Abstract
The switch from proliferation to differentiation during the terminal stages of erythropoiesis is a tightly controlled process that relies in part on transcription factor-mediated activation of cell cycle components. EKLF is a key transcription factor that is necessary for the initial establishment of the red cell phenotype. Here, we find that EKLF also plays a role during the subsequent differentiation process, as it induces p21WAF1/CIP1 expression independent of p53 to regulate the changes in the cell cycle underlying erythroid maturation. EKLF activates p21 not only by directly binding to an EKLF site within a previously characterized GC-rich region in the p21 proximal promoter but also by occupancy at a novel, phylogenetically conserved region that contains consensus CACCC core motifs located downstream from the p21 TATA box. Our findings demonstrate that EKLF, likely in coordination with other transcription factors, directly contributes to the complex set of events that occur at the final erythroid cell divisions and accentuates terminal differentiation directly by activation of CDK inhibitors such as p21.
Central to the homeostasis of the hematopoietic system is the correct balance of progenitor cell proliferation versus lineage-committed differentiation. Accordingly, impaired differentiation and unrestricted proliferation due to somatic mutations and chromosomal translocations of hematopoietic transcription factors are directly associated with the development of hematopoietic malignancies (37).
One of the defining features of the switch from the proliferative to the differentiating state is the exit from the cell cycle. Upon establishment of lineage commitment, cell cycle arrest occurs in the G1 phase as differentiation begins. Cellular proliferation is controlled through the interactions of cyclin-dependent kinases (CDKs) and their inhibitors. In contrast to Ink4 family members such as p16Ink4A and p18Ink4C that inhibit cyclin D-CDK4 complexes and act early in G1, the CIP/KIP family members p21WAF1/CIP1 and p27Kip1 regulate cell cycle transition through inhibition of cyclin E-CDK2 complexes at later stages in G1 (65) that can be p53 independent (40, 43). While p21WAF1/CIP1 expression is required in hematopoietic stem cells to maintain quiescence, its transcriptional upregulation in progenitor cells promotes differentiation (52).
Erythropoiesis has served as a useful model to elucidate the mechanisms by which hematopoietic transcription factors regulate the induction of cell cycle inhibition to promote differentiation upon lineage commitment (52, 65). Gata1 (41) and erythroid Krüppel-like factor (EKLF [KLF1]) (31) are highly expressed in the megakaryocytic erythroid progenitor and play directive roles in erythroid versus megakaryocytic commitment and differentiation (reviewed in references 5 and 16). Both are required for terminal erythroid differentiation, as the ablation of either gene leads to an arrest in maturation (10, 58). Analyses of global gene expression profiles in response to either Gata1 or EKLF activity have revealed a plethora of target genes (14, 19, 59), within which a subset is of particular relevance to the balance of proliferation versus differentiation. Gata1-dependent erythroid differentiation correlates with an inhibition of mitogenic signals (32, 47) and cell cycle progression as p18Ink4C and p27Kip1 are upregulated (47). A substantive role for EKLF in this process is suggested by its direct activation of p18Ink4C (54) and E2F2 (42, 53) and by studies showing that EKLF gain of function promotes precocious terminal differentiation (16). Consistent with these molecular effects, EKLF-null fetal livers accumulate progenitor but not Ter119-expressing erythroid cells, showing that erythroid commitment and differentiation cannot proceed in the absence of EKLF expression (19, 42). In the present study, we have used inducible cellular differentiation systems (murine erythroleukemia [MEL] and G1E-ER-Gata1 cells) to further illuminate the role of EKLF in these later stages of the erythroid terminal differentiation process. Using a combination of EKLF gain of function, RNA interference (RNAi)-mediated knockdown, gel shift analyses, cell cycle profiling, luciferase reporter assays, and chromatin immunoprecipitation, we show that p21WAF1/CIP1 activation is highly dependent on cellular EKLF levels and provides another avenue by which EKLF affects terminal differentiation; intriguingly, it performs this in part by use of a novel conserved p21WAF1/CIP1 intronic regulatory region.
MATERIALS AND METHODS
Murine erythroleukemia 745A, human erythroleukemia K562, and murine G1E-ER-Gata1 cell lines were cultured as described previously (21, 50). Stable MEL 745A clones with Flag-EKLF under the control of the zinc-inducible promoter were established as described previously (50). Luciferase assays in K562 cells were performed as described previously (50). Drosophila S2 cells were cultured and transfected as described previously (1), and a pGL3-p18 promoter reporter (54) was used as a positive control.
The following antibodies were used for Western blot analyses: anti-Flag M2-horseradish peroxidase (HRP) (A8592; Sigma), anti-EKLF 7B2, anti-p21 F-5 (sc-6246) and anti-Hsp90 H-114 (sc-7947) (both obtained from Santa Cruz Biotechnology), and anti-ERK1 (BD Biosciences). Anti-EKLF 7B2 chromatin immunoprecipitation was performed and quantified as described previously (21, 26) using G1E-ER-Gata1 cells treated either with or without 0.1 μM 17β-estradiol (Sigma) for 24 h. Anti-mouse IgG1 (Pharmingen) was used as a negative control for immunoprecipitation. Primers were designed using Primer Express software (Applied Biosystems).
For flow cytometric cell cycle analysis, MEL cells (parental and stable cell line expressing Flag-tagged wild-type [WT] EKLF) were treated with 140 μM and 160 μM ZnCl2 for 24 h or left untreated as a control. Cells (0.5 × 106) were fixed with 70% ethanol for 2 h on ice and washed twice with cold phosphate-buffered saline (PBS). Subsequently, fixed cells were resuspended in 0.5 ml of propidium iodide staining solution (20 μg/ml propidium iodide, 0.2 mg/ml RNase A, 0.1% Triton X-100) and incubated for 30 min at room temperature (RT). The stained cells were immediately analyzed on a FACScan flow cytometer (Becton Dickinson, San Jose, CA). Data were analyzed using the Dean/Jett/Fox model of FlowJo software (Tree Star) to determine cell cycle distribution.
Phylogenetic alignment and transcription factor binding site searches were performed and visualized according to reference 6, as described previously (26), with cdkn1a (p21WAF1/CIP1) locus sequences obtained from the ENSEMBL genome browser in October 2007.
Double-stranded, synthetic oligonucleotides containing the mammalian β-globin CACCC site (31) or the p21 promoter and intronic predicted EKLF binding sites 3, 4, or 6 (wild type and mutant) were used as probes or competitors for gel shift analyses (Table 1). COS7 cells (60% confluent) were transfected with 10 μg of pSG5-EKLF using DMRIE-C (Invitrogen), according to the manufacturer's protocol. Extracts were prepared after 36 h (51). Binding reactions were performed in the presence of 10 μg of whole-cell extract from COS7 cells transfected with EKLF using conditions previously described (63). Anti-EKLF antibody 4B9 was used to identify the EKLF band shift (63). The DNA-protein complexes were resolved on 8% native gels.
TABLE 1.
