Abstract
Accumulating evidence suggests that bacteria associated with periodontal disease may exert systemic immunomodulatory effects. Although the improvement in oral hygiene practices in recent decades correlates with the increased incidence of asthma in developed nations, it is not known whether diseases of the respiratory system might be influenced by the presence of oral pathogens. The present study sought to determine whether subcutaneous infection with the anaerobic oral pathogen Porphyromonas gingivalis exerts a regulatory effect on allergic airway inflammation. BALB/c mice sensitized and subsequently challenged with ovalbumin exhibited airway hyperresponsiveness to methacholine aerosol and increased airway inflammatory cell influx and Th2 cytokine (interleukin-4 [IL-4], IL-5, and IL-13) content relative to those in nonallergic controls. Airway inflammatory cell and cytokine contents were significantly reduced by establishment of a subcutaneous infection with P. gingivalis prior to allergen sensitization, whereas serum levels of ovalbumin-specific IgE and airway responsiveness were not altered. Conversely, subcutaneous infection initiated after allergen sensitization did not alter inflammatory end points but did reduce airway responsiveness in spite of increased serum IgE levels. These data provide the first direct evidence of a regulatory effect of an oral pathogen on allergic airway inflammation and responsiveness. Furthermore, a temporal importance of the establishment of infection relative to allergen sensitization is demonstrated for allergic outcomes.
A causative relationship between decreased microbial exposure and infection in recent decades and the concurrent increase in asthma prevalence in developed countries has been suggested and is thought to be attributable, at least in part, to a phenomenon known as the hygiene hypothesis (30). Originally put forth by Strachan (32), the hypothesis proposes that increased cleanliness of modern industrialized societies has resulted in decreased exposure to bacterial, viral, and other immunomodulatory organisms and their products, particularly in early life, and that this has in turn resulted in a loss of potentially protective effects of these exposures on the development of allergic diseases. Accumulating clinical and experimental evidence largely supports the hygiene hypothesis as it relates to asthma, although a consensus has not been reached. As reviewed recently (31), a variety of infections of a viral, bacterial, and parasitic nature influence the host immune response, such that regulation of the Th1-Th2 balance is modified to promote Th1 responses and impede Th2 responses, thereby reducing Th2-mediated allergic outcomes. However, this is likely a simplistic view of the effects of infections on immune system development and responses, and other factors, including host genetic makeup and timing of exposures to the infective agent relative to allergen exposure, undoubtedly contribute to the overall allergic phenotype.
In addition to the influence of environmental exposure to microbes, the potential regulation of allergic diseases by the microflora of the host is receiving increased attention. Evidence suggests that the composition of the gastrointestinal microflora differs between individuals with and without allergy (reviewed in reference 25), and disruption of the normal gut microflora by antibiotic administration leads to allergic airway responses following allergen challenge in mice not previously sensitized to the allergen (24). Moreover, although similar benefits have not yet been demonstrated in humans, the oral administration of probiotic bacteria was recently shown to decrease allergic airway inflammation in mice (8, 9).
As in the gut, the microenvironment of the oral cavity is complex and comprises hundreds of bacterial species. Porphyromonas gingivalis, a Gram-negative opportunistic periodontal pathogen, can initiate periodontal lesions in nonhuman primates when introduced into the periodontal microbiota (15) and is a major etiological agent in severe forms of periodontal disease such as chronic periodontitis (21). Interest in chronic oral infections and their potential role in adverse systemic health effects has been heightened by observations of positive associations between serum concentrations of antibodies to oral pathogens such as P. gingivalis and the incidence of cardiovascular diseases and renal dysfunction (3, 19, 28, 29). Recently, however, an inverse relationship between serum concentrations of antibodies to P. gingivalis and the prevalence of asthma, wheeze, and hay fever was observed in a representative sample of the population of the United States (2). Furthermore, a significant inverse association between periodontitis and the incidences of hay fever and allergy to house dust mites was reported for a northeast German population, with a borderline significant inverse association between periodontitis and asthma also observed (10). While limitations of these observational studies include potential recall bias pertaining to asthma symptoms and the inability to directly assess cause and effect, these findings nonetheless suggest a potential protective effect of infection with oral pathogens such as P. gingivalis on asthma pathogenesis.
In order to examine the influence of oral pathogens on the development of allergic airway disease under controlled experimental conditions, the present study sought to determine whether infection with P. gingivalis modified allergic outcomes in a murine model of asthma. To accomplish this, a subcutaneous chamber model was employed wherein mice were subjected to a local infection with live P. gingivalis either before or after sensitization to allergen, and the effects of this infection on subsequent responses to allergen challenge were assessed. The results indicate that P. gingivalis infection exerts a modulatory effect on allergic airway responses and that this effect is dependent on the timing of infection relative to allergic sensitization.
MATERIALS AND METHODS
Animals and treatments.
All studies were conducted in accordance with the principles and procedures outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals (23a) and were approved by the Animal Care and Use Committee of the National Institute of Environmental Health Sciences and by the Animal Welfare Committee and the Institutional Animal Care and Use Committee of the University of North Carolina at Chapel Hill. Female BALB/c mice (6 weeks of age; Taconic Farms, Germantown, NY) were used in an ovalbumin (OVA)-induced allergic airway inflammation model. Mice were sensitized with an intraperitoneal injection of 20 μg OVA (grade V; Sigma, St. Louis, MO) in 0.2 ml aluminum hydroxide adjuvant (Alhydrogel; Accurate Chemical, Westbury, NY) on two consecutive days. Subsequent exposure to aerosolized OVA occurred on five consecutive days (1% aerosol in phosphate-buffered saline [PBS] for 30 min per day), and assessment of respiratory mechanics and collection of bronchoalveolar lavage (BAL) fluid and tissue samples occurred 24 h following the final aerosol exposure.
