Abstract
Survival of Neisseria gonorrhoeae within host epithelial cells is expected to be important in the pathogenesis of gonococcal disease. We previously demonstrated that strain FA1090 derives iron from a host cell in a process that requires the Ton complex and a putative TonB-dependent transporter, TdfF. FA1090, however, lacks the gonococcal genetic island (GGI) that is present in the majority of strains. The GGI in strain MS11 has been partially characterized, and it encodes a type IV secretion system (T4SS) involved in DNA release. In this study we investigated the role of iron acquisition and GGI-encoded gene products in gonococcal survival within cervical epithelial cells. We demonstrated that intracellular survival of MS11 was dependent on acquisition of iron from the host cell, but unlike the findings for FA1090, expression of the Ton complex was not required. Survival was not dependent on a putative TonB-like protein encoded in the GGI but instead was directly linked to T4SS structural components in a manner independent of the ability to release or internalize DNA. These data suggest that expression of selected GGI-encoded open reading frames confers an advantage during cervical cell infection. This study provides the first link between expression of the T4SS apparatus and intracellular survival of gonococci.
Neisseria gonorrhoeae is the causative agent of the sexually transmitted infection gonorrhea. Although symptomatic urethritis is the most common presentation in men, asymptomatic infections associated with cervical acquisition in women can lead to ascension of the bacteria to the upper reproductive tract. In this context, an understanding of the immune evasion tactics employed by this organism is essential for eliminating potential downstream sequelae, including disseminated gonococcal infection, pelvic inflammatory disease, infertility, and ectopic pregnancy (23, 45). The outcome of gonococcal infections and the resulting clinical manifestations elicited are determined, in part, by strain-specific factors. The differences are expected to impact host-pathogen interactions that occur at the primary site of infection, the urogenital epithelium.
Productive gonococcal infections require that the bacteria circumvent the innate iron-withholding mechanisms employed by the human host. Moreover, epithelial cell responses to Neisseria infection, including reduced expression of the transferrin receptor gene and diminished transferrin receptor cycling, which result in further limitation of iron availability, have been described previously (8, 9, 34). As the ability to acquire iron from the human host is a key determinant in gonococcal pathogenesis (1, 15), N. gonorrhoeae has evolved numerous mechanisms to acquire this required nutrient in an iron-depleted environment. Various iron uptake systems that are thought to promote gonococcal replication in extracellular niches have been characterized (42). Specifically, these systems hijack iron from the host proteins transferrin (14), lactoferrin (7), and hemoglobin (12) in a manner that requires energy transduced to outer membrane transporters by the energizing protein TonB. TonB in complex with ExbB and ExbD (Ton complex) harnesses the proton motive force and, by an incompletely characterized mechanism, delivers this energy to specific outer membrane TonB-dependent transporters (TdTs), resulting in iron internalization. For example, expression of the TdT TbpA enables acquisition of transferrin iron. The ability of the gonococcus to express both components of the transferrin iron acquisition system, TbpA and TbpB, was shown to be necessary to establish experimental infections in human males (15), providing a link between gonococcal iron acquisition and the ability to initiate infection in vivo.
Recently, we investigated the role of iron acquisition in gonococcal intracellular survival. Localization of N. gonorrhoeae within epithelial cells has been reported by several investigators (3, 21, 44) and may contribute to host immune evasion, resulting in persistence and potential ascension to the upper genital tract. Intracellular gonococci have been described as free cells in the cytoplasm and also as cells enclosed in membranous vesicles (3, 21, 44). In a previous study, we determined that expression of the Ton complex and a putative TdT, TdfF, were required for survival of gonococcal strain FA1090 within cervical epithelial cells (25). To date, a single tonB locus in pathogenic Neisseria has been characterized, although the presence of a less efficient, alternative Ton-like system involved in the utilization of iron from hemoglobin, transferrin, and/or lactoferrin has been proposed (18). The presence of an uncharacterized open reading frame (ORF), yfd, whose product exhibits homology to the TonB protein of Anabaena variabilis, has been described for gonococcal strain MS11 (26). This ORF is part of the gonococcal genetic island (GGI) which is present in strain MS11 but not present in strain FA1090.
The GGI exhibits many features commonly associated with pathogenicity islands (24). Specifically, this island is not present in commensal Neisseria strains, is relatively large (57 kb), has a G+C content and dinucleotide frequencies that are different than those of the rest of gonococcal genome, and is flanked by direct repeats (20, 26). Such islands have been characterized in many other bacteria and have been found to encode a variety of virulence-associated traits involved in a multitude of pathogenic processes, including toxin secretion, adhesion, invasion, and type III and IV secretion. Additionally, pathogenicity islands have been found to encode iron uptake systems likely involved in promoting in vivo growth and virulence in other bacterial species, including Shigella flexneri (49), uropathogenic Escherichia coli (52), and Yersinia spp. (10).
The gonococcal genetic island has been partially characterized, and nearly one-half of the island contains genes that either exhibit no similarity to known sequences or encode proteins with unknown functions (26). The yfd ORF is in this portion of the island. Conversely, the remaining 27.5 kb encodes a type IV secretion system (T4SS) involved in chromosomal DNA excretion (20, 26). The T4SS is ancestrally related to a family of bacterial conjugation systems, which are involved in the export of distinct DNA substrates. Very diverse mechanisms for type IV secretion have been characterized and found to contribute to the pathogenesis of both extracellular and intracellular bacterial species (for a review, see reference 4). In addition to bacterial colonization, injection of virulence factors, and biofilm formation, T4SSs are also involved in exchange of genetic material, leading to increased genome plasticity and spread of conjugative plasmids that may lead to increased antibiotic resistance. The GGI-encoded T4SS has been demonstrated to mediate the release of DNA in a non-contact-dependent manner; however, a role for this secretion system or the entire genetic island in gonococcal pathogenesis or survival has yet to be established.
Since pathogenicity islands have been shown to encode proteins involved in iron acquisition and since we have established that high-affinity iron acquisition is necessary for intracellular replication of gonococci (25), we investigated the roles of iron acquisition and GGI-encoded gene products in gonococcal survival within cervical epithelial cells. In the present study, we demonstrated that intracellular survival of a GGI-containing strain was dependent on host cell-derived iron. However, in contrast to the situation in strain FA1090, which lacks the GGI, expression of the Ton complex was not required for the survival of strain MS11 (GGI+). Intracellular survival was not influenced by the putative TonB-like protein encoded on the GGI. Instead, TonB-independent intracellular survival was directly linked to T4SS structural components encoded by the GGI; survival was not impacted by the ability of the gonococcus to release or internalize DNA. Together, these findings suggest that there is a link between the presence of the GGI and intracellular survival in cervical epithelial cells. These findings also suggest that the T4SS includes products that enable intracellular survival of the gonococcus in the absence of high-affinity iron acquisition systems.
MATERIALS AND METHODS
Media, cell culture, and bacterial growth conditions.