Electrophoretic mobility shift assay oligonucleotides
| Probe | Sequence (5′-3′) |
|---|---|
| β-Globin | AGCTAGCCACACCCTAAAGCT |
| Site 3 WT | AGCTGGGGGGCGGGGCCAGCT |
| Site 3 mutant | AGCTGGGAAACGGGGCCAGCT |
| Site 4 WT | AGCTTTTGGGCGTGGAGAGCT |
| Site 4 mutant | AGCTTTTAAACGTGGAGAGCT |
| Site 6 WT | AGCTGGCGGGCGGGCAGAGCT |
| Site 6 mutant | AGCTGGCAAACGGGCAGAGCT |
| Fiz DNA | TTCGGCATCCTTGACTTTGA |
Plasmids containing the p21 promoter (bp −194 to +32), the promoter/intronic region (bp −949 to +1838), or the intronic region alone (bp +842 to +1838) were generated by PCR amplification. Amplification of the promoter utilized, as a template, the p21 promoter construct pW-2400 (bp −2369 to +31), kindly provided by Goutham Narla and Scott Friedman. The intronic regions of p21 gene were amplified using a human bacterial artificial chromosome (BAC) genomic clone (RPCIB753F1537Q; imaGenes GmbH) as a template. PCR products of the p21 promoter and intronic regions were ligated into pGL3 basic luciferase vector (Promega). Deletions were introduced with the QuikChange site-directed mutagenesis kit (Stratagene), according to the manufacturer's protocol. The pPAC and pPAC-EKLF plasmids were kindly provided by Michael Tallack and Andrew Perkins. The Renilla-TK plasmid (Promega) was cotransfected to monitor efficiency.
G1E-ER-Gata1 cells were transfected with 80 nM small interfering RNA (siRNA) (SIO1083383, SIO1083390, SIO1083397, and SIO1083404; Qiagen) targeting the EKLF gene or a nontargeting AllStars negative-control siRNA (102728; Qiagen). The conditions needed for most efficient transfection were established by flow cytometry after titration with fluorescein-conjugated siRNA (SC 36869). Transfections were carried out using RNAiFect (Qiagen) as a transfection reagent, according to the manufacturer's protocol; efficiency was 60 to 80%. Cells were harvested 24 h after transfection and resuspended in fresh media supplemented with 0.1 μM β-estradiol for differentiation. Cells were collected at 24 h after β-estradiol treatment. Western blot data from unsaturated exposures were quantified using Image J software.
ChIP and reverse transcription-quantitative PCR (RT-qPCR) primer sequences are available upon request.
RESULTS
Increased levels of EKLF are antiproliferative and stimulate expression of p21WAF1/CIP1.
We initially took a gain-of-function (GOF) approach to explore EKLF's potential role in the late stages of erythroid differentiation by attempting to establish stable constitutive expression of an ectopic Flag-tagged mouse EKLF cDNA in an MEL cell line. However, we were not able to generate any stable clones, and even pools of selected cells quickly lost Flag-EKLF expression upon further passaging (not shown). This was consistent with an earlier report showing that EKLF exerts an antiproliferative effect in erythroid cells and discourages immortalization of primary cells (10) and with our recent findings that EKLF gain of function stimulates erythroid differentiation (16). These observations prompted us to hypothesize that EKLF might transcriptionally activate the CDK inhibitor p21WAF1/CIP1, as this gene has been shown to be activated by several other KLF family members in human cell lines (KLF6 [34], KLF2 [60], KLF4 [62], and Sp1/Sp3 [43]). To test this idea, we established stable MEL lines that placed Flag-EKLF transcription under the control of a zinc-inducible promoter (38, 50).
We first analyzed p21 expression levels in response to EKLF upregulation in an MEL cell line that is deficient in p53 (27). We found that expression of Flag-WT EKLF in MEL cells directly correlates with an increase in mRNA and p21 protein levels in a concentration (not shown)- and time-dependent manner (Fig. 1A and B). Consistent with recent studies (42, 53), E2F2 is also upregulated by increased EKLF expression; however, levels of other cell cycle regulators such as c-Myc, CDK4, and p27 are not affected (Fig. 1C). The observation that the endogenous p21 gene is induced with the same kinetics as Flag-WT EKLF strongly suggests that p21 is a direct target of EKLF.
FIG. 1.
Enforced expression of EKLF activates the p21 promoter. (A) Stable Flag-EKLF MEL cells were monitored for Flag-EKLF, p21, and ERK protein expression at various times, as indicated after induction with 160 μM ZnCl2 (50). (B) Endogenous p21 mRNA levels (triplicates normalized to GAPDH [glyceraldehyde-3-phosphate dehydrogenase]) in total RNA prepared from stable Flag-EKLF MEL cell samples as shown in panel A were monitored by RT-qPCR. (C) Levels of EKLF, p21, CDK4, p27, E2F2, and c-Myc mRNA expression from total RNA collected from parental MEL or stable MEL cells treated with ZnCl2 for 18 h (coded as indicated) were monitored by quantitative RT-PCR analysis. The mRNA level of parental MEL cells without Zn induction was given an arbitrary value of 1, and all other levels were normalized to that value for comparison. EKLF primers were designed to monitor the sum of endogenous and exogenous mRNA. Expression of GAPDH was used to standardize the particular expression levels. Experiments were performed three times; relative expression values are the average values of triplicates.
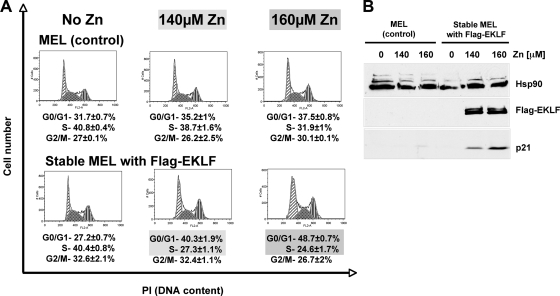
Concomitantly, zinc-mediated induction of EKLF expression in MEL cells for 24 h shifts their cell cycle profile to a more arrested state by increasing the number of cells in G0/G1 phase at the expense of the fraction of cycling cells in S phase (Fig. 2). These data are consistent with the notion that EKLF activation of p21 helps mediate cell cycle arrest in MEL cells.
FIG. 2.
EKLF induces cell cycle arrest. (A) Parental MEL cells and MEL cells stably expressing Flag-WT EKLF were cultured in the presence of ZnCl2 (0, 140, and 160 μM) for 24 h. Cells were fixed, stained, and analyzed by flow cytometry. Cell cycle analysis was conducted by utilizing the Dean/Jett/Fox model (FlowJo software; Tree Star). Numbers indicate the percentages of cells in the different phases of the cell cycle. Data are representative results from four experiments. PI, propidium iodide. (B) Whole-cell lysates from parental and stable MEL cells treated with ZnCl2 for 24 h and used for the analysis shown in panel A were analyzed for EKLF and p21 protein expression. Equal loading was assessed by probing with Hsp90 antibody.
Identification of novel phylogenetically conserved KLF sites in the p21 gene.
To determine if any EKLF responsive regulatory elements exist within the p21 locus, we comprehensively searched for such sites across the entire p21 transcription unit by creating a phylogenetic alignment of p21 genomic sequences obtained from seven mammalian species and by scanning for evolutionarily conserved transcription factor binding sites (Fig. 3) (26). Such a multispecies alignment is advantageous in identifying mammalian regulatory regions compared to a pairwise alignment (6).
FIG. 3.