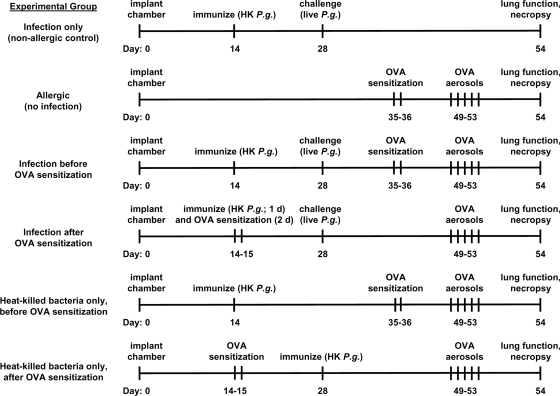
To assess the influence of infection with P. gingivalis on the development of OVA-induced pulmonary allergic responses, mice were exposed to live P. gingivalis before or after sensitization to OVA, using a previously described subcutaneous chamber model system (12, 22, 23). Briefly, a single cylindrical coiled-spring chamber made of stainless steel was surgically implanted in the dorsolumbar region of each mouse. Two weeks later, mice were immunized by intrachamber injection of 0.1 ml of suspension containing 108 heat-killed P. gingivalis organisms. Two weeks following this, chambers were injected with 0.1 ml of suspension containing 107 live P. gingivalis cells in order to establish a localized infection. Injection of chambers with live bacteria occurred either before or after sensitization to OVA in order to assess the influence of P. gingivalis infection on allergic airway responses to subsequent allergen exposure. Some mice were not sensitized or challenged with OVA but were exposed to heat-killed and live bacteria (infection only), some were not exposed to any bacteria but were sensitized and challenged with OVA (allergic group), and some were exposed only to heat-killed bacteria either before or after OVA sensitization. Details of the experimental groups and timing of the various procedures are provided in Fig. 1.
FIG. 1.
Experimental groups and timeline of study. HK, heat killed; P.g., P. gingivalis; OVA, ovalbumin. Six-week-old female BALB/c mice were cage acclimated for 1 week prior to chamber implantation surgery and thus were approximately 7 weeks old at “day 0.”
Preparation of bacterial suspensions.
A stock of P. gingivalis strain A7436 was stored in Wilkins Chalgren anaerobic broth (WC broth; DSMZ, Braunschweig, Germany) containing 10% skim milk at −80°C. Bacteria were cultivated in WC broth at 37°C in an anaerobic chamber (Coy Laboratory Products Inc., Ann Arbor, MI) with 5% H2, 10% CO2, and 85% N2. Bacterial suspensions were prepared from primary cultures at the log phase of growth. Bacterial concentration was evaluated by spectrophotometry (Cecil Instruments Ltd., Cambridge, United Kingdom), with a measured optical density at 600 nm of 1.0 corresponding to 109 bacteria/ml, and adjusted to the desired treatment concentration by dilution with broth.
Analysis of lung function and airway responsiveness.
Respiratory mechanics and airway responsiveness to aerosolized methacholine were determined on day 54 of study (24 h following the last exposure to aerosolized OVA), using invasive analysis with a FlexiVent mechanical ventilator system (SCIREQ, Montreal, Canada) as described previously (5). Total respiratory system resistance (R) was determined at baseline and in response to increasing concentrations of aerosolized methacholine (0 to 25 mg/ml in PBS), and the provocative concentration of methacholine aerosol resulting in a 200% increase (PC200) over the baseline value of R was calculated.
Tissue collection and sample analysis.
Immediately following lung function assessment, mice were removed from the ventilator and a blood sample was drawn from the abdominal aorta. Serum was subsequently extracted, frozen, and stored at −80°C. BAL was performed with two 1.0-ml aliquots of Hanks' balanced salt solution; recovery was >75% for each mouse. Recovered BAL fluid was processed and analyzed for total and differential cell counts by routine methods, and aliquots of cell-free BAL fluid were frozen and stored at −80°C. The right bronchus was ligated, and right lungs were removed, frozen, and stored at −80°C. The left lungs were then inflated with 0.4 ml paraformaldehyde that was injected slowly over approximately 10 s via a blunt needle that was inserted into the trachea. The tracheas were immediately tied off with sutures, and the lungs were submersed in 4% paraformaldehyde and used for preparation of slides for histopathological evaluation.
Histopathological evaluation of inflammation was performed on sections of left lung that were stained with hematoxylin and eosin. The evaluation was conducted by a pathologist in a blinded manner such that the treatment group and animal information were not known. An inflammatory score was calculated for each sample, based on microscopic assessment of the extent and severity of inflammation. Briefly, perivascular, peribronchial, and pleural inflammation levels were each assigned a score of 0 to 3. Perivascular inflammation was scored as follows: 0, none; 1, occasional inflamed vessels or mild inflammation only; 2, frequent inflamed vessels, but <50% of vessels inflamed, or >50% of vessels inflamed, but with predominantly mild inflammation; and 3, >50% of vessels showing moderate or marked inflammation. Peribronchial inflammation was scored as follows: 0, none; 1, occasional inflamed bronchi; 2, frequent inflamed bronchi, but <50% of bronchi inflamed, or >50% of bronchi inflamed, but with many showing very mild inflammation; 3, >50% of bronchi showed mild to moderate or marked inflammation. Pleural inflammation was scored as follows: 0, none; 1, mild and patchy inflammation; 2, inflammation was moderate in intensity and frequent in distribution; 3, either moderate and frequent inflammation with the presence of polypoid foci, the presence of foci with marked pleuritis, or diffuse involvement of the pleura. The total score (range of 0 to 9), representing the sum of the individual scores, was calculated for each animal and averaged for each experimental group.
