Abstract
Fibroblasts are ubiquitous cells essential to tissue homeostasis. Despite their nonphagocytic nature, fibroblasts restrain replication of intracellular bacterial pathogens such as Salmonella enterica serovar Typhimurium. The extent to which the entry route of the pathogen determines this intracellular response is unknown. Here, we analyzed S. Typhimurium invasion in fibroblasts obtained from diverse origins, including primary cultures and stable nontransformed cell lines derived from normal tissues. Features distinct to the invasion of epithelial cells were found in all fibroblasts tested. In some fibroblasts, bacteria lacking the type III secretion system encoded in the Salmonella pathogenicity island 1 displayed significant invasion rates and induced the formation of lamellipodia and filopodia at the fibroblast-bacteria contact site. Other bacterial invasion traits observed in fibroblasts were the requirement of phosphatidylinositol 3-kinase, mitogen-activated protein kinase MEK1, and both actin filaments and microtubules. RNA interference studies showed that different Rho family GTPases are targeted by S. Typhimurium to enter into distinct fibroblasts. Rac1 and Cdc42 knockdown affected invasion of normal rat kidney fibroblasts, whereas none of the GTPases tested (Rac1, Cdc42, RhoA, or RhoG) was essential for invasion of immortalized human foreskin fibroblasts. Collectively, these data reveal a marked diversity in the modes used by S. Typhimurium to enter into fibroblasts.
Salmonella enterica serovar Typhimurium (hereafter, S. Typhimurium) is an intracellular bacterial pathogen that causes pathologies ranging from gastroenteritis to systemic disease (35). Upon oral ingestion, S. Typhimurium preferentially associates to M cells located in the Peyer's patches of the distal ileum (47). Bacteria also cross the epithelial barrier via invasion of enterocytes or upon being captured by dendritic cells located in the intestinal lamina propria (77).
S. Typhimurium invasion of eukaryotic cells has been extensively characterized in cultured epithelial cells and is mediated by a specialized type III secretion system (TTSS) encoded in the Salmonella pathogenicity island 1 (SPI-1) (22). Mutants devoid of this secretion system are severely affected in the capacity to penetrate the intestinal epithelium of calves and streptomycin-treated mice, two extensively used animal models of S. Typhimurium enterocolitis (reviewed in references 34 and 83). The SPI-1 TTSS is composed of more than 30 proteins, including dedicated regulators, chaperons, structural proteins of the secretion apparatus, and secreted effector proteins (23). Delivery of SPI-1 effector proteins to the host cell cytosol triggers a profound rearrangement of the actin cytoskeleton in both epithelial cells and macrophages (24, 61, 67, 80). A consequence of these changes is the induction of membrane ruffling and macropinocytosis at the site of Salmonella-epithelial cell contact (20, 25, 38, 61). Membrane ruffling occurs subsequent to the activation of the Rho family GTPases Cdc42 and Rac1 by bacterial effector proteins that mimic eukaryotic guanine nucleotide exchange factors (GEFs), such as SopE and SopE2 (36, 74). S. Typhimurium injects into epithelial cells other SPI-1 effectors with the capacity to modulate actin dynamics, such as the phosphoinositide phosphatase SopB, also known as SigD. Based on the high affinity to lipids of pleckstrin homology (PH) domains present in most GEFs, it has been proposed that the activity of Cdc42 and Rac1 could be modulated via endogenous GEFs in response to changes in phosphoinositides promoted by SopB (84). In fact, recent data have shown that SopB/SigD stimulates SGEF, a RhoG-specific exchange factor (60). Other SPI-1 TTSS-secreted proteins contributing to membrane ruffling and bacterial uptake are SipA and SipC, which bind to actin and induce bundling of filamentous actin (39, 56, 85, 86). Once the invasion process has been completed, S. Typhimurium downregulates Cdc42 and Rac1 via another SPI-1 TTSS effector protein, SptP, a GTPase-activating protein (21). Important host factors implicated in S. Typhimurium invasion of epithelial cells include the N-WASP- and Scar/WAVE-Arp2/Arp3 (Arp2/3) complexes, which transmit the signal from active Cdc42 or Rac1 to the actin cytoskeleton (69, 80). A recent study revealed that S. Typhimurium invasion can also occur in the absence of functional N-WASP/WAVE activators (33). Other host factors linked to S. Typhimurium invasion include the SNARE protein VAMP8/endobrevin, the focal-adhesion kinase (FAK), the scaffolding protein p130Cas, and the multidomain protein IQGAP1 (8, 16, 68).
To date, several studies support the existence of various S. Typhimurium invasion routes in nonphagocytic cells. Thus, bacteria activate the Arp2/3 complex to enter into polarized and nonpolarized epithelial cells but Rac1, and not Cdc42, is targeted by the bacteria to invade the apical membrane of polarized cells (14, 15). Conversely, bacterial invasion of the basolateral membrane relies on a toxin B-sensitive GTPase distinct from Cdc42, Rac1, RhoA, and RhoG (15). Early studies performed in fibroblasts and epithelial cells also reported an apparent dispensability of the GTPases Ras, RhoA, and Rac1 for induction of membrane ruffling (49). More recently, a novel Arp2/3 complex activator named WASH was shown to promote a S. Typhimurium invasion route independently of membrane ruffling (33).
Our lab has focused in recent years on the characterization of the S. Typhimurium lifestyle inside fibroblasts (79). Evidence for S. Typhimurium infection of this host cell type in vivo has not been provided. However, fibroblasts are ubiquitous nonphagocytic cells that have a long life span in the connective tissue, which makes them attractive targets to be colonized by bacteria during infection. Indeed, cell tropism to fibroblasts has been shown to occur in some viral and protozoan latent infections (7, 70). Interestingly, S. Typhimurium wild-type bacteria do not proliferate inside cultured fibroblasts, and this response is orchestrated by bacterial functions such as the PhoP-PhoQ two-component system, the virulence plasmid-encoded regulator SpvR, and the alternative sigma factor RpoS (27, 79). Lack of any of these bacterial regulators leads to exacerbated intracellular bacterial growth (10). Considering this unique intracellular response, we reasoned that the mode of entry of S. Typhimurium into fibroblasts could differ to some extent from that of epithelial cells, in which wild-type bacteria proliferate extensively (2, 71). Using primary fibroblast cultures and cell lines derived from nontransformed fibroblasts of diverse origin, we show here that S. Typhimurium invasion of fibroblasts substantially differs from the entry routes previously described in epithelial cells. In addition, we show evidence for a marked heterogeneity of mechanisms used by S. Typhimurium to invade fibroblasts.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The S. Typhimurium wild-type virulent strain SL1344 (40) and the isogenic derivate strains SB169 (sipB::aphT), SB220 (sipC::aphT), MD173 (invG::aphT), MD1662 (invA/pRI203), MD1663 (invA/pIL14), MvP602 (ΔSPI-4::aphT), and MvP603 (ΔSPI-4::aphT ΔinvG) have been described elsewhere (28, 30, 50, 64). The pRI203 and pIL14 plasmids express the invasin protein (Inv) from Yersinia pseudotuberculosis (46) and the afimbrial adhesin AFA-I from uropathogenic Escherichia coli (53), respectively. The MD706 (ΔSPI-1::Kanr) strain, derived from SL1344, was constructed for this study using one-step inactivation with PCR products obtained from plasmid pKD4 as a template (17). The primers used were: SPI1-KOF (5′-CTA CCG CAA TCG GTA ACG CGC AAT TAT CGT CAG GTA CAG CAG GGT TAT GTG TGT AGG CTG GAG CTG CTT CG-3′) and SPI1-KOR (5′-TAT GGC CTT ATA AGG CTT GCA GTC TTT CAT GGC CAG CAA GTA ACG TCT GAT CAT ATG AAT ATC CTC CTT AG-3′). This mutant MD706 (ΔSPI-1::Kanr) lacks ∼34 kb of SPI-1 encompassing the region from the sprB to the invH gene. The E. coli strains MC1061 (18) and DH5α (laboratory stock) were used for comparison in the adherence/invasion assays. Bacteria were grown routinely in Luria broth (LB) at 37°C. When appropriate, kanamycin (30 μg/ml) or ampicillin (50 μg/ml) was added to the growth medium.
Fibroblast and epithelial cell lines.
Cell lines derived from normal tissues included NRK-49F normal rat kidney fibroblasts (ATCC CRL-1570) and 3T3-Swiss embryo mouse fibroblasts (ATCC CCL-92). Primary mouse intestinal fibroblasts (MIF) were isolated from C57BL/10 mice following the method of Strong et al., described for human intestinal fibroblasts (75). Human telomerase reverse transcriptase (hTERT)-immortalized BJ-5ta fibroblasts (ATCC CRL-4001), derived from BJ normal human foreskin primary fibroblasts (ATCC CRL-2522), were also used. HeLa (ATCC CCL-2) and Henle-407 (ATCC CCL-6) epithelial cells were used in invasion assays for comparison. NRK-49F fibroblasts were propagated in Dulbecco's modified Eagle's medium (DMEM) containing 5% (vol/vol) fetal bovine serum (FBS) and 4 mM l-glutamine. DMEM-10% FBS was used to propagate 3T3-Swiss fibroblasts and Henle-407 epithelial cells. Primary MIF were grown in DMEM containing 10% FBS, 4 mM l-glutamine, and 2.5% (vol/vol) HEPES. Minimum essential medium Eagle (MEM) containing 10% FBS was used to grow HeLa cells. BJ-5ta fibroblasts were propagated in a 4:1 ratio of DMEM to medium 199 containing 10% FBS, 1 mM sodium pyruvate, and 4 mM l-glutamine.
Bacterial infection assays.
Bacteria were grown for 18 h overnight without aeration (without shaking) in LB medium at 37°C. Fibroblasts and epithelial cells were seeded in 24-well plates to reach a density of ∼5 × 104 to 8 × 104 cells/well at the time of infection. Unless otherwise indicated, bacteria were used at a multiplicity of infection (MOI) of 10:1 (bacteria to eukaryotic cell) and incubated with cultured cells for 20 min. After extensive washing with Hank's buffered saline solution (HBSS), cell-associated bacteria were enumerated upon lysis of the fibroblasts in a solution containing phosphate-buffered saline (PBS), pH 7.4, 1% (vol/vol) Triton X-100, and 0.1% (wt/vol) sodium dodecyl sulfate (SDS). To determine invasion rates, the cells were infected as above and washed repeatedly with a prewarmed HBSS. Infected cells were then incubated in fresh culture medium containing 100 μg/ml gentamicin until 2 h postinfection. At this time, the culture cells were lysed as above in a PBS (pH 7.4) solution containing 1% Triton X-100 and 0.1% SDS. The number of viable intracellular bacteria was determined by plating as previously described (10).