The ME180 endocervical epithelial cell line (American Type Culture Collection) was maintained in McCoy's 5A medium (Gibco) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Gibco). Cells were maintained at 37°C in a 5% CO2 atmosphere. All gonococcal strains were maintained on GC agar (Difco) plates with Kellogg's supplement I (31) and 12 μM Fe(NO3)3. Bacterial replication was monitored in McCoy's 5A medium with 10% heat-inactivated FBS. In the absence of epithelial cells, MS11 and mutant derivatives of this strain (described below) were incapable of replication unless Fe(NO3)3 (24 μM) was added.
Bacterial strains and mutant construction.
Gonococcal strain MS11 has been described previously (47). The tonB::Ω mutant strain was constructed by transformation of MS11 with pVCU693 as described previously (25). Successful insertion in tonB was verified by PCR; the resulting transformants were not capable of growth on human transferrin as a sole iron source, as expected for a tonB mutant. The following mutant strains with the MS11 background have been described previously (26): the GGI deletion mutant ND500; the nonpolar traN mutant HH535; a traN+ complemented derivative of mutant HH535, HH578; the nonpolar traH mutant KS16; and a traH+ complemented derivative of mutant of KS16, HH576. The ΔGGI tonB, traN tonB, traN tonB/traN+, and traH tonB mutant strains were constructed by transformation of ND500, HH535, HH578, KS16, and HH576, respectively, with pVCU693, as described above. To generate the yfd tonB double mutant, the yfd gene was amplified by PCR using primers oVCU312 (5′-ATGGACTAAAGCCGTCGTCCTGTT-3′) and oVCU313 (5′-TGGTTTGACAGTGAGAGAGCAGCA-3′), and the resulting amplicons were cloned into pCR 2.1 (Invitrogen). This resulted in plasmid pVCU708, which was used to transform E. coli TOP10 cells (Invitrogen) with selection for resistance to kanamycin (50 μg/ml). Individual plasmids were isolated, and the ermC cassette was ligated into the SwaI restriction site, generating pVCU709. This construct was then transformed into chemically competent E. coli NovaBlue cells (Novagen), and transformants were selected on LB agar plates containing 250 μg/ml erythromycin. Individual plasmids were isolated, linearized, and used to transform gonococcal strain MS11tonB::Ω. To select for allelic exchange by homologous recombination, transformants were plated on GCB agar (Difco) plates containing 10 μg/ml erythromycin. A parA tonB double mutant was generated by introducing the tonB::Ω mutation in pVCU693 into the MS11 parA mutant HH545 by spot transformation as previously described (19), which generated strain ND527. Transformants were selected for spectinomycin resistance and were screened for the presence of the mutation by PCR as described above. Similarly, the tonB::Ω mutation was introduced into the MS11 traD mutant strain KL501 (39) and the MS11 traI mutant strain WSP5 (40) by transformation with pVCU693, generating ND562 (traD tonB) and ND561 (traI tonB), respectively. A pilT point mutation (K136Q) in pHH42 (H. L. Hamilton and J. P. Dillard, unpublished data) was introduced into MS11tonB::Ω by spot transformation to generate strain ND529 (pilT tonB). Potential transformants were screened for the mutation by performing PCR with primers pilT-F (5′-ATTCCGCGTCAACGCCTTCA-3′) and pilT-R (5′-CGGTTTGCAGGACGGAGTTA-3′), followed by digestion of the products with EcoRI and HaeIII. The presence of the introduced HaeIII site identified the transformants, and DNA sequencing confirmed the expected alteration in the pilT sequence. The pilT tonB double mutant was confirmed to have a transformation efficiency 1,000-fold lower than that of the wild-type strain (data not shown).
Transmission electron microscopy.
ME180 cells were seeded onto collagen-coated glass coverslips in McCoy's medium with 10% FBS at a concentration of 105 cells/well in a 24-well plate and incubated for ∼18 h at 37°C with 5% CO2. ME180 cells were then infected with gonococci suspended in phosphate-buffered saline (PBS) at a multiplicity of infection (MOI) of 200. After 4 h, infection was stopped by rinsing the coverslips with 1× PBS. The coverslips were then fixed with 2.5% glutaraldehyde-2% paraformaldehyde in 0.1 M sodium cacodylate buffer for 1 h at room temperature and rinsed with 0.2 M cacodylate buffer. Once fixed, the coverslips were embedded and processed for transmission electron microscopy.
Gentamicin protection assay.
ME180 cells were seeded into 12-well plates and grown to 70 to 80% confluence in McCoy's 5A medium supplemented with 10% FBS. Prior to infection, gonococcal strains were propagated on GC agar plates supplemented with Kellogg's supplement I (31) and 12 μM Fe(NO3)3 at 37°C in a 5% CO2 atmosphere. Intracellular survival assays were conducted as described previously (25). Briefly, ME180 monolayers were infected with suspensions of each gonococcal strain which had been resuspended in McCoy's 5A medium supplemented with 10% FBS and 24 μM Fe(NO3)3. The additional inorganic iron promoted efficient gonococcal growth and invasion, which did not occur in the presence of fetal bovine serum containing bovine transferrin. Neisseria species are exquisitely adapted to the human host and can utilize only human iron-binding proteins as sources of iron. A multiplicity of infection of 10 was used in all experiments. Epithelial cell cultures were infected for 4 h, and this was followed by one phosphate-buffered saline (PBS) wash to remove extracellular, nonadherent bacteria. Infected cells were then incubated for 1 h in McCoy's 5A medium with 10% FBS and 25 μg/ml gentamicin. The gentamicin was removed, and the cultures were washed twice with PBS. After the infection period and gentamicin treatment, McCoy's 5A medium containing 10% FBS was added to the monolayers, but no additional inorganic iron was provided in the medium. The time zero cells were immediately processed by treatment for 1 min with a saponin mixture (PBS containing 2 mM EDTA and 0.5% saponin), and then gonococci were plated for viable counting on GC agar plates. For the other infected cultures, infected monolayers were incubated for 4, 8, 12, or 24 h with McCoy's 5A medium with 10% FBS. One hour prior to collection at later time points, cells were treated a second time with gentamicin, washed, and plated as described above. Desferal (desferroxamine mesylate), which was added to some assay mixtures at a concentration of 100 μM, is a commercially available bacterial siderophore that cannot be employed by N. gonorrhoeae as an iron source. This iron chelator depletes extracellular and intracellular eukaryotic iron pools (13) and renders the ferric iron unavailable for gonococcal replication (51). For each assay, values for every time point were derived from the mean of two independently infected cell monolayers. Each assay was conducted at least three times, and the data presented below are the means of at least three independent experiments conducted on different days. The viability of epithelial cells for all Desferal concentrations, infection periods, and time points was verified using trypan blue exclusion.
Statistical analysis.
The statistical significance of intracellular survival was assessed using a two-tailed, unpaired Student t test. P values of <0.05 were considered significant.
RESULTS
Invasion and intracellular localization of gonococcal strain MS11 within cervical epithelial cells.
