Abstract
Calpains are calcium-dependent intracellular cysteine proteases, which include ubiquitously expressed μ- and m-calpains. Both calpains are heterodimers consisting of a large catalytic subunit and a small regulatory subunit. The calpain small subunit encoded by the gene Capn4 directly binds to the intracellular C-terminal tail of the receptor for the parathyroid hormone (PTH) and PTH-related peptide and modulates cellular functions in cells of the osteoblast lineage in vitro and in vivo. To investigate a physiological role of the calpain small subunit in cells of the chondrocyte lineage, we generated chondrocyte-specific Capn4 knockout mice. Mutant embryos had reduced chondrocyte proliferation and differentiation in embryonic growth plates compared with control littermates. In vitro analysis further revealed that deletion of Capn4 in cells of the chondrocyte lineage correlated with impaired cell cycle progression at the G1/S transition, reduced cyclin D gene transcription, and accumulated cell cycle proteins known as calpain substrates. Moreover, silencing of p27Kip1 rescued an impaired cell growth phenotype in Capn4 knockdown cells, and reintroducing the calpain small subunit partially normalized cell growth and accumulated cyclin D protein levels in a dose-dependent manner. Collectively, our findings suggest that the calpain small subunit is essential for proper chondrocyte functions in embryonic growth plates.
Ubiquitously expressed μ- and m-calpains belong to a family of calcium-dependent intracellular cysteine proteases (8, 13) and form heterodimers consisting of a large catalytic subunit encoded by the genes Capn1 and Capn2, respectively, and a small regulatory subunit encoded by the gene Capn4 (13). Deletion of Capn4 eliminates both μ- and m-calpain activities in embryonic fibroblasts (2), suggesting that the calpain small subunit is critical for maintenance of calpain stability and activity. Genetic ablation of Capn4 results in embryonic lethality, which demonstrates an essential role of the calpain small subunit during embryonic development (2, 53).
We previously created osteoblast-specific Capn4 knockout mice by mating mice homozygous for floxed Capn4 alleles (Capn4flox/flox) (46) with those expressing Cre driven by the osterix promoter (Osx-Cre+/−) (38) and showed that lack of the calpain small subunit in cells of the osteoblast lineage results in a significant reduction of both trabecular and cortical bone, which is associated with a severe impairment of osteoblast proliferation and differentiation (41). Interestingly, in Osx-Cre+/− transgenic mice, Cre recombinase is expressed not only in early cells of the osteoblast lineage but also in late proliferating chondrocytes (22). We, thus, speculated that the smaller size of the skeletons of Osx-Cre+/− Capn4flox/flox newborn mice could be at least partially due to abnormal growth plate development, which is associated with genetic ablation of Capn4 in chondrocytes.
m-Calpain has been reported to be expressed in hypertrophic chondrocytes in normal rat growth plates (43, 52). However, little is known about a physiological role of the calpain small subunit in cells of the chondrocyte lineage in vitro or in vivo. To investigate a role of the calpain small subunit in growth plate development, we conditionally ablated Capn4 in cells of the chondrocyte lineage. Deletion of the calpain small subunit in chondrocytes showed impaired chondrocyte proliferation and differentiation in embryonic growth plates. In vitro observations further revealed (i) impaired cell cycle transition from G1 to S phase; (ii) reduced cyclin D gene transcription; (iii) accumulated cell cycle proteins known as calpain substrates, including cyclin D, cyclin E, and cyclin-dependent kinase inhibitor 1B (p27Kip1); (iv) reduced phosphorylation of retinoblastoma protein (Rb) on threonine 821; (v) impaired phosphorylation of p27Kip1 on serine 10 and its degradation; and (vi) a rescued impaired cell growth phenotype by silencing p27Kip1 and reintroducing the calpain small subunit in Capn4 knockdown cells of the chondrocyte lineage. These findings are suggestive of a critical role of the calpain small subunit in growth plate development.
MATERIALS AND METHODS
Generation of Col.2-Cre+/− Capn4flox/flox mice.
Capn4flox/flox mice were crossed with those expressing Cre under the control of the collagen IIα1 promoter (Col.2-Cre+/−), to generate Col.2-Cre+/− Capn4flox/+ female mice (46, 51). These mice were then crossed with Capn4flox/flox male mice to generate Col.2-Cre+/− Capn4flox/flox mutant embryos and Col.2-Cre+/− Capn4flox/+ littermate controls. In some experiments, Osx-Cre+/− Capn4flox/flox mice that had been established previously were also used in this study (41). All experiments were performed in compliance with the guiding principles of the Guide for the Care and Use of Laboratory Animals and approved by the subcommittee on Research Animal Care of Massachusetts General Hospital (MGH). The genotypes of mice and embryos were determined by PCR-based strategies as described previously (41).
Analysis of bromodeoxyuridine (BrdU) incorporation.
Pregnant female mice were injected intraperitoneally with 100 μg BrdU and 12 μg fluorodeoxyuridine per gram of body weight 2 h before being sacrificed (Sigma-Aldrich Biotechnology, St. Louis, MO). To identify actively proliferating cells, nuclei that had incorporated BrdU were detected as described previously (41).
In situ hybridization.
In situ hybridization analysis was performed as described previously (26). Complementary riboprobes were transcribed from the plasmids encoding mouse collagen Iα1 (Col.1), Col.2, collagen Xα1 (Col.10), matrix metalloproteinase 13 (MMP13), and osteopontin (OP) by using Riboprobe systems from Promega (Madison, WI).
Immunohistochemistry.
Immunohistochemistry was performed using anti-rabbit p27Kip1 and cyclin D2 polyclonal antibodies (Millipore Corp., Billerica, MA, and Santa Cruz Biotechnology, Santa Cruz, CA, respectively), ABC and 3,3′-diaminobenzidine (DAB) kits (Vector Laboratory, Burlingame, CA). Counterstaining of nuclei or matrix of the growth plate was not performed. Semiquantification was performed using FluorChem SP (Alpha Innotech Corp., San Leandro, CA) by subtracting the background intensity of control samples, which were prepared similarly but without incubation with primary antibodies.
Chondrocyte isolation and culture.
Primary chondrocytes were isolated from Capn4flox/flox newborn mice. In brief, the costochondral regions of newborn Capn4flox/flox mice were dissected, rinsed with phosphate-buffered saline, and digested in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen Corp., Carlsbad, CA) supplemented with 0.25% type II collagenase (Worthington, Lakewood, NJ) for 2 h at 37°C. The digested cells were collected, washed with fresh medium, and cultured in DMEM containing 10% fetal bovine serum (FBS) (HyClone, Logan, UT) and 1% penicillin-streptomycin (Invitrogen). The medium was changed every other day. To examine the effect of Capn4 deficiency in chondrocyte differentiation, we incubated primary chondrocytes in DMEM supplemented with 10% FBS, 1% penicillin-streptomycin, and 50 μg/ml ascorbic acid (Sigma-Aldrich) for 3 days. Monolayer Capn4flox/flox chondrocytes were infected with either control adenovirus (Ad-lacZ) or Cre recombinase adenovirus (Ad-Cre) at a multiplicity of infection of 100 for most experiments, as described previously (41).
Calpain activity assay.
Calpain activity was measured as described previously with a slight modification (42). In brief, cell lysates (10 μg/sample) were incubated with 200 μM calpain substrate, N-succinyl-Leu-Leu-Val-Tyr-7-amino-4-methylcoumarin (Bachem, Torrance, CA) in a buffer containing 10 mM Tris-HCl (pH 7.5), 50 mM KCl, 1 mM EDTA, 1 mM dithiothreitol (DTT), and 3 mM Ca2+ at 37°C for 20 min. Fluorescence was measured at 460 nm with excitation at 355 nm by using a Vector3 plate reader, and data were analyzed using Wallac software (PerkinElmer Life and Analytical Science Inc., Waltham, MA). 7-Amino-4-methylcoumarine (Bachem) was used as a standard.
Real-time qPCR.
The efficiency of Cre recombinase-driven deletion of the floxed Capn4 gene was assessed using real-time quantitative PCR (qPCR) on RNA isolated from metatarsals of mutant and Capn4flox/flox embryos or primary chondrocytes infected with either Ad-Cre or Ad-lacZ. To determine RNA expression of various genes, real-time qPCR was also performed (41). Samples were run in duplicate, and the results were normalized to GAPDH expression. Primer sequences are available upon request.
Chondrocyte apoptosis assay in vitro.
Cells were stained with annexin V-phycoerythrin and 7-amino actinomycin D by using a Guava PCA Nexin kit and analyzed by a Guava personal cytometer (Guava Technology Inc., Hayward, CA) as described previously (41).
Gene silencing by RNAi.