Invasion inhibition assays.
The requirement of specific host functions for S. Typhimurium entry into fibroblasts or epithelial cells was assessed with inhibitors at the following concentrations: (i) 25 μM LY294002 (CalBiochem, Darmstadt, Germany) or 100 nM wortmannin (Sigma, St. Louis, MO) to block phosphatidylinositol-3-kinase (PI3K) and (ii) 50 μM PD98059 (CalBiochem, Darmstadt, Germany) to block MEK1 kinase. These inhibitors were added for 2 h (LY294002 and wortmannin) or 1 h (PD98059) prior to the infection. Filamentous actin was disrupted by incubating the fibroblasts with 1 μg/ml cytochalasin-D (Sigma, St. Louis, MO) for 20 min prior to bacterial infection. Microtubules were disrupted by treating the cells with 1 to 20 μg/ml nocodazole (Sigma, St. Louis, MO) for 1 h before bacterial infection. The effect on invasiveness was determined by counting viable intracellular bacteria as described above.
Gene silencing by RNAi.
Silencing gene expression of Rho family GTPases Rac1, Cdc42, RhoA, and RhoG was achieved using synthetic SMART pools (Dharmacon, Inc., Chicago, IL), each comprising four proprietary small interfering RNA (siRNA) sequences. siRNAs were transfected into NRK-49F rat kidney fibroblasts, immortalized BJ-5ta human foreskin fibroblasts, and Henle-407 human epithelial cells using HiPerfect Transfection Reagent (Qiagen GmbH, Hilden, Germany). The silencing efficiencies of these RNA interference (RNAi) constructs for their targeted mRNAs were tested by quantitative reverse transcription-PCR (RT-PCR) at 48 and 72 h after transfection on total RNA template isolated using an SV Total RNA Isolation System kit (Promega, Madison, WI). RNA samples were used to generate cDNA by means of a High-Capacity cDNA Archive kit (Applied Biosystems, Foster City, CA) according to the manufacturer's protocol. Quantitative RT-PCR was performed using a real-time detection system with Power SYBR green PCR Master Mix (Applied Biosystems, Foster City, CA) and specific primers for rat (r-) or human (h-) Rho family GTPases: r-Cdc42 (5′-TTC CCG TCG GAG TAT GTA CC-3′ and 5′-CAG GCA CCC ACT TTT CTT TC-3′), r-Rac1 (5′-AGT TAC ACG ACC AAT GCG TTC-3′ and 5′-AAT GAT GCA GGA CTC ACA AGG-3′), r-RhoA (5′-TGT GGC AGA TAT TGA AGT GGA C-3′ and 5′-CTT CTG GAG TCC ATT TTT CTG G-3′), r-RhoG (5′-ACA ACT AAT GCC TTC CCC AAG-3′ and 5′-AAC AGA TGA CGA AGA CGT TGG-3′), h-Cdc42(5′-CCT TTC TTG CTT GTT GGG ACT C-3′ and 5′-CTC CAC ATA CTT GAC AGC CTT C-3′), h-Rac1(5′-GTC CCA ACA CTC CCA TCA TCC-3′ and 5′-ACA GCA CCA ATC TCC TTA GCC-3′), h-RhoA (5′-GCA GGT AGA GTT GGC TTT GTG-3′ and 5′-GAC TTC TGG GGT CCA CTT TTC-3′), and h-RhoG (5′-TGC CTG CTC ATC TGC TAC AC-3′ and 5′-ACG AAA ACG TTG GTC TGA GG-3′). mRNA levels were normalized to GTPase mRNA in untransfected cells. RNAi knockdown efficiency and specificity were also assessed at the protein level by Western assays of cell lysates prepared at 48 and 72 h posttransfection. GTPase protein levels were determined with the mouse monoclonal antibodies anti-Cdc42 (clone 44; BD Transduction Laboratories), anti-Rac1 (clone 23A8; Upstate Biotechnology), and anti-RhoA (clone 26C4; Santa Cruz Biotechnology, Inc.). Rabbit polyclonal antibody anti-RhoG (Santa Cruz Biotechnology, Inc.) was also used. Protein depletion by RNAi was normalized to endogenous levels of tubulin using mouse monoclonal anti-α-tubulin antibody (clone DM1A; Sigma, St. Louis, MO).
Immunofluorescence microscopy.
Cell monolayers were fixed in 3% (wt/vol) paraformaldehyde, pH 7.4, for 10 min at 37°C. After cells were washed in PBS (pH 7.4), they were permeabilized in a solution containing 0.1% (wt/vol) saponin (Sigma, St. Louis, MO) and 1% (vol/vol) goat serum (Invitrogen, Carlsbad, CA). The immunostaining was performed as previously described (26). Primary antibodies and dilutions used included the following: polyclonal rabbit anti-Salmonella lipopolysaccharide (LPS), 1:2,000 (Difco, Detroit, MI); monoclonal mouse anti-Salmonella LPS, 1:200 (clone MLK33; gift of J. M. Slauch, University of Illinois, Urbana-Champaign, IL); and polyclonal rabbit anti-E. coli, 1:200 (gift of M. Vicente, CNB, Madrid, Spain). Secondary goat antibodies anti-rabbit or anti-mouse conjugated to either Alexa-Fluor 594 or Alexa-Fluor 488 (Molecular Probes, Eugene, OR) were used at a dilution of 1:1,000. Cy5-conjugated secondary antibody anti-rabbit (Jackson ImmunoResearch Lab., West Grove, PA) was used at a dilution of 1:500. For differential staining of inside versus outside bacteria (in-out staining), cells were fixed as above and stained as previously described (11). Briefly, extracellular bacteria were stained in nonpermeabilized cells with either polyclonal rabbit anti-Salmonella LPS or anti-E. coli antibodies, followed by anti-rabbit Cy5-conjugated secondary antibody. Upon permeabilization, intracellular bacteria were stained with either monoclonal mouse anti-Salmonella LPS antibody or rabbit polyclonal anti-E. coli antibody, followed by anti-mouse or anti-rabbit Alexa-Fluor 594 as secondary antibodies. Filamentous actin was stained with 1 μg/ml phalloidin conjugated to Alexa-Fluor 488 (Molecular Probes, Eugene, OR). In other experiments, 1 μg/ml phalloidin conjugated to Alexa-Fluor 594 (Molecular Probes, Eugene, OR) was also used. When required, nuclei were stained with 0.5 μg/ml DAPI (4′-6′-diamidino-2-phenylindole) for 5 min. Cells were examined using a Bio-Rad 2100 Radiance System attached to a Zeiss Axiovert 200 microscope.
Scanning electron microscopy.
Coverslips containing infected cell monolayers were fixed for 4 h at 4°C in a solution containing 2.5% (wt/vol) glutaraldehyde and 200 mM HEPES, pH 7.2, and further rinsed in 200 mM HEPES, pH 7.2, buffer. The samples were then dehydrated with a graded series of acetone and subjected to critical-point drying with CO2. The samples were further coated with gold and graphite and examined in a JEOL JM-6400 scanning electron microscope at a voltage of 40 Kv.
Statistical analysis.
Data were analyzed with GraphPad Prism, version 5.0, software (GraphPad Inc., San Diego, CA) using one-way analysis of variance (ANOVA) with a Tukey's posttest or Student's t test. A P value lower than 0.05 was considered significant.
RESULTS
S. Typhimurium entry into NRK-49F cells occurs by two alternative routes.
S. Typhimurium invades epithelial cells using a specialized type III secretion system (TTSS) encoded in the Salmonella pathogenicity island 1 (SPI-1) (22). To determine the requirement of SPI-1 TTSS in fibroblast invasion, normal rat kidney fibroblasts (NRK-49F) were infected with the S. Typhimurium wild-type strain SL1344 and isogenic mutants defective in SipB or SipC, two SPI-1-encoded invasion proteins. sipB and sipC mutants displayed a significant capacity to invade NRK-49F fibroblasts (∼30% of the rate of the wild-type strain) that was much higher than that observed in HeLa epithelial cells (∼1% of the rate of the wild-type strain) (Fig. 1A). This phenotype was confirmed with an isogenic mutant lacking the entire SPI-1 pathogenicity island, MD706 (ΔSPI-1::Kanr), which displayed an invasion rate of ∼18% compared to the wild-type parental strain (Fig. 1A). The entry exhibited by the motile nonpathogenic E. coli strain MC1061 was found to be 10-fold lower than that of the ΔSPI-1 mutant (Fig. 1A). The significant capacity of the ΔSPI-1 mutant to invade NRK-49F fibroblasts was also confirmed in other S. Typhimurium genetic backgrounds such as strains 14028s and LT2 (data not shown).
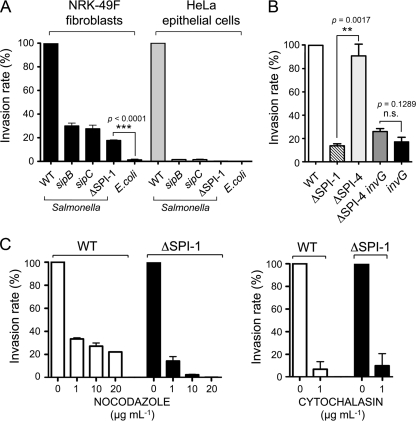
FIG. 1.
Salmonella invasion of NRK-49F fibroblasts show features distinct to the invasion of epithelial cells. (A) Invasion rates estimated by gentamicin survival assays and made relative to the rate of the wild-type bacteria. Incubation time with bacteria was 20 min. Viable intracellular bacteria were enumerated at 2 h postinfection and accounted, in the case of wild-type (WT) bacteria, for 1.04% and 3.60% of the inoculum in NRK-49F fibroblasts and HeLa epithelial cells, respectively. The S. Typhimurium strains used were SL1344 (wild-type), SB169 (sipB), SB220 (sipC), and MD706 (ΔSPI-1). The E. coli motile strain MC1061 was included for comparison. (B) Dispensability of the type I secretion system encoded by SPI-4 for bacterial entry into NRK-49F fibroblasts. The S. Typhimurium strains used included MD173 (invG), MvP602 (ΔSPI-4), and MvP603 (ΔSPI-4 invG). (C) Inhibitory effect of nocodazole and cytochalasin on the invasion of NRK-49F fibroblasts by S. Typhimurium wild-type and ΔSPI-1 strains. The invasion rate of the ΔSPI-1 strain (16% of that of wild-type strain) was normalized to 100% to highlight the differences in the drug-treated fibroblasts. Data are the means and standard deviations from three independent experiments.