Levels of interleukin-4 (IL-4), IL-5, IL-13, and granulocyte-macrophage colony-stimulating factor (GM-CSF) in BAL fluid and serum were determined with a Bio-Plex mouse cytokine kit (Bio-Rad, Hercules, CA), using fluorescently labeled microsphere beads and a Bio-Plex suspension array system (Bio-Rad) according to the manufacturer's instructions. Total serum IgE content was quantified by enzyme-linked immunosorbent assay (ELISA; BD Biosciences, San Diego, CA) according to the manufacturer's instructions. OVA-specific IgE in serum was quantified with the same ELISA kit according to the manufacturer's instructions, except for the following two modifications: plates were coated overnight with OVA (10 μg/ml PBS; 100 μl/well), and the standard curve was created using an OVA-specific IgE standard from AbD Serotec (Raleigh, NC).
Statistical analysis.
All data are expressed as group means ± standard errors of the means and were pooled from two independent experiments conducted approximately 5 months apart. Statistical comparisons were performed by one-way analysis of variance (ANOVA) followed by Newman-Keuls post hoc tests, using GraphPad Prism software (GraphPad Software Inc., San Diego, CA). In all instances, statistical significance was denoted when the P value was <0.05.
RESULTS
Allergen-induced airway hyperresponsiveness is decreased when P. gingivalis infection is established after sensitization to allergen.
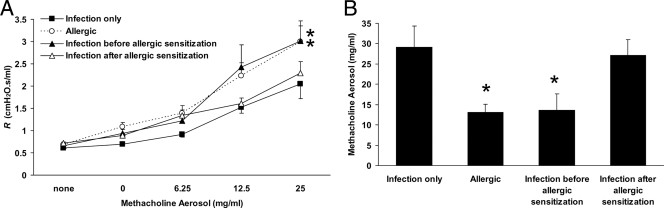
Invasive determination of R was performed to assess whether a localized P. gingivalis infection influenced the development of airway hyperresponsiveness to cholinergic stimulation, a cardinal feature of asthma and experimental allergic airway disease. No differences in baseline R values were observed among the various treatment groups (Fig. 2A). Allergic mice demonstrated increased airway responsiveness to methacholine aerosol compared to nonallergic mice (infected with P. gingivalis only), as evidenced by higher R values (Fig. 2A). The lower calculated PC200 value for R in allergic mice, indicative of increased sensitivity to methacholine, confirmed this observation (Fig. 2B). The effect of P. gingivalis infection on airway responsiveness was dependent on the timing of the infection relative to allergic sensitization. No effect was observed when P. gingivalis infection was established before OVA sensitization occurred, but infection initiated after OVA sensitization resulted in reduced airway responsiveness to methacholine aerosol (Fig. 2A) and a corresponding increase in the PC200 value for R (Fig. 2B) relative to those for allergic mice not exposed to P. gingivalis.
FIG. 2.
Airway responsiveness to methacholine aerosol in allergic mice is reduced by establishment of infection with P. gingivalis after, but not prior to, sensitization to allergen. (A) Increased airway responsiveness in allergic mice was blunted when infection with P. gingivalis occurred after, but not prior to, sensitization to OVA. *, P < 0.05 versus infection only (n = 7 to 13 per group). (B) Calculated PC200 values for methacholine (y axis) were lowest for the allergic group. Infection with P. gingivalis prior to sensitization to OVA did not alter these values, but infection established after sensitization increased the PC200 value for allergic mice. *, P < 0.05 versus infection-only group and group receiving infection after allergic sensitization (n = 7 to 13 per group).
Allergic airway inflammation is decreased when P. gingivalis infection is established before sensitization to allergen.
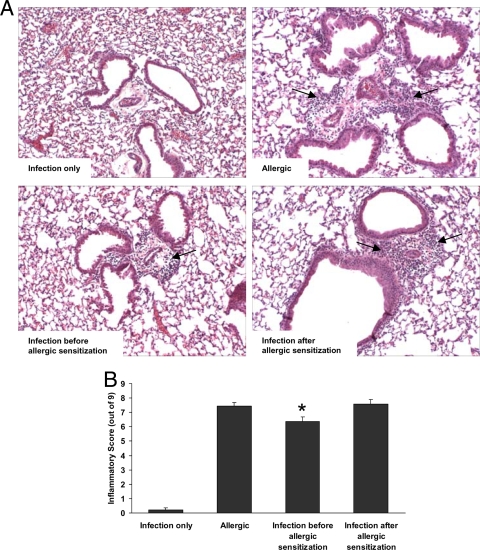
Sections of lung from the different experimental groups were prepared and stained with hematoxylin and eosin in order to allow for semiquantitative histopathological assessment of the influence of infection on allergic inflammation (representative sections are shown in Fig. 3A). As expected, the histopathological inflammatory score was higher for the allergic group than for the infection-only group (Fig. 3B). Establishment of P. gingivalis infection prior to allergic sensitization resulted in a moderate but significantly decreased inflammatory score for this group compared with that for the allergic group, whereas infection established after sensitization did not (Fig. 3B). Subanalysis of the data revealed that the decreased overall score for the group infected with P. gingivalis prior to allergic sensitization was the result of a decrease in the pleural inflammation score for this group (1.25 out of 3) compared to that for the allergic group (1.79 out of 3) (P < 0.05). No significant differences were observed among the groups in terms of perivascular or peribronchiolar inflammation scores (data not shown).
FIG. 3.