Mouse chondrogenic cells, ATDC5, were cultured in DMEM-Ham's F-12 (1:1) (DMEM-F12) (Invitrogen) supplemented with 5% FBS and 1% penicillin-streptomycin. Three independent Capn4 small interfering RNAs (siRNAs; Invitrogen) were transiently expressed using Oligofectamine as recommended by the manufacturer (Invitrogen) (41). Commercially available Capn4 microRNAs (miRNAs) were subcloned into a pcDNA6.2-GW-miR expression vector by using the Block-iT PolII miR RNA interference (RNAi) expression vector kit (Invitrogen). Four monoclonal cell lines each stably expressing either control or Capn4 miRNA were established as recommended by the manufacturer (Invitrogen). Three independent mouse p27Kip1 siRNAs purchased from Invitrogen were also used. Efficiency levels of Capn4 siRNAs, Capn4 miRNAs, and p27Kip1 siRNAs were determined by calpain activity and quantifying the intensity of the calpain small subunit and p27Kip1 protein bands by using FluorChem SP as we described previously (42).
Flow cytometry.
ATDC5 cells stably expressing control or Capn4 miRNA were serum starved (0.5% FBS) for 2 days and then stimulated by serum replacement (5% FBS) for 10 h. Cells were labeled with 10 μM BrdU for the last 1 h, harvested, and stained with anti-BrdU fluorescein isothiocyanate antibody for BrdU and propidium iodine for DNA as recommended by the manufacturer (Becton Dickinson and Company [BD], San Jose, CA). A total of 10,000 events were analyzed for each sample. The percentage of cells in G1, S, and G2/M phases of the cell cycle was determined by use of a BD LSRII analyzer, followed by analysis using FlowJo (Tree Star Inc., Ashland, OR) and ModFit (Verity Software House, Topsham, ME) software.
Western blot and immunoprecipitation analyses.
Western blot analysis was performed as described previously with antibodies against the following proteins: cyclin D1, activating transcription factor 2 (ATF2), phospho-ATF2 (Cell Signaling Technology Inc., Danvers, MA), cyclin D2, cyclin E, cyclin-dependent kinase 2 (cdk2), cdk4, cyclin-dependent kinase inhibitor 1A (p21Cip1), cyclin-dependent kinase inhibitor 1B (p27Kip1), cyclin-dependent kinase inhibitor 1C (p57Kip2) (Santa Cruz), phospho-retinoblastoma protein (threonine 821) (Invitrogen), phospho-p27Kip1 (serine 10), and phospho-p27Kip1 (T187) (Abcam Inc., Cambridge, MA) (1). In some experiments, blots were incubated in Restore Western blot stripping buffer (Thermo Scientific, Rockford, IL) and reprobed with other primary antibodies. ATDC5 cells stably expressing either control or Capn4 miRNA were harvested, and cell lysates were precleared with 0.5 μg/ml normal mouse IgG agarose (Santa Cruz) for 2 h at 4°C with agitation. Two milligrams of protein of the supernatant was incubated with 2 μg of primary antibodies to p27Kip1 and p21Cip1 for 1 h at 4°C and then with 40 μl of protein A/G agarose (Santa Cruz) overnight at 4°C. Samples were washed, dissolved in sample buffer, and resolved on a 4 to 15% or 10% SDS gel followed by Western blot analysis (1).
Reintroduction of the calpain small subunit.
Lentivirus encoding recombinant mouse 28-kDa Capn4 cDNA or control empty vector products was produced as described previously to reintroduce the calpain small subunit in Capn4 knockdown ATDC5 cells (46). Control and Capn4 knockdown ATDC5 cells were infected at a multiplicity of infection of 1 to 20 for most experiments.
Statistics.
Data were calculated from three to five independent experiments and expressed as the mean ± standard error (SE) of either duplicate or triplicate determinations unless noted. Statistical analysis was performed using the unpaired Student t test, one-way analysis of variance with Tukey's multiple comparison test, and correlation analysis. P values less than 0.05 were accepted as significant.
RESULTS
Generation of mice lacking Capn4 in cells of the chondrocyte lineage and characterization of their gross phenotype.
To investigate a role of Capn4 in chondrocytes during growth plate development, we created chondrocyte-specific Capn4 knockout embryos. Mice expressing Cre under the control of rat Col.2 promoter (Col.2-Cre+/−), a gift from T. Kobayashi at the Endocrine Unit in MGH, were crossed with Capn4flox/flox mice to produce Col.2-Cre+/− Capn4flox/+ mice, which were then mated with Capn4flox/flox mice to generate chondrocyte-specific Capn4 knockout mutant Col.2-Cre+/− Capn4flox/flox embryos. Col.2-Cre+/− Capn4flox/+ littermates were used as controls. The efficiency of Col.2-Cre-mediated ablation of Capn4 expression was approximately 80% as assessed by real-time qPCR of RNA isolated from embryonic day 15.5 (E15.5) mutant and Capn4flox/flox metatarsals.
The mutant skeleton at E18.5 and birth was apparently smaller than that of control littermates but did not exhibit any obvious patterning defect and skeletal abnormality. The mean body weight of mutant embryos was significantly reduced at E18.5 (control, 1.33 ± 0.01 g [n = 8]; mutant, 1.22 ± 0.02 g [n = 11]; P < 0.05); yet, the difference was smaller at birth (control, 1.59 ± 0.05 g [n = 17]; mutant, 1.47 ± 0.06 g [n = 10]). Mutant mice survived postnatally.
Lack of Capn4 in cells of the chondrocyte lineage reduces the proliferating but not the hypertrophic chondrocyte zone in the embryonic growth plate.
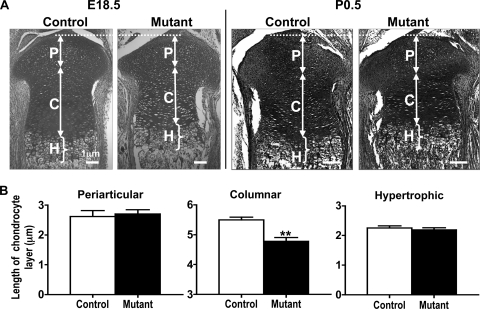
The length of proliferating and hypertrophic chondrocyte zones was measured in E18.5 control and mutant embryos to assess the effect of Capn4 deletion on growth plate development. The mean total length of the mutant proliferating chondrocyte zone was shorter than that of the controls due to the significantly reduced length of the columnar proliferating chondrocyte layer (control, 5.5 ± 0.1 μm; mutant, 4.8 ± 0.1 μm; P < 0.005) (Fig. 1A, left panel, and Fig. 1B); of note, the lengths of the periarticular and hypertrophic chondrocyte layers were indistinguishable between mutant and control embryos (Fig. 1B). The significantly shortened proliferating chondrocyte layer was also observed at birth (Fig. 1A, right panel).
FIG. 1.
Lack of Capn4 in cells of the chondrocyte lineage reduces a proliferating but not a hypertrophic chondrocyte zone in embryonic growth plates. (A) Representative images of proximal tibia at E18.5 (left panel; Safranin O stain) and birth (right panel; hematoxylin and eosin [H&E] stain). Arrows and brackets indicate the periarticular (P) and columnar (C) proliferating, and hypertrophic (H) chondrocyte layers, respectively. The scale represents 1 μm. (B) Quantitative analysis of the length of periarticular and columnar proliferating and hypertrophic chondrocyte zones of proximal tibias at E18.5 (n = 6 in each group). The mean total length of the proliferating chondrocyte zone was shorter in mutants than in controls due to the significantly reduced length of the columnar proliferating chondrocyte layer. The lengths of the periarticular and hypertrophic chondrocyte layers were indistinguishable between mutant and control embryos. **, P < 0.005.
Lack of Capn4 impairs proliferation of cells of the chondrocyte lineage.
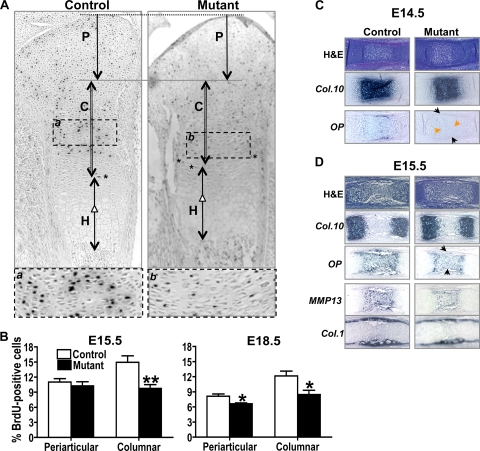
The reduced length of the proliferating chondrocyte layer observed in mutant embryos could be a consequence of either decreased proliferation, increased apoptosis, or accelerated differentiation. To test the first possibility, proliferation of cells of the chondrocyte lineage was examined by BrdU incorporation in E15.5 and E18.5 embryos. Mutant proximal tibial growth plates displayed substantially reduced numbers of BrdU-positive cells in the periarticular region at E18.5 and in the columnar proliferating region at both embryonic stages (Fig. 2A and B). No significant difference in the number of apoptotic cells as determined by a terminal deoxynucleotidyl-transferase-mediated nick end labeling staining was detected in E18.5 mutants versus controls (data not shown). Thus, these data indicate that the decreased proliferation of chondrocytes causes the reduced length of the proliferating chondrocyte zone.
