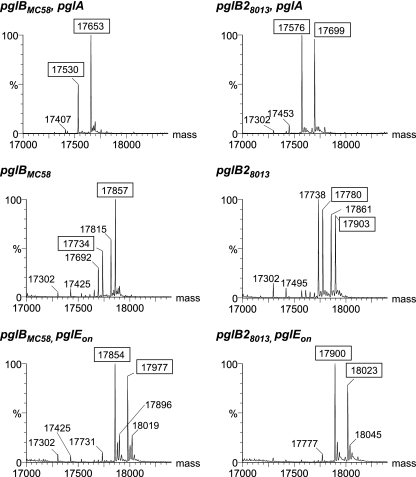
Immunoblotting of N. meningitidis strains established that there was a negative correlation between MAb reactivity and the presence of the pglB2 gene. In order to assess the nature of this association in more detail, we used an allelic replacement strategy related to that used originally to establish the connection between the GATDH proximal sugar and pglB2. We replaced the N. gonorrhoeae N400 pgl locus with the corresponding loci from N. meningitidis MC58 and 8013, which harbor pglB and pglB2, respectively, using a counterselectable marker (rpsL+) that allows gene replacement without the presence of selectable markers in the resulting recombinant strain. By carrying out these replacements with the pglA and pglEon backgrounds of N400, we also examined if the corresponding glycosyltransferases were capable of transferring the first and second hexoses onto the lipid carrier-linked basal sugars to generate the di- and trisaccharide forms of GATDH. We examined purified, intact PilE derived from the recombinants using a “top-down” electrospray infusion (ESI) MS approach developed previously (1). The reconstructed molecular mass profile of the corresponding spectrum for the pglBMC58 background revealed a distribution of species very similar to that reported for the parental background (expressing endogenous PglB) (Fig. 6). These species corresponded to PilE modified with either a 432-Da glycan moiety (O-acetylated Hex-DATDH [Ac-Hex-DATDH]) or a 391-Da glycan moiety (Hex-DATDH) and carrying either one or two phosphoethanolamine (PE) moieties (see Table S1 in the supplemental material). Likewise, in the pglA and pglEon backgrounds, PglBMC58 expression produced the MS PilE profiles observed for the parental N400 strain. In contrast, the equivalent molecular mass profiles of the corresponding spectra for the pglB28013 background were consistent with the presence of the basal GATDH sugar. In the samples from the wild-type recombinant, the predominant species corresponded to PilE modified with either a 478-Da glycan moiety (O-acetylated Hex-GATDH [Ac-Hex-GATDH]) or a 437-Da glycan moiety (Hex-GATDH) and carrying either one or two PE moieties. Identification of the GATDH sugar was further substantiated by the presence in the ESI mass spectrum of the glycan oxonium ion at m/z 479.2 and its pattern of fragmentation during subsequent analysis by collision-induced dissociation (CID) MS/MS (see Fig. S1a in the supplemental material). The O-acetylated Hex-GATDH disaccharide characterized here is different from an N-acetylhexosamine GATDH glycoform described previously despite the fact that the basic methodologies and strains employed in the two studies were the same (10). The difference can best be explained by the inability of the workers in the previous study to resolve the mass difference between O-acetylated hexose (204 Da) and N-acetylhexosamine (203 Da). In the pglA background, the profiles were indicative of modification with GATDH together with one or two PE moieties. Again, identification of the GATDH sugar was confirmed by the presence in the ESI mass spectrum of the glycan oxonium ion at m/z 275.2 and its patterns of fragmentation during subsequent analysis by collision-induced dissociation (CID) MS/MS (see Fig. S1b in the supplemental material). In the pglEon background, the profiles were indicative of modification with a Hex-Hex-GATDH trisaccharide with one or two PE moieties. These findings confirm the previously described association of PglB2 with GATDH synthesis and demonstrate for the first time that PglA and PglE are necessary for synthesizing di- and trisaccharides having GATDH at the reducing end. Furthermore, the Hex-GATDH glycoform is susceptible to O acetylation, although there were significantly lower levels of this form than there were in the DATDH-expressing background (2). Somewhat surprisingly, signals indicative of O acetylation were not detectable in the Hex-Hex-GATDH-expressing background.
FIG. 6.
MS analysis of intact PilE carrying DATDH- or GATDH-based glycan forms. ESI-MS analyses of intact PilE utilizing pili from strains carrying either pglBMC58 or pglB28013 in different pgl backgrounds were carried out to characterize the glycan structure. N400 pglBMC58 pglA produced two major signals that are enclosed in boxes and represent PilE carrying the DATDH monosaccharide with one (m/z 17530) or two (m/z 17653) PE modifications. The N400 pglB28013 pglA signals represent PilE carrying the GATDH monosaccharide with one (m/z 17576) or two (m/z 17699) PEs. For N400 pglBMC58 and N400 pglB28013, the major signals represent PilE with one or two PE modifications in conjunction with the Ac-Hex-DATDH and Ac-Hex-GATDH disaccharides, respectively (indicated by m/z values in boxes). For N400 pglBMC58 pglEon, major signals correspond to PilE carrying Hex-Hex-DATDH with one or two PE modifications (boxes). N400 pglB28013 pglEon major signals are enclosed in boxes and represent Hex-Hex-GATDH with one or two PEs. Table S1 in the supplemental material shows all of the ion species along with the m/z values and corresponding molecular weights. The strains used were KS306 (N400 pglBMC58 pglA), KS309 (N400 pglB28013 pglA), KS305 (N400 pglBMC58), KS308 (N400 pglB28013), KS307 (N400 pglBMC58 pglEon), and KS310 (N400 pglB28013 pglEon).