S. Typhimurium adhesion and penetration of polarized epithelial cell barriers is promoted by the concerted action of the coregulated type I and type III secretion systems encoded by SPI-4 and SPI-1 (29, 51, 54, 58). To test whether SPI-4 was involved in bacterial entry into fibroblasts, we used ΔSPI-4 and ΔSPI-4 invG isogenic strains, obtained from M. Hensel. InvG is an outer membrane protein absolutely essential for the correct assembly of the SPI-1 TTSS apparatus, and invG mutants are defective in type III secretion (23). Invasion assays in NRK-49F fibroblasts ruled out a contribution of SPI-4 in this infection model (Fig. 1B). Lack of SPI-4 also had no effect on the entry rate of the invG mutant (Fig. 1B). We also asked whether flagella, which facilitate invasiveness in tissue culture cells, activate innate immune responses, and promote antiapoptotic processes in epithelial cells (81), were essential for bacterial entry into NRK-49F fibroblasts. Invasion assays performed with flagellin-defective mutants in both wild-type and ΔSPI-1 backgrounds eliminated such a possibility (data not shown). Based on these observations, we next aimed to dissect the contribution of cytoskeletal proteins in the invasion of fibroblasts. This was assessed with microtubule- or actin-destabilizing drugs such as nocodazole or cytochalasin-D. Both drugs significantly reduced the entry of wild-type and ΔSPI-1 bacteria into NRK-49F fibroblasts (Fig. 1C). The effect of nocodazole was found to be more pronounced in the case of the ΔSPI-1 mutant (Fig. 1C). We next examined by microscopy the morphological alterations occurring in NRK-49F fibroblasts during bacterial uptake. Prominent membrane ruffling, macropinocytosis, and massive actin rearrangement were phenomena observed exclusively during invasion of wild-type bacteria (Fig. 2A). Unexpectedly, invading ΔSPI-1 bacteria were found associated to lamellipodium-like structures at the bacteria-host cell contact site (Fig. 2B). Although discrete accumulations of actin were visualized surrounding motile E. coli bacteria ingested by the fibroblasts, no lamellipodia were observed in this case (Fig. 2B). Scanning electron microscopy confirmed the ability of the S. Typhimurium ΔSPI-1 mutant to trigger the formation of lamellipodia and filopodia in the fibroblast-bacteria contact area (Fig. 2C). Taken together, these data demonstrate that entry of S. Typhimurium into NRK-49F fibroblasts proceeds by two distinctive routes that differ in the involvement of the SPI-1 TTSS system, the requirement of microtubules, and the extent to which the host cell membrane is remodeled during the bacterial uptake process.
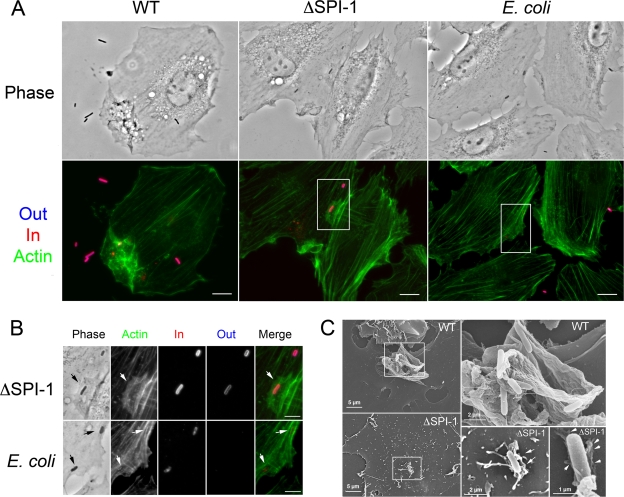
FIG. 2.
S. Typhimurium entry into NRK-49F fibroblasts occurs via two modes that differ in the extent of actin rearrangement and macropinocytosis. Extracellular (blue) and intracellular (red) bacteria were differentiated by the in-out staining procedure (see Materials and Methods). Actin was labeled with fluorescein isothiocyanate-phalloidin (green). Entry of S. Typhimurium wild-type (WT) and ΔSPI-1 bacteria was compared to that of the E. coli MC1061 motile strain. (A) Low-magnification images showing prominent membrane ruffling and macropinocytosis induced by S. Typhimurium wild-type bacteria in NRK-49F fibroblasts. Note that, unlike the few E. coli MC1061 cells observed associated to the fibroblasts, some of the S. Typhimurium ΔSPI-1 bacteria were associated to lamellipodium-like membrane extensions. Bar, 10 μm. (B) Magnifications of the areas marked in panel A. Arrows indicate a large and actin-rich lamellipodium contacting a surface-located ΔSPI-1 bacterium and discrete actin accumulations surrounding two E. coli MC1061 bacteria located intracellularly. Bar, 5 μm. (C) Scanning electron microscopy images of NRK-49F fibroblasts infected for 20 min with S. Typhimurium wild-type or ΔSPI-1 bacteria. Note the prominent membrane ruffling in the area where wild-type bacteria locate. Filopodia and lamellipodia were observed associated to ΔSPI-1 bacteria located onto the fibroblast surfaces. The ΔSPI-1 mutant was also occasionally visualized retracting the fibroblast surface underneath invading bacteria (arrows).
S. Typhimurium entry into fibroblasts derived from others sources.
To define whether the mode of entry displayed by the S. Typhimurium ΔSPI-1 mutant in NRK-49F fibroblasts was a generalized phenomenon, the behavior of this strain was examined in fibroblasts obtained from other sources. Fibroblasts obtained from normal tissues such as the 3T3-Swiss mouse embryo fibroblasts and primary fibroblasts obtained from mouse intestine (MIF) were used. Adhesion and invasion rates among the different bacteria and fibroblasts used were determined by counting both viable intracellular bacteria and cell-associated bacteria. In addition to the E. coli motile strain MC1061, a second E. coli strain displaying poor motility, DH5α, was also included to assess whether these latter bacteria could be engulfed to some extent by the fibroblasts. As noticed in pilot experiments, the most optimal infection conditions in these fibroblasts were found to be 30 min for S. Typhimurium wild-type bacteria and 60 min for the S. Typhimurium ΔSPI-1 mutant and E. coli strains. In contrast to NRK-49F fibroblasts, the invasion rate displayed by the S. Typhimurium ΔSPI-1 mutant in 3T3-Swiss fibroblasts was not different from that shown by the E. coli motile strain MC1061 (Fig. 3A). In MIF the ΔSP1 mutant exhibited a higher invasion rate than E. coli MC1061 although the difference was not statistically significant (Fig. 3A). On the other hand, the entry of ΔSPI-1 mutant bacteria into 3T3-Swiss fibroblasts and MIF was at a significantly higher rate than that of E. coli DH5α, which was negligible (Fig. 3A). Indeed, in most cases no viable intracellular E. coli DH5α was recovered from the fibroblasts. Interestingly, the adherence levels of S. Typhimurium ΔSPI-1 and the motile E. coli MC1061 strain were found to be lower (40 to 60%) than adherence shown by wild-type bacteria in 3T3-Swiss fibroblasts and MIF (Fig. 3B). Because such a difference was not evident in NRK-49F fibroblasts (Fig. 3B), the diminished adherence to the fibroblasts could be a factor contributing to the decreased invasiveness of the ΔSPI-1 mutant observed in these other fibroblasts. Taken together, these data show that the S. Typhimurium SPI-1 TTSS-independent invasion route is not a generalized phenomenon and that it occurs in fibroblasts obtained from only certain sources.
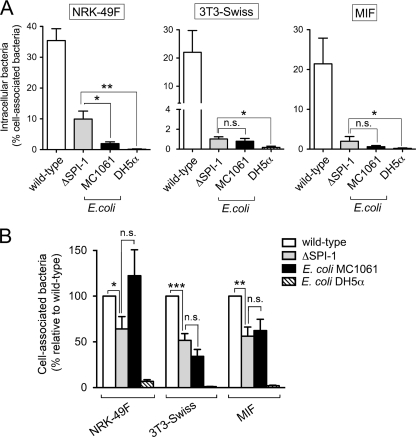
FIG. 3.
Requirement of the TTSS encoded by SPI-1 for entry into diverse fibroblast cells. The cell lines used included NRK-49F, 3T3-Swiss fibroblasts, and MIF. (A) Invasion rates of S. Typhimurium SL1344 (wild type) and MD706 (ΔSPI-1) and the E. coli strains MC1061 (motile) and DH5α (nonmotile) expressed as the ratio of intracellular bacteria versus total number of fibroblast-associated bacteria counted before gentamicin treatment. Using this parameter, the invasiveness of the ΔSPI-1 mutant relative to the wild type was 28% (NRK-49F), 4.6% (3T3-Swiss fibroblasts), and 9.2% (MIF). (B) Bacterial adhesion to the different fibroblast cell lines used. The number of cell-associated bacteria was made relative to that of wild-type strain in each of the fibroblast cell lines used. Data are the means and standard deviations from three independent experiments. *, P = 0.01 to 0.05; **, P = 0.001 to 0.01; n.s., not significant by a Student t test (A) and one-way ANOVA with Tukey's posttest (B).