FIG. 2.
Lack of Capn4 impairs proliferation and differentiation of cells of the chondrocyte lineage in vivo. (A) Incorporated BrdU was detected by immunostaining by the use of anti-BrdU antibody in proximal tibias of E18.5 embryos. Arrows indicate the periarticular (P) and columnar (C) proliferating, and hypertrophic (H) chondrocyte layers, respectively. Cells marked with asterisks were used to determine the endpoint of columnar proliferating layer. Enlarged images of selected areas, a and b, are also shown. (B) Quantitative analysis of BrdU-positive cells in the periarticular and columnar chondrocytes in the embryonic growth plate at E15.5 and E18.5 (n = 6 to 8 in each group). The number of BrdU-positive cells detected in the periarticular and column-forming proliferating region of the proximal tibia was significantly reduced in mutant embryos compared to that in controls. Histology and in situ hybridization analysis of growth plates isolated from E14.5 (C) and E15.5 (D) embryos. (C) Appearance of OP mRNA was delayed in Col.2-Cre+/−Capn4flox/flox embryos. (D) Restricted expression of Col.10, OP, and MMP13, but not Col.1, was observed in E15.5 mutant embryos. Black and orange arrowheads indicate reduced expression of OP in the perichondrium and growth plate, respectively. *, P < 0.05; **, P < 0.005.
Mutant mice have decreased expression of chondrocyte differentiation markers.
Detailed histological and in situ hybridization analyses were then performed on hind limbs isolated from E14.5 and E15.5 embryos to investigate the role of Capn4 in chondrocyte differentiation in vivo. Expression of chondrocyte markers, Col.2 (data not shown) and Col.10 (Fig. 2C) mRNAs, was indistinguishable from the controls; however, appearance of late chondrocyte differentiation marker, OP, was delayed in both the growth plate (orange arrowheads in Fig. 2C) and the perichondrium (black arrowheads in Fig. 2C) of E14.5 mutant embryos. This expected deletion of Capn4 in the mutant perichondrium region was consistent with the previous report that showed, using ROSA26 reporter mice, Col.2-Cre activity not only in chondrocytes but also in the perichondrium and osteoblasts in the bone collar (29). Spatially reduced expression of Col.10, OP, and MMP13 mRNAs was detected, while expression of the osteoblastic marker, Col.1 mRNA, was similar to that of controls, and no obvious impaired vessel invasion was observed in E15.5 mutant embryos (Fig. 2D). Thus, these results indicate that deletion of Capn4 in chondrocytes delays chondrocyte terminal differentiation. Ablation of Capn4 in the perichondrium might also play a part in this phenotype; however, it apparently showed a minimum effect on vessel invasion and bone collar formation in the mutant embryonic growth plate (Fig. 2D). Furthermore, the absence of evidence for accelerated differentiation suggests that the decrease in chondrocyte proliferation is the sole explanation for the shortened chondrocyte columns noted in Fig. 1.
Osx-Cre+/− Capn4flox/flox embryos, whose growth plate proliferation was apparently intact, also exhibited delayed late chondrocyte differentiation.
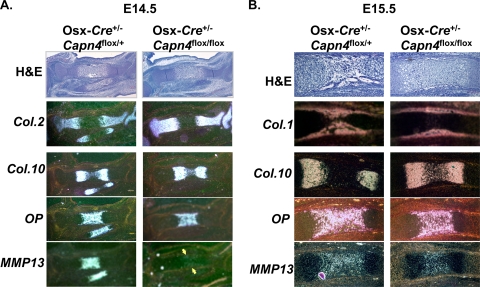
It was shown previously that in Osx-Cre+/− transgenic mice, Cre recombinase is expressed not only in early cells of the osteoblast lineage but also in late proliferating chondrocytes (22) and that no significant impairment of chondrocyte proliferation, as assessed by BrdU incorporation, was observed in Osx-Cre+/− Capn4flox/flox versus Osx-Cre+/− Capn4flox/+ littermates at 2 weeks of age (41). The latter observation was also confirmed in E18.5 embryos; the numbers of the BrdU-positive cells in the columnar chondrocyte layer were indistinguishable between Osx-Cre+/− Capn4flox/+ and Osx-Cre+/− Capn4flox/flox embryos (14.8% ± 1.6% versus 14.9% ± 1.1%, respectively). Thus, to rule out the possibility that delayed chondrocyte differentiation observed in Col.2-Cre+/− Capn4flox/flox mutant embryos could be mainly due to reduced chondrocyte proliferation, the growth plate phenotype was examined in Osx-Cre+/− Capn4flox/flox embryos and compared with that of Osx-Cre+/− Capn4flox/+ littermate embryos. Histology and in situ hybridization analysis were performed on histological sections of hind limbs isolated from E14.5, E15.5, and E18.5 Osx-Cre+/− Capn4flox/flox and Osx-Cre+/− Capn4flox/+ embryos. At E14.5, the expression patterns of Col.2 and Col.10 mRNAs were similar in Osx-Cre+/− Capn4flox/+ and Osx-Cre+/− Capn4flox/flox mice (Fig. 3A). Conversely, MMP13 mRNA expression was reduced in mutants compared to that in controls (Fig. 3A). Spatially restricted expression of Col.10, OP, and MMP13 mRNA levels was also observed in E15.5 Osx-Cre+/− Capn4flox/flox embryos compared with those in Osx-Cre+/− Capn4flox/+ littermates (Fig. 3B). The impairment of endochondral bone development was still detectable in Osx-Cre+/− Capn4flox/flox embryos at E18.5 (data not shown) but not at later postnatal time points (41). Taken together, these data suggest that terminal differentiation of chondrocytes was also delayed in Osx-Cre+/− Capn4flox/flox embryos. Moreover, unlike Col.2-Cre+/− Capn4flox/flox embryos, blood vessel invasion and formation of the primary ossification center were also delayed in Osx-Cre+/− Capn4flox/flox embryos, as shown by histology and in situ hybridization analysis of Col.1 mRNA expression (Fig. 3B), which was likely due to lack of Capn4 primarily in cells of the osteoblast lineage (41). Collectively, these results imply that lack of Capn4 in cells of the chondrocyte lineage delays chondrocyte terminal differentiation even when chondrocyte proliferation is apparently normal in vivo, as we showed in Osx-Cre+/− Capn4flox/flox mice in comparison with Osx-Cre+/− Capn4flox/+ mice. However, the main mechanisms that led to the similar phenotypes of delayed chondrocyte differentiation might be slightly different between two animal models, Osx-Cre+/− Capn4flox/flox and Col.2-Cre+/− Capn4flox/flox mice.
FIG. 3.
Terminal differentiation of chondrocytes and formation of the primary ossification center are delayed in Osx-Cre+/− Capn4flox/flox embryos. Histology and in situ hybridization analysis of proximal tibias isolated from E14.5 (A) and E15.5 (B) embryos. (A) MMP13 mRNA expression was markedly reduced in Osx-Cre+/− Capn4flox/flox embryos. Yellow arrows indicate MMP13 mRNA expression. (B) A spatially restricted expression of Col.10, OP, and MMP13 was observed in Osx-Cre+/− Capn4flox/flox in comparison with that in control littermates.
Lack of Capn4 impairs cell growth and differentiation in cells of the chondrocyte lineage in vitro.