Using strains expressing defined glycoforms in a single background, MAb reactivity was examined (Fig. 7). First, the patterns of reactivity exhibited by the strains expressing endogenous and MC58 PglB were identical for the three MAbs. This included detection of low levels of modified PilE with mono- and trisaccharide-recognizing MAbs in the disaccharide-expressing strains and of low levels of modified PilE with mono- and disaccharide-recognizing MAbs in the trisaccharide-expressing strains. For the trisaccharide reactivity in the disaccharide-expressing strains and the disaccharide reactivity in the trisaccharide-expressing strains, this could have been due to the presence of pglE phase variants that arose in the populations. For the monosaccharide reactivity in the disaccharide- and trisaccharide-expressing strains, it was unlikely due to a minor population of pglA phase-off variants as the allele in this background is not predicted to be subject to phase variability. Therefore, microheterogeneity in these cases may have reflected instances in which lipid-linked DATDH was flipped across the membrane prior to its modification by PglA.
FIG. 7.
GATDH-based glycans are antigenically distinct from DATDH-based forms. Western blots of N400 (pglB), N400 pglBMC58, and N400 pglB28013 in different pgl backgrounds were incubated with the npg1, npg2, and npg3 monoclonal antibodies. The strains used were KS141 (N400 pglA), KS306 (N400 pglBMC58 pglA), KS309 (N400 pglB28013 pglA), KS100 (N400), KS305 (N400 pglBMC58), KS308 (N400 pglB28013), KS142 (N400 pglEon), KS307 (N400 pglBMC58 pglEon), and KS310 (N400 pglB28013 pglEon).
The results for the GATDH-expressing backgrounds were strikingly distinct, and the levels of MAb reactivity were dramatically reduced. Nonetheless, weak PilE reactivity was seen for the monosaccharide-expressing strain with the monosaccharide-specific MAb and for the disaccharide-expressing strain with the disaccharide-specific MAb. It is noteworthy that moderate PilE reactivity was detected for the GATDH-based trisaccharide-expressing strain with the trisaccharide-specific MAb.
Immunochemical analysis of GATDH-based saccharides.
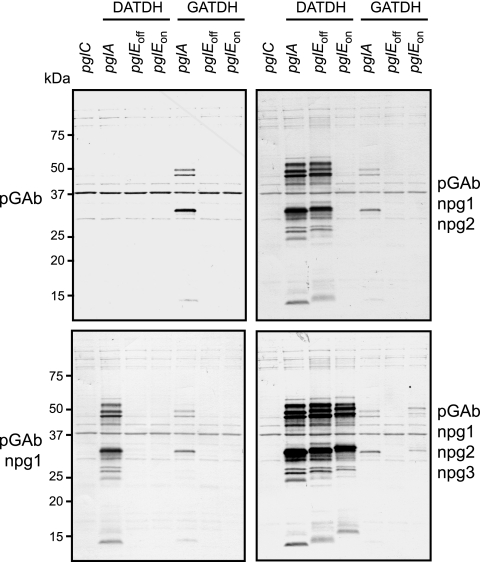
To examine GATDH immunogenicity and antigenicity, PilE bearing the GATDH monosaccharide (in the form of purified pili) was used to immunize rabbits, and the response was monitored by immunoblotting with the full suite of N. gonorrhoeae strains and recombinants (Fig. 8). The response observed was specific, and reactivity was detected only with the GATDH-expressing strain. In this case, four major immunoreactive proteins were detected, and three of them were identified as the AniA nitrite reductase and the Ng1494 and Ng1043 lipoproteins (data not shown). To compare this pattern with those observed for the other glycan-expressing strains, the same immunoblot filter was probed sequentially with the MAbs. Taking into account the minor shifts in mobilities associated with the different chain lengths of the glycoforms, the overall patterns of glycosylated proteins were remarkably similar. Moreover, npg3 detected an antigen pattern in the Hex-Hex-GATDH background analogous to that seen in the other strains, although the intensity was lower. Along with data for reactivity with the equivalently modified PilE, this confirms that npg3 has a diminished but specific ability to recognize an epitope associated with Hex-Hex-GATDH glycan. Taken together, the results show that protein-linked GATDH glycan is immunogenic and antigenic and that at least the GATDH and Hex-Hex-GATDH glycoforms can act as donor substrates in general, broad-spectrum protein glycosylation.
FIG. 8.
Protein-associated GATDH monosaccharide is both immunogenic and antigenic. An immunoblot of whole-cell lysates from strains with defined pgl backgrounds was first incubated with pGAb, a polyclonal antiserum raised against pili bearing the GATDH monosaccharide. The same filter was subsequently reprobed with the npg1, npg2, and npg3 MAbs with washing and developing steps between exposures. The strains used all had a 4/3/1 background, in which pilE was conditionally repressed, and were KS105 (4/3/1 pglC), KS122 (4/3/1 pglA), KS101 (4/3/1), KS127 (4/3/1 pglEon), KS312 (4/3/1 pglB28013 pglA), KS311 (4/3/1 pglB28013), and KS313 (4/3/1 pglB28013 pglEon).
Glycan epitopes are exposed on type IV pili.
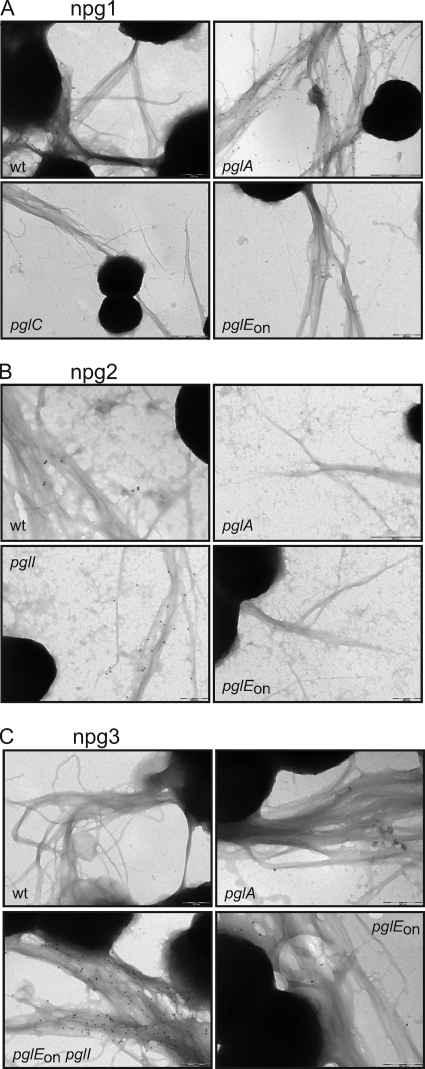
Modeling based on structural data suggested that the glycan attached at Ser63 of PilE is exposed on the pilus polymer surface (15). We directly tested this hypothesis using the MAbs and strains expressing DATDH-based glycans in transmission immunoelectron microscopy. Transmission electron micrographs demonstrated that decoration of pili with the MAbs occurred in a glycan-specific manner, with the caveat that in the case of the di- and trisaccharide-reactive MAbs the reactivity was dramatically increased in the absence of O acetylation (in the pglI background) (Fig. 9).