Phylogeny of the p21 locus. (A) A phylogenetic alignment of seven mammalian cdkn1a (p21WAF1/CIP1) loci (each sequence, 15 kb; total alignment length, about 27,400 nucleotides [nt]) identifies several conserved regulatory regions upstream of the transcription start site and within the first intron. Each sequence is represented as a black line (breaks demarcate gaps in the alignment). Repeats or low-complexity DNA are represented as blue bars, while exons are depicted either as tan bars for untranslated regions (UTR) or as red bars for translated regions. The position of a fourth exon described for the human p21 gene is indicated as Hs exon 1A. The degree of sequence conservation between species is expressed as a score (y axis) per nucleotide position (x axis; global length). Peaks of conservation above 0.6 (red line) within noncoding regions are deemed functionally significant (6). The seven conserved sites discussed in the text are numbered below their location in the alignment, as indicated by arrows. An expansion of selected regions (sites 3, 4, and 6) is shown below (based on panel B). Transcription factor consensus motifs used for putative binding site search are as follows: p53, RRRCWWGYYY (48); Sp1, CCCGCC; KLF, CACCC (60); and EKLF, (N/C)CNCNCCC (15). No conserved WGATAR motifs were found across the entire length of the p21 alignment. (B) Detailed view at nucleotide resolution of the seven regions within the phylogenetic alignment of the cdkn1a (p21WAF1/CIP1) locus that display conserved consensus binding sites of either p53 (highlighted in blue), KLF (bright green), EKLF (turquoise), or Sp1 (pink). The TATA box and predicted transcription start site(s) (highlighted in red) downstream of the GC-rich cluster are indicated as well. The respective transcription factor consensus binding motifs and names are displayed above each conserved site, with capital versus lowercase letters indicating the degree of conservation. Both strands were searched for consensus binding motifs, which are denoted 5′ to 3′ in either case. Site 3 is also alternatively highlighted (3b) to show the consensus EKLF binding sites (turquoise) that are dispersed within the gaps generated by the alignment program. Mm = Mus Musculus = mouse; Hs = Homo sapiens = human; Pt = Pan troglodytes = chimpanzee; Cf = Canis familiaris = dog; Rn = Rattus norvegicus = rat; Bt = Bos taurus = cow; MaM = Macaca mulatta = rhesus (macaque).
As expected, we find the two known, conserved p53 consensus motifs at the proximal promoter (sites 1 and 2) (Fig. 3) (48). We also find the partially conserved GC-rich elements upstream of exon 1 (site 3) (Fig. 3) within a region known to play a role in other Sp1/KLF family member effects on p21 expression (34, 43, 60, 62). Closer inspection reveals a consensus EKLF binding site (5′-CCNCNCCCN) (15, 31) embedded within this region that is readily apparent after rearrangement of the gaps introduced by the multispecies alignment (Fig. 3).
However, we also discovered four novel distinct regions within intron 1 (sites 4 to 7) (Fig. 3) that each display a high degree of sequence homology across all species and contain previously unreported putative KLF or Sp1/3 binding sites (detailed in Fig. 3B). These novel intronic CACCC elements raise the possibility that EKLF may regulate p21 expression outside the known cluster of GC-rich motifs located upstream of the TATA box. Of particular interest are sites 4 and 6, as they display the highest levels of conserved homology to the predicted EKLF binding sequence motif.
EKLF binds the p21 proximal promoter and intron regions in vitro and in vivo.
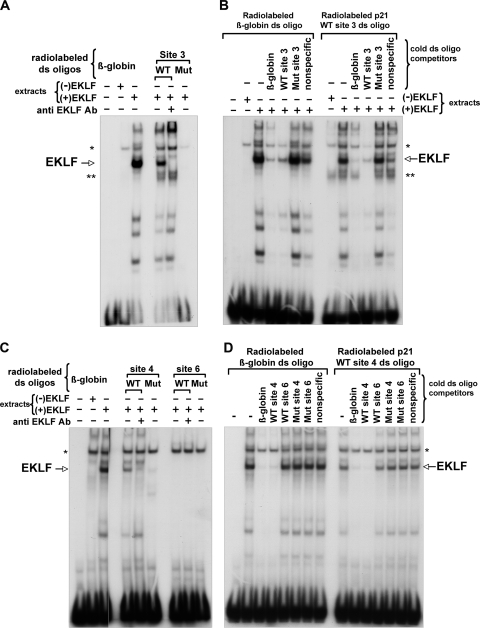
We used an in vitro gel shift assay to test the binding specificity of EKLF to p21 proximal promoter site 3 and novel intron sites 4 and 6. COS7 cells do not express EKLF, but they contain a number of other CACCC element binding proteins from the Sp and KLF families; however, these are easily distinguished from the novel complex seen upon transfection of EKLF (Fig. 4).
FIG. 4.
EKLF binds to the p21 promoter and novel intronic CACCC element sites in vitro. Gel shift assays were performed with wild-type or mutant (Mut) radiolabeled double-stranded (ds) oligonucleotides, comprising p21 promoter EKLF binding site 3 (A) and p21 intronic predicted EKLF binding sites 4 or 6 (C), after incubation with the indicated extracts from COS7 cells. A probe containing the β-globin CACCC site (31) was used as a positive binding control; anti-EKLF antibody 4B9 was included, as indicated (63). (B and D) Radiolabeled ds oligonucleotides for the β-globin CACCC site (left) and p21 WT site 3 (B, right) or p21 WT site 4 (D, right) were subjected to binding competition with a 100-fold molar excess of the indicated unlabeled (cold) ds oligonucleotides in the presence of extract from COS7 cells expressing EKLF. Asterisk, nonspecific band; double asterisk, specific band (non-EKLF) only observed with WT site 3.
We used these extracts to test for EKLF binding to radiolabeled double-stranded oligonucleotides containing p21 wild-type (WT) or mutated sites 3, 4, and 6 and included the EKLF binding site from β-globin promoter as a positive control (31). Only p21 WT sites 3 (Fig. 4A) and 4 (Fig. 4C) generate a DNA-EKLF complex similar to that seen with the β-globin site that can uniquely be disrupted by inclusion of anti-EKLF antibody, while the mutated versions of these sites, where the conserved CCC sequence is changed to TTT, do not interact with EKLF. It is worth noting that a non-EKLF-related complex is uniquely seen with the p21 site 3 probe (Fig. 4, double asterisks). In contrast, p21 WT site 6, although predicted to be a potential EKLF binding site based on phylogenetic conservation (Fig. 3), does not bind EKLF (Fig. 4C), thus suggesting that subtle sequence variances are sufficient to disrupt binding (previously seen in vitro [15] and in vivo [HbVar database] with single point mutants in the β-globin CACCC element). Competition assays further support the binding specificity of EKLF to promoter p21 WT site 3 and novel p21 WT site 4, as only the β-globin site or p21 WT sites 3 or 4 effectively antagonize each other's complex formation, unlike p21 WT site 6 or any of the mutated variants (Fig. 4B and D).
To corroborate this finding in vivo, we next examined EKLF occupancy at the p21 locus by chromatin immunoprecipitation (ChIP) using the estradiol-induced differentiation of the G1E-ER-Gata1 cell line as a model of erythroid maturation (47). G1E-ER-Gata1 cells do not express any endogenous Gata1 but express ER-Gata1 (estrogen receptor-Gata1 fusion protein) in its place, thereby keeping Gata1 localized to the cytoplasm in the absence of ER ligand and the cells thus arrested in differentiation. These cells resemble primary erythroblasts, which, upon addition of ER ligand, activate Gata1 target genes and thereby initiate erythroid maturation.