Histopathological evidence of airway inflammation is reduced in mice infected with P. gingivalis prior to but not after sensitization to allergen. (A) Representative histological sections (stained with hematoxylin and eosin) demonstrating areas of eosinophilic and lymphocytic inflammation in perivascular and peribronchiolar regions (depicted by arrows). These regions were less numerous and intense in mice infected before allergic sensitization. (B) Calculated histopathological scores revealed decreased inflammation in allergic mice infected with P. gingivalis prior to sensitization to OVA. *, P < 0.05 versus allergic group and group receiving infection after allergic sensitization (n = 9 to 16 per group).
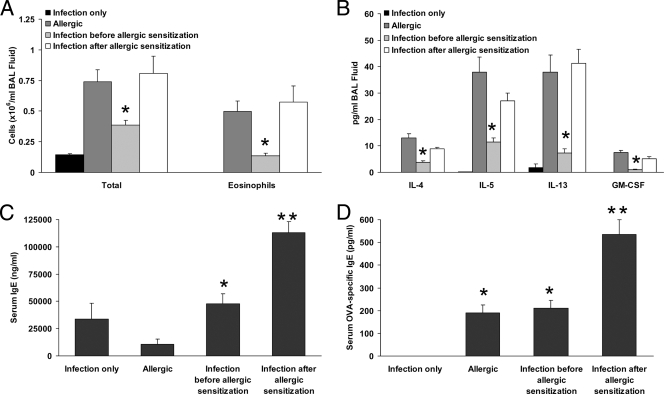
Quantification of inflammatory cell influx and cytokine content in BAL fluid was also performed. The total number of cells recovered from the airways of allergic mice was increased relative to that recovered from mice only infected with P. gingivalis (Fig. 4A), and the majority of these (∼62%) were eosinophils. Infection with P. gingivalis before sensitization to allergen resulted in an ∼50% reduction in the number of total cells and an ∼67% reduction in the number of eosinophils recovered from allergic mice (P < 0.05 for both) (Fig. 4A). Moreover, the percentage of recovered cells that were eosinophils (∼34%) was considerably decreased in this group. Conversely, infection with P. gingivalis subsequent to allergic sensitization did not significantly alter the number of total cells or eosinophils recovered (Fig. 4A) or the percentage of eosinophils in the total cell population (∼63%) relative to that found for allergic mice not infected with P. gingivalis. Levels of the Th2 cytokines IL-4, IL-5, IL-13, and GM-CSF were increased in BAL fluid of allergic mice relative to those in mice that were only infected with P. gingivalis (Fig. 4B). P. gingivalis infection established prior to OVA sensitization resulted in decreased airway levels of IL-4, IL-5, IL-13, and GM-CSF relative to those found in allergic mice not exposed to bacteria, whereas infection established after sensitization did not alter these levels (Fig. 4B). BAL fluid levels of other inflammatory cytokines (IL-6, IL-12, IL-1β, and IFN-γ) were at or near the lower limit of detection and did not differ among the groups (data not shown). In contrast to what was observed in BAL fluid samples, no consistent pattern of effect resulting from P. gingivalis infection was observed for serum cytokines. Serum IL-4 levels were not different among the groups, and IL-5, IL-13, and GM-CSF levels were generally increased in all groups relative to those in the infection-only group (data not shown).
FIG. 4.
BAL fluid inflammatory parameters and serum IgE levels in mice infected with P. gingivalis prior to or after sensitization to allergen. (A) Total cell and eosinophil populations in the airways of allergic mice were reduced when infection with P. gingivalis was established prior to sensitization to OVA. *, P < 0.05 versus allergic group and group receiving infection after allergic sensitization (n = 9 to 16 per group). (B) BAL fluid cytokine content in allergic mice was reduced when infection with P. gingivalis was established prior to sensitization to OVA. *, P < 0.05 versus allergic group and group receiving infection after allergic sensitization (n = 9 to 16 per group). (C) Serum IgE levels were higher in the infection-only group than in the allergic group, but this was not statistically significant. Infection initiated either before or after sensitization to OVA increased levels relative to those for the allergic group, with infection initiated after sensitization resulting in the highest levels. *, P < 0.05 versus allergic group; **, P < 0.05 versus all other groups (n = 9 to 15 per group). (D) OVA-specific IgE levels in serum were highest when infection was initiated after sensitization to OVA, whereas infection initiated prior to sensitization to OVA did not alter the level compared to that in the allergic group. *, P < 0.05 versus infection-only group; **, P < 0.05 versus all other groups (n = 10 to 15 per group).
Infection with P. gingivalis alone resulted in an increased trend in serum total IgE levels compared to those observed in allergic mice (Fig. 4C). Infection established before OVA sensitization resulted in a significantly increased serum total IgE level compared to that observed for the allergic group, whereas infection established after OVA sensitization resulted in the highest level of serum total IgE among all the groups (Fig. 4C). The serum OVA-specific IgE level was also highest when infection was established after OVA sensitization, whereas infection established before sensitization did not alter the OVA-specific IgE level compared to that observed for the allergic group (Fig. 4D). As expected, no OVA-specific IgE was detected in mice that were only infected with P. gingivalis (Fig. 4D).
Cumulatively, these data indicate that allergic airway inflammation was reduced by the establishment of a P. gingivalis infection prior to, but not after, allergen sensitization and that alterations in airway cytokine and serum IgE levels may have been involved in these effects.
Immunization with heat-killed P. gingivalis alone does not alter allergic airway inflammation or hyperresponsiveness.