S. Typhimurium entry into telomerase-immortalized human foreskin fibroblasts.
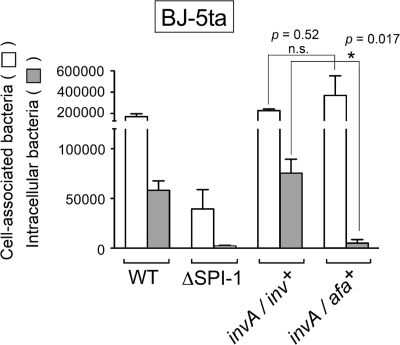
Considering the variety of results obtained in nontransformed fibroblast cell lines derived from normal tissues and primary fibroblasts (Fig. 3), we decided to optimize a new fibroblast infection model. The purpose was to bypass the disadvantages linked to the use of normal, nontransformed cell lines, stabilized by undefined mutational processes to overcome cellular aging, or of primary cultures displaying limited passage capability. To this aim, we used human telomerase reverse transcriptase (hTERT)-immortalized BJ-5ta cells derived from normal human foreskin fibroblasts (73). The expression of hTERT in normal cells extends life span while maintaining a normal, nontransformed phenotype (6, 48). Interestingly, microscopy analysis of human foreskin BJ-5ta fibroblasts infected with S. Typhimurium wild-type bacteria revealed that actin rearrangement in the bacteria-fibroblast contact area was not as pronounced as in NRK-49F normal rat kidney fibroblasts (Fig. 4 A and B). This discrete accumulation of actin in the bacteria-fibroblast contact area was also common in MIF (data not shown). Macropinocytosis was another phenomenon rarely observed in BJ-5ta fibroblasts or MIF infected with wild-type bacteria (data not shown). The invasion rates of S. Typhimurium wild-type and ΔSPI-1 strains in the immortalized human foreskin BJ-5ta fibroblasts, determined as the ratio of intracellular versus cell-associated bacteria, were 37% and 6%, respectively (Fig. 4C). For comparison, the rates calculated for the E. coli strains MC1061 and DH5α were 0.45 and 0.035%, respectively (Fig. 4C). Interestingly, the invasion rate displayed by the ΔSPI-1 mutant in the immortalized BJ-5ta fibroblasts was higher than the rates in 3T3-Swiss fibroblasts and MIF (Fig. 3A). Strikingly, the difference in invasiveness of BJ-5ta fibroblasts found between the ΔSPI-1 and the motile E. coli MC1061 (6% and 0.45%, respectively) correlated with a higher adhesion rate of the E. coli MC1061 strain (Fig. 4C). This unexpected result suggested that, at least in the immortalized BJ-5ta fibroblasts, bacterial adherence may not directly influence the efficiency of the entry process. To test this assumption, we evaluated in the immortalized BJ-5ta fibroblasts the entry rate of S. Typhimurium invA isogenic strains expressing the invasin protein from Y. pseudotuberculosis (46) or the afimbrial adhesin AFA-I from pathogenic E. coli (53). Since InvA is an essential structural protein of the S. Typhimurium SPI-1-encoded type III secretion system (23), invasion should occur via the SPI-1-independent route and, in addition, by ligand-receptor interactions following the expression of the Inv protein of Y. pseudotuberculosis. Only if bacterial adherence positively influences entry into these fibroblasts should an increase in bacterial invasion also be detected in the invA strain expressing the AFA-I adhesin. Despite the notable increase in adherence promoted by the AFA-I adhesin (Fig. 5), the invasion rate increased only upon expression of the invasin of Y. pseudotuberculosis (Fig. 5). Collectively, these data reveal that, as observed in NRK-49F fibroblasts, S. Typhimurium invades the immortalized human foreskin BJ-5ta fibroblasts by SPI-1 TTSS-dependent and -independent routes. The data obtained from these immortalized fibroblasts also permit the mechanistic separation of the adhesion and invasion processes.
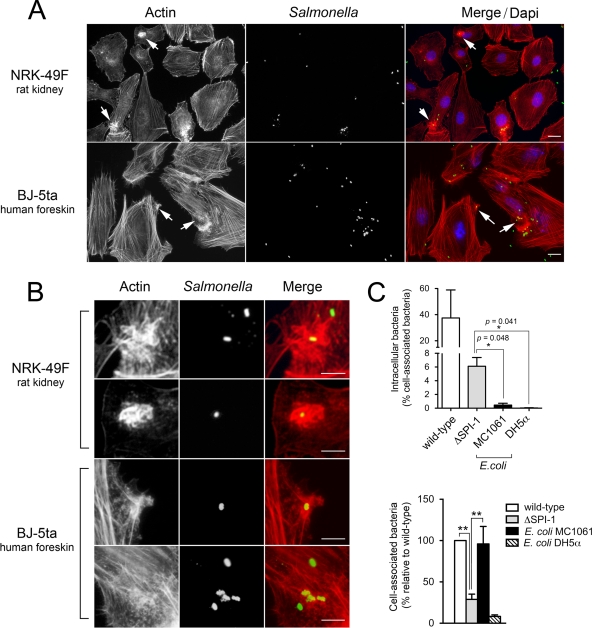
FIG. 4.
The extent of membrane ruffling induced by S. Typhimurium in fibroblasts varies depending on the source of fibroblasts used. (A) Actin distribution in NRK-49F fibroblasts and BJ-5ta fibroblasts infected with the S. Typhimurium SL1344 (wild-type) strain for 20 min. Note that the ruffles are more prominent in the infected NRK-49F fibroblasts. Actin was labeled with Alexa-Fluor 594-phalloidin (red), bacteria was labeled with primary anti-rabbit Salmonella followed by secondary Alexa-Fluor 488-conjugated goat anti-rabbit antibody (green), and the nuclei were labeled with DAPI (blue). Bar, 10 μm. (B) Enlargement of areas marked with arrows in panel A. Bar, 5 μm. (C) Invasion rates and adherence properties of the S. Typhimurium ΔSPI-1 mutant and the E. coli strains MC1061 and DH5α in the BJ-5ta fibroblasts. Data are the means and standard deviations from three independent experiments. *, P = 0.01 to 0.05; **, P = 0.001 to 0.01; n.s., not significant (Student t test).
FIG. 5.
Increased adherence to BJ-5ta fibroblasts mediated by the afimbrial adhesin AFA-I is not followed by a higher invasion rate in the SPI-1-independent entry mode. Shown are absolute numbers of fibroblast-associated bacteria counted before gentamicin treatment and those of intracellular bacteria surviving gentamicin treatment. Infection was for 20 min (wild type) or 60 min (all other strains). The S. Typhimurium strains used included SL1344 (WT), MD706 (ΔSPI-1), MD1662 (invA/inv+) and MD1663 (invA/afa+). Note that the expression of the invasin protein (Inv) from Y. pseudotuberculosis increases bacterial entry while the enhanced adherence promoted by the afimbrial adhesin AFA-I (compared to the ΔSPI-1 strain) does not. Data are the means and standard deviations from three independent experiments. *, P = 0.01 to 0.05; n.s., not significant (Student t test).
PI3K and the MAP kinase MEK1 are required for efficient bacterial entry into fibroblasts.
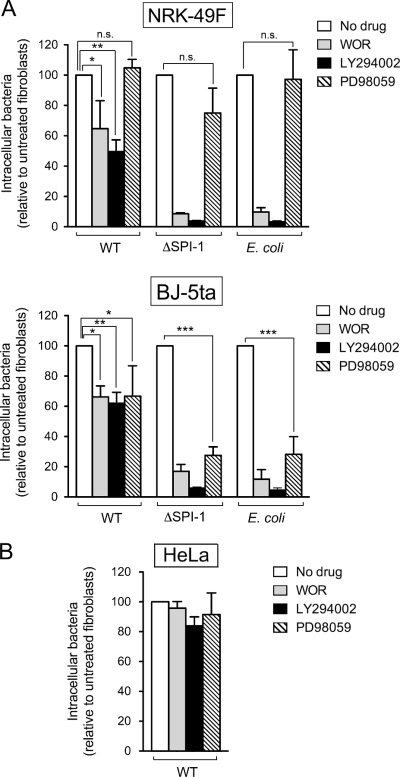
Phosphatidylinositol 3-kinase (PI3K) and mitogen-activated protein (MAP) kinase MEK1 are host functions targeted by many bacterial pathogens that invade nonphagocytic cells by the “zipper” mechanism. In this entry mode, host cell membrane surface receptors are activated independently of type III secretion systems (13, 63). In agreement with that, S. Typhimurium invasion of epithelial cells is known to be insensitive to PI3K or MEK1 kinase inhibitors (57, 65, 72, 76, 78). Based on the results obtained in NRK-49F and BJ-5ta fibroblasts, which are consistent with the existence of two alternative routes and different contributions of the SPI-1 TTSS system, we aimed to analyze whether PI3K and MEK1 were kinases required for bacterial entry into these fibroblasts. Two PI3K inhibitors, wortmannin and LY294002, were used together with PD90589, a MEK1 inhibitor. PI3K inhibition caused a significant reduction in the invasion of NRK-49F fibroblasts by both wild-type and ΔSPI-1 strains while no obvious effect was observed upon MEK1 inhibition (Fig. 6A). When inhibitors were tested in the BJ-5ta immortalized foreskin human fibroblasts, inhibition of both PI3K and MEK1 reduced the invasiveness of S. Typhimurium wild-type and ΔSPI-1 strains (Fig. 6A). Interestingly, these drugs also affected the uptake of the E. coli motile strain MC1061 to an extent similar to that observed for the S. Typhimurium ΔSPI-1 mutant (Fig. 6A). This fact may reflect some similarities in the mechanisms that operate during entry of the ΔSPI-1 mutant and uptake of the E. coli MC1061 strain. Control experiments performed in HeLa epithelial cells confirmed that neither PI3K nor MEK1 is required for entry of S. Typhimurium wild-type bacteria (Fig. 6B). No comparative invasion assay with ΔSPI-1 or E. coli MC1061 strains was possible due to the low number of gentamicin-protected bacteria recovered from untreated HeLa epithelial cells (data not shown). Altogether, these data demonstrate that PI3K is a kinase targeted by S. Typhimurium to trigger uptake by fibroblasts. In the case of MEK1, this kinase seems to play a relevant role in the SPI-1-independent entry mode and only in certain fibroblasts.
FIG. 6.
PI3K and the MAP kinase MEK1 are involved to different extents in the entry into fibroblasts of S. Typhimurium SL1344 (wild-type) and MD706 (ΔSPI-1) strains and the E. coli motile strain MC1061. The role of these two kinases was assessed in invasion assays using 100 nM wortmannin (WOR) and 25 μM LY294002 (PI3K inhibitors) or 50 μM PD98059 (MEK1 inhibitor). (A) Effect of the drugs on bacterial entry into NRK-49F fibroblasts and BJ-5ta fibroblasts. (B) Control assay performed in HeLa human epithelial cells. Note the dispensability of these two kinases in HeLa epithelial cells and their differential requirement for bacterial entry into fibroblasts depending on the fibroblast and the bacterial strain used. Data are the means and the standard deviations of three independent experiments. *, P = 0.01 to 0.05; **, P = 0.001 to 0.01; ***, P < 0.001; n.s., not significant (Student t test).
Role of Rho family GTPases in S. Typhimurium entry into fibroblasts.
S. Typhimurium targets preferentially the Rho family GTPases Rac1 and Cdc42 to trigger the cytoskeletal remodeling required for uptake by epithelial cells (11, 36, 61, 74). Cdc42 is also exploited by S. Typhimurium to induce nuclear responses such as the induction of the proinflammatory cytokine interleukin-8 (IL-8) (60). Interestingly, recent RNA interference (RNAi) studies have shown that membrane ruffling and bacterial internalization into epithelial cells could depend on the GTPases Rac1 and RhoG, with Cdc42 dispensable for these events (60). To get insights on the GTPases targeted by S. Typhimurium for entry into fibroblasts, we applied RNAi in cells responding differently to bacterial infection at the level of membrane ruffling and macropinocytosis, such as the NRK-49F and the immortalized BJ-5ta fibroblasts (Fig. 4). GTPases targeted by this RNAi treatment included Rac1, Cdc42, RhoA, and RhoG. The level and specificity of the interference were assessed by quantitative RT-PCR (data not shown) and Western analyses (see below). Both S. Typhimurium wild-type and ΔSPI-1 strains were used in the different assays.