We previously employed confocal microscopy to demonstrate that gonococcal strain FA1090 was capable of invading ME180 epithelial cells (25). In the present study, we infected monolayers of ME180 cells with piliated strain MS11 for 4 h and then processed the infected cells for transmission electron microscopy to visually assess invasion and intracellular localization. As shown in Fig. 1, MS11 was capable of invading ME180 cells under these conditions, which resulted in some intracellular diplococci that were free in the cytoplasm and other gonococci that appeared to be membrane enclosed. In order to quantitatively assess invasion by wild-type and mutant gonococci under a variety of conditions, we subsequently employed a gentamicin protection assay.
FIG. 1.
Invasion of ME180 cells by MS11: transmission electron microscopy image of ME180 cells following 4 h of invasion with piliated MS11.
Intracellular survival of MS11 within cervical epithelial cells is attenuated but not eliminated in the absence of the Ton system.
We previously demonstrated that gonococci express TonB during cervical cell infection and furthermore that this protein is critical to the survival of gonococcal strain FA1090 within cervical epithelial cells (25). To determine if the Ton system was required for growth of gonococcal strain MS11 in the ME180 cell line, we utilized a gentamicin protection assay and monitored the intracellular survival of MS11 and the corresponding isogenic tonB::Ω mutant strain over a 24-h time course (Fig. 2). The tonB::Ω polar insertion mutant does not express TonB, ExbB, or ExbD (25); thus, the insertion inactivates the entire Ton system. As shown in Fig. 2, in both backgrounds the wild-type strains and tonB mutants exhibited a decrease in viability during the first 4 h after gentamicin treatment. MS11, like FA1090, was able to recover; by 24 h, the viable counts for the wild-type strains were similar to those for the initial intracellular population at time zero. In the FA1090 background, the isogenic tonB mutant strain exhibited significantly reduced recovery (approximately 1,000-fold reduced) by 24 h (Fig. 2A). While the MS11 tonB mutant strain was capable of intracellular survival at nearly wild-type levels (Fig. 2B), the mutant was consistently attenuated at all time points. Importantly, unlike the FA1090 tonB mutant (Fig. 2A), the MS11 tonB mutant survived better than its wild-type parent in the period from 8 to 24 h, and the intracellular CFU counts were within 10-fold of the wild-type intracellular CFU counts. It is also worth noting that the levels of both MS11 and the tonB isogenic mutant (Fig. 2B) increased approximately 10-fold from the point at which the fewest intracellular counts were recovered to the 24-h time point, suggesting that tonB is not necessary for intracellular proliferation of MS11. Cumulatively, these data suggest that MS11 possesses a mechanism for intracellular survival within ME180 cervical cells that does not depend on expression of the Ton system. We also verified that gonococcal strain MS11, like FA1090 (25), was unable to grow in McCoy's 5A medium containing FBS and no additional inorganic iron (data not shown). This observation demonstrates that the medium added after the initial gentamicin treatment did not support extracellular proliferation of MS11; therefore, the increase in the number of viable counts from 4 to 24 h shown in Fig. 2B represents proliferation of wild-type strain MS11 within the ME180 cells.
FIG. 2.
Expression of a functional Ton system is not critical for the survival of gonococcal strain MS11 within ME180 cells. (A) Intracellular survival of FA1090 and the isogenic tonB mutant within ME180 cells as assessed by the gentamicin protection assay. (B) Intracellular survival of MS11 and the isogenic tonB mutant within ME180 cells as assessed by the gentamicin protection assay. The numbers of intracellular bacteria per well (IC CFU/well) were monitored for 24 h. Each bar indicates the geometric mean of four independent experiments. *, P < 0.05; **, P < 0.01.
Ferric iron chelation attenuates gonococcal intracellular survival.
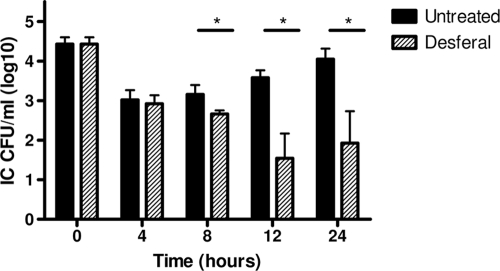
In a previous study (25), we demonstrated that treatment of epithelial cell monolayers with the ferric iron chelator Desferal ablates gonococcal iron acquisition, resulting in inhibition of the intracellular growth of strain FA1090. Desferal chelates iron from endosomal, lysosomal, and cytosolic compartments of the epithelial cells (13) and renders ferric iron inaccessible to gonococci (51). Desferal was added to MS11-infected cultures after the initial gentamicin treatment, and intracellular gonococci were enumerated over a 24-h time course. As shown in Fig. 3, significant decreases in the number of bacteria recovered from Desferal-treated cervical epithelial cells compared to the number of bacteria recovered from untreated controls were observed after 8, 12, and 24 h. The iron-dependent survival was observed despite the fact that both treated and untreated epithelial cells remained viable over the 24-h period, as evaluated by trypan blue exclusion (data not shown). These results suggest that iron is necessary for survival of gonococcal strain MS11 within epithelial cells, as we previously observed with strain FA1090 (25).
FIG. 3.
Desferal treatment inhibits replication of gonococcal strain MS11 within ME180 cells, as assessed by the gentamicin protection assay. Cultures were either not treated or treated with 100 μM Desferal during the replication phase of the experiment. Desferal chelates ferric iron both from the medium and from within the ME180 cellular compartments. The numbers of intracellular bacteria per well (IC CFU/well) were monitored for 24 h. Each bar indicates the geometric mean of three independent experiments. *, P < 0.05.
GGI promotes Ton-independent intracellular survival of MS11.
One characterized difference between gonococcal strain MS11 and strain FA1090 is the presence of the gonococcal genetic island (GGI). Similar islands have been characterized in other bacteria and have been found to contain a variety of virulence-associated genes involved in many pathogenic processes. Therefore, we investigated the role of this island in gonococcal intracellular survival within ME180 cells. This was done initially by testing an MS11 mutant lacking the GGI (26) using the gentamicin protection assay. Deletion of the GGI resulted in a growth phenotype that was statistically similar to that of MS11 at all time points, and by 24 h the absence of the GGI did not affect the recovery of the strains in ME180 cells (Fig. 4A).
FIG. 4.