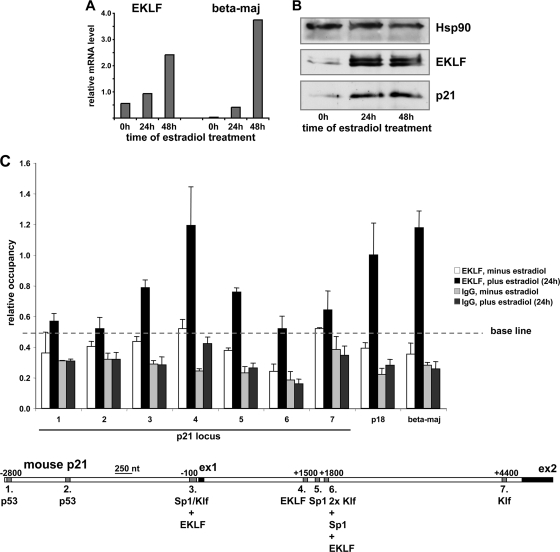
As previously reported (21), estradiol treatment of G1E-ER-Gata1 cells for up to 48 h upregulates EKLF and leads to terminal differentiation, as judged by the accumulation of β-globin mRNA levels (Fig. 5A). This increase in EKLF expression, in turn, correlates with a rise in p21 protein levels within 24 h (Fig. 5B), similar to the effect described above in MEL cells.
FIG. 5.
EKLF directly binds to the p21 promoter and intron in vivo. (A) EKLF and β-globin (beta-maj) mRNA levels as determined by RT-qPCR were monitored after treatment of G1E-ER-Gata1 cells with estradiol for up to 48 h. (B) EKLF and p21 protein levels were monitored by Western blot analysis in whole-cell lysates prepared from G1E-ER-Gata1 cells after estradiol treatment for up to 48 h. Substantial upregulation of p21 protein levels occurred in G1E-ER-Gata1 cells within 24 h of induced erythroid differentiation (middle). Hsp90 served as a loading control. (C) Chromatin immunoprecipitation analysis of EKLF occupancy at distinct sites within the p21, p18, and β-globin loci in G1E-ER-Gata1 cells was performed with anti-EKLF 7B2 antibody or its IgG isotype control before or after stimulation with estradiol for 24 h, as indicated. Sites 1 to 7 within the p21 locus correspond to the regions harboring the identified, conserved transcription factor DNA binding consensus motifs, as indicated in Fig. 3 and as depicted schematically below for the mouse p21 genomic sequence. Known EKLF binding sites within the p18 locus (54) and the β-globin (beta-maj) promoter (21) were used as positive controls.
Using binding to the two conserved p53 consensus motifs (sites 1 and 2) as negative controls (baseline) (Fig. 5C), ChIP analysis shows that, prior to erythroid differentiation (i.e., in the absence of estradiol), EKLF occupancy levels across the p21 locus (sites 1 to 7) as well as at positive-control sites in the β-globin (25) and p18 (54) loci are generally low and do not differ significantly (Fig. 5C). As expected, after 24 h of induced erythroid differentiation, EKLF occupancy at the β-globin and p18 binding sites is markedly upregulated. We also find that EKLF occupancy increases at p21 promoter site 3 upstream of exon 1 and at the Sp1 site in intron 1 (site 5). However, most notably an increase in EKLF occupancy comparable to that reached at the positive-control β-globin and p18 sites is recorded at the newly identified, conserved EKLF binding region that encompasses sites 4 and 5 in p21 intron 1. In contrast, no significant increase in EKLF occupancy upon estradiol treatment occurs at the two conserved p53 consensus motifs (sites 1 and 2). Similarly, EKLF does not bind to the conserved KLF sites further downstream in p21 intron 1 (sites 6 and 7), demonstrating that phylogenetic sequence conservation alone is not sufficient to predict in vivo occupancy (19, 21).
To corroborate EKLF's direct activation of the p21 gene as suggested by our ChIP analysis, we next examined whether murine WT EKLF transactivates the human p21 promoter in luciferase reporter assays. Given the abundance of putative Sp1 binding sites within the conserved GC-rich sequence elements in the p21 proximal promoter and newly identified intronic regions, we used Sp1-deficient Drosophila S2 cells (11) to minimize possible interference with EKLF-mediated reporter gene activation. Our preliminary testing of this line with the p18-luciferase reporter shows a strong activation by EKLF, as expected (not shown) (54). Using these cells, we first tested a construct containing the proximal promoter sequence that encompasses site 3 within the previously known GC-rich region upstream of the p21 TATA box. EKLF robustly induces reporter gene expression from this minimal p21 promoter (Fig. 6A). This transactivation is largely EKLF dependent, as deletion of the site 3 CACCC EKLF consensus sequence (the remaining Sp1/KLF sites forming the site 3 are intact) significantly reduces luciferase activity (Fig. 6A). We next tested the contribution of the intronic regulatory region containing sites 4, 5, and 6 to EKLF-dependent transactivation by using a larger construct that more closely resembles the architecture of the endogenous p21 locus (bp −949 to +1838). While the additional sequence raises the basal level of reporter gene expression, cotransfection of EKLF nonetheless results in a strong transactivation (Fig. 6B). We then used this large construct as a template for site-directed mutagenesis of each individual EKLF binding site. However, the differences among mutated GC-rich sites 4 (EKLF), 5 (Sp1), and 6 (2× Klf/Sp1/EKLF) are too subtle to be distinguished by the assay (not shown). Nevertheless, deletion of all three sites together leads to a dramatic decrease in EKLF transactivation (compare the “+EKLF” lanes in Fig. 6B).
FIG. 6.
EKLF activates the p21 gene through its interactions with upstream promoter and downstream intronic regions in Drosophila and human cells. EKLF transactivation of the p21 promoter was tested by transient cotransfection into Drosophila S2 cells (A, B) or human K562 cells (C to E) with plasmids containing the luciferase reporter gene under the control of the human p21 promoter (bp −194 to +31 or −33 to +2) that varies in the presence of the EKLF binding motif in site 3 (A, C), the human p21 promoter and the intronic region (bp −949 to +1838) (B, D), or the intronic region alone (bp +842 to +1838) (E), each of which varied as to the presence of sites 4 to 6. An EKLF expression plasmid or empty vector was included as indicated (− or +). A Renilla reporter construct was included as a normalization control for transfection efficiency. An average of triplicate results is shown (arithmetic mean ± standard deviation). The genomic layout of the promoter/intronic region of p21 is depicted at the bottom. RLU, relative light units.
Virtually the same results are obtained when we analyze the EKLF-dependent transactivation of p21 regulatory sequence-controlled luciferase constructs in K562 cells, a human erythroleukemic cell line that lacks endogenous EKLF expression (3). In contrast to Drosophila S2 cells, however, the basal level of reporter gene activation (i.e., in the absence of EKLF transfection) is higher and more variable, most likely due to the presence of Sp1 family proteins that, similar to EKLF, bind to GC-rich motifs. In spite of this, the minimal p21 promoter construct is activated in an EKLF-dependent manner that relies on the presence of site 3 (Fig. 6C). Analogously, EKLF transactivates the long reporter construct above basal levels, and this transactivation is dependent on sites 4, 5, and 6, while site 3 does not contribute in this context (Fig. 6D and E).
In summary and in concert with the in vitro and in vivo observations, the luciferase data substantiate our suggestion that the p21 promoter and intronic regions contain bona fide, functional EKLF binding sites. Taken together, these results demonstrate that EKLF directly targets specific regions within the p21 gene for activation during erythroid differentiation.
Lowered levels of EKLF lead to decreased p21 expression.