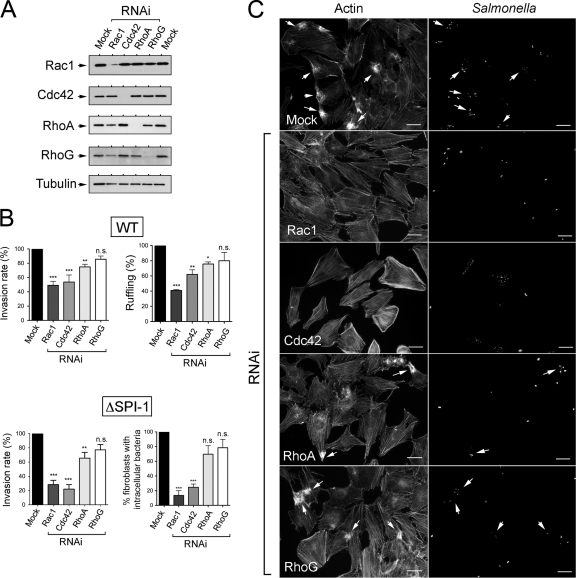
Interference of Rac1 and Cdc42 was accompanied by a significant decrease, ∼50%, in the invasion rate of wild-type bacteria in NRK-49F fibroblasts (Fig. 7A and B). Of note, this effect was observed even under conditions in which knockdown of Rac1 was not totally efficient (Fig. 7A). A slightly lower, although still statistically significant, effect on wild-type bacteria invasion was also observed upon depletion of the GTPase RhoA (Fig. 7A and B). In contrast, no appreciable effect on bacterial internalization was evident in RhoG-depleted NRK-49F fibroblasts. A similar requirement for the GTPases Rac1 and Cdc42 and, to lesser extent, RhoA was observed for entry of the ΔSPI-1 mutant (Fig. 7A and B). Taken together, these data indicate that S. Typhimurium preferentially engages Rac1 and Cdc42 to promote entry into NRK-49F fibroblasts by the SPI-1 TTSS-dependent or -independent invasion routes. Microscopy analyses supported the RNAi data. Thus, knockdown of either Rac1 or Cdc42 abrogated the capacity of wild-type bacteria to induce membrane ruffling in NRK-49F fibroblasts (Fig. 7C), with only a partial defect in membrane ruffling observed in RhoA-depleted fibroblasts and no changes observed upon depletion of the RhoG GTPase (Fig. 7C).
FIG. 7.
Requirement of Rho family GTPases for entry of S. Typhimurium SL1344 (wild type) and MD706 (ΔSPI-1) strains into NRK-49F fibroblasts. (A) Levels of Rac1, Cdc42, RhoA, and RhoG upon incubation for 72 h in the presence of their respective RNAi. The level of α-tubulin was determined as a control. (B) Invasion rate of wild-type (WT) and ΔSPI-1 bacteria in RNAi-treated NRK-49F fibroblasts. Incubation time with bacteria was of 20 min (wild type) and 40 min (ΔSPI-1). Viable intracellular bacteria were counted at 2 h postinfection. Shown are the percentages of RNAi-treated NRK-49F fibroblasts either exhibiting membrane ruffling in response to the infection with wild-type bacteria or containing intracellular bacteria upon infection with the ΔSPI-1 mutant. The analysis in the ΔSPI-1 mutant was performed with in-out staining. Data are the means and the standard deviations of three independent experiments. *, P = 0.01 to 0.05; **, P = 0.001 to 0.01; ***, P < 0.001; n.s., not significant (one-way ANOVA with Tukey's posttest). (C) Microscopy analysis showing the capacity of wild-type bacteria to induce membrane ruffling in NRK-49F fibroblasts depleted of RhoA or RhoG. Bar, 10 μm.
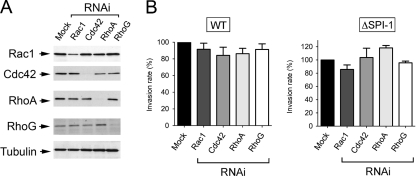
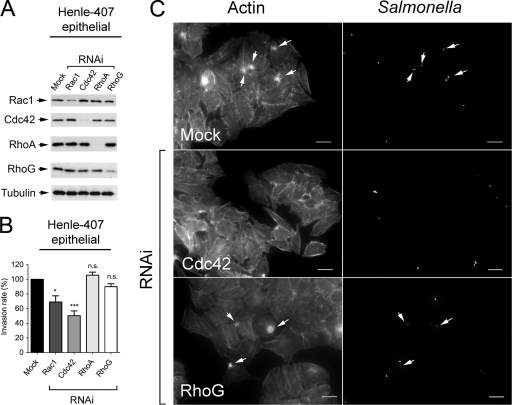
Unexpectedly, depletion of Rac1, Cdc42, RhoA, or RhoG in the BJ-5ta human foreskin fibroblasts caused no major effect on internalization of either wild-type or ΔSPI-1 strains (Fig. 8). Since, as in NRK-49F rat fibroblasts, Rac1 knockdown was not very efficient in BJ-5ta fibroblasts (Fig. 8A), it is not possible to exclude a putative contribution of this particular GTPase in bacterial entry. This “generalized” lack of effect contrasted, however, with the data obtained upon treatment of Henle-407 human epithelial cells with the same set of specific RNAi for human GTPases. In contrast to a recent study with the same epithelial cell line (60), our RNAi data indicated that S. Typhimurium could target Cdc42 to promote entry (Fig. 9 A and B). Recall that a similar level of Rac1 interference obtained in BJ-5ta human fibroblasts and Henle-407 human epithelial cells had different consequences in bacterial invasion in these two cell lines, with a negative effect observed only in the case of epithelial cells (compare Fig. 8 and 9). Taken together, these data argue for a variety of entry mechanisms that can be differentiated not only between fibroblast and epithelial cells but also among fibroblasts of distinct origins.
FIG. 8.
RNAi interference directed at Rac1, Cdc42, RhoA, or RhoG does not substantially affect the invasion of BJ-5ta fibroblasts by S. Typhimurium SL1344 (wild type) or MD706 (ΔSPI-1) strains. (A) Levels of Rac1, Cdc42, RhoA, and RhoG upon incubation for 72 h in the presence of their respective RNAi. The level of α-tubulin was determined as a control. (B) Invasion rate of wild-type (WT) and ΔSPI-1 bacteria in RNAi-treated BJ-5ta fibroblasts. Incubation times with bacteria were 20 min (wild type) and 60 min (ΔSPI-1). Viable intracellular bacteria were counted at 2 h postinfection. Data were analyzed by one-way ANOVA with Tukey's posttest. Shown are the means and the standard deviations of three independent experiments. No significant differences were found.
FIG. 9.
Rac1 and Cdc42 are targeted by S. Typhimurium SL1344 (wild type) to invade Henle-407 human epithelial cells. (A) Levels of Rac1, Cdc42, RhoA, and RhoG upon incubation for 72 h in the presence of their respective RNAi. The level of α-tubulin was determined as a control. (B) Invasion rate of wild-type bacteria in RNAi-treated Henle-407 human epithelial cells. Incubation time with bacteria was 20 min, and viable intracellular bacteria were counted at 2 h postinfection. Data are the means and the standard deviations of three independent experiments. *, P = 0.01 to 0.05; ***, P < 0.001; n.s., not significant (one-way ANOVA with Tukey's posttest). Note that although Rac1 and RhoG are not totally depleted by the RNAi treatment, a significant effect on invasion is observed in the case of only Rac1. (C) Representative microscopy images depicting the involvement of Cdc42 in the membrane ruffling triggered by wild-type bacteria in these epithelial cells. Bar, 10 μm.
DISCUSSION
Salmonella invasion of eukaryotic cells has been extensively studied in epithelial cells and macrophages, with relatively few analyses in other cell types. Prominent membrane ruffling and macropinocytosis triggered by SPI-1 effectors occur in most cases (61, 62), but exceptions to this association of events are also known. Thus, a mutant lacking the SPI-1 effectors SopE and SopE2 was shown to invade fibroblast-like COS-7 cells in the absence of membrane ruffling (74). A very recent report by Hanisch et al. (33) has also demonstrated that activation of the Arp2/3 complex by S. Typhimurium and bacterial uptake can occur in fibroblasts devoid of functional WASP and WAVE proteins, which are central components linking Cdc42 and Rac1 function to rearrangement of the actin cytoskeleton. These authors, who used the simian virus 40 (SV40)-transformed human lung fibroblast cell line VA-13, were able to separate the membrane ruffling event from bacterial invasion, with the former dependent on a WASP and WAVE connection with the Arp2/3 complex and the latter associated with the role of a new Arp2/3 complex activator named WASH (33). In this scenario, wild-type bacteria could potentially be stimulating the function of three different positive regulators of the Arp2/3 complex. Our study, based on the use of nontransformed fibroblasts derived from different sources, extends these observations to reveal that wild-type S. Typhimurium invades primary fibroblasts isolated from mouse intestine or immortalized foreskin human fibroblasts in the absence of prominent membrane ruffling and macropinocytosis (Fig. 4 and data not shown). These moderate membrane alterations occurring in nontransformed fibroblasts in response to wild-type Salmonella could reflect the particular anatomy of these cells. Fibroblasts are normally “embedded” into the connective tissue, and this fact may impede these cells from undergoing rapid and intense changes in the membrane surface, such as those observed in S. Typhimurium-infected epithelial cells facing a luminal compartment (34, 35, 83), in response to invading bacteria.
Another aspect uncovered by our study was a mode of entry into fibroblasts that takes place independently of the SPI-1 TTSS system. This observation has precedents in previous studies performed in epithelial cells. An early study reported an SPI-1 TTSS-independent invasion route in Chinese hamster ovary (CHO) epithelial cells (42), and a recent work, based on the use of a three-dimensional organotypic model of human colonic epithelium, proved the dispensability of the SPI-1 TTSS system for invasion of epithelial cells (41). Other findings include the capacity of S. Typhimurium strains deficient in SPI-1 effector proteins to invade bovine small intestine explants (66) and the efficient translocation exhibited by an S. Typhimurium SPI-1-defective mutant in an M cell in vitro model (55). Altogether, these observations suggest that S. Typhimurium may use multiple mechanisms to induce internalization by epithelial cells. The data obtained here with nontransformed fibroblasts support the concept of varied modes of bacterial entry. This assumption is further sustained by the visualization of phenomena such as filopodia and lamellipodia induced by ΔSPI-1 mutant bacteria that are intimately apposed to the fibroblast surface (Fig. 2A and B). To our knowledge, this is the first report of host cell surface remodeling promoted during bacterial entry by an S. Typhimurium strain lacking the SPI-1 TTSS apparatus.