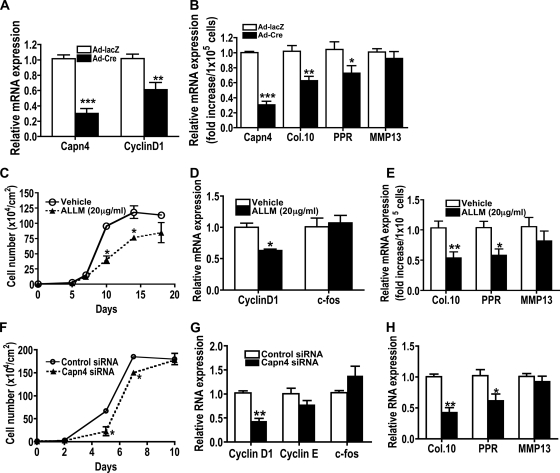
To investigate the mechanism by which the calpain small subunit modulates chondrocyte proliferation and differentiation, chondrocyte functions were next examined in vitro. Primary chondrocytes isolated from Capn4flox/flox newborn mice were infected with adenovirus expressing either Cre recombinase (Ad-Cre) or β-galactosidase (Ad-lacZ). Levels of Capn4 mRNA expression were reduced to approximately 30% in Capn4flox/flox chondrocytes infected with Ad-Cre in comparison with those infected with Ad-lacZ (Fig. 4A and B). Forty-eight hours after adenoviral infection, cells were incubated in a growth medium for 2 days and then in a serum-free medium overnight. Expression of cyclin D1 mRNA was significantly reduced in Capn4-deficient primary chondrocytes compared to that in control primary chondrocytes (Fig. 4A). There was no difference in apoptosis between control and Capn4-deficient primary chondrocytes as assessed by the Nexin assay (data not shown). Capn4-deficient primary chondrocytes showed significantly impaired mRNA expression of Col.10 and PPR, early chondrocyte differentiation markers, but not expression of MMP13, a late differentiation marker, after 3 days incubation of cells in a differentiation medium (Fig. 4B). Mouse chondrogenic cell line ATDC5 was used next. First, ATDC5 cells were treated with either a vehicle or a cell permeable calpain inhibitor, N-acetyl-Leu-Leu-Met-CHO (ALLM) (EMD Chemicals, Gibbstown, NJ). The number of ATDC5 cells treated with 20 μg/ml ALLM was significantly reduced on days 10 and 14 when cells were seeded at the concentration of 1 × 104 cells/cm2 on day 0 (Fig. 4C). Cyclin D1 mRNA expression (Fig. 4D) was markedly reduced in ATDC5 cells treated with ALLM. Reduced calpain activity significantly reduced mRNA expression of Col.10 and PPR, but not MMP13, after 3 days incubation of cells in a differentiation medium (Fig. 4E). Next, control and Capn4 siRNAs were transiently expressed in ATDC5 cells. Calpain activity of ATDC5 cells expressing Capn4 siRNAs was reduced to 40 to 60%. The number of cells was significantly reduced on day 5 and 7 but became indistinguishable by day 10 (Fig. 4F). Consistent with the observations described above, ATDC5 cells expressing Capn4 siRNA also showed reduced cyclin D1, Col.10, and PPR mRNA expression (Fig. 4G and H). Thus, these results support the hypothesis that lack of Capn4 impairs both proliferation and differentiation of cells of the chondrocyte lineage.
FIG. 4.
Primary costal chondrocytes and ATDC5 cells with reduced calpain activity exhibited impaired cell growth, reduced mRNA expression of the cyclin D1 gene and early differentiation markers of cells of the chondrocyte lineage. (A) Expression of Capn4 and cyclin D1 mRNA was significantly reduced in Capn4-null primary chondrocytes. (B) Expression of markers for chondrocyte differentiation was reduced in Capn4-null chondrocytes. Relative levels of expression of Capn4, Col.10, PPR, and MMP13 RNA were determined using real-time qPCR. PPR, PTH/PTHrP receptor. (C) Cell growth of ATDC5 cells treated with a chemical calpain inhibitor, ALLM, showed reduced cell growth. (D) Reduced calpain activity reduced cyclin D1 mRNA expression in ATDC5 cells treated with ALLM. (E) Reduced calpain activity showed reduced expression of early chondrocyte differentiation markers, Col.10 and PPR, in ATDC5 cells treated with ALLM. (F) Transient expression of Capn4 siRNA impaired cell growth in ATDC5 cells. (G) Transient expression of Capn4 siRNA showed significantly reduced cyclin D1 mRNA expression. (H) Expression of early chondrocyte differentiation markers, Col.10 and PPR, was reduced in ATDC5 cells transiently expressing Capn4 siRNA. *, P < 0.05; **, P < 0.005; and ***, P < 0.001.
Lack of Capn4 in cells of the chondrocyte lineage modulates cell cycle progression at the G1/S transition.
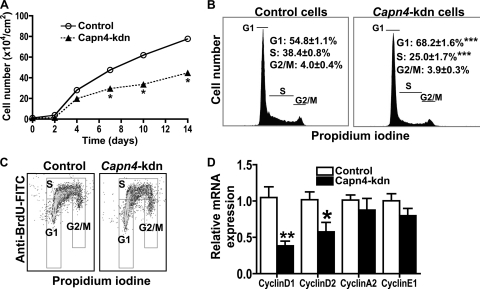
We next created the ATDC5 cell lines stably expressing either control or Capn4 miRNA. Calpain activity of Capn4 knockdown cells was reduced to approximately 20 to 40% of that of control cells. The number of Capn4 knockdown ATDC5 cells was significantly reduced on days 7 to 14 when cells were seeded at the concentration of 1 × 104 cells/cm2 on day 0 (Fig. 5A). There was no significant difference in apoptosis between control and Capn4 knockdown ATDC5 cells when cells were stained with annexin V-phycoerythrin and 7-amino actinomycin D, followed by analysis using a Guava personal cytometer (annexin V-positive apoptotic cell population for control cells, 10.4% ± 0.4%; for Capn4 knockdown cells, 9.1% ± 0.8%). We then analyzed the effect of Capn4 on the cell cycle distribution of ATDC5 cells by the use of flow cytometry analysis. Capn4 knockdown chondrocytes significantly accumulated in G1 phase, with a reduction of BrdU-positive proliferating cells in S phase (Fig. 5B and C), as we expected from our in vivo data, suggesting that Capn4 plays a critical role at the G1/S transition of the cell cycle in cells of the chondrocyte lineage.
FIG. 5.
Capn4 knockdown (kdn) ATDC5 cells stably expressing Capn4 miRNA showed impaired G1/S transition in the cell cycle. (A) Knockdown of Capn4 impaired cell growth in ATDC5 cells. (B) Knockdown of Capn4 impaired cell cycle progression at G1/S transition in ATDC5 cells. (C) Knockdown of Capn4 reduced BrdU incorporation in ATDC5 cells. (D) Knockdown of Capn4 reduced mRNA expression of cyclin D1 and cyclin D2, but not cyclin A2 and cyclin E1. *, P < 0.05; **, P < 0.005; and ***, P < 0.001.
Lack of Capn4 in cells of the chondrocyte lineage impairs cyclin D1 transcription through both PTHrP and TGF-β-mediated pathways.
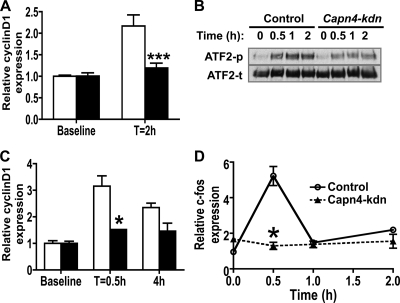
To investigate whether deletion of Capn4 modulates cyclin D1 transcription, we examined mRNA expression of various cyclins by using real-time qPCR. After 48 h serum starvation, the relative levels of expression of cyclin D1 and cyclin D2 mRNA, but not cyclin A2 and cyclin E1 mRNA, were significantly reduced in Capn4 knockdown cells compared to those in control cells (Fig. 5D), and the reduced cyclin D1 and cyclin D2 mRNA expression was restored by serum replacement for 6 h (relative cyclin D1 mRNA expression for control, 1.0 ± 0.1; for Capn4 knockdown cells, 1.1 ± 0.1). It was reported previously that cyclin D1 mRNA expression in chondrocytes is regulated by transforming growth factor β (TGF-β) and parathyroid hormone-related peptide (PTHrP) (3). To examine the mechanism that regulates cyclin D1 mRNA expression in Capn4 knockdown cells, we treated control and Capn4 knockdown ATDC5 cells with either 1 ng/ml TGF-β1 (Millipore) or 10−8 M PTHrP(1-36) (a gift from T. J. Gardella, Endocrine Unit, MGH) for various periods of time, and monitored TGF-β1- and PTHrP-stimulated cyclin D1 mRNA expression. The response of cyclin D1 mRNA levels to either TGFβ1 (Fig. 6A) or PTHrP (Fig. 6C) treatment was markedly reduced in Capn4 knockdown cells compared with control cells. Next, we tested TGFβ1-mediated phosphorylation of ATF2 to further address the mechanism of impaired TGFβ1-mediated increase of cyclin D1 mRNA levels in Capn4 knockdown cells. The intensity of the phosphorylated ATF2 protein band was significantly reduced in Capn4 knockdown cells compared with control cells at 0.5, 1, and 2 h after the treatment with TGFβ1 (Fig. 6B). PTHrP-stimulated relative c-fos mRNA expression was also blunted in Capn4 knockdown cells at 0.5 h after PTHrP treatment (Fig. 6D). These results suggest that deletion of Capn4 could modulate cell proliferative function through TGFβ1- and PTHrP-mediated CyclinD1 transcription in cells of the chondrocyte lineage.
FIG. 6.
Capn4 knockdown ATDC5 cells exhibited reduced cyclin D1 mRNA expression. (A) Knockdown of Capn4 impaired TGF-β1-mediated cyclin D1 mRNA expression in ATDC5 cells. Cells were incubated with 1 ng/ml TGF-β1 for 0 and 2 h. (B) Knockdown of Capn4 impaired TGFβ1-mediated phosphorylation of ATF2 in ATDC5 cells. Cells were incubated with 1 ng/ml TGF-β1 for 0, 0.5, 1, and 2 h. ATF2-p and ATF2-t indicate phosphorylated and total ATF2, respectively. (C) Knockdown of Capn4 impaired PTHrP-mediated cyclin D1 mRNA expression in ATDC5 cells. (D) Knockdown of Capn4 impaired PTHrP-stimulated c-fos mRNA expression in ATDC5 cells. Cells were incubated with 10−8 M PTHrP for 0, 0.5, 1, and 2 h. *, P < 0.05; ***, P < 0.001.