FIG. 9.
Glycan MAbs react specifically with intact pili as determined by immunoelectron microscopy. Immunogold labeling and transmission electron microscopy using the monoclonal antibodies (npg1, npg2, and npg3) against different N400 pgl strains demonstrated their specificity by binding to pili of the corresponding strains. The strains used were KS100 (N400) (wild type [wt]), KS141 (N400 pglA), KS104 (N400 pglC), KS142 (N400 pglEon), KS144 (N400 pglI), and KS304 (N400 pglEon pglI). Note that for the npg2 and npg3 MAbs, the levels of immunoreactivity were dramatically enhanced in the pglI background, in which glycan O acetylation was not present.
DISCUSSION
Pathogenic Neisseria species express a large number of cell surface components that are subject to intrastrain antigenic and phase variation. The evolution of the genetic systems underlying diversification of these surface molecules is likely driven by selection imposed by host adaptive and innate immunity. Previous studies have suggested that PilE-associated glycans might be immunogenic, as well as antigenically variable within a strain and between strains. Until now, however, the evidence for this has been somewhat limited as it has been obtained primarily with modified PilE as the immunogen and antigen and thus the specific contributions of polypeptide and glycan to the epitopes involved have been difficult to differentiate. Using neisserial glycoproteins modified with defined carbohydrates and immunization of rabbits, we demonstrated here that protein-linked glycans are both immunogenic and antigenic. In addition, the immune response was quite specific despite the use of structurally related glycans. The fact that the npg2 and npg3 MAb reactivities were dramatically enhanced by both the pglI null allele and chemical treatment strongly suggests that the epitopes recognized are sterically masked by O acetylation. As the pglI allele in N. gonorrhoeae N400 is not predicted to give rise to phase-off variants (70), the weak signals seen in di- and trisaccharide-expressing backgrounds with these MAbs thus likely reflect microheterogeneity due to incomplete O acetylation. Together, the results of this work prove that a single N. gonorrhoeae strain can express at least five distinct antigenic glycoforms: DATDH, Hex-DATDH, Hex-Hex-DATDH, and O-acetylated variants of the latter two glycoforms.
The MAbs recognizing defined glycan epitopes made it possible to probe other fundamental aspects of neisserial protein glycosylation. Most importantly, broad-spectrum, general O-linked protein glycosylation appears to be a common feature in the three species of Neisseria most important for humans and is not limited to N. gonorrhoeae strain N400. Given its presence in the nonpathogenic species N. lactamica, general O-linked protein glycosylation does not appear to be a virulence factor per se. Second, changes in glycan structure are manifested globally, as shown by the conservation of glycoprotein patterns in pgl variants and mutants of N. gonorrhoeae N400 and N. gonorrhoeae FA1090, as well as N. meningitidis MC58. Third, the overall repertoire of predominant glycoproteins appears to be more similar within a species than between species. Analyses of more isolates of all three species are warranted in order to corroborate and extend these findings.
Although the association between PglB2 and synthesis of the basal GATDH sugar is well established, it is not clear what effect the GATDH moiety has on glycan diversification and glycan-associated phenotypes. The pglB2 allele has been documented only for N. meningitidis strains and likely arose via import into a pglB background (48). Together with the presence of this allele in approximately 50% of isolates, it seems plausible that the prevalence of GATDH reflects the influence of positive selection. Perhaps most important in this regard is the finding that the GATDH moiety can be further modified by PglA and PglE glycosyltransferases, yielding Hex-GATDH and Hex-Hex-GATDH oligosaccharides. Since all three of these forms are antigenically distinct from the forms bearing the basal DATDH sugar, as shown by their lack of MAb reactivity, the introduction of pglB2 into N. meningitidis strains and its spread may have been due to immune selection. In addition, our data suggest that GATDH may have a negative impact on acetylation of hexoses linked to it or may promote lability of the acetate bonds. Given the manner in which the strains were constructed, we cannot formally rule out the possibility that the unique O-acetylation phenotypes are not related directly to the pglB2 allele but result from the altered gene organization and composition 3′ of pglB2. Together with the variable O acetylation of the GATDH disaccharide form, single N. meningitidis strains can express either four distinct GATDH glycoforms or five DATDH glycoforms.
Predicting the glycosylation phenotype based solely on the genotype is notoriously difficult (14). Despite this, there was a surprising degree of concordance between gene content and glycan phenotype as determined by both MAb reactivity and MS. Not only did reactivity with the MAbs correlate with the presence of pglB, pglA, and pglE, but minor levels of reactivity with the trisaccharide-recognizing MAb also correlated with the presence of phase-off alleles of pglE. Nonetheless, significant exceptions were found (Table 2). For example, on the basis of its pglB, pglAon, and pglEon genotypes, FA1090 would have been predicted to have expressed predominantly the DATDH-based trisaccharide and thus have an immunoblot pattern similar to that observed for the N400 pglEon background. Instead, it has a reactivity pattern consistent with a mixture of DATDH-based monosaccharide and trisaccharide forms. In addition, a similar pattern was seen for N. lactamica strain ST-3787, which, based on its genotype, would be expected to express the DATDH-based trisaccharide. Together with the reactivity with the trisaccharide MAb in FA1090 and ST-3787, this microheterogeneity likely reflects reduced PglA activity, which could be due to either an altered PglA structure or the presence of an alternative activity that competes with PglA for the lipid-linked DATDH substrate. Also, these two scenarios are not mutually exclusive. Furthermore, the pglB pglAon pglEoff genotype of N. meningitidis strain Z2491 resulted in the prediction that it should have an MAb reactivity pattern identical to that of MC58 and H44/76. However, unlike the results for the other two strains, no reactivity with the trisaccharide recognizing MAb was seen for Z2491 even though pglE is phase on in this strain. Further studies are needed to resolve the basis for these discrepancies.