GGI increases iron-dependent, Ton-independent intracellular survival of strain MS11. (A) Intracellular survival of wild-type strain MS11 (WT) and the isogenic tonB, ΔGGI, and ΔGGI tonB mutants as assessed by the gentamicin protection assay. (B) The defect in intracellular survival of the ΔGGI tonB double mutant was overcome when the cultures were supplemented with 24 μM Fe(NO3)3. The intracellular survival of the wild-type strain and the ΔGGI tonB double mutant was compared to the intracellular survival of parallel cultures of the wild-type strain and the ΔGGI tonB double mutant that were supplemented with iron (+Fe). Each bar indicates the geometric mean of three independent experiments. IC, intracellular bacteria. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
As pathogenicity islands have been shown to express genes involved in invasion and intracellular survival, we investigated the possibility that the TonB-independent intracellular survival of MS11 was mediated by a supplemental method for iron uptake encoded by the GGI. We generated a ΔGGI tonB double mutant and tested this derivative using the gentamicin protection assay. At time zero, the number of intracellular ΔGGI tonB double mutant bacteria was statistically similar to the number of wild-type bacteria, indicating that the mutations did not inhibit invasion of cervical cells. In contrast, the combination of the two mutations resulted in recovery of significantly fewer intracellular gonococci than the numbers observed for the wild-type strain at the 8-, 12-, and 24-h time points (Fig. 4A). The observed difference in intracellular survival was not due to a selective advantage of MS11 in the medium prior to gentamicin treatment, as the wild-type strain and the double mutant survived similarly in the absence of epithelial cells (data not shown). Likewise, the in vitro growth patterns of the tonB mutant and the double mutant lacking both the GGI and the Ton system were indistinguishable from the in vitro growth pattern of the wild-type strain in the presence and absence of iron (data not shown), suggesting that the growth defect shown in Fig. 4A for the double mutant is specific to replication within ME180 cells. These data indicate that the presence of the GGI is associated with promoting TonB-independent intracellular survival.
To determine if the inability of the ΔGGI tonB double mutant to survive within ME180 cells was directly related to an inability to acquire iron, supplemental ferric nitrate was added to cultures infected individually with either MS11 or the ΔGGI tonB double mutant. The survival defect of the double mutant was rescued by addition of supplemental iron (Fig. 4B), suggesting that the inability of the double mutant to grow within the cervical cells was due to a defect in high-affinity iron acquisition.
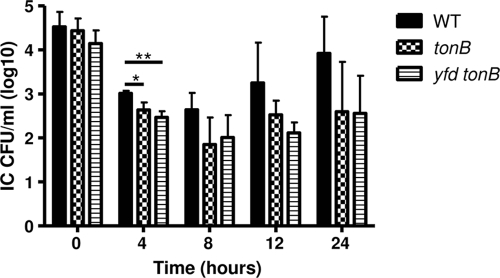
Yfd is not responsible for the Ton-independent intracellular survival promoted by the GGI.
Since the presence of the GGI was necessary for the tonB mutant strain to survive within ME180 cells, we sought to define the specific mechanism of TonB-independent intracellular survival provided by the GGI. A search of the GGI sequence for genes exhibiting homology to characterized genes encoding proteins involved in iron acquisition revealed no candidate transporter genes and only one possible tonB homolog. The predicted protein encoded by the yfd ORF exhibits limited sequence similarity to the TonB protein of A. variabilis (26). The presence of multiple TonB homologs in a genome sequence is not uncommon; thus, we investigated the possibility that yfd encoded a second TonB-like protein, providing a means to bypass the requirement for the characterized, chromosomal tonB locus. To address this possibility, a yfd tonB double mutant was constructed and tested using the gentamicin protection assay along with the tonB and MS11 parental strains. The yfd tonB double mutant exhibited survival patterns statistically similar to those of the tonB mutant at all time points (Fig. 5), demonstrating that a product encoded by yfd was not responsible for the TonB-independent mechanism encoded by the GGI.
FIG. 5.
Expression of Yfd is not critical for the intracellular survival of MS11 within ME180 cells. The mechanism of Ton-independent intracellular survival encoded by the GGI was not dependent on expression of Yfd. The intracellular survival of wild-type strain MS11 (WT), the tonB mutant, and the yfd tonB double mutant within ME180 cells was determined. Each bar indicates the geometric mean of three independent experiments. IC, intracellular bacteria. *, P < 0.05; **, P < 0.01.
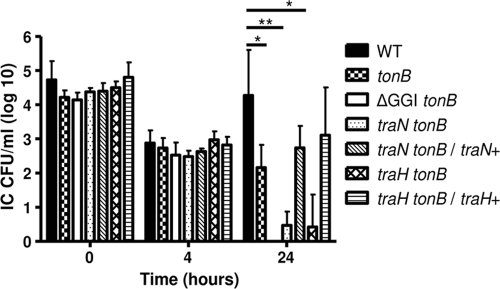
Ton-independent intracellular survival promoted by the GGI requires T4SS components.
The GGI was shown to encode a type IV secretion system (T4SS) involved in DNA release into the supernatant in a non-contact-dependent manner (20, 26). Therefore, we investigated the role of this secretion mechanism in supporting Ton-independent intracellular survival. TraN is a putative outer membrane protein encoded by the GGI that is conserved in all F-type type IV secretion systems (35). Inactivation of this protein ablates DNA secretion by gonococcal strain MS11 (26). To examine whether expression of TraN was required for TonB-independent intracellular survival, a traN tonB double mutant was constructed and tested using the gentamicin protection assay along with the isogenic parental strains with single mutations in either tonB or traN. The traN mutant strain exhibited intracellular survival patterns similar to those of the wild type, including an initial decrease in viability that was followed by the characteristic recovery by 24 h (data not shown). Conversely, the level of recovery of the traN tonB double mutant was significantly lower than the level of recovery of the wild type at 24 h (Fig. 6). The survival pattern of the traN tonB double mutant was nearly identical to that of the ΔGGI tonB double mutant (Fig. 6).
FIG. 6.
The mechanism of Ton-independent intracellular survival afforded by the GGI requires components of the T4SS. The intracellular survival of the following gonococcal strains was assessed by using the gentamicin protection assay: wild-type strain MS11 (WT) and the tonB, ΔGGI tonB, traN tonB, traN tonB/traN+, traH tonB, and traH tonB/traH+ mutants. Each bar indicates the geometric mean of at least three independent experiments. IC, intracellular bacteria. *, P < 0.05; **, P < 0.01.
To confirm the phenotype of the traN tonB double mutant, we tested a previously characterized MS11 derivative in which the traN mutation was complemented with a wild-type copy of traN inserted ectopically into the gonococcal chromosome. The wild-type copy of traN restored the DNA secretion capability of the traN mutant (26). We insertionally inactivated the tonB gene in this traN-complemented strain and tested the resulting mutant using the gentamicin protection assay. Complementation of traN restored the level of intracellular survival of gonococci to levels similar to those of the wild-type and tonB mutant strains (Fig. 6), indicating that specific inactivation of traN resulted in the defect observed. These data confirm the contribution of the T4SS in TonB-independent intracellular replication and indicate that TraN is a necessary component.
The same phenomenon was observed with a traH tonB double mutant (Fig. 6). Complementation of traH in the chromosome of the traH tonB double mutant restored intracellular survival to levels comparable to those of the tonB mutant strain (Fig. 6). TraH is homologous to a protein encoded by the E. coli F plasmid involved in pilus assembly, is required for T4SS-mediated DNA secretion (26), and may be a structural component of the pore (35). The fact that single mutations in either traN or traH in conjunction with a mutation in tonB resulted in defective intracellular survival phenotypes, similar to that observed for the ΔGGI tonB double mutant, indicated that the benefit afforded by the island was mediated by these components of the T4SS.