Lack of Capn4 in cells of the chondrocyte lineage leads to accumulation of cell cycle proteins known to be calpain substrates.
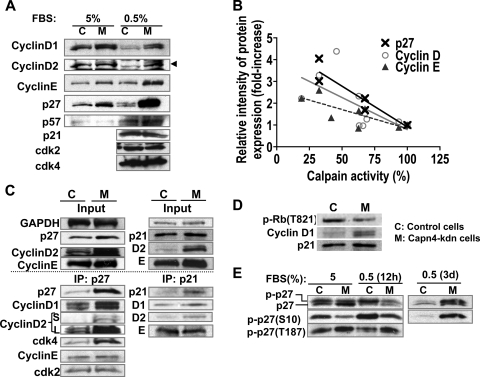
Next, we examined the expression of G1/S cell cycle proteins in Capn4 knockdown cells. Levels of protein expression of cyclin D1, cyclin D2, cyclin E, and p27Kip1 were increased in Capn4 knockdown cells compared with levels in control cells, particularly when cells were incubated in a culture medium containing 0.5% FBS compared with 5% FBS (Fig. 7A). The relative intensity of the p27Kip1, cyclin D, and cyclin E protein bands were negatively and significantly correlated with reduced calpain activity when three monoclonal stable Capn4 knockdown cell lines were used for the analysis (Fig. 7B) (Pearson's r value for p27Kip1, −0.9456 [P < 0.005]; for cyclin D, −0.6354, and for cyclin E, −0.7668 [P < 0.05]). In contrast, levels of p21Cip1, p57Kip2, cdk2, and cdk4 were almost indistinguishable between Capn4 knockdown and control ATDC5 cells (Fig. 7A). We next performed coimmune precipitation analysis with anti-p27Kip1 and p21Cip1 antibodies to examine which G1/S cell cycle proteins were assembled by p27Kip1 and p21Cip1. p27Kip1 protein predominantly assembled cyclin D and cdk4, rather than cyclin E and cdk2, in Capn4 knockdown cells (Fig. 7C, left panel). Slightly increased levels of p21Cip1 in Capn4 knockdown cells also assembled cyclin D without affecting the total binding to cyclin E (Fig. 7C, right panel), suggesting that p21Cip1 also plays a part in the inactivation of increased cyclin D; yet, its role was likely minor compared with that of p27Kip1, because p27Kip1 protein levels were concomitantly and more significantly increased with cyclin D and cyclin E than with p21Cip1 (Fig. 7A). Moreover, ablation of Capn4 reduced phosphorylation of Rb on T821 in ATDC5 cells (Fig. 7D). Lastly, phosphorylation of p27Kip1 on S10 was significantly impaired in Capn4 knockdown cells compared with that in control ATDC5 cells in a DMEM-F12 medium containing 5% FBS (Fig. 7E). Serum starvation (0.5% FBS) promoted phosphorylation of p27Kip1 on S10 in both control and Capn4 knockdown cells after 12 h of culture. Paradoxically, phosphorylated p27Kip1 (S10) protein was found substantially accumulated in Capn4 knockdown cells after 3 days of serum starvation, but not in control cells (Fig. 7E). Conversely, phosphorylation of p27Kip1 on T187 was slightly increased in Capn4 knockdown cells compared with that in control cells, and the levels of phospho-p27Kip1 (T187) were not altered by serum starvation in both control and Capn4 knockdown cells (Fig. 7E). Taken together, these results suggest that deletion of Capn4 reduces calpain activity, resulting in a significant accumulation of cyclin D, cyclin E, and p27Kip1, despite reduced cyclin D1 mRNA levels. Increased cyclin D protein levels were subsequently inactivated mainly by p27Kip1 but also by p21Cip1, which might lead to reduced phosphorylation of Rb on T821 and increased cell numbers of the chondrocyte lineage in G1 phase of cell cycle. Furthermore, deletion of Capn4 was suggested to reduce both phosphorylation of p27Kip1 on S10 and degradation of the phospho-p27Kip1 (S10) protein.
FIG. 7.
Capn4 knockdown ATDC5 cells exhibited significant accumulation of cell cycle proteins known to be calpain substrates. (A) Knockdown of Capn4 showed accumulation of cyclin D1, cyclin D2, cyclin E, and p27Kip1 in ATDC5 cells. (B) Calpain activity and relative intensity of protein expression of cyclin D, cyclin E, and p27Kip1 were negatively correlated. Three independent stable cells lines stably expressing Capn4 miRNAs, whose levels of calpain activity were approximately 30%, 65% and 90% of those of control cells were used in the experiments. (C) p27Kip1 predominantly assembled cyclin D and cdk4 rather than cyclin E and cdk2 in Capn4 knockdown ATDC5 cells. Ablation of Capn4 did not increase p21Cip1 protein levels as significantly as it did p27Kip1; however, slightly increased p21Cip1 also bound to excess cyclin D1 and cyclin D2 without altering total binding to cyclin E compared with control cells. S and L in cyclin D2 columns represent images obtained after short (S) and long (L) exposure to films. (D) Knockdown of Capn4 in ATDC5 cells showed impaired phosphorylation of Rb on T821. (E) Knockdown of Capn4 in ATDC5 cells showed impaired phosphorylation of p27Kip1 on S10 in a DMEM-F12 medium containing 5% FBS. Serum starvation for 12 h stimulated phosphorylation of p27Kip1 on S10 in both control and Capn4 knockdown cells. Serum starvation for 3 days caused significant accumulation of phospho-p27Kip1 (S10) in Capn4 knockdown ATDC5 cells.
Knockdown of p27Kip1 protein rescues an impaired cell growth phenotype in Capn4 knockdown chondrocytes in vitro.
To examine whether knockdown of p27Kip1 protein rescues an impaired cell growth phenotype in Capn4 knockdown cells, we transiently expressed three different p27Kip siRNAs and modified p27Kip1 protein levels in Capn4 knockdown cells. When p27Kip1 protein levels were reduced to the levels similar to those of control cells expressing control siRNA, cell growth became indistinguishable between Capn4 knockdown cells expressing p27Kip1 siRNA and control cells expressing control siRNA (Fig. 8A). These results suggest that accumulation of p27Kip1 in Capn4 knockdown cells is, at least in part, responsible for reduced cell growth in Capn4 knockdown cells.
FIG. 8.
Knockdown of p27Kip1 and reintroduction of the calpain small subunit normalized impaired cell growth and accumulation of cyclin D protein in Capn4 knockdown ATDC5 cells. (A) Knockdown of p27Kip1 to a level similar to that of control cells restored cell growth of Capn4 knockdown ATDC5 cells in a dose-dependent manner. Three different p27Kip1 siRNAs were transiently expressed in Capn4 knockdown ATDC5 cells. Cells were seeded at the concentration of 1 × 104 per cm2 on day 0, and cell numbers were examined on days 5 and 7. (B) Reintroduction of the calpain small subunit via lentiviral infection dose dependently restored impaired cell number and accumulation of cyclin D protein in ATDC5 cells. Control and Capn4 knockdown cells were infected with lentivirus carrying either empty vector or Capn4. Cell number was counted, and the intensity of the relative protein expression of cyclin D2 and the calpain small subunit was determined. The data marked with ovals are those of control cells infected with control lentivirus. *, P < 0.05.
Reintroduction of Capn4 restores cell number and cyclin D expression in a dose-dependent manner.
To examine whether reintroduction of the calpain small subunit restores impaired cell growth and accumulation of cyclin D protein, we reintroduced the calpain small subunit by using lentiviral transduction in Capn4 knockdown cells (46). When cell number and protein levels of the calpain small subunit and cyclin D2 in Capn4 knockdown cells were set to 1, respectively, cell number positively correlated with the relative protein expression of the calpain small subunit, and the intensity of the cyclin D2 protein band negatively correlated (Fig. 8B) (Pearson's r value for cell number, 0.9778 [P < 0.001]; for relative protein expression of cyclin D2, −0.9527 [P < 0.05]). The negative correlation between protein expression of cyclin D1 and the calpain small subunit was similarly observed to that of cyclin D2 (data now shown). Thus, reintroduction of the calpain small subunit dose dependently restores impaired cell growth and accumulated cyclin D protein phenotypes in ATDC5 cells.
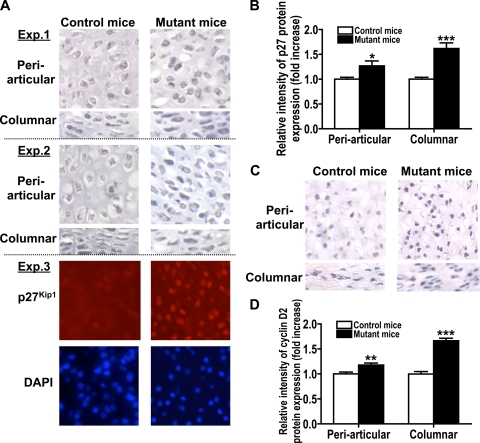
Capn4 deficiency correlates with p27Kip1 and cyclin D protein accumulation in proliferating chondrocytes in vivo.