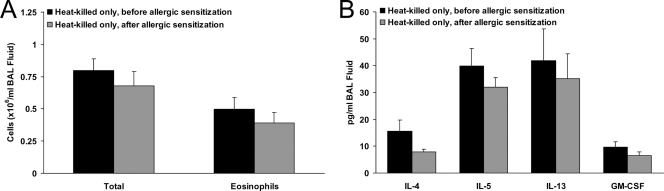
To determine if the measured allergic outcomes were influenced by the immunization procedure with heat-killed bacteria, separate groups of mice were immunized by introducing heat-killed P. gingivalis into the subcutaneous chambers but were not subsequently challenged with live bacteria. Relative to that for the allergic group not treated with any bacteria (PC200 value for R of 13.0 ± 2.0 mg/ml) (Fig. 2B), the airway hyperresponsiveness to methacholine was not significantly altered in mice only immunized with heat-killed bacteria either before or after sensitization to OVA (PC200 values for R of 17.5 ± 1.7 and 17.8 ± 1.5 mg/ml, respectively; P > 0.05 for both). Airway inflammatory cell and cytokine contents also did not differ in mice only immunized with heat-killed bacteria either before or after sensitization to OVA (Fig. 5A and B) relative to those for allergic mice not treated with any bacteria (Fig. 4A and B). Thus, the immunization procedure with heat-killed bacteria did not in and of itself exert a regulatory effect on the observed allergic outcomes.
FIG. 5.
Allergic airway inflammation is not altered by heat-killed P. gingivalis. BAL fluid cell counts (A) and cytokine levels (B) were not altered by intrachamber injection of heat-killed P. gingivalis either before or after sensitization to OVA (compare data to those in Fig. 3C and D) (n = 4 to 7 per group).
DISCUSSION
While considerable attention has been given to the influence of environmental exposures to bacterial products such as lipopolysaccharide (LPS) on asthma pathogenesis (14, 33), much less is known regarding the possible influence of bacteria and bacterial products derived from the host microflora on allergic airway disease. The gastrointestinal microflora has been proposed to exert regulatory effects on immune system development and, in particular, to affect allergic respiratory diseases (8, 9, 13, 24-26). To our knowledge, however, the potential influence of oral bacteria in an experimental model of allergic airway disease has not been reported. The purpose of this study was to determine whether allergic airway inflammation is modified by infection with the periodontal pathogen P. gingivalis in an established murine model of asthma. The results indicate that P. gingivalis exerted differential regulatory effects on allergic airway inflammation that were dependent on the timing of the establishment of infection relative to allergic sensitization.
Several studies have identified a positive association between serum levels of antibodies to P. gingivalis and cardiovascular disease risk in humans (3, 28, 29), although recent observations of inverse relationships between serum P. gingivalis antibody levels and asthma prevalence and between the incidences of periodontitis and allergic respiratory diseases (2, 10) suggest that not all extra-oral effects of this bacterium may be deleterious. Furthermore, the improvement in oral hygiene in recent decades coincides with the increase in asthma prevalence in developed nations (1, 16), suggestive of a potentially causative relationship. A major limitation of these observations, however, is the inability to discern whether the presence of oral bacteria, and of P. gingivalis in particular, promotes/inhibits the development of the diseases in question, or vice versa. The present study thus evaluated the influence of P. gingivalis infection initiated prior to or after allergic sensitization under controlled experimental conditions. With this approach, we found that P. gingivalis infection established before allergen sensitization had the most striking effects on airway inflammation, with reduced airway levels of IL-4, IL-5, IL-13, and GM-CSF, decreased histological inflammation, and decreased airway eosinophilia. Neither OVA-specific IgE in serum nor airway hyperresponsiveness was decreased in this setting. Airway hyperresponsiveness was decreased, however, when P. gingivalis infection was established after allergic sensitization occurred, although inflammation was unaffected and OVA-specific IgE levels in serum were increased. These findings highlight a complex regulatory scheme for allergic airway inflammation and functional alterations that is affected in a temporally important fashion by P. gingivalis infection.
The mechanistic basis for the reduction of allergic inflammatory responses in the lung due to P. gingivalis infection established prior to, but not after, allergic sensitization is unclear, but it does not appear to involve alterations in serum levels of total or OVA-specific IgE. Infection with P. gingivalis alone resulted in a higher total IgE level in serum than that observed in allergic mice, suggestive of a significant immunomodulatory effect of this bacterium. Supportive of this concept are data demonstrating that administration of LPS derived from P. gingivalis to newborn BALB/c mice results in increased serum IgE levels upon maturity, whereas administration of LPS derived from Actinobacillus actinomycetemcomitans or Escherichia coli does not (17). Moreover, P. gingivalis-derived LPS stimulates the release of tumor necrosis factor alpha from macrophages from Toll-like receptor 4 mutant C3H/HeJ mice (18), suggestive of an alternative Toll-like receptor or other signaling pathway for LPS derived from P. gingivalis. IgE is a recognized contributor to allergic airway inflammation and hyperresponsiveness, and asthma therapies based on targeting of IgE are now in clinical use (34). Nonetheless, serum IgE and experimental allergic outcomes are not always positively correlated (36, 37), and serum IgE levels did not appear to correlate well with the inflammatory and functional outcomes observed in the treatment groups studied here. For example, mice infected with P. gingivalis prior to allergic sensitization had reduced airway inflammatory cells and cytokines but a >4-fold increase in serum total IgE and unaltered OVA-specific IgE compared to levels in allergic mice not infected with P. gingivalis. Similarly, mice infected with P. gingivalis after allergic sensitization had airway inflammatory cell and cytokine contents comparable to those in allergic mice not infected with P. gingivalis, despite having >10-fold and nearly 3-fold higher total and OVA-specific IgE levels in serum, respectively. The fact that mice infected with P. gingivalis alone had higher (albeit not statistically significant) total IgE levels in serum than did allergic mice suggests that the presence of infection was a significant contributor to the outcomes observed, likely due in part to immunomodulatory effects reflected by the changes in total IgE.