The analysis of the cytoskeletal proteins required for S. Typhimurium invasion of fibroblasts unequivocally showed a requirement of microtubules for bacterial entry, which was more apparent in the case of the ΔSPI-1 mutant. This finding was unexpected, given the dispensability of microtubules shown for the invasion of epithelial cells by this pathogen (20). On the other hand, microtubules and microtubule motors are used by some intracellular pathogens to invade epithelial cells and modulate the intracellular vacuolar niche (37, 82). Most of these pathogens also exploit actin cytoskeleton dynamics to promote entry (5, 82). Exceptions are certain strains of Campylobacter jejuni and Citrobacter freundii, which enter epithelial cells via a microtubule-dependent but actin-independent process (59). The fact that actin and microtubules are required for S. Typhimurium entry into fibroblasts suggests that in some cases the pathogen may trigger remodeling of both cytoskeleton networks, as Shigella flexneri does in epithelial cells (82). Another distinct trait found in our study was the requirement of kinases PI3K and MEK1 for S. Typhimurium invasion of fibroblasts even though these kinases are known to be dispensable for entry into epithelial cells (57, 72, 76, 78). Upon contact with the fibroblast, S. Typhimurium may then employ mechanisms similar to those described for Listeria monocytogenes (45), group A and group B streptococci (9, 65), C. jejuni (43), Helicobacter pylori (52), and Chlamydia pneumoniae (12), which subvert PI3K and MEK1 signaling subsequent to bacterial ligand-host surface receptor interactions. Further work is required to demonstrate the apparent capacity of S. Typhimurium to exploit ligand-receptor interactions for invasion. In this line, the diminished uptake of the E. coli motile strain MC1061 in fibroblasts treated with PI3K and MEK1 inhibitors suggests that fibroblasts may be equipped with a large variety of receptors capable of promoting cytoskeletal changes upon stimulation with different bacterial products, some of which are present even in nonpathogenic E. coli. The behavior in NRK-49F and BJ-5ta fibroblasts of the E. coli motile strain MC1061 compared to that of the S. Typhimurium ΔSPI-1 mutant has, however, some clear distinctions. Examples are the different invasion rates found in NRK-49F and BJ-5ta fibroblasts (Fig. 1A and 4C) and the limited alterations of the actin cytoskeleton associated with the uptake of the E. coli MC1061 bacteria (Fig. 2B). This evidence supports the idea of a mode of bacteria entry used by S. Typhimurium independent of the SPI-1 TTSS that may rely on a pathway partially similar to that stimulated by nonpathogenic E. coli. Based on the differences in entry displayed by these two strains in some fibroblasts (NRK-49F and BJ-5ta) but not in others (3T3-Swiss fibroblasts and MIF), we favor the hypothesis that distinct fibroblast receptors could be stimulated by surface structures of these two bacteria. These distinct ligand-receptor interactions, which may induce common targets, such as the kinases PI3K and/or MEK1, could ultimately dictate the extent to which the actin/microtubules are remodeled and the ultimate efficiency of the entry process. The assays performed with a mutant devoid of either the SiiE adhesin encoded in SPI-4 (31) or the flagella rule out any involvement of these surface structures. However, many S. enterica serovars are known to encode multiple fimbriae (44). Major S. enterica serovar Typhi fimbriae were recently shown to be dispensable for adhesion and invasion of cultured epithelial cells (4). Conversely, the S. Typhimurium fimbriae Lpf and Fim influence invasion in several cultured epithelial cell lines (3). FimH-dependent internalization of S. Typhimurium into dendritic cells has also recently been shown (32). Therefore, further investigations focused on a putative contribution of these structures to fibroblast invasion need to be performed. Experiments are currently in progress in our group to select for invasion-defective mutants in an ΔSPI-1 genetic background. It was also of interest in our study to determine whether the bacterial adhesion process directs the subsequent entry into the fibroblasts. Because the motile E. coli strain MC1061 was counted in relatively high numbers as cell-associated bacteria, it was possible that the entry could be just a “consequence” of the ability of the bacteria to adhere to the fibroblast surface and a rather unspecific “phagocytic” capacity of the fibroblast. The assays performed in the BJ-5ta cells with invA strains devoid of a functional SPI-1 TTSS system but expressing either the invasin (Inv) protein of Y. pseudotuberculosis or the afimbrial adhesin AFA-I ruled out such a correlation between adherence and invasion. Based on these observations, we favor the idea that the differential entry of the S. Typhimurium ΔSPI-1 and E. coli MC1061 strains in fibroblasts such as NRK-49F and BJ-5ta depends upon distinct bacterial-host receptor interactions. Interesting aspects to address in future investigations include the infection phase in which S. Typhimurium wild-type bacteria could invade host cells independently of the SPI-1 TTSS and the benefit for the pathogen of such a mode of entry. Equally appealing is the possibility that such an SPI-1 TTSS-independent entry mode occurs stochastically as part of the inherent heterogeneity of the bacterial population that colonizes the host tissues.
We lastly aimed to decipher the panel of GTPases of the Rho family targeted by S. Typhimurium to invade fibroblasts. The role of Rac1, Cdc2, RhoA, and RhoG was assessed in NRK-49F and BJ-5ta fibroblasts exhibiting a distinct intensity in both ruffling and macropinocytosis (Fig. 4). Our data suggest that the set of Rho GTPases subverted by S. Typhimurium to trigger internalization might vary depending on the type of fibroblast tested. Massive membrane ruffling and macropinocytosis (NRK-49F fibroblasts) correlated with engagement of Cdc42, Rac1, and, to a lesser extent, RhoA. In contrast, S. Typhimurium entry in telomerase-immortalized BJ-5ta fibroblasts displaying much less cytoskeleton reorganization occurred in the absence of Cdcd42, RhoA, and RhoG or with reduced Rac1 levels (Fig. 8). These results suggest that other yet-unknown GTPase(s) of the Rho family may direct entry of S. Typhimurium into these immortalized nontransformed fibroblasts. Other Rho GTPases such as Wrch-1, RhoD, and Rif are capable of modulating cytoskeleton dynamics and promoting filopodium formation (19). Rac2 and Rac3 have also been reported to induce lamellipodium formation, and RhoB and RhoC can promote stress fiber assembly (1). It is also possible that no Rho GTPase is absolutely essential for S. Typhimurium entry into these immortalized nontransformed BJ-5ta fibroblasts. This assumption would to some extent agree with results of an early study of Jones et al. showing that membrane ruffling induced by S. Typhimurium was independent of Ras, Rac1, and RhoA (49). However, these authors reported major membrane ruffling structures visible upon Salmonella invasion in both 3T3-Swiss fibroblasts and MDCK epithelial cells, a situation clearly different from that observed in immortalized BJ-5ta fibroblasts. Finally, our RNAi analysis unequivocally linked Cdc42 to the entry of S. Typhimurium into the human epithelial cell line Henle-407 (Fig. 9). We are uncertain about the explanation for this different outcome from the study of Patel and Galán, who claimed that RhoG, and not Cdc42, is targeted by S. Typhimurium to invade the same human epithelial cell line (60). The different method used to grow the bacteria may explain such a discrepancy. Regardless of this fact, the data support our main conclusion that a marked versatility in the types of Rho GTPases targeted by S. Typhimurium depends on the origin and type of nonphagocytic cells used. Subsequent studies based on the analysis of the phosphorylation status of these GTPases in the different fibroblasts examined here and in the absence or presence of bacteria should reinforce the conclusions derived from RNAi data. Our findings also open new questions regarding the bacterial effectors and mechanisms sustaining the entry of wild-type bacteria into fibroblasts in the absence of prominent membrane ruffling and macropinocytosis. Thus, it would be of interest to address in future studies whether this mode of entry requires such key activators of the Arp2/3-complex as N-WASP and WAVE. Considering the data of Hanisch et al. in the VA-13 transformed fibroblasts (33), it is tempting to postulate that N-WASP and WAVE of immortalized BJ-5ta cells may not be targeted by wild-type bacteria during the invasion process as they are in epithelial cells. Further work directed to analyze these aspects should provide new insights into the heterogeneity of S. Typhimurium invasion routes uncovered in fibroblasts. Given the striking differences observed, we favor future studies with primary normal (nontransformed) cells that do not display limited passage capability such as by the telomerase-immortalized human foreskin fibroblasts described here. It would be of interest to develop similar models with epithelial cells since, up to now, most of the studies have been performed with immortal cancer cells derived from malignant tumors.
Acknowledgments
We are grateful to Jorge Galán and Michael Hensel for sending strains. We also thank Diana Barroso and M. Laura Navarro for their technical assistance.
This work was supported by grants BIO2007-67457-C02-01, GEN2006-27776-C2-1-E/PAT, and CSD2008-00013-INTERMODS from the Spanish Ministry of Science and Innovation. A.A. was the recipient of a fellowship from the Government of the Basque Country. M.G.P. is a tenured professor of the Universidad Autónoma de Madrid.
Editor: F. C. Fang
Footnotes
Published ahead of print on 5 April 2010.