Ton-independent intracellular survival afforded by the GGI does not require DNA uptake or secretion.
The studies described above demonstrated that the T4SS has a role in Ton-independent intracellular survival. However, the results did not allow us to discern whether the structural components of the T4SS were directly responsible for the growth promotion, whether something additional that promoted gonococcal growth was secreted by the T4SS, or whether the DNA that was secreted and subsequently internalized was involved. Ferrous iron has been shown to bind to purine nucleotide complexes (2). Therefore, it was formally possible that DNA, secreted by the T4SS, was capable of binding host cell-derived iron for subsequent internalization by the gonococcus. To investigate this hypothesis, we employed mutants that were deficient in DNA secretion (ParA) or DNA uptake (PilT); however, genes expressing the functional pore of the T4SS apparatus were not disturbed in these mutants.
Gonococcal ParA is encoded by a gene in the GGI that is not proximate to the genes encoding the T4SS structural components; however, this protein exhibits homology to chromosome partitioning proteins involved in bacterial chromosome localization during cell division. ParA expression is essential for T4SS-mediated DNA secretion, likely because it processes and/or escorts DNA to the T4SS apparatus (26). Therefore, we generated a parA tonB double-knockout strain to determine if the mechanism that allows Ton-independent intracellular survival requires DNA secretion. The parA tonB mutant exhibited intracellular survival patterns statistically similar to those of the wild-type strain at all time points (Fig. 7), indicating that Ton-independent survival was not due to DNA secretion.
FIG. 7.
Ton-independent intracellular survival promoted by the GGI is not dependent on DNA secretion and/or uptake. The intracellular survival of the following gonococcal strains was assessed by the gentamicin protection assay: wild-type strain MS11 (WT), the ΔGGI tonB double mutant, the parA tonB double mutant, and the pilT tonB double mutant. Each bar indicates the geometric mean of at least three independent experiments. IC, intracellular bacteria. *, P < 0.05; **, P < 0.01.
To investigate the role of DNA uptake in TonB-independent survival, we generated a pilT tonB double mutant. Gonococcal PilT belongs to a family of highly conserved ATPases and is required for motility, pilus retraction, and uptake of DNA (38, 50). The adherence to human epithelial cells of a pilT mutant has been demonstrated to be similar to that of the wild-type strain (36). Therefore, the pilT tonB double mutant was not expected to be deficient in adherence to the epithelial cells; however, uptake of DNA would be ablated. As was observed for the parA tonB double mutant, the pilT tonB mutant exhibited intracellular survival characteristics statistically similar to those of the wild-type strain at all time points (Fig. 7), indicating that the mechanism of Ton-independent intracellular survival was not dependent on uptake of DNA. Together, these data demonstrate that the Ton-independent promotion of intracellular survival provided by the GGI is not mediated by either DNA uptake or DNA secretion processes.
We also tested whether two nonstructural components of the type IV secretion system were required for Ton-independent survival. TraI has been ascribed relaxase functions and is required for DNA processing (40). TraD is homologous to docking proteins involved in type IV secretion of DNA, and thus it is expected that TraD is required for secretion of proteins by the T4SS. traI mutants are defective for DNA secretion (26, 40). The traD deletion mutant used here is also defective for DNA secretion (39). We generated a traD tonB double mutant, as well as a traI tonB double mutant. Both mutants exhibited intracellular survival phenotypes similar to those described above for pilT and parA mutants (data not shown). We concluded that these nonstructural components of the type IV secretion system are not required for Ton-independent intracellular survival. These observations also argue against the hypothesis that a reductase might be secreted via the type IV secretion system, since protein secretion would be expected to require TraD function for export. Conceivably, secretion of an iron reductase could make iron more accessible for intracellular survival of gonococci; however, no reductase or chelating activity was detected in the supernatants collected from GGI-containing strain MS11 (data not shown).
DISCUSSION
A direct link between bacterial iron acquisition and the ability to initiate and maintain an infection in vivo has been established. Specific TonB-dependent transporters (TdTs) are necessary for both establishment of infection in male humans (1, 15) and gonococcal survival within human cervical epithelial cells (25). Previous studies, however, were limited to gonococcal strain FA1090. In the present study, we investigated the intracellular survival of another strain, MS11, which possesses the gonococcal genetic island (GGI). As genetic islands are often associated with bacterial virulence, we examined the hypothesis that the GGI may confer an advantage during cervical cell infection. Specifically, island-encoded iron acquisition systems have been characterized in other pathogens; therefore, we investigated the contribution of the GGI to intracellular replication in the absence of high-affinity Ton-dependent iron acquisition.
In the present study, we obtained evidence that MS11 exhibits intracellular growth characteristics similar to those of strain FA1090 and that a 4-h infection period resulted in a population of intracellular gonococci that can be monitored over a 24-h period. Gonococcal survival was dependent on the presence of cervical epithelial cells, as the medium present during the survival assay did not support growth. Since this medium contains bovine transferrin, which is not an accessible iron source for the gonococcus, we reasoned that strain MS11, like FA1090, was capable of accessing host cell-derived iron, which promoted intracellular survival.
Further investigation of the role of iron acquisition revealed that similar to the results obtained for FA1090 (25) and the closely related organism Neisseria meningitidis (33), the growth of MS11 within cultured cervical epithelial cells could be pharmacologically blocked by addition of an iron chelator, Desferal. Desferal is capable of chelating iron both from the extracellular medium and from sources within the epithelial cells. Thus, the attenuation of growth observed in the presence of this inhibitor supports the hypothesis that the intracellular iron acquisition pathway utilized by this gonococcal strain is effectively short-circuited by addition of Desferal.
Unlike the findings for N. meningitidis and gonococcal strain FA1090, however, the expression of tonB was not essential for recovery of strain MS11 in cervical epithelial cells. One characterized difference between the two gonococcal strains is the presence of the gonococcal genetic island (GGI). This island was likely horizontally acquired and integrated into the gonococcal chromosome (26). Such islands often provide selective advantages, such as enhanced pathogenicity, metabolism, or ecological fitness (24). We therefore investigated the role of this island in intracellular survival of gonococci and found that growth within cervical cells was completely ablated when the GGI was deleted and the Ton complex was simultaneously inactivated. Interestingly, iron supplementation restored intracellular survival of the double mutant, suggesting that the survival defect was directly related to high-affinity iron acquisition.