In the subcutaneous chamber model used herein, immunization of mice with heat-killed P. gingivalis prior to exposure to live bacteria allowed for colonization of the chamber by the live organisms, avoidance of host clearance, and establishment of an infection (12). Immunization with heat-killed bacteria is designed to emulate the effects of chronic infection rather than acute infection. In humans, these organisms are commensal, but they are foreign to the mouse. Thus, if this strain of P. gingivalis is provided as a challenge in a mouse that has not been immunized with heat-killed bacteria, bacteria can disseminate from the chamber more broadly to cause abscess formation, acute sepsis, and death (12). Importantly, we were able to demonstrate that the immunization procedure itself did not influence allergic airway outcomes, as mice only immunized with heat-killed P. gingivalis, either before or after allergic sensitization, demonstrated a phenotype similar to those of the allergic group. A necessity for live organisms has been observed for the modulating effect of probiotics on experimental allergic airway inflammation (9). It is unclear, however, whether our observation of attenuated inflammation as a result of establishment of infection prior to allergic sensitization was dependent on the presence of live bacteria within the chamber at the time sensitization occurred. This was the case in the present study by virtue of the experimental design. Indeed, sensitization to allergen occurred 7 days after establishment of infection, well within the 14-day period in which live bacteria can be cultured from chamber fluid (12) and, based on previous experimental data using the chamber model, a time by which bacteria had likely entered the systemic circulation (22). It would therefore be interesting to determine whether establishment of infection at a time point much earlier than that utilized here (e.g., weeks to months prior to allergic sensitization) would elicit the same blunting effect on allergic inflammation.
Although beyond the scope of the present study, it also would be beneficial to ascertain whether establishment of an oral P. gingivalis infection, a protocol recently shown to accelerate experimental atherosclerosis (20), elicits the same regulatory influence on allergic airway inflammation as that we observed with a subcutaneous infection. P. gingivalis, however, does not easily or reproducibly colonize in the oral cavity of the mouse, and it is therefore difficult to uniformly provide a similar infectious dose by this route. The effects we sought to emulate were those seen under conditions where the oral organism disseminates systemically, and this is much more effectively reproduced using the chamber model. For example, the organism is detectible in the liver at low levels following challenge in the chamber model, but variably so with the oral infection model. Furthermore, although P. gingivalis is an oral pathogen, it normally colonizes the subgingival environment and is not subject to traditional mucosal immune surveillance. The subgingival biofilm is bathed in serum via the crevicular fluid, not saliva, and is exposed to serum antibody and not secretory antibody. The host response to these invasive oral pathogens is a systemic humoral response more akin to the response to a cutaneous challenge. This is in contrast to oral organisms which inhabit the tooth surface above the gum line, such as Streptococcus mutans, which do not elicit a systemic acquired immune response, but rather a mucosal immune response. Thus, the chamber model bypasses the need for oral disease, which causes ulceration of the subgingival environment and systemic invasion of the organism to trigger the systemic humoral response. Challenge within the chamber reproducibly provides a low-level systemic challenge which mimics the presence of naturally occurring oral infection and enables one to reduce the variability in the animal response by providing a more controlled challenge dosage.
The apparent discrepancy between measures of allergic airway inflammation and airway responsiveness in the different treatment groups is intriguing but not without precedent, and it suggests a differential regulation of these processes in the allergic airway. A disconnect between measures of allergic inflammation and airway responsiveness to cholinergic stimulation has been documented in several other studies (4, 6, 7, 11, 27, 35), reinforcing the notion that inflammatory outcomes and functional alterations are not always strongly correlated. What may be viewed as surprising in our study was the observation of a lack of airway hyperresponsiveness in the group receiving infection after OVA sensitization despite the presence of substantial allergic airway inflammation, as measured by BAL fluid cells and cytokines, lung histopathology, and serum IgE levels. Although great strides have been made in our understanding of the mechanisms of allergic airway disease, the identification of an immunological biomarker that correlates with airway hyperresponsiveness has remained elusive. This is due to the tremendous complexity of the disease, as evidenced by the growing variety of factors reported to differentially regulate inflammatory and functional outcomes in animal models and humans. Indeed, potential candidates reported to underlie the discordance between allergic airway inflammation and hyperresponsiveness include signaling molecules (e.g., NF-κB) (27), enzymes (e.g., cyclooxygenase-2) (11), receptors (e.g., estrogen receptor alpha, D6 chemokine receptor) (6, 35), and autonomic dysfunction (7). These and other reported candidates provide a large number of potential mechanisms and pathways through which P. gingivalis infection may differentially regulate the various aspects of allergic airway disease.
To the best of our knowledge, this is the first report describing a direct effect of an oral pathogen on allergic airway disease. While it is recognized that the route of exposure to P. gingivalis that was employed in this study does not reflect the normal route of exposure to this organism, we maintain that the results of this study reveal its potentially important regulatory influence on allergic airway responses. Delineating the mechanisms responsible for the modulatory effect(s) of P. gingivalis infection on the allergic airway inflammatory and functional processes observed in this study and more fully elucidating their temporal characteristics and importance are the subjects of ongoing investigations that we anticipate will provide further insight into the regulatory role of oral bacteria in allergic airway disease.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences (grant no. Z01 ES025043 to D.C.Z.), by a grant (Mp1-RR-00046) from the University of North Carolina General Clinical Research Center (S.O.), and by a Senior Research Training Fellowship from the American Lung Association of North Carolina (J.W.C.). This research was conducted in part at the National Institute of Environmental Health Sciences Inhalation Facility under contract to Alion Science and Technology.
We are grateful to Sandy Ward for help with cell differential counting and to Jermaine Fuller and Patrick Galloway for the animal surgery and microbiological work related to the chamber infection model. G.P.F. conducted the histopathological evaluations.