The in vitro and in vivo occupancy profiling of the p21 locus and the kinetics of p21 expression after EKLF gain of function strongly suggest that EKLF directly activates the p21 gene, and we wished to determine whether the converse loss of function was true. Although fetal liver EKLF-null erythroid cells are available, they are stalled at the CFU-erythroid (CFU-E) stage and do not mature into more differentiated cells (42, 53), making them unsuitable for studying terminal differentiation events. We therefore chose to utilize the G1E-ER-Gata1 erythroid cells (used in Fig. 5) to illuminate the effect of EKLF knockdown on p21 levels during their induced differentiation. To this end, we transiently transfected G1E-ER-Gata1 cells with four different siRNAs directed against EKLF mRNA prior to treatment with estradiol. RNAi-mediated knockdown of EKLF protein in estradiol-treated G1E-ER-Gata1 cells results in reduced levels of p21 protein compared to the levels seen with estradiol induction alone (Fig. 7A). Moreover, variations in knockdown efficiency (20 to 65%) between the four different siRNAs result in different levels of remaining EKLF protein, which in turn correlate directly to the p21 protein levels observed (Fig. 7B). These loss-of-function data show that subtle variation in EKLF concentration can have dramatic effects on target gene expression; for example, a 2.5-fold drop in EKLF leads to a 10-fold drop in p21 levels (Fig. 7B). We conclude that EKLF directly targets the p21 gene and induces its upregulation to promote the terminal differentiation of erythroid cells.
FIG. 7.
Knockdown of EKLF leads to decreased p21 expression in G1E-ER-Gata1 cells. (A) G1E-ER-Gata1 cells were transfected with 80 nM of four different anti-EKLF siRNAs or a nontargeting AllStars negative-control siRNA for 24 h and then treated with 0.1 μM β-estradiol for an additional 24 h. Cells were collected, and cell lysates were subjected to Western blot analysis with EKLF and p21 antibodies. Equal loading was assessed with Hsp90 antibody. The data are representative of results from two experiments. (B) Individual protein levels of EKLF, before and after EKLF knockdown, were quantified from three experiments and plotted against the quantified level of p21 protein derived from the same extract. A correlation coefficient of 0.85 with a linear slope of 1.02 was attained between the two sets.
DISCUSSION
In this study, we show that EKLF activates the p21 gene during the differentiation of erythroid cells, which correlates with an exit from the cell cycle and G0/G1 arrest. We identify novel conserved KLF binding motifs at the p21 locus and demonstrate EKLF occupancy in vitro and in vivo. Knockdown of EKLF protein in differentiating erythroid cells negatively affects p21 expression. We propose that EKLF promotes the differentiation and maturation of red cells by directly targeting the p21 gene to regulate cell cycle progression via p21-mediated inhibition of cyclin-dependent kinases.
Coordinate role of transcription factors in erythroid terminal differentiation.
The tightly regulated control of proliferation versus differentiation during the derivation of large numbers of terminally differentiated cells from small pools of stem and progenitor cells is crucial to the homeostasis of the hematopoietic system. One key aspect in this regard is the control of cell cycle exit as lineage commitment occurs and differentiation ensues, directing highly proliferative progenitor cells to enter G0/G1 arrest (24). Both EKLF and Gata1 are found at the nexus of cell cycle control during erythroid differentiation, as EKLF regulates p21 (this study), p18 (54), and E2F2 (42, 53), while Gata1 controls expression levels of p18, p27, Cdk6, cyclin D2, and c-myc (47).
Interestingly, the p21 locus alignment does not contain any conserved Gata factor consensus binding sites (WGATAR), arguing that the direct activation of the p21 gene by EKLF during erythroid differentiation is Gata1 (and p53) independent. This notion is supported by an earlier report identifying the CDK inhibitors p18 and p27, but not p21, as direct targets of Gata1 during the maturation of erythroid cells (47). However, as Gata1 stimulates EKLF expression upon erythroid commitment (26), it thereby indirectly also regulates p21 based on our current findings. We have located conserved EKLF consensus binding motifs within the hematopoietic enhancer region of the Gata1 gene and find that EKLF occupies the Gata1 hematopoietic enhancer in vivo upon estradiol-induced differentiation of G1E-ER-Gata1 cells (Fig. 8). These observations suggest the potential existence of a positive feedback loop between EKLF and Gata1 that reciprocally enhances the regulation of each transcription factor's target gene profile and locks in any cell cycle changes.
FIG. 8.
EKLF contributes to Gata1 activation through its binding site within the hematopoietic enhancer element. (A) Excerpt of the phylogenetic alignment of the Gata1 locus from four mammalian species (Mm = Mus musculus, or mouse; Rn = Rattus norvegicus, or rat; Hs = Homo sapiens, or human; Cf = Canis familiaris, or dog) at nucleotide resolution, as previously displayed by Lohmann and Bieker (26), showing the hematopoietic enhancer (HE) element of the Gata1 locus and a conserved region of Gata1 exon6. A known conserved WGATAR motif that is required for Gata1 expression at the progenitor stage as well as in committed erythroid cells (35, 57) is highlighted in light gray, while newly identified EKLF and KLF consensus sites are highlighted in dark gray. The transcription factor consensus binding motifs and names are displayed above each conserved site, with capital versus lowercase letters indicating the degree of conservation. Both strands were searched for consensus binding motifs, which are denoted 5′ to 3′ in either case. (B) EKLF occupancy at the Gata1 hematopoietic enhancer (HE), as analyzed by chromatin immunoprecipitation in G1E-ER-Gata1 cells treated with or without estradiol for 24 h, analogous to that shown in Fig. 5C. A conserved GC-rich element in Gata1 exon6 (ex6) serves as a negative control, while EKLF occupancy at the β-globin (beta-maj) promoter from Fig. 5C is shown for comparison.
Biological effects resulting from altered EKLF levels.
Our studies suggest that altering cellular levels of EKLF protein can change the erythroid cell cycle status from proliferation to differentiation and that this effect is mediated by its direct activation of the p21 gene. The first report of an antiproliferative effect of EKLF was published by Perkins et al., who observed an increased derivation frequency of immortal clones from EKLF−/− fetal liver cells compared with that of EKLF+/− fetal liver cells after J2 virus infection, indicating that EKLF discouraged immortalization (10). The absence of EKLF resulted in a markedly increased rate of transformation by cooperating oncogenes v-mil-raf and v-myc. This same group has recently shown that the CDK inhibitor p18(INK4c) is directly activated by EKLF (54). These data, together with our results showing that induction of EKLF immediately increases endogenous expression of the cell cycle inhibitor p21, suggest a mechanism by which an increase in the level of EKLF inhibits cellular proliferation and thus minimizes the likelihood of successfully establishing a stable cell line.
An increase in p21 protein levels correlates with erythroid terminal differentiation, as monitored in sorted primary cells (17), in differentiating Friend virus (FVA)-infected spleen-derived erythroid cells (20), and in two-stage liquid culture assay systems (39). In addition, MEL cells exhibit a biphasic mode of p21 increase after induction with hexamethylene bisacetamide (HMBA) (30). Similarly, EKLF levels increase during hematopoiesis (16), continue to do so during the terminal differentiation process (29), and also fluctuate during MEL cell differentiation (45). Our studies suggest that these observations are more than a correlation, as EKLF stimulates p21 expression by directly interacting with conserved target sequences within the p21 transcription unit.
The role of EKLF as a transcriptional activator has been most extensively analyzed at the adult β-globin promoter (2). Recruitment of BRG1 to the promoter is deficient in the absence of EKLF (4), and acetylation of EKLF K288 by P300/CBP is important for this interaction (61, 63). This mode of regulation may also be operant at the p21 promoter, as BRG1 is critical for expression of p21 (18, 22).