Although a significant portion of the GGI remains largely uncharacterized, the presence of yfd, an ORF exhibiting homology to the ORF encoding the TonB protein of A. variabilis, was reported previously (26). Therefore, we investigated the possibility that the GGI encoded a second TonB-like protein which provided a benefit for intracellular replication within cervical cells. Advances in genome sequencing have led to identification of additional tonB loci in other Gram-negative pathogens, such as Actinobacillus pleuropneumoniae (6), Vibrio cholerae (43), Vibrio anguillarum (46), and Pseudomonas (53). To date, only a single tonB locus has been characterized in the pathogenic Neisseria species, although the presence of a less efficient, alternative Ton-like system involved in the utilization of iron from hemoglobin, transferrin, and/or lactoferrin has been proposed (18). In the present study, however, we demonstrated that a putative TonB-like protein, Yfd, was not essential for the intracellular survival of gonococcal strain MS11 and, furthermore, that the Ton-independent survival benefit afforded by the GGI is not mediated through this uncharacterized protein.
In addition to the region of the GGI that exhibits little homology to previously characterized sequences, approximately one-half of the 57-kb GGI is proposed to be involved in expression of a functional T4SS that mediates chromosomal DNA secretion (26). A number of human pathogens are known to utilize type IV systems for secretion of important virulence effectors, including effectors involved in pertussis toxin production by Bordetella pertussis, pseudopodium induction by Helicobacter pylori, and promotion of intracellular survival of Legionella pneumophila within macrophages and amoebae because they encode effectors that alter phagosome trafficking (16, 30).
Interestingly, gonococcal mutants lacking either a functional TraN or TraH in conjunction with a mutation in tonB exhibited survival patterns nearly identical to those of the ΔGGI tonB double mutant. Mutations in traN or traH were previously shown to eliminate DNA secretion by N. gonorrhoeae (26, 27). Although the functions and locations of TraN and TraH in gonococci have not been determined, data are available on the homologous proteins of the related E. coli F plasmid. F plasmid-encoded TraN is an outer membrane protein required for conjugation and is hypothesized to act in the formation of the conjugative pore (32). F plasmid-encoded TraH is a periplasmic protein that is required for conjugation and F pilus formation (22). F plasmid-encoded TraH interacts with at least three other DNA transfer proteins of the conjugation system (28). Thus, it is likely that gonococcal TraN and TraH are essential parts of the secretion apparatus and that this apparatus is required for intracellular survival in the absence of the Ton system.
As individual mutations in traN and traH disrupt the T4SS pore, resulting in elimination of DNA secretion (26), it was unclear whether Ton-independent intracellular survival was mediated by the secretion pore or by DNA. Therefore, we investigated the role of DNA secretion and entry in promoting TonB-independent intracellular survival by testing the hypothesis that DNA secreted by the T4SS is capable of binding host cell-derived iron and gonococci are subsequently able to take up this complex, promoting intracellular survival of gonococci and alleviating the Ton complex requirement. Prevention of DNA secretion or DNA uptake, however, did not alter intracellular survival of a TonB mutant strain, demonstrating that these processes were not responsible for TonB-independent survival. Likewise, TraD and TraI did not appear to play critical roles in intracellular survival of the Ton system mutant.
Together, these data demonstrate that disruption of the secretion pore inhibited gonococcal intracellular survival in the absence of a functional Ton complex. We examined one possible mechanism by which the type IV secretion system might enhance iron acquisition by testing the hypothesis that MS11 secreted a molecule capable of chelating or reducing iron (such as a ferric reductase), which would allow gonococci to access intracellular iron. One gonococcal ferric reductase localized in the cytoplasm has been identified and characterized as an enzyme that reduces Fe3+ citrate (37). Surface-bound and/or secreted ferric reductases have been identified for other pathogenic intracellular bacteria, including Listeria monocytogenes (5, 17) and Mycobacterium paratuberculosis (29). This type of reductase may be more important in internalized bacteria, where the necessary cofactors are available for enzyme functionality (for a review, see reference 41). Extracellular ferric reductases can also mobilize Fe3+ from human ferritin (29), a protein which is rapidly degraded during infection with N. meningitidis (34). However, several lines of evidence argue against this hypothesis, including the data showing that the MS11 traD tonB double mutant is no more attenuated in intracellular survival than the tonB mutant. Since traD encodes the predicted coupling protein, it would be expected to be necessary for secretion of proteins by the T4SS. Thus, for this hypothesis to be correct, the T4SS would have to secrete an iron-related protein using a different, unknown coupling protein or no coupling protein at all. Furthermore, we were unable to detect any GGI-dependent secretion of reductase or chelating activities.
Two, more-favored hypotheses to explain the mechanisms by which the GGI affords Ton-independent intracellular survival are currently being examined. First, the T4SS could provide a structural pore for indiscriminate uptake of iron by the gonococcus. It has been observed that expression of a particular variant of the pilus secretin protein, PilQ, is associated with increased entry of heme and antimicrobial compounds (11), providing evidence that there is increased iron entry through a modified pore-like protein. Also, the T4SS of L. pneumophila makes this bacterium sensitive to salt in the environment, suggesting that a T4SS can act as an indiscriminate pore (48). In a similar scenario, a possible mechanism to explain our observations is that iron could enter through the structural pore of the gonococcal T4SS. The biological relevance of this possibility, however, may be questionable simply due to a lack of iron accessibility. Iron in the intracellular environment is expected to be complexed to host iron-binding molecules, such as ferritin or iron-sulfur proteins, and therefore simply providing a T4SS-mediated opening into the gonococcal cell is likely not sufficient for iron-dependent growth.
The second possibility is that the T4SS allows invasion or intracellular localization in a niche in which the Ton system and high-affinity iron acquisition are not necessary. In the wild-type GGI-carrying strain, both niches would be available for intracellular survival of gonococci. In the absence of either GGI or the Ton system, intracellular survival would be promoted in the niche provided by the intact system. However, in the absence of both T4SS and TonB-mediated iron acquisition, gonococcal survival within epithelial cells would be severely compromised. As is true for L. pneumophila (30), this third hypothesis involves the gonococcal T4SS setting up an intracellular niche, perhaps by interacting with host cell trafficking pathways, in which high-affinity acquisition of ferric iron is unnecessary. This seems to be a viable hypothesis given the known functions of other T4SSs in intracellular survival.
In summary, acquisition of host-derived iron was necessary for intracellular survival of gonococci, and, in the absence of the GGI, the Ton complex was necessary for intracellular growth. The presence of the GGI, however, increased TonB-independent intracellular survival, and this required components of the T4SS. Intracellular survival was directly linked to bacterial iron acquisition as addition of iron reversed the intracellular growth defects of the Ton and GGI mutants. To our knowledge, this study provides the first evidence that links iron acquisition, expression of T4SS components, and intracellular survival of gonococci.
Acknowledgments
Funding for this work was provided by grants AI065555, AI047141, and AI084400 to C.N.C. and by grants AI047958 and AI072605 to J.P.D. from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health. N. M. Dominguez was supported by grant T32 G088349 from the NIH.
We gratefully acknowledge Magdalene So and Shelley Payne for valuable intellectual contributions at the inception of this project.
Editor: A. J. Bäumler
Footnotes
Published ahead of print on 22 March 2010.