Editor: B. A. McCormick
Footnotes
Published ahead of print on 22 March 2010.
REFERENCES
- 1.Anderson, H. R., R. Gupta, D. P. Strachan, and E. S. Limb. 2007. 50 years of asthma: UK trends from 1955 to 2004. Thorax 62:85-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arbes, S. J., Jr., M. L. Sever, B. Vaughn, E. A. Cohen, and D. C. Zeldin. 2006. Oral pathogens and allergic disease: results from the Third National Health and Nutrition Examination Survey. J. Allergy Clin. Immunol. 118:1169-1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beck, J. D., P. Eke, G. Heiss, P. Madianos, D. Couper, D. Lin, K. Moss, J. Elter, and S. Offenbacher. 2005. Periodontal disease and coronary heart disease: a reappraisal of the exposure. Circulation 112:19-24. [DOI] [PubMed] [Google Scholar]
- 4.Brewer, J. P., A. B. Kisselgof, and T. R. Martin. 1999. Genetic variability in pulmonary physiological, cellular, and antibody responses to antigen in mice. Am. J. Respir. Crit. Care Med. 160:1150-1156. [DOI] [PubMed] [Google Scholar]
- 5.Card, J. W., M. A. Carey, J. A. Bradbury, L. M. DeGraff, D. L. Morgan, M. P. Moorman, G. P. Flake, and D. C. Zeldin. 2006. Gender differences in murine airway responsiveness and lipopolysaccharide-induced inflammation. J. Immunol. 177:621-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carey, M. A., J. W. Card, J. A. Bradbury, M. P. Moorman, N. Haykal-Coates, S. H. Gavett, J. P. Graves, V. R. Walker, G. P. Flake, J. W. Voltz, D. Zhu, E. R. Jacobs, A. Dakhama, G. L. Larsen, J. E. Loader, E. W. Gelfand, D. R. Germolec, K. S. Korach, and D. C. Zeldin. 2007. Spontaneous airway hyperresponsiveness in estrogen receptor-alpha-deficient mice. Am. J. Respir. Crit. Care Med. 175:126-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crimi, E., A. Spanevello, M. Neri, P. W. Ind, G. A. Rossi, and V. Brusasco. 1998. Dissociation between airway inflammation and airway hyperresponsiveness in allergic asthma. Am. J. Respir. Crit. Care Med. 157:4-9. [DOI] [PubMed] [Google Scholar]
- 8.Feleszko, W., J. Jaworska, R. D. Rha, S. Steinhausen, A. Avagyan, A. Jaudszus, B. Ahrens, D. A. Groneberg, U. Wahn, and E. Hamelmann. 2007. Probiotic-induced suppression of allergic sensitization and airway inflammation is associated with an increase of T regulatory-dependent mechanisms in a murine model of asthma. Clin. Exp. Allergy 37:498-505. [DOI] [PubMed] [Google Scholar]
- 9.Forsythe, P., M. D. Inman, and J. Bienenstock. 2007. Oral treatment with live Lactobacillus reuteri inhibits the allergic airway response in mice. Am. J. Respir. Crit. Care Med. 175:561-569. [DOI] [PubMed] [Google Scholar]
- 10.Friedrich, N., H. Volzke, C. Schwahn, A. Kramer, M. Junger, T. Schafer, U. John, and T. Kocher. 2006. Inverse association between periodontitis and respiratory allergies. Clin. Exp. Allergy 36:495-502. [DOI] [PubMed] [Google Scholar]
- 11.Gavett, S. H., S. L. Madison, P. C. Chulada, P. E. Scarborough, W. Qu, J. E. Boyle, H. F. Tiano, C. A. Lee, R. Langenbach, V. L. Roggli, and D. C. Zeldin. 1999. Allergic lung responses are increased in prostaglandin H synthase-deficient mice. J. Clin. Invest. 104:721-732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Genco, C. A., D. R. Kapczynski, C. W. Cutler, R. J. Arko, and R. R. Arnold. 1992. Influence of immunization on Porphyromonas gingivalis colonization and invasion in the mouse chamber model. Infect. Immun. 60:1447-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayashi, T., L. Beck, C. Rossetto, X. Gong, O. Takikawa, K. Takabayashi, D. H. Broide, D. A. Carson, and E. Raz. 2004. Inhibition of experimental asthma by indoleamine 2,3-dioxygenase. J. Clin. Invest. 114:270-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hollingsworth, J. W., G. S. Whitehead, K. L. Lin, H. Nakano, M. D. Gunn, D. A. Schwartz, and D. N. Cook. 2006. TLR4 signaling attenuates ongoing allergic inflammation. J. Immunol. 176:5856-5862. [DOI] [PubMed] [Google Scholar]
- 15.Holt, S. C., J. Ebersole, J. Felton, M. Brunsvold, and K. S. Kornman. 1988. Implantation of Bacteroides gingivalis in nonhuman primates initiates progression of periodontitis. Science 239:55-57. [DOI] [PubMed] [Google Scholar]
- 16.International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee.1998. Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. Lancet 351:1225-1232. [PubMed] [Google Scholar]
- 17.Kato, T., R. Kimizuka, and K. Okuda. 2006. Changes of immunoresponse in BALB/c mice neonatally treated with periodontopathic bacterial endotoxin. FEMS Immunol. Med. Microbiol. 47:420-424. [DOI] [PubMed] [Google Scholar]
- 18.Kirikae, T., T. Nitta, F. Kirikae, Y. Suda, S. Kusumoto, N. Qureshi, and M. Nakano. 1999. Lipopolysaccharides (LPS) of oral black-pigmented bacteria induce tumor necrosis factor production by LPS-refractory C3H/HeJ macrophages in a way different from that of Salmonella LPS. Infect. Immun. 67:1736-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kshirsagar, A. V., S. Offenbacher, K. L. Moss, S. P. Barros, and J. D. Beck. 2007. Antibodies to periodontal organisms are associated with decreased kidney function. The Dental Atherosclerosis Risk In Communities Study. Blood Purif. 25:125-132. [DOI] [PubMed] [Google Scholar]