Finally, to examine whether mutant proliferating chondrocytes in embryonic growth plates accumulate p27Kip1 and cyclin D proteins as we expected based on our in vitro observations, we performed immunohistochemistry on specimens of E18.5 control and mutant embryonic growth plates by using anti-p27Kip1 and cyclin D2 antibodies. Stronger p27Kip1 (Fig. 9A and B) and cyclin D2 (Fig. 9C and D) immunoreactivities were detected in mutant proliferating chondrocytes than in controls. Therefore, deletion of Capn4 in cells of the chondrocyte lineage reduces proliferating chondrocyte layer, which correlates with reduced BrdU-positive cells and accumulated cell cycle regulatory proteins p27Kip1 and cyclin D in proliferating chondrocytes in vivo.
FIG. 9.
Capn4 deficiency correlates with p27Kip1 and cyclin D2 protein accumulation in proliferative chondrocytes in embryonic growth plates. Immunohistochemistry using anti-p27Kip1 and cyclin D2 antibodies showed stronger immunoreactivities of p27Kip1 (A and B) and cyclin D2 (C and D) in nuclei of mutant proliferating chondrocytes than in controls in vivo. (A) Experiments 1 to 3 represent three independent experiments using control and mutant littermate samples, respectively. Experiment 3 shows immunohistochemical analysis of periarticular chondrocytes by using Alexa Fluor-labeled goat anti-rabbit IgG antibody (Invitrogen) as a secondary antibody. Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) (Vector Laboratory). (B and D) Semiquantitative representation of immunohistochemical analysis (n = 4 each group). Magnification is ×200. *, P < 0.05; **, P < 0.005; and ***, P < 0.001.
DISCUSSION
Calpains belong to a family of intracellular cysteine proteases that have been shown to cleave numerous and diverse substrates and modulate their cellular functions (13). In this study, we report that the calpain small regulatory subunit encoded by Capn4 plays a critical role in growth plate development. Targeted deletion of Capn4 in cells of the chondrocyte lineage caused reduced chondrocyte proliferation and delayed chondrocyte differentiation.
Whether calpains play a role in the cell cycle has been controversial. Embryonic fibroblasts isolated from universal Capn4 knockouts proliferate normally (2). However, several in vitro studies using calpain inhibitors suggested that calpain modulates cell cycle progression, mainly at the G1-to-S transition, by regulating proteolysis of various proteins such as cyclin D1, cyclin E, and p27Kip1 (4, 7, 24, 36, 50). Our chondrocyte-specific Capn4 knockout embryos exhibited a shorter columnar proliferating chondrocyte layer than did controls mainly due to reduced cell proliferation as determined by BrdU incorporation. These results were consistent with our previous findings that lack of Capn4 in cells of the osteoblast lineage exhibited reduced trabecular bone, most likely due to reduced osteoblast proliferation in vivo (41).
In vitro knockdown of Capn4 confirmed impaired cell cycle progression at the G1/S transition in cells of the chondrocyte lineage. To identify a mechanism of reduced proliferation of cells of the chondrocyte lineage, we examined gene transcription and protein levels of various G1/S cell cycle proteins and found that the calpain small subunit modulates both transcription/posttranscriptional modification and protein degradation of multiple cell cycle proteins. Deletion of the calpain small subunit significantly reduced cyclin D mRNA levels in cells of the chondrocyte lineage. Beier et al. reported that TGF-β1 and PTHrP regulate cyclin D1 gene transcription in chondrocytes through activation of cyclic AMP response element (CRE) and activator protein 1 (AP-1) binding sites in the promoter regions of the cyclin D1 gene (3). TGF-β1 stimulates phosphorylation of ATF2 (3) through activation of mitogen-activated protein (MAP) kinases Jun N-terminal protein kinase (JNK) and p38 (14, 15, 28, 37). Activated ATF2 directly induces CRE activity, and activated JNK induces AP-1 activity (17). Similarly, PTHrP promotes phosphorylation of the cAMP response element binding protein (CREB) (3) by protein kinase A (40) and other kinases (10, 18, 47), and activated CREB directly induces CRE activity and stimulates expression of the immediate early gene c-fos (25) to induce AP-1 binding to the cyclin D1 promoter region. TGF-β1 treatment failed to induce cyclin D1 gene transcription in Capn4 knockdown ATDC5 cells compared with controls due, at least in part, to reduced phosphorylation of ATF2. Consistent with our previous observation in Capn4 knockdown osteosarcoma cells treated with PTH (41), Capn4 knockdown ATDC5 cells displayed reduced c-fos and cyclin D1 mRNA induction upon PTHrP stimulation. Collectively, these results suggest that the reduced proliferation of cells of the chondrocyte lineage is, at least partly, due to impaired cyclin D1 gene transcription in response to TGF-β1- and PTHrP-mediated signaling.
Paradoxically, given our observation of reduced cyclin D1 mRNA expression, Capn4 knockdown ATDC5 cells significantly accumulated cyclin D1 and cyclin D2 proteins in vitro. Detailed analysis further revealed additional retention of cell cycle-related proteins, which include cyclin E and p27Kip1, but not p57Kip2, whose degradation has been shown to be mainly through the ubiquitin-regulated proteosomal pathway (49). A few reports suggested that p21Cip1 is a calpain substrate in cytomegalovirus-infected and chemical carcinogen-exposed lung and mammary cells (6, 20); however, we detected slight, if any, accumulation of p21Cip1 protein in our chondrogenic cells compared with the level of p27Kip1. It has been reported that calpain-mediated degradation of p27Kip1 plays an important role in proliferating preadipocytes by allowing cyclin D-cdk4 to phosphorylate the retinoblastoma tumor suppressor, Rb, thereby promoting the G1/S transition (31, 36). T821 of Rb is one of the known phosphorylation sites that disrupt its binding to transcription factor, E2F; this, in turn, promotes cell cycle progression at G1/S (16, 35). Thus, our data suggest that despite reduced basal mRNA expression of the cyclin D gene, deletion of the calpain small subunit causes accumulation of cyclin D protein, whose function is subsequently inactivated mainly by p27Kip1, but also by p21Cip1, resulting in reduced phosphorylation of Rb, at least, on T821. Underphosphorylated Rb likely remains actively binding to transcription factors and blocks cell cycle transition at G1/S in Capn4 knockdown cells compared with control ATDC5 cells.
The specific mechanism that regulates calpain-mediated degradation of p27Kip1 has not been fully understood. Phosphorylation of p27Kip1 on S10 has been suggested to signal a nucleocytoplasmic translocation of p27Kip1 (19, 39), and the translocation was shown to be critical for MAP kinase-dependent p27Kip1 degradation by calpain (9). Our finding further suggests the dual regulation of p27Kip1 by the calpain small subunit, phosphorylation of p27Kip1 on S10, and degradation of phospho-p27Kip1 (S10). In contrast, phosphorylation of p27Kip1 on T187 by cdk2, which is active in S to G2 phases of the cell cycle, has been shown to be critical for degradation of p27Kip1 via the proteasome pathway (5, 11, 44, 45, 48). Increased levels of phospho-p27Kip1 (T187) protein observed in Capn4 knockdown cells compared with control cells might reflect an upregulation of proteasome degradation pathway to reduce the accumulated p27Kip1 protein levels in Capn4 knockdown cells; however, further investigation will be necessary to clarify the mechanism.
p27Kip1-null newborn animals are normal in size (12, 21, 32), and very low levels of p27Kip1 mRNA and protein expression are detected compared with those of p57Kip2, which has been suggested as one of the most critical regulators of cell cycle in embryonic growth plates (30). Thus, the relatively mild growth plate phenotype of chondrocyte-specific Capn4-null embryos could be, at least partly, due to the overall low levels of expression of p27Kip1. However, the observed in vivo accumulation of p27Kip1 and cyclin D in Capn4 mutant embryonic growth plates (Fig. 9) and the in vitro evidence, which includes (i) the apparent increase in cyclin D, cyclin E, and p27Kip1 and increased association of cyclin D-cdk4 with p27Kip1 in Capn4 knockdown cells; (ii) reduced phosphorylation of Rb (T821); (iii) Capn4-mediated dual posttranscriptional regulation of p27Kip1 on S10; (iv) restored cell growth in Capn4 knockdown cells by normalizing p27Kip1 protein levels (Fig. 8A); and (v) reduction of increased cyclin D protein levels by reintroducing the calpain small subunit in a dose-dependent manner (Fig. 8B), argue that the calpain small subunit plays a critical role in regulation of the cell cycle during chondrocyte proliferation.