The present study, along with others that have analyzed the effects of manipulating EKLF expression in erythroid cells derived from differentiating embryonic stem (ES) or fetal liver cells (16, 42, 53), strongly supports the idea that altering cellular levels of EKLF protein can change the erythroid cell cycle status. However, changes in EKLF levels are probably not sufficient to explain its effects. Experiments in other differentiating systems may be instructive; for example, MyoD is present in both myoblasts and differentiating myotubes, and its positive effect on cell cycle withdrawal is dependent both on its acetylation status (28) and on the loss of its interaction with HDAC1 (44). In this sense, previous studies with EKLF showing that its association with corepressors such as Sin3a or Mi2β (both of which recruit HDAC1) is dependent on EKLF's acetylation (K302) (7) or sumoylation (K74) status (50), in addition to observations that EKLF-Sin3a interactions exhibit erythroid stage specificity (7), provide a plausible scenario to help explain how the EKLF protein may not be equivalent in all erythroid cell populations.
Function of EKLF in terminal differentiation decisions.
It is not likely that EKLF regulation of p21 levels is the sole determinant in establishing the terminal events of erythropoiesis, particularly as the role of cell cycle components in erythroid differentiation is complex and does not follow the course typically observed in other cell types for renewal divisions. For example, the level of E2F2, considered to be positively required for S-phase progression (12, 55), is increased during erythroid terminal differentiation (13). In addition, E2F4, a transcriptional repressor that typically coordinates cell cycle exit (12, 55), is required for proliferation of erythroid progenitors (23).
Recent studies have shown that E2F2 is a direct target of EKLF activation (42, 53). Although this helps explain the observation that E2F2 increases during terminal differentiation, such a simple scheme falls apart in terms of the cell cycle when also considering that EKLF directly increases p21 (this study) and p18 (54) levels during this process, two proteins that inhibit cell cycle progression. Of possibly greater relevance are observations that E2F2 limits T-cell proliferation, as E2F2-null T cells enter S phase and proliferate more extensively than the wild type (64). In addition, loss of even a single copy of E2F2 accelerates Myc-induced lymphomagenesis, suggesting that E2F2 can function as a tumor suppressor (36).
EKLF is present in renewable erythroid progenitors and their terminal differentiated progeny (16), cell types that exhibit virtually opposite cell cycle properties. EKLF-null erythroid cells also show an abnormal cell cycle profile that is stalled at G0/G1 (42, 53). As noted by others (24, 56), the proliferation (renewal) and differentiation stages of erythroid terminal differentiation may be governed by distinct cell cycle mechanisms. In addition, the role of E2Fs may be heavily dependent on the cellular context in which they are expressed (12). In support of this notion, recent studies have shown that E2F1 to -3 display a dual functionality (9), as follows: in progenitor cells, they act as transcriptional activators, but in differentiating cells, they function in a repressor complex with Rb that effectively silences E2F targets and facilitates exit from the cell cycle.
We suggest that quantitative differences in EKLF effective concentration or its posttranslational modification status (8, 49, 50) at different stages of erythroid differentiation help establish which gene is expressed and which cell cycle program is eventually executed. This is particularly pertinent, as these modifications directly affect the protein partners with which EKLF can interact (7, 50, 63).
Additionally, our discovery that EKLF directly regulates the CDK inhibitor p21 during erythroid differentiation, by analogy to results obtained with other KLF factors (e.g., KLF4 [46] and KLF6 [33]), raises the possibility that EKLF may normally behave as a tumor suppressor and that its dysregulation might contribute to hematopoietic malignancies in humans.
Acknowledgments
We thank Goutham Narla, Scott L. Friedman, Mitch Weiss, Rob Krauss, Kay Macleod, Michael Tallack, and Andrew Perkins for providing reagents and/or discussion.
This work was supported by NIH PHS grant R01 DK46865 (to J.J.B.). The Quantitative PCR Shared Research Facility is supported by MSSM.
Footnotes
Published ahead of print on 5 April 2010.
REFERENCES
- 1.Bao, S., and R. Cagan. 2005. Preferential adhesion mediated by Hibris and Roughest regulates morphogenesis and patterning in the Drosophila eye. Dev. Cell 8:925-935. [DOI] [PubMed] [Google Scholar]
- 2.Bieker, J. J. 2000. EKLF and the development of the erythroid lineage, p. 71-84. In J. D. Light and K. Ravid (ed.), Transcription factors: normal and malignant development of blood cells. Wiley-Liss, New York, NY.
- 3.Bieker, J. J. 1996. Isolation, genomic structure, and expression of human erythroid Kruppel-like factor (EKLF). DNA Cell Biol. 15:347-352. [DOI] [PubMed] [Google Scholar]
- 4.Bottardi, S., J. Ross, N. Pierre-Charles, V. Blank, and E. Milot. 2006. Lineage-specific activators affect beta-globin locus chromatin in multipotent hematopoietic progenitors. EMBO J. 25:3586-3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouilloux, F., G. Juban, N. Cohet, D. Buet, B. Guyot, W. Vainchenker, F. Louache, and F. Morle. 2008. EKLF restricts megakaryocytic differentiation at the benefit of erythrocytic differentiation. Blood 112:576-584. [DOI] [PubMed] [Google Scholar]
- 6.Chapman, M. A., I. J. Donaldson, J. Gilbert, D. Grafham, J. Rogers, A. R. Green, and B. Gottgens. 2004. Analysis of multiple genomic sequence alignments: a web resource, online tools, and lessons learned from analysis of mammalian SCL loci. Genome Res. 14:313-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, X., and J. J. Bieker. 2004. Stage-specific repression by the EKLF transcriptional activator. Mol. Cell. Biol. 24:10416-10424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, X., and J. J. Bieker. 2001. Unanticipated repression function linked to erythroid Kruppel-like factor. Mol. Cell. Biol. 21:3118-3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chong, J. L., P. L. Wenzel, M. T. Saenz-Robles, V. Nair, A. Ferrey, J. P. Hagan, Y. M. Gomez, N. Sharma, H. Z. Chen, M. Ouseph, S. H. Wang, P. Trikha, B. Culp, L. Mezache, D. J. Winton, O. J. Sansom, D. Chen, R. Bremner, P. G. Cantalupo, M. L. Robinson, J. M. Pipas, and G. Leone. 2009. E2f1-3 switch from activators in progenitor cells to repressors in differentiating cells. Nature 462:930-934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coghill, E., S. Eccleston, V. Fox, L. Cerruti, C. Brown, J. Cunningham, S. Jane, and A. Perkins. 2001. Erythroid Kruppel-like factor (EKLF) coordinates erythroid cell proliferation and hemoglobinization in cell lines derived from EKLF null mice. Blood 97:1861-1868. [DOI] [PubMed] [Google Scholar]