TABLE 2.
Protein glycosylation genotypes and phenotypes
| Strain | Genotype |
Predicted glycan based on genotype | Main glycan identifieda | |||
|---|---|---|---|---|---|---|
| pglA | pglE | pglI | pglB | |||
| N400 | On, 4Gb | Off, 14xc | On, 6Gb | pglB | Ac-Hex-DATDH | Ac-Hex-DATDH |
| FA1090 | On, 11G | Onb | On, 6Gb | pglB | Ac-Hex-Hex-DATDH | DATDH |
| MC58 | On, 11G | Off, 34x | On, 13G | pglB | Ac-Hex-DATDH | Ac-Hex-DATDH |
| H44/76 | On, 11G | Off, 23x | On, 10G | pglB | Ac-Hex-DATDH | Ac-Hex-DATDH |
| Z2491 | On, 14G | On, 25x | On, 10G | pglB | Ac-Hex-Hex-DATDH | Ac-Hex-DATDH |
| 8013 | Off, 8G | Off, 39x | On, 10G | pglB2 | GATDH | |
| FAM18 | Off, 11G | Off, 29x | Off, 14G | pglB2 | GATDH | |
| BZ 10 | Off, 12G | Off, 24x | Off, 9G | pglB2 | Hex-GATDH | |
| BZ 198 | On, 8G | Off, 22x | Off, 11G | pglB2 | Hex-GATDH | |
| ST-3787 | On, 8G | On, 16x | On, 12G | pglB | Ac-Hex-Hex-DATDH | DATDH |
| ST-640 | Off, 10G | On, 25x | On, 15G | pglB | DATDH | DATDH |
The finding that immunization with glycosylated Tfp from N. gonorrhoeae produces a humoral immune response specific to the glycan moiety is interesting in a number of ways. First, a large number of studies in the 1960s to 1990s examined the immunogenicity and antigenicity of N. gonorrhoeae pili in order to assess their potential as a gonococcal vaccine component (8, 30, 42, 60). Together with analogous studies of N. meningitidis pili, these studies provided serological evidence for pilus antigenic diversity, which was subsequently interpreted as a reflection of wholesale changes in pilin primary structure resulting from gene conversion-like events involving multiple pilin gene copies (55, 74). Although not appreciated at the time, it seems very likely that glycosylated pili were used in these studies and that antiglycan antibodies may have influenced at least some of the observations made. In this context, it is also worth noting that although a large number of MAbs and sera (including those raised against pilin-based peptides) have been used to define conserved and variable domains on pili, the surface-exposed epitopes involved have been precisely defined in only a very few instances (17, 55, 65, 66, 68). We suggest that glycan modification at a site in pilin which is both surface exposed and constrained at the level of primary structure may directly mask conserved epitopes at two levels. First, the glycan may physically block accessibility of polypeptide-directed antibodies. Second, the glycan may perturb antigen processing and peptide presentation such that humoral responses to conserved domains are diminished and instead directed toward the glycan, which is antigenically variable itself.
Glycan-directed antibodies might also exert an effect through glycoproteins other than PilE. In this context, it is interesting that other studies have reported evidence indicating that some of the glycoproteins identified in N. gonorrhoeae and now in N. meningitidis are exposed at the bacterial surface. These glycoproteins include a putative peptidyl-prolylisomerase (NGO1225) (38), GNA1946 (45), Ag473 (NGO1043) (28), and the nitrite reductase AniA (NGO1276) (37). It is also worth mentioning that the original evidence that AniA is expressed in vivo was derived from detection of AniA-reactive antibodies in diseased patients but not in normal human sera (12). Based on our findings, it is possible that the reactions observed for AniA reflect a more general antiglycan response rather than a specific AniA response.
In summary, these studies lay the foundation for detailed elucidation of the genotype-phenotype relationships underlying protein glycan diversity in important neisserial species. Given the high degree of pgl gene polymorphism and the presence of uncharacterized genes linked to known pgl-related loci, we hypothesize that the total repertoire of protein glycans may be significantly larger than that identified so far. In fact, the protein-associated glycan diversity may exceed that documented for neisserial lipooligosaccharides and meningococcal capsular polysaccharides. The findings of this study and the reagents used should also facilitate efforts to evaluate the potential presence of glycoproteins in outer membrane vesicle (OMV)-based vaccines currently in use or being tested (25) and to evaluate whether humans generate either humoral or innate immune responses to the protein glycan moieties during carriage or disease.
Supplementary Material
Acknowledgments
We thank J. Moir (York University) and M. Virji (University of Bristol) for providing the AniA and SM1 antibodies, respectively.
This research was supported in part by Research Council of Norway grants 166931, 183613, and 183814 and by funds from the Department of Molecular Biosciences and Center for Molecular Biology and Neurosciences of the University of Oslo.