REFERENCES
- 1.Aspenstrom, P., A. Fransson, and J. Saras. 2004. Rho GTPases have diverse effects on the organization of the actin filament system. Biochem. J. 377:327-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bakowski, M. A., V. Braun, and J. H. Brumell. 2008. Salmonella-containing vacuoles: directing traffic and nesting to grow. Traffic 9:2022-2031. [DOI] [PubMed] [Google Scholar]
- 3.Baumler, A. J., R. M. Tsolis, and F. Heffron. 1996. Contribution of fimbrial operons to attachment to and invasion of epithelial cell lines by Salmonella typhimurium. Infect. Immun. 64:1862-1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bishop, A., D. House, T. Perkins, S. Baker, R. A. Kingsley, and G. Dougan. 2008. Interaction of Salmonella enterica serovar Typhi with cultured epithelial cells: roles of surface structures in adhesion and invasion. Microbiology 154:1914-1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biswas, D., K. Itoh, and C. Sasakawa. 2003. Role of microfilaments and microtubules in the invasion of INT-407 cells by Campylobacter jejuni. Microbiol. Immunol. 47:469-473. [DOI] [PubMed] [Google Scholar]
- 6.Bodnar, A. G., M. Ouellette, M. Frolkis, S. E. Holt, C. P. Chiu, G. B. Morin, C. B. Harley, J. W. Shay, S. Lichtsteiner, and W. E. Wright. 1998. Extension of life-span by introduction of telomerase into normal human cells. Science 279:349-352. [DOI] [PubMed] [Google Scholar]
- 7.Bogdan, C., N. Donhauser, R. Doring, M. Rollinghoff, A. Diefenbach, and M. G. Rittig. 2000. Fibroblasts as host cells in latent leishmaniosis. J. Exp. Med. 191:2121-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown, M. D., L. Bry, Z. Li, and D. B. Sacks. 2007. IQGAP1 regulates Salmonella invasion through interactions with actin, Rac1, and Cdc42. J. Biol. Chem. 282:30265-30272. [DOI] [PubMed] [Google Scholar]
- 9.Burnham, C. A., S. E. Shokoples, and G. J. Tyrrell. 2007. Invasion of HeLa cells by group B streptococcus requires the phosphoinositide-3-kinase signalling pathway and modulates phosphorylation of host-cell Akt and glycogen synthase kinase-3. Microbiology 153:4240-4252. [DOI] [PubMed] [Google Scholar]
- 10.Cano, D. A., M. Martinez-Moya, M. G. Pucciarelli, E. A. Groisman, J. Casadesus, and F. Garcia-Del Portillo. 2001. Salmonella enterica serovar Typhimurium response involved in attenuation of pathogen intracellular proliferation. Infect. Immun. 69:6463-6474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen, L. M., S. Hobbie, and J. E. Galan. 1996. Requirement of CDC42 for Salmonella-induced cytoskeletal and nuclear responses. Science 274:2115-2118. [DOI] [PubMed] [Google Scholar]
- 12.Coombes, B. K., and J. B. Mahony. 2002. Identification of MEK- and phosphoinositide 3-kinase-dependent signalling as essential events during Chlamydia pneumoniae invasion of HEp2 cells. Cell Microbiol. 4:447-460. [DOI] [PubMed] [Google Scholar]
- 13.Cossart, P., and P. J. Sansonetti. 2004. Bacterial invasion: the paradigms of enteroinvasive pathogens. Science 304:242-248. [DOI] [PubMed] [Google Scholar]
- 14.Criss, A. K., D. M. Ahlgren, T. S. Jou, B. A. McCormick, and J. E. Casanova. 2001. The GTPase Rac1 selectively regulates Salmonella invasion at the apical plasma membrane of polarized epithelial cells. J. Cell Sci. 114:1331-1341. [DOI] [PubMed] [Google Scholar]
- 15.Criss, A. K., and J. E. Casanova. 2003. Coordinate regulation of Salmonella enterica serovar Typhimurium invasion of epithelial cells by the Arp2/3 complex and Rho GTPases. Infect. Immun. 71:2885-2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dai, S., Y. Zhang, T. Weimbs, M. B. Yaffe, and D. Zhou. 2007. Bacteria-generated PtdIns(3)P recruits VAMP8 to facilitate phagocytosis. Traffic 8:1365-1374. [DOI] [PubMed] [Google Scholar]
- 17.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Durfee, T., R. Nelson, S. Baldwin, G. Plunkett, 3rd, V. Burland, B. Mau, J. F. Petrosino, X. Qin, D. M. Muzny, M. Ayele, R. A. Gibbs, B. Csorgo, G. Posfai, G. M. Weinstock, and F. R. Blattner. 2008. The complete genome sequence of Escherichia coli DH10B: insights into the biology of a laboratory workhorse. J. Bacteriol. 190:2597-2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faix, J., D. Breitsprecher, T. E. Stradal, and K. Rottner. 2009. Filopodia: complex models for simple rods. Int. J. Biochem. Cell Biol. 41:1656-1664. [DOI] [PubMed] [Google Scholar]
- 20.Finlay, B. B., S. Ruschkowski, and S. Dedhar. 1991. Cytoskeletal rearrangements accompanying I entry into epithelial cells. J. Cell Sci. 99:283-296. [DOI] [PubMed] [Google Scholar]
- 21.Fu, Y., and J. E. Galan. 1999. A Salmonella protein antagonizes Rac-1 and Cdc42 to mediate host-cell recovery after bacterial invasion. Nature 401:293-297. [DOI] [PubMed] [Google Scholar]
- 22.Galán, J. E. 2001. Salmonella interactions with host cells: type III secretion at work. Annu. Rev. Cell Dev. Biol. 17:53-86. [DOI] [PubMed] [Google Scholar]
- 23.Galán, J. E., and H. Wolf-Watz. 2006. Protein delivery into eukaryotic cells by type III secretion machines. Nature 444:567-573. [DOI] [PubMed] [Google Scholar]
- 24.Galán, J. E., and D. Zhou. 2000. Striking a balance: modulation of the actin cytoskeleton by Salmonella. Proc. Natl. Acad. Sci. U. S. A. 97:8754-8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.García-del Portillo, F., and B. B. Finlay. 1994. Salmonella invasion of nonphagocytic cells induces formation of macropinosomes in the host cell. Infect. Immun. 62:4641-4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.García-del Portillo, F., and B. B. Finlay. 1995. Targeting of Salmonella typhimurium to vesicles containing lysosomal membrane glycoproteins bypasses compartments with mannose 6-phosphate receptors. J. Cell Biol. 129:81-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.García-del Portillo, F., C. Nunez-Hernandez, B. Eisman, and J. Ramos-Vivas. 2008. Growth control in the Salmonella-containing vacuole. Curr. Opin. Microbiol. 11:46-52. [DOI] [PubMed] [Google Scholar]
- 28.García-del Portillo, F., M. G. Pucciarelli, W. A. Jefferies, and B. B. Finlay. 1994. Salmonella typhimurium induces selective aggregation and internalization of host cell surface proteins during invasion of epithelial cells. J. Cell Sci. 107:2005-2020. [DOI] [PubMed] [Google Scholar]
- 29.Gerlach, R. G., N. Claudio, M. Rohde, D. Jackel, C. Wagner, and M. Hensel. 2008. Cooperation of Salmonella pathogenicity islands 1 and 4 is required to breach epithelial barriers. Cell. Microbiol. 10:2364-2376. [DOI] [PubMed] [Google Scholar]
- 30.Gerlach, R. G., D. Jackel, N. Geymeier, and M. Hensel. 2007. Salmonella pathogenicity island 4-mediated adhesion is coregulated with invasion genes in Salmonella enterica. Infect. Immun. 75:4697-4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gerlach, R. G., D. Jackel, B. Stecher, C. Wagner, A. Lupas, W. D. Hardt, and M. Hensel. 2007. Salmonella pathogenicity island 4 encodes a giant non-fimbrial adhesin and the cognate type 1 secretion system. Cell. Microbiol. 9:1834-1850. [DOI] [PubMed] [Google Scholar]
- 32.Guo, A., M. A. Lasaro, J. C. Sirard, J. P. Kraehenbuhl, and D. M. Schifferli. 2007. Adhesin-dependent binding and uptake of Salmonella enterica serovar Typhimurium by dendritic cells. Microbiology 153:1059-1069. [DOI] [PubMed] [Google Scholar]
- 33.Hanisch, J., J. Ehinger, M. Ladwein, M. Rohde, E. Derivery, T. Bosse, A. Steffen, D. Bumann, B. Misselwitz, W. D. Hardt, A. Gautreau, T. E. Stradal, and K. Rottner. 2010. Molecular dissection of Salmonella-induced membrane ruffling versus invasion. Cell. Microbiol. 12:84-98. [DOI] [PubMed] [Google Scholar]
- 34.Hapfelmeier, S., and W. D. Hardt. 2005. A mouse model for S. typhimurium-induced enterocolitis. Trends Microbiol. 13:497-503. [DOI] [PubMed] [Google Scholar]
- 35.Haraga, A., M. B. Ohlson, and S. I. Miller. 2008. Salmonellae interplay with host cells. Nat. Rev. Microbiol. 6:53-66. [DOI] [PubMed] [Google Scholar]
- 36.Hardt, W. D., L. M. Chen, K. E. Schuebel, X. R. Bustelo, and J. E. Galan. 1998. S. typhimurium encodes an activator of Rho GTPases that induces membrane ruffling and nuclear responses in host cells. Cell 93:815-826. [DOI] [PubMed] [Google Scholar]
- 37.Henry, T., J. P. Gorvel, and S. Meresse. 2006. Molecular motors hijacking by intracellular pathogens. Cell. Microbiol. 8:23-32. [DOI] [PubMed] [Google Scholar]
- 38.Hernandez, L. D., K. Hueffer, M. R. Wenk, and J. E. Galan. 2004. Salmonella modulates vesicular traffic by altering phosphoinositide metabolism. Science 304:1805-1807. [DOI] [PubMed] [Google Scholar]
- 39.Higashide, W., S. Dai, V. P. Hombs, and D. Zhou. 2002. Involvement of SipA in modulating actin dynamics during Salmonella invasion into cultured epithelial cells. Cell. Microbiol. 4:357-365. [DOI] [PubMed] [Google Scholar]
- 40.Hoiseth, S. K., and B. A. Stocker. 1981. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature 291:238-239. [DOI] [PubMed] [Google Scholar]
- 41.Honer zu Bentrup, K., R. Ramamurthy, C. M. Ott, K. Emami, M. Nelman-Gonzalez, J. W. Wilson, E. G. Richter, T. J. Goodwin, J. S. Alexander, D. L. Pierson, N. Pellis, K. L. Buchanan, and C. A. Nickerson. 2006. Three-dimensional organotypic models of human colonic epithelium to study the early stages of enteric salmonellosis. Microbes Infect. 8:1813-1825. [DOI] [PubMed] [Google Scholar]
- 42.Hong, K. H., and V. L. Miller. 1998. Identification of a novel Salmonella invasion locus homologous to Shigella ipgDE. J. Bacteriol. 180:1793-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hu, L., J. P. McDaniel, and D. J. Kopecko. 2006. Signal transduction events involved in human epithelial cell invasion by Campylobacter jejuni 81-176. Microb. Pathog. 40:91-100. [DOI] [PubMed] [Google Scholar]
- 44.Humphries, A., S. Deridder, and A. J. Baumler. 2005. Salmonella enterica serotype Typhimurium fimbrial proteins serve as antigens during infection of mice. Infect. Immun. 73:5329-5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ireton, K., B. Payrastre, H. Chap, W. Ogawa, H. Sakaue, M. Kasuga, and P. Cossart. 1996. A role for phosphoinositide 3-kinase in bacterial invasion. Science 274:780-782. [DOI] [PubMed] [Google Scholar]