- 11.Courey, A. J., and R. Tjian. 1988. Analysis of Sp1 in vivo reveals multiple transcriptional domains, including a novel glutamine-rich activation motif. Cell 55:887-898. [DOI] [PubMed] [Google Scholar]
- 12.Dimova, D. K., and N. J. Dyson. 2005. The E2F transcriptional network: old acquaintances with new faces. Oncogene 24:2810-2826. [DOI] [PubMed] [Google Scholar]
- 13.Dirlam, A., B. T. Spike, and K. F. Macleod. 2007. Deregulated E2f-2 underlies cell cycle and maturation defects in retinoblastoma null erythroblasts. Mol. Cell. Biol. 27:8713-8728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drissen, R., M. von Lindern, A. Kolbus, S. Driegen, P. Steinlein, H. Beug, F. Grosveld, and S. Philipsen. 2005. The erythroid phenotype of EKLF-null mice: defects in hemoglobin metabolism and membrane stability. Mol. Cell. Biol. 25:5205-5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng, W. C., C. M. Southwood, and J. J. Bieker. 1994. Analyses of beta-thalassemia mutant DNA interactions with erythroid Kruppel-like factor (EKLF), an erythroid cell-specific transcription factor. J. Biol. Chem. 269:1493-1500. [PubMed] [Google Scholar]
- 16.Frontelo, P., D. Manwani, M. Galdass, H. Karsunky, F. Lohmann, P. G. Gallagher, and J. J. Bieker. 2007. Novel role for EKLF in megakaryocyte lineage commitment. Blood 110:3871-3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goardon, N., J. A. Lambert, P. Rodriguez, P. Nissaire, S. Herblot, P. Thibault, D. Dumenil, J. Strouboulis, P. H. Romeo, and T. Hoang. 2006. ETO2 coordinates cellular proliferation and differentiation during erythropoiesis. EMBO J. 25:357-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hendricks, K. B., F. Shanahan, and E. Lees. 2004. Role for BRG1 in cell cycle control and tumor suppression. Mol. Cell. Biol. 24:362-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hodge, D., E. Coghill, J. Keys, T. Maguire, B. Hartmann, A. McDowall, M. Weiss, S. Grimmond, and A. Perkins. 2006. A global role for EKLF in definitive and primitive erythropoiesis. Blood 107:3359-3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsieh, F. F., L. A. Barnett, W. F. Green, K. Freedman, I. Matushansky, A. I. Skoultchi, and L. L. Kelley. 2000. Cell cycle exit during terminal erythroid differentiation is associated with accumulation of p27(Kip1) and inactivation of cdk2 kinase. Blood 96:2746-2754. [PubMed] [Google Scholar]
- 21.Im, H., J. A. Grass, K. D. Johnson, S.-I. Kim, M. E. Boyer, A. N. Imbalzano, J. J. Bieker, and E. H. Bresnick. 2005. Chromatin domain activation via GATA-1 utilization of a small subset of dispersed GATA motifs within a broad chromosomal region. Proc. Natl. Acad. Sci. U. S. A. 102:17065-17070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang, H., K. Cui, and K. Zhao. 2004. BRG1 controls the activity of the retinoblastoma protein via regulation of p21CIP1/WAF1/SDI. Mol. Cell. Biol. 24:1188-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kinross, K. M., A. J. Clark, R. M. Iazzolino, and P. O. Humbert. 2006. E2f4 regulates fetal erythropoiesis through the promotion of cellular proliferation. Blood 108:886-895. [DOI] [PubMed] [Google Scholar]
- 24.Koury, M. J., S. T. Sawyer, and S. J. Brandt. 2002. New insights into erythropoiesis. Curr. Opin. Hematol. 9:93-100. [DOI] [PubMed] [Google Scholar]
- 25.Letting, D. L., C. Rakowski, M. J. Weiss, and G. A. Blobel. 2003. Formation of a tissue-specific histone acetylation pattern by the hematopoietic transcription factor GATA-1. Mol. Cell. Biol. 23:1334-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lohmann, F., and J. J. Bieker. 2008. Activation of Eklf expression during hematopoiesis by Gata2 and Smad5 prior to erythroid commitment. Development 135:2071-2082. [DOI] [PubMed] [Google Scholar]
- 27.Macleod, K. F., N. Sherry, G. Hannon, D. Beach, T. Tokino, K. Kinzler, B. Vogelstein, and T. Jacks. 1995. p53-dependent and independent expression of p21 during cell growth, differentiation, and DNA damage. Genes Dev. 9:935-944. [DOI] [PubMed] [Google Scholar]
- 28.Mal, A., M. Sturniolo, R. L. Schiltz, M. K. Ghosh, and M. L. Harter. 2001. A role for histone deacetylase HDAC1 in modulating the transcriptional activity of MyoD: inhibition of the myogenic program. EMBO J. 20:1739-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marinkovic, D., X. Zhang, S. Yalcin, J. P. Luciano, C. Brugnara, T. Huber, and S. Ghaffari. 2007. Foxo3 is required for the regulation of oxidative stress in erythropoiesis. J. Clin. Invest. 117:2133-2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matushansky, I., F. Radparvar, and A. I. Skoultchi. 2000. Manipulating the onset of cell cycle withdrawal in differentiated erythroid cells with cyclin-dependent kinases and inhibitors. Blood 96:2755-2764. [PubMed] [Google Scholar]
- 31.Miller, I. J., and J. J. Bieker. 1993. A novel, erythroid cell-specific murine transcription factor that binds to the CACCC element and is related to the Kruppel family of nuclear proteins. Mol. Cell. Biol. 13:2776-2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Munugalavadla, V., L. C. Dore, B. L. Tan, L. Hong, M. Vishnu, M. J. Weiss, and R. Kapur. 2005. Repression of c-kit and its downstream substrates by GATA-1 inhibits cell proliferation during erythroid maturation. Mol. Cell. Biol. 25:6747-6759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Narla, G., A. Difeo, H. L. Reeves, D. J. Schaid, J. Hirshfeld, E. Hod, A. Katz, W. B. Isaacs, S. Hebbring, A. Komiya, S. K. McDonnell, K. E. Wiley, S. J. Jacobsen, S. D. Isaacs, P. C. Walsh, S. L. Zheng, B. L. Chang, D. M. Friedrichsen, J. L. Stanford, E. A. Ostrander, A. M. Chinnaiyan, M. A. Rubin, J. Xu, S. N. Thibodeau, S. L. Friedman, and J. A. Martignetti. 2005. A germline DNA polymorphism enhances alternative splicing of the KLF6 tumor suppressor gene and is associated with increased prostate cancer risk. Cancer Res. 65:1213-1222. [DOI] [PubMed] [Google Scholar]
- 34.Narla, G., K. E. Heath, H. L. Reeves, D. Li, L. E. Giono, A. C. Kimmelman, M. J. Glucksman, J. Narla, F. J. Eng, A. M. Chan, A. C. Ferrari, J. A. Martignetti, and S. L. Friedman. 2001. KLF6, a candidate tumor suppressor gene mutated in prostate cancer. Science 294:2563-2566. [DOI] [PubMed] [Google Scholar]
- 35.Nishimura, S., S. Takahashi, T. Kuroha, N. Suwabe, T. Nagasawa, C. Trainor, and M. Yamamoto. 2000. A GATA box in the GATA-1 gene hematopoietic enhancer is a critical element in the network of GATA factors and sites that regulate this gene. Mol. Cell. Biol. 20:713-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Opavsky, R., S. Y. Tsai, M. Guimond, A. Arora, J. Opavska, B. Becknell, M. Kaufmann, N. A. Walton, J. A. Stephens, S. A. Fernandez, N. Muthusamy, D. W. Felsher, P. Porcu, M. A. Caligiuri, and G. Leone. 2007. Specific tumor suppressor function for E2F2 in Myc-induced T cell lymphomagenesis. Proc. Natl. Acad. Sci. U. S. A. 104:15400-15405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Orkin, S. H., and L. I. Zon. 2008. Hematopoiesis: an evolving paradigm for stem cell biology. Cell 132:631-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ouyang, L., X. Chen, and J. J. Bieker. 1998. Regulation of erythroid Kruppel-like factor (EKLF) transcriptional activity by phosphorylation of a protein kinase casein kinase II site within its interaction domain. J. Biol. Chem. 273:23019-23025. [DOI] [PubMed] [Google Scholar]