Lack of Capn4 in chondrocytes significantly reduced expression of differentiation markers, such as Col.10, OP, and MMP13 mRNAs, in embryonic growth plates and the perichondrium in two independent Capn4-null animal models; the first model, which used Col.2-Cre to drive Capn4 deletion, correlated with reduced chondrocyte proliferation, and the second model, which used Osx-Cre, did not. There were also some differences in their growth plate phenotypes; severely impaired vessel invasion was observed in Osx-Cre+/− Capn4flox/flox embryos but not in Col2-Cre+/− Capn4flox/flox embryos at E15.5, likely due to additional ablation of Capn4 in osteoblasts in the bone collar and/or cells of the perichondrium of Osx-Cre+/− Capn4flox/flox embryos. It has been well documented that the perichondrium releases several paracrine factors such as bone morphogenetic proteins and fibroblast growth factors that play critical roles in chondrocyte proliferation and differentiation (23, 27, 33, 34). Because of the complexity of calpain molecular functions and differentiation mechanisms of growth plate, further studies will be required to identify specific molecular mechanisms by which the calpain small subunit regulates chondrocyte differentiation. However, our findings provide in vivo evidence that Capn4 is an essential modulator of terminal cell differentiation in cells of the chondrocyte lineage and that the shortened proliferating chondrocyte layer is not due to accelerated chondrocyte differentiation.
In summary, lack of Capn4 in cells of the chondrocyte lineage impairs proliferation and differentiation in embryonic growth plates. Our in vitro findings critically provide mechanistic insights of the characteristic in vivo phenotype of impaired cell proliferation of the chondrocyte lineage. Our data thus indicate that the calpain small subunit is a critical regulator of embryonic growth plate development.
Acknowledgments
We thank Henry M. Kronenberg for critical review of the manuscript and helpful discussion and Andrew P. McMahon (Department of Molecular and Cellular Biology, Harvard University) for a generous gift of Osx-Cre+/− transgenic mice. We also thank Kimberly Atkin and Kerline Cepoudy at the Endocrine Unit and Laura B. Prickett-Rice at the Center for Regenerative Medicine in MGH for technical support.
This work was supported by grants from the National Institutes of Health Grant R01 DK072102 (M.S.) and the Canadian Institute of Health Research MOP-81189 (P.A.G.).
Footnotes
Published ahead of print on 5 April 2010.
REFERENCES
- 1.Aikawa, T., G. V. Segre, and K. Lee. 2001. Fibroblast growth factor inhibits chondrocytic growth through induction of p21 and subsequent inactivation of cyclin E-Cdk2. J. Biol. Chem. 276:29347-29352. [DOI] [PubMed] [Google Scholar]
- 2.Arthur, J. S., J. S. Elce, C. Hegadorn, K. Williams, and P. A. Greer. 2000. Disruption of the murine calpain small subunit gene, Capn4: calpain is essential for embryonic development but not for cell growth and division. Mol. Cell. Biol. 20:4474-4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beier, F., Z. Ali, D. Mok, A. C. Taylor, T. Leask, C. Albanese, R. G. Pestell, and P. LuValle. 2001. TGFbeta and PTHrP control chondrocyte proliferation by activating cyclin D1 expression. Mol. Biol. Cell 12:3852-3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carragher, N. O., M. A. Westhoff, D. Riley, D. A. Potter, P. Dutt, J. S. Elce, P. A. Greer, and M. C. Frame. 2002. v-Src-induced modulation of the calpain-calpastatin proteolytic system regulates transformation. Mol. Cell. Biol. 22:257-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carrano, A. C., E. Eytan, A. Hershko, and M. Pagano. 1999. SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat. Cell Biol. 1:193-199. [DOI] [PubMed] [Google Scholar]
- 6.Chen, Z., E. Knutson, A. Kurosky, and T. Albrecht. 2001. Degradation of p21cip1 in cells productively infected with human cytomegalovirus. J. Virol. 75:3613-3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi, Y. H., S. J. Lee, P. Nguyen, J. S. Jang, J. Lee, M. L. Wu, E. Takano, M. Maki, P. A. Henkart, and J. B. Trepel. 1997. Regulation of cyclin D1 by calpain protease. J. Biol. Chem. 272:28479-28484. [DOI] [PubMed] [Google Scholar]
- 8.Cong, J., D. E. Goll, A. M. Peterson, and H. P. Kapprell. 1989. The role of autolysis in activity of the Ca2+-dependent proteinases (mu-calpain and m-calpain). J. Biol. Chem. 264:10096-10103. [PubMed] [Google Scholar]
- 9.Delmas, C., N. Aragou, S. Poussard, P. Cottin, J. M. Darbon, and S. Manenti. 2003. MAP kinase-dependent degradation of p27Kip1 by calpains in choroidal melanoma cells. Requirement of p27Kip1 nuclear export. J. Biol. Chem. 278:12443-12451. [DOI] [PubMed] [Google Scholar]
- 10.Du, K., and M. Montminy. 1998. CREB is a regulatory target for the protein kinase Akt/PKB. J. Biol. Chem. 273:32377-32379. [DOI] [PubMed] [Google Scholar]
- 11.Feldman, R. M., C. C. Correll, K. B. Kaplan, and R. J. Deshaies. 1997. A complex of Cdc4p, Skp1p, and Cdc53p/cullin catalyzes ubiquitination of the phosphorylated CDK inhibitor Sic1p. Cell 91:221-230. [DOI] [PubMed] [Google Scholar]
- 12.Fero, M. L., M. Rivkin, M. Tasch, P. Porter, C. E. Carow, E. Firpo, K. Polyak, L. H. Tsai, V. Broudy, R. M. Perlmutter, K. Kaushansky, and J. M. Roberts. 1996. A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27(Kip1)-deficient mice. Cell 85:733-744. [DOI] [PubMed] [Google Scholar]
- 13.Goll, D. E., V. F. Thompson, H. Li, W. Wei, and J. Cong. 2003. The calpain system. Physiol. Rev. 83:731-801. [DOI] [PubMed] [Google Scholar]
- 14.Gupta, S., T. Barrett, A. J. Whitmarsh, J. Cavanagh, H. K. Sluss, B. Derijard, and R. J. Davis. 1996. Selective interaction of JNK protein kinase isoforms with transcription factors. EMBO J. 15:2760-2770. [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta, S., D. Campbell, B. Derijard, and R. J. Davis. 1995. Transcription factor ATF2 regulation by the JNK signal transduction pathway. Science 267:389-393. [DOI] [PubMed] [Google Scholar]
- 16.Gützkow, K. B., S. Naderi, and H. K. Blomhoff. 2002. Forskolin-mediated G1 arrest in acute lymphoblastic leukaemia cells: phosphorylated pRB sequesters E2Fs. J. Cell Sci. 115:1073-1082. [DOI] [PubMed] [Google Scholar]
- 17.Hocevar, B. A., T. L. Brown, and P. H. Howe. 1999. TGF-beta induces fibronectin synthesis through a c-Jun N-terminal kinase-dependent, Smad4-independent pathway. EMBO J. 18:1345-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iordanov, M., K. Bender, T. Ade, W. Schmid, C. Sachsenmaier, K. Engel, M. Gaestel, H. J. Rahmsdorf, and P. Herrlich. 1997. CREB is activated by UVC through a p38/HOG-1-dependent protein kinase. EMBO J. 16:1009-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishida, N., T. Hara, T. Kamura, M. Yoshida, K. Nakayama, and K. I. Nakayama. 2002. Phosphorylation of p27Kip1 on serine 10 is required for its binding to CRM1 and nuclear exports. J. Biol. Chem. 277:14355-14358. [DOI] [PubMed] [Google Scholar]
- 20.Khan, Q. A., A. Dipple, and L. M. Anderson. 2002. Protease inhibitor-induced stabilization of p21(waf1/cip1) and cell-cycle arrest in chemical carcinogen-exposed mammary and lung cells. Mol. Carcinog. 33:1-8. [DOI] [PubMed] [Google Scholar]
- 21.Kiyokawa, H., R. D. Kineman, K. O. Manova-Todorova, V. C. Soares, E. S. Hoffman, M. Ono, D. Khanam, A. C. Hayday, L. A. Frohman, and A. Koff. 1996. Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27(Kip1). Cell 85:721-732. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi, T., J. Lu, B. S. Cobb, S. J. Rodda, A. P. McMahon, E. Schipani, M. Merkenschlager, and H. M. Kronenberg. 2008. Dicer-dependent pathways regulate chondrocyte proliferation and differentiation. Proc. Natl. Acad. Sci. U. S. A. 105:1949-1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kronenberg, H. M. 2007. The role of the perichondrium in fetal bone development. Ann. N. Y. Acad. Sci. 1116:59-64. [DOI] [PubMed] [Google Scholar]