Footnotes
Published ahead of print on 2 April 2010.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Aas, F. E., W. Egge-Jacobsen, H. C. Winther-Larsen, C. Lovold, P. G. Hitchen, A. Dell, and M. Koomey. 2006. Neisseria gonorrhoeae type IV pili undergo multisite, hierarchical modifications with phosphoethanolamine and phosphocholine requiring an enzyme structurally related to lipopolysaccharide phosphoethanolamine transferases. J. Biol. Chem. 281:27712-27723. [DOI] [PubMed] [Google Scholar]
- 2.Aas, F. E., A. Vik, J. Vedde, M. Koomey, and W. Egge-Jacobsen. 2007. Neisseria gonorrhoeae O-linked pilin glycosylation: functional analyses define both the biosynthetic pathway and glycan structure. Mol. Microbiol. 65:607-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aas, F. E., H. C. Winther-Larsen, M. Wolfgang, S. Frye, C. Lovold, N. Roos, J. P. van Putten, and M. Koomey. 2007. Substitutions in the N-terminal alpha helical spine of Neisseria gonorrhoeae pilin affect type IV pilus assembly, dynamics and associated functions. Mol. Microbiol. 63:69-85. [DOI] [PubMed] [Google Scholar]
- 4.Banerjee, A., R. Wang, S. L. Supernavage, S. K. Ghosh, J. Parker, N. F. Ganesh, P. G. Wang, S. Gulati, and P. A. Rice. 2002. Implications of phase variation of a gene (pgtA) encoding a pilin galactosyl transferase in gonococcal pathogenesis. J. Exp. Med. 196:147-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennett, J. S., D. T. Griffiths, N. D. McCarthy, K. L. Sleeman, K. A. Jolley, D. W. Crook, and M. C. Maiden. 2005. Genetic diversity and carriage dynamics of Neisseria lactamica in infants. Infect. Immun. 73:2424-2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bentley, S. D., G. S. Vernikos, L. A. Snyder, C. Churcher, C. Arrowsmith, T. Chillingworth, A. Cronin, P. H. Davis, N. E. Holroyd, K. Jagels, M. Maddison, S. Moule, E. Rabbinowitsch, S. Sharp, L. Unwin, S. Whitehead, M. A. Quail, M. Achtman, B. Barrell, N. J. Saunders, and J. Parkhill. 2007. Meningococcal genetic variation mechanisms viewed through comparative analysis of serogroup C strain FAM18. PLoS Genet. 3:e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benz, I., and M. A. Schmidt. 2001. Glycosylation with heptose residues mediated by the aah gene product is essential for adherence of the AIDA-I adhesin. Mol. Microbiol. 40:1403-1413. [DOI] [PubMed] [Google Scholar]
- 8.Buchanan, T. M. 1975. Antigenic heterogeneity of gonococcal pili. J. Exp. Med. 141:1470-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carlin, N. I., T. Wehler, and A. A. Lindberg. 1986. Shigella flexneri O-antigen epitopes: chemical and immunochemical analyses reveal that epitopes of type III and group 6 antigens are identical. Infect. Immun. 53:110-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chamot-Rooke, J., B. Rousseau, F. Lanternier, G. Mikaty, E. Mairey, C. Malosse, G. Bouchoux, V. Pelicic, L. Camoin, X. Nassif, and G. Dumenil. 2007. Alternative Neisseria spp. type IV pilin glycosylation with a glyceramido acetamido trideoxyhexose residue. Proc. Natl. Acad. Sci. U. S. A. 104:14783-14788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charbonneau, M. E., V. Girard, A. Nikolakakis, M. Campos, F. Berthiaume, F. Dumas, F. Lepine, and M. Mourez. 2007. O-linked glycosylation ensures the normal conformation of the autotransporter adhesin involved in diffuse adherence. J. Bacteriol. 189:8880-8889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clark, V. L., J. S. Knapp, S. Thompson, and K. W. Klimpel. 1988. Presence of antibodies to the major anaerobically induced gonococcal outer membrane protein in sera from patients with gonococcal infections. Microb. Pathog. 5:381-390. [DOI] [PubMed] [Google Scholar]
- 13.Comstock, L. E., and D. L. Kasper. 2006. Bacterial glycans: key mediators of diverse host immune responses. Cell 126:847-850. [DOI] [PubMed] [Google Scholar]
- 14.Coutinho, P. M., E. Deleury, G. J. Davies, and B. Henrissat. 2003. An evolving hierarchical family classification for glycosyltransferases. J. Mol. Biol. 328:307-317. [DOI] [PubMed] [Google Scholar]
- 15.Craig, L., N. Volkmann, A. S. Arvai, M. E. Pique, M. Yeager, E. H. Egelman, and J. A. Tainer. 2006. Type IV pilus structure by cryo-electron microscopy and crystallography: implications for pilus assembly and functions. Mol. Cell 23:651-662. [DOI] [PubMed] [Google Scholar]
- 16.DiGiandomenico, A., M. J. Matewish, A. Bisaillon, J. R. Stehle, J. S. Lam, and P. Castric. 2002. Glycosylation of Pseudomonas aeruginosa 1244 pilin: glycan substrate specificity. Mol. Microbiol. 46:519-530. [DOI] [PubMed] [Google Scholar]
- 17.Edwards, M., R. L. McDade, G. Schoolnik, J. B. Rothbard, and E. C. Gotschlich. 1984. Antigenic analysis of gonococcal pili using monoclonal antibodies. J. Exp. Med. 160:1782-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faridmoayer, A., M. A. Fentabil, M. F. Haurat, W. Yi, R. Woodward, P. G. Wang, and M. F. Feldman. 2008. Extreme substrate promiscuity of the Neisseria oligosaccharyl transferase involved in protein O-glycosylation. J. Biol. Chem. 283:34596-34604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fletcher, C. M., M. J. Coyne, O. F. Villa, M. Chatzidaki-Livanis, and L. E. Comstock. 2009. A general O-glycosylation system important to the physiology of a major human intestinal symbiont. Cell 137:321-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freitag, N. E., H. S. Seifert, and M. Koomey. 1995. Characterization of the pilF-pilD pilus-assembly locus of Neisseria gonorrhoeae. Mol. Microbiol. 16:575-586. [DOI] [PubMed] [Google Scholar]