- 20.Lalla, E., I. B. Lamster, M. A. Hofmann, L. Bucciarelli, A. P. Jerud, S. Tucker, Y. Lu, P. N. Papapanou, and A. M. Schmidt. 2003. Oral infection with a periodontal pathogen accelerates early atherosclerosis in apolipoprotein E-null mice. Arterioscler. Thromb. Vasc. Biol. 23:1405-1411. [DOI] [PubMed] [Google Scholar]
- 21.Lamont, R. J., and H. F. Jenkinson. 1998. Life below the gum line: pathogenic mechanisms of Porphyromonas gingivalis. Microbiol. Mol. Biol. Rev. 62:1244-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin, D., M. A. Smith, C. Champagne, J. Elter, J. Beck, and S. Offenbacher. 2003. Porphyromonas gingivalis infection during pregnancy increases maternal tumor necrosis factor alpha, suppresses maternal interleukin-10, and enhances fetal growth restriction and resorption in mice. Infect. Immun. 71:5156-5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin, D., M. A. Smith, J. Elter, C. Champagne, C. L. Downey, J. Beck, and S. Offenbacher. 2003. Porphyromonas gingivalis infection in pregnant mice is associated with placental dissemination, an increase in the placental Th1/Th2 cytokine ratio, and fetal growth restriction. Infect. Immun. 71:5163-5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23a.National Research Council. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, DC.
- 24.Noverr, M. C., N. R. Falkowski, R. A. McDonald, A. N. McKenzie, and G. B. Huffnagle. 2005. Development of allergic airway disease in mice following antibiotic therapy and fungal microbiota increase: role of host genetics, antigen, and interleukin-13. Infect. Immun. 73:30-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noverr, M. C., and G. B. Huffnagle. 2005. The ‘microflora hypothesis’ of allergic diseases. Clin. Exp. Allergy 35:1511-1520. [DOI] [PubMed] [Google Scholar]
- 26.Noverr, M. C., R. M. Noggle, G. B. Toews, and G. B. Huffnagle. 2004. Role of antibiotics and fungal microbiota in driving pulmonary allergic responses. Infect. Immun. 72:4996-5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poynter, M. E., R. Cloots, T. van Woerkom, K. J. Butnor, P. Vacek, D. J. Taatjes, C. G. Irvin, and Y. M. Janssen-Heininger. 2004. NF-kappa B activation in airways modulates allergic inflammation but not hyperresponsiveness. J. Immunol. 173:7003-7009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pussinen, P. J., G. Alfthan, J. Tuomilehto, S. Asikainen, and P. Jousilahti. 2004. High serum antibody levels to Porphyromonas gingivalis predict myocardial infarction. Eur. J. Cardiovasc. Prev. Rehabil. 11:408-411. [DOI] [PubMed] [Google Scholar]
- 29.Pussinen, P. J., P. Jousilahti, G. Alfthan, T. Palosuo, S. Asikainen, and V. Salomaa. 2003. Antibodies to periodontal pathogens are associated with coronary heart disease. Arterioscler. Thromb. Vasc. Biol. 23:1250-1254. [DOI] [PubMed] [Google Scholar]
- 30.Racila, D. M., and J. N. Kline. 2005. Perspectives in asthma: molecular use of microbial products in asthma prevention and treatment. J. Allergy Clin. Immunol. 116:1202-1205. [DOI] [PubMed] [Google Scholar]
- 31.Schaub, B., R. Lauener, and E. von Mutius. 2006. The many faces of the hygiene hypothesis. J. Allergy Clin. Immunol. 117:969-977. [DOI] [PubMed] [Google Scholar]
- 32.Strachan, D. P. 1989. Hay fever, hygiene, and household size. BMJ 299:1259-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thorne, P. S., K. Kulhankova, M. Yin, R. Cohn, S. J. Arbes, Jr., and D. C. Zeldin. 2005. Endotoxin exposure is a risk factor for asthma: the national survey of endotoxin in United States housing. Am. J. Respir. Crit. Care Med. 172:1371-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walters, E. H., J. A. Walters, and R. Wood-Baker. 2007. Anti-IgE and chemotherapy: a critical appraisal of treatment options for severe asthma. Expert Opin. Pharmacother. 8:585-592. [DOI] [PubMed] [Google Scholar]
- 35.Whitehead, G. S., T. Wang, L. M. DeGraff, J. W. Card, S. A. Lira, G. J. Graham, and D. N. Cook. 2007. The chemokine receptor D6 has opposing effects on allergic inflammation and airway reactivity. Am. J. Respir. Crit. Care Med. 175:243-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilder, J. A., D. D. Collie, B. S. Wilson, D. E. Bice, C. R. Lyons, and M. F. Lipscomb. 1999. Dissociation of airway hyperresponsiveness from immunoglobulin E and airway eosinophilia in a murine model of allergic asthma. Am. J. Respir. Cell Mol. Biol. 20:1326-1334. [DOI] [PubMed] [Google Scholar]
- 37.Yang, X., Y. Fan, S. Wang, X. Han, J. Yang, L. Bilenki, and L. Chen. 2002. Mycobacterial infection inhibits established allergic inflammatory responses via alteration of cytokine production and vascular cell adhesion molecule-1 expression. Immunology 105:336-343. [DOI] [PMC free article] [PubMed] [Google Scholar]