- 46.Isberg, R. R., and S. Falkow. 1985. A single genetic locus encoded by Yersinia pseudotuberculosis permits invasion of cultured animal cells by Escherichia coli K-12. Nature 317:262-264. [DOI] [PubMed] [Google Scholar]
- 47.Jepson, M. A., and M. A. Clark. 2001. The role of M cells in Salmonella infection. Microbes Infect. 3:1183-1190. [DOI] [PubMed] [Google Scholar]
- 48.Jiang, X. R., G. Jimenez, E. Chang, M. Frolkis, B. Kusler, M. Sage, M. Beeche, A. G. Bodnar, G. M. Wahl, T. D. Tlsty, and C. P. Chiu. 1999. Telomerase expression in human somatic cells does not induce changes associated with a transformed phenotype. Nat. Genet. 21:111-114. [DOI] [PubMed] [Google Scholar]
- 49.Jones, B. D., H. F. Paterson, A. Hall, and S. Falkow. 1993. Salmonella typhimurium induces membrane ruffling by a growth factor-receptor-independent mechanism. Proc. Natl. Acad. Sci. U. S. A. 90:10390-10394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaniga, K., S. Tucker, D. Trollinger, and J. E. Galan. 1995. Homologs of the Shigella IpaB and IpaC invasins are required for Salmonella typhimurium entry into cultured epithelial cells. J. Bacteriol. 177:3965-3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kiss, T., E. Morgan, and G. Nagy. 2007. Contribution of SPI-4 genes to the virulence of Salmonella enterica. FEMS Microbiol. Lett. 275:153-159. [DOI] [PubMed] [Google Scholar]
- 52.Kwok, T., S. Backert, H. Schwarz, J. Berger, and T. F. Meyer. 2002. Specific entry of Helicobacter pylori into cultured gastric epithelial cells via a zipper-like mechanism. Infect. Immun. 70:2108-2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Labigne-Roussel, A. F., D. Lark, G. Schoolnik, and S. Falkow. 1984. Cloning and expression of an afimbrial adhesin (AFA-I) responsible for P blood group-independent, mannose-resistant hemagglutination from a pyelonephritic Escherichia coli strain. Infect. Immun. 46:251-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Main-Hester, K. L., K. M. Colpitts, G. A. Thomas, F. C. Fang, and S. J. Libby. 2008. Coordinate regulation of Salmonella pathogenicity island 1 (SPI1) and SPI4 in Salmonella enterica serovar Typhimurium. Infect. Immun. 76:1024-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martinez-Argudo, I., and M. A. Jepson. 2008. Salmonella translocates across an in vitro M cell model independently of SPI-1 and SPI-2. Microbiology 154:3887-3894. [DOI] [PubMed] [Google Scholar]
- 56.McGhie, E. J., R. D. Hayward, and V. Koronakis. 2001. Cooperation between actin-binding proteins of invasive Salmonella: SipA potentiates SipC nucleation and bundling of actin. EMBO J. 20:2131-2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mecsas, J., B. Raupach, and S. Falkow. 1998. The Yersinia Yops inhibit invasion of Listeria, Shigella and Edwardsiella but not Salmonella into epithelial cells. Mol. Microbiol. 28:1269-1281. [DOI] [PubMed] [Google Scholar]
- 58.Morgan, E., A. J. Bowen, S. C. Carnell, T. S. Wallis, and M. P. Stevens. 2007. SiiE is secreted by the Salmonella enterica serovar Typhimurium pathogenicity island 4-encoded secretion system and contributes to intestinal colonization in cattle. Infect. Immun. 75:1524-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oelschlaeger, T. A., P. Guerry, and D. J. Kopecko. 1993. Unusual microtubule-dependent endocytosis mechanisms triggered by Campylobacter jejuni and Citrobacter freundii. Proc. Natl. Acad. Sci. U. S. A. 90:6884-6888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Patel, J. C., and J. E. Galan. 2006. Differential activation and function of Rho GTPases during Salmonella-host cell interactions. J. Cell Biol. 175:453-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Patel, J. C., and J. E. Galan. 2005. Manipulation of the host actin cytoskeleton by Salmonella-all in the name of entry. Curr. Opin. Microbiol. 8:10-15. [DOI] [PubMed] [Google Scholar]
- 62.Patel, J. C., O. W. Rossanese, and J. E. Galan. 2005. The functional interface between Salmonella and its host cell: opportunities for therapeutic intervention. Trends Pharmacol. Sci. 26:564-570. [DOI] [PubMed] [Google Scholar]
- 63.Pizarro-Cerda, J., and P. Cossart. 2006. Bacterial adhesion and entry into host cells. Cell 124:715-727. [DOI] [PubMed] [Google Scholar]
- 64.Pucciarelli, M. G., and F. García-del Portillo. 2003. Protein-peptidoglycan interactions modulate the assembly of the needle complex in the Salmonella invasion-associated type III secretion system. Mol. Microbiol. 48:573-585. [DOI] [PubMed] [Google Scholar]
- 65.Purushothaman, S. S., B. Wang, and P. P. Cleary. 2003. M1 protein triggers a phosphoinositide cascade for group A Streptococcus invasion of epithelial cells. Infect. Immun. 71:5823-5830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Raffatellu, M., R. P. Wilson, D. Chessa, H. Andrews-Polymenis, Q. T. Tran, S. Lawhon, S. Khare, L. G. Adams, and A. J. Baumler. 2005. SipA, SopA, SopB, SopD, and SopE2 contribute to Salmonella enterica serotype Typhimurium invasion of epithelial cells. Infect. Immun. 73:146-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schlumberger, M. C., and W. D. Hardt. 2006. Salmonella type III secretion effectors: pulling the host cell's strings. Curr. Opin. Microbiol. 9:46-54. [DOI] [PubMed] [Google Scholar]
- 68.Shi, J., and J. E. Casanova. 2006. Invasion of host cells by Salmonella typhimurium requires focal adhesion kinase and p130Cas. Mol. Biol. Cell 17:4698-4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shi, J., G. Scita, and J. E. Casanova. 2005. WAVE2 signaling mediates invasion of polarized epithelial cells by Salmonella typhimurium. J. Biol. Chem. 280:29849-29855. [DOI] [PubMed] [Google Scholar]
- 70.Sinzger, C., M. Digel, and G. Jahn. 2008. Cytomegalovirus cell tropism. Curr. Top. Microbiol. Immunol. 325:63-83. [DOI] [PubMed] [Google Scholar]
- 71.Steele-Mortimer, O. 2008. The Salmonella-containing vacuole: moving with the times. Curr. Opin. Microbiol. 11:38-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Steele-Mortimer, O., J. H. Brumell, L. A. Knodler, S. Meresse, A. Lopez, and B. B. Finlay. 2002. The invasion-associated type III secretion system of Salmonella enterica serovar Typhimurium is necessary for intracellular proliferation and vacuole biogenesis in epithelial cells. Cell. Microbiol. 4:43-54. [DOI] [PubMed] [Google Scholar]
- 73.Steinert, S., J. W. Shay, and W. E. Wright. 2000. Transient expression of human telomerase extends the life span of normal human fibroblasts. Biochem. Biophys. Res. Commun. 273:1095-1098. [DOI] [PubMed] [Google Scholar]
- 74.Stender, S., A. Friebel, S. Linder, M. Rohde, S. Mirold, and W. D. Hardt. 2000. Identification of SopE2 from Salmonella typhimurium, a conserved guanine nucleotide exchange factor for Cdc42 of the host cell. Mol. Microbiol. 36:1206-1221. [DOI] [PubMed] [Google Scholar]
- 75.Strong, S. A., T. T. Pizarro, J. S. Klein, F. Cominelli, and C. Fiocchi. 1998. Proinflammatory cytokines differentially modulate their own expression in human intestinal mucosal mesenchymal cells. Gastroenterology 114:1244-1256. [DOI] [PubMed] [Google Scholar]
- 76.Tafazoli, F., K. E. Magnusson, and L. Zheng. 2003. Disruption of epithelial barrier integrity by Salmonella enterica serovar Typhimurium requires geranylgeranylated proteins. Infect. Immun. 71:872-881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tam, M. A., A. Rydstrom, M. Sundquist, and M. J. Wick. 2008. Early cellular responses to Salmonella infection: dendritic cells, monocytes, and more. Immunol. Rev. 225:140-162. [DOI] [PubMed] [Google Scholar]
- 78.Tang, P., C. L. Sutherland, M. R. Gold, and B. B. Finlay. 1998. Listeria monocytogenes invasion of epithelial cells requires the MEK-1/ERK-2 mitogen-activated protein kinase pathway. Infect. Immun. 66:1106-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tierrez, A., and F. García-del Portillo. 2005. New concepts in Salmonella virulence: the importance of reducing the intracellular growth rate in the host. Cell. Microbiol. 7:901-909. [DOI] [PubMed] [Google Scholar]
- 80.Unsworth, K. E., M. Way, M. McNiven, L. Machesky, and D. W. Holden. 2004. Analysis of the mechanisms of Salmonella-induced actin assembly during invasion of host cells and intracellular replication. Cell. Microbiol. 6:1041-1055. [DOI] [PubMed] [Google Scholar]
- 81.Vijay-Kumar, M., H. Wu, R. Jones, G. Grant, B. Babbin, T. P. King, D. Kelly, A. T. Gewirtz, and A. S. Neish. 2006. Flagellin suppresses epithelial apoptosis and limits disease during enteric infection. Am. J. Pathol. 169:1686-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yoshida, S., and C. Sasakawa. 2003. Exploiting host microtubule dynamics: a new aspect of bacterial invasion. Trends Microbiol. 11:139-143. [DOI] [PubMed] [Google Scholar]
- 83.Zhang, S., R. A. Kingsley, R. L. Santos, H. Andrews-Polymenis, M. Raffatellu, J. Figueiredo, J. Nunes, R. M. Tsolis, L. G. Adams, and A. J. Baumler. 2003. Molecular pathogenesis of Salmonella enterica serotype Typhimurium-induced diarrhea. Infect. Immun. 71:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhou, D., L. M. Chen, L. Hernandez, S. B. Shears, and J. E. Galan. 2001. A Salmonella inositol polyphosphatase acts in conjunction with other bacterial effectors to promote host cell actin cytoskeleton rearrangements and bacterial internalization. Mol. Microbiol. 39:248-259. [DOI] [PubMed] [Google Scholar]
- 85.Zhou, D., M. S. Mooseker, and J. E. Galan. 1999. An invasion-associated Salmonella protein modulates the actin-bundling activity of plastin. Proc. Natl. Acad. Sci. U. S. A. 96:10176-10181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhou, D., M. S. Mooseker, and J. E. Galan. 1999. Role of the S. typhimurium actin-binding protein SipA in bacterial internalization. Science 283:2092-2095. [DOI] [PubMed] [Google Scholar]