- 21.Glover, K. J., E. Weerapana, and B. Imperiali. 2005. In vitro assembly of the undecaprenylpyrophosphate-linked heptasaccharide for prokaryotic N-linked glycosylation. Proc. Natl. Acad. Sci. U. S. A. 102:14255-14259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hegge, F. T., P. G. Hitchen, F. E. Aas, H. Kristiansen, C. Lovold, W. Egge-Jacobsen, M. Panico, W. Y. Leong, V. Bull, M. Virji, H. R. Morris, A. Dell, and M. Koomey. 2004. Unique modifications with phosphocholine and phosphoethanolamine define alternate antigenic forms of Neisseria gonorrhoeae type IV pili. Proc. Natl. Acad. Sci. U. S. A. 101:10798-10803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hendrixson, D. R., and V. J. DiRita. 2004. Identification of Campylobacter jejuni genes involved in commensal colonization of the chick gastrointestinal tract. Mol. Microbiol. 52:471-484. [DOI] [PubMed] [Google Scholar]
- 24.Hollis, D. G., G. L. Wiggins, and R. E. Weaver. 1969. Neisseria lactamicus sp. n., a lactose-fermenting species resembling Neisseria meningitidis. Appl. Microbiol. 17:71-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holst, J., D. Martin, R. Arnold, C. C. Huergo, P. Oster, J. O'Hallahan, and E. Rosenqvist. 2009. Properties and clinical performance of vaccines containing outer membrane vesicles from Neisseria meningitidis. Vaccine 27(Suppl. 2):B3-B12. [DOI] [PubMed] [Google Scholar]
- 26.Holten, E. 1979. Serotypes of Neisseria meningitidis isolated from patients in Norway during the first six months of 1978. J. Clin. Microbiol. 9:186-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horzempa, J., T. K. Held, A. S. Cross, D. Furst, M. Qutyan, A. N. Neely, and P. Castric. 2008. Immunization with a Pseudomonas aeruginosa 1244 pilin provides O-antigen-specific protection. Clin. Vaccine Immunol. 15:590-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsu, C. A., W. R. Lin, J. C. Li, Y. L. Liu, Y. T. Tseng, C. M. Chang, Y. S. Lee, and C. Y. Yang. 2008. Immunoproteomic identification of the hypothetical protein NMB1468 as a novel lipoprotein ubiquitous in Neisseria meningitidis with vaccine potential. Proteomics 8:2115-2125. [DOI] [PubMed] [Google Scholar]
- 29.Jennings, M. P., M. Virji, D. Evans, V. Foster, Y. N. Srikhanta, L. Steeghs, P. van der Ley, and E. R. Moxon. 1998. Identification of a novel gene involved in pilin glycosylation in Neisseria meningitidis. Mol. Microbiol. 29:975-984. [DOI] [PubMed] [Google Scholar]
- 30.Johnson, S. C., R. C. Chung, C. D. Deal, J. W. Boslego, J. C. Sadoff, S. W. Wood, C. C. Brinton, Jr., and E. C. Tramont. 1991. Human immunization with Pgh 3-2 gonococcal pilus results in cross-reactive antibody to the cyanogen bromide fragment-2 of pilin. J. Infect. Dis. 163:128-134. [DOI] [PubMed] [Google Scholar]
- 31.Johnston, D. M., and J. G. Cannon. 1999. Construction of mutant strains of Neisseria gonorrhoeae lacking new antibiotic resistance markers using a two gene cassette with positive and negative selection. Gene 236:179-184. [DOI] [PubMed] [Google Scholar]
- 32.Kahler, C. M., L. E. Martin, Y. L. Tzeng, Y. K. Miller, K. Sharkey, D. S. Stephens, and J. K. Davies. 2001. Polymorphisms in pilin glycosylation locus of Neisseria meningitidis expressing class II pili. Infect. Immun. 69:3597-3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klee, S. R., X. Nassif, B. Kusecek, P. Merker, J. L. Beretti, M. Achtman, and C. R. Tinsley. 2000. Molecular and biological analysis of eight genetic islands that distinguish Neisseria meningitidis from the closely related pathogen Neisseria gonorrhoea e. Infect. Immun. 68:2082-2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knight, K. L. October 1997. Immortalized rabbit hybridoma fusion partner. U.S. patent 5,675,0630.
- 35.Knudsen, S. K., A. Stensballe, M. Franzmann, U. B. Westergaard, and D. E. Otzen. 2008. Effect of glycosylation on the extracellular domain of the Ag43 bacterial autotransporter: enhanced stability and reduced cellular aggregation. Biochem. J. 412:563-577. [DOI] [PubMed] [Google Scholar]
- 36.Kowarik, M., N. M. Young, S. Numao, B. L. Schulz, I. Hug, N. Callewaert, D. C. Mills, D. C. Watson, M. Hernandez, J. F. Kelly, M. Wacker, and M. Aebi. 2006. Definition of the bacterial N-glycosylation site consensus sequence. EMBO J. 25:1957-1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ku, S. C., B. L. Schulz, P. M. Power, and M. P. Jennings. 2009. The pilin O-glycosylation pathway of pathogenic Neisseria is a general system that glycosylates AniA, an outer membrane nitrite reductase. Biochem. Biophys. Res. Commun. 378:84-89. [DOI] [PubMed] [Google Scholar]
- 38.Leuzzi, R., L. Serino, M. Scarselli, S. Savino, M. R. Fontana, E. Monaci, A. Taddei, G. Fischer, R. Rappuoli, and M. Pizza. 2005. Ng-MIP, a surface-exposed lipoprotein of Neisseria gonorrhoeae, has a peptidyl-prolyl cis/trans isomerase (PPIase) activity and is involved in persistence in macrophages. Mol. Microbiol. 58:669-681. [DOI] [PubMed] [Google Scholar]