REFERENCES
- 1.Anderson, J. E., M. M. Hobbs, G. D. Biswas, and F. Sparling. 2003. Opposing selective forces for expression of the gonococcal lactoferrin receptor. Mol. Microbiol. 48:1325-1337. [DOI] [PubMed] [Google Scholar]
- 2.Aoki, K. 1996. Metal ions in biological systems, vol. 32. Marcel Dekker, Inc., New York, NY.
- 3.Apicella, M. A., M. Ketterer, F. K. N. Lee, D. Zhou, P. A. Rice, and M. S. Blake. 1996. The pathogenesis of gonococcal urethritis in men: confocal and immunoelectron microscopic analysis of urethral exudates from men infected with Neisseria gonorrhoeae. J. Infect. Dis. 173:636-646. [DOI] [PubMed] [Google Scholar]
- 4.Backert, S., and T. F. Meyer. 2006. Type IV secretion systems and their effectors in bacterial pathogenesis. Curr. Opin. Microbiol. 9:207-217. [DOI] [PubMed] [Google Scholar]
- 5.Barchini, E., and R. E. Cowart. 1996. Extracellular iron reductase activity produced by Listeria monocytogenes. Arch. Microbiol. 166:51-57. [DOI] [PubMed] [Google Scholar]
- 6.Beddek, A. J., B. J. Sheehan, J. T. Bosse, A. N. Rycroft, J. S. Kroll, and P. R. Langford. 2004. Two TonB systems in Actinobacillus pleuropneumoniae: their roles in iron acquisition and virulence. Infect. Immun. 72:701-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biswas, G. D., and P. F. Sparling. 1995. Characterization of lbpA, the structural gene for a lactoferrin receptor in Neisseria gonorrhoeae. Infect. Immun. 63:2958-2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonnah, R. A., S. W. Lee, B. L. Vasquez, C. A. Enns, and M. So. 2000. Alteration of epithelial cell transferrin-iron homeostasis by Neisseria meningitidis and Neisseria gonorrhoeae. Cell. Microbiol. 2:207-218. [DOI] [PubMed] [Google Scholar]
- 9.Bonnah, R. A., M. U. Muckenthaler, H. Carlson, B. Minana, C. A. Enns, M. W. Hentze, and M. So. 2004. Expression of epithelial cell iron-related genes upon infection by Neisseria meningitidis. Cell. Microbiol. 6:473-484. [DOI] [PubMed] [Google Scholar]
- 10.Buchrieser, C., R. Brosch, S. Bach, A. Guiyoule, and E. Carniel. 1998. The high-pathogenicity island of Yersinia pseudotuberculosis can be inserted into any of the three chromosomal asn tRNA genes. Mol. Microbiol. 30:965-978. [DOI] [PubMed] [Google Scholar]
- 11.Chen, C.-J., D. M. Tobiason, C. E. Thomas, W. M. Shafer, H. S. Seifert, and P. F. Sparling. 2004. A mutant form of the Neisseria gonorrhoeae pilus secretin protein PilQ allows increased entry of heme and antimicrobial compounds. J. Bacteriol. 186:730-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen, C., P. Sparling, L. Lewis, D. Dyer, and C. Elkins. 1996. Identification and purification of a hemoglobin-binding outer membrane protein from Neisseria gonorrhoeae. Infect. Immun. 64:5008-5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooper, C. E., G. R. Lynagh, K. P. Hoyes, R. C. Hider, R. Cammack, and J. B. Porter. 1996. The relationship of intracellular iron chelation to the inhibition and regeneration of human ribonucleotide reductase. J. Biol. Chem. 271:20291-20299. [DOI] [PubMed] [Google Scholar]
- 14.Cornelissen, C. N., G. D. Biswas, J. Tsai, D. K. Paruchuri, S. A. Thompson, and P. F. Sparling. 1992. Gonococcal transferrin-binding protein 1 is required for transferrin utilization and is homologous to TonB-dependent outer membrane receptors. J. Bacteriol. 174:5788-5797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cornelissen, C. N., M. Kelley, M. M. Hobbs, J. E. Anderson, J. G. Cannon, M. S. Cohen, and P. F. Sparling. 1998. The transferrin receptor expressed by gonococcal strain FA1090 is required for the experimental infection of human male volunteers. Mol. Microbiol. 27:611-616. [DOI] [PubMed] [Google Scholar]
- 16.Covacci, A., J. L. Telford, G. D. Giudice, J. Parsonnet, and R. Rappuoli. 1999. Helicobacter pylori virulence and genetic geography. Science 284:1328-1333. [DOI] [PubMed] [Google Scholar]
- 17.Deneer, H. G., V. Healey, and I. Boychuk. 1995. Reduction of exogenous ferric iron by a surface-associated ferric reductase of Listeria spp. Microbiology 141:1985-1992. [DOI] [PubMed] [Google Scholar]
- 18.Desai, P. J., E. Garges, and C. A. Genco. 2000. Pathogenic Neisseriae can use hemoglobin, transferrin, and lactoferrin independently of the tonB locus. J. Bacteriol. 182:5586-5591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dillard, J. 2006. Genetic manipulation of Neisseria gonorrhoeae, p. 4A.2.1-4A.2.19. In R. Coico, T. Kowalik, J. Quarles, B. Stevenson, R. Taylor, and A. Simon (ed.), Current protocols in microbiology. John Wiley and Sons, New York, NY. [DOI] [PubMed]
- 20.Dillard, J. P., and H. S. Seifert. 2001. A variable genetic island specific for Neisseria gonorrhoeae is involved in providing DNA for natural transformation and is found more often in disseminated infection isolates. Mol. Microbiol. 41:263-277. [DOI] [PubMed] [Google Scholar]
- 21.Edwards, J. L., J. Q. Shao, K. A. Ault, and M. A. Apicella. 2000. Neisseria gonorrhoeae elicits membrane ruffling and cytoskeletal rearrangements upon infection of primary human endocervical and ectocervical cells. Infect. Immun. 68:5354-5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frost, L. S., K. Ippen-Ihler, and R. A. Skurray. 1994. Analysis of the sequence and gene products of the transfer region of the F sex factor. Microbiol. Mol. Biol. Rev. 58:162-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerbase, A. C., J. T. Rowley, and T. E. Mertens. 1998. Global epidemiology of sexually transmitted diseases. Lancet 351:2-4. [DOI] [PubMed] [Google Scholar]
- 24.Hacker, J., and J. B. Kaper. 2000. Pathogenicity islands and the evolution of microbes. Annu. Rev. Microbiol. 54:641-679. [DOI] [PubMed] [Google Scholar]
- 25.Hagen, T. A., and C. N. Cornelissen. 2006. Neisseria gonorrhoeae requires expression of TonB and the putative transporter TdfF to replicate within cervical epithelial cells. Mol. Microbiol. 62:1144-1157. [DOI] [PubMed] [Google Scholar]
- 26.Hamilton, H. L., N. M. Domínguez, K. J. Schwartz, K. T. Hackett, and J. P. Dillard. 2005. Neisseria gonorrhoeae secretes chromosomal DNA via a novel type IV secretion system. Mol. Microbiol. 55:1704-1721. [DOI] [PubMed] [Google Scholar]