- 39.Panzenbock, B., P. Bartunek, M. Y. Mapara, and M. Zenke. 1998. Growth and differentiation of human stem cell factor/erythropoietin-dependent erythroid progenitor cells in vitro. Blood 92:3658-3668. [PubMed] [Google Scholar]
- 40.Parker, S. B., G. Eichele, P. Zhang, A. Rawls, A. T. Sands, A. Bradley, E. N. Olson, J. W. Harper, and S. J. Elledge. 1995. p53-independent expression of p21Cip1 in muscle and other terminally differentiating cells. Science 267:1024-1027. [DOI] [PubMed] [Google Scholar]
- 41.Pevny, L., M. C. Simon, E. Robertson, W. H. Klein, S. F. Tsai, V. D'Agati, S. H. Orkin, and F. Costantini. 1991. Erythroid differentiation in chimaeric mice blocked by a targeted mutation in the gene for transcription factor GATA-1. Nature 349:257-260. [DOI] [PubMed] [Google Scholar]
- 42.Pilon, A. M., M. O. Arcasoy, H. K. Dressman, S. E. Vayda, Y. D. Maksimova, J. I. Sangerman, P. G. Gallagher, and D. M. Bodine. 2008. Failure of terminal erythroid differentiation in EKLF-deficient mice is associated with cell cycle perturbation and reduced expression of E2F2. Mol. Cell. Biol. 28:7394-7401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prowse, D. M., L. Bolgan, A. Molnar, and G. P. Dotto. 1997. Involvement of the Sp3 transcription factor in induction of p21Cip1/WAF1 in keratinocyte differentiation. J. Biol. Chem. 272:1308-1314. [DOI] [PubMed] [Google Scholar]
- 44.Puri, P. L., S. Iezzi, P. Stiegler, T. T. Chen, R. L. Schiltz, G. E. Muscat, A. Giordano, L. Kedes, J. Y. Wang, and V. Sartorelli. 2001. Class I histone deacetylases sequentially interact with MyoD and pRb during skeletal myogenesis. Mol. Cell 8:885-897. [DOI] [PubMed] [Google Scholar]
- 45.Quadrini, K. J., E. Gruzglin, and J. J. Bieker. 2008. Non-random subcellular distribution of variant EKLF in erythroid cells. Exp. Cell Res. 314:1595-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rowland, B. D., and D. S. Peeper. 2006. KLF4, p21 and context-dependent opposing forces in cancer. Nat. Rev. Cancer 6:11-23. [DOI] [PubMed] [Google Scholar]
- 47.Rylski, M., J. J. Welch, Y. Y. Chen, D. L. Letting, J. A. Diehl, L. A. Chodosh, G. A. Blobel, and M. J. Weiss. 2003. GATA-1-mediated proliferation arrest during erythroid maturation. Mol. Cell. Biol. 23:5031-5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saramaki, A., C. M. Banwell, M. J. Campbell, and C. Carlberg. 2006. Regulation of the human p21(waf1/cip1) gene promoter via multiple binding sites for p53 and the vitamin D3 receptor. Nucleic Acids Res. 34:543-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sengupta, T., K. Chen, E. Milot, and J. J. Bieker. 2008. Acetylation of EKLF is essential for epigenetic modification and transcriptional activation of the beta-globin locus. Mol. Cell. Biol. 28:6160-6170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Siatecka, M., L. Xue, and J. J. Bieker. 2007. Sumoylation of EKLF promotes transcriptional repression and is involved in inhibition of megakaryopoiesis. Mol. Cell. Biol. 27:8547-8560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Southwood, C. M., K. M. Downs, and J. J. Bieker. 1996. Erythroid Kruppel-like factor exhibits an early and sequentially localized pattern of expression during mammalian erythroid ontogeny. Dev. Dyn. 206:248-259. [DOI] [PubMed] [Google Scholar]
- 52.Steinman, R. A. 2002. Cell cycle regulators and hematopoiesis. Oncogene 21:3403-3413. [DOI] [PubMed] [Google Scholar]
- 53.Tallack, M. R., J. R. Keys, P. O. Humbert, and A. C. Perkins. 2009. EKLF/KLF1 controls cell cycle entry via direct regulation of E2f2. J. Biol. Chem. 284:20966-20974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tallack, M. R., J. R. Keys, and A. C. Perkins. 2007. Erythroid Kruppel-like factor regulates the G1 cyclin dependent kinase inhibitor p18INK4c. J. Mol. Biol. 369:313-321. [DOI] [PubMed] [Google Scholar]
- 55.Trimarchi, J. M., and J. A. Lees. 2002. Sibling rivalry in the E2F family. Nat. Rev. Mol. Cell Biol. 3:11-20. [DOI] [PubMed] [Google Scholar]
- 56.von Lindern, M. 2006. Cell-cycle control in erythropoiesis. Blood 108:781-782. [Google Scholar]
- 57.Vyas, P., M. A. McDevitt, A. B. Cantor, S. G. Katz, Y. Fujiwara, and S. H. Orkin. 1999. Different sequence requirements for expression in erythroid and megakaryocytic cells within a regulatory element upstream of the GATA-1 gene. Development 126:2799-2811. [DOI] [PubMed] [Google Scholar]
- 58.Weiss, M. J., G. Keller, and S. H. Orkin. 1994. Novel insights into erythroid development revealed through in vitro differentiation of GATA-1 embryonic stem cells. Genes Dev. 8:1184-1197. [DOI] [PubMed] [Google Scholar]
- 59.Welch, J. J., J. A. Watts, C. R. Vakoc, Y. Yao, H. Wang, R. C. Hardison, G. A. Blobel, L. A. Chodosh, and M. J. Weiss. 2004. Global regulation of erythroid gene expression by transcription factor GATA-1. Blood 104:3136-3147. [DOI] [PubMed] [Google Scholar]
- 60.Wu, J., and J. B. Lingrel. 2004. KLF2 inhibits Jurkat T leukemia cell growth via upregulation of cyclin-dependent kinase inhibitor p21WAF1/CIP1. Oncogene 23:8088-8096. [DOI] [PubMed] [Google Scholar]
- 61.Zhang, W., and J. J. Bieker. 1998. Acetylation and modulation of erythroid Kruppel-like factor (EKLF) activity by interaction with histone acetyltransferases. Proc. Natl. Acad. Sci. U. S. A. 95:9855-9860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang, W., D. E. Geiman, J. M. Shields, D. T. Dang, C. S. Mahatan, K. H. Kaestner, J. R. Biggs, A. S. Kraft, and V. W. Yang. 2000. The gut-enriched Kruppel-like factor (Kruppel-like factor 4) mediates the transactivating effect of p53 on the p21WAF1/Cip1 promoter. J. Biol. Chem. 275:18391-18398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang, W., S. Kadam, B. M. Emerson, and J. J. Bieker. 2001. Site-specific acetylation by p300 or CREB binding protein regulates erythroid Kruppel-like factor transcriptional activity via its interaction with the SWI-SNF complex. Mol. Cell. Biol. 21:2413-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhu, J. W., S. J. Field, L. Gore, M. Thompson, H. Yang, Y. Fujiwara, R. D. Cardiff, M. Greenberg, S. H. Orkin, and J. DeGregori. 2001. E2F1 and E2F2 determine thresholds for antigen-induced T-cell proliferation and suppress tumorigenesis. Mol. Cell. Biol. 21:8547-8564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhu, L., and A. I. Skoultchi. 2001. Coordinating cell proliferation and differentiation. Curr. Opin. Genet. Dev. 11:91-97. [DOI] [PubMed] [Google Scholar]