- 39.Linton, D., N. Dorrell, P. G. Hitchen, S. Amber, A. V. Karlyshev, H. R. Morris, A. Dell, M. A. Valvano, M. Aebi, and B. W. Wren. 2005. Functional analysis of the Campylobacter jejuni N-linked protein glycosylation pathway. Mol. Microbiol. 55:1695-1703. [DOI] [PubMed] [Google Scholar]
- 40.Logan, S. M. 2006. Flagellar glycosylation—a new component of the motility repertoire? Microbiology 152:1249-1262. [DOI] [PubMed] [Google Scholar]
- 41.Logan, S. M., J. F. Kelly, P. Thibault, C. P. Ewing, and P. Guerry. 2002. Structural heterogeneity of carbohydrate modifications affects serospecificity of Campylobacter flagellins. Mol. Microbiol. 46:587-597. [DOI] [PubMed] [Google Scholar]
- 42.McChesney, D., E. C. Tramont, J. W. Boslego, J. Ciak, J. Sadoff, and C. C. Brinton. 1982. Genital antibody response to a parenteral gonococcal pilus vaccine. Infect. Immun. 36:1006-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Orskov, F., I. Orskov, A. Sutton, R. Schneerson, W. Lin, W. Egan, G. E. Hoff, and J. B. Robbins. 1979. Form variation in Escherichia coli K1: determined by O-acetylation of the capsular polysaccharide. J. Exp. Med. 149:669-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parkhill, J., M. Achtman, K. D. James, S. D. Bentley, C. Churcher, S. R. Klee, G. Morelli, D. Basham, D. Brown, T. Chillingworth, R. M. Davies, P. Davis, K. Devlin, T. Feltwell, N. Hamlin, S. Holroyd, K. Jagels, S. Leather, S. Moule, K. Mungall, M. A. Quail, M. A. Rajandream, K. M. Rutherford, M. Simmonds, J. Skelton, S. Whitehead, B. G. Spratt, and B. G. Barrell. 2000. Complete DNA sequence of a serogroup A strain of Neisseria meningitidis Z2491. Nature 404:502-506. [DOI] [PubMed] [Google Scholar]
- 45.Pizza, M., V. Scarlato, V. Masignani, M. M. Giuliani, B. Arico, M. Comanducci, G. T. Jennings, L. Baldi, E. Bartolini, B. Capecchi, C. L. Galeotti, E. Luzzi, R. Manetti, E. Marchetti, M. Mora, S. Nuti, G. Ratti, L. Santini, S. Savino, M. Scarselli, E. Storni, P. Zuo, M. Broeker, E. Hundt, B. Knapp, E. Blair, T. Mason, H. Tettelin, D. W. Hood, A. C. Jeffries, N. J. Saunders, D. M. Granoff, J. C. Venter, E. R. Moxon, G. Grandi, and R. Rappuoli. 2000. Identification of vaccine candidates against serogroup B meningococcus by whole-genome sequencing. Science 287:1816-1820. [DOI] [PubMed] [Google Scholar]
- 46.Power, P. M., S. C. Ku, K. Rutter, M. J. Warren, E. A. Limnios, J. W. Tapsall, and M. P. Jennings. 2007. The phase-variable allele of the pilus glycosylation gene pglA is not strongly associated with strains of Neisseria gonorrhoeae isolated from patients with disseminated gonococcal infection. Infect. Immun. 75:3202-3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Power, P. M., L. F. Roddam, M. Dieckelmann, Y. N. Srikhanta, Y. C. Tan, A. W. Berrington, and M. P. Jennings. 2000. Genetic characterization of pilin glycosylation in Neisseria meningitidis. Microbiology 146:967-979. [DOI] [PubMed] [Google Scholar]
- 48.Power, P. M., L. F. Roddam, K. Rutter, S. Z. Fitzpatrick, Y. N. Srikhanta, and M. P. Jennings. 2003. Genetic characterization of pilin glycosylation and phase variation in Neisseria meningitidis. Mol. Microbiol. 49:833-847. [DOI] [PubMed] [Google Scholar]
- 49.Qutyan, M., M. Paliotti, and P. Castric. 2007. PilO of Pseudomonas aeruginosa 1244: subcellular location and domain assignment. Mol. Microbiol. 66:1444-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sartain, M. J., and J. T. Belisle. 2009. N-terminal clustering of the O-glycosylation sites in the Mycobacterium tuberculosis lipoprotein SodC. Glycobiology 19:38-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sherlock, O., U. Dobrindt, J. B. Jensen, R. Munk Vejborg, and P. Klemm. 2006. Glycosylation of the self-recognizing Escherichia coli Ag43 autotransporter protein. J. Bacteriol. 188:1798-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spieker-Polet, H., P. Sethupathi, P. C. Yam, and K. L. Knight. 1995. Rabbit monoclonal antibodies: generating a fusion partner to produce rabbit-rabbit hybridomas. Proc. Natl. Acad. Sci. U. S. A. 92:9348-9352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Steiner, K., R. Novotny, D. B. Werz, K. Zarschler, P. H. Seeberger, A. Hofinger, P. Kosma, C. Schaffer, and P. Messner. 2008. Molecular basis of S-layer glycoprotein glycan biosynthesis in Geobacillus stearothermophilus. J. Biol. Chem. 283:21120-21133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stimson, E., M. Virji, K. Makepeace, A. Dell, H. R. Morris, G. Payne, J. R. Saunders, M. P. Jennings, S. Barker, M. Panico, et al. 1995. Meningococcal pilin: a glycoprotein substituted with digalactosyl 2,4-diacetamido-2,4,6-trideoxyhexose. Mol. Microbiol. 17:1201-1214. [DOI] [PubMed] [Google Scholar]
- 55.Swanson, J., K. Robbins, O. Barrera, D. Corwin, J. Boslego, J. Ciak, M. Blake, and J. M. Koomey. 1987. Gonococcal pilin variants in experimental gonorrhea. J. Exp. Med. 165:1344-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Taguchi, F., K. Takeuchi, E. Katoh, K. Murata, T. Suzuki, M. Marutani, T. Kawasaki, M. Eguchi, S. Katoh, H. Kaku, C. Yasuda, Y. Inagaki, K. Toyoda, T. Shiraishi, and Y. Ichinose. 2006. Identification of glycosylation genes and glycosylated amino acids of flagellin in Pseudomonas syringae pv. tabaci. Cell. Microbiol. 8:923-938. [DOI] [PubMed] [Google Scholar]