- 27.Hamilton, H. L., K. J. Schwartz, and J. P. Dillard. 2001. Insertion-duplication mutagenesis of Neisseria: use in characterization of DNA transfer genes in the gonococcal genetic island. J. Bacteriol. 183:4718-4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harris, R. L., and P. M. Silverman. 2004. Tra proteins characteristic of F-like type IV secretion systems constitute an interaction group by yeast two-hybrid analysis. J. Bacteriol. 186:5480-5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Homuth, M., P. Valentin-Weigand, M. Rohde, and G. Gerlach. 1998. Identification and characterization of a novel extracellular ferric reductase from Mycobacterium paratuberculosis. Infect. Immun. 66:710-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Isberg, R., T. O'Connor, and M. Heidtman. 2009. The Legionella pneumophila replication vacuole: making a cosy niche inside host cells. Nat. Rev. Microbiol. 7:13-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kellogg, D. S., Jr., W. L. Peacock, Jr., W. E. Deacon, L. Brown, and C. I. Pirkle. 1963. Neisseria gonorrhoeae. I. Virulence genetically linked to clonal variation. J. Bacteriol. 85:1274-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klimke, W. A., C. D. Rypien, B. Klinger, R. A. Kennedy, J. M. Rodriguez-Maillard, and L. S. Frost. 2005. The mating pair stabilization protein, TraN, of the F plasmid is an outer-membrane protein with two regions that are important for its function in conjugation. Microbiology 151:3527-3540. [DOI] [PubMed] [Google Scholar]
- 33.Larson, J. A., D. L. Higashi, I. Stojiljkovic, and M. So. 2002. Replication of Neisseria meningitidis within epithelial cells requires TonB-dependent acquisition of host cell iron. Infect. Immun. 70:1461-1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Larson, J. A., H. L. Howie, and M. So. 2004. Neisseria meningitidis accelerates ferritin degradation in host epithelial cells to yield an essential iron source. Mol. Microbiol. 53:807-820. [DOI] [PubMed] [Google Scholar]
- 35.Lawley, T. D., W. A. Klimke, M. J. Gubbins, and L. S. Frost. 2003. F factor conjugation is a true type IV secretion system. FEMS Microbiol. Lett. 224:1-15. [DOI] [PubMed] [Google Scholar]
- 36.Lee, S. W., D. L. Higashi, A. Snyder, A. J. Merz, L. Potter, and M. So. 2005. PilT is required for PI(3,4,5)P3-mediated crosstalk between Neisseria gonorrhoeae and epithelial cells. Cell. Microbiol. 7:1271-1284. [DOI] [PubMed] [Google Scholar]
- 37.LeFaou, A. E., and S. A. Morse. 1991. Characterization of a solube ferric reductase from Neisseria gonorrhoeae. Biol. Met. 4:126-131. [DOI] [PubMed] [Google Scholar]
- 38.Merz, A. J., and M. So. 2000. Interactions of pathogenic Neisseriae with epithelial cell membranes. Annu. Rev. Cell Dev. Biol. 16:423-457. [DOI] [PubMed] [Google Scholar]
- 39.Salgado-Pabón, W., Y. Du, K. T. Hackett, K. M. Lyons, C. G. Arvidson, and J. P. Dillard. 2010. Increased expression of the type IV secretion system in piliated Neisseria gonorrhoeae variants. J. Bacteriol. 192:1912-1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salgado-Pabón, W., S. Jain, N. Turner, C. van der Does, and J. Dillard. 2007. A novel relaxase homologue is involved in chromosomal DNA processing for type IV secretion in Neisseria gonorrhoeae. Mol. Microbiol. 66:930-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schroder, I., E. Johnson, and S. deVries. 2003. Microbial ferric iron reductases. FEMS Microbiol. Rev. 27:427-447. [DOI] [PubMed] [Google Scholar]
- 42.Schryvers, A., and I. Stojiljkovic. 1999. Iron acquisition systems in the pathogenic Neisseria. Mol. Microbiol. 32:1117-1123. [DOI] [PubMed] [Google Scholar]
- 43.Seliger, S., A. Mey, A. Valle, and S. Payne. 2001. The two TonB systems of Vibrio cholerae: redundant and specific functions. Mol. Microbiol. 39:801-812. [DOI] [PubMed] [Google Scholar]
- 44.Shaw, J. H., and S. Falkow. 1988. Model of invasion of human tissue culture cells by Neisseria gonorrhoeae. Infect. Immun. 56:1625-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sparling, P. F. 2000. Sexually transmitted diseases, p. 433-449. In K. K. Homes, P. F. Sparling, S. M. Lemon, W. E. Stamm, P. Piot, and J. N. Wasserheit (ed.), Biology of Neisseria gonorrhoeae, 2nd ed. McGraw Hill, New York, NY.
- 46.Stork, M., M. Di Lorenzo, S. Mourino, C. R. Osorio, M. L. Lemos, and J. H. Crosa. 2004. Two tonB systems function in iron transport in Vibrio anguillarum, but only one is essential for virulence. Infect. Immun. 72:7326-7329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Swanson, J. 1972. Studies on gonococcus infection. II. Freeze-fracture, freeze-etch studies on gonococci. J. Exp. Med. 136:1258-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vogel, J., and R. Isberg. 1999. Cell biology of Legionella pneumophila. Curr. Opin. Microbiol. 2:30-34. [DOI] [PubMed] [Google Scholar]
- 49.Vokes, S. A., S. A. Reeves, A. G. Torres, and S. M. Payne. 1999. The aerobactin iron transport system genes in Shigella flexneri are present within a pathogenicity island. Mol. Microbiol. 33:63-73. [DOI] [PubMed] [Google Scholar]
- 50.Wolfgang, M., P. Lauer, H.-S. Park, L. Brossay, J. Hébert, and M. Koomey. 1998. PilT mutations lead to simultaneous defects in competence for natural transformation and twitching motility in piliated Neisseria gonorrhoeae. Mol. Microbiol. 29:321-330. [DOI] [PubMed] [Google Scholar]
- 51.Yancey, R. J., and R. A. Finkelstein. 1981. Siderophore production by pathogenic Neisseria spp. Infect. Immun. 32:600-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ye, C., and J. Xu. 2001. Prevalence of iron transport gene on pathogenicity-associated island of uropathogenic Escherichia coli in E. coli O157:H7 containing Shiga toxin gene. J. Clin. Microbiol. 39:2300-2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao, Q., and K. Poole. 2000. A second tonB gene in Pseudomonas aeruginosa is linked to the exbB and exbD genes. FEMS Microbiol. Lett. 184:127-132. [DOI] [PubMed] [Google Scholar]