- 24.Le, X. F., F. X. Claret, A. Lammayot, L. Tian, D. Deshpande, R. LaPushin, A. M. Tari, and R. C. Bast, Jr. 2003. The role of cyclin-dependent kinase inhibitor p27Kip1 in anti-HER2 antibody-induced G1 cell cycle arrest and tumor growth inhibition. J. Biol. Chem. 278:23441-23450. [DOI] [PubMed] [Google Scholar]
- 25.Lee, C., T. J. Gardella, A. B. Abou-Samra, S. R. Nussbaum, G. V. Segre, J. T. Potts, Jr., H. M. Kronenberg, and H. Juppner. 1994. Role of the extracellular regions of the parathyroid hormone (PTH)/PTH-related peptide receptor in hormone binding. Endocrinology 135:1488-1495. [DOI] [PubMed] [Google Scholar]
- 26.Lee, K., B. Lanske, A. C. Karaplis, J. D. Deeds, H. Kohno, R. A. Nissenson, H. M. Kronenberg, and G. V. Segre. 1996. Parathyroid hormone-related peptide delays terminal differentiation of chondrocytes during endochondral bone development. Endocrinology 137:5109-5118. [DOI] [PubMed] [Google Scholar]
- 27.Liu, Z., J. Xu, J. S. Colvin, and D. M. Ornitz. 2002. Coordination of chondrogenesis and osteogenesis by fibroblast growth factor 18. Genes Dev. 16:859-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Livingstone, C., G. Patel, and N. Jones. 1995. ATF-2 contains a phosphorylation-dependent transcriptional activation domain. EMBO J. 14:1785-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Long, F., U. I. Chung, S. Ohba, J. McMahon, H. M. Kronenberg, and A. P. McMahon. 2004. Ihh signaling is directly required for the osteoblast lineage in the endochondral skeleton. Development 131:1309-1318. [DOI] [PubMed] [Google Scholar]
- 30.MacLean, H. E., J. Guo, M. C. Knight, P. Zhang, D. Cobrinik, and H. M. Kronenberg. 2004. The cyclin-dependent kinase inhibitor p57(Kip2) mediates proliferative actions of PTHrP in chondrocytes. J. Clin. Invest. 113:1334-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsushime, H., M. E. Ewen, D. K. Strom, J. Y. Kato, S. K. Hanks, M. F. Roussel, and C. J. Sherr. 1992. Identification and properties of an atypical catalytic subunit (p34PSK-J3/cdk4) for mammalian D type G1 cyclins. Cell 71:323-334. [DOI] [PubMed] [Google Scholar]
- 32.Nakayama, K., N. Ishida, M. Shirane, A. Inomata, T. Inoue, N. Shishido, I. Horii, D. Y. Loh, and K. Nakayama. 1996. Mice lacking p27(Kip1) display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell 85:707-720. [DOI] [PubMed] [Google Scholar]
- 33.Ohbayashi, N., M. Shibayama, Y. Kurotaki, M. Imanishi, T. Fujimori, N. Itoh, and S. Takada. 2002. FGF18 is required for normal cell proliferation and differentiation during osteogenesis and chondrogenesis. Genes Dev. 16:870-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ornitz, D. M., and P. J. Marie. 2002. FGF signaling pathways in endochondral and intramembranous bone development and human genetic disease. Genes Dev. 16:1446-1465. [DOI] [PubMed] [Google Scholar]
- 35.Parada, Y., L. Banerji, J. Glassford, N. C. Lea, M. Collado, C. Rivas, J. L. Lewis, M. Y. Gordon, N. S. Thomas, and E. W. Lam. 2001. BCR-ABL and interleukin 3 promote haematopoietic cell proliferation and survival through modulation of cyclin D2 and p27Kip1 expression. J. Biol. Chem. 276:23572-23580. [DOI] [PubMed] [Google Scholar]
- 36.Patel, Y. M., and M. D. Lane. 2000. Mitotic clonal expansion during preadipocyte differentiation: calpain-mediated turnover of p27. J. Biol. Chem. 275:17653-17660. [DOI] [PubMed] [Google Scholar]
- 37.Raingeaud, J., S. Gupta, J. S. Rogers, M. Dickens, J. Han, R. J. Ulevitch, and R. J. Davis. 1995. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J. Biol. Chem. 270:7420-7426. [DOI] [PubMed] [Google Scholar]
- 38.Rodda, S. J., and A. P. McMahon. 2006. Distinct roles for Hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development 133:3231-3244. [DOI] [PubMed] [Google Scholar]
- 39.Rodier, G., A. Montagnoli, L. Di Marcotullio, P. Coulombe, G. F. Draetta, M. Pagano, and S. Meloche. 2001. p27 cytoplasmic localization is regulated by phosphorylation on Ser10 and is not a prerequisite for its proteolysis. EMBO J. 20:6672-6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schipani, E., K. Kruse, and H. Juppner. 1995. A constitutively active mutant PTH-PTHrP receptor in Jansen-type metaphyseal chondrodysplasia. Science 268:98-100. [DOI] [PubMed] [Google Scholar]
- 41.Shimada, M., P. A. Greer, A. P. McMahon, M. L. Bouxsein, and E. Schipani. 2008. In vivo targeted deletion of calpain small subunit, capn4, in cells of the osteoblast lineage impairs cell proliferation, differentiation, and bone formation. J. Biol. Chem. 283:21002-21010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shimada, M., M. J. Mahon, P. A. Greer, and G. V. Segre. 2005. The receptor for parathyroid hormone and parathyroid hormone-related peptide is hydrolyzed and its signaling properties are altered by directly binding the calpain small subunit. Endocrinology 146:2336-2344. [DOI] [PubMed] [Google Scholar]
- 43.Shimizu, K., T. Hamamoto, T. Hamakubo, W. J. Lee, K. Suzuki, Y. Nakagawa, T. Murachi, and T. Yamamuro. 1991. Immunohistochemical and biochemical demonstration of calcium-dependent cysteine proteinase (calpain) in calcifying cartilage of rats. J. Orthop. Res. 9:26-36. [DOI] [PubMed] [Google Scholar]
- 44.Skowyra, D., K. L. Craig, M. Tyers, S. J. Elledge, and J. W. Harper. 1997. F-box proteins are receptors that recruit phosphorylated substrates to the SCF ubiquitin-ligase complex. Cell 91:209-219. [DOI] [PubMed] [Google Scholar]
- 45.Sutterlüty, H., E. Chatelain, A. Marti, C. Wirbelauer, M. Senften, U. Muller, and W. Krek. 1999. p45SKP2 promotes p27Kip1 degradation and induces S phase in quiescent cells. Nat. Cell Biol. 1:207-214. [DOI] [PubMed] [Google Scholar]
- 46.Tan, Y., N. Dourdin, C. Wu, T. De Veyra, J. S. Elce, and P. A. Greer. 2006. Conditional disruption of ubiquitous calpains in the mouse. Genesis 44:297-303. [DOI] [PubMed] [Google Scholar]
- 47.Tan, Y., J. Rouse, A. Zhang, S. Cariati, P. Cohen, and M. J. Comb. 1996. FGF and stress regulate CREB and ATF-1 via a pathway involving p38 MAP kinase and MAPKAP kinase-2. EMBO J. 15:4629-4642. [PMC free article] [PubMed] [Google Scholar]
- 48.Tsvetkov, L. M., K. H. Yeh, S. J. Lee, H. Sun, and H. Zhang. 1999. p27(Kip1) ubiquitination and degradation is regulated by the SCF(Skp2) complex through phosphorylated Thr187 in p27. Curr. Biol. 9:661-664. [DOI] [PubMed] [Google Scholar]
- 49.Urano, T., H. Yashiroda, M. Muraoka, K. Tanaka, T. Hosoi, S. Inoue, Y. Ouchi, and H. Toyoshima. 1999. p57(Kip2) is degraded through the proteasome in osteoblasts stimulated to proliferation by transforming growth factor beta1. J. Biol. Chem. 274:12197-12200. [DOI] [PubMed] [Google Scholar]
- 50.Wang, X. D., J. L. Rosales, A. Magliocco, R. Gnanakumar, and K. Y. Lee. 2003. Cyclin E in breast tumors is cleaved into its low molecular weight forms by calpain. Oncogene 22:769-774. [DOI] [PubMed] [Google Scholar]
- 51.Yamada, Y., T. Miyashita, P. Savagner, W. Horton, K. S. Brown, J. Abramczuk, H. X. Xie, K. Kohno, M. Bolander, and L. Bruggeman. 1990. Regulation of the collagen II gene in vitro and in transgenic mice. Ann. N. Y. Acad. Sci. 580:81-87. [DOI] [PubMed] [Google Scholar]
- 52.Yasuda, T., K. Shimizu, Y. Nakagawa, S. Yamamoto, H. Niibayashi, and T. Yamamuro. 1995. m-Calpain in rat growth plate chondrocyte cultures: its involvement in the matrix mineralization process. Dev. Biol. 170:159-168. [DOI] [PubMed] [Google Scholar]
- 53.Zimmerman, U. J., L. Boring, J. H. Pak, N. Mukerjee, and K. K. Wang. 2000. The calpain small subunit gene is essential: its inactivation results in embryonic lethality. IUBMB Life 50:63-68. [DOI] [PubMed] [Google Scholar]