- 57.Takeuchi, K., F. Taguchi, Y. Inagaki, K. Toyoda, T. Shiraishi, and Y. Ichinose. 2003. Flagellin glycosylation island in Pseudomonas syringae pv. glycinea and its role in host specificity. J. Bacteriol. 185:6658-6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tettelin, H., N. J. Saunders, J. Heidelberg, A. C. Jeffries, K. E. Nelson, J. A. Eisen, K. A. Ketchum, D. W. Hood, J. F. Peden, R. J. Dodson, W. C. Nelson, M. L. Gwinn, R. DeBoy, J. D. Peterson, E. K. Hickey, D. H. Haft, S. L. Salzberg, O. White, R. D. Fleischmann, B. A. Dougherty, T. Mason, A. Ciecko, D. S. Parksey, E. Blair, H. Cittone, E. B. Clark, M. D. Cotton, T. R. Utterback, H. Khouri, H. Qin, J. Vamathevan, J. Gill, V. Scarlato, V. Masignani, M. Pizza, G. Grandi, L. Sun, H. O. Smith, C. M. Fraser, E. R. Moxon, R. Rappuoli, and J. C. Venter. 2000. Complete genome sequence of Neisseria meningitidis serogroup B strain MC58. Science 287:1809-1815. [DOI] [PubMed] [Google Scholar]
- 59.Tonjum, T., N. E. Freitag, E. Namork, and M. Koomey. 1995. Identification and characterization of pilG, a highly conserved pilus-assembly gene in pathogenic Neisseria. Mol. Microbiol. 16:451-464. [DOI] [PubMed] [Google Scholar]
- 60.Tramont, E. C., J. C. Sadoff, J. W. Boslego, J. Ciak, D. McChesney, C. C. Brinton, S. Wood, and E. Takafuji. 1981. Gonococcal pilus vaccine. Studies of antigenicity and inhibition of attachment. J. Clin. Invest. 68:881-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Kooyk, Y., and G. A. Rabinovich. 2008. Protein-glycan interactions in the control of innate and adaptive immune responses. Nat. Immunol. 9:593-601. [DOI] [PubMed] [Google Scholar]
- 62.van Sorge, N. M., N. M. Bleumink, S. J. van Vliet, E. Saeland, W. L. van der Pol, Y. van Kooyk, and J. P. van Putten. 2009. N-glycosylated proteins and distinct lipooligosaccharide glycoforms of Campylobacter jejuni target the human C-type lectin receptor MGL. Cell. Microbiol. 11:1768-1781. [DOI] [PubMed] [Google Scholar]
- 63.Verma, A., S. K. Arora, S. K. Kuravi, and R. Ramphal. 2005. Roles of specific amino acids in the N terminus of Pseudomonas aeruginosa flagellin and of flagellin glycosylation in the innate immune response. Infect. Immun. 73:8237-8246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vik, A., F. E. Aas, J. H. Anonsen, S. Bilsborough, A. Schneider, W. Egge-Jacobsen, and M. Koomey. 2009. Broad spectrum O-linked protein glycosylation in the human pathogen Neisseria gonorrhoeae. Proc. Natl. Acad. Sci. U. S. A. 106:4447-4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Virji, M., and J. E. Heckels. 1983. Antigenic cross-reactivity of Neisseria pili: investigations with type- and species-specific monoclonal antibodies. J. Gen. Microbiol. 129:2761-2768. [DOI] [PubMed] [Google Scholar]
- 66.Virji, M., and J. E. Heckels. 1984. The role of common and type-specific pilus antigenic domains in adhesion and virulence of gonococci for human epithelial cells. J. Gen. Microbiol. 130:1089-1095. [DOI] [PubMed] [Google Scholar]
- 67.Virji, M., J. E. Heckels, W. J. Potts, C. A. Hart, and J. R. Saunders. 1989. Identification of epitopes recognized by monoclonal antibodies SM1 and SM2 which react with all pili of Neisseria gonorrhoeae but which differentiate between two structural classes of pili expressed by Neisseria meningitidis and the distribution of their encoding sequences in the genomes of Neisseria spp. J. Gen. Microbiol. 135:3239-3251. [DOI] [PubMed] [Google Scholar]
- 68.Virji, M., J. E. Heckels, and P. J. Watt. 1983. Monoclonal antibodies to gonococcal pili: studies on antigenic determinants on pili from variants of strain P9. J. Gen. Microbiol. 129:1965-1973. [DOI] [PubMed] [Google Scholar]
- 69.Wacker, M., D. Linton, P. G. Hitchen, M. Nita-Lazar, S. M. Haslam, S. J. North, M. Panico, H. R. Morris, A. Dell, B. W. Wren, and M. Aebi. 2002. N-linked glycosylation in Campylobacter jejuni and its functional transfer into E. coli. Science 298:1790-1793. [DOI] [PubMed] [Google Scholar]
- 70.Warren, M. J., L. F. Roddam, P. M. Power, T. D. Terry, and M. P. Jennings. 2004. Analysis of the role of pglI in pilin glycosylation of Neisseria meningitidis. FEMS Immunol. Med. Microbiol. 41:43-50. [DOI] [PubMed] [Google Scholar]
- 71.Wolfgang, M., J. P. van Putten, S. F. Hayes, D. Dorward, and M. Koomey. 2000. Components and dynamics of fiber formation define a ubiquitous biogenesis pathway for bacterial pili. EMBO J. 19:6408-6418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu, H., S. Bu, P. Newell, Q. Chen, and P. Fives-Taylor. 2007. Two gene determinants are differentially involved in the biogenesis of Fap1 precursors in Streptococcus parasanguis. J. Bacteriol. 189:1390-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Young, N. M., J. R. Brisson, J. Kelly, D. C. Watson, L. Tessier, P. H. Lanthier, H. C. Jarrell, N. Cadotte, F. St. Michael, E. Aberg, and C. M. Szymanski. 2002. Structure of the N-linked glycan present on multiple glycoproteins in the Gram-negative bacterium, Campylobacter jejuni. J. Biol. Chem. 277:42530-42539. [DOI] [PubMed] [Google Scholar]
- 74.Zhang, Q. Y., D. DeRyckere, P. Lauer, and M. Koomey. 1992. Gene conversion in Neisseria gonorrhoeae: evidence for its role in pilus antigenic variation. Proc. Natl. Acad. Sci. U. S. A. 89:5366-5370. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.